化工进展 ›› 2019, Vol. 38 ›› Issue (06): 2905-2914.DOI: 10.16085/j.issn.1000-6613.2018-2126
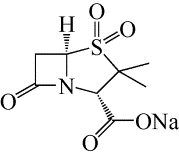
舒巴坦钠溶析结晶工艺优化
- 河北科技大学化学与制药工程学院,河北 石家庄 050018
-
收稿日期:2018-10-30出版日期:2019-06-05发布日期:2019-06-05 -
通讯作者:孙华 -
作者简介:李丽(1992—),女,硕士研究生,研究方向为工业结晶、药物分离与纯化。E-mail:<email>lili_L0815@163.com</email>。
Optimization of anti-solvent crystallization process of sulbactam sodium
Li LI( ),Baoshu LIU,Xueming ZHENG,Hua SUN(
),Baoshu LIU,Xueming ZHENG,Hua SUN( )
)
- College of Chemical and Pharmaceutical Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, Hebei, China
-
Received:2018-10-30Online:2019-06-05Published:2019-06-05 -
Contact:Hua SUN
摘要:
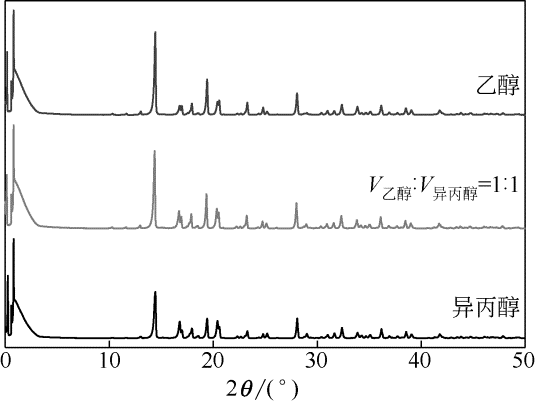
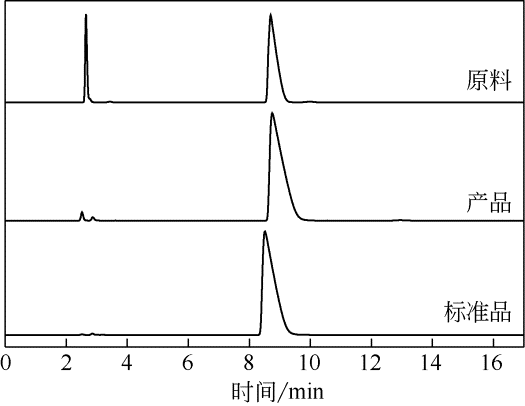
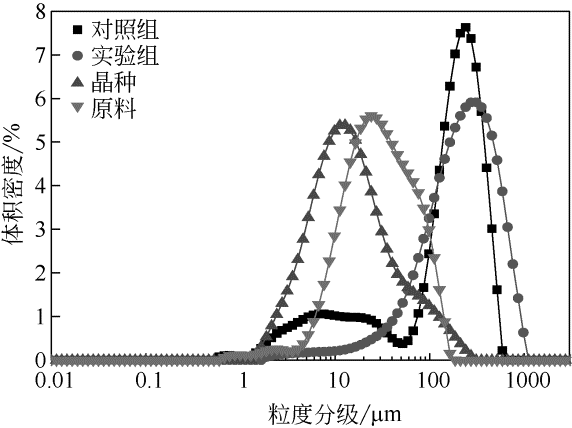
目前舒巴坦钠粗品的提纯采用还原成舒巴坦酸后再经反应结晶合成舒巴坦钠的方式,耗时耗力且所需药品、试剂种类较多,不节能环保。针对该问题,采用正交实验
中图分类号:
引用本文
李丽, 刘宝树, 郑学明, 孙华. 舒巴坦钠溶析结晶工艺优化[J]. 化工进展, 2019, 38(06): 2905-2914.
Li LI, Baoshu LIU, Xueming ZHENG, Hua SUN. Optimization of anti-solvent crystallization process of sulbactam sodium[J]. Chemical Industry and Engineering Progress, 2019, 38(06): 2905-2914.
