化工进展 ›› 2023, Vol. 42 ›› Issue (6): 3167-3176.DOI: 10.16085/j.issn.1000-6613.2022-1431
以级联反应为基础的多酶共固定载体研究进展
- 江南大学生物工程学院,工业生物技术教育部重点实验室,江苏 无锡 214122
-
收稿日期:2022-08-01修回日期:2023-01-23出版日期:2023-06-25发布日期:2023-06-29 -
通讯作者:陈鹏程 -
作者简介:张耀丹(1995—),女,硕士研究生,研究方向为生物与医药。E-mail: zhangyaodanjy@163.com。 -
基金资助:江南大学2021年本科教育教学改革研究项目(JG2021056)
Advances of multi-enzyme co-immobilization carrier based on cascade reactions
ZHANG Yaodan( ), SUN Ruoxi, CHEN Pengcheng(
), SUN Ruoxi, CHEN Pengcheng( )
)
- Key Laboratory of Industrial Biotechnology, Ministry of Education, School of Biotechnology, Jiangnan University, Wuxi 214122, Jiangsu, China
-
Received:2022-08-01Revised:2023-01-23Online:2023-06-25Published:2023-06-29 -
Contact:CHEN Pengcheng
摘要:
多酶共固定体系以级联反应体系为基础,将两种或两种以上的酶固定在同一载体上,具有较高的原子经济性和可持续利用性,已成为材料科学、生命科学、生物医药等多种领域的研究热点。选择适宜的载体材料是提高多酶共固定体系催化效率最基本也是最重要的途径。本文以葡萄糖氧化酶和辣根过氧化物酶构建的级联反应作为研究对象,分别从随机固定、分区域固定以及定向固定这三种不同的载体和酶之间的作用形式入手,概述了目前用于多酶共固定体系的载体研究进展。最后分析了多酶共固定领域目前存在的局限和挑战,以期为更多多酶共固定体系提供研究思路。
中图分类号:
引用本文
张耀丹, 孙若溪, 陈鹏程. 以级联反应为基础的多酶共固定载体研究进展[J]. 化工进展, 2023, 42(6): 3167-3176.
ZHANG Yaodan, SUN Ruoxi, CHEN Pengcheng. Advances of multi-enzyme co-immobilization carrier based on cascade reactions[J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3167-3176.
| 共固定策略 | 固定材料 | 固定化结果 |
|---|---|---|
| 随机固定 | SBA-15介孔材料[ | (GOD/SBA-15)-(HRP/SBA-15)比活力为(0.4±0.1)μmol/(min·mg) |
| 二氧化硅[ | 7天后吸光度为初始样品的90% | |
| PPCS纳米颗粒[ | 核/壳纳米粒子(PPCS NPs)使活性提高20% | |
| SnO2多孔纳米纤维[ | 检测线性响应范围为5~100μmol/L,检测极限1.8μmol/L | |
| 玻碳电极[ | 检测线性响应范围为0.022~7.0mmol/L | |
| 碳纳米管[ | 15天后酶活为初始样品酶活的64% | |
| Cu3(PO4)2·3H2O杂化纳米花[ | 检测线性响应范围为0.1~10mmol/L,检测极限为25μmol/L | |
| 氧化石墨烯[ | 2个月后酶活为初始样品酶活的85% | |
| UIO-66金属有机框架[ | 在室温下超过1个月的长期稳定性 | |
| 分区域固定 | Fe3O4磁性纳米粒子[ | 重复使用8次,固定体系酶活为初始样品酶活的67.21% |
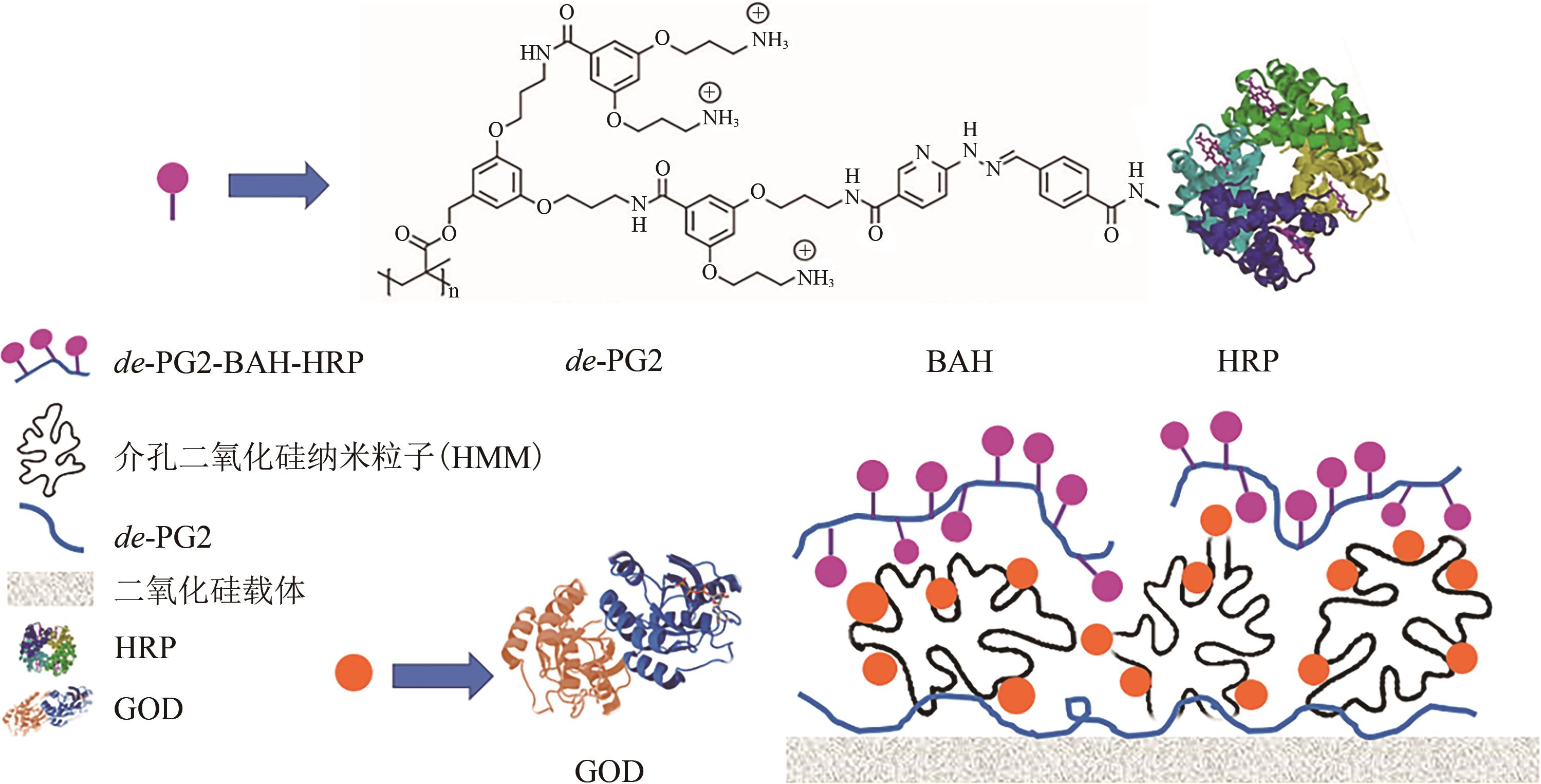
| de-PG2聚阳离子树枝状聚合物[ | 2周后酶活为初始样品酶活的70%~75% | |
| DNA“折纸技术”[ | 酶分子之间距离为10nm时,酶活性超出游离酶的15倍 | |
| 定向固定 | PANI聚合物构建支架[ | 催化效率是游离酶的24倍 |
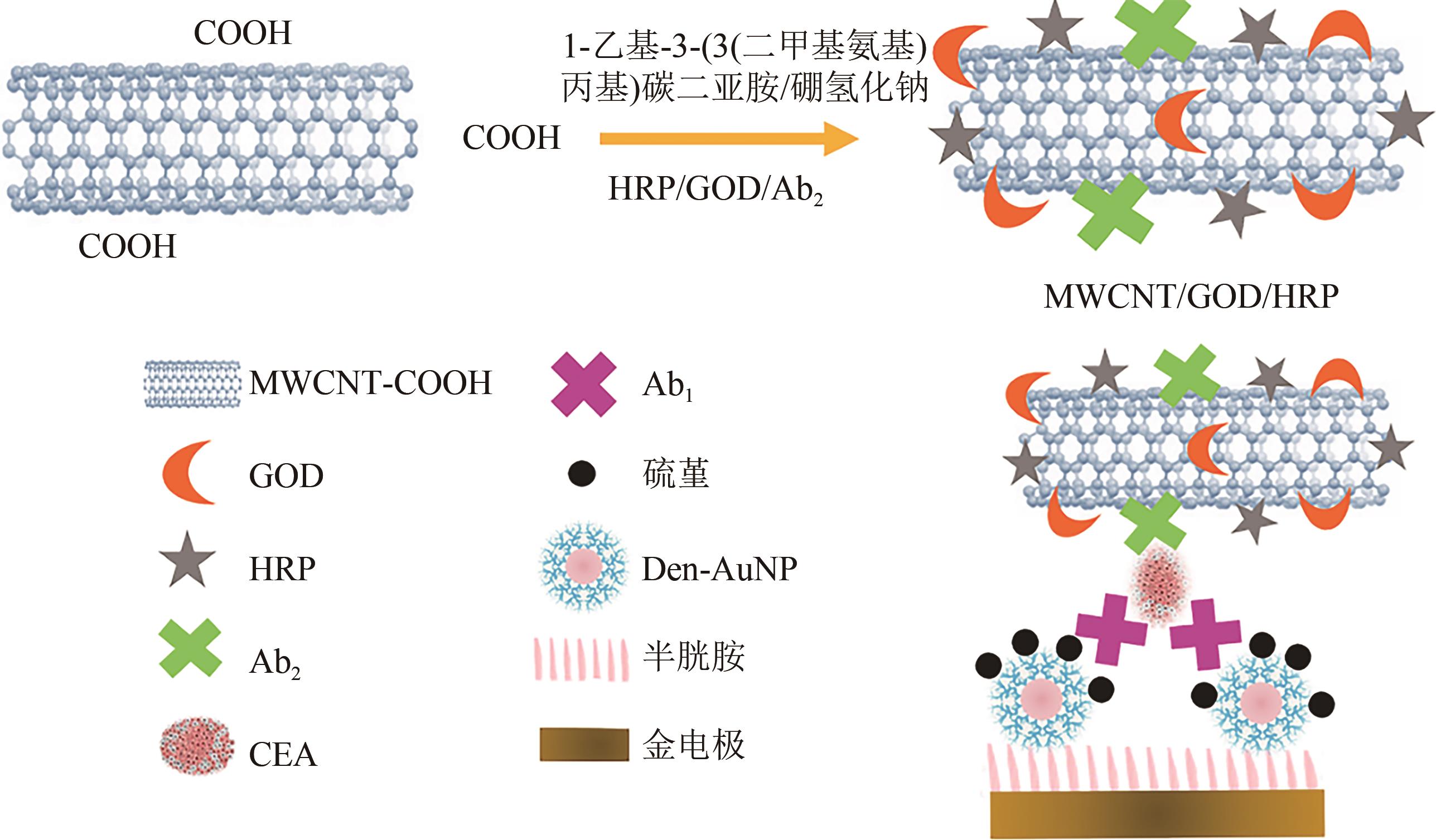
| 多壁碳纳米管[ | 构建的生物传感器灵敏度比构建的单酶生物传感器灵敏度高5.2倍 | |
| P1-P2/DM/MP DNA结构共价结合[ | 酶活比GOD/HRP游离体系酶活高了24.7% |
表1 列举典型的固定化材料
| 共固定策略 | 固定材料 | 固定化结果 |
|---|---|---|
| 随机固定 | SBA-15介孔材料[ | (GOD/SBA-15)-(HRP/SBA-15)比活力为(0.4±0.1)μmol/(min·mg) |
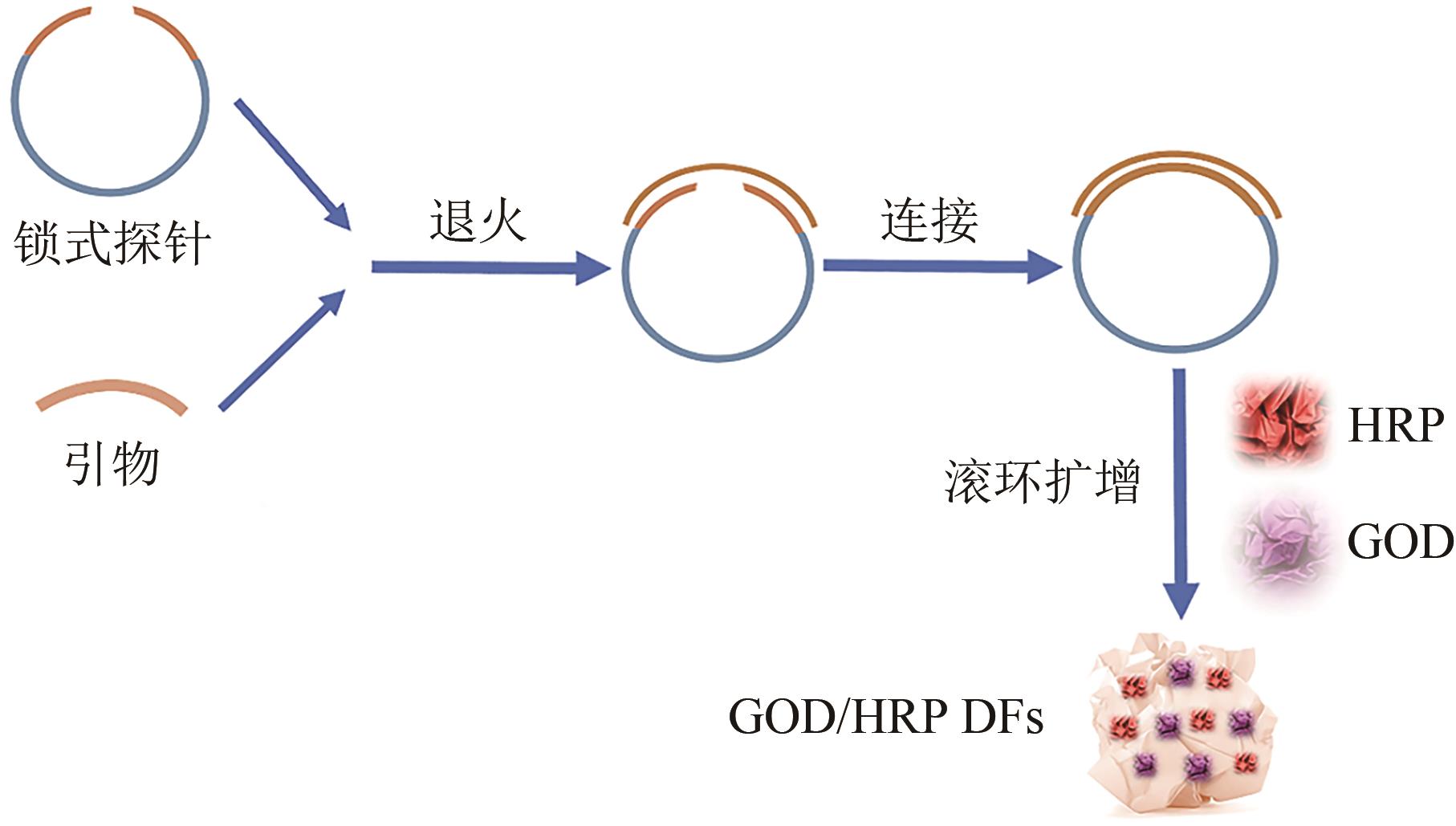
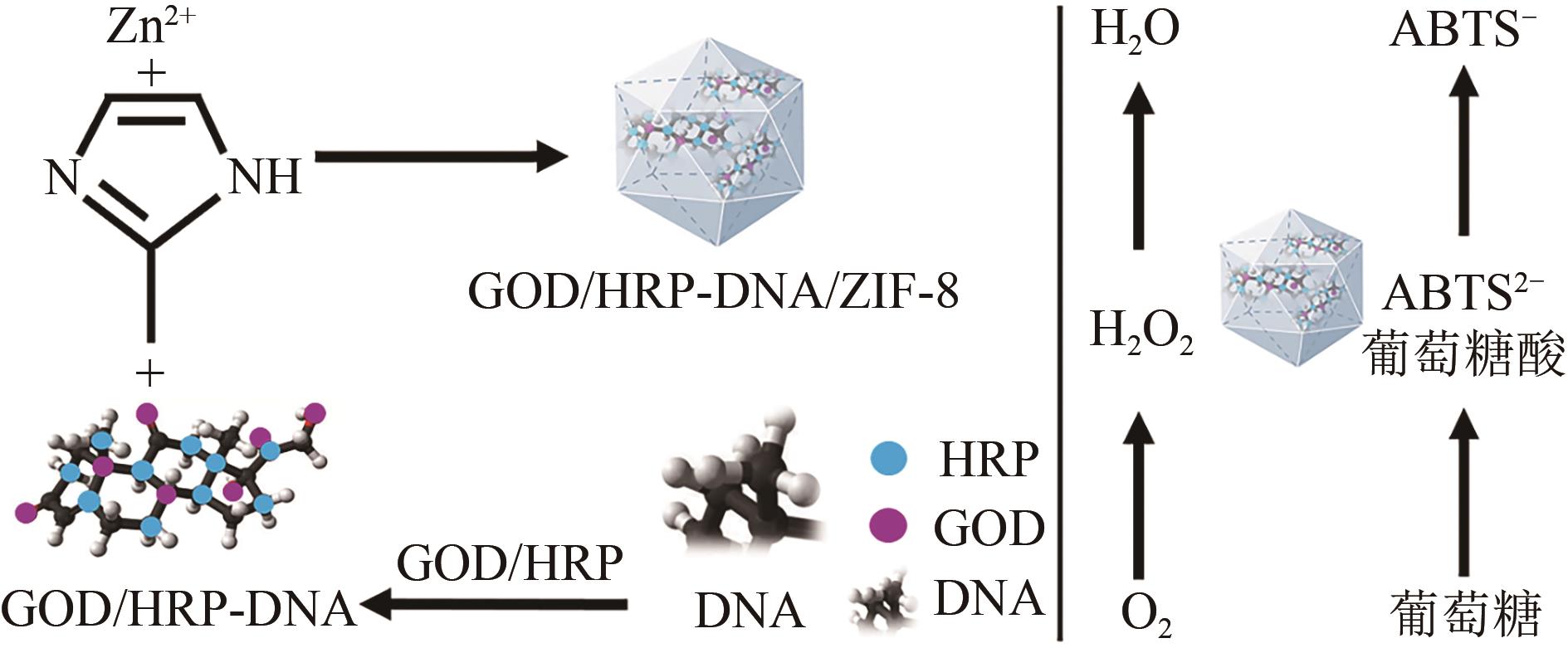
| 二氧化硅[ | 7天后吸光度为初始样品的90% | |
| PPCS纳米颗粒[ | 核/壳纳米粒子(PPCS NPs)使活性提高20% | |
