化工进展 ›› 2021, Vol. 40 ›› Issue (4): 2188-2205.DOI: 10.16085/j.issn.1000-6613.2020-1045
磷化钴材料在电化学能源领域的研究进展
- 兰州理工大学石油化工学院,甘肃 兰州 730050
-
收稿日期:2020-06-19出版日期:2021-04-05发布日期:2021-04-14 -
通讯作者:赵鹬 -
作者简介:赵鹬(1981—),副教授,硕士生导师,研究方向为多相催化及新材料。E-mail:yzhao@lut.edu.cn |周飞(1995—),男,硕士研究生,研究方向为电化学储能。E-mail:1440907127@qq.com 。 -
基金资助:国家自然科学基金(21763016)
Research progress of cobalt phosphide materials in the field of electrochemical energy
ZHAO Yu( ), ZHOU Fei(
), ZHOU Fei( ), ZHANG Weiwei, LI Ning, LI Shiyou, LI Guixian
), ZHANG Weiwei, LI Ning, LI Shiyou, LI Guixian
- School of Petrochemical Engineering, Lanzhou University of Technology, Lanzhou 730050, Gansu, China
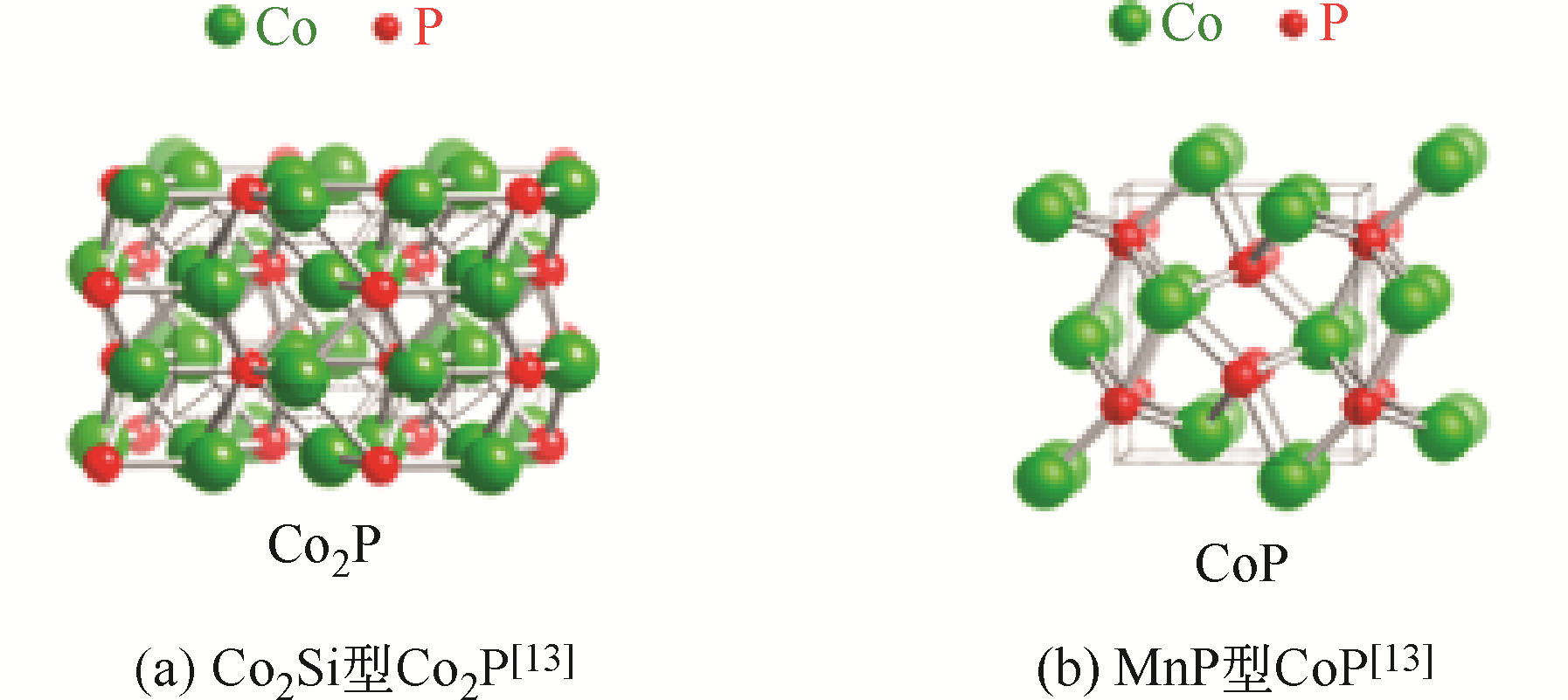
-
Received:2020-06-19Online:2021-04-05Published:2021-04-14 -
Contact:ZHAO Yu
摘要:
当今时代,人类社会的发展日益依赖巨量的能源,这与不可再生资源有限的储量相矛盾,发展绿色清洁、高效可持续的可再生能源和能源利用技术迫在眉睫。磷化钴材料作为过渡金属磷化物的重要一员,由于其对氢的优异吸/脱附性能和特殊的晶体结构,广泛应用于电解水、超级电容器和二次电池等电化学能量储存与转化领域。然而目前磷化钴材料的应用还存在很多缺陷,在电解水反应中活性组分易分解,结构稳定性差;在超级电容器使用中活性位点暴露不足、电导率偏低;作为锂/钠离子电池电极材料在充放电过程中存在巨大的体积变化而导致循环稳定性降低等。本文对磷化钴的晶体结构、制备方法及改良方法作了总结,对其应用于电解水、超级电容器和锂/钠离子电池的生效机理和发展近况进行概述。最后提出了存在的问题和未来的发展方向。
中图分类号:
引用本文
赵鹬, 周飞, 张伟伟, 李宁, 李世友, 李贵贤. 磷化钴材料在电化学能源领域的研究进展[J]. 化工进展, 2021, 40(4): 2188-2205.
ZHAO Yu, ZHOU Fei, ZHANG Weiwei, LI Ning, LI Shiyou, LI Guixian. Research progress of cobalt phosphide materials in the field of electrochemical energy[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2188-2205.
