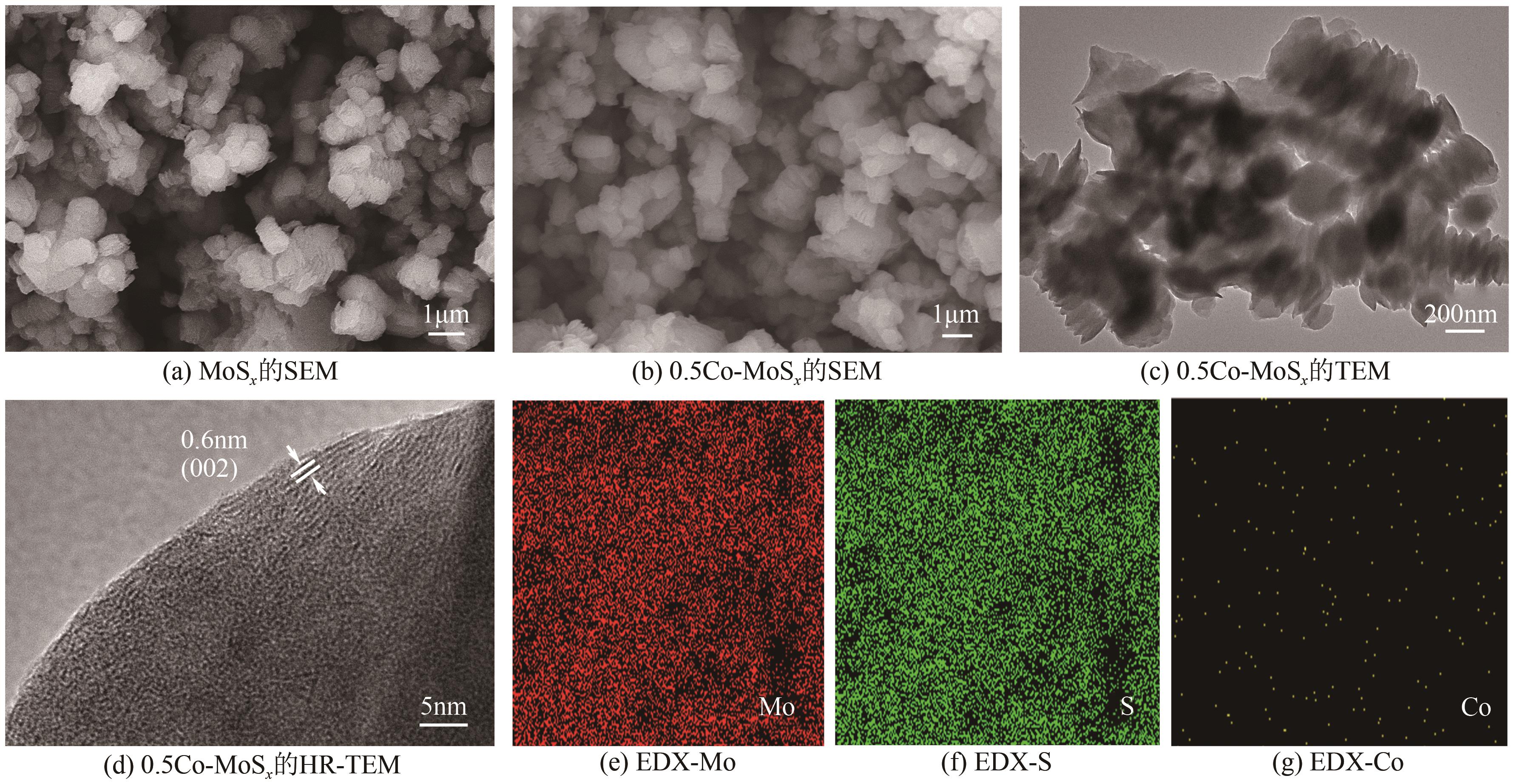
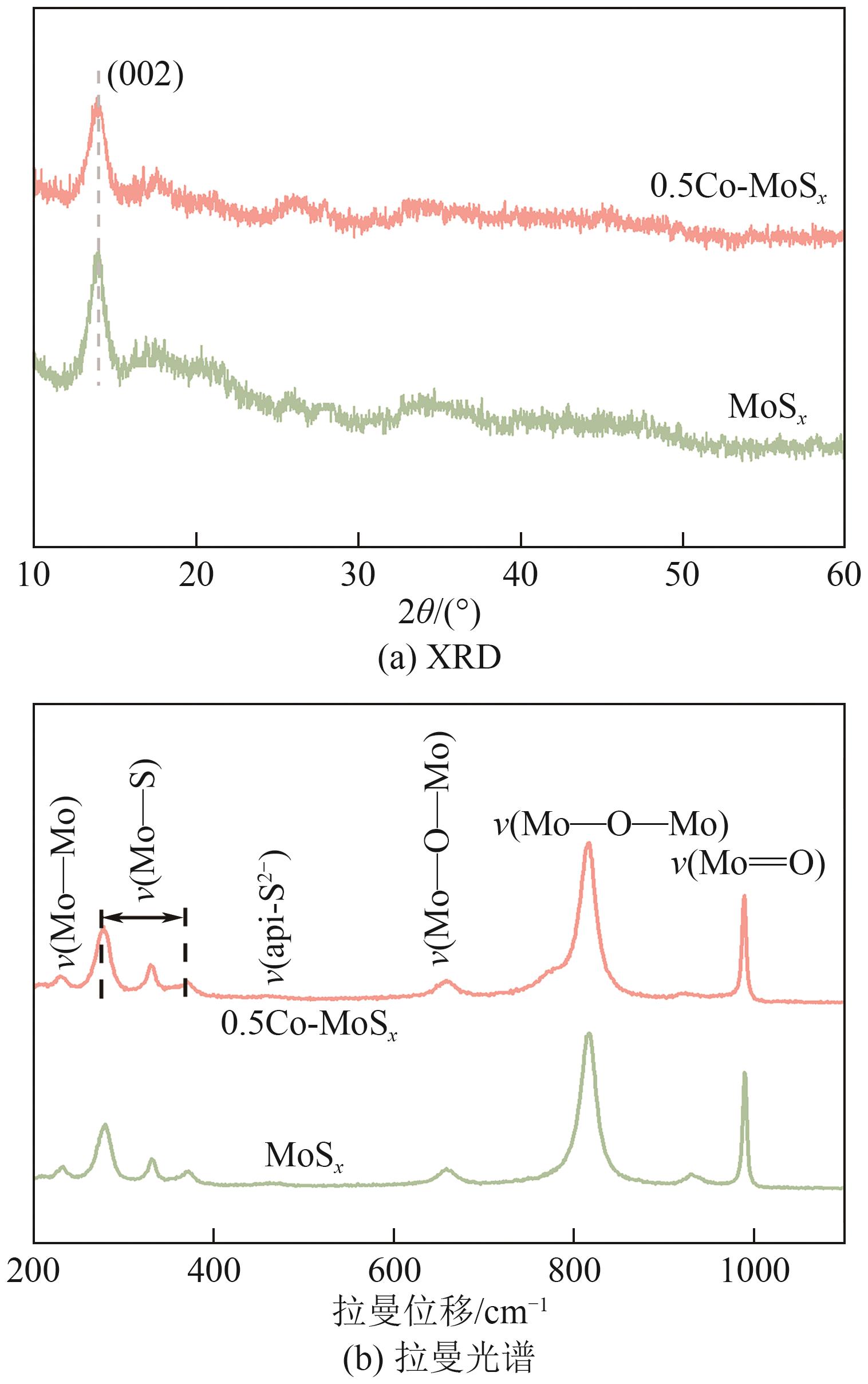
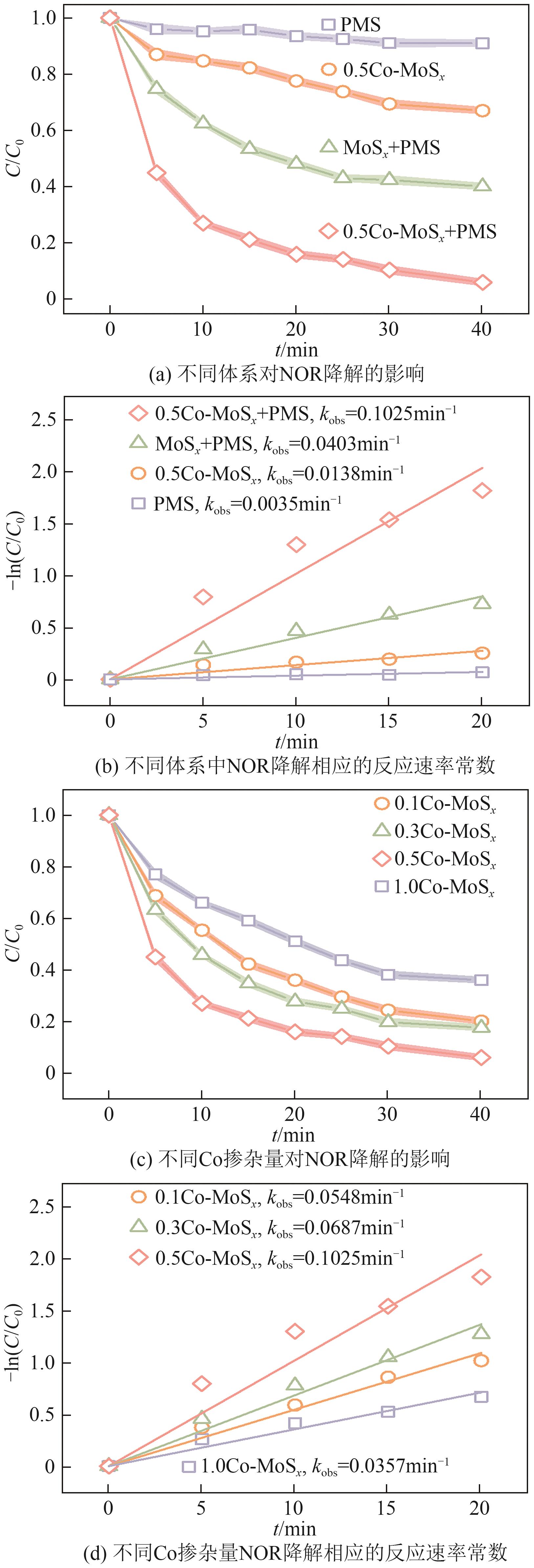
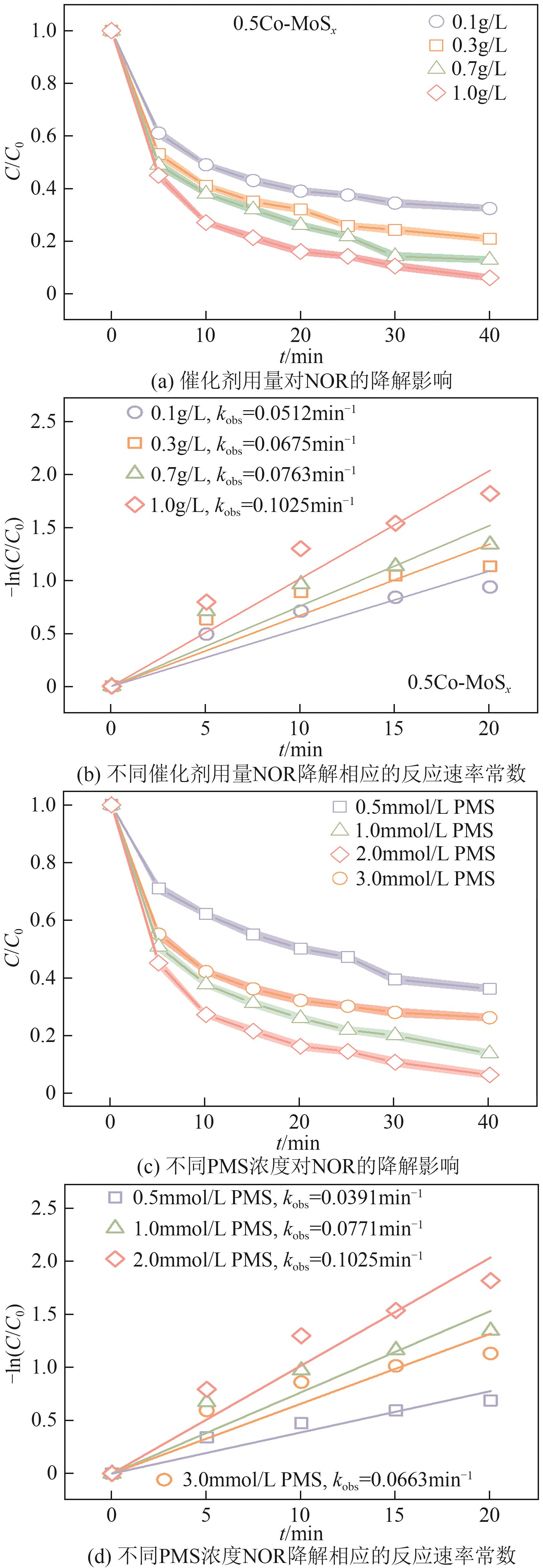
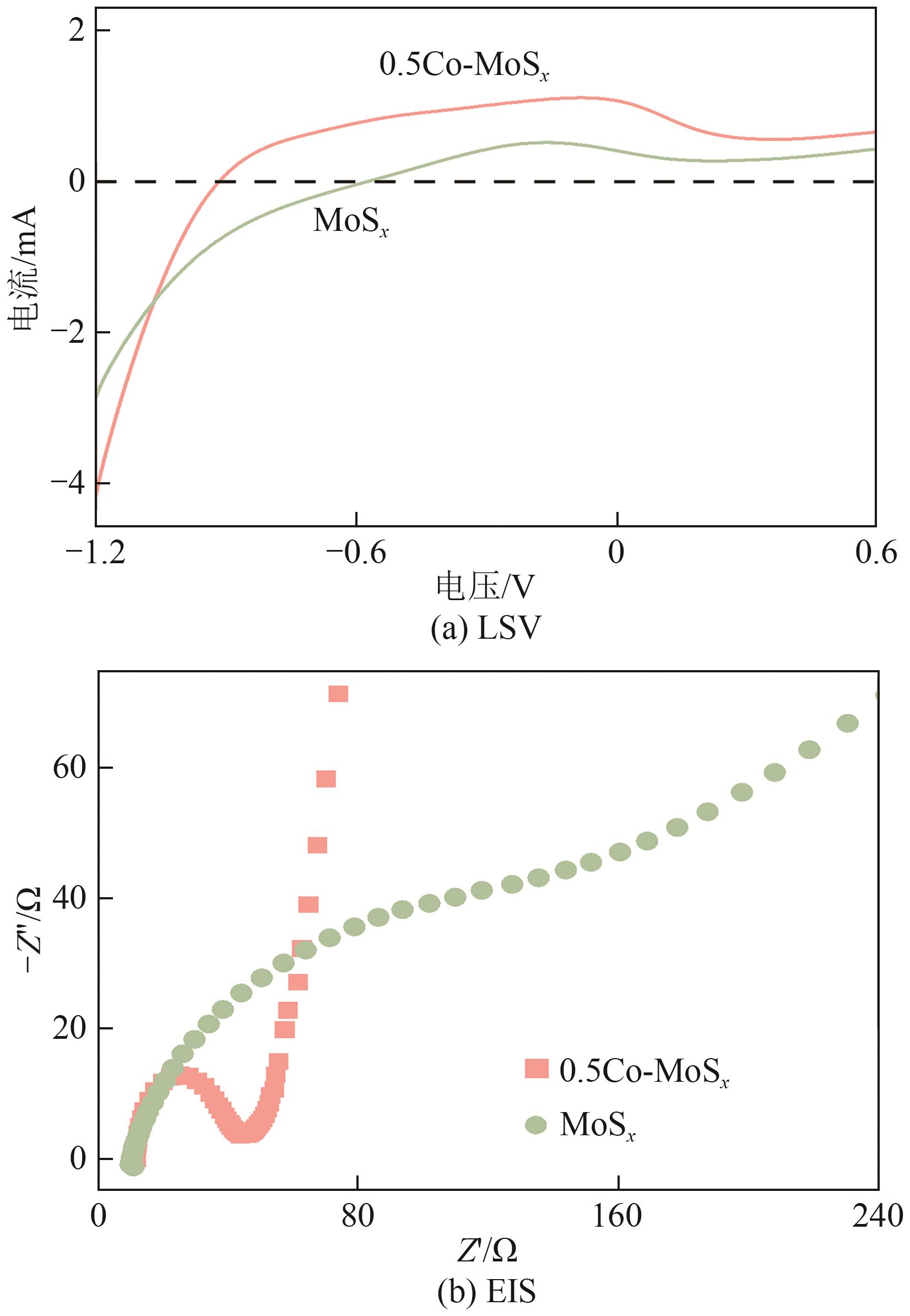
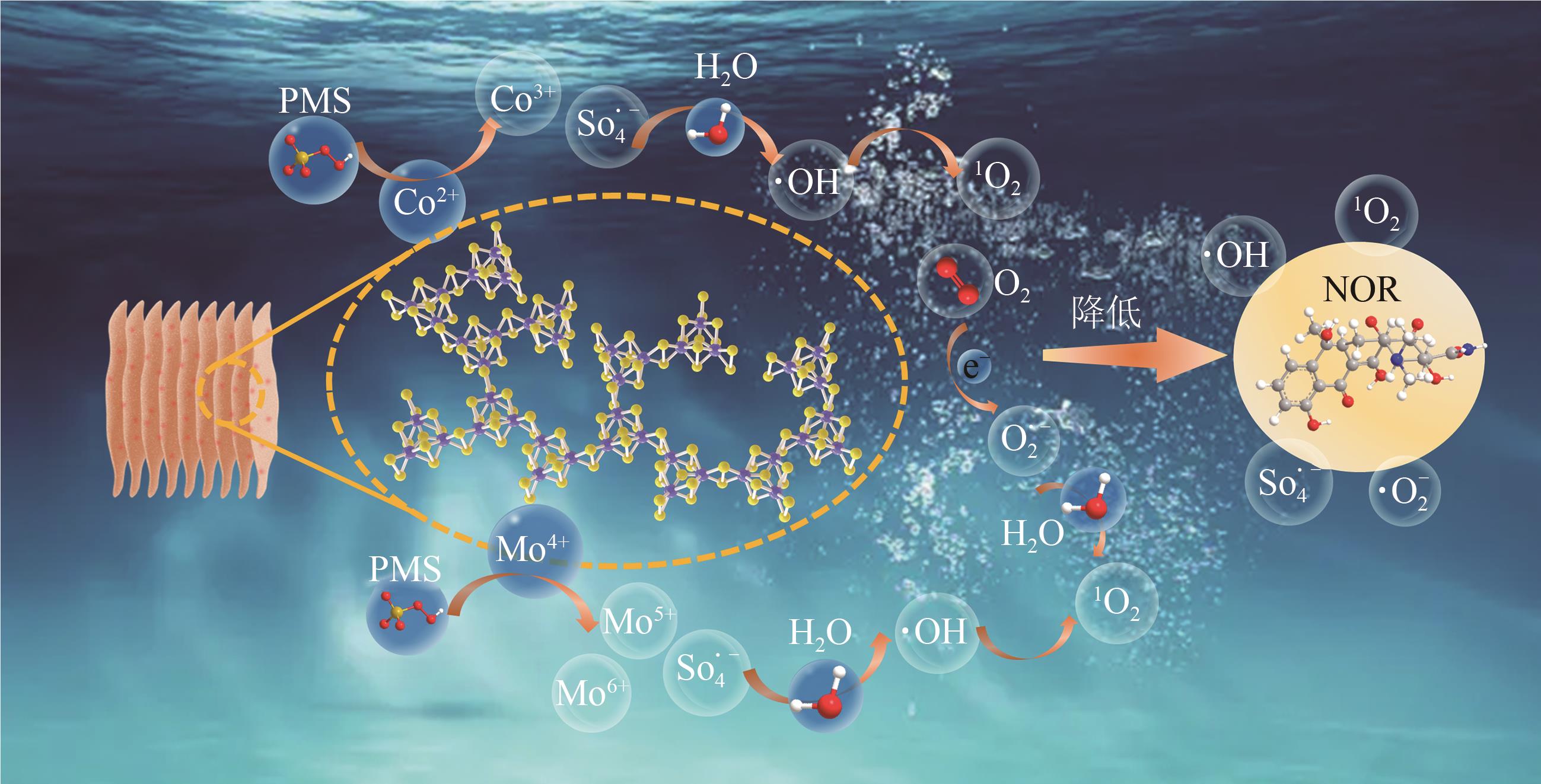