| SnO2多孔纳米纤维[ | 检测线性响应范围为5~100μmol/L,检测极限1.8μmol/L | |
| 玻碳电极[ | 检测线性响应范围为0.022~7.0mmol/L | |
| 碳纳米管[ | 15天后酶活为初始样品酶活的64% | |
| Cu3(PO4)2·3H2O杂化纳米花[ | 检测线性响应范围为0.1~10mmol/L,检测极限为25μmol/L | |
| 氧化石墨烯[ | 2个月后酶活为初始样品酶活的85% | |
| UIO-66金属有机框架[ | 在室温下超过1个月的长期稳定性 | |
| 分区域固定 | Fe3O4磁性纳米粒子[ | 重复使用8次,固定体系酶活为初始样品酶活的67.21% |
| de-PG2聚阳离子树枝状聚合物[ | 2周后酶活为初始样品酶活的70%~75% | |
| DNA“折纸技术”[ | 酶分子之间距离为10nm时,酶活性超出游离酶的15倍 | |
| 定向固定 | PANI聚合物构建支架[ | 催化效率是游离酶的24倍 |
| 多壁碳纳米管[ | 构建的生物传感器灵敏度比构建的单酶生物传感器灵敏度高5.2倍 | |
| P1-P2/DM/MP DNA结构共价结合[ | 酶活比GOD/HRP游离体系酶活高了24.7% |
| 1 | BILAL Muhammad, HUSSAIN Nazim, AMÉRICO-PINHEIRO Juliana Heloisa Pinê, et al. Multi-enzyme co-immobilized nano-assemblies: Bringing enzymes together for expanding bio-catalysis scope to meet biotechnological challenges[J]. International Journal of Biological Macromolecules, 2021, 186: 735-749. |
| 2 | NASEER Sidra, OUYANG Jie, CHEN Xing, et al. Immobilization of β-glucosidase by self-catalysis and compared to crosslinking with glutaraldehyde[J]. International Journal of Biological Macromolecules, 2020, 154: 1490-1495. |
| 3 | XIAO Qinggui, TAO Xia, CHEN Jianfeng. Silica nanotubes based on needle-like calcium carbonate: fabrication and immobilization for glucose oxidase[J]. Industrial & Engineering Chemistry Research, 2007, 46(2): 459-463. |
| 4 | 刘雪凌, 林贝. 载体固定化酶的应用及前景展望[J]. 广州化工, 2020, 48(9): 22-24. |
| LIU Xueling, LIN Bei. Application and prospect of carrier immobilized enzyme[J]. Guangzhou Chemical Industry, 2020, 48(9): 22-24. | |
| 5 | LEE Chan Hee, LEE Hye Sun, LEE Jae Won, et al. Evaluating enzyme stabilizations in calcium carbonate: Comparing in situ and crosslinking mediated immobilization[J]. International Journal of Biological Macromolecules, 2021, 175: 341-350. |
| 6 | Mahmut ÖZACAR, MEHDE Atheer Awad, MEHDI Wesen Adel, et al. The novel multi cross-linked enzyme aggregates of protease, lipase, and catalase production from the sunflower seeds, characterization and application[J]. Colloids and Surfaces B: Biointerfaces, 2019, 173: 58-68. |
| 7 | QUIN M B, WALLIN K K, ZHANG G, et al. Spatial organization of multi-enzyme biocatalytic cascades[J].Organic & Biomolecular Chemistry, 2017, 15(20): 4260-4271. |