| 产物 | 钴源 | 磷源 | 溶液 | 温度/℃ | 时间 | 形貌 | 文献 |
|---|---|---|---|---|---|---|---|
| CoP | 八羰基钴 | TOP | 1-十八烯、油胺 | 330 | 60min | 纳米颗粒 | [ |
| Co2P | 八羰基钴 | TOP | 1-十八烯 | 290 | 40min | 纳米颗粒 | [ |
| Co2P | 乙酰丙酮钴 | TPP | 油胺、甲苯 | 280 | 2h | 中空纳米花 | [ |
| Co2P | 八羰基钴 | TOP | 1-十八烯,油胺 | 300 | 1h | 纳米颗粒 | [ |
| CoP | 八羰基钴 | TOP | 油胺 | 350 | 2.5h | 纳米颗粒 | [ |
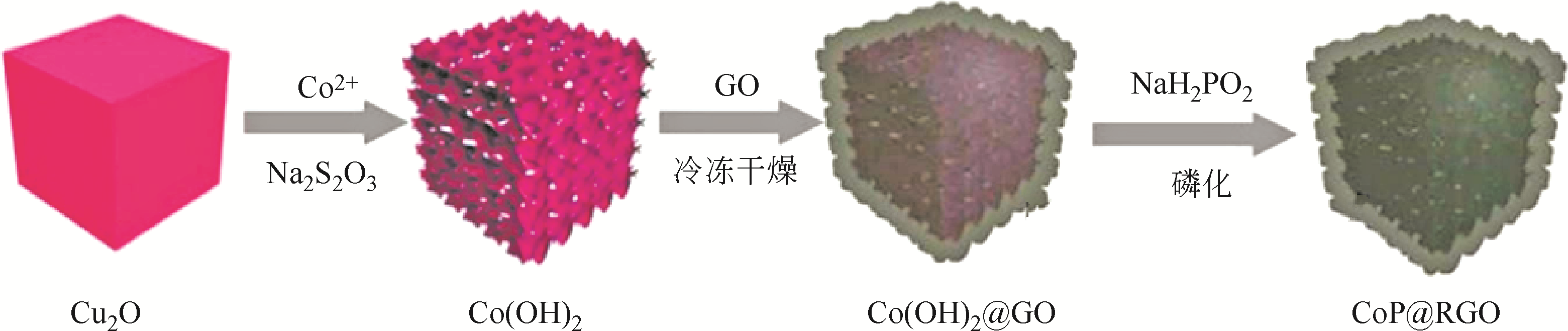
| CoP | 乙酰丙酮钴 | TOP | 1-十八烯,油胺 | 320 | 2h | 单分散纳米晶 | [ |
| Co2P | 乙酰丙酮钴 | TOP | 1-十八烯,油胺 | 280 | 2h | 单分散纳米晶 | [ |
| CoP | 乙酰丙酮钴 | TPP | 油胺、甲苯 | 300 | 3h | 升温20min纳米棒,25min纳米花 | [ |
| Co2P | 乙酸钴 | TPP | 石墨烯分散于油胺 | 280 | 3h | Co2P-Co/石墨烯中空纳米复合材料 | [ |
| Co2P | 硬脂酸钴 | TOP | 油胺、油酸、三辛胺 | 310 | 1h | 纳米球 | [ |
| CoP | 乙酰丙酮钴 | TOP | 1-十八烯,油胺 | 330 | 1h | 纳米棒 | [ |
| Co2P | 乙酰丙酮钴 | TPP | 油胺 | 320 | 2h | 纳米微球 | [ |
表1 有机金属分解法合成多种形貌磷化钴的条件总结
| 产物 | 钴源 | 磷源 | 溶液 | 温度/℃ | 时间 | 形貌 | 文献 |
|---|---|---|---|---|---|---|---|
| CoP | 八羰基钴 | TOP | 1-十八烯、油胺 | 330 | 60min | 纳米颗粒 | [ |
| Co2P | 八羰基钴 | TOP | 1-十八烯 | 290 | 40min | 纳米颗粒 | [ |
| Co2P | 乙酰丙酮钴 | TPP | 油胺、甲苯 | 280 | 2h | 中空纳米花 | [ |
| Co2P | 八羰基钴 | TOP | 1-十八烯,油胺 | 300 | 1h | 纳米颗粒 | [ |
| CoP | 八羰基钴 | TOP | 油胺 | 350 | 2.5h | 纳米颗粒 | [ |
| CoP | 乙酰丙酮钴 | TOP | 1-十八烯,油胺 | 320 | 2h | 单分散纳米晶 | [ |
| Co2P | 乙酰丙酮钴 | TOP | 1-十八烯,油胺 | 280 | 2h | 单分散纳米晶 | [ |
| CoP | 乙酰丙酮钴 | TPP | 油胺、甲苯 | 300 | 3h | 升温20min纳米棒,25min纳米花 | [ |
| Co2P | 乙酸钴 | TPP | 石墨烯分散于油胺 | 280 | 3h | Co2P-Co/石墨烯中空纳米复合材料 | [ |
| Co2P | 硬脂酸钴 | TOP | 油胺、油酸、三辛胺 | 310 | 1h | 纳米球 | [ |
| CoP | 乙酰丙酮钴 | TOP | 1-十八烯,油胺 | 330 | 1h | 纳米棒 | [ |
| Co2P | 乙酰丙酮钴 | TPP | 油胺 | 320 | 2h | 纳米微球 | [ |
| 催化剂 | 钴源 | 磷源 | 特征尺寸 | 比表面积 /m2·g-1 | 用途 | 电解液 | 过电势/电流密度 | 文献 |
|---|---|---|---|---|---|---|---|---|
| 中空的Co2P纳米颗粒 | 八羰基钴 | TOP | 约11nm的纳米颗粒 | HER | 0.5mol·L-1 H2SO4 | 95mV/10mA·cm-2 | [ | |
| 中空的CoP纳米颗粒 | 八羰基钴 | TOP | 约12nm的纳米颗粒 | HER | 0.5mol·L-1 H2SO4 | 75mV/10mA·cm-2 | [ | |
| Co2P/WC异质结 | 多金属氧酸盐 (Co7P6W18) | 多金属氧酸盐 (Co7P6W18) | 4.7nm的异质结 | 226 | HER | 0.5mol·L-1 H2SO4 | 91mV/10mA·cm-2 | [ |
| Co2P-CoN核壳纳米 颗粒 | 硝酸钴 | TPP | 174.81 | HER | 0.5mol·L-1 H2SO4 | 98mV/10mA·cm-2 | [ | |
| Co-Co2P纳米颗粒 | 酵母菌 | 酵母菌 | 约104.8nm的纳米颗粒 | 110.2 | HER | 0.5mol·L-1 H2SO4 | 61.5mV/10mA·cm-2 | [ |
| Ag@CoxP核壳结构 | 乙酸钴 | TPP | 约23.7nm的纳米球 | OER | 1mol·L-1 KOH | 310mV/10mA·cm-2 | [ | |
| 针状窄六方相Co2P | 氯化钴 | 磷化氢 | 长约70nm、宽约5nm的纳米针 | OER | 1mol·L-1 KOH | 310mV/10mA·cm-2 | [ | |
| O、Fe共掺杂的Co2P 纳米线 | 硝酸钴 | TPP | HER | 1mol·L-1 KOH | 87.5mV/10mA·cm-2 | [ | ||
| OER | 274.5mV/10mA·cm-2 | |||||||
| 分层的花状CoP/CoP2/ Al2O3复合物 | 硝酸钴 | 红磷 | 约12.9nm的颗粒 | 25.8 | HER | 1mol·L-1 KOH | 138mV/10mA·cm-2 | [ |
| OER | 300mV/10mA·cm-2 | |||||||
| 全水解 | 1.65V/10mA·cm-2 | |||||||
| 碳纳米片/纳米管封 装的Co2P颗粒 | 硝酸钴 | 磷酸 | 平均2.3nm的颗粒 | 199.94 | HER | 1mol·L-1 KOH | 154mV/10mA·cm-2 | [ |
| OER | 280mV/10mA·cm-2 | |||||||
| 全水解 | 1.64V/10mA·cm-2 | |||||||
| 碳骨架封装的Cu-Co 双金属磷化物 | BCP-ZIF | 次磷酸钠 | HER | 1mol·L-1 KOH | 190mV/10mA·cm-2 | [ | ||
| OER | 220mV/10mA·cm-2 | |||||||
| 全水解 | 1.74V/70mA·cm-2 |
表2 各种磷化钴电解水催化剂达到相应电流密度所需过电势汇总
| 催化剂 | 钴源 | 磷源 | 特征尺寸 | 比表面积 /m2·g-1 | 用途 | 电解液 | 过电势/电流密度 | 文献 |
|---|---|---|---|---|---|---|---|---|
| 中空的Co2P纳米颗粒 | 八羰基钴 | TOP | 约11nm的纳米颗粒 | HER | 0.5mol·L-1 H2SO4 | 95mV/10mA·cm-2 | [ | |
| 中空的CoP纳米颗粒 | 八羰基钴 | TOP | 约12nm的纳米颗粒 | HER | 0.5mol·L-1 H2SO4 | 75mV/10mA·cm-2 | [ | |
| Co2P/WC异质结 | 多金属氧酸盐 (Co7P6W18) | 多金属氧酸盐 (Co7P6W18) | 4.7nm的异质结 | 226 | HER | 0.5mol·L-1 H2SO4 | 91mV/10mA·cm-2 | [ |
| Co2P-CoN核壳纳米 颗粒 | 硝酸钴 | TPP | 174.81 | HER | 0.5mol·L-1 H2SO4 | 98mV/10mA·cm-2 | [ | |
| Co-Co2P纳米颗粒 | 酵母菌 | 酵母菌 | 约104.8nm的纳米颗粒 | 110.2 | HER | 0.5mol·L-1 H2SO4 | 61.5mV/10mA·cm-2 | [ |
| Ag@CoxP核壳结构 | 乙酸钴 | TPP | 约23.7nm的纳米球 | OER | 1mol·L-1 KOH | 310mV/10mA·cm-2 | [ | |
| 针状窄六方相Co2P | 氯化钴 | 磷化氢 | 长约70nm、宽约5nm的纳米针 | OER | 1mol·L-1 KOH | 310mV/10mA·cm-2 | [ | |
| O、Fe共掺杂的Co2P 纳米线 | 硝酸钴 | TPP | HER | 1mol·L-1 KOH | 87.5mV/10mA·cm-2 | [ | ||
| OER | 274.5mV/10mA·cm-2 | |||||||
| 分层的花状CoP/CoP2/ Al2O3复合物 | 硝酸钴 | 红磷 | 约12.9nm的颗粒 | 25.8 | HER | 1mol·L-1 KOH | 138mV/10mA·cm-2 | [ |
| OER | 300mV/10mA·cm-2 | |||||||
| 全水解 | 1.65V/10mA·cm-2 | |||||||
| 碳纳米片/纳米管封 装的Co2P颗粒 | 硝酸钴 | 磷酸 | 平均2.3nm的颗粒 | 199.94 | HER | 1mol·L-1 KOH | 154mV/10mA·cm-2 | [ |
| OER | 280mV/10mA·cm-2 | |||||||
| 全水解 | 1.64V/10mA·cm-2 | |||||||
| 碳骨架封装的Cu-Co 双金属磷化物 | BCP-ZIF | 次磷酸钠 | HER | 1mol·L-1 KOH | 190mV/10mA·cm-2 | [ | ||
| OER | 220mV/10mA·cm-2 | |||||||
| 全水解 | 1.74V/70mA·cm-2 |
| 材料 | 钴源 | 磷源 | 比表面积 /m2·g-1 | 微观形态 | 电流密度/比容量 | 用作ASC电极时功率密度 /能量密度 | 文献 |
|---|---|---|---|---|---|---|---|
| Co2P纳米花 | 乙酰丙酮钴 | TPP | 29 | 直径10~20nm的纳米晶 | 1A·g-1/416F·g-1 | 0.3kW·kg-1/24W·h·kg-1 | [ |
| Co2P中空纳米花 | 乙酰丙酮钴 | TPP | 19.37 | 约110nm的纳米球 | 1A·g-1/412.7F·g-1 | 0.85kW·kg-1/30.5W·h·kg-1 | [ |
