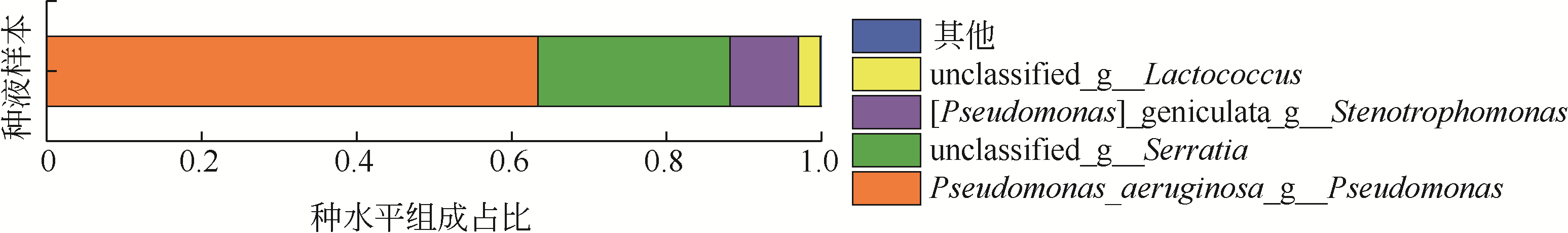化工进展 ›› 2021, Vol. 40 ›› Issue (5): 2882-2892.DOI: 10.16085/j.issn.1000-6613.2020-1258
固定化生物吸附剂对Cd(Ⅱ)的去除性能及机理
余关龙1,2,3( ), 彭海渊1,2, 王世涛1,2, 汪国梁1,2, 陈宏1,3, 杜春艳1,3, 刘媛媛1, 孙士权1,3(
), 彭海渊1,2, 王世涛1,2, 汪国梁1,2, 陈宏1,3, 杜春艳1,3, 刘媛媛1, 孙士权1,3( ), 禹丽娥1,3, 王建武1,3
), 禹丽娥1,3, 王建武1,3
- 1.长沙理工大学水利工程学院,湖南 长沙 410114
2.湖南省环境保护河湖污染控制工程技术中心,湖南 长沙 410114
3.洞庭湖水环境治理与生态修复湖南省重点实验室,湖南 长沙 410114
-
收稿日期:2020-07-06出版日期:2021-05-06发布日期:2021-05-24 -
通讯作者:孙士权 -