| 8 | YU Mingan, LIU Duqiang, SUN Lili, et al. Facile fabrication of 3D porous hybrid sphere by co-immobilization of multi-enzyme directly from cell lysates as an efficient and recyclable biocatalyst for asymmetric reduction with coenzyme regeneration in situ [J]. International Journal of Biological Macromolecules, 2017, 103: 424-434. |
| 9 | SU Li, FENG Jie, ZHOU Ximin, et al. Colorimetric detection of urine glucose based ZnFe2O4 magnetic nanoparticles[J]. Analytical Chemistry, 2012, 84(13): 5753-5758. |
| 10 | MAFRA Agnes, ULRICH Letícia, KORNECKI Jakub, et al. Combi-CLEAs of glucose oxidase and catalase for conversion of glucose to gluconic acid eliminating the hydrogen peroxide to maintain enzyme activity in a bubble column reactor[J]. Catalysts, 2019, 9(8): 657. |
| 11 | 黄磊, 程振民. 无机材料在酶固定化中的应用[J]. 化工进展, 2006, 25(11): 1245-1250. |
| HUANG Lei, CHENG Zhenmin. Application of inorganic materials in enzyme immobilization[J]. Chemical Industry and Engineering Progress, 2006, 25(11): 1245-1250. | |
| 12 | XU Songwei, LU Yang, LI Jian, et al. Efficient conversion of CO2 to methanol catalyzed by three dehydrogenases co-encapsulated in an alginate-silica (ALG-SiO2) hybrid gel[J]. Industrial & Engineering Chemistry Research, 2006, 45(13): 4567-4573. |
| 13 | CHO Eun Jin, JUNG Sera, KIM Hyun Joo, et al. Co-immobilization of three cellulases on Au-doped magnetic silica nanoparticles for the degradation of cellulose[J]. Chemical Communications, 2012, 48(6): 886-888. |
| 14 | PITZALIS Federica, MONDUZZI Maura, SALIS Andrea. A bienzymatic biocatalyst constituted by glucose oxidase and Horseradish peroxidase immobilized on ordered mesoporous silica[J]. Microporous and Mesoporous Materials, 2017, 241: 145-154. |
| 15 | YANG Hao, WEI Wei, LIU Songqin. Monodispersed silica nanoparticles as carrier for co-immobilization of bi-enzyme and its application for glucose biosensing[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2014, 125: 183-188. |
| 16 | AKTER Rashida, RHEE Choong Kyun, RAHMAN Md Aminur. A highly sensitive quartz crystal microbalance immunosensor based on magnetic bead-supported bienzymes catalyzed mass enhancement strategy[J]. Biosensors and Bioelectronics, 2015, 66: 539-546. |
| 17 | ZORE Omkar V, PATTAMMATTEL Ajith, GNANAGURU Shailaja, et al. Bienzyme–polymer–graphene oxide quaternary hybrid biocatalysts: Efficient substrate channeling under chemically and thermally denaturing conditions[J]. ACS Catalysis, 2015, 5(9): 4979-4988. |