| 水平 | A 养晶时间 /h | B 溶析剂 用量/mL | C 溶析剂中乙醇的体积分数/% | D 滴加速率 /mL·min-1 | E 搅拌速度 /r·min-1 | F 温度/℃ |
|---|---|---|---|---|---|---|
| 1 | 1 | 300.0 | 0 | 6.0 | 237~253 | 5.0 |
| 2 | 2 | 375.0 | 50 | 7.5 | 300~330 | 15.0 |
| 3 | 3 | 450.0 | 100 | 9.0 | 350~380 | 25.0 |
表1 因素与水平表
| 水平 | A 养晶时间 /h | B 溶析剂 用量/mL | C 溶析剂中乙醇的体积分数/% | D 滴加速率 /mL·min-1 | E 搅拌速度 /r·min-1 | F 温度/℃ |
|---|---|---|---|---|---|---|
| 1 | 1 | 300.0 | 0 | 6.0 | 237~253 | 5.0 |
| 2 | 2 | 375.0 | 50 | 7.5 | 300~330 | 15.0 |
| 3 | 3 | 450.0 | 100 | 9.0 | 350~380 | 25.0 |
| 序号 | A | B | A×B | A×B | C | A×C | A×C | B×C | D | 空白 | B×C | E | F | D[4,3]/μm | x/% | 综合评分 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 77.50 | 99.1576 | 76.74 |
| 2 | 1 | 1 | 1 | 1` | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 125.00 | 95.3440 | 78.18 |
| 3 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 21.70 | 97.1945 | 70.50 |
| 4 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 145.00 | 99.0681 | 82.55 |
| 5 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 135.00 | 94.0519 | 78.13 |
| 6 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 2 | 2 | 2 | 158.00 | 93.9219 | 80.04 |
| 7 | 1 | 3 | 3 | 3 | 1 | 1 | 1 | 3 | 3 | 3 | 2 | 2 | 2 | 141.00 | 98.6865 | 81.93 |
| 8 | 1 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 3 | 3 | 3 | 183.00 | 96.4820 | 84.02 |
| 9 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 174.00 | 94.6185 | 81.93 |
| 10 | 2 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 178.00 | 94.3386 | 82.08 |
| 11 | 2 | 1 | 2 | 3 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 114.00 | 99.1497 | 79.91 |
| 12 | 2 | 1 | 2 | 3 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 64.90 | 96.1809 | 73.54 |
| 13 | 2 | 2 | 3 | 1 | 1 | 2 | 3 | 2 | 3 | 1 | 3 | 1 | 2 | 345.00 | 98.8351 | 99.77 |
| 14 | 2 | 2 | 3 | 1 | 2 | 3 | 1 | 3 | 1 | 2 | 1 | 2 | 3 | 142.00 | 96.2244 | 80.28 |
| 15 | 2 | 2 | 3 | 1 | 3 | 1 | 2 | 1 | 2 | 3 | 2 | 3 | 1 | 138.00 | 96.5311 | 80.15 |
| 16 | 2 | 3 | 1 | 2 | 1 | 2 | 3 | 3 | 1 | 2 | 2 | 3 | 1 | 205.00 | 98.9170 | 87.66 |
| 17 | 2 | 3 | 1 | 2 | 2 | 3 | 1 | 1 | 2 | 3 | 3 | 1 | 2 | 181.00 | 94.5988 | 82.52 |
| 18 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 2 | 3 | 1 | 1 | 2 | 3 | 140.00 | 97.5186 | 81.02 |
| 19 | 3 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 164.00 | 99.0285 | 84.17 |
| 20 | 3 | 1 | 3 | 2 | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 95.40 | 95.7996 | 75.93 |
| 21 | 3 | 1 | 3 | 2 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | 147.00 | 94.7566 | 79.68 |
| 22 | 3 | 2 | 1 | 3 | 1 | 3 | 2 | 2 | 1 | 3 | 3 | 2 | 1 | 159.00 | 98.9166 | 83.66 |
| 23 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 3 | 2 | 1 | 1 | 3 | 2 | 218.00 | 94.5893 | 85.73 |
| 24 | 3 | 2 | 1 | 3 | 3 | 2 | 1 | 1 | 3 | 2 | 2 | 1 | 3 | 125.00 | 97.4810 | 79.69 |
| 25 | 3 | 3 | 2 | 1 | 1 | 3 | 2 | 3 | 2 | 1 | 2 | 1 | 3 | 185.00 | 94.2366 | 82.61 |
| 26 | 3 | 3 | 2 | 1 | 2 | 1 | 3 | 1 | 3 | 2 | 3 | 2 | 1 | 216.00 | 93.9306 | 85.09 |
| 27 | 3 | 3 | 2 | 1 | 3 | 2 | 1 | 2 | 1 | 3 | 1 | 3 | 2 | 110.00 | 97.5640 | 78.44 |
表2 设计及实验结果
| 序号 | A | B | A×B | A×B | C | A×C | A×C | B×C | D | 空白 | B×C | E | F | D[4,3]/μm | x/% | 综合评分 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 77.50 | 99.1576 | 76.74 |
