化工进展 ›› 2023, Vol. 42 ›› Issue (1): 1-15.DOI: 10.16085/j.issn.1000-6613.2022-1795
可再生能源驱动的生物催化固定CO2的研究进展
- 北京化工大学生命科学与技术学院,北京 100029
-
收稿日期:2022-09-26修回日期:2022-12-02出版日期:2023-01-25发布日期:2023-02-20 -
通讯作者:谭天伟 -
作者简介:刘艳辉(1978—),女,博士,副教授,研究方向为生物化工。E-mail:liuyh@mail.buct.edu.cn。 -
基金资助:国家重点研发计划(2021YFC2103500)
Recent advances on the bio-fixation of CO2 driven by renewable energy
LIU Yanhui( ), ZHOU Mingfang, MA Ming, WANG Kai, TAN Tianwei(
), ZHOU Mingfang, MA Ming, WANG Kai, TAN Tianwei( )
)
- College of Life Science and Techonogy, Beijing University of Chemical Technology, Beijing 100029, China
-
Received:2022-09-26Revised:2022-12-02Online:2023-01-25Published:2023-02-20 -
Contact:TAN Tianwei
摘要:
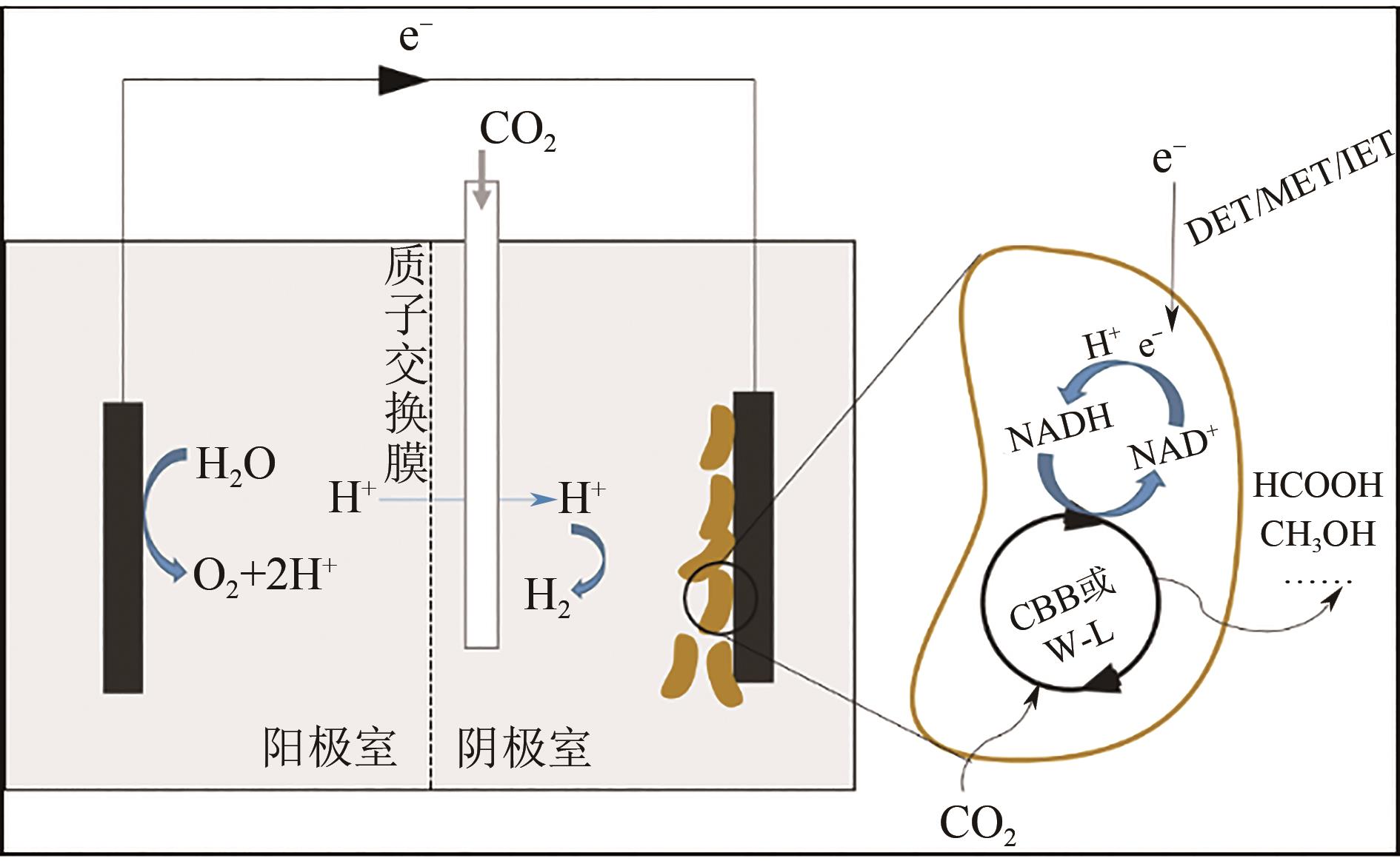
工业发展带来了经济效益的同时,伴随着化石燃料的日益枯竭和CO2的大量排放,加剧了温室效应,出现了全球变暖的问题。我国在倡导节能减排的同时,大力发展CO2固定技术,将大气中丰富的CO2转化为可以供人们利用的化工原料、燃料甚至更高附加值产品,不仅能够保护环境,同时还可提高经济效益。当前全球可再生能源规模的不断扩大缓解了传统能源消耗的压力,同时可再生能源可以为固定CO2提供可持续的、清洁的驱动能量,并且随着分子生物学的发展,生物法固碳技术越发成熟,同时较其他固定CO2方法而言,生物法固碳具有条件温和、选择性高、产品多样等优势,因此利用可再生能源耦合生物催化进行CO2的固定逐渐成为了这一领域的研究重点。本文总结了近年来生物催化与电化学、光化学反应耦合固定CO2的研究,包括光、电催化与酶催化偶联以及光、电催化与全细胞催化偶联对CO2的利用,简述了其耦合催化原理与研究进展,并总结了目前研究需要突破的关键技术及提高CO2催化转化效率的方法。
中图分类号:
引用本文
刘艳辉, 周明芳, 马铭, 王凯, 谭天伟. 可再生能源驱动的生物催化固定CO2的研究进展[J]. 化工进展, 2023, 42(1): 1-15.
LIU Yanhui, ZHOU Mingfang, MA Ming, WANG Kai, TAN Tianwei. Recent advances on the bio-fixation of CO2 driven by renewable energy[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 1-15.
