化工进展 ›› 2019, Vol. 38 ›› Issue (04): 1887-1893.DOI: 10.16085/j.issn.1000-6613.2018-1428
卡维地洛滴丸的制备工艺与表征
- 太原工业学院化学与化工系,山西 太原 030008
Preparation technology and characterization of carvedilol dropping pills
Rongqian MENG( ),Shengjuan SHAO,Huifang WANG
),Shengjuan SHAO,Huifang WANG
- Department of Chemistry and Chemical Engineering, Taiyuan Institute of Technology, Taiyuan 030008,Shanxi,China
摘要:
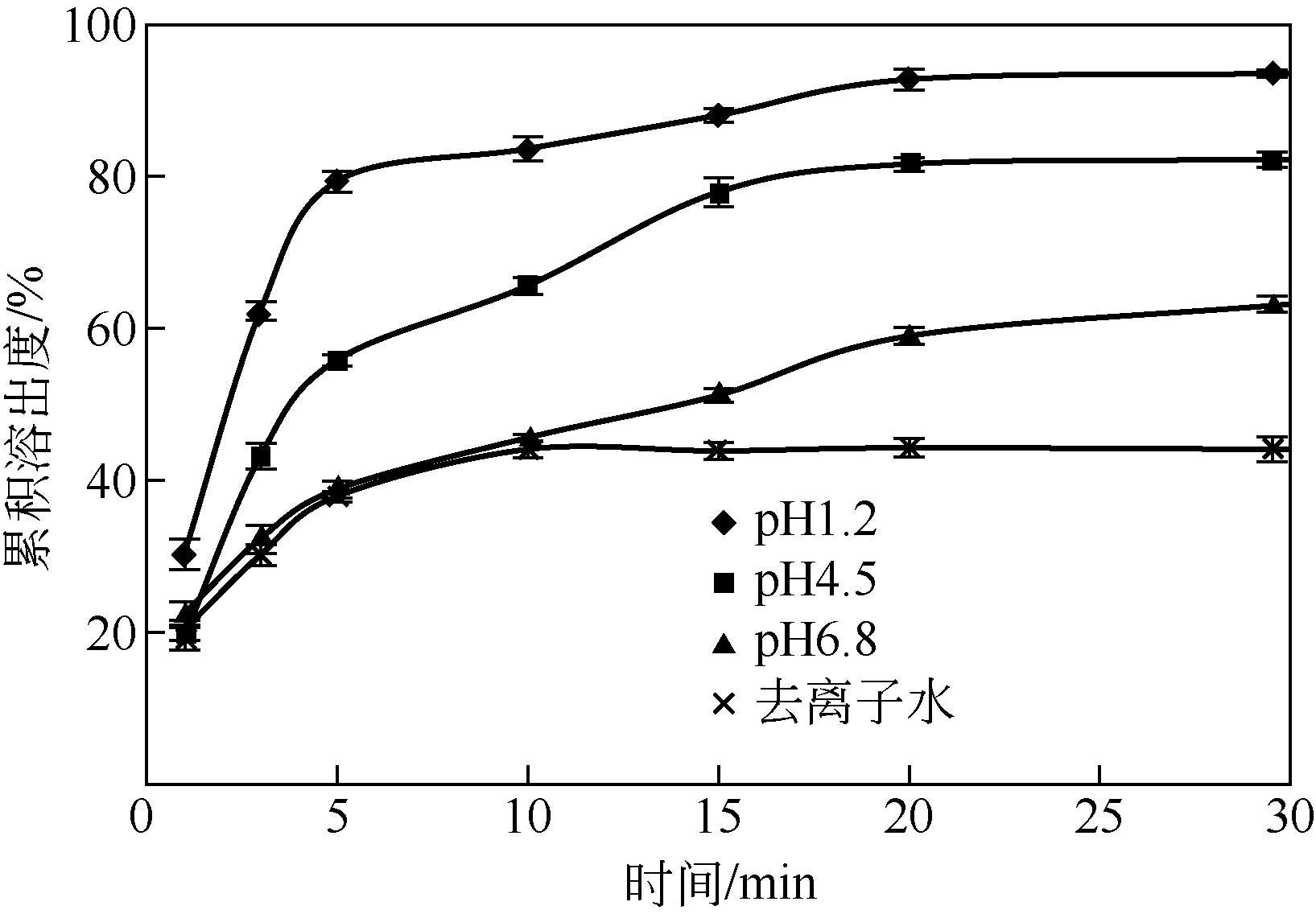