| 18 | ZHANG Xingyue, LIU Shigang, ZHANG Wenjie, et al. Photoelectrochemical platform for glucose sensing based on g-C3N4/ZnIn2S4 composites coupled with bi-enzyme cascade catalytic in situ precipitation[J]. Sensors and Actuators B: Chemical, 2019, 297: 126818. |
| 19 | 张伟, 甄生航, 谢国明, 等. 电化学极化玻碳电极构建的葡萄糖生物传感器[J]. 传感器与微系统, 2011, 30(8): 110-112, 119. |
| ZHANG Wei, ZHEN Shenghang, XIE Guoming, et al. Biosensor for glucose based on electrochemical polarization glassy carbon electrode[J]. Transducer and Microsystem Technologies, 2011, 30(8): 110-112, 119. | |
| 20 | CHRISTWARDANA Marcelinus, CHUNG Yongjin, KIM Do-Heyoung, et al. Glucose biofuel cells using the two-step reduction reaction of bienzyme structure as cathodic catalyst[J]. Journal of Industrial and Engineering Chemistry, 2019, 71: 435-444. |
| 21 | BONGJIN Jeong, RASHIDA Akter, HAN Oc Hee, et al. Increased electrocatalyzed performance through dendrimer-encapsulated gold nanoparticles and carbon nanotube-assisted multiple bienzymatic labels: Highly sensitive electrochemical immunosensor for protein detection[J]. Analytical Chemistry, 2013, 85(3): 1784-1791. |
| 22 | LEE Seung Woo, CHEON Seon Ah, KIM Moon Il, et al. Organic–inorganic hybrid nanoflowers: Types, characteristics, and future prospects[J]. Journal of Nanobiotechnology, 2015, 13(1): 54. |
| 23 | SUN Jiayu, GE Jiechao, LIU Weimin, et al. Multi-enzyme co-embedded organic-inorganic hybrid nanoflowers: Synthesis and application as a colorimetric sensor[J]. Nanoscale, 2014, 6(1): 255-262. |
| 24 | 戈钧, 卢滇楠, 朱晶莹, 等. 纳米酶催化剂制备方法研究进展[J]. 化工学报, 2014, 65(7): 2668-2675. |
| GE Jun, LU Diannan, ZHU Jingying, et al. Advances in preparation of nanostructured enzyme catalysts[J]. CIESC Journal, 2014, 65(7): 2668-2675. | |
| 25 | ARIZA-AVIDAD M, SALINAS-CASTILLO A, CAPITÁN-VALLVEY L F. A 3D µPAD based on a multi-enzyme organic-inorganic hybrid nanoflower reactor[J]. Biosensors and Bioelectronics, 2016, 77: 51-55. |
| 26 | 徐亚楠, 周全, 吕永康. 固定化多酶策略及易分离的载体材料研究进展[J]. 化学通报, 2022, 85(10): 1170-1176, 1218. |
| XU Yanan, ZHOU Quan, Yongkang LYU. Research progress in immobilized multi-enzyme strategy and easily separated support materials[J]. Chemistry, 2022, 85(10): 1170-1176, 1218. | |
| 27 | LOCKHART Jacob N, HMELO Anthony B, HARTH Eva. Electron beam lithography of poly(glycidol) nanogels for immobilization of a three-enzyme cascade[J]. Polymer Chemistry, 2018, 9(5): 637-645. |
