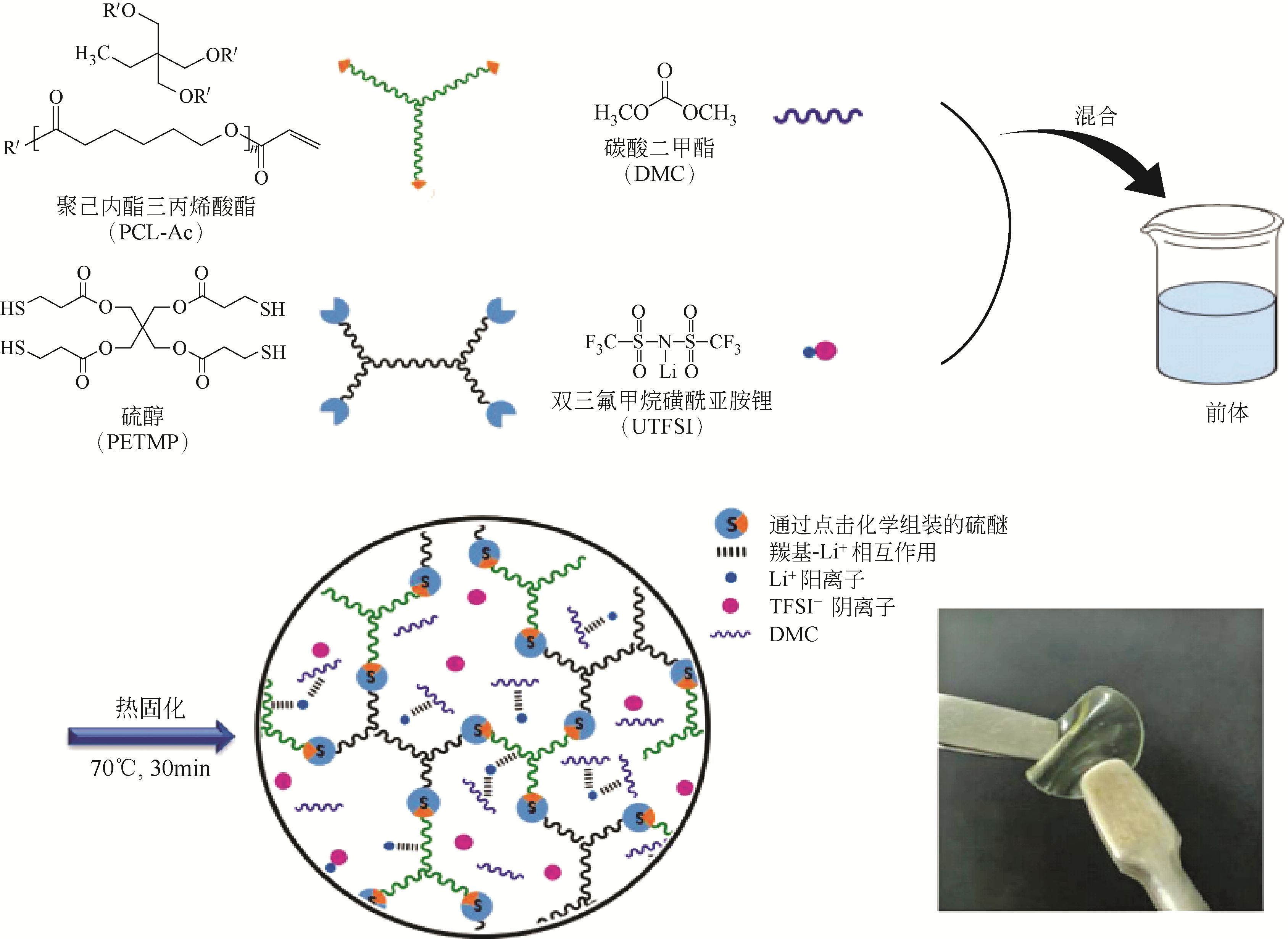
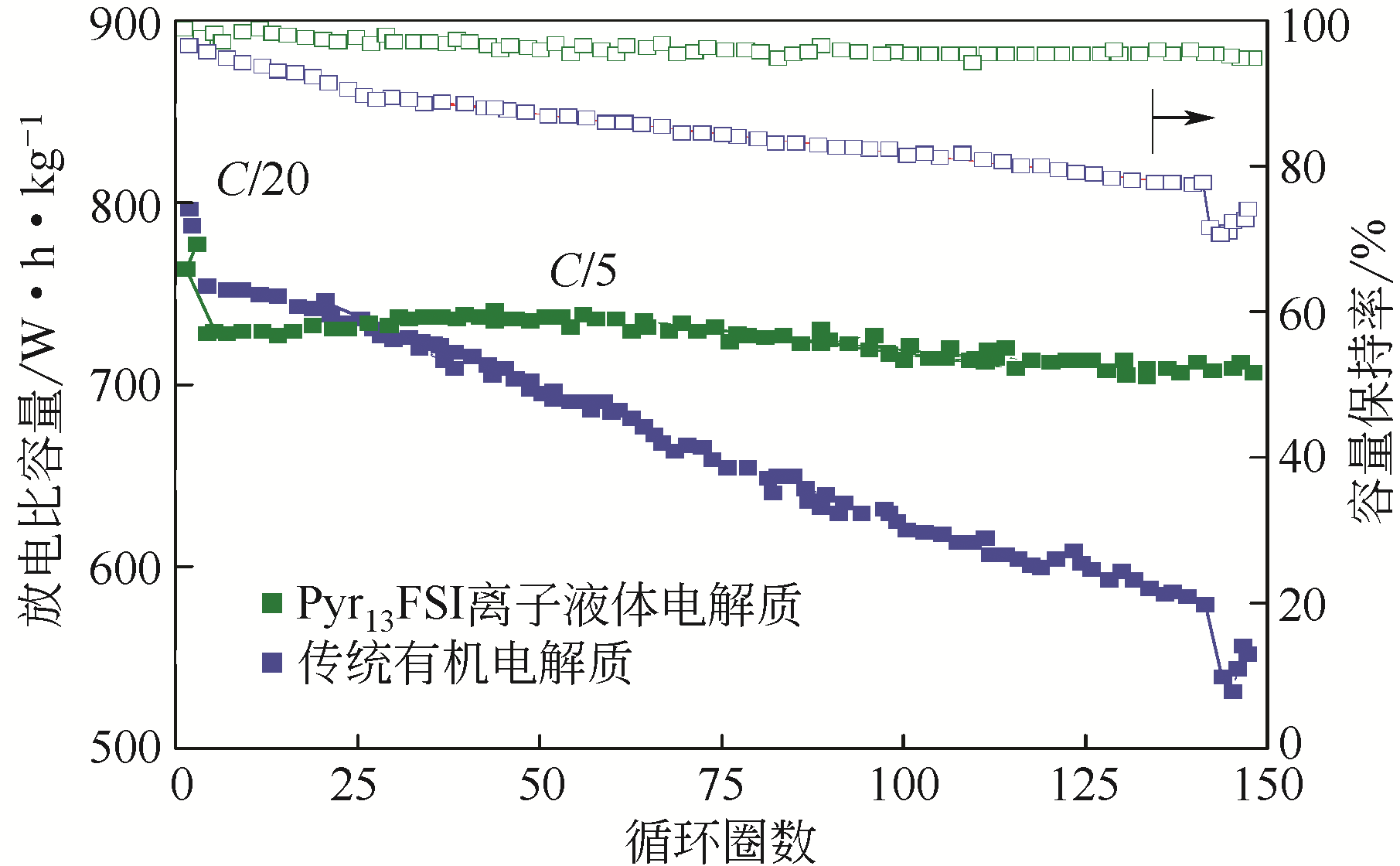
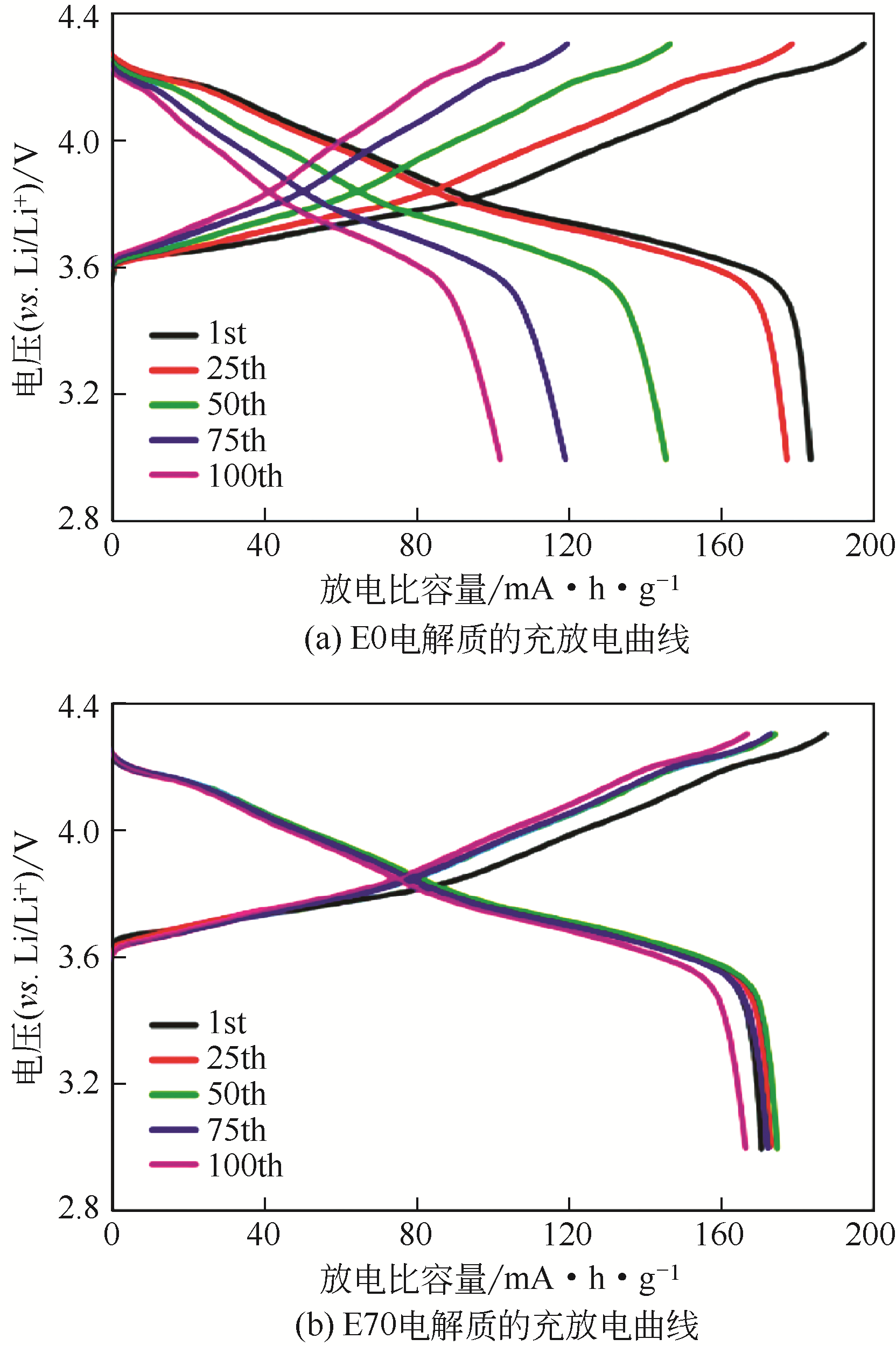
化工进展 ›› 2021, Vol. 40 ›› Issue (4): 2175-2187.DOI: 10.16085/j.issn.1000-6613.2020-0973
高镍锂离子电池三元材料NCM电解质的应用
张文林( ), 刘雪娇, 马青查, 杨双丞, 张永康, 李春利
), 刘雪娇, 马青查, 杨双丞, 张永康, 李春利
- 河北工业大学化工学院,化工节能过程集成与资源利用国家地方联合工程实验室,天津 300310
-
收稿日期:2020-06-01出版日期:2021-04-05发布日期:2021-04-14 -
通讯作者:张文林 -
作者简介:张文林(1968—),男,博士,教授,主要从事分离与纯化技术以及绿色化工方面的研究。E-mail:ctstzwl@163.com 。 -
基金资助:河北省高等学校科学技术研究项目(ZD2015118)
Application of NCM electrolyte for nickel-rich lithium ion battery