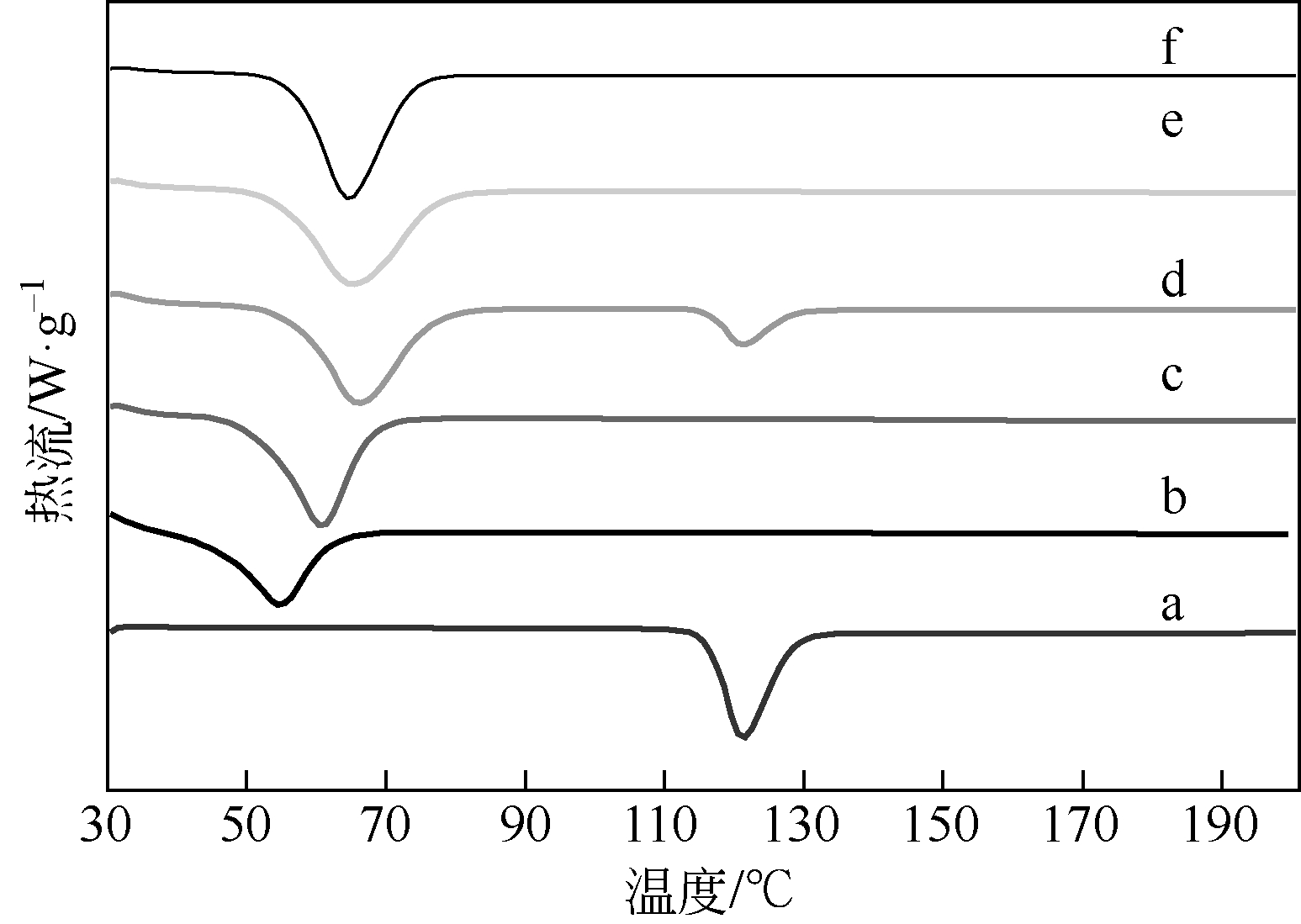
本文开发了一种可提高新剂型的可提高难溶性药物卡维地洛溶出的制备方法并进行表征。采用固体分散技术法制备滴丸,通过响应面试验法,按照归一值法优化指标,优选最佳工艺并验证,考察自制滴丸体外溶出度。采用差示扫描量热法(DSC)、粉末 X射线衍射法和红外吸收光谱法鉴定药物在滴丸中的存在状态。结果表明:最优制备工艺为卡维地洛与基质质量比为1∶7,PEG6000与PEG4000质量比为1∶3,滴速为54min-1,药液温度为75℃,所建模型显著。滴丸在pH1.2的盐酸溶液中30min内释药最快,达到90%以上,其次为pH4.5、pH6.8、去离子水。所制3批滴丸重现性好,体外溶出相似(f 2>85),稳定性好。药物在滴丸中主要以无定形状态存在,可提高难溶性药物的溶出,为卡维地洛新的口服速释剂型的开发提供参考。
中图分类号: