化工进展 ›› 2025, Vol. 44 ›› Issue (1): 583-595.DOI: 10.16085/j.issn.1000-6613.2024-0062
磷钼酸-Fe3O4球磨共改性污泥基生物炭对四环素的吸附特性
赵丽阳1( ), 李倩1, 何佩熹1, 潘鸿辉1,2(
), 李倩1, 何佩熹1, 潘鸿辉1,2( ), 刘艳1, 刘细祥1,2(
), 刘艳1, 刘细祥1,2( )
)
- 1.广西民族大学材料与环境学院,广西先进结构材料与碳中和重点实验室,广西高校环境友好材料及碳中和 新技术重点实验室,土壤与地下水环境研究中心,广西 南宁 530006
2.广西化工研究院有限公司,广西 南宁 530001
-
收稿日期:2024-01-09修回日期:2024-03-04出版日期:2025-01-15发布日期:2025-02-13 -
通讯作者:潘鸿辉,刘细祥 -
作者简介:赵丽阳(1996—),女,硕士研究生,研究方向为污泥生物炭制备及应用。E-mail: 240946929@qq.com。 -
基金资助:国家自然科学基金(41967030);广西科技基地和人才专项(桂科AD23026173);广西自然科学基金(2020GXNSFAA159170);广西民族大学相思湖青年学者创新团队(2022);广西民族大学人才启动项目(2022KJQD29);大学生创新创业训练(202310608026)
Tetracycline adsorption properties of sludge-based biochar ball-milled co-modified by phosphomolybdic acid-Fe3O4
ZHAO Liyang1( ), LI Qian1, HE Peixi1, PAN Honghui1,2(