| 2 | 1 | 1 | 1 | 1` | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 125.00 | 95.3440 | 78.18 |
| 3 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 21.70 | 97.1945 | 70.50 |
| 4 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 145.00 | 99.0681 | 82.55 |
| 5 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 135.00 | 94.0519 | 78.13 |
| 6 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 1 | 1 | 1 | 2 | 2 | 2 | 158.00 | 93.9219 | 80.04 |
| 7 | 1 | 3 | 3 | 3 | 1 | 1 | 1 | 3 | 3 | 3 | 2 | 2 | 2 | 141.00 | 98.6865 | 81.93 |
| 8 | 1 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 3 | 3 | 3 | 183.00 | 96.4820 | 84.02 |
| 9 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 174.00 | 94.6185 | 81.93 |
| 10 | 2 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 178.00 | 94.3386 | 82.08 |
| 11 | 2 | 1 | 2 | 3 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 114.00 | 99.1497 | 79.91 |
| 12 | 2 | 1 | 2 | 3 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 64.90 | 96.1809 | 73.54 |
| 13 | 2 | 2 | 3 | 1 | 1 | 2 | 3 | 2 | 3 | 1 | 3 | 1 | 2 | 345.00 | 98.8351 | 99.77 |
| 14 | 2 | 2 | 3 | 1 | 2 | 3 | 1 | 3 | 1 | 2 | 1 | 2 | 3 | 142.00 | 96.2244 | 80.28 |
| 15 | 2 | 2 | 3 | 1 | 3 | 1 | 2 | 1 | 2 | 3 | 2 | 3 | 1 | 138.00 | 96.5311 | 80.15 |
| 16 | 2 | 3 | 1 | 2 | 1 | 2 | 3 | 3 | 1 | 2 | 2 | 3 | 1 | 205.00 | 98.9170 | 87.66 |
| 17 | 2 | 3 | 1 | 2 | 2 | 3 | 1 | 1 | 2 | 3 | 3 | 1 | 2 | 181.00 | 94.5988 | 82.52 |
| 18 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 2 | 3 | 1 | 1 | 2 | 3 | 140.00 | 97.5186 | 81.02 |
| 19 | 3 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 164.00 | 99.0285 | 84.17 |
| 20 | 3 | 1 | 3 | 2 | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 95.40 | 95.7996 | 75.93 |
| 21 | 3 | 1 | 3 | 2 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | 147.00 | 94.7566 | 79.68 |
| 22 | 3 | 2 | 1 | 3 | 1 | 3 | 2 | 2 | 1 | 3 | 3 | 2 | 1 | 159.00 | 98.9166 | 83.66 |
| 23 | 3 | 2 | 1 | 3 | 2 | 1 | 3 | 3 | 2 | 1 | 1 | 3 | 2 | 218.00 | 94.5893 | 85.73 |
| 24 | 3 | 2 | 1 | 3 | 3 | 2 | 1 | 1 | 3 | 2 | 2 | 1 | 3 | 125.00 | 97.4810 | 79.69 |
| 25 | 3 | 3 | 2 | 1 | 1 | 3 | 2 | 3 | 2 | 1 | 2 | 1 | 3 | 185.00 | 94.2366 | 82.61 |
| 26 | 3 | 3 | 2 | 1 | 2 | 1 | 3 | 1 | 3 | 2 | 3 | 2 | 1 | 216.00 | 93.9306 | 85.09 |
| 27 | 3 | 3 | 2 | 1 | 3 | 2 | 1 | 2 | 1 | 3 | 1 | 3 | 2 | 110.00 | 97.5640 | 78.44 |
| 因素 | | | | |
|---|---|---|---|---|
| A | 128.91 | 167.54 | 157.711 | 7256.007 |
| B | 109.72 | 173.89 | 170.556 | 23487.500 |
| A×B | 139.13 | 145.100 | 169.93 | 4802.807 |
| A×B | 151.133 | 152.27 | 150.77 | 11.007 |
| C | 177.72 | 156.600 | 119.844 | 15440.869 |
| A×C | 137.311 | 172.556 | 144.300 | 6268.176 |
| A×C | 131.39 | 143.767 | 179.011 | 10989.769 |
| B×C | 157.833 | 156.378 | 139.96 | 1774.269 |
| D | 132.756 | 165.667 | 155.744 | 5130.242 |
| 空白 | 174.167 | 151.211 | 128.79 | 9266.569 |
| B×C | 148.722 | 142.93 | 162.511 | 1820.802 |
| E | 153.644 | 156.222 | 144.300 | 708.309 |
| F | 151.722 | 167.43 | 135.011 | 4731.902 |
表3 粒度D[4,3]的实验结果计算表[33]
| 因素 | | | | |
|---|---|---|---|---|
| A | 128.91 | 167.54 | 157.711 | 7256.007 |
| B | 109.72 | 173.89 | 170.556 | 23487.500 |