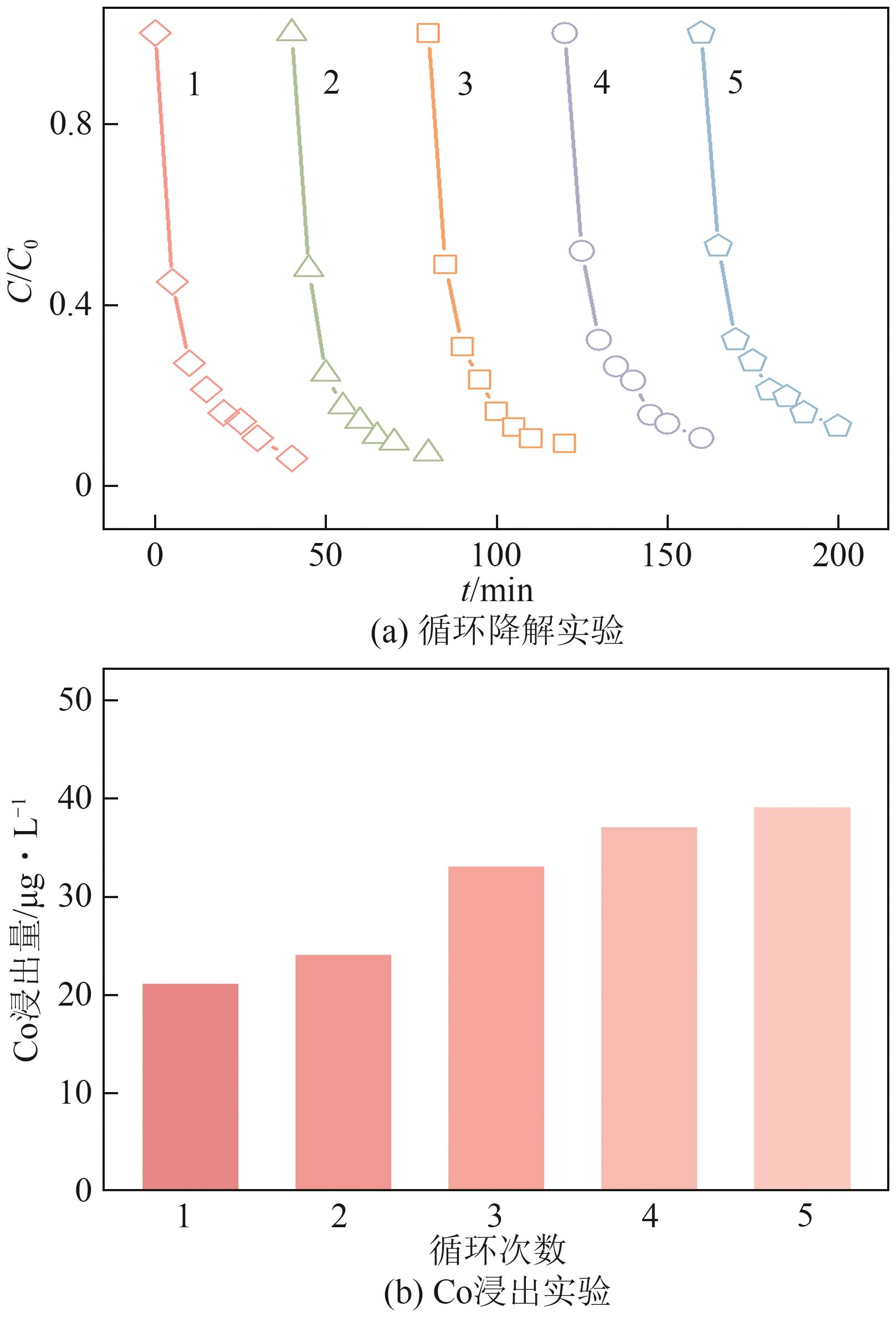
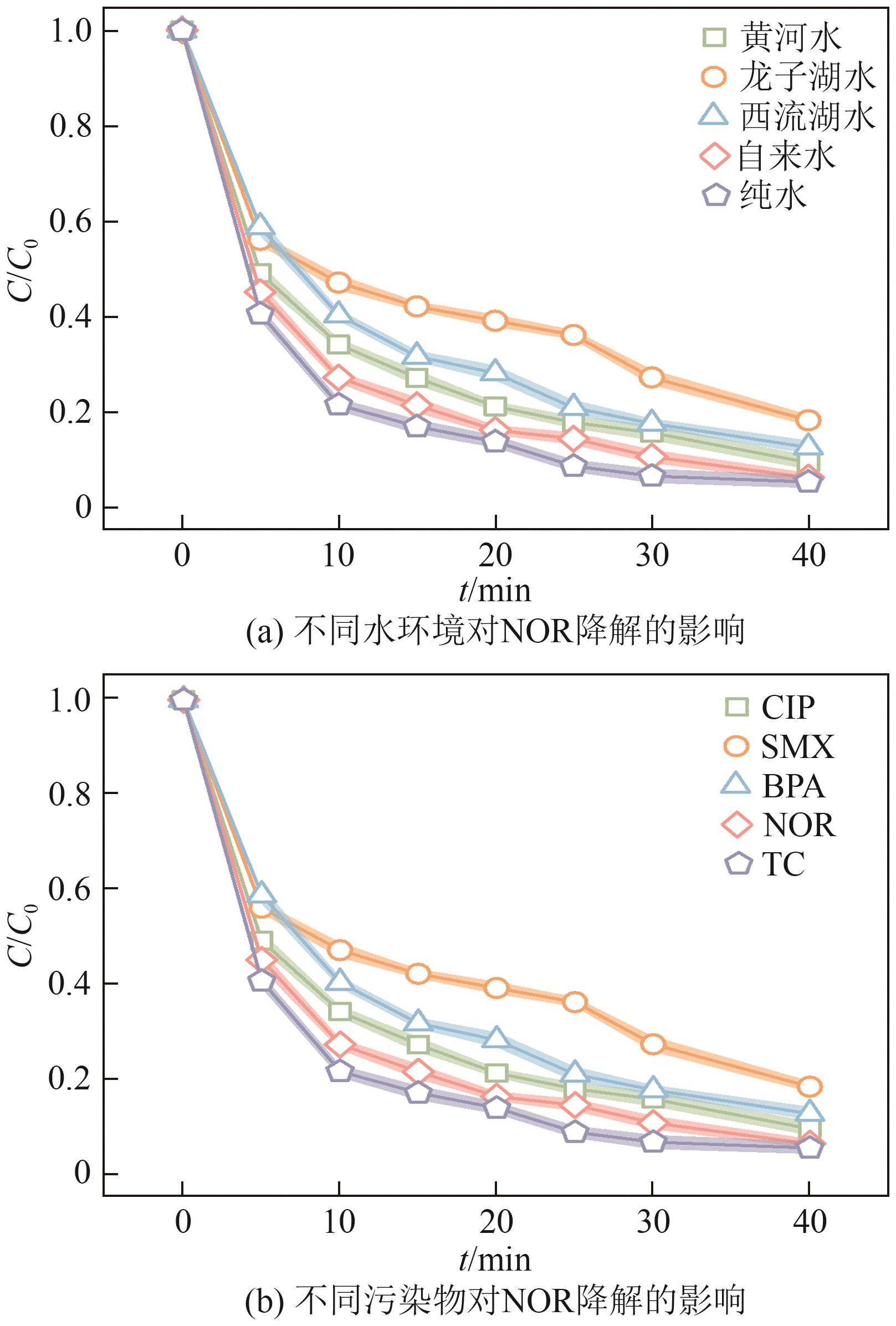
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (10): 6004-6015.DOI: 10.16085/j.issn.1000-6613.2024-1275
• Resources and environmental engineering • Previous Articles