| 绒球状CoP中空微球 | 硝酸钴 | 次磷酸钠 | 24.6 | 最宽500nm的纳米针 | 1A·g-1/449.4F·g-1 | 0.3749kW·kg-1/22.2W·h·kg-1 | [ |
| CoP纳米线阵列 | 硝酸钴 | 次磷酸钠 | 3~8μm长的CoP纳米线阵列 | 3mA·cm-2/1.89F·cm-2 | [ | ||
| CoP纳米线阵列 | 硝酸钴 | 次磷酸钠 | 1mA·cm-2/571.3mF·cm-2 | 10.15mW·cm-3/0.69mW·h·cm-3 | [ | ||
| CoP纳米线 | 硝酸钴 | 次磷酸钠 | 直径约60nm的纳米线 | 1mA·cm-2/8.66F·cm-2 | 0.193kW·kg-1/35.21W·h·kg-1 | [ | |
| CoP纳米管阵列 | 硝酸钴 | 次磷酸钠 | 472.22 | CoP微晶尺寸约18.5nm | 1A·g-1/610F·g-1 | 0.8kW·kg-1/39W·h·kg-1 | [ |
表3 各种磷化钴材料用于超级电容器性能数据汇总
| 材料 | 钴源 | 磷源 | 比表面积 /m2·g-1 | 微观形态 | 电流密度/比容量 | 用作ASC电极时功率密度 /能量密度 | 文献 |
|---|---|---|---|---|---|---|---|
| Co2P纳米花 | 乙酰丙酮钴 | TPP | 29 | 直径10~20nm的纳米晶 | 1A·g-1/416F·g-1 | 0.3kW·kg-1/24W·h·kg-1 | [ |
| Co2P中空纳米花 | 乙酰丙酮钴 | TPP | 19.37 | 约110nm的纳米球 | 1A·g-1/412.7F·g-1 | 0.85kW·kg-1/30.5W·h·kg-1 | [ |
| 绒球状CoP中空微球 | 硝酸钴 | 次磷酸钠 | 24.6 | 最宽500nm的纳米针 | 1A·g-1/449.4F·g-1 | 0.3749kW·kg-1/22.2W·h·kg-1 | [ |
| CoP纳米线阵列 | 硝酸钴 | 次磷酸钠 | 3~8μm长的CoP纳米线阵列 | 3mA·cm-2/1.89F·cm-2 | [ | ||
| CoP纳米线阵列 | 硝酸钴 | 次磷酸钠 | 1mA·cm-2/571.3mF·cm-2 | 10.15mW·cm-3/0.69mW·h·cm-3 | [ | ||
| CoP纳米线 | 硝酸钴 | 次磷酸钠 | 直径约60nm的纳米线 | 1mA·cm-2/8.66F·cm-2 | 0.193kW·kg-1/35.21W·h·kg-1 | [ | |
| CoP纳米管阵列 | 硝酸钴 | 次磷酸钠 | 472.22 | CoP微晶尺寸约18.5nm | 1A·g-1/610F·g-1 | 0.8kW·kg-1/39W·h·kg-1 | [ |
| 电池类型 | 磷化钴材料 | 钴源 | 磷源 | 比表面积 /m2·g-1 | 尺寸 | 电流密度(A·g-1) /循环次数 | 比容量 /mA·h·g-1 | 文献 |
|---|---|---|---|---|---|---|---|---|
锂离子电池 | 锚定在石墨烯上的Co2P纳米棒 | 硬脂酸钴 | TOP | — | 数个Co2P纳米棒组成100~200nm海胆状球体 | 0.1/180 | 888 | [ |
| Co2P-Co中空纳米球用石墨烯修饰 | 乙酸钴 | TPP | 25.4 | 直径200~300nm的空心纳米球 | 0.1/200 | 929 | [ | |
| 石墨烯包裹的CoP纳米笼 | 氯化钴 | 次磷酸钠 | 112.29 | 平均直径约650nm的纳米笼 | 0.1/500 | 546.6 | [ | |
| 限制在分层多维碳基质中的CoP | 氯化钴 | 次磷酸钠 | 129 | 5~15nm的纳米颗粒 | 0.25/300 | 754 | [ | |
| 生长在石墨烯上的分层Co2P微球 | 乙酸钴 | 次磷酸钠 | 28.91 | — | 0.2/500 | 698 | [ | |
| 生长在石墨烯上的CoP纳米线 | 氯化钴 | 次磷酸钠 | 29.1 | 直径200~300nm的纳米线 | 0.2/200 | 960 | [ | |
| 嵌入到石墨烯纳米片网络的CoP | 硝酸钴 | 次磷酸钠 | 97.8 | 约500nm的颗粒 | 2/2000 | 808 | [ | |
| 生长的碳纳米管中的Co2P | ZIF-67 | 次磷酸钠 | — | 数纳米的纳米颗粒 | 0.1/300 | 857 | [ | |
| 分布在碳纳米管缠绕的碳立方体中的CoP | ZIF-67 | 次磷酸钠 | 396.3 | — | 0.1/100 | 691.9 | [ | |
| 镶嵌在N掺杂碳基质里的CoP | ZIF-67 | 次磷酸钠 | — | 纳米颗粒镶嵌在1.5μm的菱形十二面体上 | 0.2/750 | 522.6 | [ | |
| CoxP纳米颗粒镶嵌在多孔碳多面体中 | ZIF-67 | 红磷 | 326.5 | 纳米颗粒分散在400~800nm的碳多面体中 | 0.1/100 | 1224 | [ | |
钠离子电池 | 封装了Co2P纳米的碳纳米片网 | 硝酸钴 | 次磷酸钠 | 453 | 5~25nm的Co2P颗粒 | 0.05/100 | 306 | [ |
| 多面体碳基质复合的Co2P纳米颗粒 | ZIF-67 | 次磷酸钠 | — | 约120nm的颗粒 | 0.05/100 | 225 | [ | |
| 掺杂在碳纳米片中的CoP纳米颗粒 | 硝酸钴 | 红磷 | 102.19 | 约11.3nm的颗粒 | 1/900 | 386 | [ | |
| 石墨烯连接的FeP/CoP核壳立方体 | 乙酸钴 | 次磷酸钠 | 55.6 | 直径500nm立方体表面的一层约50nm厚的壳 | 0.1/200 | 456.2 | [ | |
| CoP/碳多面体核壳结构锚定在石墨烯上 | ZIF-67 | 次磷酸钠 | — | 约10nm的颗粒 | 0.1/100 | 473.1 | [ |
表4 各种磷化钴复合纳米材料及其用于锂/钠离子电池性能表现汇总
| 电池类型 | 磷化钴材料 | 钴源 | 磷源 | 比表面积 /m2·g-1 | 尺寸 | 电流密度(A·g-1) /循环次数 | 比容量 /mA·h·g-1 | 文献 |
|---|---|---|---|---|---|---|---|---|
锂离子电池 | 锚定在石墨烯上的Co2P纳米棒 | 硬脂酸钴 | TOP | — | 数个Co2P纳米棒组成100~200nm海胆状球体 | 0.1/180 | 888 | [ |
| Co2P-Co中空纳米球用石墨烯修饰 | 乙酸钴 | TPP | 25.4 | 直径200~300nm的空心纳米球 | 0.1/200 | 929 | [ | |
| 石墨烯包裹的CoP纳米笼 | 氯化钴 | 次磷酸钠 | 112.29 | 平均直径约650nm的纳米笼 | 0.1/500 | 546.6 | [ | |
| 限制在分层多维碳基质中的CoP | 氯化钴 | 次磷酸钠 | 129 | 5~15nm的纳米颗粒 | 0.25/300 | 754 | [ | |
| 生长在石墨烯上的分层Co2P微球 | 乙酸钴 | 次磷酸钠 | 28.91 | — | 0.2/500 | 698 | [ | |
| 生长在石墨烯上的CoP纳米线 | 氯化钴 | 次磷酸钠 | 29.1 | 直径200~300nm的纳米线 | 0.2/200 | 960 | [ | |
| 嵌入到石墨烯纳米片网络的CoP | 硝酸钴 | 次磷酸钠 | 97.8 | 约500nm的颗粒 | 2/2000 | 808 | [ | |
| 生长的碳纳米管中的Co2P | ZIF-67 | 次磷酸钠 | — | 数纳米的纳米颗粒 | 0.1/300 | 857 | [ | |
| 分布在碳纳米管缠绕的碳立方体中的CoP | ZIF-67 | 次磷酸钠 | 396.3 | — | 0.1/100 | 691.9 | [ | |
| 镶嵌在N掺杂碳基质里的CoP | ZIF-67 | 次磷酸钠 | — | 纳米颗粒镶嵌在1.5μm的菱形十二面体上 | 0.2/750 | 522.6 | [ | |
| CoxP纳米颗粒镶嵌在多孔碳多面体中 | ZIF-67 | 红磷 | 326.5 | 纳米颗粒分散在400~800nm的碳多面体中 | 0.1/100 | 1224 | [ | |
钠离子电池 | 封装了Co2P纳米的碳纳米片网 | 硝酸钴 | 次磷酸钠 | 453 | 5~25nm的Co2P颗粒 | 0.05/100 | 306 | [ |
| 多面体碳基质复合的Co2P纳米颗粒 | ZIF-67 | 次磷酸钠 | — | 约120nm的颗粒 | 0.05/100 | 225 | [ | |
| 掺杂在碳纳米片中的CoP纳米颗粒 | 硝酸钴 | 红磷 | 102.19 | 约11.3nm的颗粒 | 1/900 | 386 | [ | |
| 石墨烯连接的FeP/CoP核壳立方体 | 乙酸钴 | 次磷酸钠 | 55.6 | 直径500nm立方体表面的一层约50nm厚的壳 | 0.1/200 | 456.2 | [ | |
| CoP/碳多面体核壳结构锚定在石墨烯上 | ZIF-67 | 次磷酸钠 | — | 约10nm的颗粒 | 0.1/100 | 473.1 | [ |
| 1 | OYAMA S T. Handbook of heterogeneous catalysis: transition metal carbides, nitrides, and phosphides[M]. Weinheim: Wiley-VCH, 2008: 342-356. |
| 2 | SUN M, LIU H, QU J, et al. Earth-rich transition metal phosphide for energy conversion and storage[J]. Advanced Energy Materials, 2016, 6(13): 1600087. |
| 3 | OYAMA S T, GOTT T, ZHAO H, et al. Transition metal phosphide hydroprocessing catalysts: a review[J]. Catalysis Today, 2009, 143(1): 94-107. |
