化工进展 ›› 2022, Vol. 41 ›› Issue (12): 6213-6225.DOI: 10.16085/j.issn.1000-6613.2021-2238
吸附法碳捕集固体胺吸附剂成型技术研究进展
雷婷1( ), 喻树楠1, 周昶安1, 宋磊1, 马奎1, 李子鹏2, 岳海荣1,3(
), 喻树楠1, 周昶安1, 宋磊1, 马奎1, 李子鹏2, 岳海荣1,3( )
)
- 1.四川大学化学工程学院,四川 成都 610065
2.湖北省地质调查院,湖北 武汉 430034
3.四川大学新能源与 低碳技术研究院,四川 成都 610207
-
收稿日期:2021-11-01修回日期:2022-08-27出版日期:2022-12-20发布日期:2022-12-29 -
通讯作者:岳海荣 -
作者简介:雷婷(1983—),女,硕士研究生,研究方向为碳捕集与矿物加工。E-mail:leit@newhope.cn。 -
基金资助:国家重点研发计划(2022YFC2904700);湖北省地质调查院合作项目(22H0479)
Research progress on the shaping technology of solid amine adsorbents for CO2 capture by adsorption method
LEI Ting1( ), YU Shunan1, ZHOU Chang’an1, SONG Lei1, MA Kui1, LI Zipeng2, YUE Hairong1,3(
), YU Shunan1, ZHOU Chang’an1, SONG Lei1, MA Kui1, LI Zipeng2, YUE Hairong1,3( )
)
- 1.School of Chemical Engineering, Sichuan University, Chengdu 610065, Sichuan, China
2.Hubei Geological Survey, Wuhan 430034, Hubei, China
3.Institute of New Energy and Low-Carbon Technology, Sichuan University, Chengdu 610207, Sichuan, China
-
Received:2021-11-01Revised:2022-08-27Online:2022-12-20Published:2022-12-29 -
Contact:YUE Hairong
摘要:
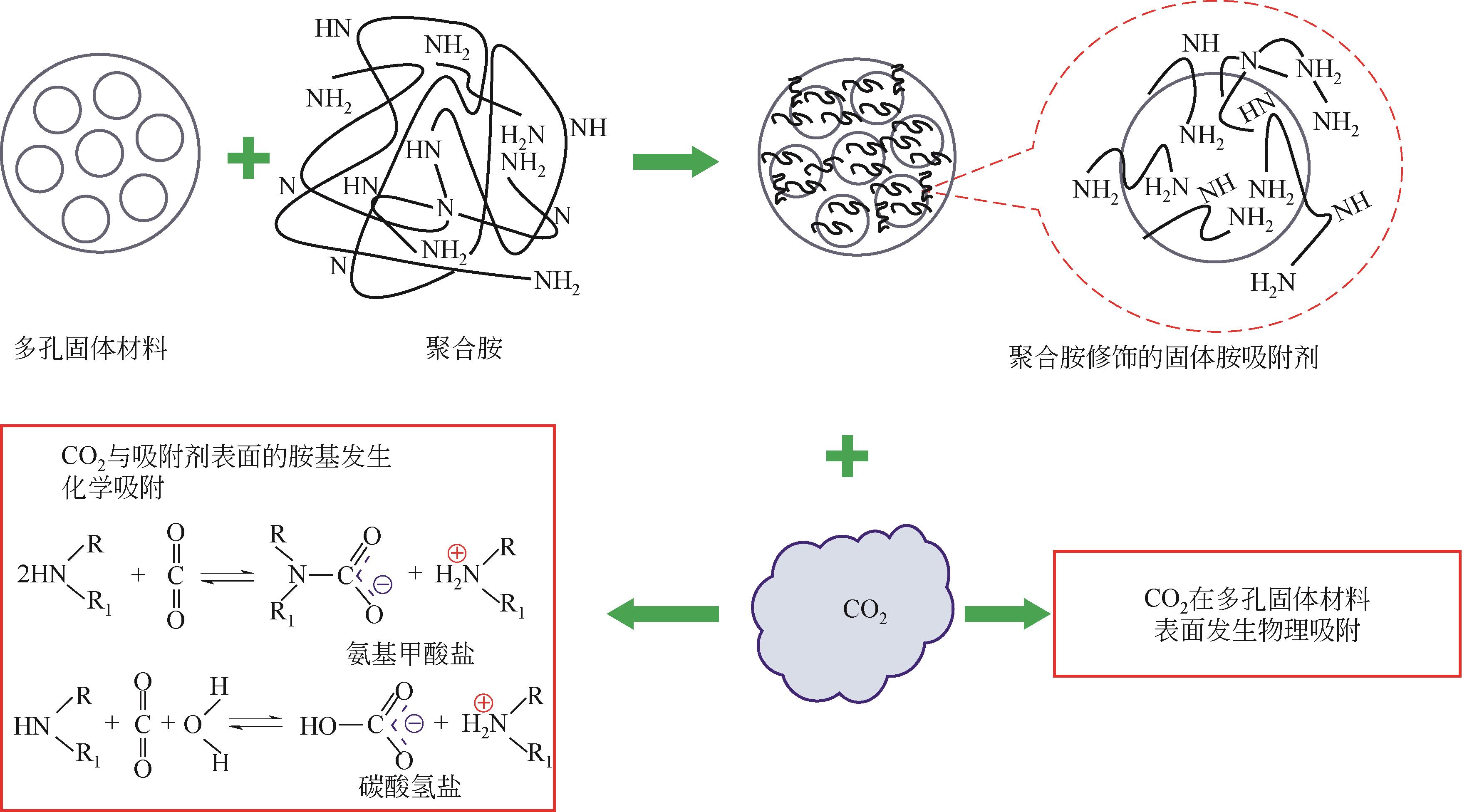
吸附法碳捕集技术是实现工业过程或大气中CO2分离与脱除的重要途径之一,高性能吸附剂的开发是该技术的关键。固体胺吸附剂由于其优异的CO2吸附量、选择性以及较低的再生能耗,近年来受到了广泛的关注,但用于工业的成型吸附剂仍面临机械强度低、稳定性差和胺流失严重等关键难题,难以在工业中大范围的推广应用。本文分析了固体胺成型吸附剂制备面临的主要难题,重点总结了近年来国内外吸附剂成型技术的研发进展,并对固体胺工业吸附剂的发展方向进行了展望。未来固体胺吸附法碳捕集技术的研发重点在于立足吸附反应机理和工业烟气的特性,创新成型固体胺吸附剂制备技术,提升吸附剂的CO2吸附量、胺效率、机械与循环稳定性,研发低能耗的配套吸附工艺和核心装置。
中图分类号:
引用本文
雷婷, 喻树楠, 周昶安, 宋磊, 马奎, 李子鹏, 岳海荣. 吸附法碳捕集固体胺吸附剂成型技术研究进展[J]. 化工进展, 2022, 41(12): 6213-6225.