Degradation of norfloxacin with peroxymonosulfate activated by amorphous MoS x doped with single atom cobalt
FU Linna1( ), HAO Hongdan1, ZHANG Jinxiao2, HAN Jiajun2
), HAO Hongdan1, ZHANG Jinxiao2, HAN Jiajun2
- 1.College of Food and chemical Engineering, Zhengzhou University of Technology, Zhengzhou 450044, Henan, China
2.School of Materials Engineering, Henan University of Engineering, Zhengzhou 451191, Henan, China
-
Received:2024-08-03Revised:2025-01-28Online:2025-11-10Published:2025-10-25 -
Contact:FU Linna
单原子钴掺杂非晶态MoS x 活化过一硫酸盐降解诺氟沙星
- 1.郑州工程技术学院食品与化工学院,河南 郑州 450044
2.河南工程学院材料工程学院,河南 郑州 451191
-
通讯作者:符林娜 -
作者简介:符林娜(1994—),女,硕士,研究方向为污水资源化利用。E-mail:Destiny_AFu@126.com。
CLC Number:
Cite this article
FU Linna, HAO Hongdan, ZHANG Jinxiao, HAN Jiajun. Degradation of norfloxacin with peroxymonosulfate activated by amorphous MoS x doped with single atom cobalt[J]. Chemical Industry and Engineering Progress, 2025, 44(10): 6004-6015.