作者简介:余关龙(1978—),男,博士,研究方向为水污染控制及水环境修复。E-mail:ygl079@csust.edu.cn 。 -
基金资助:国家自然科学基金(51308069);湖南省教育厅重点项目(19A032);长沙理工大学助推项目(2019QJCZ026)
Performance and mechanism of immobilized biological adsorbent for Cd(
YU Guanlong1,2,3( ), PENG Haiyuan1,2, WANG Shitao1,2, WANG Guoliang1,2, CHEN Hong1,3, DU Chunyan1,3, LIU Yuanyuan1, SUN Shiquan1,3(
), PENG Haiyuan1,2, WANG Shitao1,2, WANG Guoliang1,2, CHEN Hong1,3, DU Chunyan1,3, LIU Yuanyuan1, SUN Shiquan1,3( ), YU Li’e1,3, WANG Jianwu1,3
), YU Li’e1,3, WANG Jianwu1,3
- 1.School of Hydraulic Engineering, Changsha University of Science and Technology, Changsha 410114, Hunan, China
2.Engineering and Technical Center of Hunan Provincial Environmental Protection for River-Lake Dredging Pollution Control, Changsha 410114, Hunan, China
3.Key Laboratory of Dongting Lake Aquatic Eco-Environmental Control and Restoration of Hunan Province, Changsha 410114, Hunan, China
-
Received:2020-07-06Online:2021-05-06Published:2021-05-24 -
Contact:SUN Shiquan
摘要:
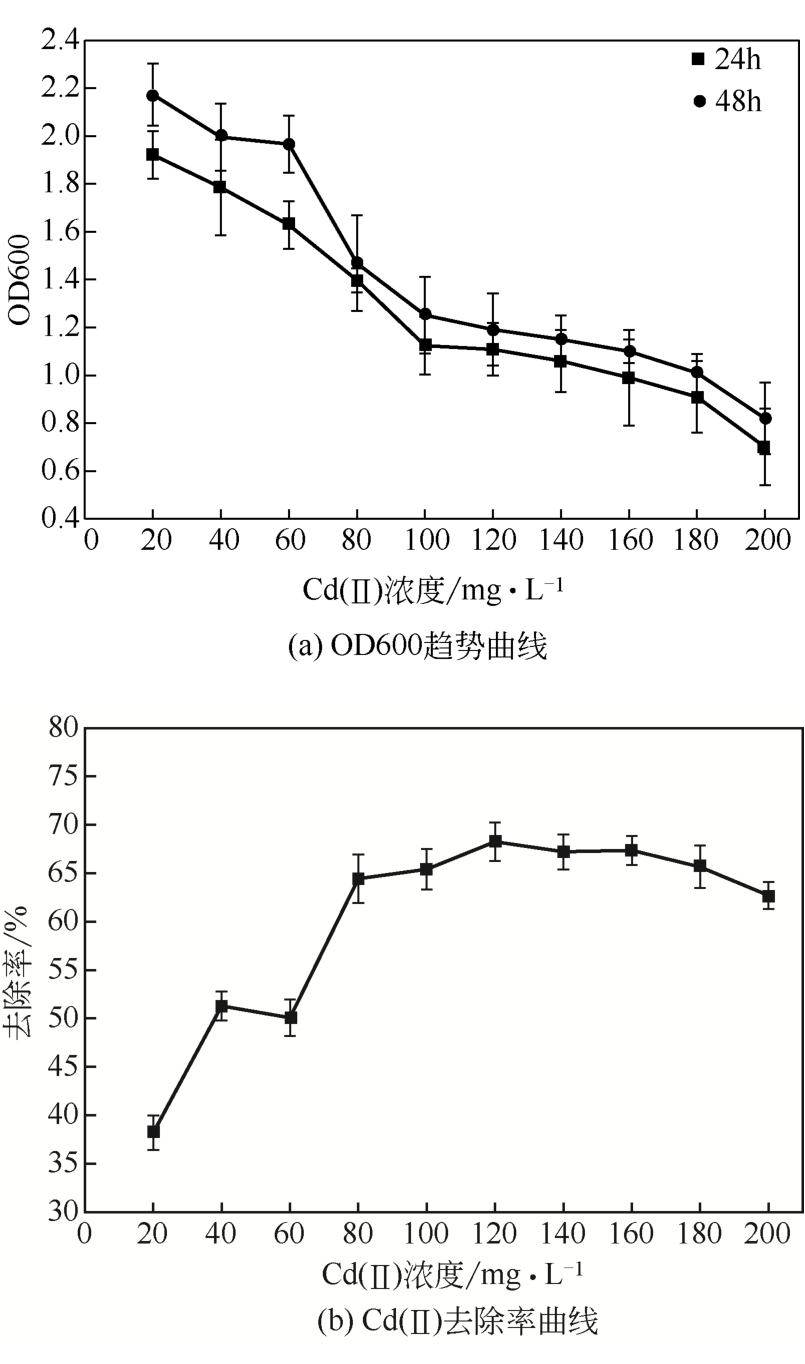
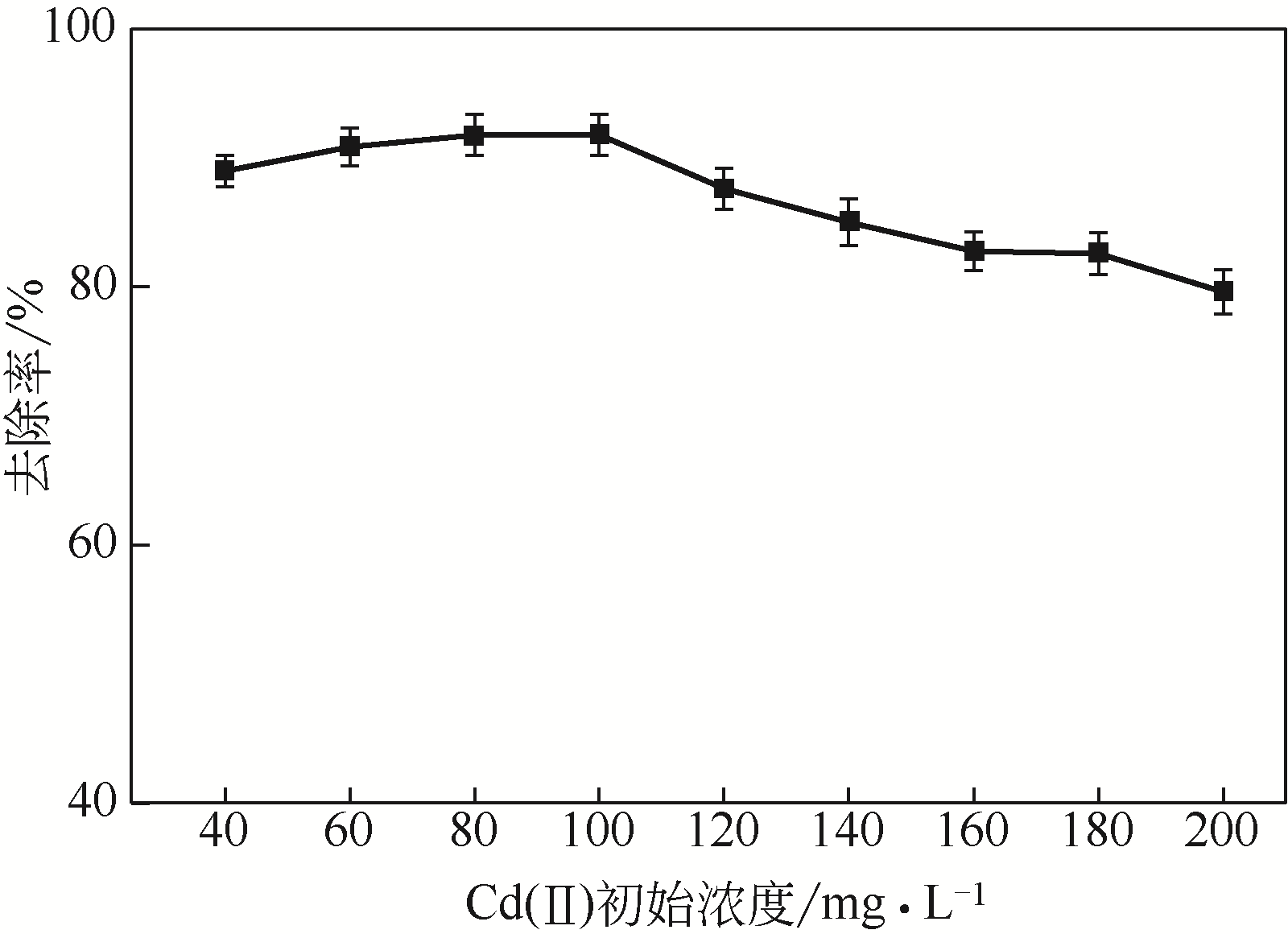
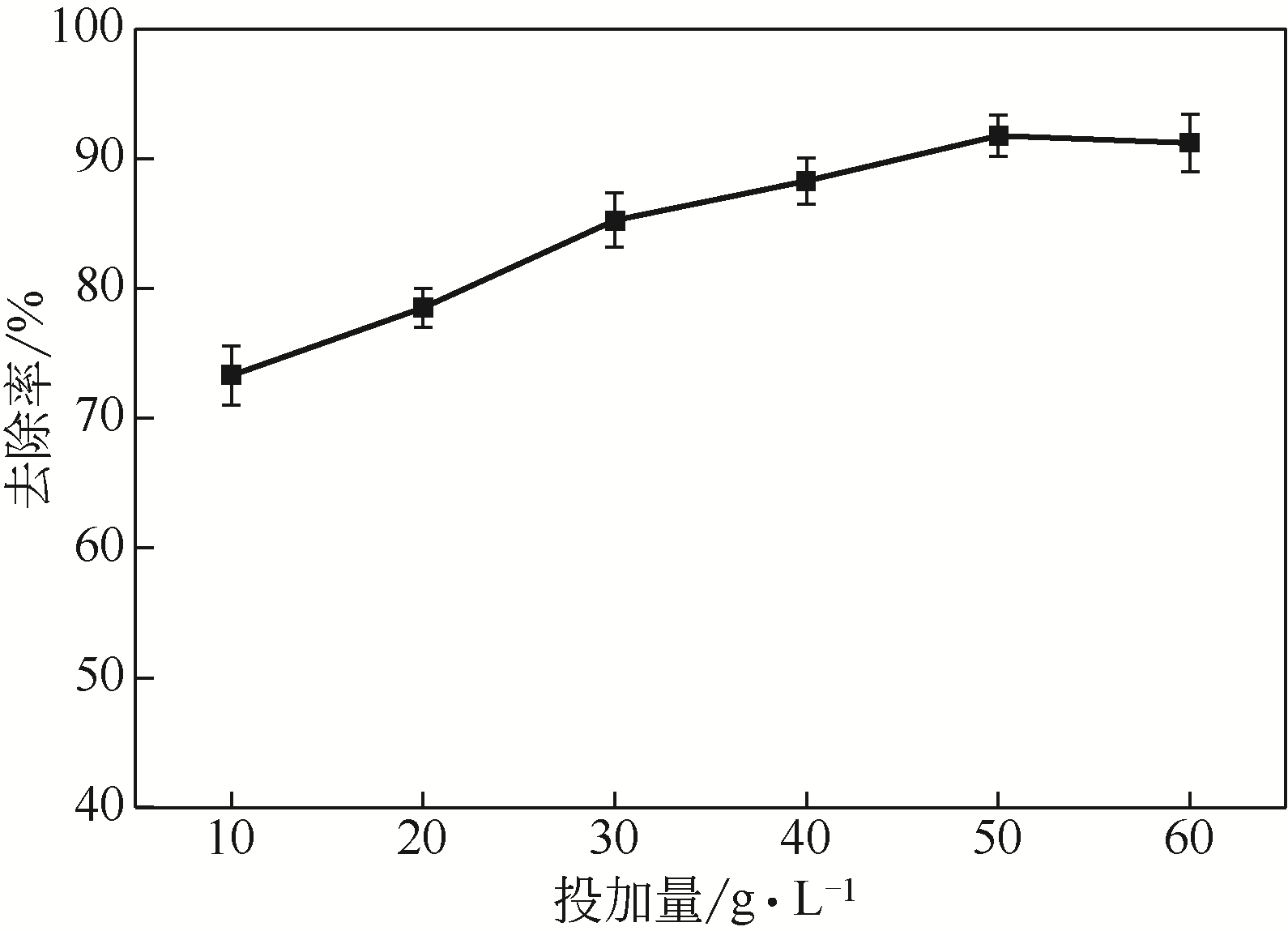
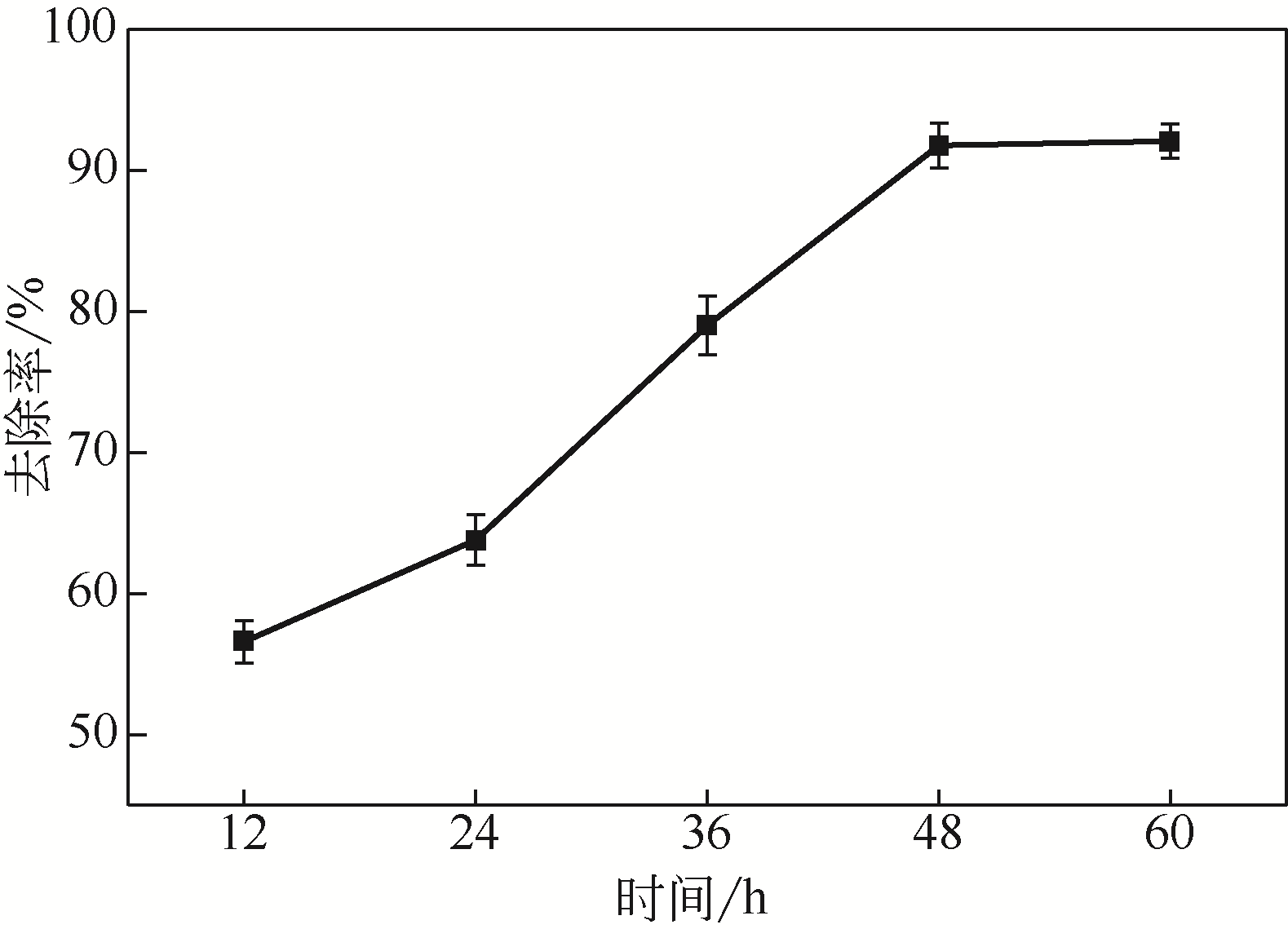
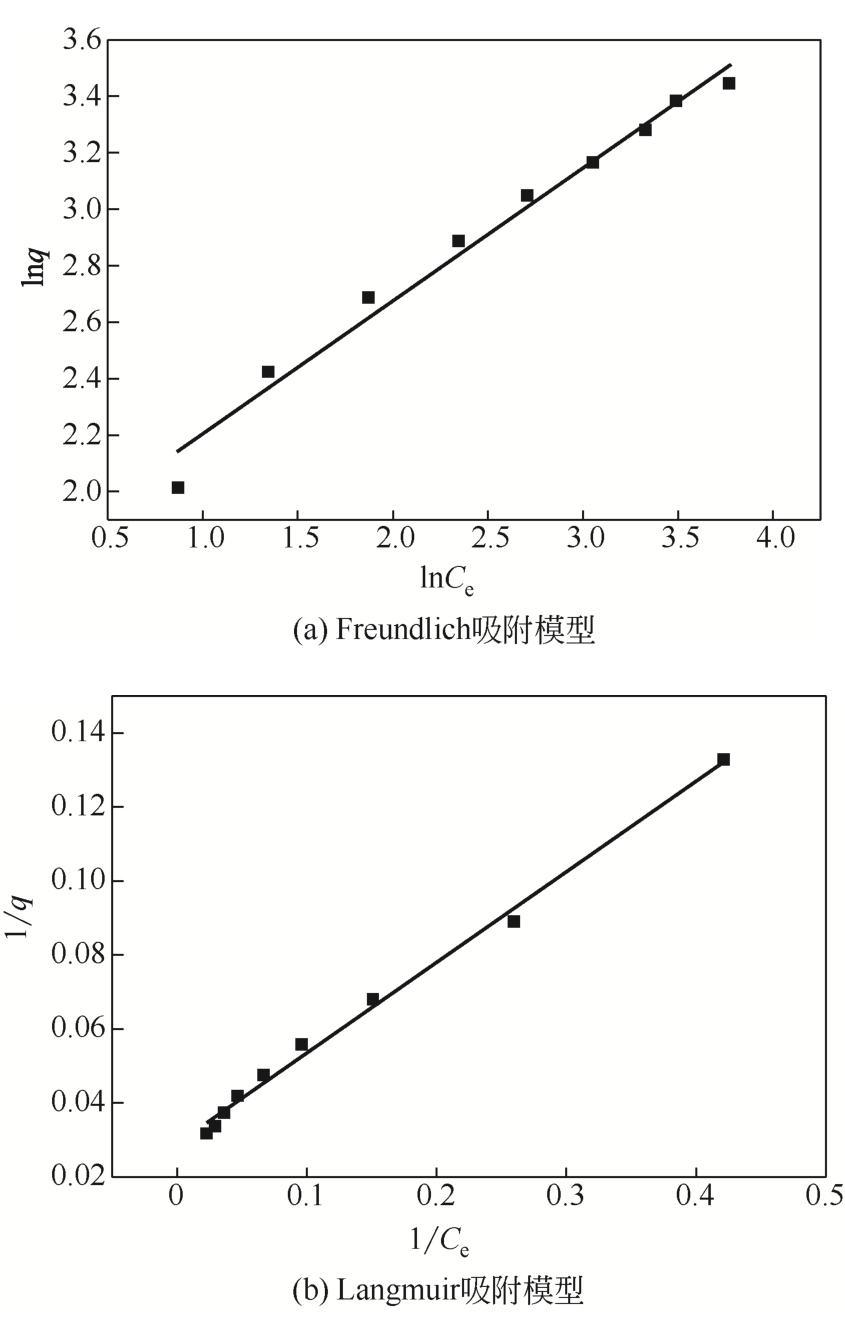
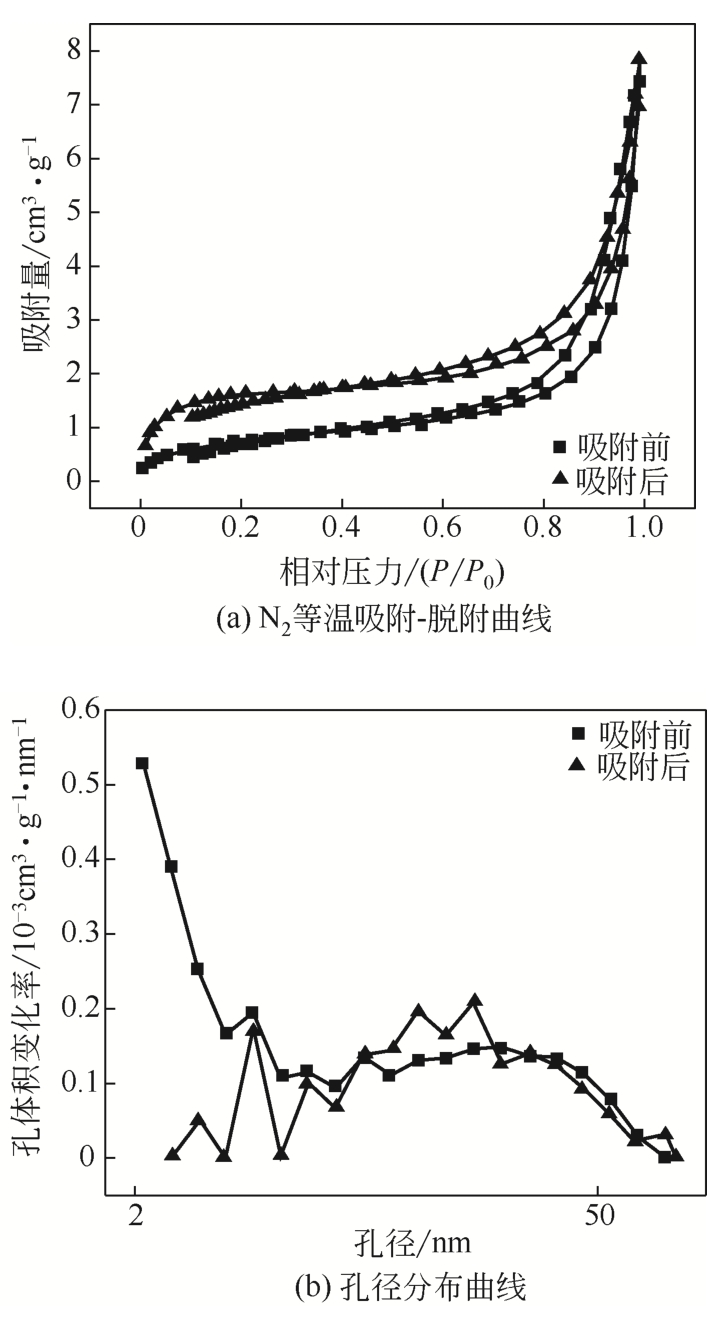
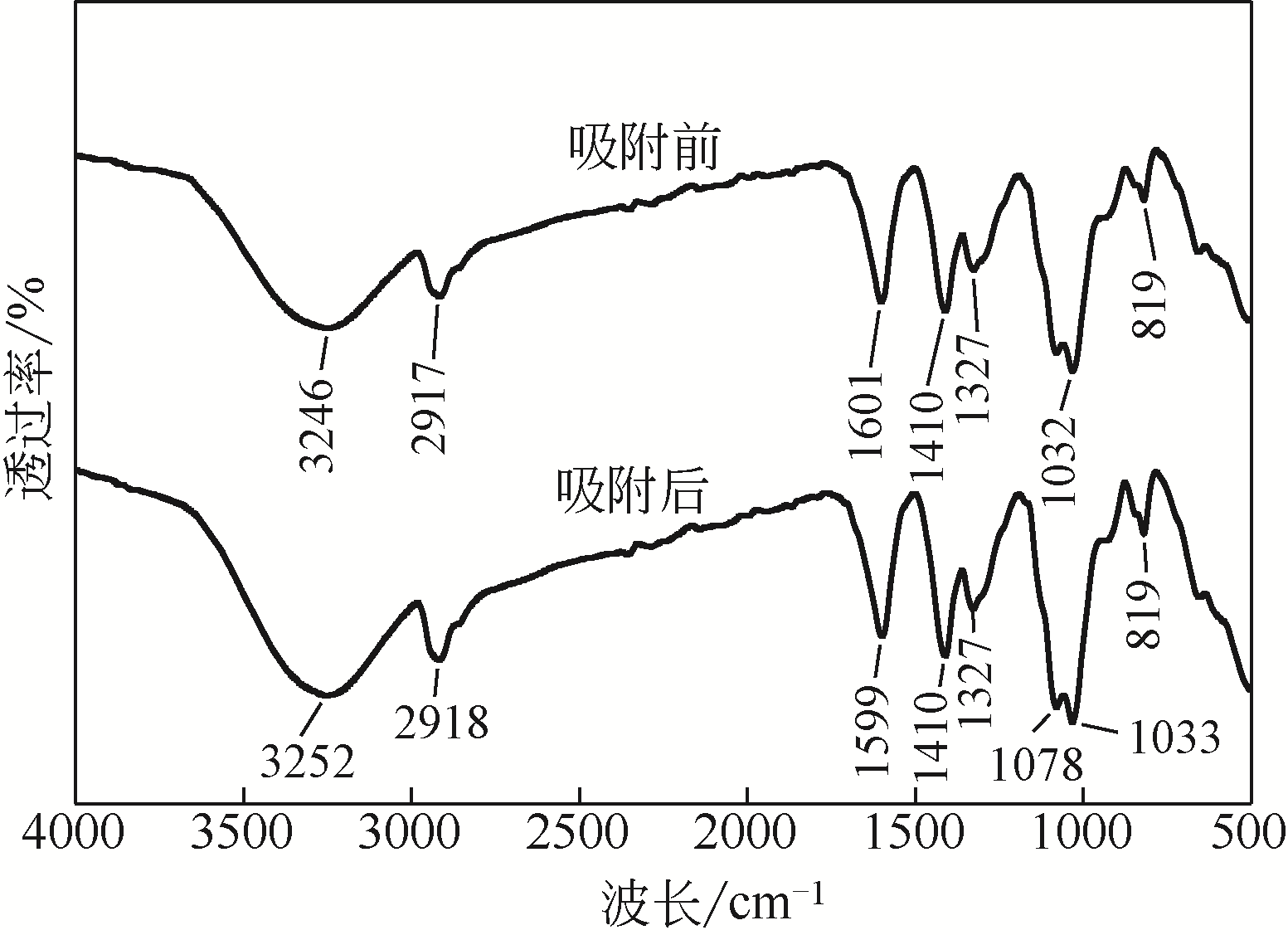
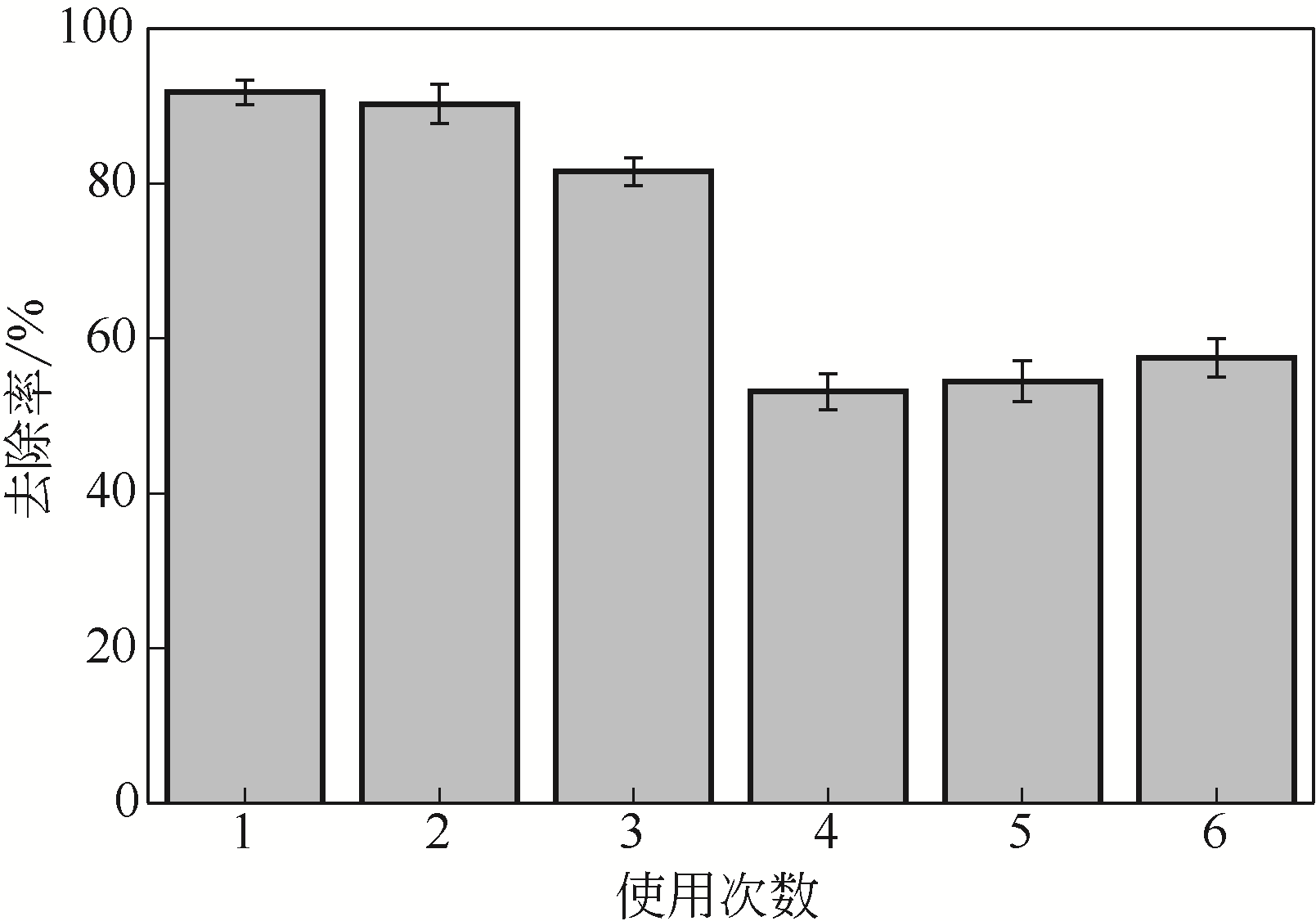
从处理含Cd(Ⅱ)废水的人工湿地基质层中提取微生物,经过重金属浓度梯度筛选后,通过聚合酶链式反应(PCR)技术分析发现筛选后的菌群对Cd(Ⅱ)有较好的耐受性和吸附能力,菌种丰度依次为Lactococcus< Stenotrophomonas< Serratia< Pseudomona。