LEI Ting, YU Shunan, ZHOU Chang’an, SONG Lei, MA Kui, LI Zipeng, YUE Hairong. Research progress on the shaping technology of solid amine adsorbents for CO2 capture by adsorption method[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6213-6225.
| 捕集技术 | 吸收/吸附剂 |  |  | 文献 |
|---|---|---|---|---|
| 溶剂吸收法 | MEA | 3900 | 62.8 | [ |
| 固体吸附法 | PEI/silica | — | 48.1~75.2 | [ |
| 固体吸附法 | TEPA/MCM-41 | 1800 | 29.68 | [ |
| 固体吸附法 | EB-PEI/SiO2 | 22300 | — | [ |
| 固体吸附法 | amine polymers | 2440~2650 | — | [ |
表1 CO2捕集能耗和成本对比
| 捕集技术 | 吸收/吸附剂 |  |  | 文献 |
|---|---|---|---|---|
| 溶剂吸收法 | MEA | 3900 | 62.8 | [ |
| 固体吸附法 | PEI/silica | — | 48.1~75.2 | [ |
| 固体吸附法 | TEPA/MCM-41 | 1800 | 29.68 | [ |
| 固体吸附法 | EB-PEI/SiO2 | 22300 | — | [ |
| 固体吸附法 | amine polymers | 2440~2650 | — | [ |
| 吸附剂 | 载体材料 | 负载胺 | 负载量 | 测试条件 | 吸附量/mg·g-1 | 文献 |
|---|---|---|---|---|---|---|
| 1 | Silica CS-2129 | PEI | 32%(质量分数) | 75℃、60kPa、60mL/min | 85.8 | [ |
| 2 | γ-Al2O3(6.9MPa) | PEI | 4.3mmol N/g | 35℃、10kPa、90mL/min | 26.4 | [ |
| 3 | γ-Al2O3(34.5MPa) | PEI | 4.3mmol N/g | 35℃、10kPa、90mL/min | 22.0 | [ |
| 4 | PD-09024(6.9MPa) | PEI | 4.3mmol N/g | 35℃、10kPa、90mL/min | 31.2 | [ |
| 5 | PD-09024(34.5MPa) | PEI | 4.3mmol N/g | 35℃、10kPa、90mL/min | 28.2 | [ |
| 6 | SBA-15(6.9MPa) | PEI | 4.2mmol N/g | 35℃、10kPa、90mL/min | 17.6 | [ |
| 7 | SBA-15(34.5MPa) | PEI | 4.2mmol N/g | 35℃、10kPa、90mL/min | 10.6 | [ |
| 8 | γ-Al2O3(17.2~20.7MPa) | PEI | 7.81mmol N/g | 30℃、0.4Pa、60mL/min | 75.2 | [ |
| 9 | Silica PD-09024 | TEPA | 11.5mmol N/g | 25℃、10kPa、60mL/min | 98.1 | [ |
| 10 | Silica PD-09024 | PEI | 13.2mmol N/g | 25℃、10kPa、60mL/min | 53.7 | [ |
| 11 | MIL-101 | TEPA | 3.5mmol N/g | 25℃、3Pa、60mL/min | 70.4 | [ |
| 12 | MIL-101 | PEI | 5.5mmol N/g | 25℃、3Pa、60mL/min | 61.6 | [ |
表2 挤条法、压柱法和3D打印法制备成型固体胺吸附剂的吸附性能
| 吸附剂 | 载体材料 | 负载胺 | 负载量 | 测试条件 | 吸附量/mg·g-1 | 文献 |
|---|---|---|---|---|---|---|
| 1 | Silica CS-2129 | PEI | 32%(质量分数) | 75℃、60kPa、60mL/min | 85.8 | [ |
| 2 | γ-Al2O3(6.9MPa) | PEI | 4.3mmol N/g | 35℃、10kPa、90mL/min | 26.4 | [ |
| 3 | γ-Al2O3(34.5MPa) | PEI | 4.3mmol N/g | 35℃、10kPa、90mL/min | 22.0 | [ |
| 4 | PD-09024(6.9MPa) | PEI | 4.3mmol N/g | 35℃、10kPa、90mL/min | 31.2 | [ |
| 5 | PD-09024(34.5MPa) | PEI | 4.3mmol N/g | 35℃、10kPa、90mL/min | 28.2 | [ |
| 6 | SBA-15(6.9MPa) | PEI | 4.2mmol N/g | 35℃、10kPa、90mL/min | 17.6 | [ |
| 7 | SBA-15(34.5MPa) | PEI | 4.2mmol N/g | 35℃、10kPa、90mL/min | 10.6 | [ |
| 8 | γ-Al2O3(17.2~20.7MPa) | PEI | 7.81mmol N/g | 30℃、0.4Pa、60mL/min | 75.2 | [ |
| 9 | Silica PD-09024 | TEPA | 11.5mmol N/g | 25℃、10kPa、60mL/min | 98.1 | [ |
| 10 | Silica PD-09024 | PEI | 13.2mmol N/g | 25℃、10kPa、60mL/min | 53.7 | [ |
| 11 | MIL-101 | TEPA | 3.5mmol N/g | 25℃、3Pa、60mL/min | 70.4 | [ |
| 12 | MIL-101 | PEI | 5.5mmol N/g | 25℃、3Pa、60mL/min | 61.6 | [ |
| 吸附剂 | 载体 | 改性胺 | 负载质量分数/% | 测试条件 | 吸附量/mg·g-1 | 制备周期/d | 文献 |
|---|---|---|---|---|---|---|---|
| 1 | SiO2整体柱 | PEI | 50 | 75℃、100kPa、70mL/min | 70.84 | 5 | [ |
| 2 | SiO2整体柱 | TEPA | 65 | 75℃、5kPa、30mL/min | 260.00 | 6 | [ |
| 3 | SiO2整体柱 | PEI | 60 | 75℃、100kPa、20mL/min | 183.04 | 12 | [ |
| 4 | 硅气凝胶 | APS | — | 25℃、100kPa、0.3mL/min | 283.80 | 1 | [ |