| 28 | BILAL El-Zahab, DUSTIN Donnelly, WANG Ping. Particle-tethered NADH for production of methanol from CO2 catalyzed by coimmobilized enzymes[J]. Biotechnology and Bioengineering, 2008, 99(3): 508-514. |
| 29 | Javier ROCHA-MARTIN, Susana VELASCO-LOZANO, GUISÁN José M, et al. Oxidation of phenolic compounds catalyzed by immobilized multi-enzyme systems with integrated hydrogen peroxide production[J]. Green Chemistry, 2014, 16(1): 303-311. |
| 30 | NEUBAUEROVA Katrin, CARNEIRO Mariana C C G, RODRIGUES Lígia R, et al. Nanocellulose-based biosensor for colorimetric detection of glucose[J]. Sensing and Bio-Sensing Research, 2020, 29: 100368. |
| 31 | YAN Yongcun, QIAO Zhenjie, Xin HAI, et al. Versatile electrochemical biosensor based on bi-enzyme cascade biocatalysis spatially regulated by DNA architecture[J]. Biosensors and Bioelectronics, 2021, 174: 112827. |
| 32 | KILIAN Vogele, JONATHAN List, SIMMEL Friedrich C, et al. Enhanced efficiency of an enzyme cascade on DNA-activated silica surfaces[J]. Langmuir, 2018, 34(49): 14780-14786. |
| 33 | MATHEW M, SANDHYARANI N. Detection of glucose using immobilized bienzyme on cyclic bisureas–gold nanoparticle conjugate[J]. Analytical Biochemistry, 2014, 459: 31-38. |
| 34 | SINGH K, SINGH B P, CHAUHAN R, et al. Fabrication of amperometric bienzymatic glucose biosensor based on MWCNT tube and polypyrrole multilayered nanocomposite[J]. Journal of Applied Polymer Science, 2012, 125(S1): E235-E246. |
| 35 | SEUNG Hoon Baek, JIHYEOK Roh, CHAN Yeong Park, et al. Cu-nanoflower decorated gold nanoparticles-graphene oxide nanofiber as electrochemical biosensor for glucose detection[J]. Materials Science & Engineering C: Materials for Biological Applications, 2020, 107: 110273. |
| 36 | LI Feng, WANG Zhen, CHEN Wei, et al. A simple strategy for one-step construction of bienzyme biosensor by in situ formation of biocomposite film through electrodeposition[J]. Biosensors and Bioelectronics, 2009, 24(10): 3030-3035. |
| 37 | 侯晨, 陈文强, 付琳慧, 等. 共价有机框架材料在固定化酶及模拟酶领域的应用[J]. 化学进展, 2020, 32(7): 895-905. |
| HOU Chen, CHEN Wenqiang, FU Linhui, et al. Covalent organic frameworks(COFs) materials in enzyme immobilization and mimic enzymes[J]. Progress in Chemistry, 2020, 32(7): 895-905. | |
| 38 | 白云岫, 曹逊, 戈钧. 高分子修饰/无机晶体固定化酶研究进展[J]. 生物加工过程, 2018, 16(1): 12-18. |