| A×B | 139.13 | 145.100 | 169.93 | 4802.807 |
| A×B | 151.133 | 152.27 | 150.77 | 11.007 |
| C | 177.72 | 156.600 | 119.844 | 15440.869 |
| A×C | 137.311 | 172.556 | 144.300 | 6268.176 |
| A×C | 131.39 | 143.767 | 179.011 | 10989.769 |
| B×C | 157.833 | 156.378 | 139.96 | 1774.269 |
| D | 132.756 | 165.667 | 155.744 | 5130.242 |
| 空白 | 174.167 | 151.211 | 128.79 | 9266.569 |
| B×C | 148.722 | 142.93 | 162.511 | 1820.802 |
| E | 153.644 | 156.222 | 144.300 | 708.309 |
| F | 151.722 | 167.43 | 135.011 | 4731.902 |
| 方差来源 | Sj | f | | Fj | F-theory | 显著性 |
|---|---|---|---|---|---|---|
| A | 7256.007 | 2 | 3628.003 | 1.84 | 2.62-3.54-4.56 | |
| B | 23487.500 | 2 | 11743.750 | 5.95 | ** | |
| C | 15440.869 | 2 | 7720.434 | 3.91 | * | |
| D △ | 5130.242 | 2 | 2565.121 | 1.30 | ||
| E △ | 708.309 | 2 | 354.154 | 0.18 | ||
| F △ | 4731.902 | 2 | 2365.951 | 1.20 | ||
| A×B △ | 4813.813 | 4 | 1203.453 | 0.61 | 2.29-2.93-3.61 | |
| A×C | 17257.944 | 4 | 4314.486 | 2.19 | ||
| B×C △ | 3595.071 | 4 | 898.768 | 0.46 | ||
| e | 9266.569 | 2 | 4633.284 | 2.35 | ||
| | 35501.913 | 18 | 1972.329 |
表4 粒度的方差分析
| 方差来源 | Sj | f | | Fj | F-theory | 显著性 |
|---|---|---|---|---|---|---|
| A | 7256.007 | 2 | 3628.003 | 1.84 | 2.62-3.54-4.56 | |
| B | 23487.500 | 2 | 11743.750 | 5.95 | ** | |
| C | 15440.869 | 2 | 7720.434 | 3.91 | * | |
| D △ | 5130.242 | 2 | 2565.121 | 1.30 | ||
| E △ | 708.309 | 2 | 354.154 | 0.18 | ||
| F △ | 4731.902 | 2 | 2365.951 | 1.20 | ||
| A×B △ | 4813.813 | 4 | 1203.453 | 0.61 | 2.29-2.93-3.61 | |
| A×C | 17257.944 | 4 | 4314.486 | 2.19 | ||
| B×C △ | 3595.071 | 4 | 898.768 | 0.46 | ||
| e | 9266.569 | 2 | 4633.284 | 2.35 | ||
| | 35501.913 | 18 | 1972.329 |
| 因素 | | | | |
|---|---|---|---|---|
| A | 96.50 | 96.92 | 96.26 | 2.039 |
| B | 96.77 | 96.62 | 96.28 | 1.130 |
| A×B | 97.08 | 95.83 | 96.77 | 7.678 |
| A×B | 96.56 | 96.41 | 96.72 | 0.430 |
| C | 97.91 | 95.57 | 96.20 | 26.319 |
| A×C | 96.83 | 96.42 | 96.43 | 0.978 |
| A×C | 97.41 | 96.48 | 95.79 | 11.842 |
| B×C | 96.16 | 97.42 | 96.09 | 10.094 |
| D | 97.02 | 95.34 | 97.32 | 20.426 |
| 空白 | 96.52 | 96.75 | 96.41 | 0.564 |
| B×C | 96.34 | 96.67 | 96.66 | 0.634 |
| E | 96.11 | 95.96 | 97.61 | 15.087 |
| F | 96.67 | 96.53 | 96.48 | 0.172 |
表5 纯度的实验结果计算表
| 因素 | | | | |
|---|---|---|---|---|
| A | 96.50 | 96.92 | 96.26 | 2.039 |
| B | 96.77 | 96.62 | 96.28 | 1.130 |
| A×B | 97.08 | 95.83 | 96.77 | 7.678 |
| A×B | 96.56 | 96.41 | 96.72 | 0.430 |
| C | 97.91 | 95.57 | 96.20 | 26.319 |
| A×C | 96.83 | 96.42 | 96.43 | 0.978 |
| A×C | 97.41 | 96.48 | 95.79 | 11.842 |
| B×C | 96.16 | 97.42 | 96.09 | 10.094 |
| D | 97.02 | 95.34 | 97.32 | 20.426 |
| 空白 | 96.52 | 96.75 | 96.41 | 0.564 |
| B×C | 96.34 | 96.67 | 96.66 | 0.634 |
| E | 96.11 | 95.96 | 97.61 | 15.087 |
| F | 96.67 | 96.53 | 96.48 | 0.172 |
| 方差来源 | S | f | | Fj | F-theory | 显著性 |
|---|---|---|---|---|---|---|
| A | 2.039 | 2 | 1.019 | 3.28 | 5.14-7.26-10.90 | |
| B△ | 1.130 | 2 | 0.565 | |||
| C | 26.319 | 2 | 13.160 | 42.32 | ** | |
| D | 20.426 | 2 | 10.213 | 32.84 | ** | |
| E | 15.087 | 2 | 7.543 | 24.26 | ** | |
| F△ | 0.172 | 2 | 0.086 | |||
| A×B | 8.108 | 4 | 2.027 | 6.52 | 4.53-6.23-9.15 | * |
| A×C | 12.820 | 4 | 3.205 | 10.31 | ** | |