| 5 | 硅气凝胶 | APS | — | 25℃、100kPa、2mL/min | 96.80 | 2 | [ |
| 6 | 碳球 | PEI | 50 | 75℃、100kPa、100mL/min | 163.40 | 6 | [ |
| 7 | 聚合物气凝胶 | APS | — | 30℃、1kPa、300mL/min | 157.08 | 3 | [ |
| 8 | SiO2整体柱 | TEPA | 39 | 75℃、100kPa | 171 | 3 | [ |
| 9 | SiO2整体柱 | PEI | 50 | 80℃、100kPa、40mL/min | 167 | 19 | [ |
| 10 | 蜂窝式陶瓷纤维 | PEI | — | 75℃、10kPa、75mL/min | 45.1 | — | [ |
| 11 | 蜂窝式氧化铝 | PEI | — | 7℃、0.4Pa、90mL/min | 30.8 | — | [ |
| 12 | 聚丙烯聚烯烃聚合物泡沫 | PEHA | — | 50℃、10kPa、75mL/min | 7.18cm3/g | — | [ |
| 13 | 分级多孔SiO2 | APS | — | 28℃、100kPa | 66.44 | — | [ |
| 14 | MCM-41载体 | TEPA | 70 | 75℃、100kPa、60mL/min | 151 | 2 | [ |
| 15 | 3D打印载体 | TEPA | 43 | 25℃、100kPa | 98.1 | — | [ |
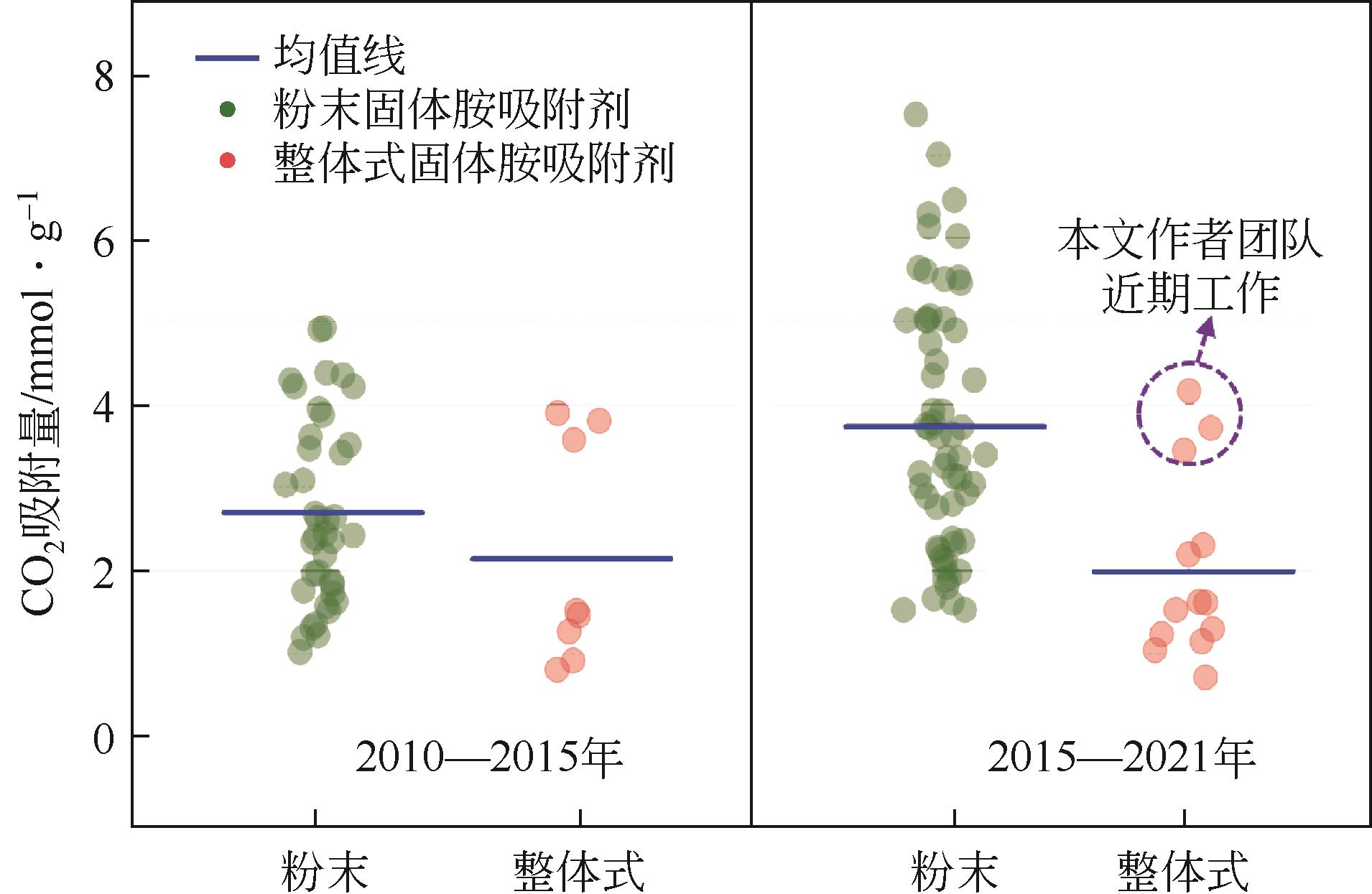
表3 不同方法制备得到的成型固体胺吸附剂性能比较
| 吸附剂 | 载体 | 改性胺 | 负载质量分数/% | 测试条件 | 吸附量/mg·g-1 | 制备周期/d | 文献 |
|---|---|---|---|---|---|---|---|
| 1 | SiO2整体柱 | PEI | 50 | 75℃、100kPa、70mL/min | 70.84 | 5 | [ |
| 2 | SiO2整体柱 | TEPA | 65 | 75℃、5kPa、30mL/min | 260.00 | 6 | [ |
| 3 | SiO2整体柱 | PEI | 60 | 75℃、100kPa、20mL/min | 183.04 | 12 | [ |
| 4 | 硅气凝胶 | APS | — | 25℃、100kPa、0.3mL/min | 283.80 | 1 | [ |
| 5 | 硅气凝胶 | APS | — | 25℃、100kPa、2mL/min | 96.80 | 2 | [ |
| 6 | 碳球 | PEI | 50 | 75℃、100kPa、100mL/min | 163.40 | 6 | [ |
| 7 | 聚合物气凝胶 | APS | — | 30℃、1kPa、300mL/min | 157.08 | 3 | [ |
| 8 | SiO2整体柱 | TEPA | 39 | 75℃、100kPa | 171 | 3 | [ |
| 9 | SiO2整体柱 | PEI | 50 | 80℃、100kPa、40mL/min | 167 | 19 | [ |
| 10 | 蜂窝式陶瓷纤维 | PEI | — | 75℃、10kPa、75mL/min | 45.1 | — | [ |
| 11 | 蜂窝式氧化铝 | PEI | — | 7℃、0.4Pa、90mL/min | 30.8 | — | [ |
| 12 | 聚丙烯聚烯烃聚合物泡沫 | PEHA | — | 50℃、10kPa、75mL/min | 7.18cm3/g | — | [ |
| 13 | 分级多孔SiO2 | APS | — | 28℃、100kPa | 66.44 | — | [ |
| 14 | MCM-41载体 | TEPA | 70 | 75℃、100kPa、60mL/min | 151 | 2 | [ |
| 15 | 3D打印载体 | TEPA | 43 | 25℃、100kPa | 98.1 | — | [ |
| 1 | HOFFMAN James S, HAMMACHE Sonia, GRAY McMahan L, et al. Parametric study for an immobilized amine sorbent in a regenerative carbon dioxide capture process[J]. Fuel Processing Technology, 2014, 126: 173-187. |
| 2 | ZHANG Wenbin, LIU Hao, SUN Yuan, et al. Parametric study on the regeneration heat requirement of an amine-based solid adsorbent process for post-combustion carbon capture[J]. Applied Energy, 2016, 168: 394-405. |
| 3 | TSUDA T, FUJIWARA T, TAKETANI Y, et al. Amino silica gels acting as a carbon dioxide absorbent [J]. Chemistry Letters, 1992, 21(11): 2161-2164. |
| 4 | Rodrigo SERNA-GUERRERO, BELMABKHOUT Youssef, SAYARI Abdelhamid. Triamine-grafted pore-expanded mesoporous silica for CO2 capture: effect of moisture and adsorbent regeneration strategies[J]. Adsorption, 2010, 16(6): 567-575. |
| 5 | LI Kaimin, JIANG Jianguo, TIAN Sicong, et al. Polyethyleneimine-nano silica composites: a low-cost and promising adsorbent for CO2 capture[J]. Journal of Materials Chemistry A, 2015, 3(5): 2166-2175. |
| 6 | 丁明月, 刘梦龙, 岳海荣, 等. TEPA/MCM-41固体胺吸附剂的制备及其CO2吸附性能研究[J]. 应用化工, 2019, 48(11): 2533-2537. |
| DING Mingyue, LIU Menglong, YUE Hairong, et al. Preparation and performance of TEPA/MCM-41 solid amine adsorbents for CO2 adsorption[J]. Applied Chemical Industry, 2019, 48(11): 2533-2537. | |