ZHANG Wenlin( ), LIU Xuejiao, MA Qingcha, YANG Shuangcheng, ZHANG Yongkang, LI Chunli
), LIU Xuejiao, MA Qingcha, YANG Shuangcheng, ZHANG Yongkang, LI Chunli
- National-Local Joint Engineering Laboratory for Energy Conservation of Chemical Process Integration and Resources Utilization, School of Chemical Engineering, Hebei University of Technology, Tianjin 300130, China
-
Received:2020-06-01Online:2021-04-05Published:2021-04-14 -
Contact:ZHANG Wenlin
摘要:
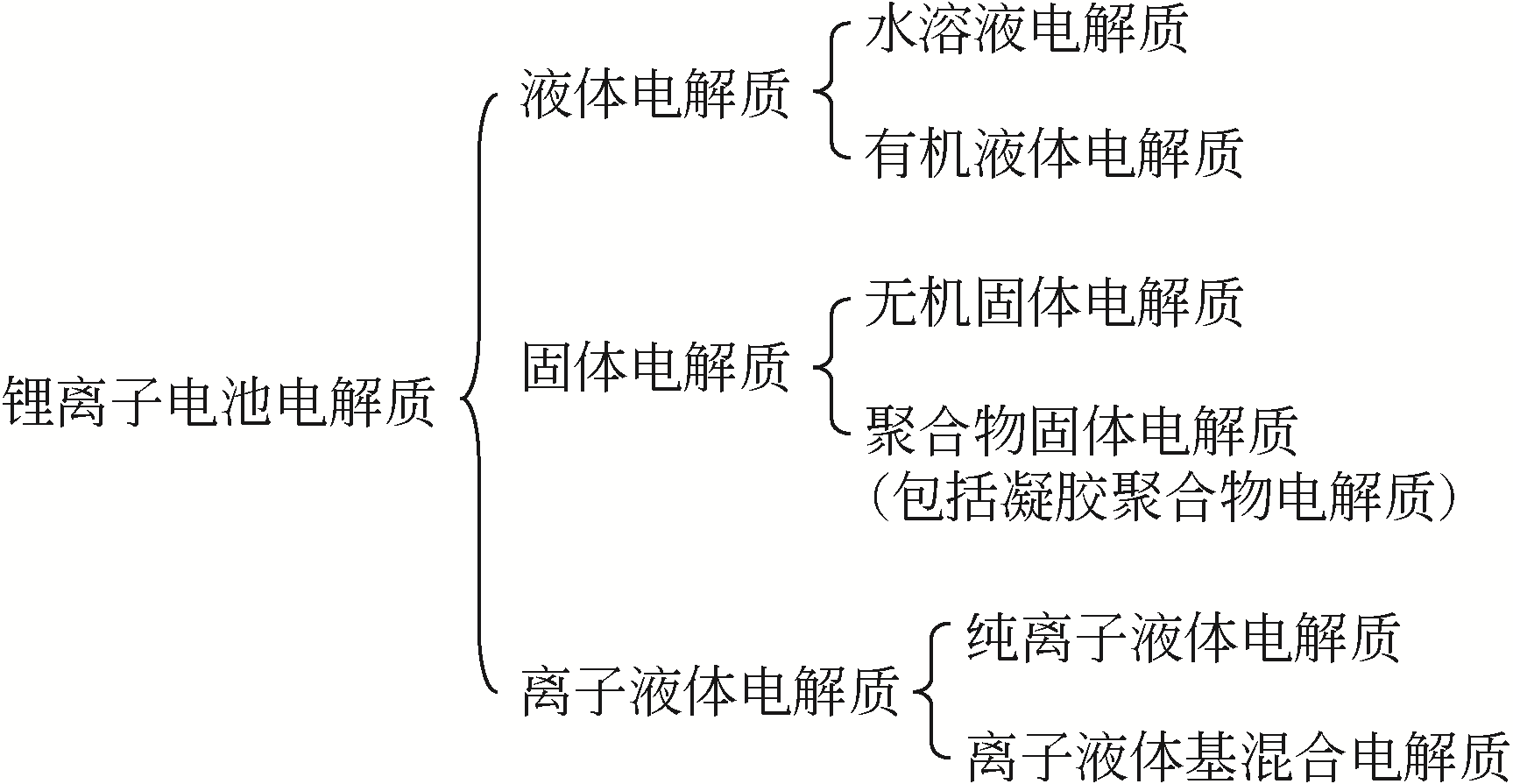
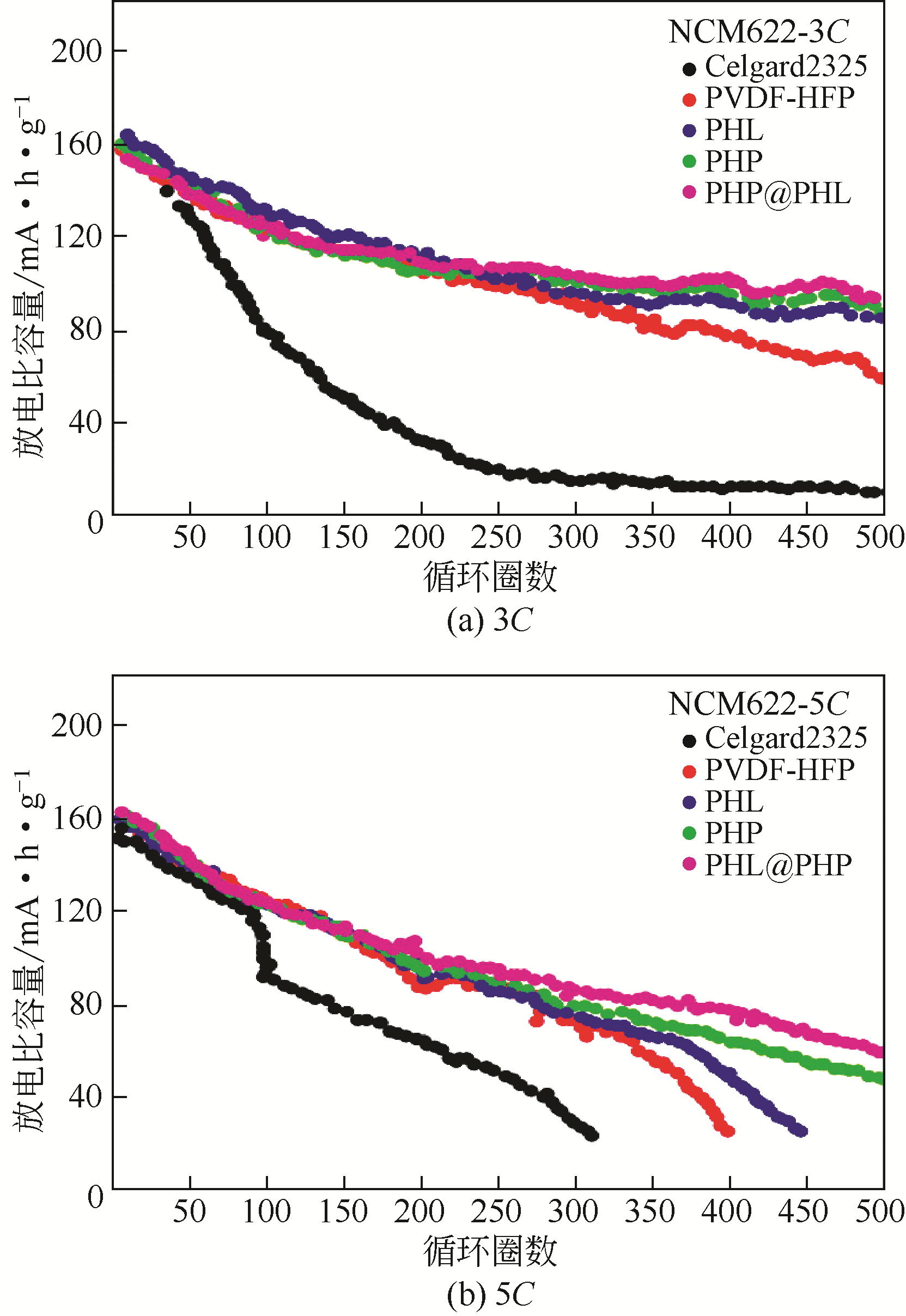