| 酶 | 反应 | 固碳机制 | 优点 | 缺点 | 参考文献 |
|---|---|---|---|---|---|
| 碳酸酐酶(CA) | 与Zn2+活性位点络合的OH-亲核进攻CO2生成HCO | 转化效率高,不需要辅助因子 | 价格昂贵,可重用性低 | [ | |
| 一氧化碳脱氢酶(CODH) | CODH与CO2分子结合,通过铁硫团簇(Fe4S4)催化CO2生成CO | 活性高,产品可转化性高 | 稳定性差,氧气敏感 | [ | |
| 重构固氮酶 | Fe蛋白将电子传递给相应的催化中心 | 产物谱较大 | 氧敏感 | [ | |
| 甲酸脱氢酶(FDH) | 利用活性中心还原的金属团簇作为氢化物供体,通过氢化物转移催化CO2还原 | 选择性高 | 部分氧敏感,依赖辅助因子 | [ |
表1 常见碳还原相关酶
| 酶 | 反应 | 固碳机制 | 优点 | 缺点 | 参考文献 |
|---|---|---|---|---|---|
| 碳酸酐酶(CA) | 与Zn2+活性位点络合的OH-亲核进攻CO2生成HCO | 转化效率高,不需要辅助因子 | 价格昂贵,可重用性低 | [ | |
| 一氧化碳脱氢酶(CODH) | CODH与CO2分子结合,通过铁硫团簇(Fe4S4)催化CO2生成CO | 活性高,产品可转化性高 | 稳定性差,氧气敏感 | [ | |
| 重构固氮酶 | Fe蛋白将电子传递给相应的催化中心 | 产物谱较大 | 氧敏感 | [ | |
| 甲酸脱氢酶(FDH) | 利用活性中心还原的金属团簇作为氢化物供体,通过氢化物转移催化CO2还原 | 选择性高 | 部分氧敏感,依赖辅助因子 | [ |
| 阳极 | 阴极 | 酶 | 转化产物 | 效率 | 参考文献 |
|---|---|---|---|---|---|
| Pt | 石墨棒 | FDH | HCOOH | 928.8μmol·L-1·mg Enzyme-1·h-1 | [ |
| Co-Pi/α-Fe2O3 | ITO | TsFDH | 6.4μmol·h-1 | [ | |
| TK/TiO2 | CH3V(CH2)9COOH | FDH | 0.01µmol·h-1 | [ | |
| IO-TiO2|dpp|POs | IO-TiO2 | W-FDH | (0.185 ± 0.017)μmol·cm-2 | [ | |
| FeOOH/BiVO4 | 3D TiN | ClFDH | 0.78µmol·h-1 | [ | |
| Pt | 碳布@DA2+ | FDH | 3500µmol·L-1·h-1 | [ | |
| Co-Pi/α-Fe2O3 | BiFeO3 | FDH,FaldDH,ADH | CH3OH | 220μmol·h-1 | [ |
| Pt | ZIF-8@ [Cp*RhCl2]2 | FDH,FaldDH,ADH | 822μmol·g-1·h-1 | [ | |
| Pt | Rh合物 | FDH,FaldDH,ADH | 480μmol·L-1·h-1 | [ |
表2 不同电酶耦合催化相关参数汇总
| 阳极 | 阴极 | 酶 | 转化产物 | 效率 | 参考文献 |
|---|---|---|---|---|---|
| Pt | 石墨棒 | FDH | HCOOH | 928.8μmol·L-1·mg Enzyme-1·h-1 | [ |
| Co-Pi/α-Fe2O3 | ITO | TsFDH | 6.4μmol·h-1 | [ | |
| TK/TiO2 | CH3V(CH2)9COOH | FDH | 0.01µmol·h-1 | [ | |
| IO-TiO2|dpp|POs | IO-TiO2 | W-FDH | (0.185 ± 0.017)μmol·cm-2 | [ | |
| FeOOH/BiVO4 | 3D TiN | ClFDH | 0.78µmol·h-1 | [ | |
| Pt | 碳布@DA2+ | FDH | 3500µmol·L-1·h-1 | [ | |
| Co-Pi/α-Fe2O3 | BiFeO3 | FDH,FaldDH,ADH | CH3OH | 220μmol·h-1 | [ |
| Pt | ZIF-8@ [Cp*RhCl2]2 | FDH,FaldDH,ADH | 822μmol·g-1·h-1 | [ | |
| Pt | Rh合物 | FDH,FaldDH,ADH | 480μmol·L-1·h-1 | [ |
| 光催化剂 | 酶 | 催化条件 | 转化产物 | 效率 | 参考文献 |
|---|---|---|---|---|---|
| CCG-IP | FDH,FaldDH,ADH | 可见光 | CH3OH | 6.8μmol·L-1·h-1 | [ |
| CNA | [Cp*Rh(bpy)H2O]2+,TEOA,可见光 | 45μmol·h-1 | [ | ||
| H2TPPS | TEOA,MV2+,可见光 | 4.08μmol·L-1·h-1 | [ | ||
| ZnTPyPBr | [Cp*Rh(bpy)H2O]2+,TEOA,氙灯照射 | 143.3μmol·L-1·h-1 | [ | ||
| PTi,Cds QDs | FDH FDH,FaldDH,ADH | [Cp*Rh(bpy)H2O]2+,TEOA,可见光 | HCOOH CH3OH | 1500μmol·L-1·h-1 99μmol·L-1·h-1 | [ |
| C60聚合物 | FDH | Rh[Cp*Rh(bpy)H2O]2+,可见光 | HCOOH | 119.73μmol·h-1 | [ |
| TiO2 | 紫外光 | 51.3μmol·L-1·h-1 | [ | ||
| TPE-C3N4 | Rh,TEOA,MAF-8,可见光 | 1861μmol·L-1·h-1 | [ | ||
| g-C3N4 | CA,FateDH | ZIF-8,可见光 | HCOOH | 48.6μmol·h-1 | [ |
表3 不同光酶耦合催化相关参数汇总
| 光催化剂 | 酶 | 催化条件 | 转化产物 | 效率 | 参考文献 |
|---|---|---|---|---|---|
| CCG-IP | FDH,FaldDH,ADH | 可见光 | CH3OH | 6.8μmol·L-1·h-1 | [ |
| CNA | [Cp*Rh(bpy)H2O]2+,TEOA,可见光 | 45μmol·h-1 | [ | ||
| H2TPPS | TEOA,MV2+,可见光 | 4.08μmol·L-1·h-1 | [ | ||
| ZnTPyPBr | [Cp*Rh(bpy)H2O]2+,TEOA,氙灯照射 | 143.3μmol·L-1·h-1 | [ | ||
| PTi,Cds QDs | FDH FDH,FaldDH,ADH | [Cp*Rh(bpy)H2O]2+,TEOA,可见光 | HCOOH CH3OH | 1500μmol·L-1·h-1 99μmol·L-1·h-1 | [ |
| C60聚合物 | FDH | Rh[Cp*Rh(bpy)H2O]2+,可见光 | HCOOH | 119.73μmol·h-1 | [ |
| TiO2 | 紫外光 | 51.3μmol·L-1·h-1 | [ | ||
| TPE-C3N4 | Rh,TEOA,MAF-8,可见光 | 1861μmol·L-1·h-1 | [ | ||
| g-C3N4 | CA,FateDH | ZIF-8,可见光 | HCOOH | 48.6μmol·h-1 | [ |
| 菌株 | 培养类型 | 产物 | 效率 | 阴极材料 | 参考文献 |
|---|---|---|---|---|---|
| 真罗氏菌LH74D | 纯培养 | 甲酸 | 0.73mmol·L-1·d-1 | Pt(多孔陶瓷杯) | [ |
| 莫氏热自养菌 | 甲酸 乙酸 | 63.2mmol·m-2·d-1 58.2mmol·m-2·d-1 | 碳纳米颗粒 | [ | |
| 大肠杆菌 | 丙酮酸 | 10mmol·L-1·d-1 | 铟片 | [ | |
| 大肠杆菌 | 琥珀酸 | 4.36mmol·L-1·d-1 | Pt+碳布 | [ | |
| 大肠杆菌 | 甲酸 | 15.64mmol·L-1·d-1 | 酞菁铁分散碳化物衍生碳FePc-CDC | [ | |
| 富养罗尔斯通氏菌 | 番茄红素 | 8.06×10-4mmol·L-1·d-1 | 不锈钢网 | [ | |
| 富养罗尔斯通氏菌 | α-葎草烯 | 0.0077mmol·L-1·d-1 | 不锈钢网 | [ | |
| 醋酸杆菌、乙酰乙酸菌等 | 混合培养 | 乙酸 丁酸 异丙醇 | 21g·m-2·d-1 3.7g·m-2·d-1 3.3g·m-2·d-1 | 碳毡 | [ |
| 嗜热产甲烷菌 | 甲烷 | 0.34mmol·L-1·d-1 | 碳化椰子壳 | [ | |
| 厌氧菌群 | 乙酸 异丁酸 丙酸 | 0.81mmol·L-1·d-1 0.63mmol·L-1·d-1 0.44mmol·L-1·d-1 | 石墨毡 | [ | |
| 梭菌、脱硫弧菌等 | 乙酸 | 35.8g·m-2·d-1 | 石墨毡 | [ | |
| 硫酸盐还原菌、铁还原菌 | 甲醇、乙醇等 | 0.74g·L-1·d-1 | 石墨板 | [ | |
| 厌氧菌群 | 乙酸 正丁酸 丙酸 | 163mmol·L-1·d-1 64.69mmol·L-1·d-1 27mmol·L-1·d-1 | 碳毡,石墨颗粒 | [ | |
| 甲烷球菌 | 甲烷 | (8.81 ± 0.51)mmol·m-2·d-1 | Pt | [ | |
| 厌氧甲烷菌 | 甲烷 | 1.392mmol·L-1·d-1 | Ti+碳毡 | [ | |
| 产甲烷菌 | 甲烷 | 47.86mL·L-1·d-1 | 石墨毡 | [ | |
| 厚壁菌、变形菌放线菌、拟杆菌等构成的微生物群落 | 丁酸 | (0.428±0.026)mmol·L-1·d-1 | 空心纤维膜和碳毡复合 | [ | |
| 变形菌门和热菌门为主的生物群落 | 丁酸 | 约0.76mmol·L-1·d-1 | 镍铁氧体(NiFe2O4@CF)修饰碳毡 | [ | |
| 醋酸杆菌等 | 己酸 | (2.05±0.102)mmol·L-1·d-1 | 碳毡 | [ |
表4 不同微生物电合成发酵利用CO2相关参数汇总
| 菌株 | 培养类型 | 产物 | 效率 | 阴极材料 | 参考文献 |
|---|---|---|---|---|---|
| 真罗氏菌LH74D | 纯培养 | 甲酸 | 0.73mmol·L-1·d-1 | Pt(多孔陶瓷杯) | [ |
| 莫氏热自养菌 | 甲酸 乙酸 | 63.2mmol·m-2·d-1 58.2mmol·m-2·d-1 | 碳纳米颗粒 | [ | |
| 大肠杆菌 | 丙酮酸 | 10mmol·L-1·d-1 | 铟片 | [ | |
| 大肠杆菌 | 琥珀酸 | 4.36mmol·L-1·d-1 | Pt+碳布 | [ | |
| 大肠杆菌 | 甲酸 | 15.64mmol·L-1·d-1 | 酞菁铁分散碳化物衍生碳FePc-CDC | [ | |
| 富养罗尔斯通氏菌 | 番茄红素 | 8.06×10-4mmol·L-1·d-1 | 不锈钢网 | [ | |
| 富养罗尔斯通氏菌 | α-葎草烯 | 0.0077mmol·L-1·d-1 | 不锈钢网 | [ | |
| 醋酸杆菌、乙酰乙酸菌等 | 混合培养 | 乙酸 丁酸 异丙醇 | 21g·m-2·d-1 3.7g·m-2·d-1 3.3g·m-2·d-1 | 碳毡 | [ |
| 嗜热产甲烷菌 | 甲烷 | 0.34mmol·L-1·d-1 | 碳化椰子壳 | [ | |
| 厌氧菌群 | 乙酸 异丁酸 丙酸 | 0.81mmol·L-1·d-1 0.63mmol·L-1·d-1 0.44mmol·L-1·d-1 | 石墨毡 | [ | |
| 梭菌、脱硫弧菌等 | 乙酸 | 35.8g·m-2·d-1 | 石墨毡 | [ | |
| 硫酸盐还原菌、铁还原菌 | 甲醇、乙醇等 | 0.74g·L-1·d-1 | 石墨板 | [ | |
| 厌氧菌群 | 乙酸 正丁酸 丙酸 | 163mmol·L-1·d-1 64.69mmol·L-1·d-1 27mmol·L-1·d-1 | 碳毡,石墨颗粒 | [ | |
| 甲烷球菌 | 甲烷 | (8.81 ± 0.51)mmol·m-2·d-1 | Pt | [ | |
| 厌氧甲烷菌 | 甲烷 | 1.392mmol·L-1·d-1 | Ti+碳毡 | [ | |
| 产甲烷菌 | 甲烷 | 47.86mL·L-1·d-1 | 石墨毡 | [ | |
| 厚壁菌、变形菌放线菌、拟杆菌等构成的微生物群落 | 丁酸 | (0.428±0.026)mmol·L-1·d-1 | 空心纤维膜和碳毡复合 | [ | |
| 变形菌门和热菌门为主的生物群落 | 丁酸 | 约0.76mmol·L-1·d-1 | 镍铁氧体(NiFe2O4@CF)修饰碳毡 | [ | |
| 醋酸杆菌等 | 己酸 | (2.05±0.102)mmol·L-1·d-1 | 碳毡 | [ |
| 催化剂 | 微生物 | 固碳途径 | 产物 | 固碳效率(较非耦合而言) | 参考文献 |
|---|---|---|---|---|---|