将筛选后的微生物用包埋固定化技术制成固定化生物吸附剂,在pH=4~5、吸附时间48h、吸附剂用量(湿重)50g/L、Cd(Ⅱ)初始浓度100mg/L时,对Cd(Ⅱ)的最大去除率可达91%±2%。通过吸附平衡研究发现吸附过程符合准一级动力学模型和Langmuir模型,对Cd(Ⅱ)的最大单分子层吸附量为34.4mg/g。BET分析结果显示,固定化生物吸附剂具有介孔结构且比表面积大,有利于吸附作用的进行;傅里叶变换衰减全反射红外光谱法(FTIR-ATR)分析结果说明固定化生物吸附剂具有丰富的重金属结合点 位,—COOH、—OH、—NH和—CH基团参与了Cd(Ⅱ)的吸附过程。固定化生物吸附剂重复使用3次能保持较好的吸附效果,显示出较高的经济实用性。水环境中常见阳离子对Cd(Ⅱ)竞争吸附影响顺序依次为Na+<K+<Ca2+。
中图分类号:
引用本文
余关龙, 彭海渊, 王世涛, 汪国梁, 陈宏, 杜春艳, 刘媛媛, 孙士权, 禹丽娥, 王建武. 固定化生物吸附剂对Cd(Ⅱ)的去除性能及机理[J]. 化工进展, 2021, 40(5): 2882-2892.
