化工进展 ›› 2025, Vol. 44 ›› Issue (6): 3041-3052.DOI: 10.16085/j.issn.1000-6613.2024-1146
• 专栏:化工生态环境前沿交叉新技术 • 上一篇
纳米管状Co-N-C活化过碳酸盐降解四环素
石秀顶1,2( ), 王永全3, 曾静3, 苏畅3, 洪俊明1,2(
), 王永全3, 曾静3, 苏畅3, 洪俊明1,2( )
)
- 1.华侨大学化工学院,福建 厦门 361021
2.福建省工业废水生化处理工程技术研究中心,福建 厦门 361021
3.厦门烟草工业有限责任公司,福建 厦门 361021
-
收稿日期:2024-07-17修回日期:2025-01-15出版日期:2025-06-25发布日期:2025-07-08 -
通讯作者:洪俊明 -
作者简介:石秀顶(1996—),男,博士研究生,研究方向为水污染处理工程。E-mail:2429462654@qq.com。 -
基金资助:国家自然科学基金(51978291);福建省科技项目基金(2021J01311);福建省科技项目基金(2022I0030)
Nanotubular Co-N-C activated percarbonate for tetracycline degradation
SHI Xiuding1,2( ), WANG Yongquan3, ZENG Jing3, SU Chang3, HONG Junming1,2(
), WANG Yongquan3, ZENG Jing3, SU Chang3, HONG Junming1,2( )
)
- 1.College of Chemical Engineering, Huaqiao University, Xiamen 361021, Fujian, China
2.Fujian Province Engineering Research Center of Industrial Wastewater Biochemical Treatment, Xiamen 361021, Fujian, China
3.Xiamen Tobacco Industrial Company Limited, Xiamen 361021, Fujian, China
-
Received:2024-07-17Revised:2025-01-15Online:2025-06-25Published:2025-07-08 -
Contact:HONG Junming
摘要:
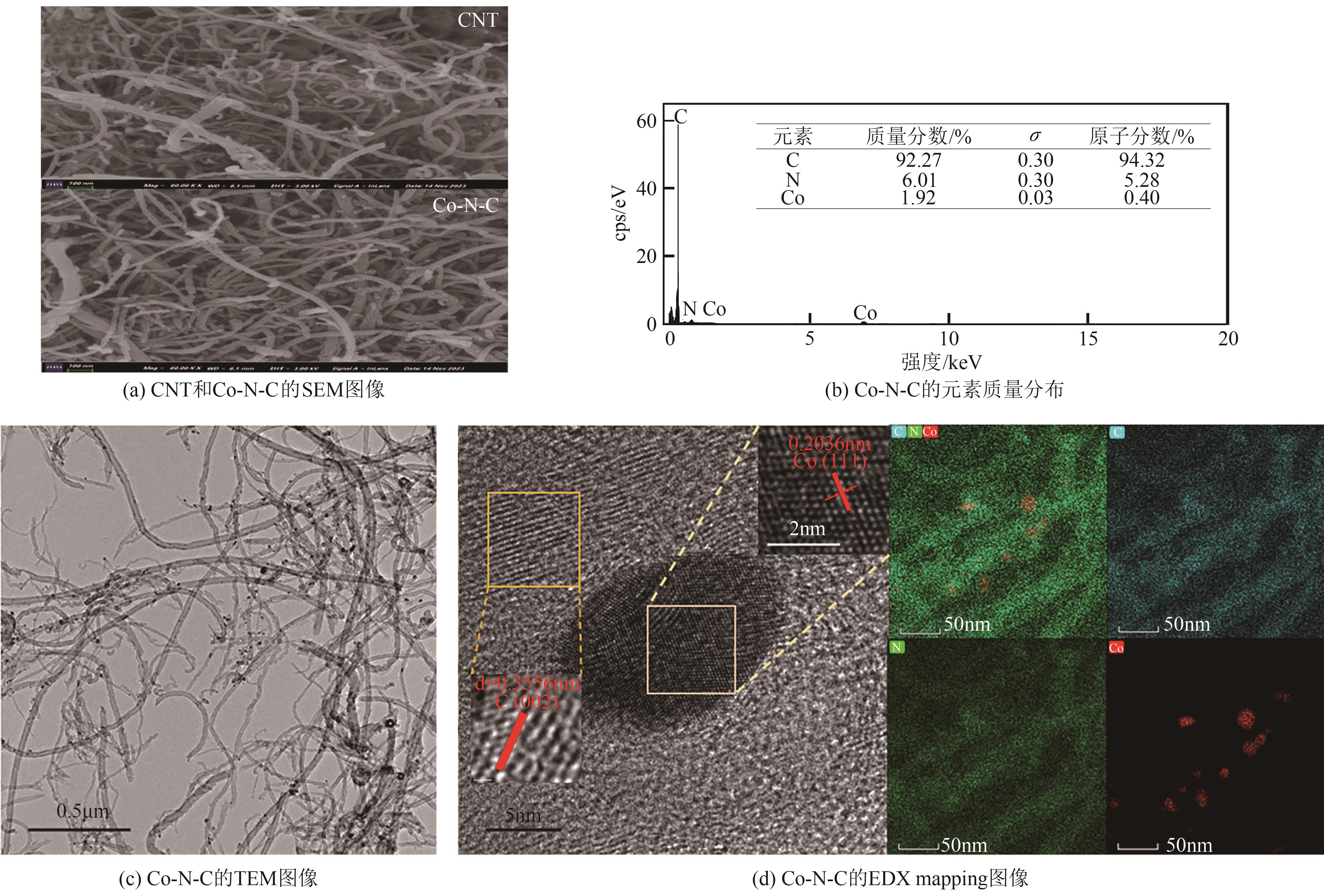
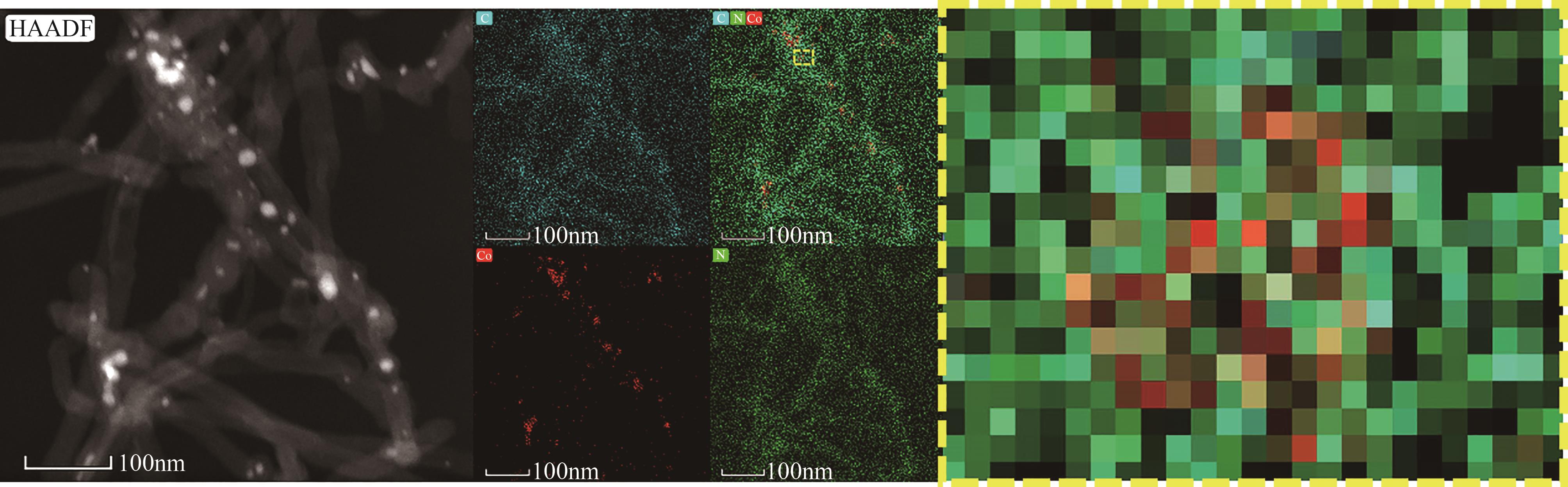
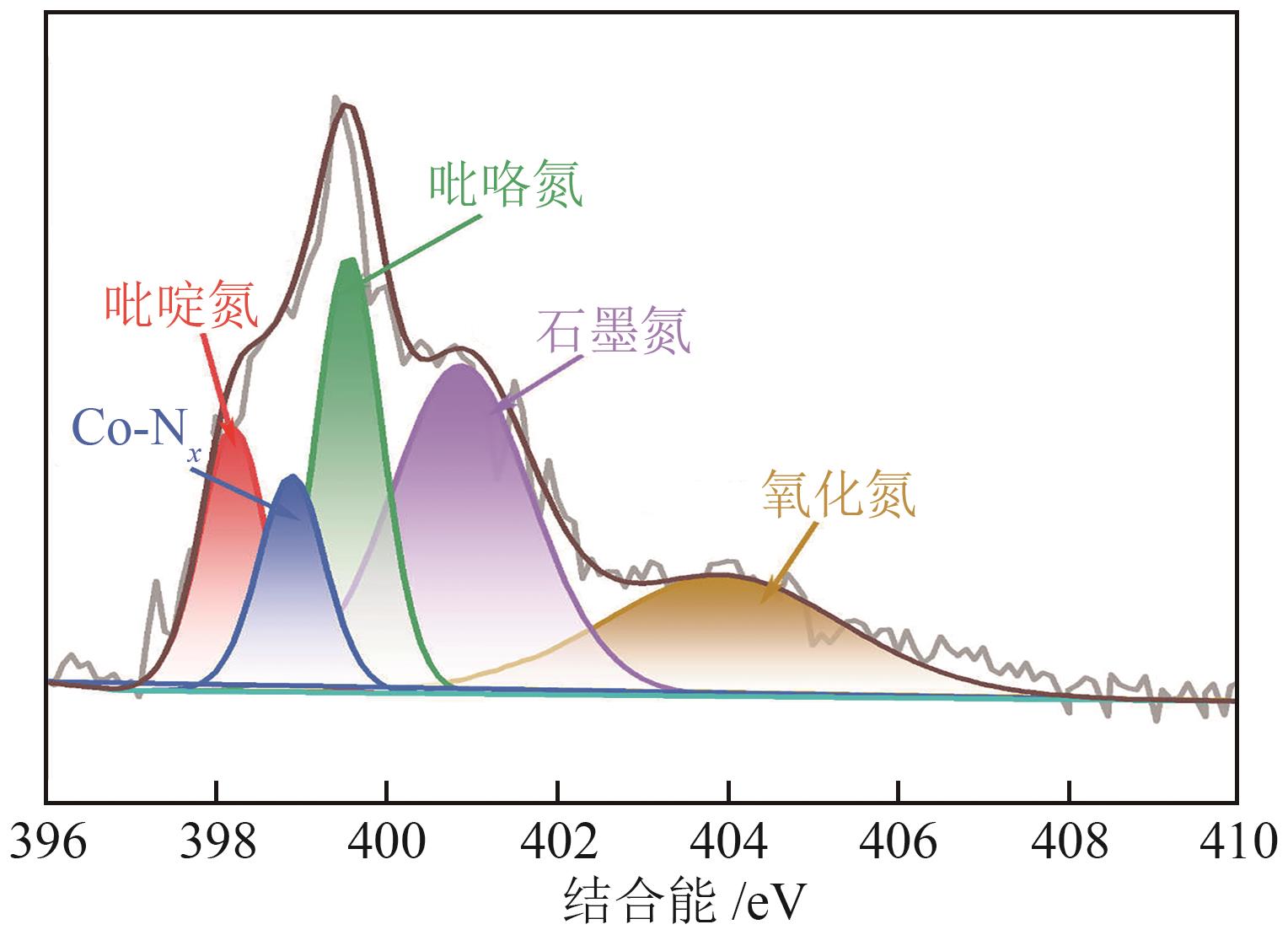
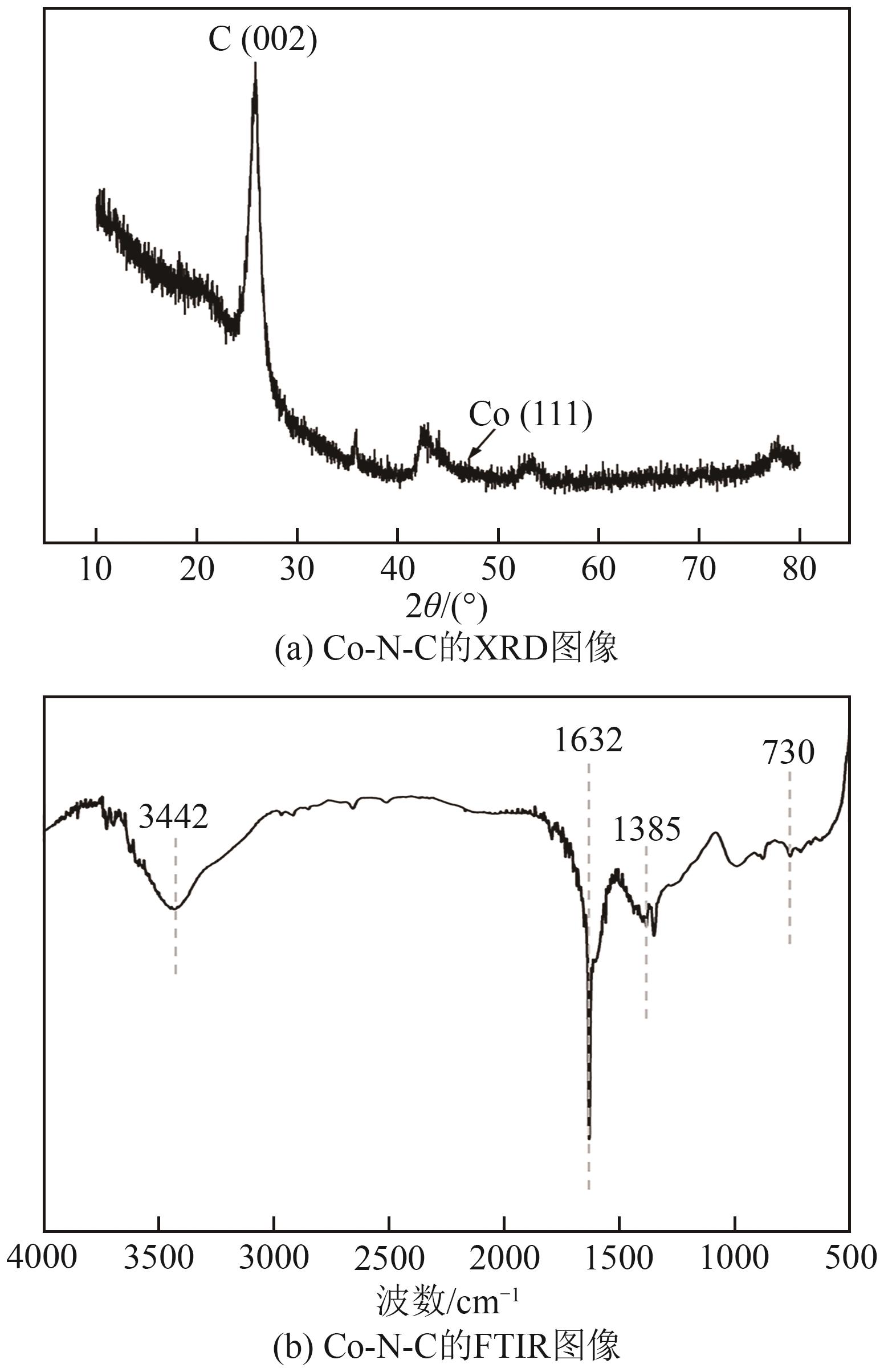
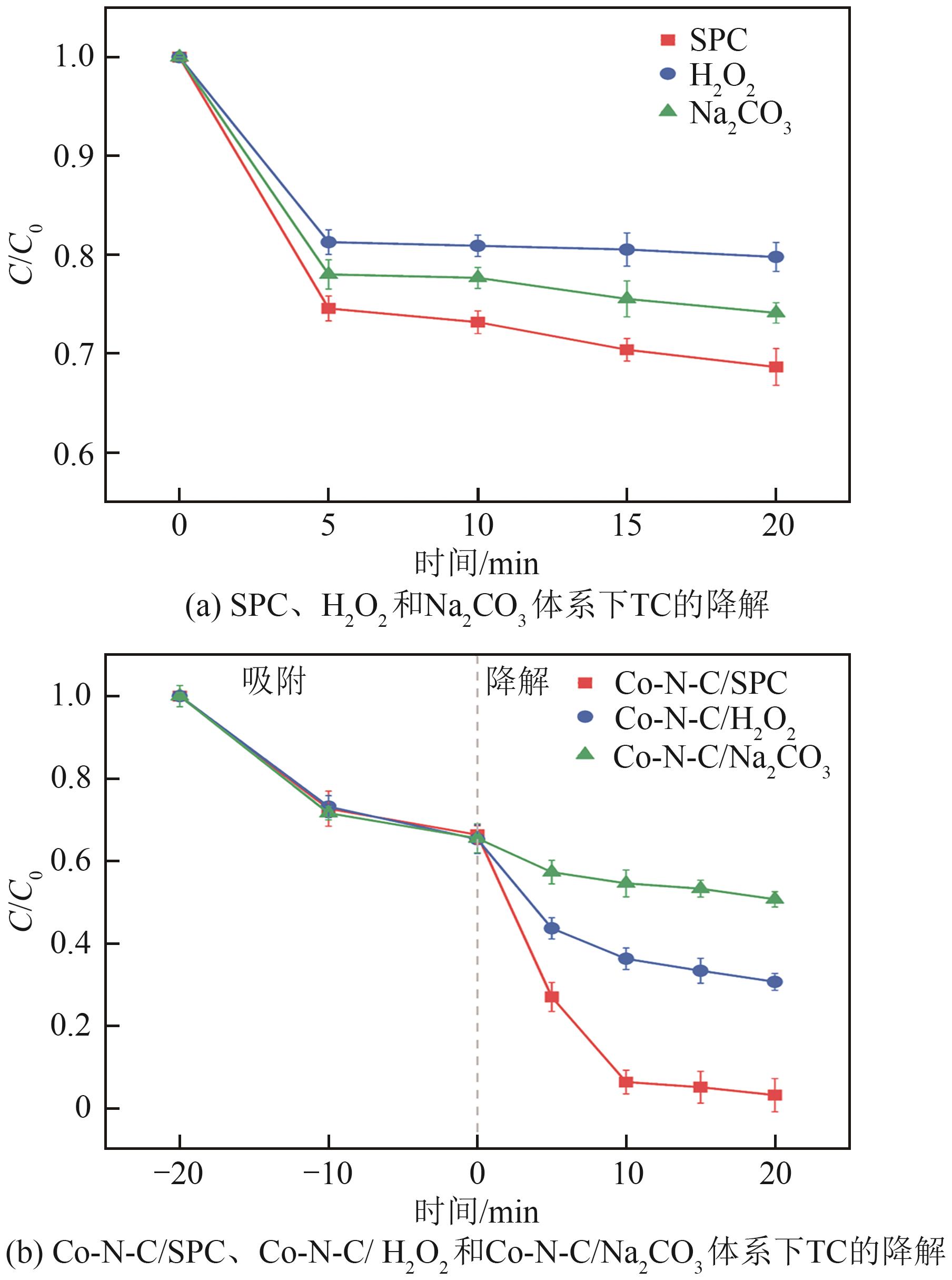
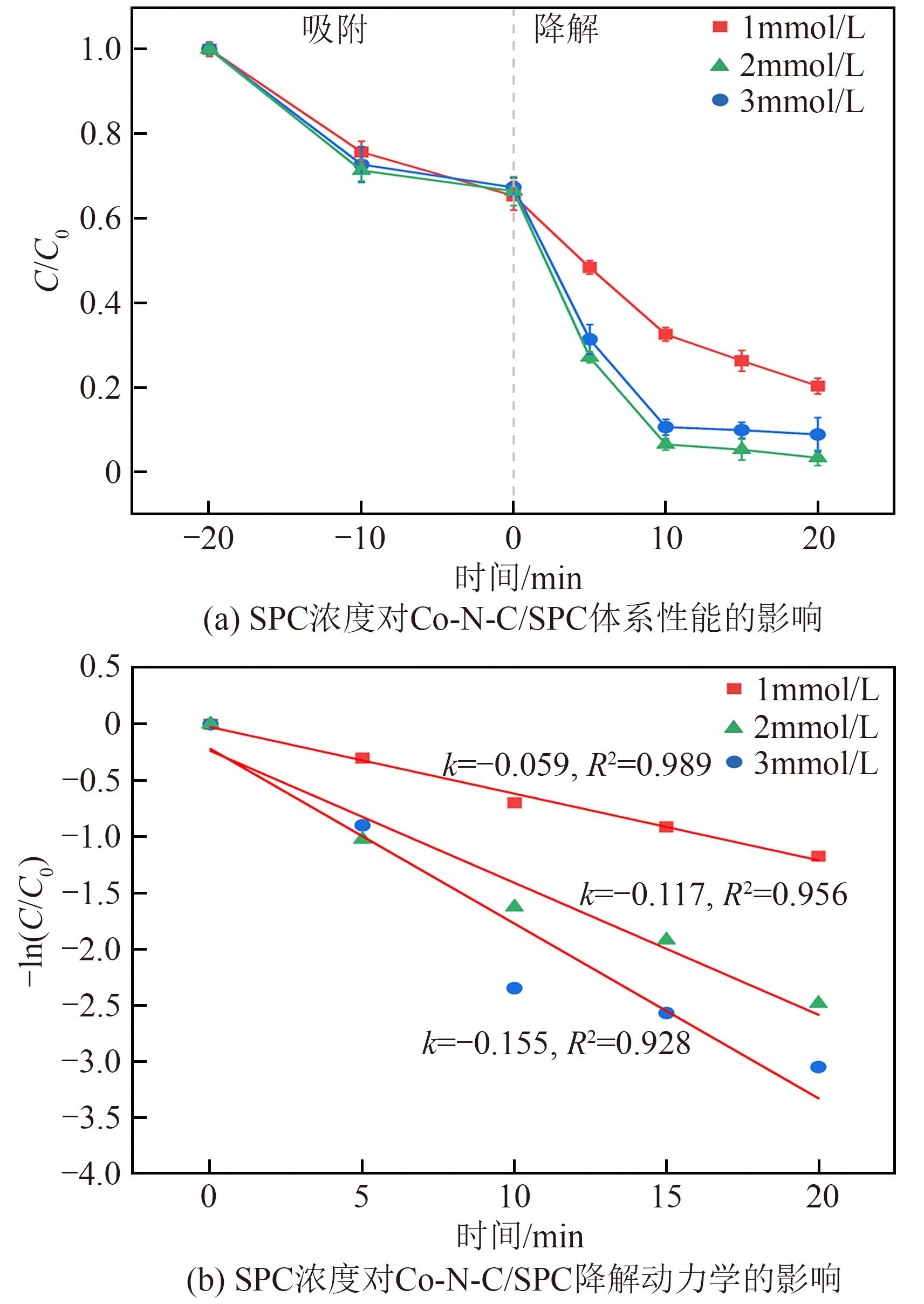
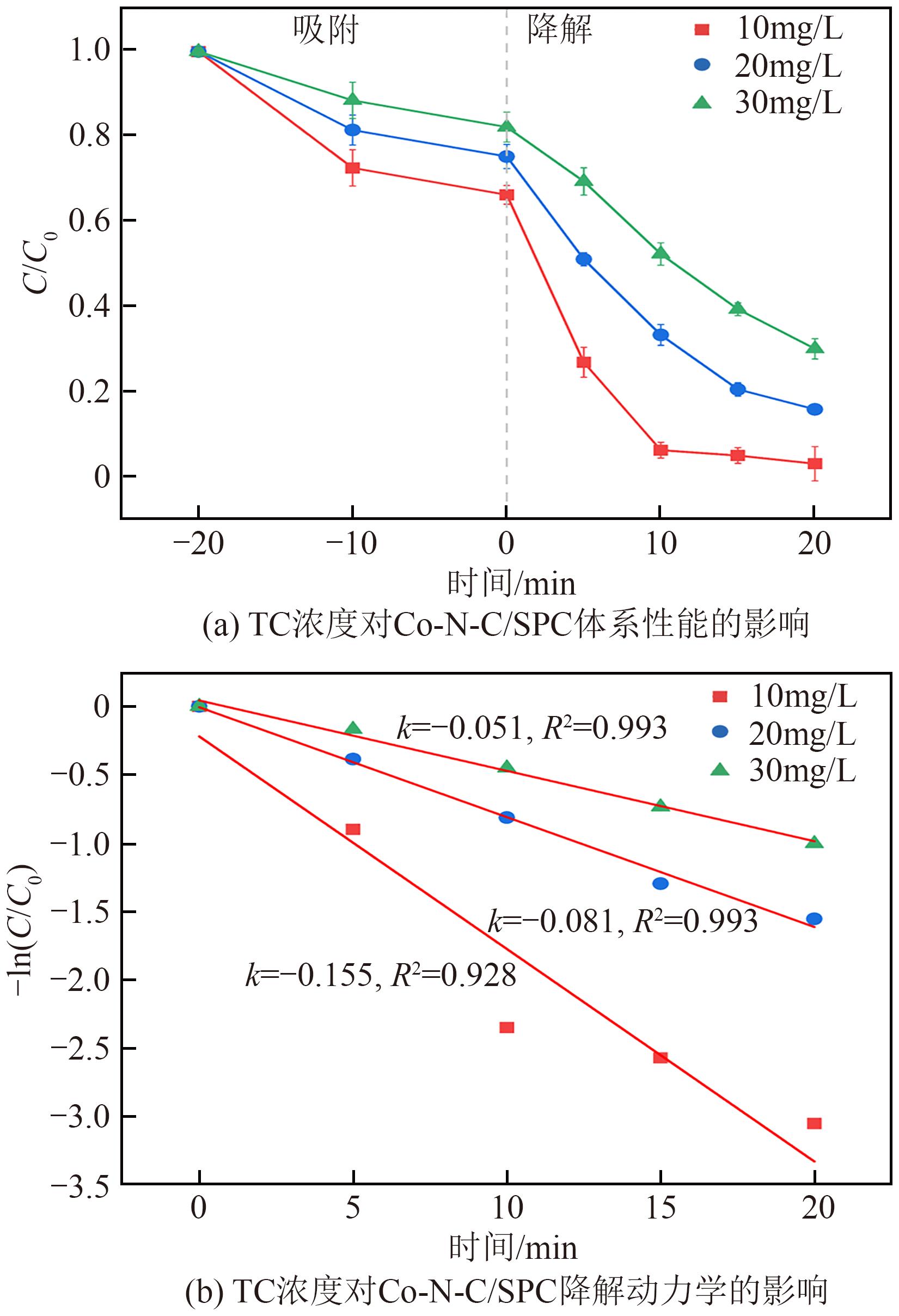
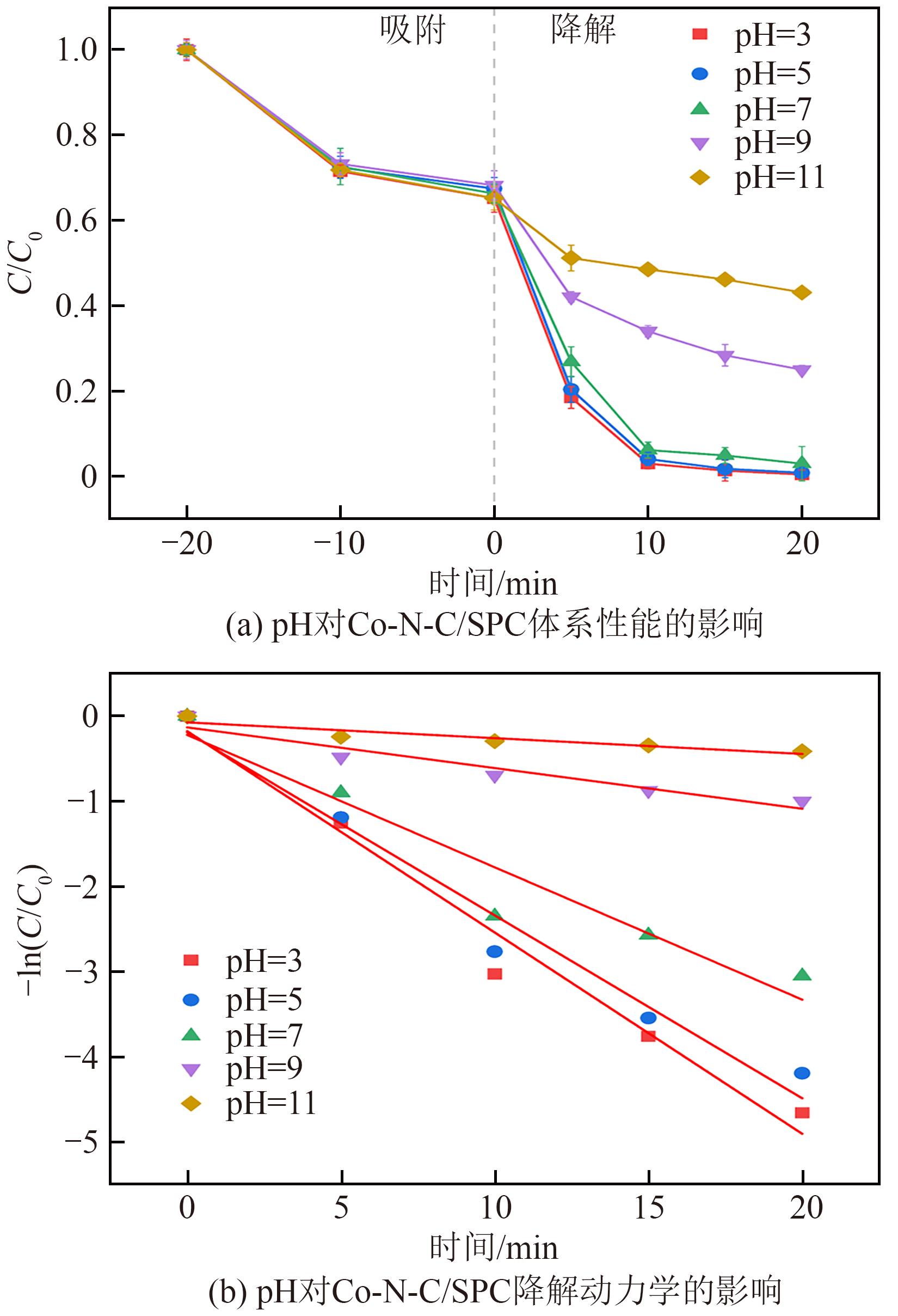
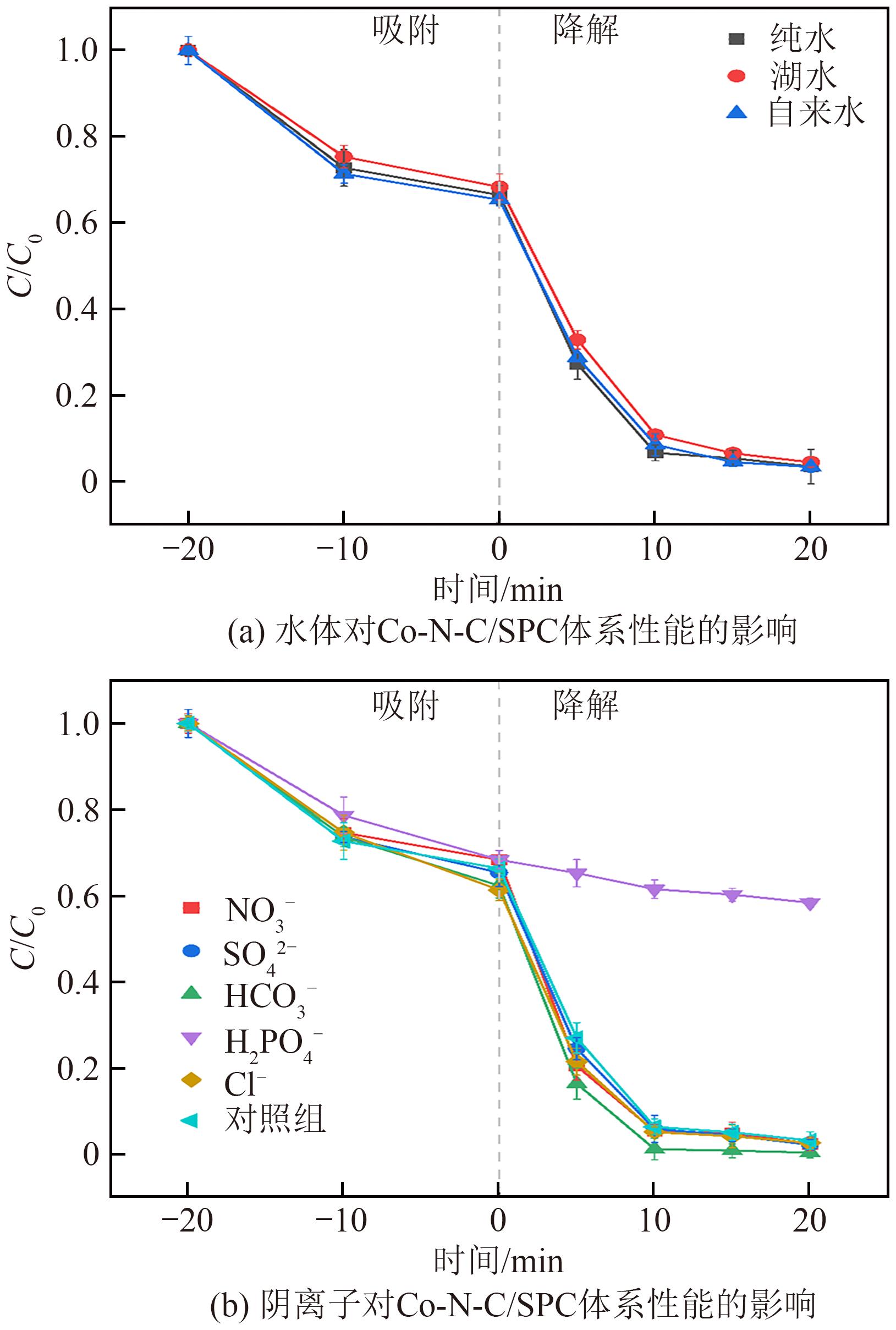
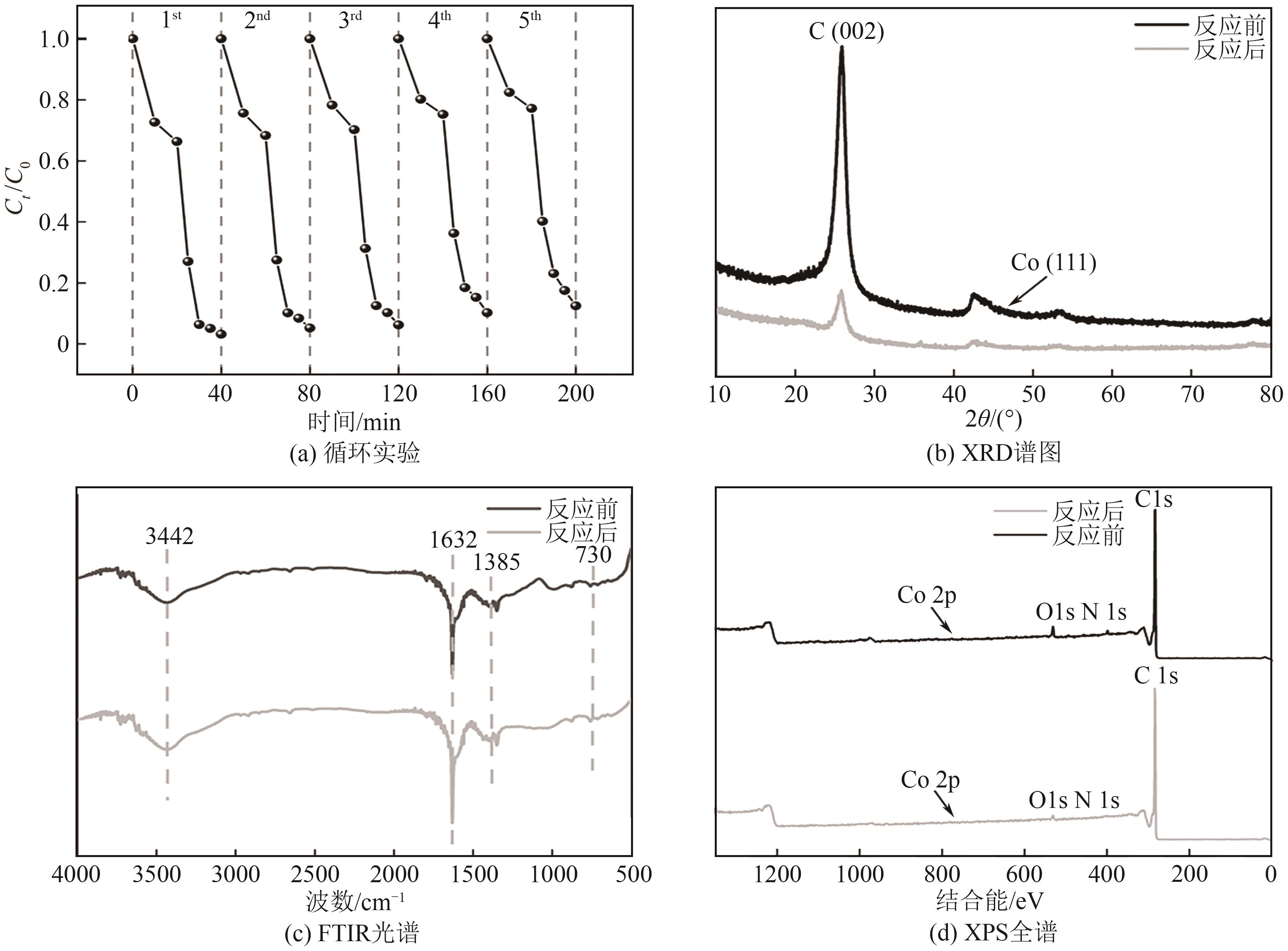
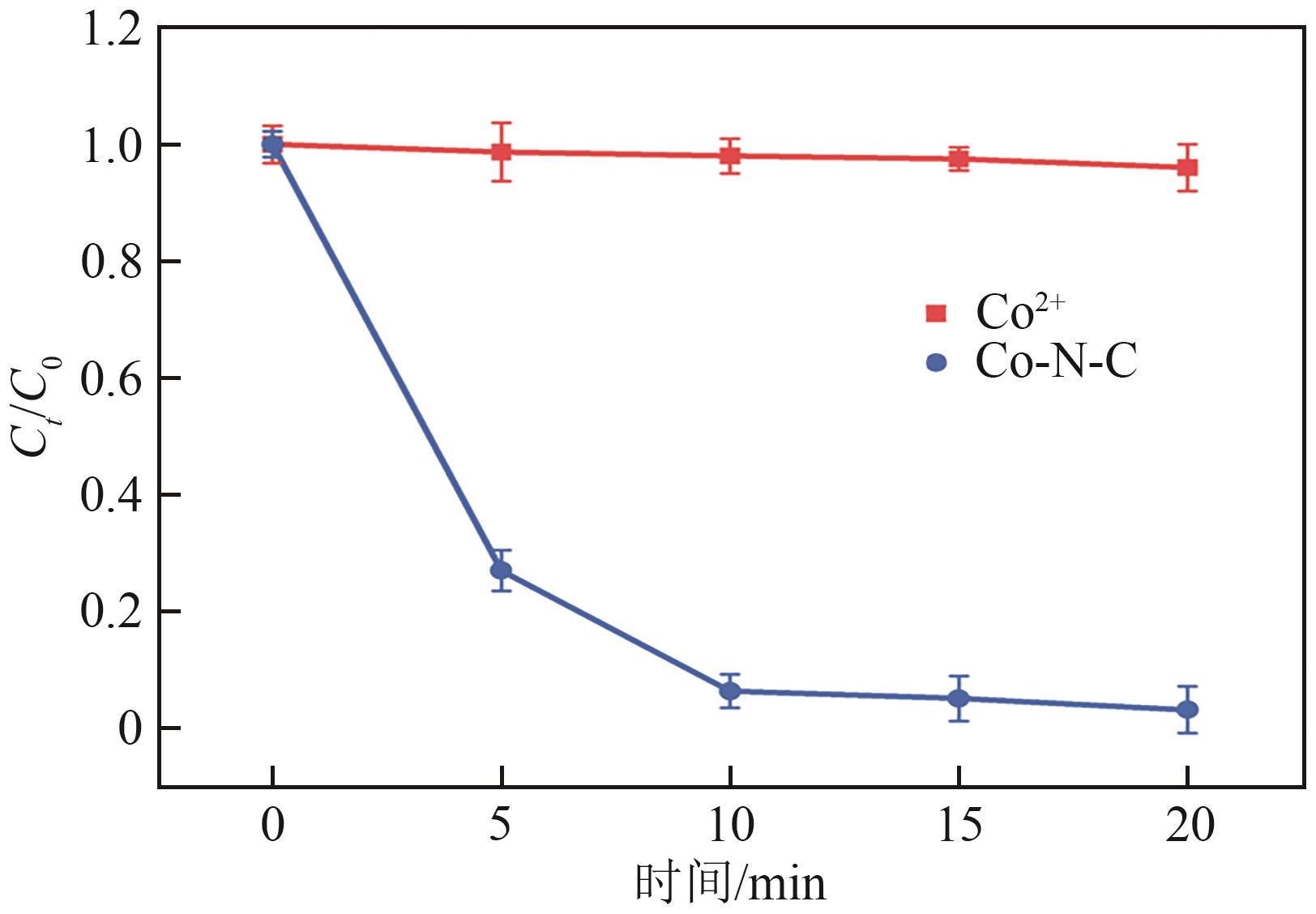
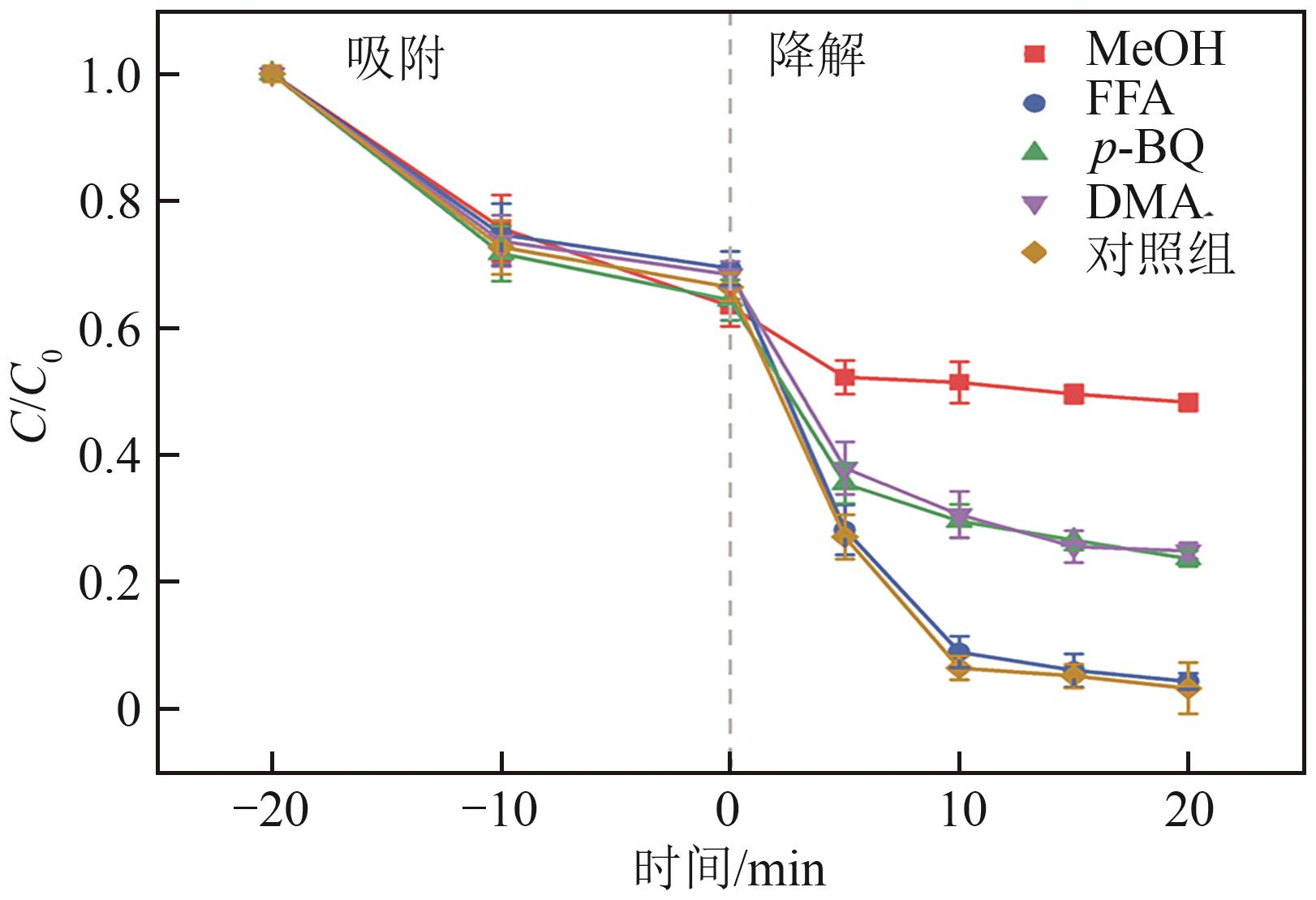
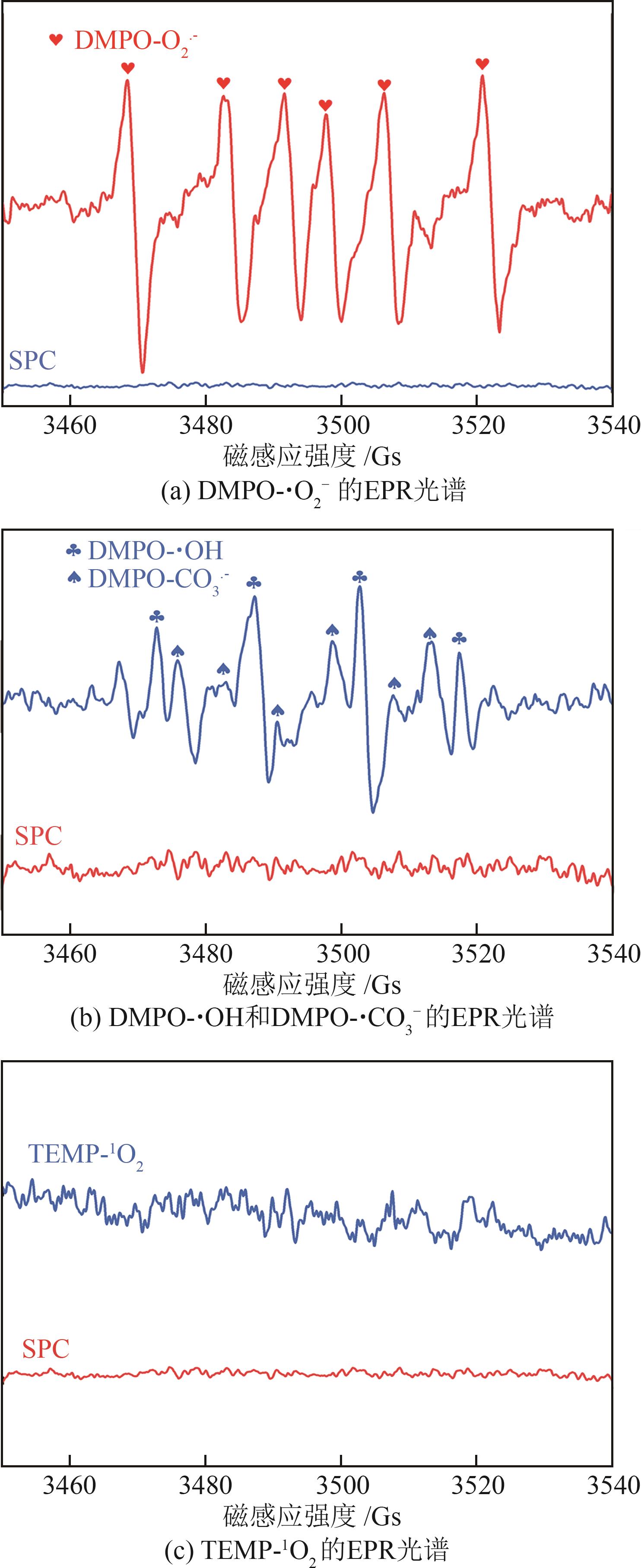
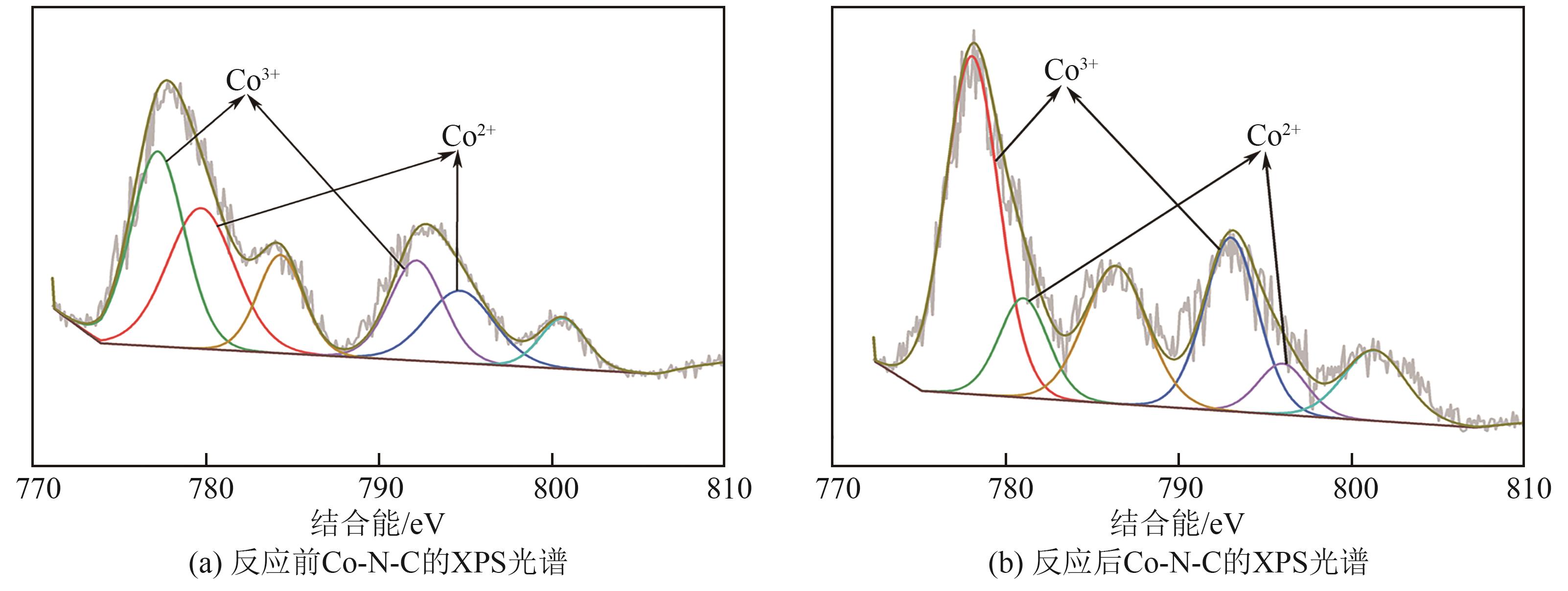
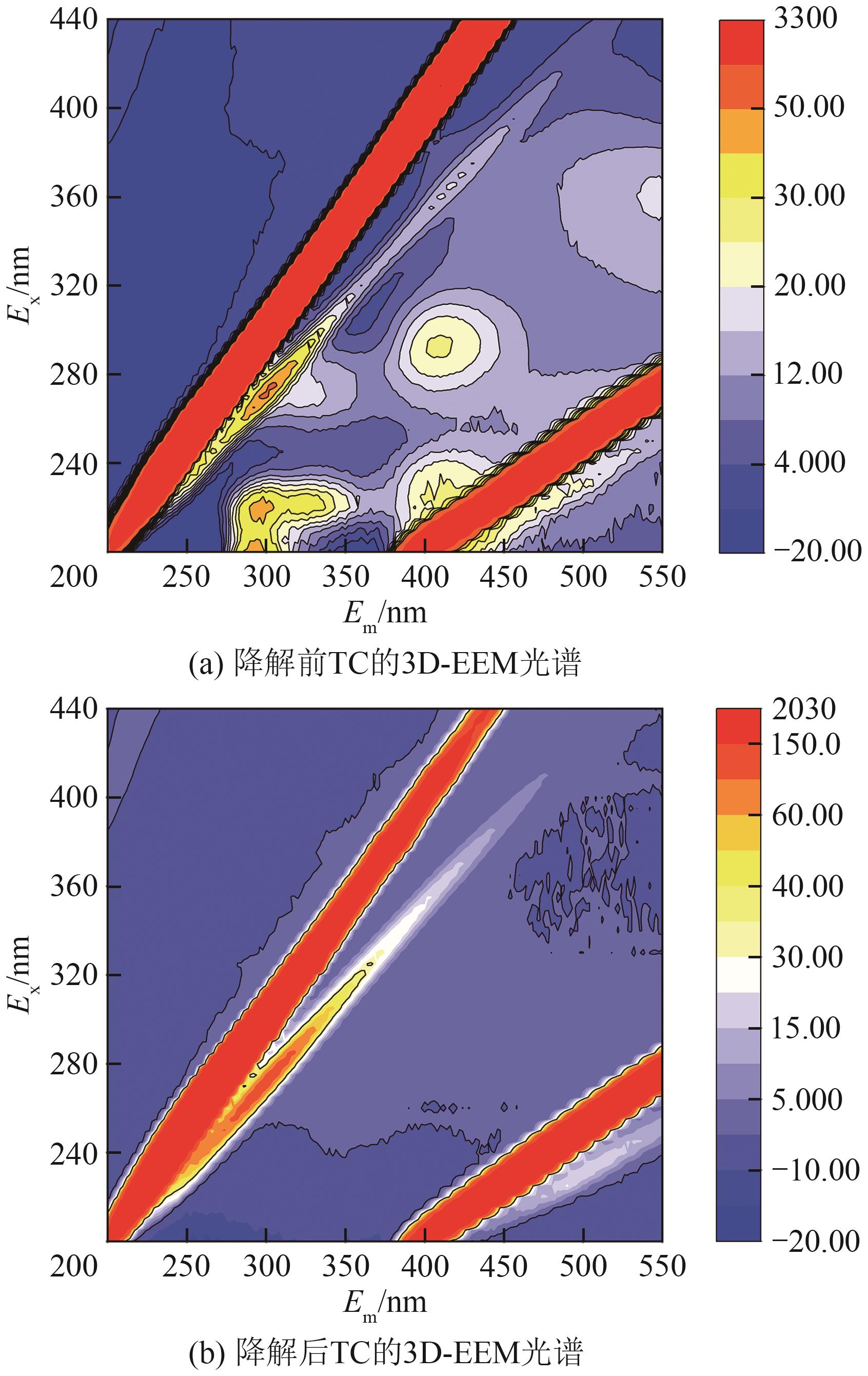