| 7 | 张杰, 郭伟, 张博, 等. 空气中直接捕集CO2技术研究进展[J]. 洁净煤技术, 2021, 27(2): 57-68. |
| ZHANG Jie, GUO Wei, ZHANG Bo, et al. Research progress on direct capture of CO2 from air[J]. Clean Coal Technology, 2021, 27(2): 57-68. | |
| 8 | CHEN Chao, XU Huifang, JIANG Qingbin, et al. Rational design of silicas with meso-macroporosity as supports for high-performance solid amine CO2 adsorbents[J]. Energy, 2021, 214: 119093. |
| 9 | ZHAO Peiyu, ZHANG Guojie, XU Ying, et al. Amine functionalized hierarchical bimodal mesoporous silicas as a promising nanocomposite for highly efficient CO2 capture[J]. Journal of CO2 Utilization, 2019, 34: 543-557. |
| 10 | LOU Feijian, ZHANG Anfeng, ZHANG Guanghui, et al. Enhanced kinetics for CO2 sorption in amine-functionalized mesoporous silica nanosphere with inverted cone-shaped pore structure[J]. Applied Energy, 2020, 264: 114637. |
| 11 | 何凯武, 唐思扬, 刘长军, 等. 有机胺功能化介孔固体吸附剂吸附分离CO2性能研究[J]. 化工学报, 2018, 69(9): 3887-3895. |
| HE Kaiwu, TANG Siyang, LIU Changjun, et al. Performance of amine functionalized mesoporous adsorbents for CO2 adsorption[J]. CIESC Journal, 2018, 69(9): 3887-3895. | |
| 12 | SUNG Siyoung, Myunghyun Paik SUH. Highly efficient carbon dioxide capture with a porous organic polymer impregnated with polyethylenimine[J]. Journal of Materials Chemistry A, 2014, 2(33): 13245-13249. |
| 13 | XU Xiaochun, SONG Chunshan, ANDRÉSEN John M, et al. Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer-modified mesoporous molecular sieve MCM-41[J]. Microporous and Mesoporous Materials, 2003, 62(1/2): 29-45. |
| 14 | CAPLOW M. Kinetics of carbamate formation and breakdown[J]. Journal of American Chemical Society, 1968, 90(24): 6795-6803. |
| 15 | LI K M, JIANG J G, CHEN X J, et al. Research on urea linkages formation of amine functional adsorbents during CO2 capture process: two key factors analysis, temperature and moisture[J]. Journal of Physical Chemistry C, 2016, 120(45): 25892-25902. |
| 16 | DANCKWERTS P V. The reaction of CO2 with ethanolamines[J]. Chemical Engineering Science, 1979, 34(4): 443-446. |
| 17 | JUNG Wonho, PARK Junhyung, WON Wangyun, et al. Simulated moving bed adsorption process based on a polyethylenimine-silica sorbent for CO2 capture with sensible heat recovery[J]. Energy, 2018, 150: 950-964. |
| 18 | PARK Junhyung, WON Wangyun, JUNG Wonho, et al. One-dimensional modeling of a turbulent fluidized bed for a sorbent-based CO2 capture process with solid-solid sensible heat exchange[J]. Energy, 2019, 168: 1168-1180. |
| 19 | JUNG Wonho, PARK Sunghyun, LEE Kwang Soon, et al. Rapid thermal swing adsorption process in multi-beds scale with sensible heat recovery for continuous energy-efficient CO2 capture[J]. Chemical Engineering Journal, 2020, 392: 123656. |
| 20 | ZHAO Wenying, ZHANG Zhi, LI Zhenshan, et al. Continuous CO2 capture in dual fluidized beds using silica supported amine[J]. Energy Procedia, 2013, 37: 89-98. |
| 21 | ZHAO Wenying, VENEMAN Rens, CHEN Denggao, et al. Post-combustion CO2 capture demonstration using supported amine sorbents: design and evaluation of 200kWth pilot[J]. Energy Procedia, 2014, 63: 2374-2383. |