| BAI Yunxiu, CAO Xun, GE Jun. Advances in enzyme-polymer conjugates and enzyme-inorganic crystal composites[J]. Chinese Journal of Bioprocess Engineering, 2018, 16(1): 12-18. | |
| 39 | MEMON Amjad Hussain, DING Runsheng, YUAN Qipeng, et al. Coordination of GMP ligand with Cu to enhance the multiple enzymes stability and substrate specificity by co-immobilization process[J]. Biochemical Engineering Journal, 2018, 136: 102-108. |
| 40 | LIANG Hao, SUN Shanshan, ZHOU Yan, et al. In-situ self-assembly of zinc/adenine hybrid nanomaterials for enzyme immobilization[J]. Catalysts, 2017, 7(11): 327. |
| 41 | MUNIRAH Mohammad, AMIR Razmjou, LIANG Kang, et al. Metal-organic-framework-based enzymatic microfluidic biosensor via surface patterning and biomineralization[J]. ACS Applied Materials & Interfaces, 2019, 11(2): 1807-1820. |
| 42 | LI Wenjun, CHEN Siyu, YANG Yue, et al. Ultrasensitive electrochemical immunosensor based on the signal amplification strategy of the competitive reaction of Zn2+ and ATP ions to construct a “signal on” mode GOx-HRP enzyme cascade reaction[J]. Mikrochimica Acta, 2021, 188(2): 61. |
| 43 | SONG Jiayi, HE Wenting, SHEN Hao, et al. Construction of multiple enzyme metal-organic frameworks biocatalyst via DNA scaffold: A promising strategy for enzyme encapsulation[J]. Chemical Engineering Journal, 2019, 363: 174-182. |
| 44 | LIANG Huihui, WANG Linyu, YANG Yuxi, et al. A novel biosensor based on multienzyme microcapsules constructed from covalent-organic framework[J]. Biosensors and Bioelectronics, 2021, 193: 113553. |
| 45 | Bäumler HANS, RADOSTINA Georgieva. Coupled enzyme reactions in multicompartment microparticles[J]. Biomacromolecules, 2010, 11(6): 1480-1487. |
| 46 | ZHANG Lei, SHI Jiafu, JIANG Zhongyi, et al. Bioinspired preparation of polydopamine microcapsule for multienzyme system construction[J]. Green Chemistry, 2011, 13(2): 300-306. |
| 47 | 陈思佳. 细胞外纳米多酶催化与生物合成[D]. 北京: 北京化工大学, 2018. |
| CHEN Sijia. Catalysis and biosynthesis of extracellular nanometer polyenzymes[D]. Beijing: Beijing University of Chemical Technology, 2018. | |
| 48 | HANNA Gustafsson, Küchler ANDREAS, KRISTER Holmberg, et al. Co-immobilization of enzymes with the help of a dendronized polymer and mesoporous silica nanoparticles[J]. Journal of Materials Chemistry B, 2015, 3(30): 6174-6184. |