过碳酸钠(SPC,Na2CO3·1.5H2O2)作为固体过氧化氢,近年来受到越来越多的关注。在此,通过简单的方法制备了具有丰富活性位点的纳米管状钴氮碳催化剂(Co-N-C),并用于活化SPC以有效去除四环素(TC)。通过透射电子显微镜(TEM)、扫描电子显微镜(SEM)对材料形貌进行表征。通过X射线粉末衍射仪(XRD)、傅里叶变换红外光谱仪(FTIR)和X射线光电子能谱(XPS)分析元素分布和价态变化。表征结果表明合成了具有丰富活性位点的纳米管状Co-N-C。在TC浓度10mg/L,SPC浓度为2mmol/L,Co-N-C含量为0.2g/L,pH为7的条件下,在20min内几乎实现了TC的100%降解。自由基淬灭实验和EPR测试表明Co-N-C/SPC系统中的 ·OH、·CO3-和·O2-为TC降解的主要活性物质。反应前后钴元素价态变化以及Co2+/Co3+循环过程促进了SPC的活化,三维荧光光谱分析表明TC在Co-N-C/SPC体系中被降解。
中图分类号:
引用本文
石秀顶, 王永全, 曾静, 苏畅, 洪俊明. 纳米管状Co-N-C活化过碳酸盐降解四环素[J]. 化工进展, 2025, 44(6): 3041-3052.
SHI Xiuding, WANG Yongquan, ZENG Jing, SU Chang, HONG Junming. Nanotubular Co-N-C activated percarbonate for tetracycline degradation[J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3041-3052.
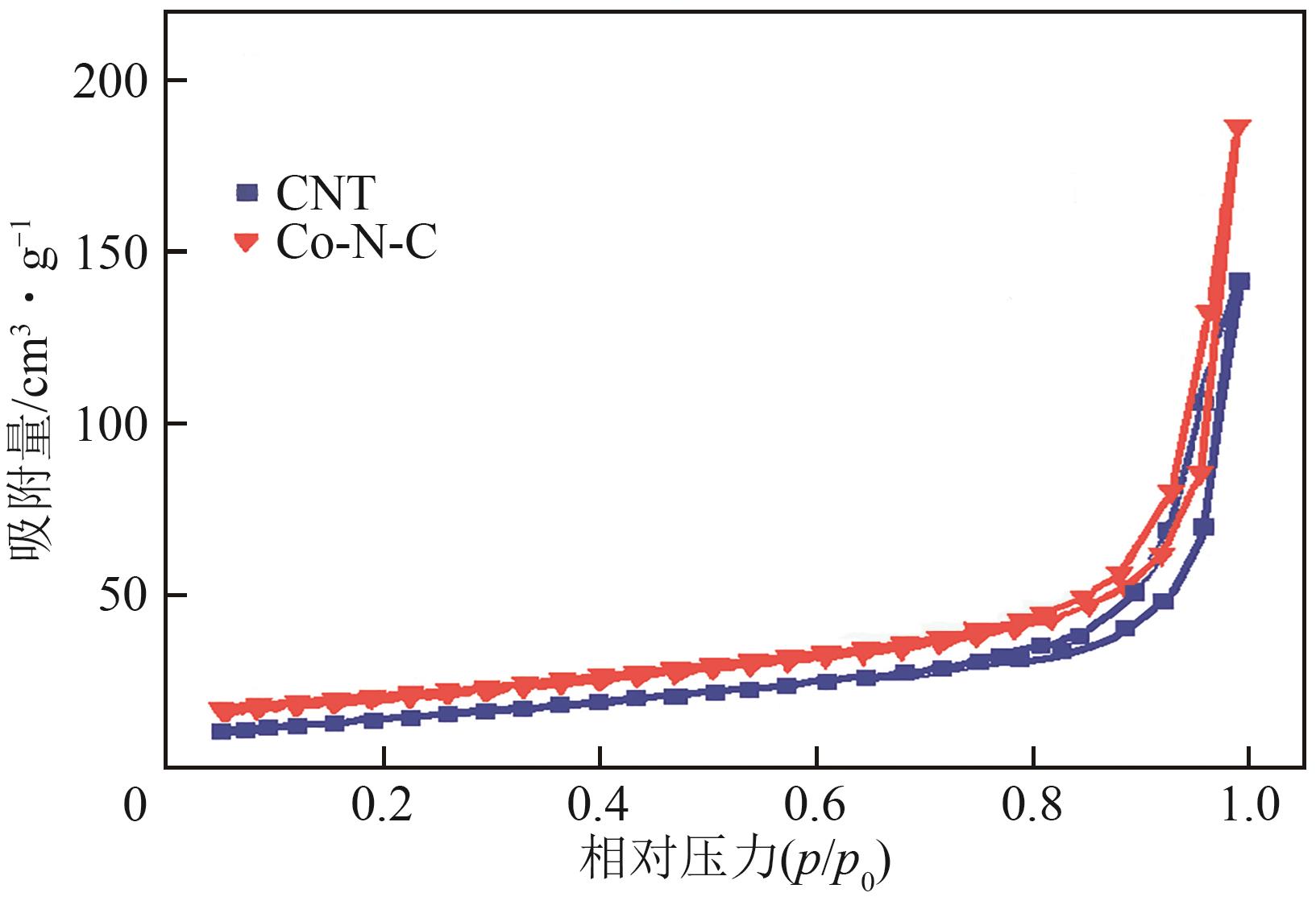
| 样品 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| CNT | 50.14 | 0.13 | 5.50 |
| Co-N-C | 68.52 | 0.18 | 5.35 |
表1 不同样品的相对比表面积、平均孔容、平均孔径
| 样品 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| CNT | 50.14 | 0.13 | 5.50 |
| Co-N-C | 68.52 | 0.18 | 5.35 |
| 催化剂 | 污染物 | 氧化剂 | 实验条件 | 反应时间/min | 去除率/% | 参考文献 |
|---|---|---|---|---|---|---|
| Fe3O4@α-MnO2 | RBK5 | PMS | Cat.=1.2g/L;PMS=4mmol/L;pH=7;RBK5=30mg/L | 60 | 91 | [ |
| Mn2O3@α-Fe3O4 | RBK5 | SPC | Cat.=0.3g/L;SPC=1mmol/L;pH=3;RBK5=10mg/L | 90 | 88 | [ |
| FeS2 | TC | SPC | Cat.=0.5g/L;SPC=8mmol/L;pH=5;TC=10mg/L | 60 | 78.86 | [ |
| Cu3(MoO4)2(OH)2 | OTC | SPC | Cat.=0.25g/L;SPC=0.2g/L;pH=3;TC=20mg/L | 40 | 80.01 | [ |
| CuCoFe-LDHs | CIP | SPC | Cat.=0.5g/L;SPC=0.3mmol/L;pH=5;CIP=20mg/L | 60 | 66 | [ |
| Co-N-C | TC | SPC | Cat.=0.2g/L;SPC=0.2mmol/L;pH=7;TC=10mg/L | 20 | 99.7 | 本文 |
表2 不同体系下污染物的降解效果
| 催化剂 | 污染物 | 氧化剂 | 实验条件 | 反应时间/min | 去除率/% | 参考文献 |
|---|---|---|---|---|---|---|
| Fe3O4@α-MnO2 | RBK5 | PMS | Cat.=1.2g/L;PMS=4mmol/L;pH=7;RBK5=30mg/L | 60 | 91 | [ |
| Mn2O3@α-Fe3O4 | RBK5 | SPC | Cat.=0.3g/L;SPC=1mmol/L;pH=3;RBK5=10mg/L | 90 | 88 | [ |
| FeS2 | TC | SPC | Cat.=0.5g/L;SPC=8mmol/L;pH=5;TC=10mg/L | 60 | 78.86 | [ |