| 4 | CECILIA J A, INFANTES-MOLINA A, RODRÍGUEZ-CASTELLÓN E, et al. Dibenzothiophene hydrodesulfurization over cobalt phosphide catalysts prepared through a new synthetic approach: effect of the support[J]. Applied Catalysis B: Environmental, 2009, 92(1): 100-113. |
| 5 | BERENGUER A, THANGARAJU S, GOMEZ G, et al. Evaluation of transition metal phosphides supported on ordered mesoporous materials as catalysts for phenol hydrodeoxygenation[J]. Green Chemistry, 2015, 18(7): 1938-1951. |
| 6 | CHEN J, SHI H, LI L, et al. Deoxygenation of methyl laurate as a model compound to hydrocarbons on transition metal phosphide catalysts [J]. Applied Catalysis B: Environmental, 2014, 144: 870-884. |
| 7 | HALL J W, MEMBRENO N, WU J, et al. Low-temperature synthesis of amorphous FeP2 and its use as anodes for Li ion batteries[J]. Journal of the American Chemical Society, 2012, 134(12): 5532-5535. |
| 8 | ZHANG Y, GAO L, HENSEN E J M, et al. Evaluating the stability of Co2P electrocatalysts in the hydrogen evolution reaction for both acidic and alkaline electrolytes[J]. ACS Energy Letters, 2018, 3(6): 1360-1365. |
| 9 | NOZAKI F, TOKUMI M. Hydrogenation activity of metal phosphides and promoting effect of oxygen[J]. Journal of Catalysis, 1983, 79(1): 207-210. |
| 10 | FENG L G, XUE H G. Advances in transition-metal phosphide applications in electrochemical energy storage and catalysis[J]. Chemelectrochem, 2017, 4(1): 20-34. |
| 11 | HU Y, LIU M, YANG Q, et al. Facile synthesis of high electrical conductive CoP via solid-state synthetic routes for supercapacitors[J]. Journal of Energy Chemistry, 2017, 26(1): 49-55. |
| 12 | YANG Z, LIU L, WANG X, et al. Stability and electronic structure of the Co-P compounds from first-principle calculations[J]. Journal of Alloys and Compounds, 2011, 509(1): 165-171. |
| 13 | CALLEJAS J F, READ C G, POPCZUN E J, et al. Nanostructured Co2P electrocatalyst for the hydrogen evolution reaction and direct comparison with morphologically equivalent CoP[J]. Chemistry of Materials, 2015, 27(10): 3769-3774. |
| 14 | LI H, LI Q, WEN P, et al. Colloidal cobalt phosphide nanocrystals as trifunctional electrocatalysts for overall water splitting powered by a zinc-air battery[J]. Advanced Materials, 2018, 30(9): 1705796. |
| 15 | DENG J, DENG D H, BAO X H. Robust catalysis on 2D materials encapsulating metals: concept, application, and perspective[J]. Advanced Materials, 2017, 29(43): 1606967. |
| 16 | LI J, LIU G Y, LIU B B, et al. An extremely facile route to Co2P encased in N,P-codoped carbon layers: highly efficient bifunctional electrocatalysts for ORR and OER[J]. International Journal of Hydrogen Energy, 2018, 43(3): 1365-1374. |
| 17 | NITTA N, YUSHIN G. High-capacity anode materials for lithium-ion batteries: choice of elements and structures for active particles[J]. Particle & Particle Systems Characterization, 2014, 31(3): 317-336. |
| 18 | KWON H T, KIM J H, JEON K J, et al. CoxP compounds: electrochemical conversion/partial recombination reaction and partially disproportionated nanocomposite for Li-ion battery anodes[J]. RSC Advances, 2014, 4(81): 43227-43234. |
| 19 | GUAN J, WANG Y, QIN M L, et al. Synthesis of transition-metal phosphides from oxidic precursors by reduction in hydrogen plasma[J]. Journal of Solid State Chemistry, 2009, 182(6): 1550-1555. |
| 20 | YAO Z W, HAI H, LAI Z C, et al. A novel carbothermal synthesis route for carbon nanotube supported Fe2P nanoparticles[J]. Topics in Catalysis, 2012, 55(14/15): 1040-1045. |
| 21 | OYAMA S T, WANG X, LEE Y K, et al. Effect of phosphorus content in nickel phosphide catalysts studied by XAFS and other techniques[J]. Journal of Catalysis, 2002, 210(1): 207-217. |
| 22 | ZUZANIUK V, PRINS R. Synthesis and characterization of silica-supported transition-metal phosphides as HDN catalysts[J]. Journal of Catalysis, 2003, 219(1): 85-96. |
| 23 | CECILIA J, INFANTES-MOLINA A, RODRÍGUEZ-CASTELLÓN E, et al. Gas phase catalytic hydrodechlorination of chlorobenzene over cobalt phosphide catalysts with different P contents[J]. Journal of Hazardous Materials, 2013, 260: 167-175. |
| 24 | DAS D, DAS A, REGHUNATH M, et al. Phosphine-free avenue to Co2P nanoparticle encapsulated N,P co-doped CNTs: a novel non-enzymatic glucose sensor and an efficient electrocatalyst for oxygen evolution reaction[J]. Green Chemistry, 2017, 19(5): 1327-1335. |
| 25 | LI Y G, GAO Y, LANG Z L, et al. A Co2P/WC nano-heterojunction covered with N-doped carbon as highly efficient electrocatalyst for hydrogen evolution reaction[J]. ChemSusChem, 2018, 11(6): 1082-1091. |