), LI Qian1, HE Peixi1, PAN Honghui1,2( ), LIU Yan1, LIU Xixiang1,2(
), LIU Yan1, LIU Xixiang1,2( )
)
- 1.Research Center for Soil and Groundwater Environment, Guangxi Colleges and Universities Key Laboratory of Environmental-friendly Materials and New Technology for Carbon Neutralization, Guangxi Key Laboratory of Advanced Structural Materials and Carbon Neutralization, School of Materials and Environment, Guangxi Minzu University, Nanning 530006, Guangxi, China
2.Guangxi Research Institute of Chemical Industry Co. , Ltd. , Nanning 530001, Guangxi, China
-
Received:2024-01-09Revised:2024-03-04Online:2025-01-15Published:2025-02-13 -
Contact:PAN Honghui, LIU Xixiang
摘要:
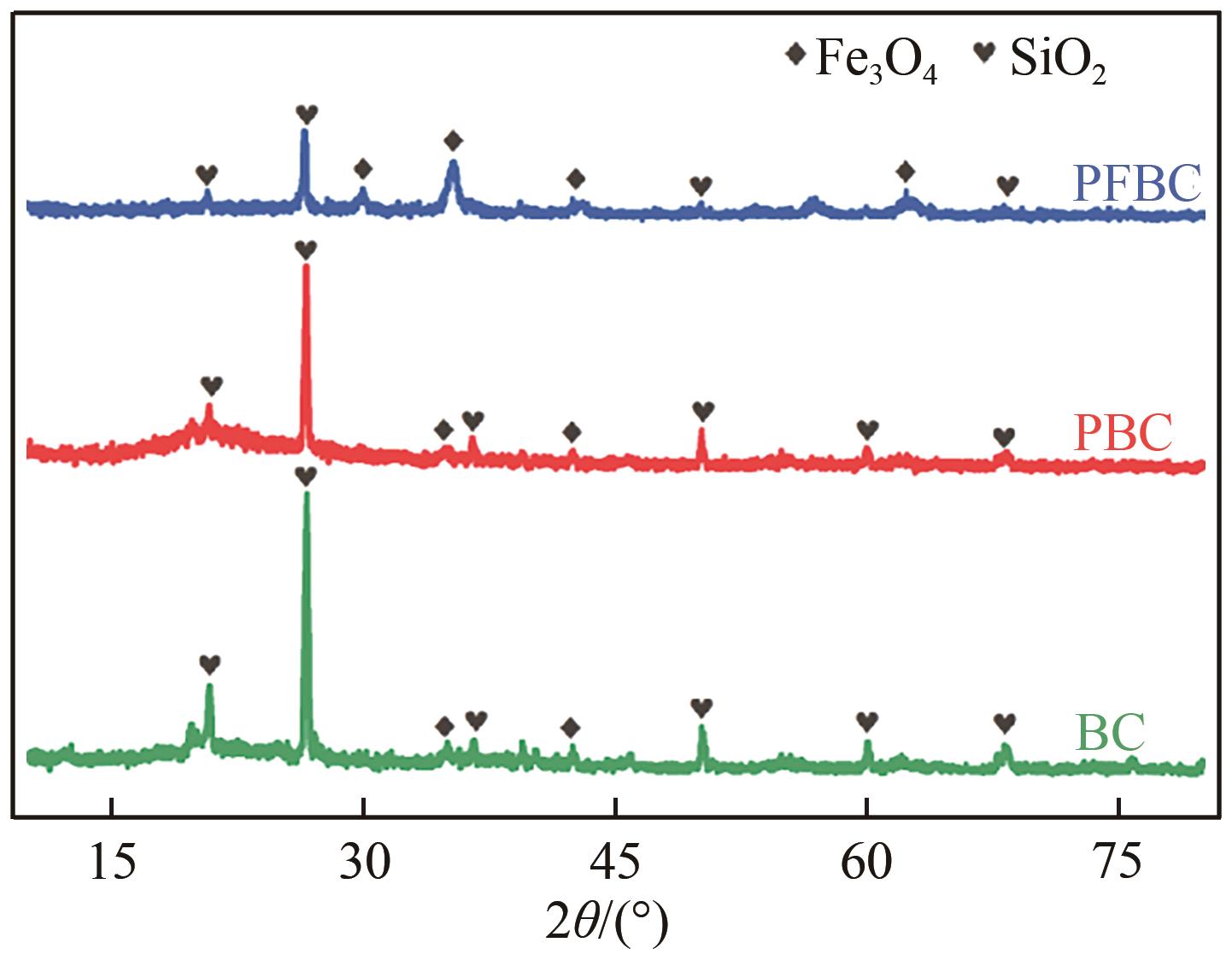
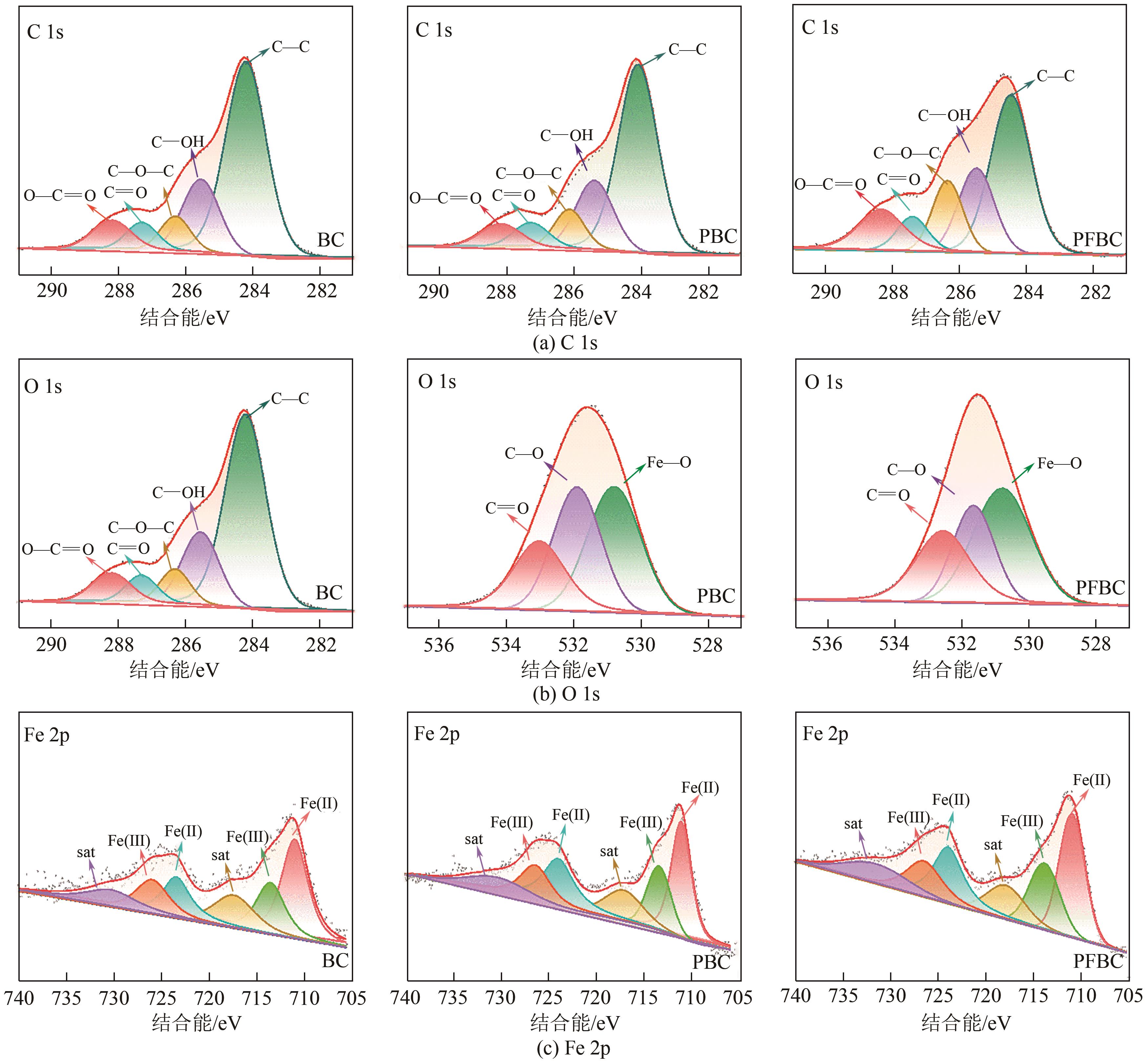
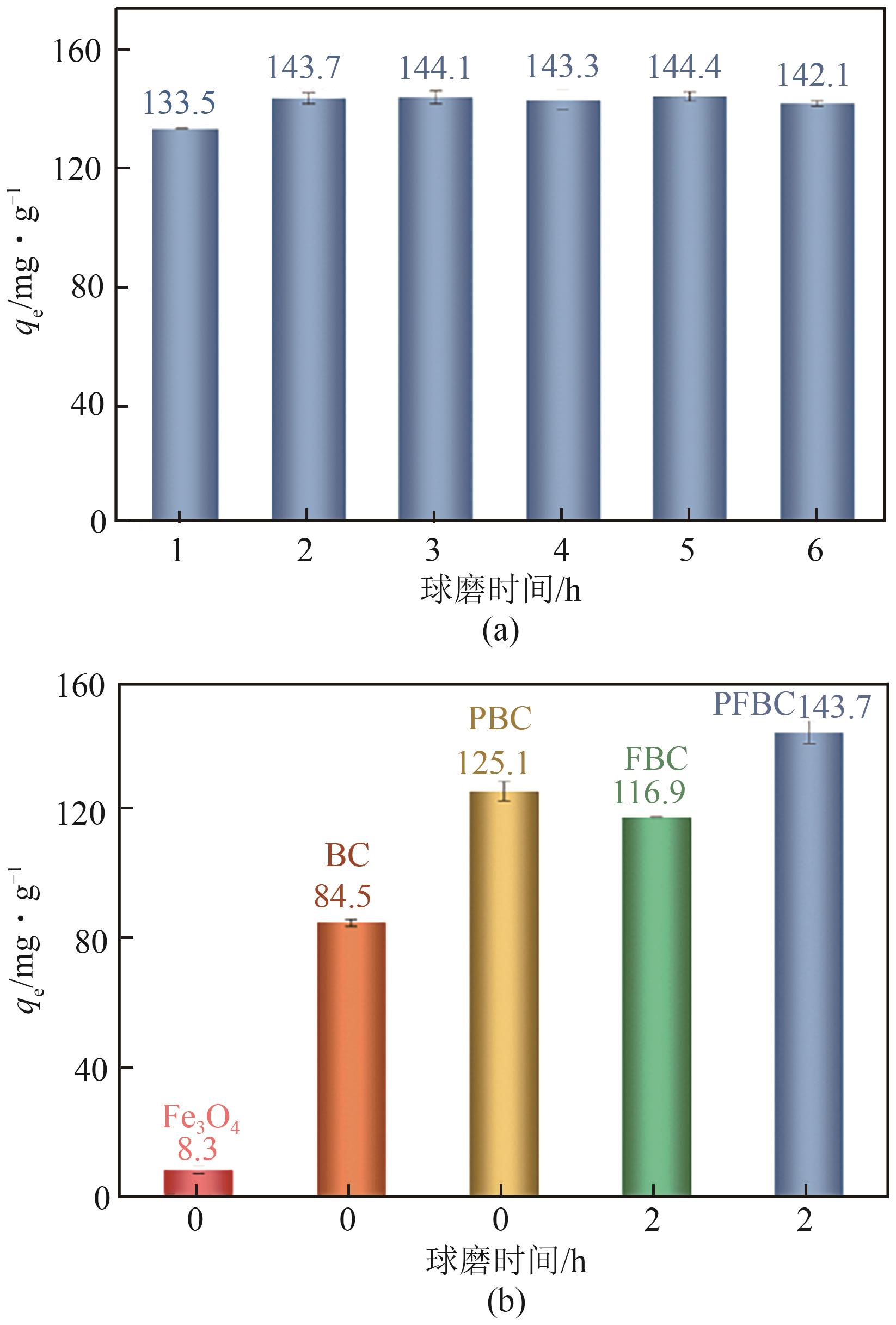
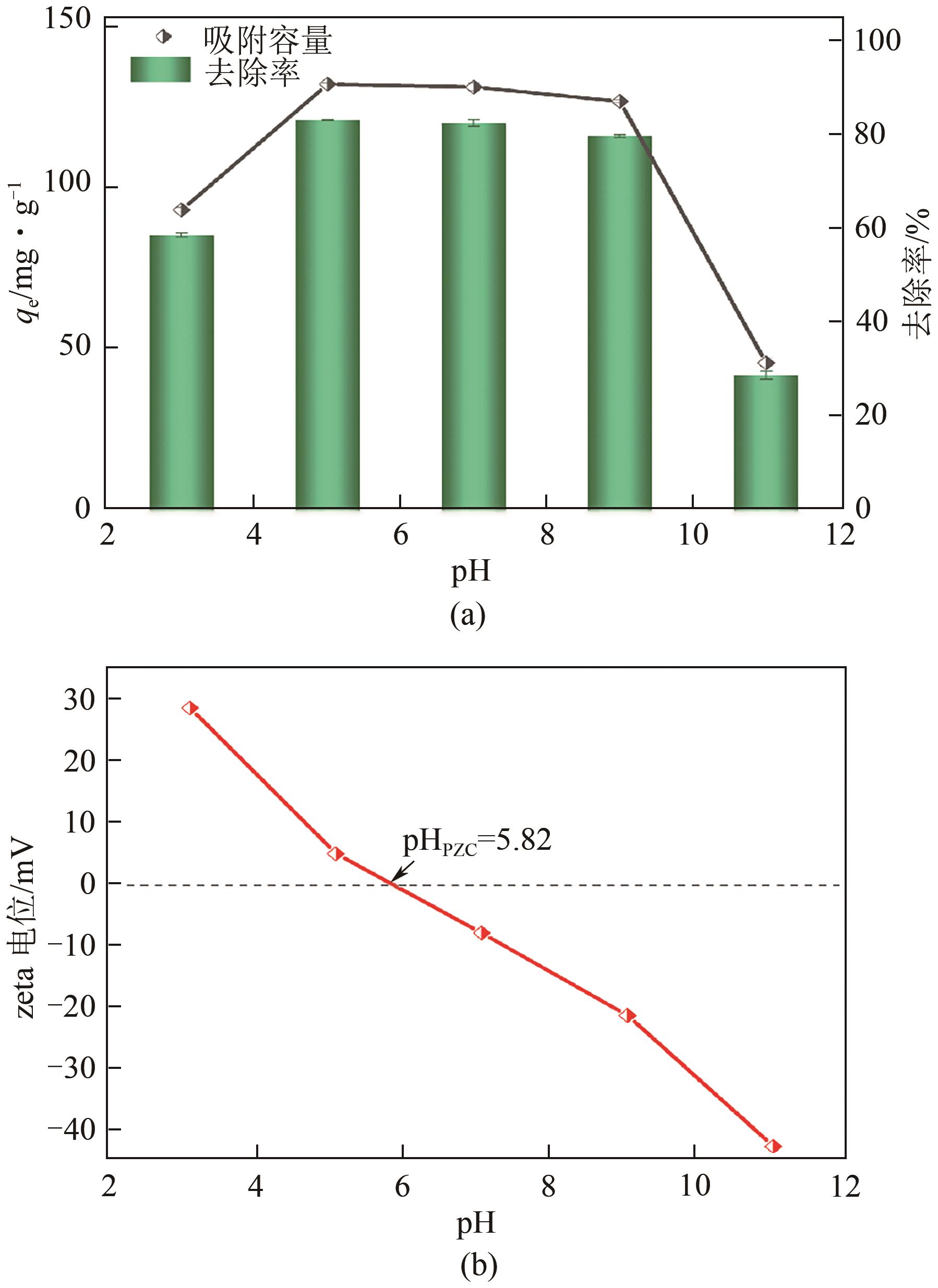