| 硫化镉(CdS) | Rhodopseudomonaspalustris | 卡尔文循环 | 类胡萝卜素、PHB和甲烷 | 生物量提高了39%,产品产量提高了1.17~1.35倍 | [ |
| Rhodopseudomonaspalustris | 对其Mo Fe固氮酶进行突变,使其具有固碳产甲烷的能力 | 甲烷 | 产量提高了1.7倍 | [ | |
| Clostridiumautoethanogenum | Wood-Ljungdahl | 乙酸 | 产量提高了2~3倍 | [ | |
| Escherichia coli | 异源构建HWLS (half-Wood-Ljungdahl-formolase)途径 | L-苹果酸、丁酸 | 生物量提高了1.5倍,L-苹果酸、丁酸产率分别比对照组菌株高出8%和18%~25% | [ | |
| CoPi、Co-P | Ralstonia eutropha | 卡尔文循环 | 异丙醇、异丁醇和异戊醇 | 固碳效率超过了最高产植物的太阳能-生物质效率 | [ |
| NCNCNx | Methanosarcina barkeri | 脱氢酶、参与卡尔文循环的各种酶组成的固碳途径[ | 甲烷 | 量子产率为50.3%,选择性为92.3% | [ |
| AuNCs | Moorella thermoacetica | Wood-Ljungdahl | 乙酸 | 量子效率比CdS耦合的高出33%,可持续性更强 | [ |
| g-C3N4 | Ralstonia eutropha | 卡尔文循环 | 生物塑料聚羟基丁酸酯(polyhydroxybutyrate,PHB) | 产量提高了约2倍 | [ |
| CuO/g-C3N4 | 微生物群落 | — | 乙酸 | 产量为5.1g/L,且具有长期稳定性 | [ |
| 有机半导体苝二酰亚胺衍生物(perylene diimide derivative, PDI)和聚芴-联苯(fluorene-co-phenylene, PFP) | Moorella thermoacetica | Wood-Ljungdahl | 乙酸 | 效率为约1.6%,与报道的无机生物杂交种系统相当 | [ |
| 沼泽红假单胞菌 | Methanosarcina barkeri | 脱氢酶、参与卡尔文循环的各种酶组成的固碳途径 | 甲烷 | 略高于典型半导体-生物杂交体系的产甲烷速率 | [ |
表5 人工光合作用的催化剂与固碳微生物总结
| 催化剂 | 微生物 | 固碳途径 | 产物 | 固碳效率(较非耦合而言) | 参考文献 |
|---|---|---|---|---|---|
| 硫化镉(CdS) | Rhodopseudomonaspalustris | 卡尔文循环 | 类胡萝卜素、PHB和甲烷 | 生物量提高了39%,产品产量提高了1.17~1.35倍 | [ |
| Rhodopseudomonaspalustris | 对其Mo Fe固氮酶进行突变,使其具有固碳产甲烷的能力 | 甲烷 | 产量提高了1.7倍 | [ | |
| Clostridiumautoethanogenum | Wood-Ljungdahl | 乙酸 | 产量提高了2~3倍 | [ | |
| Escherichia coli | 异源构建HWLS (half-Wood-Ljungdahl-formolase)途径 | L-苹果酸、丁酸 | 生物量提高了1.5倍,L-苹果酸、丁酸产率分别比对照组菌株高出8%和18%~25% | [ | |
| CoPi、Co-P | Ralstonia eutropha | 卡尔文循环 | 异丙醇、异丁醇和异戊醇 | 固碳效率超过了最高产植物的太阳能-生物质效率 | [ |
| NCNCNx | Methanosarcina barkeri | 脱氢酶、参与卡尔文循环的各种酶组成的固碳途径[ | 甲烷 | 量子产率为50.3%,选择性为92.3% | [ |
| AuNCs | Moorella thermoacetica | Wood-Ljungdahl | 乙酸 | 量子效率比CdS耦合的高出33%,可持续性更强 | [ |
| g-C3N4 | Ralstonia eutropha | 卡尔文循环 | 生物塑料聚羟基丁酸酯(polyhydroxybutyrate,PHB) | 产量提高了约2倍 | [ |
| CuO/g-C3N4 | 微生物群落 | — | 乙酸 | 产量为5.1g/L,且具有长期稳定性 | [ |
| 有机半导体苝二酰亚胺衍生物(perylene diimide derivative, PDI)和聚芴-联苯(fluorene-co-phenylene, PFP) | Moorella thermoacetica | Wood-Ljungdahl | 乙酸 | 效率为约1.6%,与报道的无机生物杂交种系统相当 | [ |
| 沼泽红假单胞菌 | Methanosarcina barkeri | 脱氢酶、参与卡尔文循环的各种酶组成的固碳途径 | 甲烷 | 略高于典型半导体-生物杂交体系的产甲烷速率 | [ |
| 1 | LIU Zihe, WANG Kai, CHEN Yun, et al. Third-generation biorefineries as the means to produce fuels and chemicals from CO2 [J]. Nature Catalysis, 2020, 3(3): 274-288. |
| 2 | MA Wenchao, HE Xiaoyang, WANG Wei, et al. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts[J]. Chemical Society Reviews, 2021, 50(23): 12897-12914. |
| 3 | HE Jie, Csaba JANÁKY. Recent advances in solar-driven carbon dioxide conversion: expectations versus reality[J]. ACS Energy Letters, 2020, 5(6): 1996-2014. |
| 4 | KIM Soo Rin, KIM Soo-Jung, KIM Sun-Ki, et al. Yeast metabolic engineering for carbon dioxide fixation and its application[J]. Bioresource Technology, 2022, 346: 126349. |
| 5 | HU Guipeng, LI Zehong, MA Danlei, et al. Light-driven CO2 sequestration in Escherichia coli to achieve theoretical yield of chemicals[J]. Nature Catalysis, 2021, 4(5): 395-406. |
| 6 | YU Senshen, Pengfei LYU, XUE Pan, et al. Light-driven enzymatic nanosystem for highly selective production of formic acid from CO2 [J]. Chemical Engineering Journal, 2021, 420: 127649. |
| 7 | CHEN Xiaoli, CAO Yingxiu, LI Feng, et al. Enzyme-assisted microbial electrosynthesis of poly(3-hydroxybutyrate) via CO2 bioreduction by engineered Ralstonia eutropha [J]. ACS Catalysis, 2018, 8(5): 4429-4437. |
| 8 | 李丽, 石永霞, 侯曼, 等. 铜基材料电催化二氧化碳还原反应的研究进展[J]. 稀有金属, 2022, 46(6): 681-694. |
| LI Li, SHI Yongxia, HOU Man, et al. Research progress of copper-based materials for electrocatalytic CO2 reduction reaction[J]. Chinese Journal of Rare Metals, 2022, 46(6): 681-694. | |
| 9 | 寇佳伟, 程淑艳, 程芳琴. 类水滑石基催化剂光催化二氧化碳还原研究进展[J]. 化工进展, 2022, 41(S1): 190-198. |
| KOU Jiawei, CHENG Shuyan, CHENG Fangqin. Research advance of hydrotalcite-based catalysts in photocatalytic reduction of carbon dioxide[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 190-198. | |
| 10 | BADGETT Alex, FEISE Alison, STAR Andrew. Optimizing utilization of point source and atmospheric carbon dioxide as a feedstock in electrochemical CO2 reduction[J]. iScience, 2022, 25(5): 104270. |
| 143 | LIAO James C, MI Luo, PONTRELLI Sammy, et al. Fuelling the future: microbial engineering for the production of sustainable biofuels[J]. Nature Reviews Microbiology, 2016, 14(5): 288-304. |
| 144 | ZHANG Junli, LIU Guoxia, CARVAJAL Alonso I, et al. Discovery of a readily heterologously expressed Rubisco from the deep sea with potential for CO2 capture[J]. Bioresources and Bioprocessing, 2021, 8: 1-16. |
| 145 | YANG Fan, ZHANG Junli, CAI Zhen, et al. Exploring the oxygenase function of Form Ⅱ Rubisco for production of glycolate from CO2 [J]. AMB Express, 2021, 11(1): 65. |
| 146 | STEFFENS Lydia, PETTINATO Eugenio, STEINER Thomas M, et al. High CO2 levels drive the TCA cycle backwards towards autotrophy[J]. Nature, 2021, 592(7856): 784-788. |
| 147 | SAKIMOTO Kelsey K, WONG Andrew Barnabas, YANG Peidong. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production[J]. Science, 2016, 351(6268): 74-77. |
| 148 | Stefano CESTELLOS-BLANCO, ZHANG Hao, KIM Ji Min, et al. Photosynthetic semiconductor biohybrids for solar-driven biocatalysis[J]. Nature Catalysis, 2020, 3(3): 245-255. |
| 149 | GUO Junling, Miguel SUÁSTEGUI, SAKIMOTO Kelsey K, et al. Light-driven fine chemical production in yeast biohybrids[J]. Science, 2018, 362(6416): 813-816. |
| 11 | 顾洪宾, 范慧璞, 谢越韬, 等. 双碳背景下全球可再生能源领域发展机遇展望[J]. 国际工程与劳务, 2022(9): 22-25. |
| GU Hongbin, FAN Huipu, XIE Yuetao, et al. Prospects for development opportunities in the field of global renewable energy under the Dual Carbon background [J]. International Project Contracting & Labour Service, 2022(9): 22-25. | |