高镍三元正极材料成本低、比容量高,符合锂离子电池可持续发展的理念,被认为是下一代的主流正极材料。但是,高镍材料需搭配合适的电解质才能有效发挥其性能,而这一研究很少被关注。因此,总结并选择适配的电解质对于高镍锂离子电池来说格外重要。本文简述了锂离子电池电解质的一般组成及其产生的电解质类型,重点综述了有机液体电解质、固体电解质及离子液体基电解质在高镍三元材料电池中的应用,并通过电解质的量化计算进行了验证总结。分析表明,离子液体-有机溶剂混合电解质在高镍三元材料(NCM)电池中具备更好的循环效果,同时满足了电池安全稳定的要求,更适合作为高镍材料电池的电解质。最后,针对混合电解质各溶剂间的相互作用机理及Li+传输等分子动力学研究进行了展望。
中图分类号:
引用本文
张文林, 刘雪娇, 马青查, 杨双丞, 张永康, 李春利. 高镍锂离子电池三元材料NCM电解质的应用[J]. 化工进展, 2021, 40(4): 2175-2187.
ZHANG Wenlin, LIU Xuejiao, MA Qingcha, YANG Shuangcheng, ZHANG Yongkang, LI Chunli. Application of NCM electrolyte for nickel-rich lithium ion battery[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2175-2187.