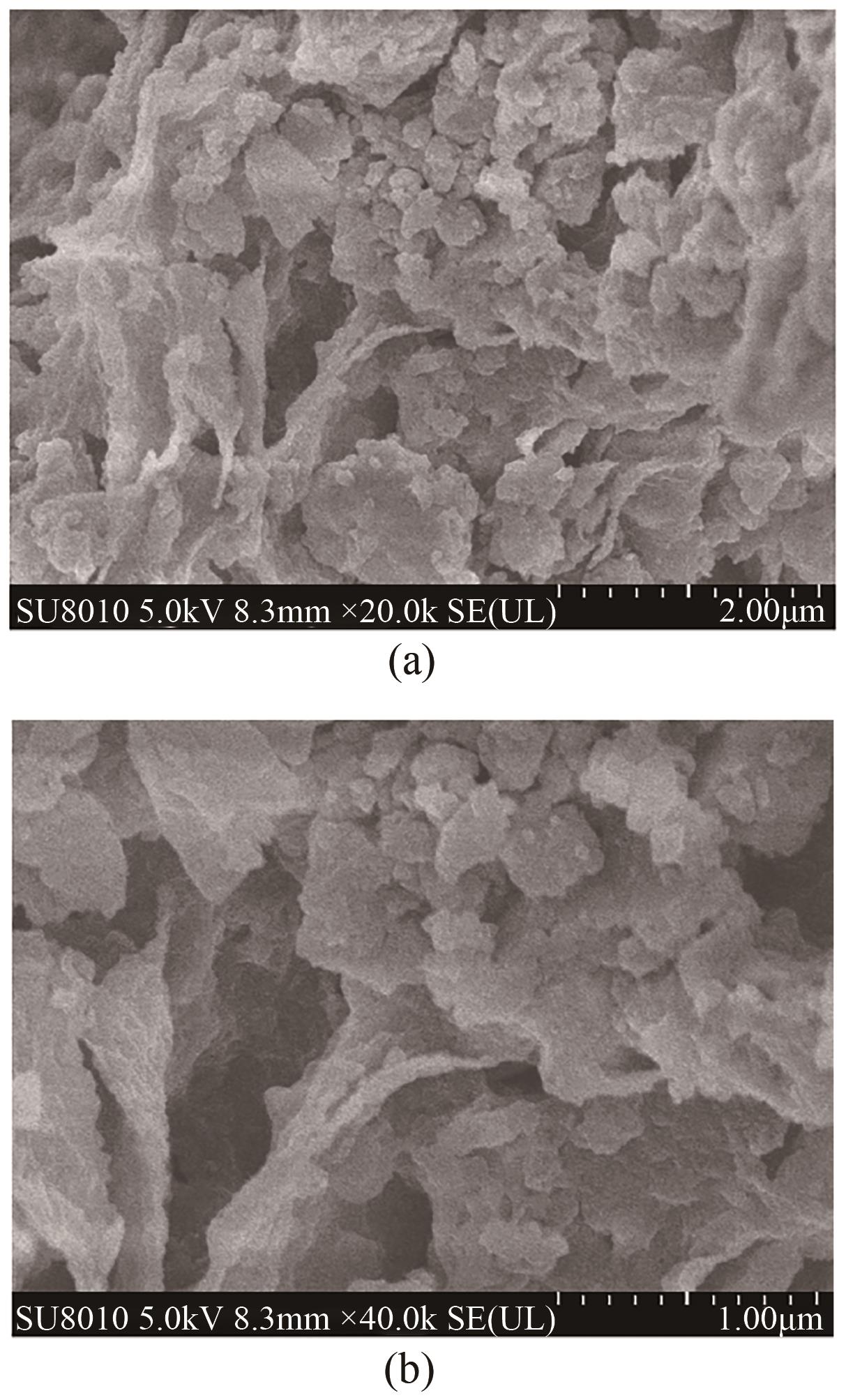
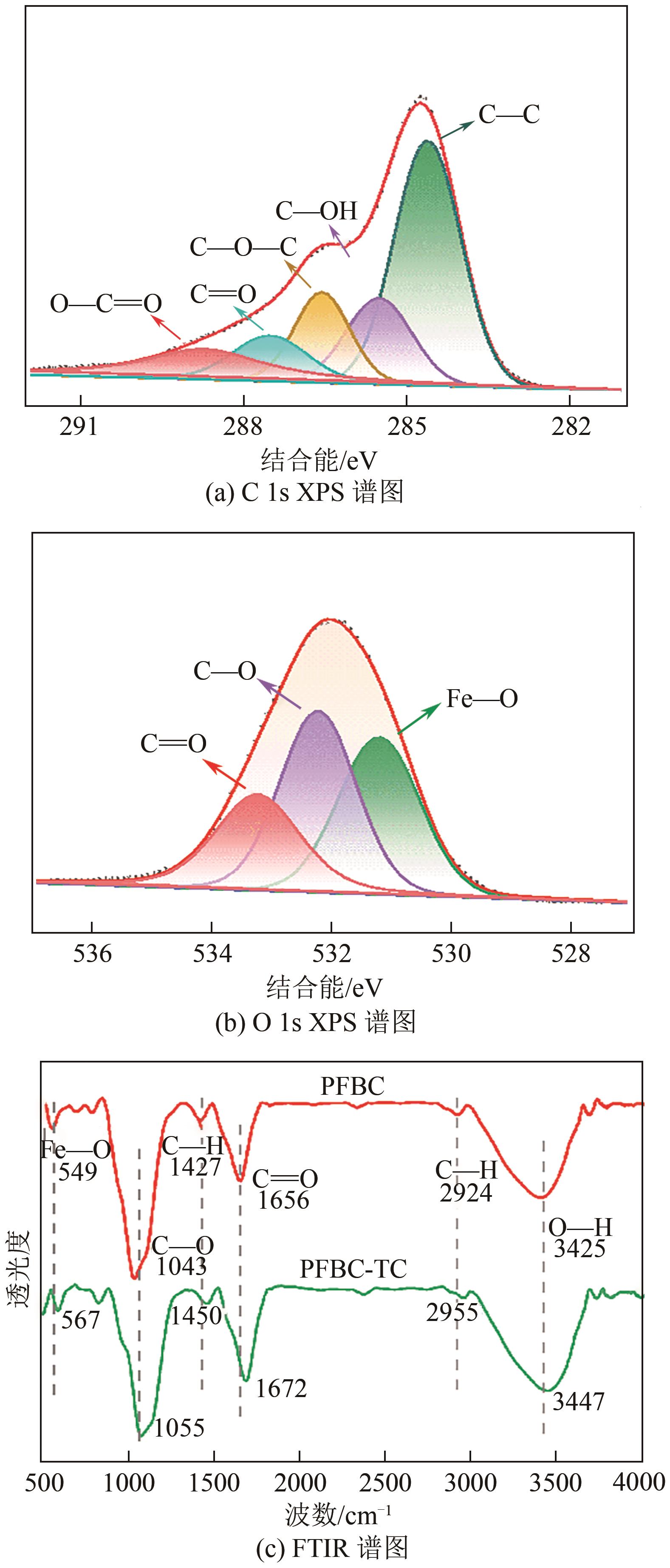
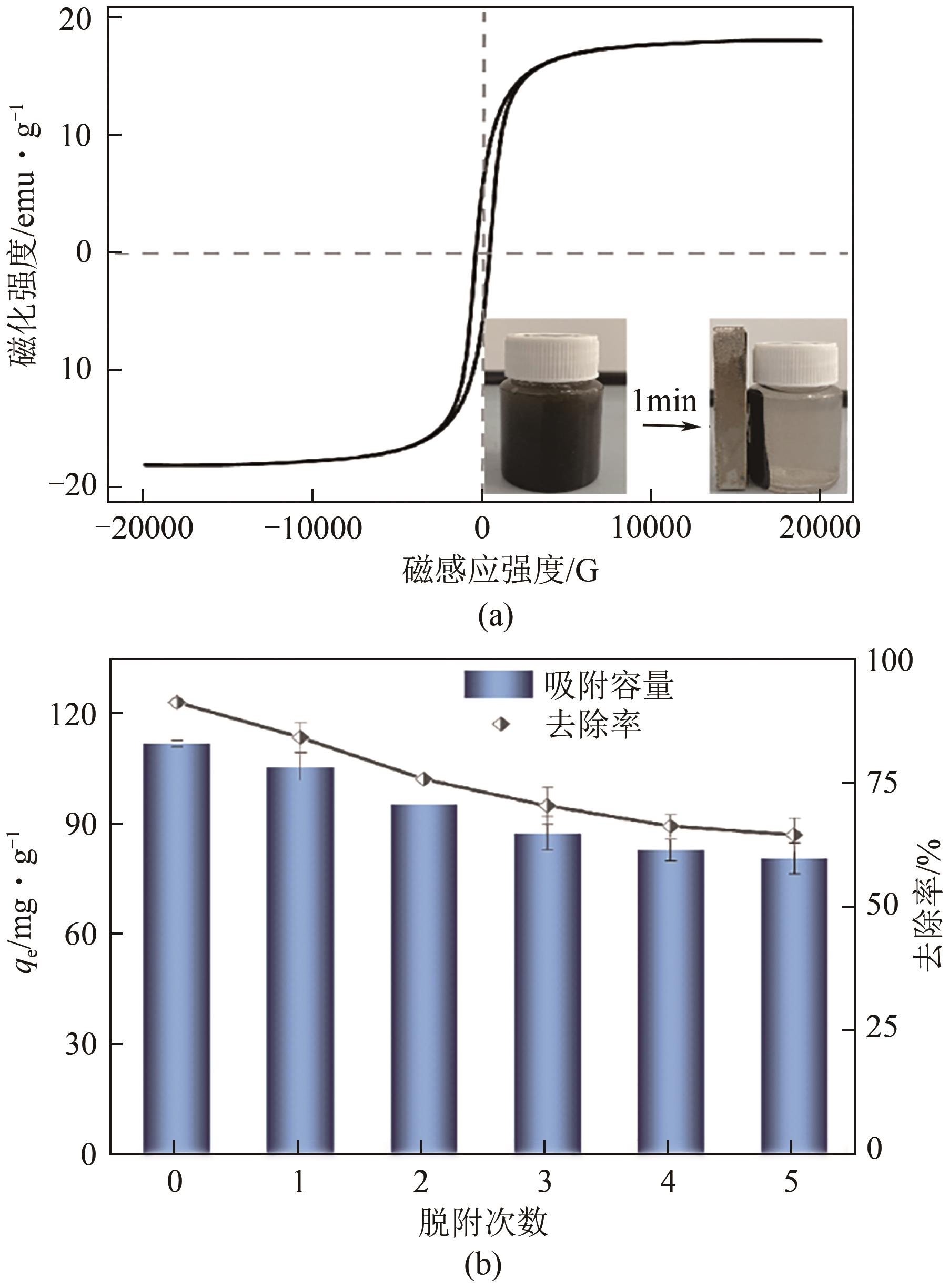
以剩余污泥为原料,在200℃的热解温度下,通过磷钼酸(PMA)改性和球磨法负载Fe3O4,分别制备原始生物炭(BC)、球磨污泥生物炭(FBC)、磷钼酸改性生物炭(PBC)、磷钼酸-Fe3O4球磨共改性生物炭(PFBC),用以吸附水体中的四环素(TC)。结果表明,PFBC具有更大的比表面积、孔体积和更小的粒径,共改性后表面含氧官能团含量明显增强,XRD和XPS表征结果表明PFBC成功负载Fe3O4。TC初始浓度为30mg/L、PFBC投加量为0.25g/L、pH=7时,PFBC对TC的去除率可达到88.7%。