| Cu3(MoO4)2(OH)2 | OTC | SPC | Cat.=0.25g/L;SPC=0.2g/L;pH=3;TC=20mg/L | 40 | 80.01 | [ |
| CuCoFe-LDHs | CIP | SPC | Cat.=0.5g/L;SPC=0.3mmol/L;pH=5;CIP=20mg/L | 60 | 66 | [ |
| Co-N-C | TC | SPC | Cat.=0.2g/L;SPC=0.2mmol/L;pH=7;TC=10mg/L | 20 | 99.7 | 本文 |
| pH | k/min-1 | R2 |
|---|---|---|
| 3 | 0.236 | 0.976 |
| 5 | 0.214 | 0.972 |
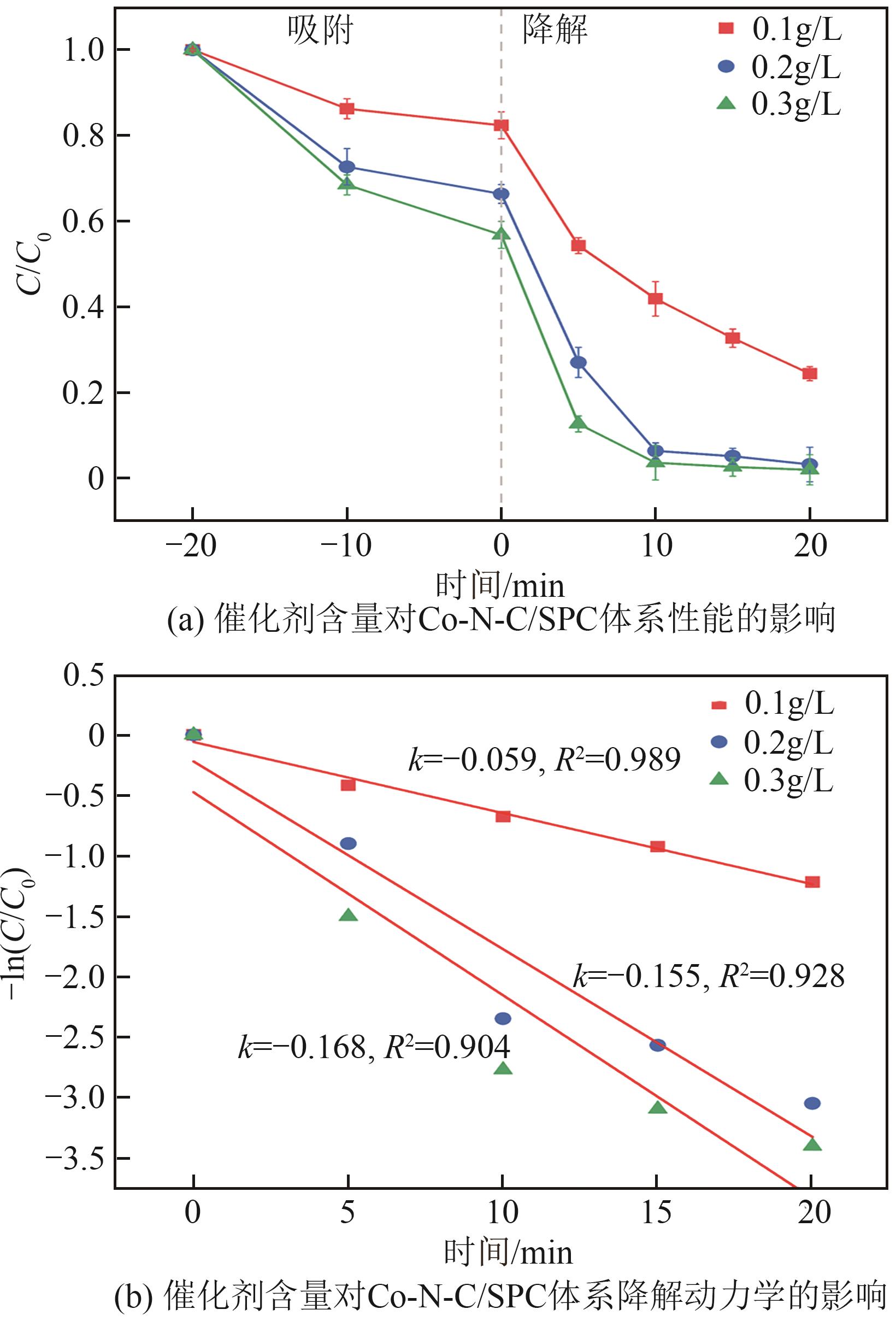
| 7 | 0.155 | 0.928 |
| 9 | 0.132 | 0.926 |
| 11 | 0.018 | 0.865 |
表3 不同pH下Co-N-C/SPC体系的降解动力学参数
| pH | k/min-1 | R2 |
|---|---|---|
| 3 | 0.236 | 0.976 |
| 5 | 0.214 | 0.972 |
| 7 | 0.155 | 0.928 |
| 9 | 0.132 | 0.926 |
| 11 | 0.018 | 0.865 |
| 时间/min | Co2+浸出/mg·L-1 |
|---|---|
| 3 | 0.046 |
| 5 | 0.078 |
| 10 | 0.083 |
| 15 | 0.097 |
| 20 | 0.103 |
表4 钴离子的浸出量
| 时间/min | Co2+浸出/mg·L-1 |
|---|---|
| 3 | 0.046 |
| 5 | 0.078 |
| 10 | 0.083 |
| 15 | 0.097 |
| 20 | 0.103 |
| [1] | LIN Zhong, CHEN Yijie, LI Gaoyang, et al. Change of tetracycline speciation and its impacts on tetracycline removal efficiency in vermicomposting with epigeic and endogeic earthworms[J]. Science of the Total Environment, 2023, 881: 163410. |
| [2] | YU Kan, LI Xiaoyong, QIU Yushu, et al. Low-dose effects on thyroid disruption in zebrafish by long-term exposure to oxytetracycline[J]. Aquatic Toxicology, 2020, 227: 105608. |
| [3] | HAN Chee-Hun, PARK Hee-Deung, KIM Song-Bae, et al. Oxidation of tetracycline and oxytetracycline for the photo-Fenton process: Their transformation products and toxicity assessment[J]. Water Research, 2020, 172: 115514. |
| [4] | HAN Q F, ZHAO S, ZHANG X R, et al. Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea, North China[J]. Environment International, 2020, 138: 105551. |
| [5] | LIU Xiaohui, LIU Ying, LU Shaoyong, et al. Occurrence of typical antibiotics and source analysis based on PCA-MLR model in the East Dongting Lake, China[J]. Ecotoxicology and Environmental Safety, 2018, 163: 145-152. |