| B×C | 10.727 | 4 | 2.682 | 8.62 | * | |
| e | 0.546 | 2 | 0.282 | |||
| | 1.866 | 6 | 0.311 |
表6 纯度的方差分析表
| 方差来源 | S | f | | Fj | F-theory | 显著性 |
|---|---|---|---|---|---|---|
| A | 2.039 | 2 | 1.019 | 3.28 | 5.14-7.26-10.90 | |
| B△ | 1.130 | 2 | 0.565 | |||
| C | 26.319 | 2 | 13.160 | 42.32 | ** | |
| D | 20.426 | 2 | 10.213 | 32.84 | ** | |
| E | 15.087 | 2 | 7.543 | 24.26 | ** | |
| F△ | 0.172 | 2 | 0.086 | |||
| A×B | 8.108 | 4 | 2.027 | 6.52 | 4.53-6.23-9.15 | * |
| A×C | 12.820 | 4 | 3.205 | 10.31 | ** | |
| B×C | 10.727 | 4 | 2.682 | 8.62 | * | |
| e | 0.546 | 2 | 0.282 | |||
| | 1.866 | 6 | 0.311 |
| 项目 | C 1 | C 2 | C 3 | KiA | ai |
|---|---|---|---|---|---|
| A 1 | D 11=296.91 | D 12=285.88 | D 13=285.73 | 868.53 | -0.06 |
| A 2 | D 21=292.09 | D 22=289.97 | D 23=290.23 | 872.29 | 0.36 |
| A 3 | D 31=292.18 | D 32=284.32 | D 33=289.80 | 866.30 | -0.30 |
| KiC | 881.18 | 860.17 | 865.77 | 2607.12 | |
| cj | 1.35 | -0.99 | -0.36 |
表7 因素A,C的二元表
| 项目 | C 1 | C 2 | C 3 | KiA | ai |
|---|---|---|---|---|---|
| A 1 | D 11=296.91 | D 12=285.88 | D 13=285.73 | 868.53 | -0.06 |
| A 2 | D 21=292.09 | D 22=289.97 | D 23=290.23 | 872.29 | 0.36 |
| A 3 | D 31=292.18 | D 32=284.32 | D 33=289.80 | 866.30 | -0.30 |
| KiC | 881.18 | 860.17 | 865.77 | 2607.12 | |
| cj | 1.35 | -0.99 | -0.36 |
| 项目 | C 1 | C 2 | C 3 |
|---|---|---|---|
| A 1 | 1.21 | -0.22 | -0.89 |
| A 2 | -0.91 | 0.72 | 0.19 |
| A 3 | -0.21 | -0.50 | 0.71 |
表8 纯度的 a c i j 值表
| 项目 | C 1 | C 2 | C 3 |
|---|---|---|---|
| A 1 | 1.21 | -0.22 | -0.89 |
| A 2 | -0.91 | 0.72 | 0.19 |
| A 3 | -0.21 | -0.50 | 0.71 |
| 项目 | C 1 | C 2 | C 3 | KiB | bi |
|---|---|---|---|---|---|
| B 1 | 292.52 | 290.29 | 288.13 | 870.95 | 0.21 |
| B 2 | 296.82 | 284.87 | 287.93 | 869.62 | 0.06 |
| B 3 | 291.84 | 285.01 | 289.70 | 866.55 | -0.28 |
| KiC | 881.18 | 860.17 | 865.77 | 2607.12 | |
| cj | 1.35 | -0.99 | -0.36 |
表9 因素B,C的二元表
| 项目 | C 1 | C 2 | C 3 | KiB | bi |
|---|---|---|---|---|---|
| B 1 | 292.52 | 290.29 | 288.13 | 870.95 | 0.21 |
| B 2 | 296.82 | 284.87 | 287.93 | 869.62 | 0.06 |
| B 3 | 291.84 | 285.01 | 289.70 | 866.55 | -0.28 |
| KiC | 881.18 | 860.17 | 865.77 | 2607.12 | |
| cj | 1.35 | -0.99 | -0.36 |
| 项目 | C 1 | C 2 | C 3 |
|---|---|---|---|
| B 1 | -0.61 | 0.98 | -0.36 |
| B 2 | 0.97 | -0.68 | -0.28 |
| B 3 | -0.35 | -0.29 | 0.65 |
表10 B,C交互作用 b c i j 表
| 项目 | C 1 | C 2 | C 3 |
|---|---|---|---|
| B 1 | -0.61 | 0.98 | -0.36 |
| B 2 | 0.97 | -0.68 | -0.28 |
| B 3 | -0.35 | -0.29 | 0.65 |
| 因素 | | | | |
|---|---|---|---|---|
| A | 79.34 | 82.99 | 81.67 | 61.63 |
| B | 77.86 | 83.33 | 82.80 | 164.14 |
| A×B | 80.63 | 80.27 | 83.09 | 42.58 |
| A×B | 81.31 | 81.30 | 81.39 | 0.04 |
| C | 84.57 | 81.09 | 78.33 | 176.14 |
| A×C | 80.30 | 83.07 | 80.62 | 41.40 |
| A×C | 80.19 | 80.61 | 83.19 | 47.54 |
| B×C | 81.61 | 82.37 | 80.01 | 26.28 |
| D | 80.03 | 81.71 | 82.25 | 23.98 |
| 空白 | 83.28 | 81.45 | 79.26 | 72.98 |
| B×C | 80.95 | 80.68 | 82.37 | 14.92 |
| E | 81.21 | 81.33 | 81.46 | 0.28 |
| F | 81.44 | 82.70 | 79.85 | 36.73 |
表11 综合评分的计算表
| 因素 | | | | |
|---|---|---|---|---|
| A | 79.34 | 82.99 | 81.67 | 61.63 |
| B | 77.86 | 83.33 | 82.80 | 164.14 |
| A×B | 80.63 | 80.27 | 83.09 | 42.58 |