| 22 | VAN PAASEN Sander, INFANTINO Melina, YAO Joseph, et al. Development of the solid sorbent technology for post combustion CO2 capture towards commercial prototype[J]. International Journal of Greenhouse Gas Control, 2021, 109: 103368. |
| 23 | JUNG Wonho, LEE Jinwon. Economic evaluation for four different solid sorbent processes with heat integration for energy-efficient CO2 capture based on PEI-silica sorbent[J]. Energy, 2022, 238: 121864. |
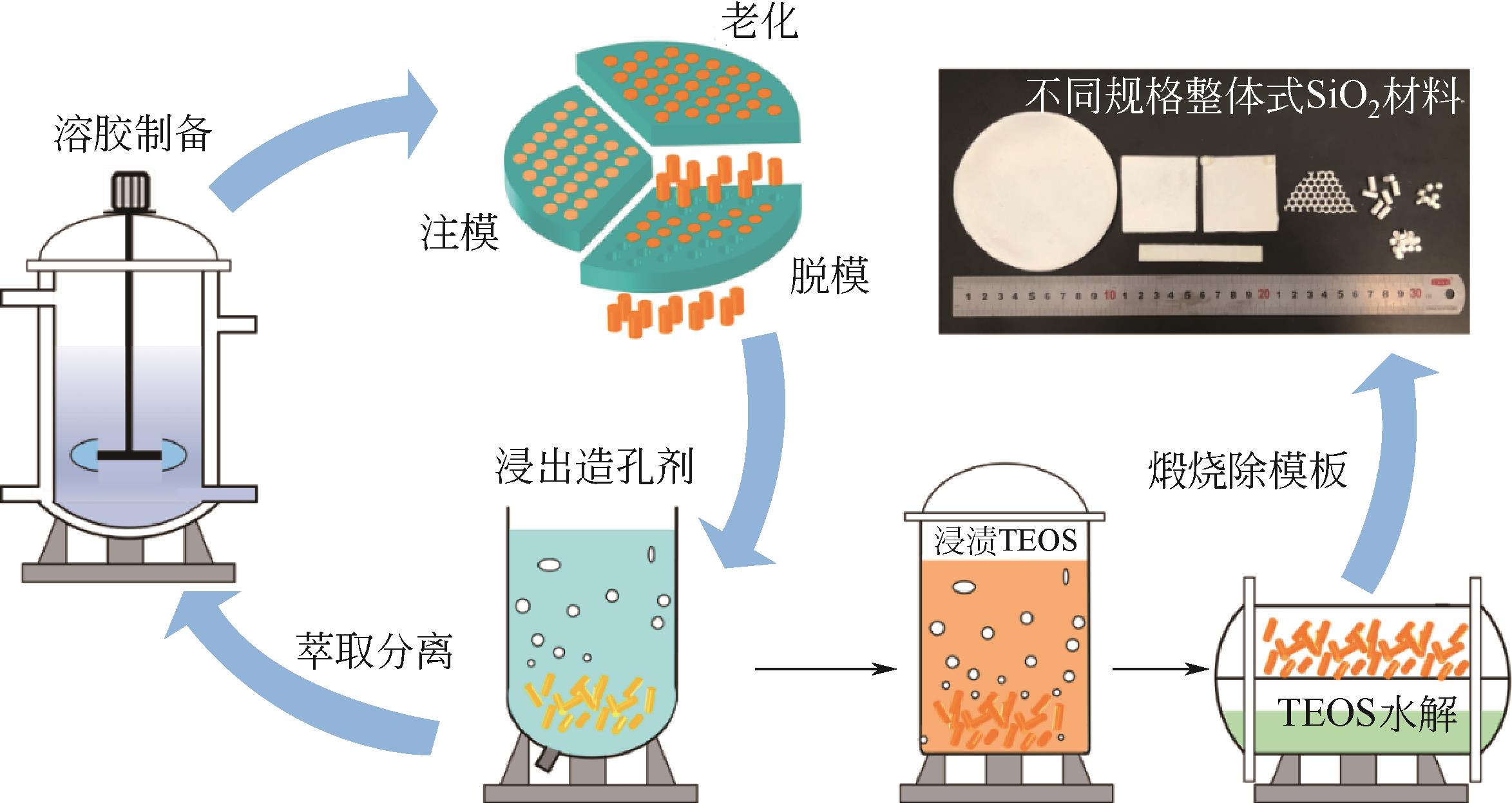
| 24 | 岳海荣, 喻树楠, 马奎, 等. 整体式固体胺吸附剂及其制备方法: CN114522669A[P]. 2022-05-24. |
| YUE Hairong, YU Shunan, MA Kui, et al. Monolithic solid amine adsorbent and preparation method thereof: CN114522669A[P]. 2022-05-24. | |
| 25 | JUNG Wonho, LEE Jinwon. Pseudo counter-current turbulent fluidized bed process with sensible heat recovery for energy-efficient CO2 capture using an amine-functionalized solid sorbent[J]. Energy, 2022, 240: 122803. |
| 26 | CHOI Woosung, PARK Jongbeom, KIM Chaehoon, et al. Structural effects of amine polymers on stability and energy efficiency of adsorbents in post-combustion CO2 capture[J]. Chemical Engineering Journal, 2021, 408: 127289. |
| 27 | GUO Yangyang, LUO Lei, ZHENG Yang, et al. Optimization of CO2 adsorption on solid-supported amines and thermal regeneration mode comparison[J]. ACS Omega, 2020, 5(17): 9641-9648. |
| 28 | GOEPPERT Alain, ZHANG Hang, Raktim SEN, et al. Oxidation-resistant, cost-effective epoxide-modified polyamine adsorbents for CO2 capture from various sources including air[J]. ChemSusChem, 2019, 12(8): 1712-1723. |
| 29 | WILFONG W C, GRAY M L, KAIL B W, et al. Pelletization of immobilized amine carbon dioxide sorbents with fly ash and poly(vinyl chloride)[J]. Energy Technology, 2016, 4(5): 610-619. |
| 30 | WILFONG Walter Christopher, KAIL Brian W, HOWARD Bret H, et al. Robust immobilized amine CO2 sorbent pellets utilizing a poly(chloroprene) polymer binder and fly ash additive[J]. Energy Technology, 2017, 5(2): 228-233. |
| 31 | WILFONG Walter C, KAIL Brian W, WANG Qiuming, et al. Scale-up of immobilized amine sorbent pellets for landfill gas upgrading, using benchtop and pilot equipment[J]. Powder Technology, 2022, 395: 243-254. |
| 32 | LI K M, JIANG J G, TIAN S C, et al. Influence of silica types on synthesis and performance of amine-silica hybrid materials used for CO2 capture [J]. Journal of Physical Chemistry C, 2014, 118(5): 2454-2462. |
| 33 | AHMED Sohail, RAMLI Anita, YUSUP Suzana, et al. Adsorption behavior of tetraethylenepentamine-functionalized Si-MCM-41 for CO2 adsorption[J]. Chemical Engineering Research and Design, 2017, 122: 33-42. |
| 34 | ISENBERG M, CHUANG S S C. The nature of adsorbed CO2 and amine sites on the immobilized amine sorbents regenerated by industrial boiler steam[J]. Industrial & Engineering Chemistry Research, 2013, 52(35): 12530-12539. |
| 35 | KLINTHONG W, HUANG C H, TAN C S. Polyallylamine and NaOH as a novel binder to pelletize amine-functionalized mesoporous silicas for CO2 capture[J]. Microporous and Mesoporous Materials, 2014, 197: 278-287. |
| 36 | HAN Yosep, HWANG Gukhwa, KIM Hyunjung, et al. Amine-impregnated millimeter-sized spherical silica foams with hierarchical mesoporous-macroporous structure for CO2 capture[J]. Chemical Engineering Journal, 2015, 259: 653-662. |