| 12 | SADOK Rachel, BENVENISTE Gabriela, WANG Ligang, et al. Life cycle assessment of power-to-gas applications via co-electrolysis of CO2 and H2O[J]. Journal of Physics: Energy, 2020, 2(2): 024006. |
| 13 | LIM Fatin Nasreen Ahmad Rizal, MARPANI Fauziah, DILOL Victoria Eliz Anak, et al. A review on the design and performance of enzyme-aided catalysis of carbon dioxide in membrane, electrochemical cell and photocatalytic reactors[J]. Membranes, 2021, 12(1): 28. |
| 14 | FU Jingwei, HUANG Yun, LIAO Qiang, et al. Photo-bioreactor design for microalgae: a review from the aspect of CO2 transfer and conversion[J]. Bioresource Technology, 2019, 292: 121947. |
| 15 | Mandy Ching Man YAU, HAYES Martin, KALATHIL Shafeer. Biocatalytic conversion of sunlight and carbon dioxide to solar fuels and chemicals[J]. RSC Advances, 2022, 12(26): 16396-16411. |
| 16 | Aişe ÜNLÜ, DUMAN-ÖZDAMAR Zeynep Efsun, Buse ÇALOĞLU, et al. Enzymes for efficient CO2 conversion[J]. The Protein Journal, 2021, 40(4): 489-503. |
| 17 | LIU Guanzhang, YUAN Hang, LI Xiaobo, et al. Tailoring the properties of self-assembled carbonic anhydrase supraparticles for CO2 capture[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(37): 12374-12385. |
| 18 | COHEN Steven E, Mehmet CAN, WITTENBORN Elizabeth C, et al. Crystallographic characterization of the carbonylated A-cluster in carbon monoxide dehydrogenase/acetyl-CoA synthase[J]. ACS Catalysis, 2020, 10(17): 9741-9746. |
| 19 | ZHANG Liyun, Mehmet CAN, RAGSDALE Stephen W, et al. Fast and selective photoreduction of CO2 to CO catalyzed by a complex of carbon monoxide dehydrogenase, TiO2, and Ag nanoclusters[J]. ACS Catalysis, 2018, 8(4): 2789-2795. |
| 20 | MENEGHELLO Marta, Christophe LÉGER, FOURMOND Vincent. Electrochemical studies of CO2-reducing metalloenzymes[J]. Chemistry: a European Journal, 2021, 27(70): 17542-17553. |
| 21 | HU Bo, HARRIS Derek F, DEAN Dennis R,et al. Electrocatalytic CO2 reduction catalyzed by nitrogenase MoFe and FeFe proteins[J]. Bioelectrochemistry, 2018, 120: 104-109. |
| 22 | STIEBRITZ Martin T, HILLER Caleb J, SICKERMAN Nathaniel S, et al. Ambient conversion of CO2 to hydrocarbons by biogenic and synthetic [Fe4S4] clusters[J]. Nature Catalysis, 2018, 1(6): 444-451. |
| 23 | MAIA Luisa B, FONSECA Luis, MOURA Isabel, et al. Reduction of carbon dioxide by a molybdenum-containing formate dehydrogenase: A kinetic and mechanistic study[J]. Journal of the American Chemical Society, 2016, 138(28): 8834-8846. |
| 24 | Saadet ALPDAĞTAŞ, TURUNEN Ossi, VALJAKKA Jarkko, et al. The challenges of using NAD+-dependent formate dehydrogenases for CO2 conversion[J]. Critical Reviews in Biotechnology, 2022, 42(6): 953-972. |
| 25 | 于洋, 刘琦, 吕静, 等. 碳酸酐酶固定及在二氧化碳捕集应用研究进展[J]. 洁净煤技术, 2021, 27(2): 69-78. |
| YU Yang, LIU Qi, Jing LYU, et al. Research progress on the immobilization of carbonic anhydrase and its application in carbon dioxide capture[J]. Clean Coal Technology, 2021, 27(2): 69-78. | |
| 26 | JEOUNG Jae Hun, MARTINS Berta M, DOBBEK Holger. Carbon monoxide dehydrogenases[M]//Methods in Molecular Biology. New York, NY: Springer New York, 2018: 37-54. |
| 27 | HOWARD James B, REES Douglas C. Structural basis of biological nitrogen fixation[J]. Chemical Reviews, 1996, 96(7): 2965-2982. |
| 28 | REBELEIN Johannes G, STIEBRITZ Martin T, LEE Chi Chung, et al. Activation and reduction of carbon dioxide by nitrogenase iron proteins[J]. Nature Chemical Biology, 2017, 13(2): 147-149. |
| 29 | RETTBERG Lee A, KANG Wonchull, STIEBRITZ Martin T, et al. Structural analysis of a nitrogenase iron protein from methanosarcina acetivorans: Implications for CO2 capture by a surface-exposed [Fe4S4] cluster[J]. mBio, 2019, 10(4): e01497-e01419. |
| 30 | ZHENG Yanning, HARRIS Derek F, YU Zheng, et al. A pathway for biological methane production using bacterial iron-only nitrogenase[J]. Nature Microbiology, 2018, 3(3): 281-286. |
| 31 | OEHLMANN Niels N, REBELEIN Johannes G. The conversion of carbon monoxide and carbon dioxide by nitrogenases[J]. Chembiochem: a European Journal of Chemical Biology, 2022, 23(8): e202100453. |
| 32 | MOON Myounghoon, PARK Gwon Woo, LEE Joon Pyo. Recent progress in formate dehydrogenase (FDH) as a non-photosynthetic CO2 utilizing enzyme: a short review[J]. Journal of CO2 Utilization, 2020, 42: 101353. |
| 33 | Mervan ÇAKAR M, Juan MANGAS-SANCHEZ, BIRMINGHAM William R, et al. Discovery of a new metal and NAD+-dependent formate dehydrogenase from Clostridium ljungdahlii [J]. Preparative Biochemistry & Biotechnology, 2018, 48(4): 327-334. |
| 34 | MIN Kyoungseon, MOON Myounghoon, PARK Gwon Woo, et al. Newly explored formate dehydrogenases from Clostridium species catalyze carbon dioxide to formate[J]. Bioresource Technology, 2022, 348: 126832. |
| 35 | Su Keun KUK, GOPINATH Krishnasamy, SINGH Raushan K, et al. NADH-free electroenzymatic reduction of CO2 by conductive hydrogel-conjugated formate dehydrogenase[J]. ACS Catalysis, 2019, 9(6): 5584-5589. |
| 36 | Concepción IÑIGUEZ, Pere AGUILÓ-NICOLAU, Jeroni GALMÉS. Improving photosynthesis through the enhancement of Rubisco carboxylation capacity[J]. Biochemical Society Transactions, 2021, 49(5): 2007-2019. |
| 37 | ASHIDA Hiroki, MIZOHATA Eiichi, YOKOTA Akiho. Learning RuBisCO’s birth and subsequent environmental adaptation[J]. Biochemical Society Transactions, 2019, 47(1): 179-185. |
| 38 | WILSON Robert H, Manajit HAYER-HARTL. Complex chaperone dependence of rubisco biogenesis[J]. Biochemistry, 2018, 57(23): 3210-3216. |
| 39 | BRACHER Andreas, WHITNEY Spencer M, Ulrich HARTL F, et al. Biogenesis and metabolic maintenance of rubisco[J]. Annual Review of Plant Biology, 2017, 68: 29-60. |
| 40 | LIU Yuwan, JIANG Huifeng. Directed evolution of propionyl-CoA carboxylase for succinate biosynthesis[J]. Trends in Biotechnology, 2021, 39(4): 330-331. |
| 41 | JIANG Wei, VILLAMOR David Hernández, PENG Huadong, et al. Metabolic engineering strategies to enable microbial utilization of C1 feedstocks[J]. Nature Chemical Biology, 2021, 17(8): 845-855. |
| 42 | SONG Ping, WANG Mengli, ZHENG Qingyang, et al. Isocitrate dehydrogenase 1 from acinetobacter baummanii (AbIDH1) enzymatic characterization and its regulation by phosphorylation[J]. Biochimie, 2021, 181: 77-85. |
| 43 | CHENG Hsieh-Ting-Yang, Shou-Chen LO, HUANG Chieh-Chen, et al. Detailed profiling of carbon fixation of in silico synthetic autotrophy with reductive tricarboxylic acid cycle and Calvin-Benson-Bassham cycle in Esherichia coli using hydrogen as an energy source[J]. Synthetic and Systems Biotechnology, 2019, 4(3): 165-172. |
| 44 | CHEN Yijing, LI Peng, Hyunho NOH, et al. Stabilization of formate dehydrogenase in a metal-organic framework for bioelectrocatalytic reduction of CO2 [J]. Angewandte Chemie International Edition, 2019, 58(23): 7682-7686. |
| 45 | SRIKANTH Sandipam, MAESEN Miranda, Xochitl DOMINGUEZ-BENETTON, et al Enzymatic electrosynthesis of formate through CO 2 sequestration/reduction in a bioelectrochemical system (BES)[J]. Bioresource Technology, 2014, 165: 350-354. |
| 46 | Dong Heon NAM, Su Keun KUK, CHOE Hyunjun, et al. Enzymatic photosynthesis of formate from carbon dioxide coupled with highly efficient photoelectrochemical regeneration of nicotinamide cofactors[J]. Green Chemistry, 2016, 18(22): 5989-5993. |
| 47 | AMAO Y, FUJIMURA M, MIYAZAKI M, et al. A visible-light driven electrochemical biofuel cell with the function of CO2 conversion to formic acid: Coupled thylakoid from microalgae and biocatalyst immobilized electrodes[J]. New Journal of Chemistry, 2018, 42(11): 9269-9280. |
| 48 | SOKOL Katarzyna P, ROBINSON William E, OLIVEIRA Ana R, et al. Photoreduction of CO2 with a formate dehydrogenase driven by photosystem Ⅱ using a semi-artificial Z-scheme architecture[J]. Journal of the American Chemical Society, 2018, 140(48): 16418-16422. |
| 49 | Su Keun KUK, Youngjin HAM, GOPINATH Krishnasamy, et al. Continuous 3D titanium nitride nanoshell structure for solar-driven unbiased biocatalytic CO2 reduction[J]. Advanced Energy Materials, 2019, 9(25): 1900029. |
| 50 | ZHANG Zhibo, VASILIU Tudor, LI Fangfang, et al. Electrochemically driven efficient enzymatic conversion of CO2 to formic acid with artificial cofactors[J]. Journal of CO2 Utilization, 2021, 52: 101679. |
| 51 | Su Keun KUK, SINGH Raushan K, Dong Heon NAM, et al. Photoelectrochemical reduction of carbon dioxide to methanol through a highly efficient enzyme cascade[J]. Angewandte Chemie International Edition, 2017, 56(14): 3827-3832. |
| 52 | ZHANG Zhibo, LI Jiajia, JI Mingbo, et al. Encapsulation of multiple enzymes in a metal-organic framework with enhanced electro-enzymatic reduction of CO2 to methanol[J]. Green Chemistry, 2021, 23(6): 2362-2371. |
| 53 | ZHANG Zhibo, WANG Hui, NIE Yi, et al. Natural deep eutectic solvents enhanced electro-enzymatic conversion of CO2 to methanol[J]. Frontiers in Chemistry, 2022, 10: 894106. |
| 54 | QUADIR Mohd Golam Abdul, GOSWAMI Pranab, SARMA Mrinal Kumar. Photoelectrochemical and photosynthetic material-based biosensors[M]//Advanced Materials and Techniques for Biosensors and Bioanalytical Applications. Boca Raton: CRC Press, 2020: 183-210. |
| 55 | MICHELIN Clément, HOFFMANN Norbert. Photocatalysis applied to organic synthesis —A green chemistry approach[J]. Current Opinion in Green and Sustainable Chemistry, 2018, 10: 40-45. |
| 56 | ZHANG Shaohua, LIU Shusong, SUN Yiying, et al. Enzyme-photo-coupled catalytic systems[J]. Chemical Society Reviews, 2021, 50(24): 13449-13466. |
| 57 | WOOLERTON Thomas W, SHEARD Sally, REISNER Erwin, et al. Efficient and clean photoreduction of CO2 to CO by enzyme-modified TiO2 nanoparticles using visible light[J]. Journal of the American Chemical Society, 2010, 132(7): 2132-2133. |
| 58 | WOOLERTON Thomas W, SHEARD Sally, PIERCE Elizabeth, et al. CO2 photoreduction at enzyme-modified metal oxidenanoparticles[J]. Energy & Environmental Science, 2011, 4(7): 2393-2399. |
| 59 | YADAV Rajesh K, Gyu Hwan OH, PARK No Joong, et al. Highly selective solar-driven methanol from CO2 by a photocatalyst/biocatalyst integrated system[J]. Journal of the American Chemical Society, 2014, 136(48): 16728-16731. |
| 60 | LIU Jian, CAZELLES Rémi, CHEN Zupeng, et al. The bioinspired construction of an ordered carbon nitride array for photocatalytic mediated enzymatic reduction[J]. Physical Chemistry Chemical Physics, 2014, 16(28): 14699-14705. |
| 61 | AMAO Yutaka, KATAOKA Ryota. Methanol production from CO2 with the hybrid system of biocatalyst and organo-photocatalyst[J]. Catalysis Today, 2018, 307: 243-247. |
| 62 | ZHANG Zhibo, TONG Jiahuan, MENG Xianglei, et al. Development of an ionic porphyrin-based platform as a biomimetic light-harvesting agent for high-performance photoenzymatic synthesis of methanol from CO2 [J]. ACS Sustainable Chemistry & Engineering, 2021, 9(34): 11503-11511. |
| 63 | ZHANG Shaohua, SHI Jiafu, SUN Yiying, et al. Artificial thylakoid for the coordinated photoenzymatic reduction of carbon dioxide[J]. ACS Catalysis, 2019, 9(5): 3913-3925. |
| 64 | YADAV Dolly, YADAV Rajesh K, KUMAR Abhishek, et al. Fullerene polymer film as a highly efficient photocatalyst for selective solar fuel production from CO2 [J]. Journal of Applied Polymer Science, 2020, 137(14): 48536. |
| 65 | GU Fengjuan WANG Yanzi, MENG Zihui, et al. A coupled photocatalytic/enzymatic system for sustainable conversion of CO2 to formate[J]. Catalysis Communications, 2020, 136: 105903. |
| 66 | TIAN Yao, ZHOU Yinuo, ZONG Yongchao, et al. Construction of functionally compartmental inorganic photocatalyst-enzyme system via imitating chloroplast for efficient photoreduction of CO2 to formic acid[J]. ACS Applied Materials & Interfaces, 2020, 12(31): 34795-34805. |