| A×B | 81.31 | 81.30 | 81.39 | 0.04 |
| C | 84.57 | 81.09 | 78.33 | 176.14 |
| A×C | 80.30 | 83.07 | 80.62 | 41.40 |
| A×C | 80.19 | 80.61 | 83.19 | 47.54 |
| B×C | 81.61 | 82.37 | 80.01 | 26.28 |
| D | 80.03 | 81.71 | 82.25 | 23.98 |
| 空白 | 83.28 | 81.45 | 79.26 | 72.98 |
| B×C | 80.95 | 80.68 | 82.37 | 14.92 |
| E | 81.21 | 81.33 | 81.46 | 0.28 |
| F | 81.44 | 82.70 | 79.85 | 36.73 |
| 方差来源 | S | f | | F j | F-theory | 显著性 |
|---|---|---|---|---|---|---|
| A | 61.63 | 2 | 30.82 | 2.62-3.54-4.56 | ||
| B | 164.14 | 2 | 82.07 | 5.29 | * | |
| C | 176.14 | 2 | 88.07 | 5.67 | * | |
| D | 23.98 | 2 | 11.99 | |||
| E | 0.28 | 2 | 0.14 | |||
| F | 36.73 | 2 | 18.37 | |||
| A×B | 42.62 | 4 | 10.65 | 2.29-2.93-3.61 | ||
| A×C | 88.94 | 4 | 22.24 | 1.43 | 不显著 | |
| B×C | 41.20 | 4 | 10.30 | |||
| e | 72.98 | 2 | 36.49 | |||
| | 279.43 | 18 | 15.52 |
表12 综合评分的方差分析
| 方差来源 | S | f | | F j | F-theory | 显著性 |
|---|---|---|---|---|---|---|
| A | 61.63 | 2 | 30.82 | 2.62-3.54-4.56 | ||
| B | 164.14 | 2 | 82.07 | 5.29 | * | |
| C | 176.14 | 2 | 88.07 | 5.67 | * | |
| D | 23.98 | 2 | 11.99 | |||
| E | 0.28 | 2 | 0.14 | |||
| F | 36.73 | 2 | 18.37 | |||
| A×B | 42.62 | 4 | 10.65 | 2.29-2.93-3.61 | ||
| A×C | 88.94 | 4 | 22.24 | 1.43 | 不显著 | |
| B×C | 41.20 | 4 | 10.30 | |||
| e | 72.98 | 2 | 36.49 | |||
| | 279.43 | 18 | 15.52 |
| 1 | 程晓军, 刘安祥, 宋守忠 . β-内酰胺类抗生素发展浅述[J]. 中国药业, 2001, 10(2): 64-66. |
| 2 | CHENG X J , LIU A X , SONG S Z . The development of β-lactam antibiotics[J]. China Pharmaceuticals, 2001, 10(2): 64-66. |
| 3 | 蔡文辉, 张文莉, 付英梅, 等 . β-内酰胺酶抑制剂研究进展[J]. 中国抗生素杂志, 2013, 38(11): 805-809. |
| CAI W H , ZHANG W L , FU Y M , et al . Advances in β-lactamase inhibitors[J]. Chinese Journal of Antibiotics, 2013, 38(11): 805-809. | |
| 4 | 茹仁萍, 武谦虎 . 抗感染药物临床合理应用手册[M]. 北京: 中国医药科技出版社, 2016: 246. |
| RU R P , WU Q H . Handbook of clinical rational use of anti-infective drugs[M]. Beijing: Chinese Medical Science and Technology Press, 2016: 246. | |
| 5 | 陈兴娟, 侯秀梅, 曹欣祥, 等 . 舒巴坦的工艺优化研究[J]. 化学中间体, 2007 (2): 30-36. |
| CHEN X J , HOU X M , CAO X X , et al . Research of the optimizing process of sulbactam[J]. Chemical Intermediate, 2007 (2): 30-36. | |
| 6 | 邱方利, 潘富友 . 舒巴坦钠的合成[J]. 化学世界, 2006, 47(2): 99-101. |
| QIU F L , PAN F Y . Synthesis of sulbactam sodium[J]. Chemical World, 2006, 47(2): 99-101. | |
| 7 | 程宏辉, 谢朝晖 . 舒巴坦钠合成工艺的研究[J]. 化学设计通讯, 2001, 27(2): 61-62. |
| CHENG H H , XIE Z H . Study of the synthesis of sulbactam sodium[J]. Chemical Design Communications, 2001, 27(2): 61-62. | |
| 8 | 姜浩伟 . 舒巴坦钠结晶工艺的改进[J]. 制剂技术, 2011, 20(8): 48. |
| JIANG H W . Improvement of sulbactam sodium crystallization process[J]. Formulation Technology, 2011, 20(8): 48. | |
| 9 | 吕烨, 刘彦 . 舒巴坦钠结晶工艺的改进[J]. 黑龙江医药, 2006, 19(3): 185-186. |
| LÜ Y , LIU Y . Improvement of crystallization of sulbactam sodium[J]. Heilongjiang Medicine Jourrnal, 2006, 19(3): 185-186. | |
| 10 | 淄博鑫泉医药技术服务有限公司 . 舒巴坦钠的制备方法: CN10669974[P]. 2017-05-24. |
| Zibo Xinquan Pharmaceutical Technology Service Co., Ltd. . Process for preparation of sulbactam sodium: CN106699774[P]. 2017-05-24. | |
| 11 | 杜海生, 李凤侠, 赵振华, 等 . 反应结晶制备高质量的舒巴坦钠[J]. 山东化工, 2009, 38(4): 18-19. |
| DU H S , LI F X , ZHAO Z H , et al . A prosess of preparing high quanlity sulbactam sodium by reacting crystallization[J]. Shandong Chemical Industry, 2009, 38(4): 18-19. | |