| 37 | DUTCHER Bryce, FAN Maohong, RUSSELL Armistead G. Amine-based CO2 capture technology development from the beginning of 2013—a review[J]. ACS Applied Materials & Interfaces, 2015, 7(4): 2137-2148. |
| 38 | GELLES Teresa, LAWSON Shane, ROWNAGHI Ali A, et al. Recent advances in development of amine functionalized adsorbents for CO2 capture[J]. Adsorption, 2020, 26(1): 5-50. |
| 39 | MUKHERJEE Satyajit, AKSHAY, SAMANTA Amar N. Amine-impregnated MCM-41 in post-combustion CO2 capture: synthesis, characterization, isotherm modelling[J]. Advanced Powder Technology, 2019, 30(12): 3231-3240. |
| 40 | LEE J J, SIEVERS C, JONES C W. Silica-supported hindered aminopolymers for CO2 capture [J]. Industrial & Engineering Chemistry Research, 2019, 58(50): 22551-22560. |
| 41 | ZHAO P, ZHANG G, XU Y, et al. Development of amine-functionalized silica foams with hierarchical pore structure for CO2 capture [J]. Energy & Fuels, 2019, 33(4): 3357-3369. |
| 42 | ÜNVEREN Elif Erdal, MONKUL Bahar Özmen, Şerife SARıOĞLAN, et al. Solid amine sorbents for CO2 capture by chemical adsorption: a review[J]. Petroleum, 2017, 3(1): 37-50. |
| 43 | CHEN Chao, ZHANG Siqian, Kyung Ho ROW, et al. Amine-silica composites for CO2 capture: a short review[J]. Journal of Energy Chemistry, 2017, 26(5): 868-880. |
| 44 | REN Yanping, DING Ruiyu, YUE Hairong, et al. Amine-grafted mesoporous copper silicates as recyclable solid amine sorbents for post-combustion CO2 capture[J]. Applied Energy, 2017, 198: 250-260. |
| 45 | MELLO Marília R, PHANON Delphine, SILVEIRA Gleiciani Q, et al. Amine-modified MCM-41 mesoporous silica for carbon dioxide capture[J]. Microporous and Mesoporous Materials, 2011, 143(1): 174-179. |
| 46 | HARLICK P J E, SAYARI A. Applications of pore-expanded mesoporous silica. 5. triamine grafted material with exceptional CO2 dynamic and equilibrium adsorption performance[J]. Industrial & Engineering Chemistry Research, 2007, 46(2): 446-458. |
| 47 | XU X C, SONG C S, ANDRESEN J M, et al. Novel polyethylenimine-modified mesoporous molecular sieve of MCM-41 type as high-capacity adsorbent for CO2 capture[J]. Energy & Fuels, 2002, 16(6): 1463. |
| 48 | ZHANG Guojie, ZHAO Peiyu, HAO Lanxia, et al. Amine-modified SBA-15(P): a promising adsorbent for CO2 capture[J]. Journal of CO2 Utilization, 2018, 24: 22-33. |
| 49 | JAHANDAR LASHAKI Masoud, KHIAVI Soheil, SAYARI Abdelhamid. Stability of amine-functionalized CO2 adsorbents: a multifaceted puzzle[J]. Chemical Society Reviews, 2019, 48(12): 3320-3405. |
| 50 | ZHAO Yiying, ZHOU Jizhi, FAN Lili, et al. Indoor CO2 control through mesoporous amine-functionalized silica monoliths[J]. Industrial & Engineering Chemistry Research, 2019, 58(42): 19465-19474. |
| 51 | KLINTHONG Worasaung, HUANG Chih Hung, TAN Chung Sung. One-pot synthesis and pelletizing of polyethylenimine-containing mesoporous silica powders for CO2 capture[J]. Industrial & Engineering Chemistry Research, 2016, 55(22): 6481-6491. |