YU Guanlong, PENG Haiyuan, WANG Shitao, WANG Guoliang, CHEN Hong, DU Chunyan, LIU Yuanyuan, SUN Shiquan, YU Li’e, WANG Jianwu. Performance and mechanism of immobilized biological adsorbent for Cd(
| 测序区域 | 引物名称 | 引物序列 |
|---|---|---|
| 338F 806R | 338F | ACTCCTACGGGAGGCAGCAG |
| 806R | GGACTACHVGGGTWTCTAAT |
表1 PCR扩增引物设计
| 测序区域 | 引物名称 | 引物序列 |
|---|---|---|
| 338F 806R | 338F | ACTCCTACGGGAGGCAGCAG |
| 806R | GGACTACHVGGGTWTCTAAT |
| 培养液 | 亚细胞结构 | |||
|---|---|---|---|---|
| 细胞外膜 | 细胞壁 | 细胞内膜 | 细胞质 | |
| 24.8 | 22.6 | 18.0 | 32.0 | 2.58 |
表2 亚细胞结构的Cd(Ⅱ)吸附量(质量分数,%)
| 培养液 | 亚细胞结构 | |||
|---|---|---|---|---|
| 细胞外膜 | 细胞壁 | 细胞内膜 | 细胞质 | |
| 24.8 | 22.6 | 18.0 | 32.0 | 2.58 |
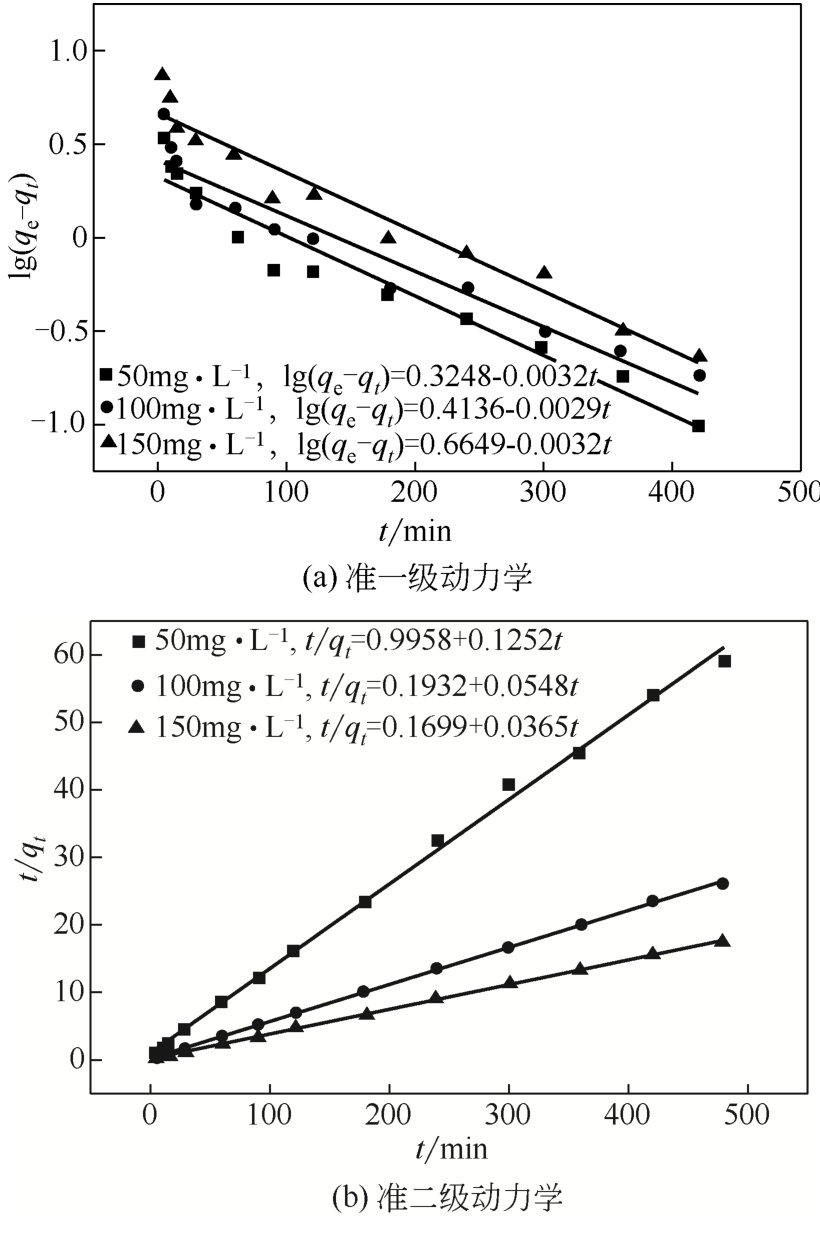
初始浓度 /mg·L-1 | 准一级动力学 | 准二级动力学 | |||
|---|---|---|---|---|---|
| K1 | R2 | K2 | R2 | ||
| 50 | 0.0073 | 0.9380 | 0.0152 | 0.9922 | |
| 100 | 0.0068 | 0.9223 | 0.0151 | 0.9995 | |
| 150 | 0.0060 | 0.9451 | 0.0078 | 0.9997 | |
表3 Cd(Ⅱ)动力学模型参数
初始浓度 /mg·L-1 | 准一级动力学 | 准二级动力学 | |||
|---|---|---|---|---|---|
| K1 | R2 | K2 | R2 | ||
| 50 | 0.0073 | 0.9380 | 0.0152 | 0.9922 | |
| 100 | 0.0068 | 0.9223 | 0.0151 | 0.9995 | |
| 150 | 0.0060 | 0.9451 | 0.0078 | 0.9997 | |
| Freundlich | Langmuir | |||||
|---|---|---|---|---|---|---|
| 1/n | kF | R2 | qmax | kL | R2 | |
| 0.47007 | 5.6624 | 0.9826 | 34.36 | 0.190 | 0.9945 | |
表4 等温吸附模型参数
| Freundlich | Langmuir | |||||
|---|---|---|---|---|---|---|
| 1/n | kF | R2 | qmax | kL | R2 | |
| 0.47007 | 5.6624 | 0.9826 | 34.36 | 0.190 | 0.9945 | |
| 样品 | 平均孔径/nm | 孔体积/cm3·g-1 | 比表面积/m2·g-1 |
|---|---|---|---|
| 吸附前 | 16.03 | 0.0114 | 2.853 |
| 吸附后 | 8.36 | 0.012 | 5.785 |
表5 材料孔结构数据
| 样品 | 平均孔径/nm | 孔体积/cm3·g-1 | 比表面积/m2·g-1 |
|---|---|---|---|
| 吸附前 | 16.03 | 0.0114 | 2.853 |
| 吸附后 | 8.36 | 0.012 | 5.785 |
| 1 | 刘江龙, 郭焱, 席艺慧. FeCl3和十六烷基三甲基溴化铵改性赤泥对水中铜离子的吸附性能和机理[J]. 化工进展, 2020, 39(2): 776-789. |
| LIU Jianglong, GUO Yan, XI Yihui. Adsorption and mechanism of copper ions in water by red mud modified with FeCl3 and hexadecyl trimethyl ammonium bromide (CTAB)[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 776-789. | |
| 2 | YIN K, WANG Q N, LV M, et al. Microorganism remediation strategies towards heavy metals[J]. Chemical Engineering Journal, 2019, 360: 1553-1563. |
| 3 | KHADIVINIA E, SHARAFI H, HADI F, et al. Cadmium biosorption by a glyphosate-degrading bacterium, a novel biosorbent isolated from pesticide-contaminated agricultural soils[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(6): 4304-4310. |
| 4 | 曹健华,刘凌沁,黄亚继,等. 原料种类和热解温度对生物炭吸附Cd2+的影响[J]. 化工进展, 2019, 38(9): 4183-4190. |
| CAO Jianhua, LIU Lingqin, HUANG Yaji. et al. Effects of feedstock type and pyrolysis temperature on Cd2+ adsorption by biochar[J]. Chemical Industry and Engineering Progress, 2019, 38(9): 4183-4190. | |
| 5 | 杨培,石先阳. 耐镉功能内生菌的筛选及其固定化处理含镉废水[J]. 生物学杂志, 2019, 36(5): 96-100, 103. |
| YANG Pei, SHI Xianyang. Screening of cadmium resistant endophytic bacteria and immobilized for treating cadmium of wastewater[J]. Journal of Biology, 2019, 36(5): 96-100, 103. | |
| 6 | 张慧,李宁,戴友芝. 重金属污染的生物修复技术[J]. 化工进展, 2004, 23(5): 562-565. |
| ZHANG Hui, LI Ning, DAI Youzhi. Research about the bioremediation of heavy metal pollution[J]. Chemical Industry and Engineering Progress, 2004, 23(5): 562-565. | |
| 7 | PENG W H, LI X M, SONG J X, et al. Bioremediation of cadmium- and zinc-contaminated soil using Rhodobacter sphaeroides[J]. Chemosphere, 2018, 197: 33-41. |
| 8 | LI J, LIU Y R, ZHANG L M, et al. Sorption mechanism and distribution of cadmium by different microbial species[J]. Journal of Environmental Management, 2019, 237: 552-559. |
| 9 | MA L L, CHEN N, FENG C P, et al. Feasibility and mechanism of microbial-phosphorus minerals-alginate immobilized particles in bioreduction of hexavalent chromium and synchronous removal of trivalent chromium[J]. Bioresource Technology, 2019, 294: 122213. |