| 67 | SCHLAGER Stefanie, DUMITRU Liviu Mihai, HABERBAUER Marianne, et al. Electrochemical reduction of carbon dioxide to methanol by direct injection of electrons into immobilized enzymes on a modified electrode[J]. ChemSusChem, 2016, 9(6): 631-635. |
| 68 | ZHOU Junhui, TIAN Xinyu, YANG Qian, et al. Three multi-enzyme cascade pathways for conversion of C1 to C2/C4 compounds[J]. Chem Catalysis, 2022, 2(10): 2675-2690. |
| 69 | MILLER Tarryn E, BENEYTON Thomas, SCHWANDER Thomas, et al. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts[J]. Science, 2020, 368(6491): 649-654. |
| 70 | Naiara HERNÁNDEZ-IBÁÑEZ, Alicia GOMIS-BERENGUER, MONTIEL Vicente, et al. Fabrication of a biocathode for formic acid production upon the immobilization of formate dehydrogenase from Candida boidinii on a nanoporous carbon[J]. Chemosphere, 2022, 291(Pt 3): 133117. |
| 71 | CHEN Yijing, LI Peng, ZHOU Jiawang, et al. Integration of enzymes and photosensitizers in a hierarchical mesoporous metal-organic framework for light-driven CO2 reduction[J]. Journal of the American Chemical Society, 2020, 142(4): 1768-1773. |
| 72 | LIN Gang, ZHANG Yuanyuan, HUA Yutao, et al. Bioinspired metalation of the metal-organic framework MIL-125-NH2 for photocatalytic NADH regeneration and gas-liquid-solid three-phase enzymatic CO2 reduction[J]. Angewandte Chemie International Edition, 2022, 61(31): e202206283. |
| 73 | RASOULI Hannaneh, ILIUTA Ion, BOUGIE Francis, et al. Hybrid enzymatic CO2 capture process in intensified flat sheet membrane contactors with immobilized carbonic anhydrase[J]. Separation and Purification Technology, 2022, 287: 120505. |
| 74 | LI Huaiguang, BUESEN Darren, DEMENTIN Sébastien, et al. Complete protection of O2-sensitive catalysts in thin films[J]. Journal of the American Chemical Society, 2019, 141(42): 16734-16742. |
| 75 | SCHLAGER Stefanie, DIBENEDETTO Angela, ARESTA Michele, et al. Biocatalytic and bioelectrocatalytic approaches for the reduction of carbon dioxide using enzymes[J]. Energy Technology (Weinheim, Germany), 2017, 5(6): 812-821. |
| 76 | YADAV Rajesh K, BAEG Jin Ook, KUMAR Abhishek, et al. Graphene-BODIPY as a photocatalyst in the photocatalytic-biocatalytic coupled system for solar fuel production from CO2 [J]. Journal of Materials Chemistry A, 2014, 2(14): 5068-5076. |
| 77 | OLIVEIRA Ana Rita, MOTA Cristiano, MOURATO Cláudia, et al. Toward the mechanistic understanding of enzymatic CO2 reduction[J]. ACS Catalysis, 2020, 10(6): 3844-3856. |
| 78 | CHENG Yuqing, SHI Jiafu, WU Yizhou, et al. Intensifying electron utilization by surface-anchored Rh complex for enhanced nicotinamide cofactor regeneration and photoenzymatic CO2 reduction[J]. Research (Washington D C), 2021, 2021: 8175709. |
| 79 | LEE Soo Youn, You-Kwan OH, LEE Sangmin, et al. Recent developments and key barriers to microbial CO2 electrobiorefinery[J]. Bioresource Technology, 2021, 320(Pt A): 124350. |
| 80 | Paolo DESSÌ, Laura ROVIRA-ALSINA, Carlos SÁNCHEZ, et al. Microbial electrosynthesis: Towards sustainable biorefineries for production of green chemicals from CO2 emissions[J]. Biotechnology Advances, 2021, 46: 107675. |
| 81 | 王凯, 刘子鹤, 陈必强, 等. 微生物利用二氧化碳合成燃料及化学品——第三代生物炼制[J]. 合成生物学, 2020, 1(1): 60-70. |
| WANG Kai, LIU Zihe, CHEN Biqiang, et al. Microbial utilization of carbon dioxide to synthesize fuels and chemicals—Third-generation biorefineries[J]. Synthetic Biology Journal, 2020, 1(1): 60-70. | |
| 82 | CHEN Hui, DONG Fangyuan, MINTEER Shelley D. The progress and outlook of bioelectrocatalysis for the production of chemicals, fuels and materials[J]. Nature Catalysis, 2020, 3(3): 225-244. |
| 83 | MAYER Florian, ENZMANN Franziska, LOPEZ Antonio Martinez, et al. Performance of different methanogenic species for the microbial electrosynthesis of methane from carbon dioxide[J]. Bioresource Technology, 2019, 289: 121706. |
| 84 | 由紫暄, 李锋, 宋浩. 电能细胞的合成生物学设计构建[J]. 合成生物学, 2022, 3(5): 1031-1059. |
| YOU Zixuan, LI Feng, SONG Hao. Design and construction of electroactive cells by synthetic biology strategies[J]. Synthetic Biology Journal, 2022, 3(5): 1031-1059. | |
| 85 | JANG Jungho, JEON Byoung Wook, KIM Yong Hwan. Bioelectrochemical conversion of CO2 to value added product formate using engineered Methylobacterium extorquens [J]. Scientific Reports, 2018, 8: 7211. |
| 86 | LI Han, OPGENORTH Paul H, WERNICK David G, et al. Integrated electromicrobial conversion of CO2 to higher alcohols[J]. Science, 2012, 335(6076): 1596. |
| 87 | YU Linpeng, YUAN Yong, TANG Jiahuan, et al. Thermophilic moorella thermoautotrophica-immobilized cathode enhanced microbial electrosynthesis of acetate and formate from CO2 [J]. Bioelectrochemistry, 2017, 117: 23-28. |
| 88 | TASHIRO Yohei, HIRANO Shinichi, MATSON Morgan M, et al. Electrical-biological hybrid system for CO2 reduction[J]. Metabolic Engineering, 2018, 47: 211-218. |
| 89 | WU Zaiqiang, WANG Junsong, LIU Jun, et al. Engineering an electroactive escherichia coli for the microbial electrosynthesis of succinate from glucose and CO2 [J]. Microbial Cell Factories, 2019, 18(1): 15. |
| 90 | SINGH Shiv, NOORI Mohammad T, VERMA Nishith. Efficient bio-electroreduction of CO2 to formate on a iron phthalocyanine-dispersed CDC in microbial electrolysis system[J]. Electrochimica Acta, 2020, 338: 135887. |
| 91 | WU Haoliang, PAN Haojie, LI Zhongjian, et al. Efficient production of lycopene from CO2 via microbial electrosynthesis[J]. Chemical Engineering Journal, 2022, 430: 132943. |
| 92 | KRIEG Thomas, SYDOW Anne, FAUST Sonja, et al. CO2 to terpenes: Autotrophic and electroautotrophic α-humulene production with Cupriavidus necator [J]. Angewandte Chemie International Edition, 2018, 57(7): 1879-1882. |