| 52 | REZAEI Fateme, SAKWA-NOVAK Miles A, BALI Sumit, et al. Shaping amine-based solid CO2 adsorbents: effects of pelletization pressure on the physical and chemical properties[J]. Microporous and Mesoporous Materials, 2015, 204: 34-42. |
| 53 | AKHTAR Farid, ANDERSSON Linnéa, OGUNWUMI Steven, et al. Structuring adsorbents and catalysts by processing of porous powders[J]. Journal of the European Ceramic Society, 2014, 34(7): 1643-1666. |
| 54 | CHEN J, LIU X, TIAN Y, et al. 3D-printed anisotropic polymer materials for functional applications[J]. Advanced Materials, 2022, 34(5): e2102877. |
| 55 | LAWSON S, GRIFFIN C, RAPP K, et al. Amine-functionalized MIL-101 monoliths for CO2 removal from enclosed environments [J]. Energy & Fuels, 2019, 33(3): 2399-2407. |
| 56 | THAKKAR Harshul, EASTMAN Stephen, Ahmed AL-MAMOORI, et al. Formulation of aminosilica adsorbents into 3D-printed monoliths and evaluation of their CO2 capture performance[J]. ACS Applied Materials & Interfaces, 2017, 9(8): 7489-7498. |
| 57 | SAKWA-NOVAK Miles A, JONES Christopher W. Steam induced structural changes of a poly(ethylenimine) impregnated γ-alumina sorbent for CO2 extraction from ambient air[J]. ACS Applied Materials & Interfaces, 2014, 6(12): 9245-9255. |
| 58 | BINGRE R, LOUIS B, NGUYEN P. An overview on zeolite shaping technology and solutions to overcome diffusion limitations[J]. Catalysts, 2018, 8(4): 163. |
| 59 | WANG Wei, LONG Haibo, LI Tao, et al. Hierarchical trimodal macro-mesoporous silica monoliths with co-continuous macrostructures and isotropic skeletons constructed by randomly oriented SBA-15-type primary particles[J]. Microporous and Mesoporous Materials, 2018, 258: 262-268. |
| 60 | SINGH Jasminder, BHUNIA Haripada, BASU Soumen. Synthesis of porous carbon monolith adsorbents for carbon dioxide capture: breakthrough adsorption study[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 89: 140-150. |
| 61 | CHEN Chao, YANG Seung Tae, Wha Seung AHN, et al. Amine-impregnated silica monolith with a hierarchical pore structure: Enhancement of CO2 capture capacity[J]. Chemical Communications, 2009(24): 3627-3629. |
| 62 | MARESZ Katarzyna, Agnieszka CIEMIĘGA, MALINOWSKI Janusz J, et al. Effect of support structure and polyamine type on CO2 capture in hierarchically structured monolithic sorbents[J]. Chemical Engineering Journal, 2020, 383: 123175. |
| 63 | FAN Hongyu, WU Zhanjun, XU Qiaoqi, et al. Flexible, amine-modified silica aerogel with enhanced carbon dioxide capture performance[J]. Journal of Porous Materials, 2016, 23(1): 131-137. |
| 64 | SHAO Zaidong, CHENG Xuan, ZHENG Yuming. Facile co-precursor sol-gel synthesis of a novel amine-modified silica aerogel for high efficiency carbon dioxide capture[J]. Journal of Colloid and Interface Science, 2018, 530: 412-423. |
| 65 | KONG Yong, SHEN Xiaodong, CUI Sheng, et al. Development of monolithic adsorbent via polymeric sol-gel process for low-concentration CO2 capture[J]. Applied Energy, 2015, 147: 308-317. |
| 66 | WEN Jingjia, GU Fangna, WEI Feng, et al. One-pot synthesis of the amine-modified meso-structured monolith CO2 adsorbent[J]. Journal of Materials Chemistry, 2010, 20(14): 2840-2846. |