| 10 | 王彩冬,黄兵,罗欢. 固定化微生物技术及其应用研究进展[J]. 云南化工, 2007, 34(4): 79-82, 91. |
| WANG Caidong, HUANG Bing, LUO Huan. Immobilized microbe technology and its application[J]. Yunnan Chemical Technology, 2007, 34(4): 79-82, 91. | |
| 11 | LI X, WU Y E, ZHANG C, et al. Immobilizing of heavy metals in sediments contaminated by nonferrous metals smelting plant sewage with sulfate reducing bacteria and micro zero valent iron[J]. Chemical Engineering Journal, 2016, 306: 393-400. |
| 12 | TODOROVA K, VELKOVA Z, STOYTCHEVA M, et al. Novel composite biosorbent from Bacillus cereus for heavy metals removal from aqueous solutions[J]. Biotechnology & Biotechnological Equipment, 2019, 33(1): 730-738. |
| 13 | 文晓凤,杜春艳,袁瀚宇,等. 改性磁性纳米颗粒固定内生菌Bacillus nealsonii吸附废水中Cd2+的特性研究[J]. 环境科学学报, 2016, 36(12): 4376-4383. |
| WEN Xiaofeng, DU Chunyan, YUAN Hanyu, et al. Adsorption of Cd2+ in wastewater through modified magnetic nanoparticles immobilizing endogenous bacterium Bacillus nealsonii[J]. Acta Scientiae Circumstantiae, 2016, 36(12): 4376-4383. | |
| 14 | ZHANG M L, WANG H X, HAN X M. Preparation of metal-resistant immobilized sulfate reducing bacteria beads for acid mine drainage treatment[J]. Chemosphere, 2016, 154: 215-223. |
| 15 | WEN X F, DU C Y, ZENG G M, et al. A novel biosorbent prepared by immobilized Bacillus licheniformis for lead removal from wastewater[J]. Chemosphere, 2018, 200: 173-179. |
| 16 | MATA Y N, BLÁZQUEZ M L, BALLESTER A, et al. Biosorption of cadmium, lead and copper with calcium alginate xerogels and immobilized Fucus vesiculosus[J]. Journal of Hazardous Materials, 2009, 163(2/3): 555-562. |
| 17 | TSEKOVA K, TODOROVA D, DENCHEVA V, et al. Biosorption of copper(Ⅱ) and cadmium(Ⅱ) from aqueous solutions by free and immobilized biomass of Aspergillus niger[J]. Bioresource Technology, 2010, 101(6): 1727-1731. |
| 18 | KUMAR M, UPRETI R K. Impact of lead stress and adaptation in Escherichia coli[J]. Ecotoxicology and Environmental Safety, 2000, 47(3): 246-252. |
| 19 | SHENG Y, WANG Y, YANG X, et al. Cadmium tolerant characteristic of a newly isolated Lactococcus lactis subsp. lactis[J]. Environmental Toxicology and Pharmacology, 2016, 48: 183-190. |
| 20 | SHENG Y, YANG X, LIAN Y Y, et al. Characterization of a cadmium resistance Lactococcus lactis subsp. lactis strain by antioxidant assays and proteome profiles methods[J]. Environmental Toxicology and Pharmacology, 2016, 46: 286-291. |
| 21 | CHEN H G, ZHONG C Y, BERKHOUSE H, et al. Removal of cadmium by bioflocculant produced by Stenotrophomonas maltophilia using phenol-containing wastewater[J]. Chemosphere, 2016, 155: 163-169. |
| 22 | LIU H K, XIE Y L, LI J J, et al. Effect of Serratia sp. K3 combined with organic materials on cadmium migration in soil-Vetiveria Zizanioides L. system and bacterial community in contaminated soil[J]. Chemosphere, 2020, 242: 125164. |
| 23 | BHATTACHARYA A, NAIK S N, KHARE S K. Harnessing the bio-mineralization ability of urease producing Serratia marcescens and Enterobacter cloacae EMB19 for remediation of heavy metal cadmium(Ⅱ)[J]. Journal of Environmental Management, 2018, 215: 143-152. |
| 24 | CHEN Y K, ZHU Q F, DONG X Z, et al. How Serratia marcescens HB-4 absorbs cadmium and its implication on phytoremediation[J]. Ecotoxicology and Environmental Safety, 2019, 185: 109723. |
| 25 | VULLO D L, CERETTI H M, DANIEL M A, et al. Cadmium, zinc and copper biosorption mediated by Pseudomonas veronii 2E[J]. Bioresource Technology, 2008, 99(13): 5574-5581. |
| 26 | XU S Z, XING Y H, LIU S, et al. Characterization of Cd2+ biosorption by Pseudomonas sp. strain 375, a novel biosorbent isolated from soil polluted with heavy metals in Southern China[J]. Chemosphere, 2020, 240: 124893. |