| 组成 | 作用(功能) | 要求 | 常见类型 |
|---|---|---|---|
| 锂盐 | 为电解质提供Li+,影响电解质的物理化学性能 | 具备低解离能和较高的溶解度 具备较好的稳定性 具备良好的SEI成膜性能 价格低廉,无毒无公害 | LiBF4、LiPF6、LiTFSI |
| 溶剂 | 提供电解质环境,溶解锂盐,促使电解质具备较高电导率 | 化学性能稳定 黏度低,介电常数高 熔点低 安全性好,毒性低 | H2O、EC、DMC、DEC、EMC、咪唑类、 哌啶类、吡咯类、PEO、PVDF、PVC |
| 添加剂 | 针对性地改善电解质的电化学性能,提高电解质的利用率 | 较少用量即能改善电解质性能 不对LIBs性能造成负面影响 价格较低 无毒或毒性较小 | VC、PS、SN、ILs添加剂 |
表1 电解质的一般组成及其特征
| 组成 | 作用(功能) | 要求 | 常见类型 |
|---|---|---|---|
| 锂盐 | 为电解质提供Li+,影响电解质的物理化学性能 | 具备低解离能和较高的溶解度 具备较好的稳定性 具备良好的SEI成膜性能 价格低廉,无毒无公害 | LiBF4、LiPF6、LiTFSI |
| 溶剂 | 提供电解质环境,溶解锂盐,促使电解质具备较高电导率 | 化学性能稳定 黏度低,介电常数高 熔点低 安全性好,毒性低 | H2O、EC、DMC、DEC、EMC、咪唑类、 哌啶类、吡咯类、PEO、PVDF、PVC |
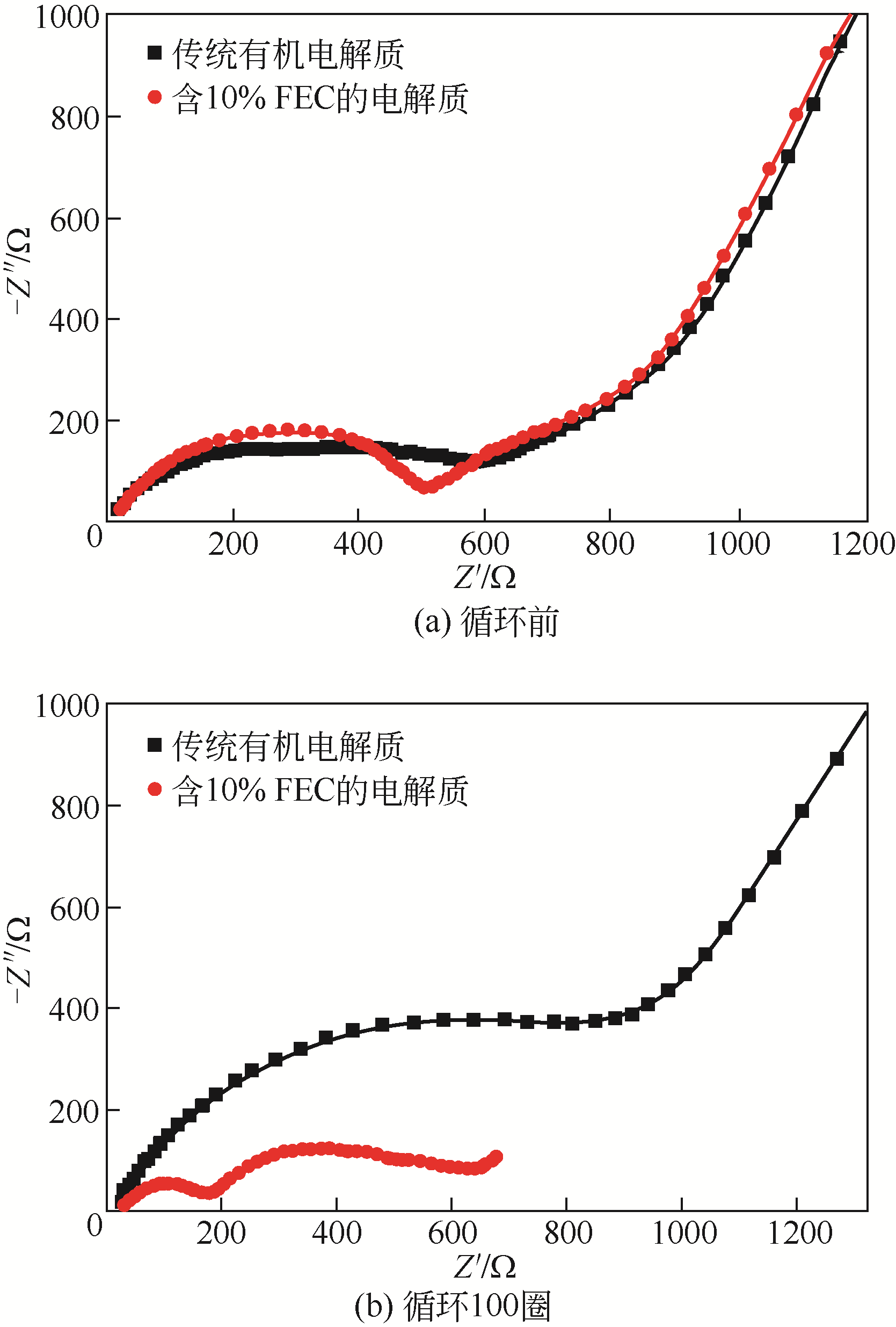
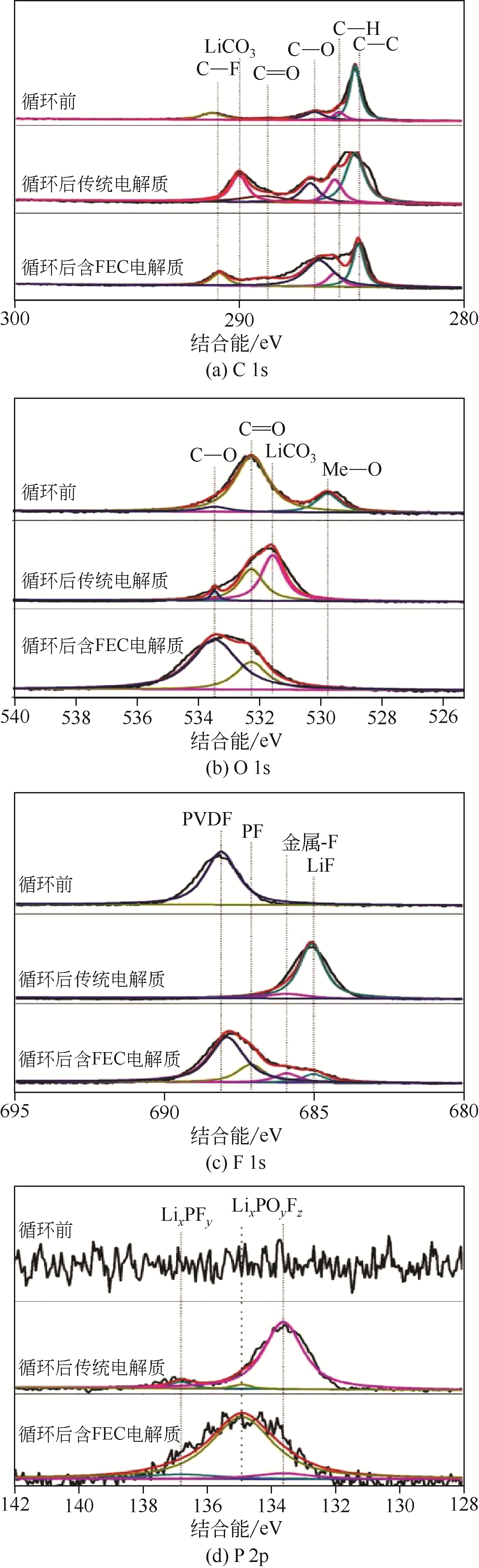
| 添加剂 | 针对性地改善电解质的电化学性能,提高电解质的利用率 | 较少用量即能改善电解质性能 不对LIBs性能造成负面影响 价格较低 无毒或毒性较小 | VC、PS、SN、ILs添加剂 |
| 类别 | 优势 | 弊端 |
|---|---|---|
| 有机液体电解质(含添加剂) | 成本低,流动性好,电导率高,电极成膜性好,在NCM电池中具有较高的放电比容量 | 易燃,安全性低 |
| 固体电解质 | 具有较高的机械稳定性,安全不易燃,能有效稳定NCM电极结构 | 离子电导率低,电化学窗口较低,不利于高压NCM材料电池循环 |
| 纯离子液体电解质 | 电化学稳定性和热稳定性好,不易燃,电化学窗口宽,离子电导率较高 | 黏度大,与NCM电极润湿性差,循环能力弱,ILs成本高 |
| 离子液体-有机溶剂混合电解质 | 安全稳定性较高,黏度较低,与NCM电极相容性好,循环性高 | ILs成本高 |
表2 高镍NCM电池电解质类型及特点
| 类别 | 优势 | 弊端 |
|---|---|---|
| 有机液体电解质(含添加剂) | 成本低,流动性好,电导率高,电极成膜性好,在NCM电池中具有较高的放电比容量 | 易燃,安全性低 |
| 固体电解质 | 具有较高的机械稳定性,安全不易燃,能有效稳定NCM电极结构 | 离子电导率低,电化学窗口较低,不利于高压NCM材料电池循环 |
| 纯离子液体电解质 | 电化学稳定性和热稳定性好,不易燃,电化学窗口宽,离子电导率较高 | 黏度大,与NCM电极润湿性差,循环能力弱,ILs成本高 |
| 离子液体-有机溶剂混合电解质 | 安全稳定性较高,黏度较低,与NCM电极相容性好,循环性高 | ILs成本高 |
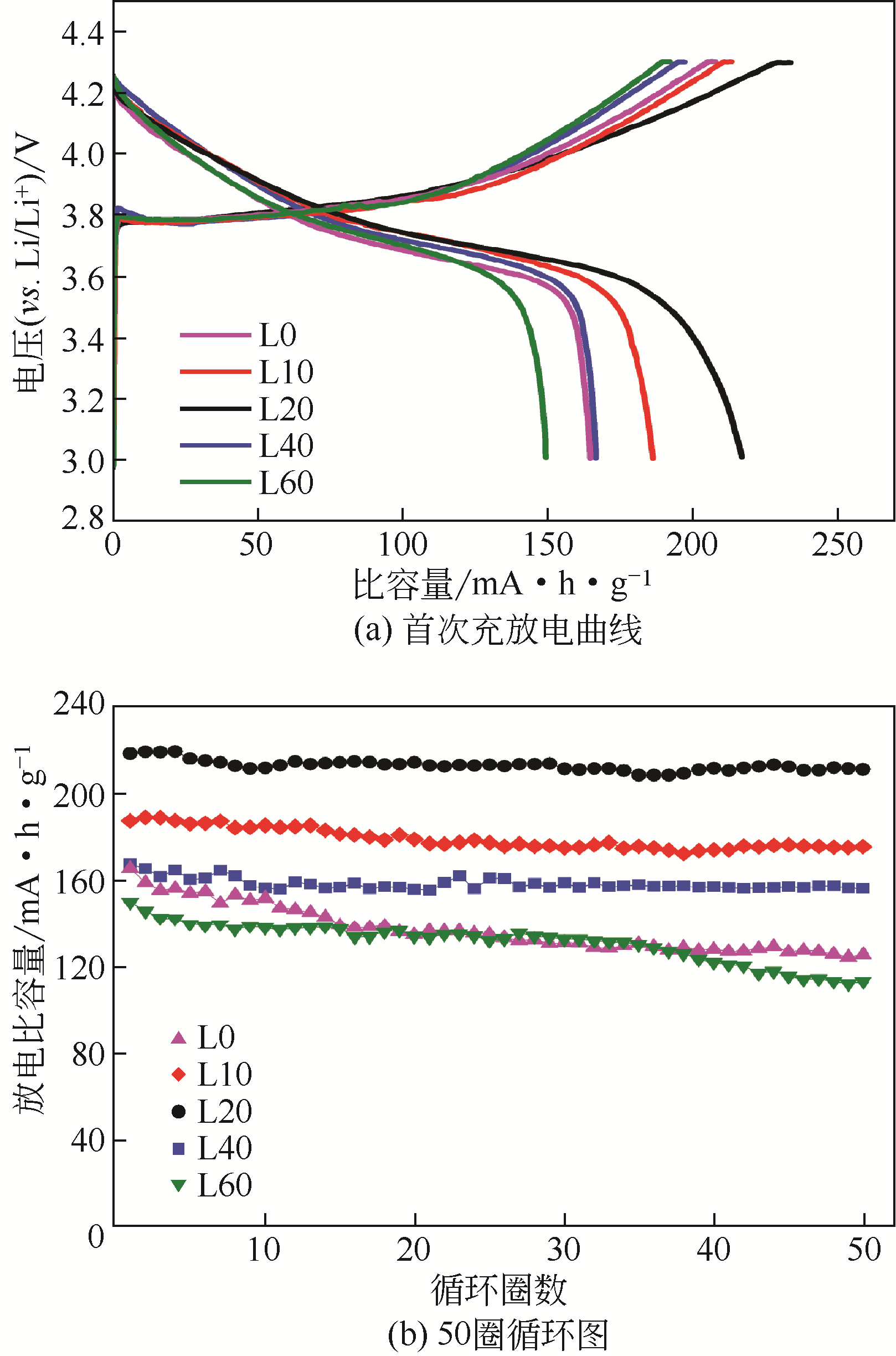
| 类别 | 电导率 /mS·cm-1 | 电化学窗口(vs.Li/Li+) /V | 首次充放电循环(0.1C) /mA·h·g-1 | 界面阻抗(100圈循环后) /Ω |
|---|---|---|---|---|
| 有机液体电解质(含添加剂) | 9.3 | 5.0 | 186.8 | 58.3 |
| 固体电解质 | 0.6 | 3.2 | 144.3 | 37.6 |
| 纯离子液体电解质 | 1.4 | 7.0 | 146.6 | 38.2 |
| 离子液体-有机溶剂混合电解质 | 9.8 | 5.6 | 190.6 | 32.9 |
表3 室温(25℃)下几种电解质在高镍三元锂电池中的电化学性能
| 类别 | 电导率 /mS·cm-1 | 电化学窗口(vs.Li/Li+) /V | 首次充放电循环(0.1C) /mA·h·g-1 | 界面阻抗(100圈循环后) /Ω |
|---|---|---|---|---|
| 有机液体电解质(含添加剂) | 9.3 | 5.0 | 186.8 | 58.3 |
| 固体电解质 | 0.6 | 3.2 | 144.3 | 37.6 |
| 纯离子液体电解质 | 1.4 | 7.0 | 146.6 | 38.2 |
| 离子液体-有机溶剂混合电解质 | 9.8 | 5.6 | 190.6 | 32.9 |
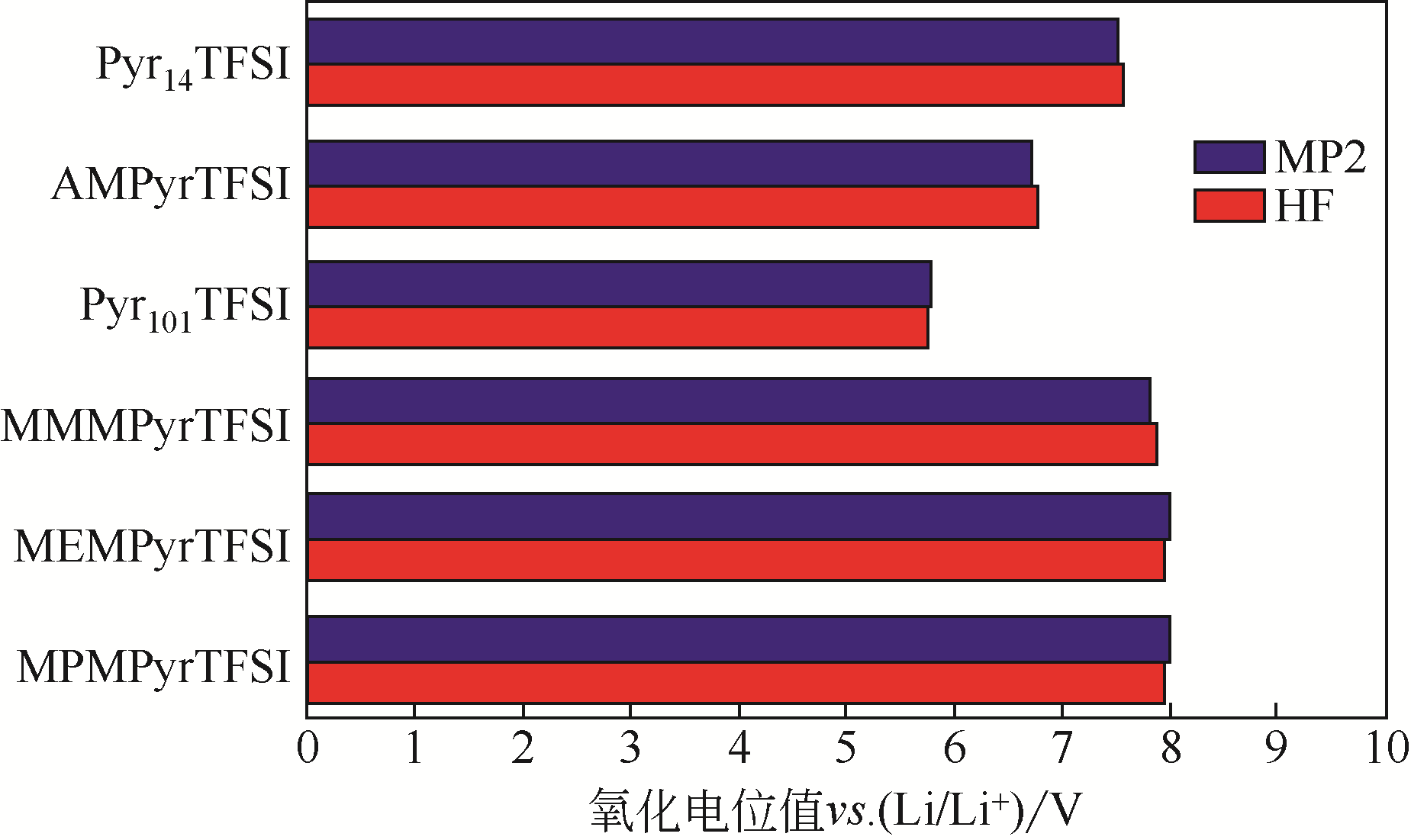
| 分子名称 | 分子结构 | HOMO/eV | LOMO/eV |
|---|---|---|---|
| EC |  | -8.468 | -0.604 |
| DEC |  | -8.055 | -0.268 |
| DMC |  | -8.179 | -0.370 |
| EMC |  | -8.133 | -0.256 |
表4 传统有机碳酸酯类溶剂的HOMO与LOMO值
| 分子名称 | 分子结构 | HOMO/eV | LOMO/eV |
|---|---|---|---|
| EC |  | -8.468 | -0.604 |
| DEC |  | -8.055 | -0.268 |
| DMC |  | -8.179 | -0.370 |
| EMC |  | -8.133 | -0.256 |
| 1 | WU Z W, CAO C H, YAN X D, et al. Effects of charge cut-off voltage on the performances of monocrystalline LiNi0.5Co0.2Mn0.3O2/graphite Li-ion cells[J]. Electrochimica Acta, 2019, 302: 153-160. |
| 2 | 肖忠良, 周乘风, 宋刘斌, 等. 富镍锂离子电池三元材料NCM的研究进展[J].化工进展, 2020, 39(1): 216-223. |
| XIAO Z L, ZHOU C F, SONG L B, et al. Research progress of ternary material NCM for nickel-rich lithium ion battery[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 216-223. | |
| 3 | ZHANG J P, XUE L L, LI Y J, et al. Suppressing nickel dissolution in Ni-rich layered oxide cathodes using NiF2 as electrolyte additive[J]. ChemElectroChem, 2019, 6: 3125-3131. |
| 4 | SON H B, JEONG M, HAN J, et al. Effect of reductive cyclic carbonate additives and linear carbonate cosolvents on fast chargeability of LiNi0.6Co0.2Mn0.2O2/graphite cells[J]. Journal of Power Sources, 2018, 400: 147-156. |
| 5 | 周丹, 梁风, 姚耀春, 等. 锂离子电池电解液负极成膜添加剂的研究进展[J]. 化工进展, 2016, 35(5): 1477-1483. |
| ZHOU D, LIANG F, YAO Y C, et al. Research progress of lithium ion battery electrolyte anode film-forming additives[J].Chemical Industry and Engineering Progress, 2016, 35(5): 1477-1483. | |
| 6 | ELIA G A, ULISSI U, MUELLER F, et al. A long-life lithium ion battery with enhanced electrode/electrolyte interface by using an ionic liquid solution[J]. Chemistry: a European Journal, 2016, 22: 6808-6814. |
| 7 | WANG W L, YANG T X, LI S, et al. 1-Ethyl-3-methylimidazolium tetrafluoroborate (EMI-BF4) as an ionic liquid-type electrolyte additive to enhance the low-temperature performance of LiNi0.5Co0.2Mn0.3O2/graphite batteries[J]. Electrochimica Acta, 2019, 317: 146-154. |
| 8 | LIU S, WU H, HUANG L, et al. Synthesis of Li2Si2O5-coated LiNi0.6Co0.2Mn0.2O2 cathode materials with enhanced high-voltage electrochemical properties for lithium-ion batteries[J]. Journal of Alloys and Compounds, 2016, 674: 447-454. |
| 9 | ZHANG H, ZHAO H, KHAN M A, et al. Recent progresses in advanced electrode materials, separators and electrolytes for lithium-ion batteries[J]. Journal of Materials Chemistry, 2018, 6: 20564-20620. |
| 10 | LIU W, LI X, XIONG D, et al. Significantly improving cycling performance of cathodes in lithium ion batteries: the effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2[J]. Nano Energy, 2018, 44: 111-120. |
| 11 | ZHANG J Y, YAO X H, MISRA R K, et al. Progress in electrolytes for beyond-lithium-ion batteries[J]. Journal of Materials Science & Technology, 2020, 44: 237-257. |
| 12 | YANG G, SONG Y D, WANG Q, et al. Review of ionic liquids containing, polymer/inorganic hybrid electrolytes for lithium metal batteries[J]. Materials and Design, 2020, 190: 108563. |
| 13 | DONG L, LIANG F X, WANG D, et al. Safe ionic liquid-sulfolane/LiDFOB electrolytes for high voltage Li1.15(Ni0.36Mn0.64)0.85O2 lithium ion battery at elevated temperatures[J]. Electrochimica Acta, 2018, 270: 426-433. |
| 14 | ZHU Y R, YI T F, et al. Recent progress in the electrolytes for improving the cycling stability of LiNi0.5Mn1.5O4 high-voltage cathode[J]. Ionics, 2016, 22: 1759-1774. |
| 15 | KANG Y S, PARK M S, PARK I, et al. Tetrathiafulvalene as a conductive film-making additive on high-voltage cathode[J]. ACS Applied Materials & Interfaces, 2017, 9: 3590-3595. |
| 16 | LEI Q F, YANG T X, ZHAO X Y, et al. Lithium difluorophosphate as a multi-functional electrolyte additive for 4.4V LiNi0.5Co0.2Mn0.3O2/graphite lithium ion batteries[J]. Journal of Electroanalytical Chemistry, 2019, 846: 113141. |
| 17 | 蒲文静, 芦伟, 谢凯, 等. 宽温型锂离子电池有机电解液的研究进展[J]. 材料导报, 2020, 34(4): 07036-07044. |
| PU W J, LU W, XIE K, et al. Progress on the carbonate-based electrolyte designed for lithium-ion batteries with wide operating temperature range[J]. Materials Reports, 2020, 34(4): 07036-07044. | |
| 18 | JANOSCHKA T, MARTIN U, MARTIN C, et al. An aqueous, polymer-based redox-flow battery using non-corrosive, safe, and low-cost materials[J]. Nature, 2015, 527: 78-81. |
| 19 | HOU Z G, ZHANG X Q, LI X N, et al. Surfactant widens the electrochemical window of an aqueous electrolyte for better rechargeable aqueous sodium/zinc battery[J]. Journal of Materials Chemistry A, 2017, 5: 730-738. |
| 20 | LEE J, JACKEL N, KIM D, et al. Porous carbon as a quasi-reference electrode in aqueous electrolytes[J]. Electrochimica Acta, 2016, 222: 1800-1805. |
| 21 | YANG Q W, ZHANG Z Q, SUN X G, et al. Ionic liquids and derived materials for lithium and sodium batteries[J]. Chemical Society Reviews, 2018, 47: 2020-2064. |