| [6] | YU Xiaolong, JIN Xu, LI Meng, et al. Mechanism and security of UV driven sodium percarbonate for sulfamethoxazole degradation using DFT and metabolomic analysis[J]. Environmental Pollution, 2023, 323: 121352. |
| [7] | QI Juanjuan, LIU Juzhe, SUN Fengbin, et al. High active amorphous Co(OH)2 nanocages as peroxymonosulfate activator for boosting acetaminophen degradation and DFT calculation[J]. Chinese Chemical Letters, 2021, 32(5): 1814-1818. |
| [8] | CHEN Haoyun, YUAN Xingzhong, JIANG Longbo, et al. Intramolecular modulation of iron-based metal organic framework with energy level adjusting for efficient photocatalytic activity[J]. Applied Catalysis B: Environmental, 2022, 302: 120823. |
| [9] | Kwasi KYERE-YEBOAH, QIAO Xiuchen. Non-thermal plasma activated peroxide and percarbonate for tetracycline and oxytetracycline degradation: Synergistic performance, degradation pathways, and toxicity evaluation[J]. Chemosphere, 2023, 336: 139246. |
| [10] | XU Ximeng, ZHOU Yuhui, LI Shasha, et al. Activation of sodium percarbonate with Fe3O4@MXene composite for the efficient removal of bisphenol A[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108702. |
| [11] | MOHAMMADI Samira, MOUSSAVI Gholamreza, YAGHMAEIAN Kamyar, et al. Development of a percarbonate-enhanced vacuum UV process for simultaneous fluoroquinolone antibiotics removal and fecal bacteria inactivation under a continuous flow mode of operation[J]. Chemical Engineering Journal, 2022, 431: 134064. |
| [12] | LI Yangju, DONG Haoran, LI Long, et al. Efficient degradation of sulfamethazine via activation of percarbonate by chalcopyrite[J]. Water Research, 2021, 202: 117451. |