| 12 | 国家药典委员会 . 中华人民共和国药典[M]. 北京:中国医药科技出版社, 2015:1423. |
| National Pharmacopoeia Commission . People’s Republic of China pharmacopoeia[M]. Beijing: Chinese Medical Science and Technology Press, 2015: 1423. | |
| 13 | 巴艳, 林悦凤 . 基础医学与临床.2[M]. 济南: 山东大学出版社, 2016: 188. |
| BA Y , LIN Y F . Basic medicine and clinical.2[M]. Jinan: Shandong University Press, 2016:188. | |
| 14 | 胡延维, 孙芸 . 舒巴坦钠结晶过程中的影响因素分析[J]. 苏州大学学报(医学版), 2003, 23(6): 674-676. |
| HU Y W , SUN Y . Analysis of factors influencing sulbactam sodium crystallization process[J]. Suzhou University Journal of Medical Science, 2003, 23(6): 674-676. | |
| 15 | 颜青, 肖永红, 何绥平 . 必须重视β-内酰胺类抗生素与其酶抑制剂复方制剂的科学开发[J]. 中国临床药学杂志, 2007, 16(4): 259-261 . |
| YAN Q , XIAO Y H , HE S P . Scientific development of compound preparations of β-lactam antibiotics and their enzyme inhibitors[J]. Chinese Journal of Clinical Pharmacy, 2007, 16(4): 259-261. | |
| 16 | 龚俊波, 陈明洋, 黄翠, 等 . 面向清洁生产的制药结晶[J]. 化工学报, 2015, 66(9): 3271-3278. |
| GONG J B , CHEN M Y , HUANG C , et al . Clean production of pharmaceutical crystallization[J]. CIESC Journal, 2015, 66(9): 3271-3278. | |
| 17 | MULLIN J W . Crystallization[M]. 4th ed. Oxford: Reed Educational and Professional Publishing, 2001. |
| 18 | KIM H J, YEO S D . Liquid antisolvent crystallization of griseofulvin from organic solutions[J]. Chemical Engineering Research and Design, 2015, 97: 68-76. |
| 19 | 刘敏, 李玉兰, 杨敏, 等 . 高效液相色谱法测定舒巴坦钠的有关物质[J]. 中国药师, 2005, 8(6): 469-472. |
| LIU M , LI Y L , YANG M , et al . HPLC determination of related substances in sulbactam sodium[J]. China Pharmacist, 2005, 8(6): 469-472. | |
| 20 | 李玉兰,刘敏, 王玉 . 舒巴坦钠中舒巴坦青霉胺杂质含量测定方法的建立[J]. 中国药师, 2005, 8(6): 467-469. |
| LI Y L , LIU M , WANG Y . Establishment of a method for the impurity determination of sulbactam penicillamine in sulbactam sodium[J]. China Pharmacist, 2005, 8(6): 467-469. | |
| 21 | 胡大为, 胡小芳, 林丽莹 . 粉体粒度分布分形维数与流动性及硬脂酸镁改进流动性关系[J]. 中国粉体技术, 2007, 13(4): 1-4. |
| HU D W , HU X F , LIN L Y . Correlation of powders’ particle size distribution fractal with flow ability and flow addition reagent magnesium stearate[J]. China Powder Technology, 2007, 13(4): 1-4. | |
| 22 | 刘瑞江, 张业旺, 闻崇炜 . 正交试验设计和分析方法研究[J]. 实验技术与管理, 2010, 27(9): 52-55. |
| LIU R J , ZHANG Y W , WEN C W . Study on the design and analysis methods of orthogonal experiment[J]. Experimental Technology and Management, 2010, 27(9): 52-55. | |
| 23 | 马博爱, 龚俊波 . 阿奇霉素二水合物溶析结晶工艺优化[J]. 化学工业与工程, 2015, 32(1): 37-40. |
| 24 | MA B A, GONG J B . Optimization of crystallization process of azithromyc in dihydrate[J]. Chemical Industryand Engineering, 2015, 32(1): 37-40. |
| 25 | 王海蓉, 张春桃, 王永莉 . 加晶种控制头孢曲松钠溶析结晶产品粒度分布的研究[J]. 中国抗生素杂志, 2009, 34(6): 337-340. |
| WANG H R , ZHANG C T , WANG Y L . Stuudies on the control of crystal size distribution of ceftriaxone sodium in dilution crystallization by seeding[J]. Chinese Journal of Antibiotics, 2009, 34(6): 337-340. | |
| 26 | 尹永恒, 鲍颖, 王永莉, 等 . 诱导头孢噻肟钠结晶过程研究[J]. 化学工业与工程, 2014, 31(2): 48-53. |
| YIN Y H , BAO Y , WANG Y L , et al . Induced crystallization of cefotaxime sodium[J]. Chemical Industry and Engineering, 2014, 31(2): 48-53. | |