符林娜, 郝红丹, 张锦晓, 韩佳骏. 单原子钴掺杂非晶态MoS x 活化过一硫酸盐降解诺氟沙星[J]. 化工进展, 2025, 44(10): 6004-6015.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1275
| [1] | LIU Wei, DONG Yingbo, YANG Dongsheng, et al. Unraveling the highly efficient synergy of adsorption and degradation for norfloxacin elimination by Mo/Fe anchored carbon fiber aerogel via peroxydisulfate activation[J]. Chemical Engineering Journal, 2023, 464: 142667. |
| [2] | WANG Chen, SHI Peng, WANG Zhaobo, et al. Efficient wastewater disinfection through FeOOH-mediated photo-Fenton reaction: A review[J]. Journal of Environmental Chemical Engineering, 2023, 11(6): 111269. |
| [3] | LI Yixiang, YAO Bin, CHEN Yuxin, et al. Metal-organic frameworks (MOFs) as efficient catalysts for electro-Fenton (EF) reactions: Current progress and prospects[J]. Chemical Engineering Journal, 2023, 463: 142287. |
| [4] | LI Chenxi, LUO Gang, LIU Yan. Comparison on the formation and toxicity for chlorinated products during the oxidation of acetic acid (CH3COOH) by three widely used advanced oxidation processes (AOPs) at the presence of Cl- [J]. Journal of Environmental Chemical Engineering, 2023, 11(6): 111501. |
| [5] | MOORE Nathan, WANG Chengjin, ANDREWS Susan, et al. On the increasing competitiveness of UV/Cl to UV/H2O2 advanced oxidation as the organic carbon concentration increases[J]. Water Research, 2023, 242: 120227. |
| [6] | QU Xinghong, ZENG Hongxue, GAO Yongsheng, et al. Phosphate enhanced thermal activation of peroxymonosulfate for acyclovir removal: Process comparison and DFT calculation[J]. Journal of Environmental Chemical Engineering, 2023, 11(6): 111525. |
| [7] | LIU Lu, LI Yinghua, ZHU Chaoqun, et al. Degradation of levofloxacin hydrochloride by Bi2O3/BiFeO3 activated peroxymonosulfate driven by visible light[J]. Optical Materials, 2023, 143: 114200. |
| [8] | LEE Sang Hoon, ANNAMALAI Sivasankar, SHIN Won Sik. Engineered ball-milled colloidal activated carbon material for advanced oxidation process of ibuprofen: Influencing factors and insights into the mechanism[J]. Environmental Pollution, 2023, 322: 121023. |
| [9] | LU Xiaowei, WU Lieshan, LIANG Liuling, et al. Levofloxacin degradation by porous Co x /CN activated peroxymonosulfate: Investigation of efficiency, mechanism, and degradation pathways[J]. Journal of Water Process Engineering, 2023, 56: 104427. |
| [10] | WANG Le, ZHENG Xiaoyu, YAN Liangguo, et al. Multiphasic MoS2 activates peroxymonosulfate for efficient removal of oxytetracycline: The dominant role of surface reactive species[J]. Separation and Purification Technology, 2023, 317: 123907. |
| [11] | ZHU Lingli, JI Jiahui, LIU Jun, et al. Designing 3D-MoS2 sponge as excellent cocatalysts in advanced oxidation processes for pollutant control[J]. Angewandte Chemie International Edition, 2020, 59(33): 13968-13976. |
| [12] | LI Xi, ZHANG Wenwen, LIU Zhenxue, et al. Effective removal of tetracycline from water by catalytic peroxymonosulfate oxidation over Co@MoS2: Catalytic performance and degradation mechanism[J]. Separation and Purification Technology, 2022, 294: 121139. |
| [13] | BALOGLOU Aristeidis, PLATTNER Manuel, Milan ONČÁK, et al. [Mo3S13]2- as a model system for hydrogen evolution catalysis by MoS x : Probing protonation sites in the gas phase by infrared multiple photon dissociation spectroscopy[J]. Angewandte Chemie International Edition, 2021, 60(10): 5074-5077. |
| [14] | TING Louisa Rui Lin, DENG Yilin, MA Liang, et al. Catalytic activities of sulfur atoms in amorphous molybdenum sulfide for the electrochemical hydrogen evolution reaction[J]. ACS Catalysis, 2016, 6(2): 861-867. |
| [15] | NIE Chunyang, WANG Jinlong, CAI Bihai, et al. Multifunctional roles of MoS2 in persulfate-based advanced oxidation processes for eliminating aqueous organic pollutants: A review[J]. Applied Catalysis B: Environmental, 2024, 340: 123173. |
| [16] | SHANG Yanan, XU Xing, GAO Baoyu, et al. Thiomolybdate [Mo3S13]2- nanoclusters anchored on reduced graphene oxide-carbon nanotube aerogels for efficient electrocatalytic hydrogen evolution[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(10): 8908-8917. |
| [17] | TRAN Phong D, TRAN Thu V, ORIO Maylis, et al. Coordination polymer structure and revisited hydrogen evolution catalytic mechanism for amorphous molybdenum sulfide[J]. Nature Materials, 2016, 15(6): 640-646. |
| [18] | WANG Hongzhi, ZHOU Haibin, ZHANG Weiguo, et al. Urea-assisted synthesis of amorphous molybdenum sulfide on P-doped carbon nanotubes for enhanced hydrogen evolution[J]. Journal of Materials Science, 2018, 53(12): 8951-8962. |
| [19] | Daniel ESCALERA-LÓPEZ, LOU Zhiheng, REES Neil V. Benchmarking the activity, stability, and inherent electrochemistry of amorphous molybdenum sulfide for hydrogen production[J]. Advanced Energy Materials, 2019, 9(8): 1802614. |
| [20] | CHEN Peng, GOU Yejing, NI Jiaming, et al. Efficient ofloxacin degradation with Co(Ⅱ)-doped MoS2 nano-flowers as PMS activator under visible-light irradiation[J]. Chemical Engineering Journal, 2020, 401: 125978. |