| 22 | LIANG F X, YU J L, WANG D, et al. Exceptional cycling performance of a graphite/Li1.1Ni0.25Mn0.65O2 battery at high voltage with ionic liquid-based electrolyte[J]. Electrochimica Acta, 2019, 307: 83-91. |
| 23 | PONROUCH A, MONTI D, BOSCHIN A, et al. Non-aqueous electrolytes for sodium-ion batteries[J]. Journal of Materials Chemistry A, 2015, 3: 22-42. |
| 24 | ESHETU G G, MARTINZE-IBANEZ M, SANCHEZ-DIEZ E, et al. Electrolyte additives for room temperature sodium-based rechargeable batteries[J]. Chemistry: an Asian Journal, 2018, 13: 2770-2780. |
| 25 | CAI Y J, XU T H, SOLMS N V, et al. Multifunctional imidazolium-based ionic liquid as additive for silicon/carbon lithium ion batteries[J]. Electrochimica Acta, 2020, 340: 135990. |
| 26 | YAN G C, LI X H, WANG Z X, et al. Fluorinated solvents for high-voltage electrolyte in lithium-ion battery[J]. Journal of Solid State Electrochemistry, 2017, 21: 1589-1597. |
| 27 | SU C, HE M, REDFERN P C, et al. Oxidatively stable fluorinated sulfone electrolytes for high voltage high energy lithium-ion batteries[J]. Energy & Environmental Science, 2017, 10: 900-904. |
| 28 | HENG S, WANG Y, QIU Q T, et al. Fluoro-ether as a bifunctional interphase electrolyte additive with graphite/LiNi0.5Co0.2Mn0.3O2 full cell[J]. ACS Applied Energy Materials, 2019, 2: 6404-6416. |
| 29 | ZHANG Q Q, LIU K, DING F, et al. Enhancing the high voltage interface compatibility of LiNi0.5Co0.2Mn0.3O2 in the succinonitrile-based electrolyte[J]. Electrochimica Acta, 2019, 298: 818-826. |
| 30 | LIN Y C, YUE X P, ZHANG H, et al. Using phenyl methanesulfonate as an electrolyte additive to improve performance of LiNi0.5Co0.2Mn0.3O2/graphite cells at low temperature[J]. Electrochimica Acta, 2019, 300: 202-207. |
| 31 | HAN S Y, ZHANG H, FAN C J, et al. 1,4-Dicyanobutane as a film-forming additive for high-voltage in lithium-ion batteries[J]. Solid State Ionics, 2019, 337: 63-69. |
| 32 | QIN Z M, HONG S, HONG B, et al. Triisopropyl borate as an electrolyte additive for improving the high voltage stability of LiNi0.6Co0.2Mn0.2O2 cathode[J]. Journal of Electroanalytical Chemistry, 2019, 854: 113506-113511. |
| 33 | LIU L L, WANG S L, ZHANG Z Y, et al. Fluoroethylene carbonate as an electrolyte additive for improving interfacial stability of high-voltage LiNi0.6Co0.2Mn0.2O2 cathode[J]. Ionics, 2019, 3: 1035-1043. |
| 34 | WANG L N, LIU S Q, ZHAO K M, et al. Improving the rate performance and stability of LiNi0.6Co0.2Mn0.2O2 in high voltage lithium-ion battery by using fluoroethylene carbonate as electrolyte additive[J]. Ionics, 2018, 24: 3337-3346. |
| 35 | PRAKASHA K R, MADASAMY K, KATHIRESAN M, et al. Ethylviologen hexafluorophosphate as electrolyte additive for high-voltage nickel-rich layered cathode[J]. The Journal of Physical Chemistry C, 2019, 123: 28604-28610. |
| 36 | LAN G Y, ZHOU H B, XING L D, et al. Insight into the interaction between Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode and BF4--introducing electrolyte at 4.5V high voltage[J]. Journal of Energy Chemistry, 2019, 39: 235-243. |
| 37 | 邓邦为, 孙大明, 万琦, 等. 锂离子电池三元正极材料电解液添加剂的研究进展[J]. 化学学报, 2018, 76(4): 259-277. |
| DENG B W, SUN D M, WAN Q, et al. Review of electrolyte additives for ternary cathode lithium-ion battery[J]. Acta Chimica Sinica, 2018, 76(4): 259-277. | |
| 38 | ZHANG D Z, REN Y Y, HU Y, et al. Ionic liquid/poly(ionic liquid)-based semi-solid state electrolytes for lithium-ion batteries[J]. Chinese Journal of Polymer Science, 2020, 38(5): 506-513. |