| 67 | WITOON Thongthai, CHAREONPANICH Metta. Synthesis of hierarchical meso-macroporous silica monolith using chitosan as biotemplate and its application as polyethyleneimine support for CO2 capture[J]. Materials Letters, 2012, 81: 181-184. |
| 68 | WU Junye, ZHU Xuancan, YANG Fan, et al. Easily-synthesized and low-cost amine-functionalized silica sol-coated structured adsorbents for CO2 capture[J]. Chemical Engineering Journal, 2021, 425: 131409. |
| 69 | SAKWA-NOVAK Miles A, YOO Chun-Jae, TAN Shuai, et al. Poly(ethylenimine)-functionalized monolithic alumina honeycomb adsorbents for CO2 capture from air[J]. ChemSusChem, 2016, 9(14): 1859-1868. |
| 70 | YANG Chuanruo, XIONG Yuxin, CHEN Jian, et al. Amine-functionalized micron-porous polymer foams with high CO2 adsorption efficiency and exceptional stability in PSA process[J]. Chemical Engineering Journal, 2021, 420: 129555. |
| 71 | Young Gun KO, LEE Hyun Jeong, KIM Jae Yong, et al. Hierarchically porous aminosilica monolith as a CO2 adsorbent[J]. ACS Applied Materials & Interfaces, 2014, 6(15): 12988-12996. |
| 72 | GUO Xingzhong, DING Li, KANAMORI Kazuyoshi, et al. Functionalization of hierarchically porous silica monoliths with polyethyleneimine (PEI) for CO2 adsorption[J]. Microporous and Mesoporous Materials, 2017, 245: 51-57. |
| 73 | WANG Mei, YAO Liwen, WANG Jitong, et al. Adsorption and regeneration study of polyethylenimine-impregnated millimeter-sized mesoporous carbon spheres for post-combustion CO2 capture[J]. Applied Energy, 2016, 168: 282-290. |
| 74 | WU Q, MA Y, WANG S, et al. 110th anniversary: sustainable synthesis of zeolites: from fundamental research to industrial production[J]. Industrial & Engineering Chemistry Research, 2019, 58(27): 11653-11658. |
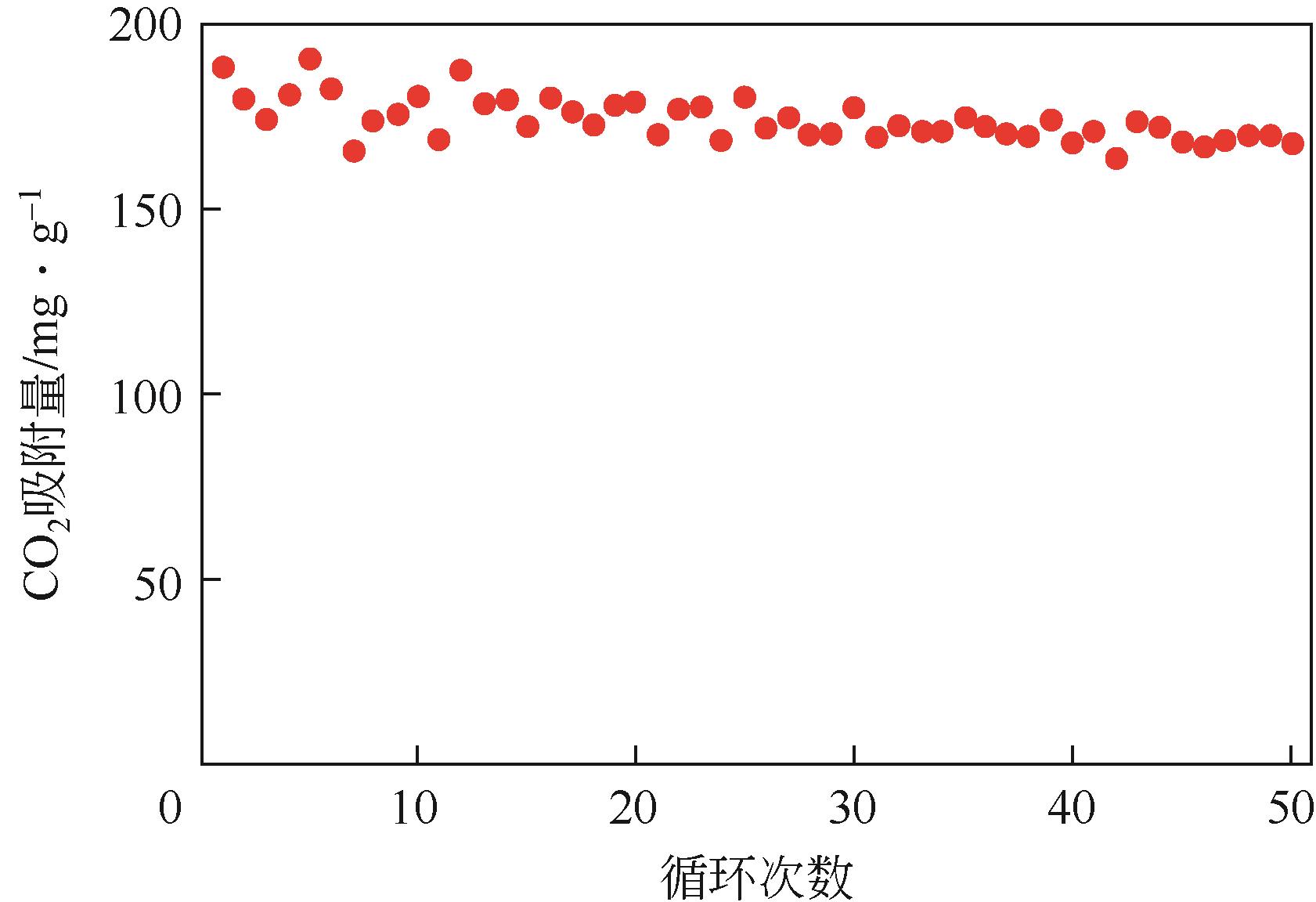
| 75 | ZHOU Changan, YU Shunan, MA Kui, et al. Amine-functionalized mesoporous monolithic adsorbents for post-combustion carbon dioxide capture[J]. Chemical Engineering Journal, 2021, 413: 127675. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [3] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [4] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [5] | 符乐, 杨阳, 徐文青, 耿錾卜, 朱廷钰, 郝润龙. 新型相变有机胺吸收捕集CO2技术研究进展[J]. 化工进展, 2023, 42(4): 2068-2080. |
| [6] | 尚玉, 肖满, 崔秋芳, 涂特, 晏水平. CO2捕集工艺中热再生气余热的PVDF/BN-OH平板复合膜回收特性[J]. 化工进展, 2023, 42(3): 1618-1628. |
| [7] | 沈天绪, 沈来宏. 基于3kW塔式串行流化床差异燃料的化学链燃烧解析[J]. 化工进展, 2023, 42(1): 138-147. |
| [8] | 王璐, 张磊, 都健. 机器学习高效筛选用于CO2/N2选择性吸附分离的沸石材料[J]. 化工进展, 2023, 42(1): 148-158. |
| [9] | 王一茹, 宋小三, 水博阳, 王三反. 胺功能化介孔二氧化硅捕集CO2的研究进展[J]. 化工进展, 2022, 41(S1): 536-544. |
| [10] | 周红军, 周颖, 徐春明. 中国碳达峰碳中和目标下炼化一体化新路径与实践[J]. 化工进展, 2022, 41(4): 2226-2230. |
| [11] | 张卫风, 周武, 王秋华. 相变吸收捕集烟气中CO2技术的发展现状[J]. 化工进展, 2022, 41(4): 2090-2101. |
| [12] | 孔祥宇, 谢亮, 王延民, 翟尚鹏, 王建国. CO2的捕集及资源化利用[J]. 化工进展, 2022, 41(3): 1187-1198. |
| [13] | 郑鹏, 李蔚玲, 郭亚飞, 孙健, 王瑞林, 赵传文. 鼓泡床中电石渣加速碳酸化分析与响应面优化[J]. 化工进展, 2022, 41(3): 1528-1538. |
| [14] | 张凡, 王树众, 李艳辉, 杨健乔, 孙圣瀚. 中国制造业碳排放问题分析与减排对策建议[J]. 化工进展, 2022, 41(3): 1645-1653. |
| [15] | 田原宇, 乔英云, 张永宁. 碳中和约束下绿色减排体系的构建[J]. 化工进展, 2022, 41(2): 1078-1084. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||