| 93 | ARENDS Jan B A, PATIL Sunil A, ROUME Hugo, et al. Continuous long-term electricity-driven bioproduction of carboxylates and isopropanol from CO2 with a mixed microbial community[J]. Journal of CO2 Utilization, 2017, 20: 141-149. |
| 94 | NAKASUGI Yasuhito, HIMENO Masanori, KOBAYASHI Hajime, et al. Experimental and mathematical analyses of bio-electrochemical conversion of carbon dioxide to methane[J]. Energy Procedia, 2017, 114: 7133-7140. |
| 95 | Sovik DAS, CHATTERJEE Pritha, GHANGREKAR M M. Increasing methane content in biogas and simultaneous value added product recovery using microbial electrosynthesis[J]. Water Science and Technology, 2018, 77(5): 1293-1302. |
| 96 | DEL PILAR ANZOLA ROJAS Mélida, MATEOS Raúl, SOTRES Ana, et al. Microbial electrosynthesis (MES) from CO2 is resilient to fluctuations in renewable energy supply[J]. Energy Conversion and Management, 2018, 177: 272-279. |
| 97 | SRIKANTH Sandipam, KUMAR Manoj, SINGH Dheer, et al. Long-term operation of electro-biocatalytic reactor for carbon dioxide transformation into organic molecules[J]. Bioresource Technology, 2018, 265: 66-74. |
| 98 | JOURDIN Ludovic, WINKELHORST Marijn, RAWLS Brian, et al. Enhanced selectivity to butyrate and caproate above acetate in continuous bioelectrochemical chain elongation from CO2: Steering with CO2 loading rate and hydraulic retention time[J]. Bioresource Technology Reports, 2019, 7: 100284. |
| 99 | YANG Hou-Yun, WANG Yi-Xuan, HE Chuan-Shu, et al. Redox mediator-modified biocathode enables highly efficient microbial electro-synthesis of methane from carbon dioxide[J]. Applied Energy, 2020, 274: 115292. |
| 100 | QI Xuejiao, JIA Xuan, WANG Yong, et al. Development of a rapid startup method of direct electron transfer-dominant methanogenic microbial electrosynthesis[J]. Bioresource Technology, 2022, 358: 127385. |
| 101 | WU Yun, LI Weichao, WANG Lutian, et al. Enhancing the selective synthesis of butyrate in microbial electrosynthesis system by gas diffusion membrane composite biocathode[J]. Chemosphere, 2022, 308(Pt 1): 136088. |
| 102 | TAHIR Khurram, MIRAN Waheed, JANG Jiseon, et al. Enhanced product selectivity in the microbial electrosynthesis of butyrate using a nickel ferrite-coated biocathode[J]. Environmental Research, 2021, 196: 110907. |
| 103 | LI Zhigang, CAI Jiayi, GAO Yu, et al. Efficient production of medium chain fatty acids in microbial electrosynthesis with simultaneous bio-utilization of carbon dioxide and ethanol[J]. Bioresource Technology, 2022, 352: 127101. |
| 104 | YE Jie, HU Andong, REN Guoping, et al. Enhancing sludge methanogenesis with improved redox activity of extracellular polymeric substances by hematite in red mud[J]. Water Research, 2018, 134: 54-62. |
| 105 | WANG Ruiwen, LI Huidong, SUN Jinzhi, et al. Nanomaterials facilitating microbial extracellular electron transfer at interfaces[J]. Advanced Materials (Deerfield Beach, Fla), 2021, 33(6): e2004051. |
| 106 | LUO Haiping, QI Jiaxin, ZHOU Meizhou, et al. Enhanced electron transfer on microbial electrosynthesis biocathode by polypyrrole-coated acetogens[J]. Bioresource Technology, 2020, 309: 123322. |
| 107 | KRACKE Frauke, DEUTZMANN Jörg S, JAYATHILAKE Buddhinie S, et al. Efficient hydrogen delivery for microbial electrosynthesis via 3D-printed cathodes[J]. Frontiers in Microbiology, 2021, 12: 696473. |
| 108 | TAN Xinyi, NIELSEN Jens. The integration of bio-catalysis and electrocatalysis to produce fuels and chemicals from carbon dioxide[J]. Chemical Society Reviews, 2022, 51(11): 4763-4785. |
| 109 | SHI Xiaochen, TREMBLAY Pier-Luc, WAN Lulu, et al. Improved robustness of microbial electrosynthesis by adaptation of a strict anaerobic microbial catalyst to molecular oxygen[J]. The Science of the Total Environment, 2021, 754: 142440. |
| 110 | LI Tao, YANG Xiaoli, CHEN Qiaoling, et al. Enhanced performance of microbial fuel cells with electron mediators from anthraquinone/polyphenol-abundant herbal plants[J]. ACS Sustainable Chemistry & Engineering, 2020, 8: 11263-11275. |
| 111 | LI Tao, SONG Hailiang, XU Han, et al. Biological detoxification and decolorization enhancement of azo dye by introducing natural electron mediators in MFCs[J]. Journal of Hazardous Materials, 2021, 416: 125864. |
| 112 | LABELLE Edward V, MARSHALL Christopher W, Harold D MAY. Microbiome for the electrosynthesis of chemicals from carbon dioxide[J]. Accounts of Chemical Research, 2020, 53(1): 62-71. |
| 113 | HENGSBACH Jan-Niklas, Björn SABEL-BECKER, ULBER Roland, et al. Microbial electrosynthesis of methane and acetate-comparison of pure and mixed cultures[J]. Applied Microbiology and Biotechnology, 2022, 106(12): 4427-4443. |
| 114 | NANGLE Shannon N, ZIESACK Marika, BUCKLEY Sarabeth, et al. Valorization of CO2 through lithoautotrophic production of sustainable chemicals in Cupriavidus necator [J]. Metabolic Engineering, 2020, 62: 207-220. |
| 115 | BRIGHAM Christopher. Perspectives for the biotechnological production of biofuels from CO2 and H2 using Ralstonia eutropha and other ‘Knallgas’ bacteria[J]. Applied Microbiology and Biotechnology, 2019, 103(5): 2113-2120. |
| 116 | LIU Xiutao, FENG Xinjun, DING Yamei, et al. Characterization and directed evolution of propionyl-CoA carboxylase and its application in succinate biosynthetic pathway with two CO2 fixation reactions[J]. Metabolic Engineering, 2020, 62: 42-50. |
| 117 | BOURGADE Barbara, MINTON Nigel P, AHSANUL ISLAM M. Genetic and metabolic engineering challenges of C1-gas fermenting acetogenic chassis organisms[J]. FEMS Microbiology Reviews, 2021, 45(2): fuab008. |
| 118 | SONG Yoseb, Jiyun BAE, JIN Sangrak, et al. Development of highly characterized genetic bioparts for efficient gene expression in CO2-fixing Eubacterium limosum [J]. Metabolic Engineering, 2022, 72: 215-226. |
