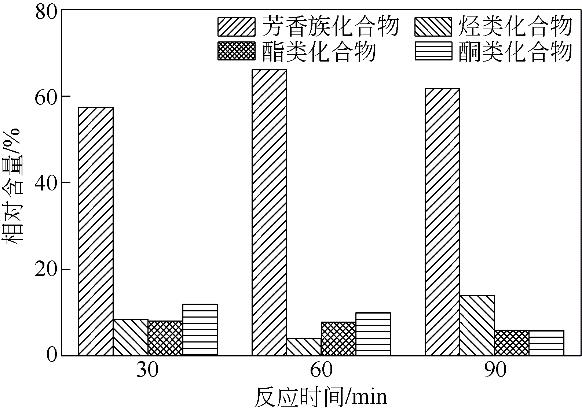
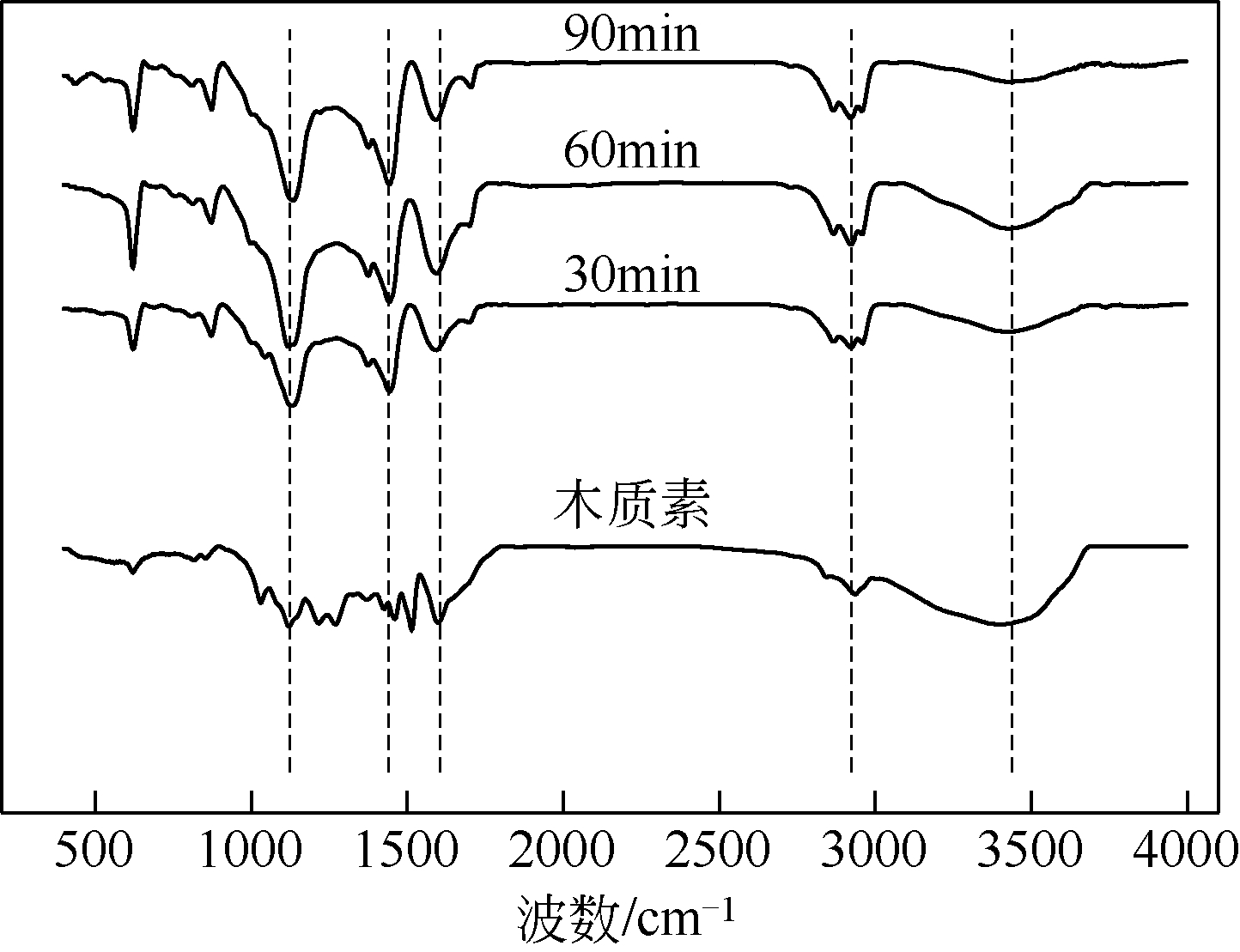
| 1 |
COCEROM J, ÁCABEZA, ABADN, et al. Understanding biomass fractionation in subcritical & supercritical water[J]. The Journal of Supercritical Fluids, 2018, 133: 550-565.
|
| 2 |
崔玉虎, 王奇, 苟光俊, 等. 木质素催化降解液化的研究进展[J]. 材料导报, 2017, 31(5): 112-116.
|
|
CUIY H, WANGQ, GOUG J, et al. Advances in catalytic degradation liquefaction of lignin[J]. Materials Review, 2017, 31(5): 112-116.
|
| 3 |
罗显峰, 金庆辉, 孙雄康. 木质素催化液化用催化剂的研究进展[J]. 广州化工, 2017, 45(22): 4-5.
|
|
LUOX F, JINQ H, SUNX K. Research progress on catalysts applied in catalytic liquefaction of lignin[J]. Guangzhou Chemical Industry, 2017, 45(22): 4-5.
|
| 4 |
SHEND, LIUG, ZHAOJ, et al. Thermo-chemical conversion of lignin to aromatic compounds: effect of lignin source and reaction temperature[J]. Journal of Analytical & Applied Pyrolysis, 2015, 112: 56-65.