| 27 | ALKAN H, GUL-GUVEN R, GUVEN K, et al. Biosorption of Cd2+, Cu2+, and Ni2+ ions by a Thermophilic Haloalkalitolerant Bacterial Strain (KG9) immobilized on Amberlite XAD-4[J]. Polish Journal of Environmental Studies, 2015, 24: 1903-1910. |
| 28 | LIN C, LAI Y. Adsorption and recovery of lead (Ⅱ) from aqueous solutions by immobilized Pseudomonas Aeruginosa PU21 beads[J]. Journal of Hazardous Materials. 2006, 137(1): 99-105. |
| 29 | HUANG F, DANG Z, GUO C, et al. Biosorption of Cd(Ⅱ) by live and dead cells of Bacillus cereus RC-1 isolated from cadmium-contaminated soil[J]. Colloids and Surfaces B: Biointerfaces, 2013, 107: 11-18. |
| 30 | BAI H J, ZHANG Z M, YANG G E, et al. Bioremediation of cadmium by growing Rhodobacter sphaeroides: kinetic characteristic and mechanism studies[J]. Bioresource Technology, 2008, 99(16): 7716-7722. |
| 31 | PANWICHIAN S, KANTACHOTE D, WITTAYAWEERASAK B, et al. Factors affecting immobilization of heavy metals by purple Nonsulfur bacteria isolated from contaminated shrimp ponds[J]. World Journal of Microbiology and Biotechnology, 2010, 26(12): 2199-2210. |
| 32 | ZHOU W Z, LIU D S, ZHANG H O, et al. Bioremoval and recovery of Cd(Ⅱ) by Pseudoalteromonas sp. SCSE709-6: comparative study on growing and grown cells[J]. Bioresource Technology, 2014, 165: 145-151. |
| 33 | 张理元,由耀辉,刘义武,等. 无机沉淀胶溶法制备钛锂离子筛及其吸附性能研究[J]. 材料导报, 2019, 33(24): 4056-4061. |
| ZHANG Liyuan, YOU Yaohui, LIU Yiwu, et al. Preparation of titanium-lithium ion sieve by the inorganic precipitation-peptization method and its adsorption performance[J]. Materials Reports, 2019, 33(24): 4056-4061. | |
| 34 | 冯克,徐丹华,成卓韦,等. 一种负载功能型微生物的营养缓释填料的制备及性能评价[J]. 环境科学, 2019, 40(1): 504-512. |
| FENG Ke, XU Danhua, CHENG Zhuowei, et al. Preparation of a nutritional slow-release packing material with function microorganisms and its characteristics evaluation[J]. Environmental Science, 2019, 40(1): 504-512. | |
| 35 | 李骅,姜灿烂,丁大虎,等. 海藻酸钠-生物炭联合固定化菌株降解2-羟基-1,4-萘醌[J].南京农业大学学报,2016,39(5):800-806. |
| LI Hua, TIANG Canlan, DING Dahu, et al. Alginate-biochar joint immobilization strains technique for 2-hydroxy-1,4-naphthoquinone(lawsone) degradation[J]. Journal of Nanjing Agricultural University, 2016, 39(5): 800-806. | |
| 36 | 王青松,柳永,王新,等. 基于质构量化分析的净水菌胶囊制备及其性能研究[J]. 浙江大学学报(农业与生命科学版), 2015, 41(6): 712-722. |
| WANG Qingsong, LIU Yong, WANG Xin, et al. Preparation and performance study of water purification bacteria-embedded solid capsules based on texture profile analysis[J]. Journal of Zhejiang University (Agriculture and Life Sciences), 2015, 41(6): 712-722. | |
| 37 | 霍凯利. 固定化重金属耐受菌对废水中铅离子生物吸附的研究[D]. 呼和浩特: 内蒙古工业大学, 2018. |
| HUO Kaili. Study on biosorption of Pb2+ from wastewater by immobilized heavy metal tolerant bacteria[D]. Huhhot: Inner Mongolia University of Technology, 2018. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [3] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [4] | 陈翔宇, 卞春林, 肖本益. 温度分级厌氧消化工艺的研究进展[J]. 化工进展, 2023, 42(9): 4872-4881. |
| [5] | 李卫华, 于倩雯, 尹俊权, 吴寅凯, 孙英杰, 王琰, 王华伟, 杨玉飞, 龙於洋, 黄启飞, 葛燕辰, 何依洋, 赵灵燕. 酸雨环境下填埋飞灰吨袋破损后重金属的溶出行为[J]. 化工进展, 2023, 42(9): 4917-4928. |
| [6] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [7] | 高聪, 陈城虎, 陈修来, 刘立明. 代谢工程改造微生物合成生物基单体的进展与挑战[J]. 化工进展, 2023, 42(8): 4123-4135. |
| [8] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [9] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [10] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [11] | 鲁少杰, 刘佳, 冀芊竹, 李萍, 韩月阳, 陶敏, 梁文俊. 硅藻土基复合填料制备及滴滤塔去除二甲苯的性能[J]. 化工进展, 2023, 42(7): 3884-3892. |
| [12] | 张杉, 仲兆平, 杨宇轩, 杜浩然, 李骞. 磷酸盐改性高岭土对生活垃圾热解过程中重金属的富集[J]. 化工进展, 2023, 42(7): 3893-3903. |
| [13] | 蒋博龙, 崔艳艳, 史顺杰, 常嘉城, 姜楠, 谭伟强. 过渡金属Co3O4/ZnO-ZIF氧还原催化剂Co/Zn-ZIF模板法制备及其产电性能[J]. 化工进展, 2023, 42(6): 3066-3076. |
| [14] | 张耀丹, 孙若溪, 陈鹏程. 以级联反应为基础的多酶共固定载体研究进展[J]. 化工进展, 2023, 42(6): 3167-3176. |
| [15] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||