| [13] | LIAO Gaozu, QING Xiaojiao, XU Peng, et al. Synthesis of single atom cobalt dispersed on 2D carbon nanoplate and degradation of acetaminophen by peroxymonosulfate activation[J]. Chemical Engineering Journal, 2022, 427: 132027. |
| [14] | 张廷, 曾静, 叶校圳, 等. 纳米立方体PBA-Fe1Mn2活化过一硫酸盐降解偶氮有机物[J]. 中国环境科学, 2023, 43(7): 3533-3544. |
| ZHANG Ting, ZENG Jing, YE Xiaozhen, et al. Nanoscale PBA-Fe1Mn2 activated peroxymonosulfate for degradation of azo organic[J]. China Environmental Science, 2023, 43(7): 3533-3544. | |
| [15] | CHEN Shaona, LIANG Yanhua, LI Bo, et al. Facile synthesis of graphene oxide-supported CoO x nanoparticles for efficient degradation of antibiotics via percarbonate activation: Performance, degradation pathway and mechanism[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 675: 131996. |
| [16] | YANG Zhao, WANG Zhaowei, LIANG Guiwei, et al. Catalyst bridging-mediated electron transfer for nonradical degradation of bisphenol A via natural manganese ore-cornstalk biochar composite activated peroxymonosulfate[J]. Chemical Engineering Journal, 2021, 426: 131777. |
| [17] | XU Baokang, ZHANG Xiao, ZHANG Yue, et al. Enhanced electron transfer-based nonradical activation of peroxymonosulfate by CoN x sites anchored on carbon nitride nanotubes for the removal of organic pollutants[J]. Chemical Engineering Journal, 2023, 466: 143155. |
| [18] | XU Haodan, JIANG Ning, WANG Da, et al. Improving PMS oxidation of organic pollutants by single cobalt atom catalyst through hybrid radical and non-radical pathways[J]. Applied Catalysis B: Environmental, 2020, 263: 118350. |
| [19] | GUO Xu, ZHANG Qicheng, HE Hongwei, et al. Wastewater flocculation substrate derived three-dimensional ordered macroporous Co single-atom catalyst for singlet oxygen-dominated peroxymonosulfate activation[J]. Applied Catalysis B: Environmental, 2023, 335: 122886. |
| [20] | XU Zhenyang, ZHANG Yimei, WANG Fei, et al. Co single-atom confined in N-doped hollow carbon sphere with superb stability for rapid degradation of organic pollutants[J]. Chemical Engineering Journal, 2023, 452: 139229. |
| [21] | 董正玉, 吴丽颖, 王霁, 等. 新型Fe3O4@α-MnO2活化过一硫酸盐降解水中偶氮染料[J]. 中国环境科学, 2018, 38(8): 3003-3010. |
| DONG Zhengyu, WU Liying, WANG Ji, et al. Novel Fe3O4@α-MnO2 activated peroxymonosulfate degradation of azo dyes in aqueous solution[J]. China Environmental Science, 2018, 38(8): 3003-3010. | |
| [22] | 徐铭骏, 郭兆春, 李立, 等. 纳米片状Mn2O3@α-Fe3O4活化过碳酸盐降解偶氮染料[J]. 化工进展, 2022, 41(2): 1043-1053. |
| XU Mingjun, GUO Zhaochun, LI Li, et al. Degradation of azo dyes by sodium percarbonate activated with nanosheet Mn2O3@α-Fe3O4 [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 1043-1053. | |
| [23] | GUO Liu, NIE Ziqiu, WEN Lijia, et al. Insights into the effects of natural pyrite-activated sodium percarbonate on tetracycline removal from groundwater: Mechanism, pathways, and column studies[J]. Science of the Total Environment, 2023, 902: 165883. |
| [24] | JIN Xiaotao, WANG Yanlan, HUANG Yingping, et al. Percarbonate activation catalyzed by nanoblocks of basic copper molybdate for antibiotics degradation: High performance, degradation pathways and mechanism[J]. Chinese Chemical Letters, 2024, 35(10): 109499. |
| [25] | CHEN Kang, LI Ting, ZHANG Xue, et al. CuCoFe-LDHs activated sodium percarbonate (SPC) for the degradation of ciprofloxacin[J]. Journal of Environmental Chemical Engineering, 2023, 11(5): 110651. |
| [26] | WU Liying, ZHANG Qian, HONG Junming, et al. Degradation of bisphenol A by persulfate activation via oxygen vacancy-rich CoFe2O4- x [J]. Chemosphere, 2019, 221: 412-422. |
| [27] | DUAN Xiaoguang, SU Chao, ZHOU Li, et al. Surface controlled generation of reactive radicals from persulfate by carbocatalysis on nanodiamonds[J]. Applied Catalysis B: Environmental, 2016, 194: 7-15. |
| [28] | LIN Kun-Yi Andrew, LIN Jyun-Ting, LIN Yifeng. Heterogeneous catalytic activation of percarbonate by ferrocene for degradation of toxic amaranth dye in water[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 78: 144-149. |
| [29] | BAI Rui, YAN Weifu, XIAO Yong, et al. Acceleration of peroxymonosulfate decomposition by a magnetic MoS2/CuFe2O4 heterogeneous catalyst for rapid degradation of fluoxetine[J]. Chemical Engineering Journal, 2020, 397: 125501. |
| [30] | SHAH Noor S, KHAN Javed Ali, SAYED Murtaza, et al. Hydroxyl and sulfate radical mediated degradation of ciprofloxacin using nano zerovalent manganese catalyzed S2O8 2- [J]. Chemical Engineering Journal, 2019, 356: 199-209. |
| [31] | WANG Jianlong, WANG Shizong. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants[J]. Chemical Engineering Journal, 2021, 411: 128392. |
| [32] | 廖兵, 胥雯, 叶秋月. 活化过碳酸盐及过氧碳酸氢盐在水处理领域中的研究进展[J]. 化工进展, 2022, 41(6): 3235-3248. |
| LIAO Bing, XU Wen, YE Qiuyue. A review of activated percarbonate and peroxymonocarbonate in the field of water treatment[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3235-3248 | |
| [33] | MO Zhihua, TAN Zexing, LIANG Jialin, et al. Iron-rich sludge biochar triggers sodium percarbonate activation for robust sulfamethoxazole removal: Collaborative roles of reactive oxygen species and electron transfer[J]. Chemical Engineering Journal, 2023, 457: 141150. |
| [34] | LI Li, GUO Ruoning, ZHANG Sai, et al. Sustainable and effective degradation of aniline by sodium percarbonate activated with UV in aqueous solution: Kinetics, mechanism and identification of reactive species[J]. Environmental Research, 2022, 207: 112176. |
| [35] | ASIF Abdul Hannan, RAFIQUE Nasir, HIRANI Rajan Arjan Kalyan, et al. Heterogeneous activation of peroxymonosulfate by Co-doped Fe2O3 nanospheres for degradation of p-hydroxybenzoic acid[J]. Journal of Colloid and Interface Science, 2021, 604: 390-401. |