| 26 | ZHOU D, FAN L Z. Co2P nanoparticles encapsulated in 3D porous N-doped carbon nanosheet networks as an anode for high-performance sodium-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(5): 2139-2147. |
| 27 | CHEN M H, ZHOU W W, QI M L, et al. Exploring highly porous Co2P nanowire arrays for electrochemical energy storage[J]. Journal of Power Sources, 2017, 342: 964-969. |
| 28 | HUANG X K, WU D F, CHENG D J. Porous Co2P nanowires as high efficient bifunctional catalysts for 4-nitrophenol reduction and sodium borohydride hydrolysis[J]. Journal of Colloid and Interface Science, 2017, 507: 429-436. |
| 29 | JIN R, LI X F, SUN Y X, et al. Metal organic frameworks-derived Co2P@N-C@rGO with dual protection layers for improved sodium storage[J]. ACS Applied Materials & Interfaces, 2018, 10(17): 14641-14648. |
| 30 | LEI C J, WANG F, YANG J, et al. Embedding Co2P nanoparticles in N-doped carbon nanotubes grown on porous carbon polyhedra for high-performance lithium-ion batteries[J]. Industrial & Engineering Chemistry Research, 2018, 57(39): 13019-13025. |
| 31 | SONG J, ZHU C, XU B Z, et al. Bimetallic cobalt-based phosphide zeolitic imidazolate framework: CoPx phase-dependent electrical conductivity and hydrogen atom adsorption energy for efficient overall water splitting[J]. Advanced Energy Materials, 2017, 7(2): 1601555. |
| 32 | GE X L, LI Z Q, YIN L W. Metal-organic frameworks derived porous core/shell CoP@C polyhedrons anchored on 3D reduced graphene oxide networks as anode for sodium-ion battery[J]. Nano Energy, 2016, 32: 117-124. |
| 33 | PAN Y, LIN Y, Chen Y J, et al. Cobalt phosphide-based electrocatalysts: synthesis and phase catalytic activity comparison for hydrogen evolution[J]. Journal of Materials Chemistry A, 2016, 4(13): 4745-4754. |
| 34 | XIE Q S, ZENG D Q, GONG P Y, et al. One-pot fabrication of graphene sheets decorated Co2P-Co hollow nanospheres for advanced lithium ion battery anodes[J]. Electrochimica Acta, 2017, 232: 465-473. |
| 35 | CHEN X J, CHENG M, CHEN D, et al. Shape-controlled synthesis of Co2P nanostructures and their application in supercapacitors[J]. ACS Applied Materials & Interfaces, 2016, 8(6): 3892-3900. |
| 36 | CHENG M, FAN H S, XU Y Y, et al. Hollow Co2P nanoflowers assembled from nanorods for ultralong cycle-life supercapacitors[J]. Nanoscale, 2017, 9(37): 14162-14171. |
| 37 | HOU Y H, LIU Y H, GAO R Q, et al. Ag@CoxP core-shell heterogeneous nanoparticles as efficient oxygen evolution reaction catalysts[J]. ACS Catalysis, 2017, 7(10): 7038-7042. |
| 38 | HA D H, MOREAU L M, BEALING C R, et al. The structural evolution and diffusion during the chemical transformation from cobalt to cobalt phosphide nanoparticles[J]. Journal of Materials Chemistry, 2011, 21(31): 11498-11510. |
| 39 | HUANG H B, LUO S H, LIU C L, et al. High-surface-area and porous Co2P nanosheets as cost-effective cathode catalysts for Li-O2 batteries[J]. ACS Applied Materials & Interfaces, 2018, 10(25): 21281-21290. |
| 40 | LIANG F, HUANG L, TIAN L, et al. Microwave-assisted hydrothermal synthesis of cobalt phosphide nanostructures for advanced supercapacitor electrodes[J]. Crystengcomm, 2018, 20(17): 2413-2420. |
| 41 | JIANG H, LI C, SHEN H B, et al. Supramolecular gel-assisted synthesis Co2P particles anchored in multielement co-doped graphene as efficient bifunctional electrocatalysts for oxygen reduction and evolution[J]. Electrochimica Acta, 2017, 231: 344-353. |
| 42 | LI G X, YU J Y, JIA J, et al. Cobalt-cobalt phosphide nanoparticles@nitrogen-phosphorus doped carbon/graphene derived from cobalt ions adsorbed saccharomycete yeasts as an efficient, stable, and large-current-density electrode for hydrogen evolution reactions[J]. Advanced Functional Materials, 2018, 28(40): 1801332. |
| 43 | LI T Q, JIN H R, LIANG Z, et al. Synthesis of single crystalline two-dimensional transition-metal phosphides via a salt-templating method[J]. Nanoscale, 2018, 10(15): 6844-6849. |
| 44 | GUO Z Y, LI C, LI W Y, et al. Ruthenium oxide coated ordered mesoporous carbon nanofiber arrays: a highly bifunctional oxygen electrocatalyst for rechargeable Zn-air batteries[J]. Journal of Materials Chemistry A, 2016, 4(17): 6282-6289. |
| 45 | DUAN J J, CHEN S, VASILEFF A, et al. Anion and cation modulation in metal compounds for bifunctional overall water splitting[J]. ACS Nano, 2016, 10(9): 8738-8745. |
| 46 | CHARLES C L M, SUHD J, JONAS C P, et al. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction[J]. Journal of the American Chemical Society, 2013, 45(135): 16977-16987. |
| 47 | PING L, RODRIGUEZ J A. Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni2P(001) surface: the importance of ensemble effect[J]. Journal of the American Chemical Society, 2005, 127(42): 14871-14878. |