| 49 | ZHOU Xiao, LIU Yan, YUAN Qipeng, et al. Preparation of multi-enzyme co-immobilized nanoparticles by bis-aryl hydrazone bond conjugation[J]. Biotechnology and Applied Biochemistry, 2016, 63(2): 214-219. |
| 50 | GOUSIA Begum, GOODWIN Brandon W, DEGLEE Ben M, et al. Compartmentalisation of enzymes for cascade reactions through biomimetic layer-by-layer mineralization[J]. Journal of Materials Chemistry B, 2015, 3(26): 5232-5240. |
| 51 | FU Jinglin, LIU Minghui, LIU Yan, et al. Interenzyme substrate diffusion for an enzyme cascade organized on spatially addressable DNA nanostructures[J]. Journal of the American Chemical Society, 2012, 134(12): 5516-5519. |
| 52 | VEIKKO Linko, MARIKA Eerikäinen, KOSTIAINEN Mauri A. A modular DNA origami-based enzyme cascade nanoreactor[J]. Chemical Communications, 2015, 51(25): 5351-5354. |
| 53 | 胡玲玲. 基于辅基法的多酶体系定向共固定化研究[D]. 杭州: 浙江理工大学, 2016. |
| HU Lingling. Oriented co-immobilization of multi-enzyme systems based on prosthetic group affinity[D]. Hangzhou: Zhejiang Sci-Tech University, 2016. | |
| 54 | LIU Yang, DU Juanjuan, YAN Ming, et al. Biomimetic enzyme nano complexes and their use as antidotes and preventive measures for alcohol intoxication[J]. Nature Nanotechnology, 2013, 8(3): 187-192. |
| 55 | CHEN Huan, XI Fengna, GAO Xia, et al. Bienzyme bionanomultilayer electrode for glucose biosensing based on functional carbon nanotubes and sugar–lectin biospecific interaction[J]. Analytical Biochemistry, 2010, 403(1/2): 36-42. |
| 56 | ZHANG Yifei, YONG You, GE Jun, et al. Lectin agglutinated multienzyme catalyst with enhanced substrate affinity and activity[J]. ACS Catalysis, 2016, 6(6): 3789-3795. |
| 57 | ORTIZ Elvis, GALLAY Pablo, GALICIA Laura, et al. Nanoarchitectures based on multi-walled carbon nanotubes non-covalently functionalized with Concanavalin A: A new building-block with supramolecular recognition properties for the development of electrochemical biosensors[J]. Sensors and Actuators B: Chemical, 2019, 292: 254-262. |
| 58 | YANG Ye, ZHANG Ruiqi, ZHOU Bingnan, et al. High activity and convenient ratio control: DNA-directed co-immobilization of multiple enzymes on multifunctionalized magnetic nanoparticles[J]. ACS Applied Materials & Interfaces, 2017, 9(42): 37254-37263. |