| 27 | 刘宝树 . 5'-鸟苷酸二钠溶析结晶过程研究[D]. 天津: 天津大学, 2007. |
| LIU B S . Study on crystallization process of disodium 5'-guanylate[D]. Tianjin: Tianjin University, 2007. | |
| 28 | WANG H Y , WARD J D . Seeding and optimization of batch reactive crystallization[J]. Industrial and Engineering Chemistry Research, 2015, 54(38): 9360-9368. |
| 29 | 李永曙, 刘继阳, 梅丽琴, 等 . 注射用美洛西林钠溶析结晶工艺研究[J]. 中国抗生素杂志, 2010, 35(5):353-356. |
| LI Y S , LIU J Y , MEI L Q , et al . Study on solvent-out crystallization process of mezlocillin sodium for injection[J]. Chinese Journal of Antibiotics, 2010, 35(5): 353-356. | |
| 30 | 鲍颖 . 盐酸大观霉素溶析结晶过程研究[D]. 天津:天津大学, 2003. |
| BAO Y . Study on the process of dilution crystallization of spectinomycin dihydrochloride[D]. Tianjin: Tianjin University, 2003. | |
| 31 | 孙华 . 洛伐他汀结晶过程研究[D]. 天津: 天津大学, 2006. |
| SUN H . Study on the crystallization process of lovastatin[D]. Tianjin: Tianjin University, 2006. | |
| 32 | 庄楚强, 何春雄 . 应用数理统计基础[M]. 广州: 华南理工大学出版社, 2011: 222-284. |
| ZHUANG C Q , HE C X . Applied mathematical statistics foundation[M]. Guangzhou: South China University of Technology Publishing House, 2011: 222-284. | |
| 33 | 王雪雁 . 一种治疗传染性疾病的舒巴坦钠化合物及其制备方法: CN10486946[P]. 2015-04-30. |
| WANG X Y . Sulbactam sodium compound for treating infectious disease and its preparing : CN10486946[P]. 2015-04-30. | |
| 34 | 张丕德, 龙晓英 . 正交设计与数据分析在药学研究中的应用(Ⅱ)[J]. 广东药学院学报, 2009, 25(6): 648-657. |
| ZHANG P D , LONG X Y . Application of orthogonal design and data analysis in pharmaceutical research (Ⅱ)[J]. Journal of Guangdong Pharmaceutical College, 2009, 25(6): 648-657. | |
| 35 | 陈翔, 梁卫玖 . 建立Excel宏快速处理正交试验设计数据[J]. 中国卫生检验杂志, 2009, 19(11): 2696-2697. |
| CHEN X , LIANG W J . Establish Excel macro to quickly process orthogonal experimental design data[J]. Chinese Journal of Health Laboratory Technology, 2009, 19(11): 2696-2697. |
| [1] | 陈佳昆, 汤健, 夏恒, 乔俊飞. 城市固废炉排炉焚烧过程二𫫇英排放浓度数值仿真[J]. 化工进展, 2023, 42(2): 1061-1072. |
| [2] | 郑瑾, 韩瑞瑞, 李丹丹, 王馨妤, 高春阳, 杜显元, 张晓飞, 邹德勋. 过氧化尿素与微生物联合修复石油污染土壤[J]. 化工进展, 2022, 41(9): 5085-5093. |
| [3] | 丁兴江, 章学来, 朱嘉豪, 毛发, 房满庭, 冯天平. 三水合乙酸钠复合相变材料过冷特性实验统计分析[J]. 化工进展, 2022, 41(11): 5946-5960. |
| [4] | 李春利, 程永辉, 李浩. 精馏-吸附-膜分离耦合工艺制备高纯度酒精流程模拟[J]. 化工进展, 2021, 40(3): 1354-1361. |
| [5] | 刘少斌, 齐宏, 余智强, 何明键, 于喜奎. 基于Taguchi方法的微小通道性能分析与参数优化[J]. 化工进展, 2021, 40(12): 6409-6422. |
| [6] | 盛磊, 脱凌晗, 姜晓滨, 贺高红. 有机膜精确调控传质的新型溶析结晶及过程强化[J]. 化工进展, 2020, 39(5): 1692-1700. |
| [7] | 曲晏利, 姜跃佳, 程景才, 杨超. 反应与溶析结晶过程强化及数值模拟研究进展[J]. 化工进展, 2020, 39(12): 4970-4982. |
| [8] | 黄炎, 孙海龙, 孟子超, 唐忠利, 王靖涛. 溶析结晶在医药领域的研究进展[J]. 化工进展, 2019, 38(05): 2380-2388. |
| [9] | 王治红, 丁晓明, 吴明鸥, 沈晓燕. 有机朗肯循环在多品位余热发电中的应用[J]. 化工进展, 2019, 38(05): 2189-2196. |
| [10] | 郭睿, 宋博, 郭煜, 李云鹏, 土瑞香, 王映月, 马兰. 木质素磺酸盐接枝共聚物的合成及工艺优化[J]. 化工进展, 2018, 37(05): 1962-1967. |
| [11] | 刘忠慧, 于旷世, 张海霞, 朱治平. 基于Aspen Plus的循环流化床工业气化炉模拟[J]. 化工进展, 2018, 37(05): 1709-1717. |
| [12] | 程琛, 闫正. 复频超声降解土霉素废水[J]. 化工进展, 2015, 34(04): 1143-1146,1164. |
| [13] | 刘军霞, 姚庆鑫, 杨金, 周磊, 谢建军. 多指标正交实验优化可发性三聚氰胺甲醛树脂的制备[J]. 化工进展, 2015, 34(02): 474-478. |
| [14] | 古蒙蒙1,涂文辉1,桂绍庸1,蔡卫权1,林元虹1,李玉军1,曹宏2?. 环保型高效稠油垢弱酸性水基清洗剂的研制[J]. 化工进展, 2014, 33(06): 1563-1566. |
| [15] | 冉二艳,张宝昌,周如彬,赵莹,刘扬. 氨基聚醚共改性硅油的合成与性能[J]. 化工进展, 2014, 33(02): 445-452. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||