| 48 | POPCZUN E J, MCKONE J R, READ C G, et al. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction[J]. Journal of the American Chemical Society, 2013, 135(25): 9267-9270. |
| 49 | HUANG Z P, CHEN Z Z, CHEN Z B, et al. Cobalt phosphide nanorods as an efficient electrocatalyst for the hydrogen evolution reaction[J]. Nano Energy, 2014, 9: 373-382. |
| 50 | JIN Z, LI P, XIAO D. Metallic Co2P ultrathin nanowires distinguished from CoP as robust electrocatalysts for overall water-splitting[J]. Green Chemistry, 2015, 18(6): 1459-1464. |
| 51 | MEN Y N, LI P, ZHOU J H, et al. Tailoring the electronic structure of Co2P by N doping for boosting hydrogen evolution reaction at all pH values[J]. ACS Catalysis, 2019, 9(4): 3744-3752. |
| 52 | YIN Z X, ZHU C L, LI C Y, et al. Hierarchical nickel-cobalt phosphide yolk-shell spheres as highly active and stable bifunctional electrocatalysts for overall water splitting[J]. Nanoscale, 2016, 8(45): 19129-19138. |
| 53 | LI D, BAYDOUN H, VERANI C N, et al. Efficient water oxidation using CoMnP nanoparticles[J]. Journal of the American Chemical Society, 2016, 138(12): 4006-4009. |
| 54 | GUO Y Y, YUAN P F, ZHANG J N, et al. Co2P-CoN double active centers confined in N-doped carbon nanotube: heterostructural engineering for trifunctional catalysis toward HER, ORR, OER, and Zn-air batteries driven water splitting[J]. Advanced Functional Materials, 2018, 28(51): 1805641. |
| 55 | DUTTA A, SAMANTARA A K, DUTTA S K, et al. Surface-oxidized dicobalt phosphide nanoneedles as a nonprecious, durable, and efficient OER catalyst[J]. ACS Energy Letters, 2016, 1(1): 169-174. |
| 56 | LI W, ZHANG S L, FAN Q L, et al. Hierarchically scaffolded CoP/CoP2 nanoparticles: controllable synthesis and their application as a well-matched bifunctional electrocatalyst for overall water splitting[J]. Nanoscale, 2017, 9(17): 5677-5685. |
| 57 | KIBSGAARD J, TSAI C, CHAN K, et al. Designing an improved transition metal phosphide catalyst for hydrogen evolution using experimental and theoretical trends[J]. Energy & Environmental Science, 2015, 8(10): 3022-3029. |
| 58 | CHEN G, MA T Y, LIU Z, et al. Efficient and stable bifunctional electrocatalysts Ni/NixMy (M= P, S) for overall water splitting[J]. Advanced Functional Materials, 2016, 26(19): 3314-3323. |
| 59 | JIAO L, ZHOU Y X, JIANG H L. Metal-organic framework-based CoP/reduced graphene oxide: high-performance bifunctional electrocatalyst for overall water splitting[J]. Chemical Science, 2016, 7(3): 1690-1695. |
| 60 | LI X Z, FANG Y Y, LI F, et al. Ultrafine Co2P nanoparticles encapsulated in nitrogen and phosphorus dual-doped porous carbon nanosheet/carbon nanotube hybrids: high-performance bifunctional electrocatalysts for overall water splitting[J]. Journal of Materials Chemistry A, 2016, 4(40): 15501-15510. |
| 61 | LI X, WANG X L, ZHOU J, et al. Ternary hybrids as efficient bifunctional electrocatalysts derived from bimetallic metal-organic-frameworks for overall water splitting[J]. Journal of Materials Chemistry A, 2018, 6(14): 5789-5796. |
| 62 | YANG Y Y, LIANG X Y, LI F, et al. Encapsulating Co2P@C core-shell nanoparticles in a porous carbon sandwich as dual-doped electrocatalyst for hydrogen evolution[J]. ChemSusChem, 2018, 11(2): 376-388. |
| 63 | LI L L,WANG Z, PENG S J, et al. Cobalt nanoparticles encapsulated in carbon nanotube-grafted nitrogen and sulfur co-doped multichannel carbon fibers as efficient bifunctional oxygen electrocatalysts[J]. Journal of Materials Chemistry A, 2017, 5(10): 4949-4961. |
| 64 | ZHANG C, PU Z, AMIINU I, et al. Co2P quantum dot embedded N, P dual-doped carbon self-supported electrodes with flexible and binder-free properties for efficient hydrogen evolution reaction[J]. Nanoscale, 2018, 10: 2902-2907. |
| 65 | WANG Y G, SONG Y F, XIA Y Y. Electrochemical capacitors: mechanism, materials, systems, characterization and applications[J]. Chemical Society Reviews, 2016, 45(21): 5925-5950. |
| 66 | KANG J L, HIRATA A, KANG L J, et al. Enhanced supercapacitor performance of MnO2 by atomic doping[J]. Angewandte Chemie International Edition, 2013, 52(6): 1664-1667. |
| 67 | WANG F X, XIAO S Y, HOU Y Y, et al. Electrode materials for aqueous asymmetric supercapacitors[J]. RSC Advances, 2013, 3(32): 13059-13084. |
| 68 | LONG J W, BÉLANGER D, BROUSSE T, et al. Asymmetric electrochemical capacitors—stretching the limits of aqueous electrolytes[J]. MRS Bulletin, 2011, 36: 513-522. |
| 69 | YANG P H, MAI W J. Flexible solid-state electrochemical supercapacitors[J]. Nano Energy, 2014, 8: 274-290. |