| [21] | FAN Yan, LIU Yanru, HU Xiang, et al. Preparation of metal organic framework derived materials CoFe2O4@NC and its application for degradation of norfloxacin from aqueous solutions by activated peroxymonosulfate[J]. Chemosphere, 2021, 275: 130059. |
| [22] | SUDHIR EKANDE Onkar, KUMAR Mathava. Self-powered piezoelectric NaNbO3 induced band position rearrangement and electrocatalysis in MoS2/NaNbO3 heterojunction for generation of reactive oxygen species for organic pollutant removal[J]. Chemical Engineering Journal, 2023, 458: 141454. |
| [23] | WANG Ning, WANG Wei, QI Dan, et al. Development of efficient and economic Bi2O3/BN/Co3O4 composite photocatalyst: Degradation mechanism, pathway and toxicity study of norfloxacin[J]. Chemosphere, 2024, 352: 141481. |
| [24] | GHANBARI Farshid, MORADI Mahsa. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review[J]. Chemical Engineering Journal, 2017, 310: 41-62. |
| [25] | HU Peidong, LONG Mingce. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications[J]. Applied Catalysis B: Environmental, 2016, 181: 103-117. |
| [26] | JIA Jialin, LIU Dongmei, WANG Songxue, et al. Visible-light-induced activation of peroxymonosulfate by TiO2 nano-tubes arrays for enhanced degradation of bisphenol A[J]. Separation and Purification Technology, 2020, 253: 117510. |
| [1] | HAO Yaling, LI Chunli, ZHOU Nan, CHENG Jiahao, WANG Jiarui, HUO Rong, WANG Delong, YANG Peng. Preparation and electrochemical performance of graphene electrode materials doped with different nitrogen sources [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5150-5160. |
| [2] | CHENG Jingwen, CHEN Qingcai, YU Bo, LIU Huan, XU Tengfei, HU Yukun, LIU Sitong. Detection performance and mechanism of VOCs by different metal-doped SnO2-based gas sensors [J]. Chemical Industry and Engineering Progress, 2025, 44(9): 5140-5149. |
| [3] | JIANG Rongyuan, LI Simin, CHEN Zhiqiang, WANG Guilong, CHEN Juntao, LIN Guanfeng, LU Beili, HUANG Biao, CHEN Yandan. Preparation of phosphorus-doped hard carbon/MnO x composite and its electrochemical properties [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 4039-4049. |
| [4] | YU Ning, WANG Qiuyue, WANG Zhicai, GAO Ziyi, CHAI Yongming, DONG Bin. Double-sites synergistic regulation for boosting water oxidation of La1-x Ni1-y Fe y O3‑δ [J]. Chemical Industry and Engineering Progress, 2025, 44(7): 3976-3984. |
| [5] | WANG Yuhao, JIANG Qinli, XU Ximeng. Degradation of organic pollutants via non-radical pathway by surface modified FeOCl activating persulfate [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3101-3111. |
| [6] | WANG Yuting, WANG Mengxiang, LI Wenwen, LI Gang, WANG Yajun. Photo-Fenton synergistic degradation of tetracycline by Fe(Ⅲ)/3D conjugated carbon nitride system [J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3072-3083. |
| [7] | CHEN Yuhang, LI Qiaoyan, LIANG Meisheng, SONG Tianyuan, WANG Yue, LI Simeng, ZHOU Yuxuan. Role of the Sn dopant on Cu/CeZrO2/γ-Al2O3 three-way catalyst: Enhancement of low-temperature activity and sulfur resistance [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1368-1377. |
| [8] | FU Tao, LI Li, GAO Lining, ZHU Fuwei, CAO Weiye, CHEN Huaxin. Cement-based boron-doped graphite phase carbon nitride material degrades NO [J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4403-4410. |
| [9] | LI Yingying, LIU An, JIANG Leyan, LI Hui, CHEN Chunyu, JU Dianchun. Progress in the preparation and electrochemical properties of transition metal sulfide Co9S8 [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3114-3127. |
| [10] | LI Yanan, GUO Kai, WANG Jiaqi, WU Yaning. Comparison of phenol degradation by persulfate and peroxymonosulfate activated with coal gasification slag [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3503-3512. |
| [11] | GUI Xin, CHEN Huiyong, BAI Boyang, JIA Yongliang, MA Xiaoxun. Catalytic hydrogenation of pyrene over Mo-doped NiC/Al-MCM-41 [J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2386-2395. |
| [12] | WANG Debin, LIN Mengyu, YANG Xue, DONG Dianquan. Preparation and adsorption properties of zinc-doped titanium-based cesium ion sieves [J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1953-1961. |
| [13] | ZENG Xiangchu, NING Haichao, WU Zhe, WEI Ruisong, YIN Xiuju. Coupled homogeneous/heterogeneous Fenton-like system for enhanced inactivating of tetracycline-resistance Salmonella typhi [J]. Chemical Industry and Engineering Progress, 2024, 43(12): 7078-7094. |
| [14] | MA Chao, SUN Zhihua, WANG Lei, JI Yu, CHEN Cuizhong, WANG Jiankang, ZHAO Chun. Degradation of reactive yellow K-RN by electricity/potassium permanganate/peroxymonosulfate system and its mechanism [J]. Chemical Industry and Engineering Progress, 2024, 43(10): 5958-5968. |
| [15] | YANG Chenggong, HUANG Rong, WANG Dong’e, TIAN Zhijian. Electrocatalytic hydrogen evolution performance of nitrogen-doped molybdenum disulfide nanocatalysts [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 465-472. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||