| 39 | ZENG Z Q, MURUGESAN V, HAN K S, et al. Non-flammable electrolytes with high salt-to-solvent ratios for Li-ion and Li-metal batteries[J]. Nature Energy, 2018, 3: 674-681. |
| 40 | ZENG G F, XIONG S L, QIAN Y T, et al. Non-flammable phosphate electrolyte with high salt-to-solvent ratios for safe potassium-ion battery[J]. Journal of The Electrochemical Society, 2019, 166: A1217-A1222. |
| 41 | YUE L P, MA J, ZHANG J J, et al. All solid-state polymer electrolytes for high-performance lithium ion batteries[J]. Energy Storage Materials, 2016, 5: 139-164. |
| 42 | MA Q, QI X G, TONG B, et al. Novel Li[(CF3SO2)(n-C4F9SO2)N]-based polymer electrolytes for solid state lithium batteries with superior electrochemical performance[J]. ACS Applied Materials & Interfaces, 2016, 8: 29705-29712. |
| 43 | WU J H, ZUO X X, CHEN Q Y, et al. Functional composite polymer electrolytes with imidazole modified SiO2 nanoparticles for high-voltage cathode lithium ion batteries[J]. Electrochimica Acta, 2019, 320: 134567-134577. |
| 44 | LIU X X, REN Y F, ZHANG L, et al. Functional ionic liquid modified core-shell structured fibrous gel polymer electrolyte for safe and efficient fast charging lithium-ion batteries[J]. Frontiers in Chemistry, 2019, 7: 421-431. |
| 45 | PARK S, JEONG B, LIM D A, et al. Quasi-solid-state electrolyte synthesized using a thiol-ene click chemistry for rechargeable lithium metal batteries with enhanced safety[J]. ACS Applied Materials & Interfaces, 2020, 12: 19553-19562. |
| 46 | HAN L, WANG Z Q, KONG D F, et al. An ordered mesoporous silica framework based electrolyte with nanowetted interfaces for solid state lithium batteries[J]. Journal of Materials Chemistry A, 2018, 6: 21280-21286. |
| 47 | ZHAO C Z, ZHANG X Q, CHENG X B, et al. An anion-immobilized composite electrolyte for dendrite-free lithium metal anodes[J]. PNAS, 2017, 114(42): 11069-11074. |
| 48 | KARUPPASAMY K, THEERTHAGIRI J, VIKRAMAN D, et al. Ionic liquid-based electrolytes for energy storage devices: a brief review on their limits and applications[J]. Polymers, 2020, 12: 918-954. |
| 49 | HOFFKNECHT J P, DREWS M, HE X, et al. Investigation of the N-butyl-N-methyl pyrrolidinium trifluoromethanesulfonyl-N-cyanoamide (PYR14TFSAM) ionic liquid as electrolyte for Li-ion battery[J]. Electrochimica Acta, 2017, 250: 25-34. |
| 50 | HEIST A, HAFNER S, LEE S H, et al. High-energy nickel-rich layered cathode stabilized by ionic liquid electrolyte[J]. Journal of the Electrochemical Society, 2019, 166(6): A873-A879. |
| 51 | LI Q, CHEN J, FAN L, et al. Progress in electrolytes for rechargeable Li-based batteries and beyond[J]. Green Energy & Environment, 2016, 1: 18-42. |
| 52 | PATRA J, WANG C H, LEE T C, et al. Mixed ionic liquid/organic carbonate electrolytes for LiNi0.8Co0.15Al0.05O2 electrodes at various temperatures[J]. RSC Advances, 2015, 5: 106824-106831. |
| 53 | MA C C, WANG D, YANG Y, et al. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode with an ionic liquid-based electrolyte[J]. Journal of the Electrochemical Society, 2019, 166(14): A3441-A3447. |
| 54 | 李田田. 量化计算在锂电池电解液中的应用[J]. 电源技术, 2015, 39(1): 196-198. |
| LI T T. Application of quantum chemistry in decomposition mechanisms of electrolyte for lithium ion batteries[J]. Chinese Journal of Power Sources, 2015, 39(1): 196-198. | |