| 70 | ZHANG G Q, LI B, LIU M C, et al. Liquid phase synthesis of CoP nanoparticles with high electrical conductivity for advanced energy storage[J]. Journal of Nanomaterials, 2017, 2017: 9728591. |
| 71 | DING L, ZHANG K X, CHEN L, et al. Formation of three-dimensional hierarchical pompon-like cobalt phosphide hollow microspheres for asymmetric supercapacitor with improved energy density[J]. Electrochimica Acta, 2019, 299: 62-71. |
| 72 | ZHANG G Z, FANG J, SUN L J, et al. Cobalt phosphide nanowire arrays grown on carbon cloth as novel electrode material for supercapacitors[J]. Materials Science in Semiconductor Processing, 2017, 66: 140-143. |
| 73 | ZHENG Z, RETANA M, HU X B, et al. Three-dimensional cobalt phosphide nanowire arrays as negative electrode material for flexible solid-state asymmetric supercapacitors[J]. ACS Applied Materials & Interfaces, 2017, 9(20): 16986-16994. |
| 74 | ZHU G L, YANG L, WANG W Y, et al. Hierarchical three-dimensional manganese doped cobalt phosphide nanowire decorated nanosheet cluster arrays for high-performance electrochemical pseudocapacitor electrodes[J]. Chemical Communications, 2018, 54(66): 9234-9237. |
| 75 | ELSHAHAWY AM, GUAN C, LI X, et al. Sulfur-doped cobalt phosphide nanotube arrays for highly stable hybrid supercapacitor[J]. Nano Energy, 2017, 39: 162-171. |
| 76 | HU Y M, LIU M C, HU Y X, et al. One-pot hydrothermal synthesis of porous nickel cobalt phosphides with high conductivity for advanced energy conversion and storage[J]. Electrochimica Acta, 2016, 215: 114-125. |
| 77 | MAROM R, AMALRAJ SF, LEIFER N, et al. A review of advanced and practical lithium battery materials[J]. Journal of Materials Chemistry, 2011, 21(27): 9938-9954. |
| 78 | TANG Y X, ZHANG Y Y, LI W L, et al. Rational material design for ultrafast rechargeable lithium-ion batteries[J]. Chemical Society Reviews, 2015, 44(17): 5926-5940. |
| 79 | HOU H S, QIU X Q, WEI W F, et al. Carbon anode materials for advanced sodium-ion batteries[J]. Advanced Energy Materials, 2017, 7(24): 1602898. |
| 80 | LOU P L, CUI Z H, JIA Z Q, et al. Monodispersed carbon coated cubic NiP2 nanoparticles anchored on carbon nanotubes as ultra-long life anodes for reversible lithium storage[J]. ACS Nano, 2017, 11(4): 3705-3715. |
| 81 | FENG X Y, TANG M X, O’NEILL S, et al. In situ synthesis and in operando NNR studies of a high-performance Ni5P4-nanosheet anode[J]. Journal of Materials Chemistry A, 2018, 6(44): 22240-22247. |
| 82 | PRALONG V, SOUZA D C S, LEUNG K T, et al. Reversible lithium uptake by CoP3 at low potential: role of the anion[J]. Electrochemistry Communications, 2002, 4: 516-520. |
| 83 | GAO H, YANG F H, ZHENG Y, et al. Three-dimensional porous cobalt phosphide nanocubes encapsulated in a graphene aerogel as an advanced anode with high coulombic efficiency for high-energy lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(5): 5373-5379. |
| 84 | VILLEVIEILLE C, ROBERT F, TABERNA P L, et al. The good reactivity of lithium with nanostructured copper phosphide[J]. Journal of Materials Chemistry, 2008, 18(48): 5956-5960. |
| 85 | YANG D, ZHU J X, RUI X H, et al. Synthesis of cobalt phosphides and their application as anodes for lithium ion batteries[J]. ACS Applied Materials & Interfaces, 2013, 5(3): 1093-1099. |
| 86 | CABANA J, MONCONDUIT L, LARCHER D, et al. Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions[J]. Advanced Materials, 2010, 22(35): E170-E192. |
| 87 | LU A L, ZHANG X Q, CHEN Y Z, et al. Synthesis of Co2P/graphene nanocomposites and their enhanced properties as anode materials for lithium ion batteries[J]. Journal of Power Sources, 2015, 295: 329-335. |
| 88 | XI B J, MAO H Z, LIN Y, et al. One-step construction of N,P-codoped porous carbon sheets/CoP hybrids with enhanced lithium and potassium storage[J]. Advanced Materials, 2018, 30: 1802310. |
| 89 | WANG B, RU Q, GUO Q, et al. Fabrication of one-dimensional mesoporous CoP nanorods as anode materials for lithium-ion batteries[J]. European Journal of Inorganic Chemistry, 2017(31): 3729-3735. |
| 90 | ALCÁNTARA R, TIRADO J, JUMAS J, et al. Electrochemical reaction of lithium with CoP3[J]. Journal of Power Sources, 2002, 109: 308-312. |
| 91 | QIN J, HE C N, ZHAO N Q, et al. Graphene networks anchored with Sn@Graphene as lithium ion battery anode[J]. ACS Nano, 2014, 8(2): 1728-1738. |
| 92 | LI W J, YANG Q R, CHOU S L, et al. Cobalt phosphide as a new anode material for sodium storage[J]. Journal of Power Sources, 2015, 294: 627-632. |