| 59 | SONG Jiayi, HE Wenting, SHEN Hao, et al. Exquisitely designed magnetic DNA nanocompartment for enzyme immobilization with adjustable catalytic activity and improved enzymatic assay performance[J]. Chemical Engineering Journal, 2020, 390: 124488. |
| 60 | NISARAPORN Suthiwangcharoen, LI Tao, WU Laying, et al. Facile co-assembly process to generate core-shell nanoparticles with functional protein corona[J]. Biomacromolecules, 2014, 15(3): 948-956. |
| 61 | ALIM Samiul, KAFI A K M, RAJAN Jose, et al. Application of polymerized multiporous nanofiber of SnO2 for designing a bienzyme glucose biosensor based on HRP/GOx [J]. International Journal of Biological Macromolecules, 2019, 123: 1028-1034. |
| 62 | YANG Han. A glucose biosensor based on horseradish peroxidase and glucose oxidase co-entrapped in carbon nanotubes modified electrode[J]. International Journal of Electrochemical Science, 2017, 12: 4958-4969. |
| 63 | ELOUARZAKI K, BOUROUROU M, HOLZINGER M, et al. Freestanding HRP-GOx redox buckypaper as an oxygen-reducing biocathode for biofuel cell applications[J]. Energy & Environmental Science, 2015, 8(7): 2069-2074. |
| 64 | ZHU Xueli, HUANG Jin, LIU Jinwen, et al. A dual enzyme-inorganic hybrid nanoflower incorporated microfluidic paper-based analytic device (μPAD) biosensor for sensitive visualized detection of glucose[J]. Nanoscale, 2017, 9(17): 5658-5663. |
| 65 | MOTILAL Mathesh, LIU Jingquan, BARROW Colin J, et al. Graphene-oxide-based enzyme nanoarchitectonics for substrate channeling[J]. Chemistry, 2017, 23(2): 304-311. |
| 66 | AHMAD Raneem, SHANAHAN Jordan, RIZALDO Sydnie, et al. Co-immobilization of an enzyme system on a metal-organic framework to produce a more effective biocatalyst[J]. Catalysts, 2020, 10(5): 499. |
| 67 | JOSEP Garcia, ZHANG Yue, HANNAH Taylor, et al. Multilayer enzyme-coupled magnetic nanoparticles as efficient, reusable biocatalysts and biosensors[J]. Nanoscale, 2011, 3(9): 3721-3730. |
| [1] | 唐婷, 周文凤, 王志, 朱晨杰, 许敬亮, 庄伟, 应汉杰, 欧阳平凯. 多酶共固定化技术在糖类催化中的研究进展[J]. 化工进展, 2022, 41(5): 2636-2648. |
| [2] | 张彦, 汪伟, 谢锐, 巨晓洁, 刘壮, 褚良银. 负载酶@ZIF-8复合物的聚合物微颗粒可控制备[J]. 化工进展, 2022, 41(4): 2022-2028. |
| [3] | 李云飞, 王致鹏, 段磊, 陈良, 徐守冬, 张鼎, 段东红, 刘世斌. 质子交换膜燃料电池有序化膜电极研究进展[J]. 化工进展, 2021, 40(S1): 101-110. |
| [4] | 马智, 李英乾, 丁彤, 董俊杰, 秦永宁. 埃洛石纳米管在生物医学应用中的研究进展[J]. 化工进展, 2017, 36(08): 3032-3039. |
| [5] | 陶维红,杨立荣,徐 刚,邰玉蕾,王 立,吴坚平. 磁性多孔微粒对脂肪酶的固定化 [J]. 化工进展, 2011, 30(7): 1584-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||