|
| 5 |
CAPORGNOM P, PRUVOSTJ, LEGRANDJ, et al. Hydrothermal liquefaction of nannochloropsis oceanica in different solvents[J]. Bioresource Technology, 2016, 214: 404-410.
|
| 6 |
许文茸, 张洁, 郑凤昳, 等. 纤维素与甲壳素常压酸催化液化生成小分子化学品的机理研究进展[J]. 化工学报, 2018, 69(4): 1288-1298.
|
|
XUW R, ZHANGJ, ZHENGF D, et al. Research progress on mechanisms of acid-catalyzed cellulose and chitin liquefaction to small molecular chemicals under atmospheric pressure[J]. CIESC Journal, 2018, 69(4): 1288-1298.
|
| 7 |
陈梦薇, 郭大亮, 王林芳, 等. 碱木质素超/亚临界乙醇体系解聚机理研究[J]. 燃料化学学报, 2016, 44(10): 1203-1210.
|
|
CHENM W, GUOD L,WANGL F, et al. Depolymerization mechanism of alkali lignin in sub-and supercritical ethanol[J]. Journal of Fuel Chemistry and Technology, 2016, 44(10): 1203-1210.
|
| 8 |
LIQ, DONGL, HOUX, et al. Hydro-liquefaction of microcrystalline cellulose, xylan and industrial lignin in different supercritical solvents[J]. Bioresoure Technology, 2016, 219: 281-288.
|
| 9 |
HUANGX, KORÁNYIT I, BOOTM D, et al. Catalytic depolymerization of lignin in supercritical ethanol[J]. ChemSusChem, 2014, 7(8): 2276-2288.
|
| 10 |
HUANGX, KORÁNYIT I, BOOTM D, et al. Ethanol as capping agent and formaldehyde scavenger for efficient depolymerization of lignin to aromatics. [J]. Green Chemistry, 2015, 17(11): 4941-4950.
|
| 11 |
HUANGX, ATAYC, ZHUJ, et al. Catalytic depolymerization of lignin and woody biomass in supercritical ethanol: Influence of reaction temperature and feedstock[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 10864-10874.
|
| 12 |
叶俊. 木质素加氢液化制芳烃的研究[D]. 淮南: 安徽理工大学, 2011.
|
|
YEJ. Research of aromatic preparation by hydrogenation of lignin liquefaction[D]. Huainan: Anhui University of Science and Technology, 2011.
|
| 13 |
程辉, 余剑, 姚梅琴, 等. 木质素慢速热解机理[J]. 化工学报, 2013, 64(5): 1757-1765.
|
|
CHENGH, YUJ, YAOM Q, et al. Mechanism analysis of lignin slow pyrolysis[J]. CIESC Journal, 2013, 64(5): 1757-1765.
|
| 14 |
陈磊, 陈汉平, 陆强, 等. 木质素结构及热解特性[J]. 化工学报, 2014, 65(9): 3626-3633.
|
|
CHENL, CHENH P, LUQ, et al. Characterization of structure and pyrolysis behavior of lignin[J]. CIESC Journal, 2014, 65(9): 3626-3633.
|
| 15 |
孙娇, 王娅莉, 解新安, 等. 纤维素在亚/超临界甲醇中液化条件对主要化合物产物的影响[J]. 燃料化学学报, 2017, 45(6): 660-668.
|
|
SUNJ, WANGY L, XIEX A, et al. Effect of liquefaction parameters of cornstalk cellulose in sub-supercritical methanol on dominant chemical products[J]. Journal of Fuel Chemistry and Technology, 2017, 45(6): 660-668.
|
| 16 |
BRANDS, JAEHOONK. Liquefaction of major lignocellulosic biomass constituents in supercritical ethanol[J]. Energy, 2015, 80: 64-74.
|
| 17 |
黎巍, 解新安, 汤成正, 等. 亚/超临界乙醇中羟基和氢自由基作用下的纤维素液化行为[J]. 燃料化学学报, 2016, 44(4): 415-421.
|
|
LIW, XIEX A, TANGC Z, et al. Effects of hydroxyl and hydrogen free radicals on the liquefaction of cellulose in sub /supercritical ethanol[J]. Journal of Fuel Chemistry and Technology, 2016, 44(4): 415-421.
|
| 18 |
GÜVENATAMB, HEERESE H J, PIDKOE A, et al. Lewis-acid catalyzed depolymerization of protobind lignin in supercritical water and ethanol[J]. Catalysis Today, 2014, 259: 460-466.
|
| 19 |
GÜVENATAMB, HEERESE H J, PIDKOE A, et al. Lewis acid-catalyzed depolymerization of soda lignin in supercritical ethanol/water mixtures[J]. Catalysis Today, 2016, 269: 9-20.
|
| 20 |
FAND, XIEX, LIY, et al. Aromatic compounds from lignin liquefaction over ZSM-5 catalysts in supercritical ethanol[J]. Chemical Engineering & Technology, 2018, 41(3): 509-516.
|
| 21 |
CAPRARIISB D, FILIPPISP D, PETRULLOA, et al. Hydrothermal liquefaction of biomass: influence of temperature and biomass composition on the bio-oil production[J]. Fuel, 2017, 208: 618-625.
|
| 22 |
ZHAOJ, WANGX, HUJ, et al. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS[J]. Polymer Degradation & Stability, 2014, 108: 133-138.
|
 ),Xin’an XIE(
),Xin’an XIE( ),Lu LI,Yan LI,Di FAN,Jiao SUN,Xin WANG
),Lu LI,Yan LI,Di FAN,Jiao SUN,Xin WANG