| 93 | DU M M, QIU B C, ZHU Q H, et al. Cobalt phosphide nanocages encapsulated with graphene as ultralong cycle life anodes for reversible lithium storage[J]. Research on Chemical Intermediates, 2018, 44(12): 7847-7859. |
| 94 | WANG B B, CHEN K, WANG G, et al. A multidimensional and hierarchical carbon-confined cobalt phosphide nanocomposite as an advanced anode for lithium and sodium storage[J]. Nanoscale, 2019, 11(3): 968-985. |
| 95 | ZHANG C, JIAO G H, KONG F J, et al. Hierarchical Co2P microspheres assembled from nanorods grown on reduced graphene oxide as anode material for lithium-ion batteries[J]. Applied Surface Science, 2018, 459: 665-671. |
| 96 | YANG J, ZHANG Y, et al. Graphene and cobalt phosphide nanowire composite as an anode material for high performance lithium-ion batteries[J]. Nano Research, 2016, 9(3): 612-621. |
| 97 | YANG Y, JIANG Y F, FU W B, et al. Cobalt phosphide embedded in graphene nanosheet network as a high-performance anode for Li-ion batteries[J]. Dalton Transactions, 2019, 48(22): 7778-7785. |
| 98 | ZHU P P, ZHANG Z, ZHAO P F, et al. Rational design of intertwined carbon nanotubes threaded porous CoP@carbon nanocubes as anode with superior lithium storage[J]. Carbon, 2019, 142: 269-277. |
| 99 | ZHU K J, LIU J, LI S T, et al. Ultrafine cobalt phosphide nanoparticles embedded in nitrogen-doped carbon matrix as a superior anode material for lithium ion batteries[J]. Advanced Materials Interfaces, 2017, 4(19): 1700377. |
| 100 | XIA G L, SU J W, LI M S, et al. A MOF-derived self-template strategy toward cobalt phosphide electrodes with ultralong cycle life and high capacity[J]. Journal of Materials Chemistry A, 2017, 5(21): 10321-10327. |
| 101 | WALTER M, BODNARCHUK M, KRAVCHYK K, et al. Evaluation of metal phosphide nanocrystals as anode materials for Na-ion batteries[J]. Chimia International Journal for Chemistry, 2015, 69: 724-728. |
| 102 | LUO W, SHEN F, BOMMIER C, et al. Na-ion battery anodes: materials and electrochemistry[J]. Accounts of Chemical Research, 2016, 49(2): 231-240. |
| 103 | CHEN C J, WEN Y W, HU X L, et al. Na+ intercalation pseudocapacitance in graphene-coupled titanium oxide enabling ultra-fast sodium storage and long-term cycling[J]. Nature Communications, 2015, 6(1): 6929. |
| 104 | ZHANG K, PARK M, ZHANG J, et al. Cobalt phosphide nanoparticles embedded in nitrogen-doped carbon nanosheets: promising anode material with high rate capability and long cycle life for sodium-ion batteries[J]. Nano Research, 2017, 10(12): 4337-4350. |
| 105 | LI Z Q, ZHANG L Y, GE X L, et al. Core-shell structured CoP/FeP porous microcubes interconnected by reduced graphene oxide as high performance anodes for sodium ion batteries[J]. Nano Energy, 2017, 32: 494-502. |
| 106 | XING Y M, ZHANG X H, LIU D H, et al. Porous amorphous Co2P/ N,B-co-doped carbon composite as an improved anode material for sodium-ion batteries[J]. Chemelectrochem, 2017, 4(6): 1395-1401. |
| 107 | WANG X, KIM H M, XIAO Y, et al. Nanostructured metal phosphide-based materials for electrochemical energy storage[J]. Journal of Materials Chemistry A, 2016, 4(39): 14915-14931. |
| 108 | LIU N, LU Z D, ZHAO J, et al. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes[J]. Nature Nanotechnology, 2014, 9(3): 187-192. |
| 109 | ZHANG X X, QU H N, JI W X, et al. Fast and controllable prelithiation of hard carbon anodes for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(10): 11589-11599. |
| 110 | FORNEY M W, GANTER M J, STAUB J W, et al. Prelithiation of silicon-carbon nanotube anodes for lithium ion batteries by stabilized lithium metal powder (SLMP)[J]. Nano Letters, 2013, 13(9): 4158-4163. |
| [1] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [4] | 雷伟, 姜维佳, 王玉高, 和明豪, 申峻. N、S共掺杂煤基碳量子点的电化学氧化法制备及用于Fe3+检测[J]. 化工进展, 2023, 42(9): 4799-4807. |
| [5] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [6] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [7] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [8] | 王帅晴, 杨思文, 李娜, 孙占英, 安浩然. 元素掺杂生物质炭材料在电化学储能中的研究进展[J]. 化工进展, 2023, 42(8): 4296-4306. |
| [9] | 张耀杰, 张传祥, 孙悦, 曾会会, 贾建波, 蒋振东. 煤基石墨烯量子点在超级电容器中的应用[J]. 化工进展, 2023, 42(8): 4340-4350. |
| [10] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [11] | 冯江涵, 宋钫. 阴离子交换膜电解池的研究进展[J]. 化工进展, 2023, 42(7): 3501-3509. |
| [12] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| [13] | 朱薇, 齐鹏刚, 苏银海, 张书平, 熊源泉. 生物油分级多孔碳超级电容器电极材料的制备及性能[J]. 化工进展, 2023, 42(6): 3077-3086. |
| [14] | 张鹏, 潘原. 单原子催化剂在电催化氧还原直接合成过氧化氢中的研究进展[J]. 化工进展, 2023, 42(6): 2944-2953. |
| [15] | 陈少华, 王义华, 胡强飞, 胡坤, 陈立爱, 李洁. 电化学修饰电极在检测Cr(Ⅵ)中的研究进展[J]. 化工进展, 2023, 42(5): 2429-2438. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||