| [36] | SHE Yuecheng, WEI Wenxuan, AI Xiaohuan, et al. Synergistic pretreatment of CaO and freezing/thawing to enhance volatile fatty acids recycling and dewaterability of waste activated sludge via anaerobic fermentation[J]. Chemosphere, 2021, 280: 130939. |
| [1] | 宋祎祺, 李雪, 叶茂, 刘中民. 基于格子Boltzmann方法的吸热反应双颗粒沉降模拟[J]. 化工进展, 2025, 44(5): 2984-2996. |
| [2] | 戴月明, 周梅芳, 沈建华, 姜海波, 李春忠. TiO2纳米颗粒烧结机制分子动力学模拟[J]. 化工进展, 2025, 44(4): 2202-2214. |
| [3] | 王沛淦, 李乐利, 谢颂专, 宋冰冰, 孔巧平, 刘改革, 麻微微, 施雪卿. 污泥基FeCa-ALE复合材料对磷酸盐的吸附机制[J]. 化工进展, 2025, 44(4): 2365-2373. |
| [4] | 金志浩, 王云帆, 田振玉. 火箭发动机尾焰复燃反应动力学分析策略[J]. 化工进展, 2025, 44(3): 1776-1780. |
| [5] | 冯鹏, 徐东海, 何冰, 刘欢腾, 杨立杰, 王攀, 刘青山. 亚/超临界水中典型硫酸盐Na2SO4和K2SO4的溶解特性及机理[J]. 化工进展, 2025, 44(3): 1706-1715. |
| [6] | 左骥, 罗莉, 谢永锴, 陈文尧, 钱刚, 周兴贵, 段学志. 甲醇无氧脱氢制甲醛Cu催化剂的粒径效应[J]. 化工进展, 2025, 44(3): 1347-1354. |
| [7] | 白依冉, 翟玉玲, 戴晶慧, 李舟航. 微纳尺度池沸腾表面润湿性的气泡成核及强化传热机制[J]. 化工进展, 2025, 44(2): 743-751. |
| [8] | 赵鹬, 石翎, 张栋强, 李宁. 沉淀法合成氧化镁吸附剂及其对氟化物的吸附机理[J]. 化工进展, 2025, 44(2): 971-981. |
| [9] | 林梅, 雷雨, 李萍, 张强. 石墨烯/橡胶复合改性沥青-集料界面黏附性能及机理[J]. 化工进展, 2025, 44(2): 991-1002. |
| [10] | 李琢宇, 余美琪, 陈孝彦, 胡若晖, 王庆宏, 陈春茂, 詹亚力. 炼油废催化剂吸附去除水中硝基苯的特性与机制[J]. 化工进展, 2025, 44(2): 1076-1087. |
| [11] | 赵丽阳, 李倩, 何佩熹, 潘鸿辉, 刘艳, 刘细祥. 磷钼酸-Fe3O4球磨共改性污泥基生物炭对四环素的吸附特性[J]. 化工进展, 2025, 44(1): 583-595. |
| [12] | 张强, 孙楠, 郑俊杰, 吴强, 刘传海, 李元吉. 混合热力学促进剂对水合物法分离回收瓦斯的影响[J]. 化工进展, 2025, 44(1): 192-201. |
| [13] | 游小银, 汪楚乔, 刘才华, 彭小明. Z型CN/NGBO/BV催化剂体系的构筑及光类芬顿降解四环素性能[J]. 化工进展, 2025, 44(1): 286-296. |
| [14] | 李依梦, 陈运全, 何畅, 张冰剑, 陈清林. 基于物理信息神经网络的甲烷无氧芳构化反应的正反问题[J]. 化工进展, 2024, 43(9): 4817-4823. |
| [15] | 何海霞, 万亚萌, 李帆帆, 牛心雨, 张静雯, 李涛, 任保增. 盐酸萘甲唑啉在甲醇-乙酸乙酯体系中的动力学及结晶工艺[J]. 化工进展, 2024, 43(8): 4230-4245. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||