| 55 | SHAO N, SUN X G, DAI S, et al. Electrochemical windows of sulfone-based electrolytes for high-voltage Li-ion batteries[J]. The Journal of Physical Chemistry B, 2011, 115: 12120-12125. |
| 56 | FORD T A. The structural, vibrational and electronic properties of the trielbonded complexes of boron trifluoride with some cyclic ethers—An ab initio study[J]. Journal of Molecular Structure, 2020, 1210: 128020-128025. |
| 57 | RAKHI R, SURESH C H. A DFT study on 1,4-dihydro-1,4-azaborinine annulated linear polyacenes: absorption spectra, singlet triplet energy gap, aromaticity, and HOMO-LUMO energy modulation[J]. Journal of Computational Chemistry, 2017, 38(26): 2232-2240. |
| 58 | 贺晓东, 郑浩, 林敏, 等. 腈类高电压电解液的量子化学计算研究[J]. 电源技术, 2018, 42(8):1148-1160. |
| HE X D, ZHENG H, LIN M, et al. Quantum chemical calculation of nitrile-based high-voltage electrolytes[J]. Chinese Journal of Power Sources, 2018, 42(8):1148-1160. | |
| 59 | WU F, ZHOU H, BAI Y, et al. Toward 5V Li-ion batteries: quantum chemical calculation and electrochemical characterization of sulfonebased high-voltage electrolytes[J]. ACS Applied Materials & Interfaces, 2015, 7(27): 15098-15107. |
| 60 | 李南, 马国强, 车海英, 等. 密度泛函理论在高电压电解液设计中的应用[J]. 化工进展, 2019, 38(7): 3253-3264. |
| LI N, MA G Q, CHE H Y, et al. Application of density functional theory in the design of high potential electrolyte[J]. Chemical Industry and Engineering Progress, 2019, 38(7): 3253-3264. | |
| 61 | CAO X, ROSER S, REZAEIRAD B, et al. Ester modified pyrrolidinium based ionic liquids as electrolyte component candidates in rechargeable lithium batteries[J]. Journal of Inorganic and General Chemistry, 2015, 641(14): 2536-2542. |
| 62 | 张文林, 霍宇, 李功伟, 等. 离子液体作为高压锂离子电池电解液添加剂的研究[D]. 天津: 河北工业大学, 2019. |
| ZHANG W L, HUO Y, LI G W, et al. The study on ionic liquid as electrolyte additives for high voltage lithium-ion batteries[D]. Tianjin: Hebei University of Technology, 2019. | |
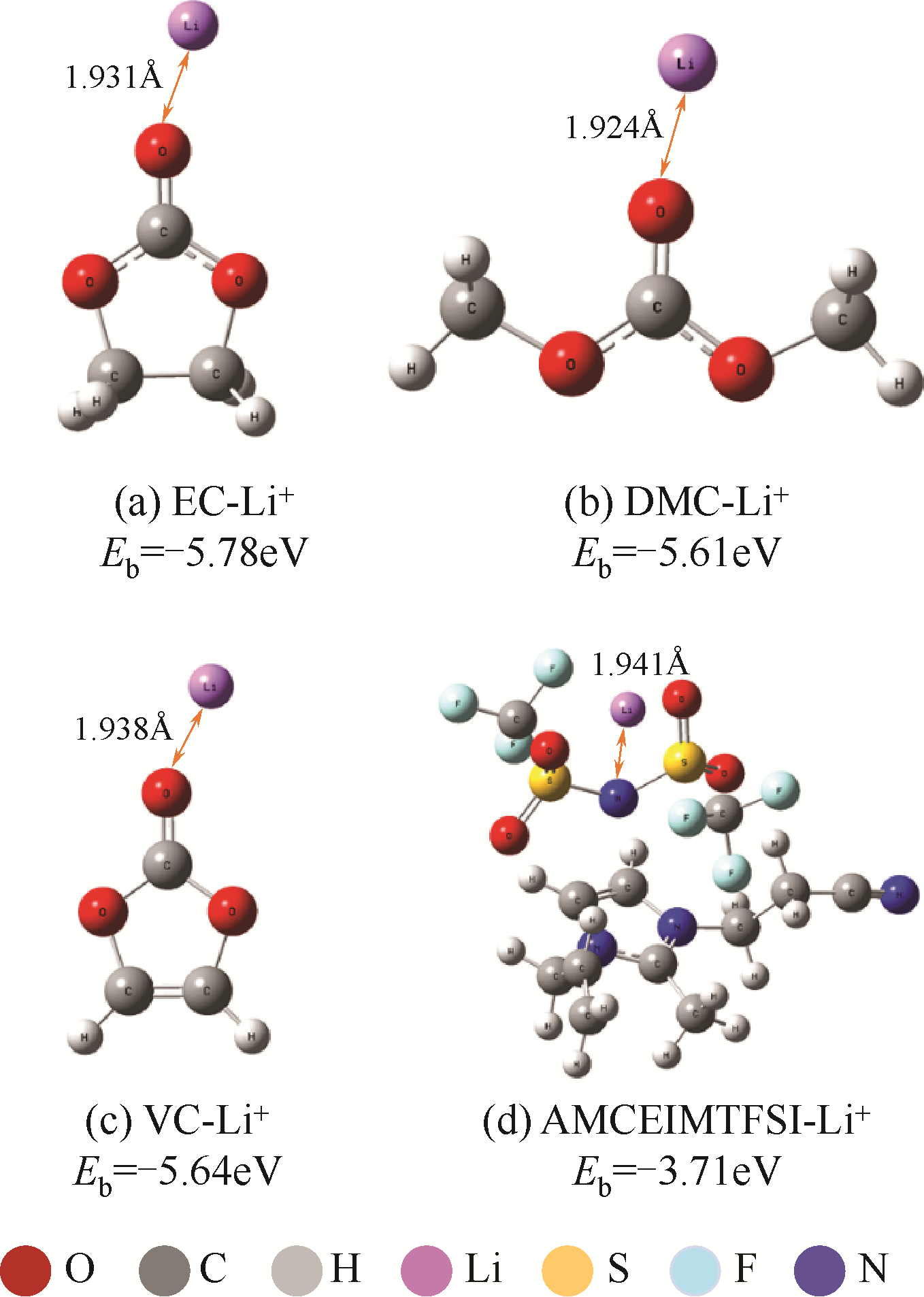
| 63 | PARK M H, LEE Y S, LEE H, et al. Low Li+ binding affinity: an important characteristic for additives to form solid electrolyte interphases in Li-ion batteries[J]. Journal of Power Sources, 2011, 196: 5109-5114. |
| [1] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [2] | 吴亚, 赵丹, 方荣苗, 李婧瑶, 常娜娜, 杜春保, 王文珍, 史俊. 用于复杂原油乳液的高效破乳剂开发及应用研究进展[J]. 化工进展, 2023, 42(8): 4398-4413. |
| [3] | 杨许召, 李庆, 袁康康, 张盈盈, 韩敬莉, 吴诗德. 含Gemini离子液体低共熔溶剂热力学性质[J]. 化工进展, 2023, 42(6): 3123-3129. |
| [4] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| [5] | 王昊, 霍进达, 曲国瑞, 杨家琪, 周世伟, 李博, 魏永刚. 退役锂电池正极材料资源化回收技术研究进展[J]. 化工进展, 2023, 42(5): 2702-2716. |
| [6] | 于捷, 张文龙. 锂离子电池隔膜的发展现状与进展[J]. 化工进展, 2023, 42(4): 1760-1768. |
| [7] | 张艺璇, 胡伟, 刘梦瑶, 鞠敬鸽, 赵义侠, 康卫民. 聚合物电解质在锌离子电池中的研究进展[J]. 化工进展, 2023, 42(3): 1397-1410. |
| [8] | 马文杰, 姚卫棠. 共价有机框架(COFs)在锂离子电池中的应用[J]. 化工进展, 2023, 42(10): 5339-5352. |
| [9] | 陈钰, 刘冲, 邱于荟, 贲梓欣, 牟天成. 离子液体和低共熔溶剂绿色回收废旧锂离子电池的研究进展[J]. 化工进展, 2022, 41(S1): 485-496. |
| [10] | 华渠成, 段庆华. 离子液体极压抗磨剂的研究进展[J]. 化工进展, 2022, 41(S1): 331-339. |
| [11] | 陈二军, 张玉玲, 路少磊, 段海洋, 靳文章. 空气纳米气泡的稳定性及理化特性[J]. 化工进展, 2022, 41(9): 4673-4681. |
| [12] | 韩明阳, 乔慧, 付佳铭, 马泽雯, 王妍, 欧阳嘉. 非水溶剂预处理木质纤维原料研究进展[J]. 化工进展, 2022, 41(8): 4086-4097. |
| [13] | 关浩然, 朱丽娜, 朱凌岳, 苑丹丹, 张雨晴, 王宝辉. 利用不同氢源及氮源电化学合成氨研究进展与挑战[J]. 化工进展, 2022, 41(8): 4098-4110. |
| [14] | 王玥, 郑晓洪, 陶天一, 刘秀庆, 李丽, 孙峙. 废锂离子电池正极材料中锂元素选择性回收的研究进展[J]. 化工进展, 2022, 41(8): 4530-4543. |
| [15] | 单清雯, 张娟, 王亚娟, 刘文强. 聚合离子液体的合成及其吸附脱硫性能[J]. 化工进展, 2022, 41(8): 4571-4579. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||