| 119 | LAKATOS Gergely Ernő, Karolína RANGLOVÁ, MANOEL João Câmara, et al. Bioethanol production from microalgae polysaccharides[J]. Folia Microbiologica, 2019, 64(5): 627-644. |
| 120 | 崔金玉, 张爱娣, 栾国栋, 等. 微藻光驱固碳合成技术的发展现状与未来展望[J]. 合成生物学, 2022, 3(5): 884-900. |
| CUI Jinyu, ZHANG Aidi, LUAN Guodong, et al. Engineering microalgae for photosynthetic biosynthesis: progress and prospect[J]. Synthetic Biology Journal, 2022, 3(5): 884-900. | |
| 121 | LIU Xufeng, XIE Hao, ROUSSOU Stamatina, et al. Current advances in engineering cyanobacteria and their applications for photosynthetic butanol production[J]. Current Opinion in Biotechnology, 2022, 73: 143-150. |
| 122 | KHAMOUSHI Atefeh, TAFAKORI Vida, ZAHED Mohammad Ali, et al. Augmenting the expression of accD and rbcL genes using optimized iron concentration to achieve higher biomass and biodiesel in Chlorella vulgaris [J]. Biotechnology Letters, 2020, 42(12): 2631-2641. |
| 123 | ZAHED Mohammad Ali, MOVAHED Elaheh, KHODAYARI Arezoo, et al. Biotechnology for carbon capture and fixation: Critical review and future directions[J]. Journal of Environmental Management, 2021, 293: 112830. |
| 124 | CHEN Jih-Heng, KATO Yuichi, MATSUDA Mami, et al. Lutein production with Chlorella sorokiniana MB-1-M12 using novel two-stage cultivation strategies-metabolic analysis and process improvement[J]. Bioresource Technology, 2021, 334: 125200. |
| 125 | 王松, 吴莎, 江亚男, 等. 微藻光合作用的优化升级助力“双碳”目标[J]. 合成生物学, 2022, 3(5): 915-931. |
| WANG Song, WU Sha, JIANG Yanan, et al. Optimization and upgradation of microalgal photosynthesis for carbon peak and carbon neutrality goals[J]. Synthetic Biology Journal, 2022, 3(5): 915-931. | |
| 126 | WANG Bo, JIANG Zhifeng, YU Jimmy C, et al. Enhanced CO2 reduction and valuable C2+ chemical production by a CdS-photosynthetic hybrid system[J]. Nanoscale, 2019, 11(19): 9296-9301. |
| 127 | 陈梦园. 沼泽红假单胞菌/硫化镉杂合体系构建及其光催化固定二氧化碳产甲烷性能的研究[D]. 镇江: 江苏大学, 2021. |
| CHEN Mengyuan. Photocatalytic methane production from carbon dioxide by a newly constructed rhodopseudomonas palustris/CdS biohybrid system[D]. Zhenjiang: Jiangsu University, 2021. | |
| 128 | JIN Sangrak, JEON Yale, JEON Min Soo, et al. Acetogenic bacteria utilize light-driven electrons as an energy source for autotrophic growth[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(9): e2020552118. |
| 129 | DOGUTAN Dilek K, NOCERA Daniel G. Artificial photosynthesis at efficiencies greatly exceeding that of natural photosynthesis[J]. Accounts of Chemical Research, 2019, 52(11): 3143-3148. |
| 130 | HUANG Lingyan, LIU Xing, ZHANG Zhishuai, et al. Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri in an electric syntrophic coculture[J]. The ISME Journal, 2022, 16(2): 370-377. |
| 131 | HU Andong, YE Jie, REN Guoping, et al. Metal-free semiconductor-based bio-nano hybrids for sustainable CO2-to-CH4 conversion with high quantum yield[J]. Angewandte Chemie International Edition, 2022, 61(35): e202206508. |
| 132 | ZHANG Hao, LIU Hao, TIAN Zhiquan, et al. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production[J]. Nature Nanotechnology, 2018, 13(10): 900-905. |
| 133 | TREMBLAY Pier-Luc, XU Mengying, CHEN Yiming, et al. Nonmetallic abiotic-biological hybrid photocatalyst for visible water splitting and carbon dioxide reduction[J]. iScience, 2020, 23(1): 100784. |
| 134 | SONG Tianshun, LI Tao, TAO Ran, et al. CuO/g-C3N4 heterojunction photocathode enhances the microbial electrosynthesis of acetate through CO2 reduction[J]. Science of the Total Environment, 2022, 818: 151820. |
| 135 | GAI Panpan, YU Wen, ZHAO Hao, et al. Solar-powered organic semiconductor-bacteria biohybrids for CO2 reduction into acetic acid[J]. Angewandte Chemie (International Ed in English), 2020, 59(18): 7224-7229. |
| 136 | Stefano CESTELLOS-BLANCO, KIM Ji Min, WATANABE Nicholas George, et al. Molecular insights and future frontiers in cell photosensitization for solar-driven CO2 conversion[J]. iScience, 2021, 24(9): 102952. |
| 137 | Jung-Ho JO, Dae-Yeon JO, LEE Sun-Hyoung, et al. InP-based quantum dots having an InP core, composition-gradient ZnSeS inner shell, and ZnS outer shell with sharp, bright emissivity and blue absorptivity for display devices[J]. ACS Applied Nano Materials, 2020, 3(2): 1972-1980. |
| 138 | SU Yude, Stefano CESTELLOS-BLANCO, KIM Ji Min, et al. Close-packed nanowire-bacteria hybrids for efficient solar-driven CO2 fixation[J]. Joule, 2020, 4(4): 800-811. |
| 139 | Stefano CESTELLOS-BLANCO, CHAN Rachel R, SHEN Yuexiao, et al. Photosynthetic biohybrid coculture for tandem and tunable CO2 and N2 fixation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(26): e2122364119. |
| 140 | WANG Xiaodong, SABA Tony, Humphrey H P YIU, et al. Cofactor NAD(P)H regeneration inspired by heterogeneous pathways[J]. Chem, 2017, 2(5): 621-654. |
| 141 | XU Yangyang, RAMANATHAN Veerabhadran, VICTOR David G. Global warming will happen faster than we think[J]. Nature, 2018, 564(7734): 30-32. |
| 142 | SULTANA Sabiha, CHANDRA SAHOO Prakash, MARTHA Satyabadi, et al. A review of harvesting clean fuels from enzymatic CO2 reduction[J]. RSC Advances, 2016, 6(50): 44170-44194. |
| [1] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [4] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [7] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [8] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [9] | 徐若思, 谭蔚. C形管池沸腾两相流流场模拟与流固耦合分析[J]. 化工进展, 2023, 42(S1): 47-55. |
| [10] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [11] | 耿源泽, 周俊虎, 张天佑, 朱晓宇, 杨卫娟. 部分填充床燃烧器中庚烷均相/异相耦合燃烧[J]. 化工进展, 2023, 42(9): 4514-4521. |
| [12] | 雷伟, 姜维佳, 王玉高, 和明豪, 申峻. N、S共掺杂煤基碳量子点的电化学氧化法制备及用于Fe3+检测[J]. 化工进展, 2023, 42(9): 4799-4807. |
| [13] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [14] | 吕程远, 张函, 杨明旺, 杜健军, 樊江莉. 生物成像用二氧杂环丁烷余辉发光体系的研究进展[J]. 化工进展, 2023, 42(8): 4108-4122. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||