PFBC对TC的吸附可以很好地用Langmuir吸附等温线和准二级吸附动力学模型拟合,表明吸附过程为单分子层的化学吸附,饱和吸附容量在25℃达到500.0mg/g。在重复利用5次后,PFBC仍能去除溶液中63.3%的TC,其饱和磁化强度达18.1emu/g,可在外加磁场作用下简易回收。PFBC对TC的吸附包括孔隙填充、π-π交互作用、静电吸引、络合作用。本研究为改性污泥生物炭在废水抗生素治理中的应用提供了一种新策略。
中图分类号:
引用本文
赵丽阳, 李倩, 何佩熹, 潘鸿辉, 刘艳, 刘细祥. 磷钼酸-Fe3O4球磨共改性污泥基生物炭对四环素的吸附特性[J]. 化工进展, 2025, 44(1): 583-595.
ZHAO Liyang, LI Qian, HE Peixi, PAN Honghui, LIU Yan, LIU Xixiang. Tetracycline adsorption properties of sludge-based biochar ball-milled co-modified by phosphomolybdic acid-Fe3O4[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 583-595.
| 样品 | 参数 | O | Si | P | Ca | Fe | Mo |
|---|---|---|---|---|---|---|---|
| BC | 质量分数/% | 64 | 31.2 | 1.6 | 0.3 | 0.9 | 2 |
| 原子分数/% | 76.9 | 21.3 | 1 | 0.3 | 0.1 | 0.4 | |
| PBC | 质量分数/% | 52.5 | 20.8 | 7.3 | 1.6 | 5.9 | 11.9 |
| 原子分数/% | 72.5 | 16.3 | 5.2 | 0.9 | 2.4 | 2.7 | |
| PFBC | 质量分数/% | 41.5 | 8.3 | 2.8 | 0.9 | 38 | 8.5 |
| 原子分数/% | 68.8 | 7.8 | 2.4 | 0.5 | 18.1 | 2.4 |
表1 生物炭的EDS元素分析
| 样品 | 参数 | O | Si | P | Ca | Fe | Mo |
|---|---|---|---|---|---|---|---|
| BC | 质量分数/% | 64 | 31.2 | 1.6 | 0.3 | 0.9 | 2 |
| 原子分数/% | 76.9 | 21.3 | 1 | 0.3 | 0.1 | 0.4 | |
| PBC | 质量分数/% | 52.5 | 20.8 | 7.3 | 1.6 | 5.9 | 11.9 |
| 原子分数/% | 72.5 | 16.3 | 5.2 | 0.9 | 2.4 | 2.7 | |
| PFBC | 质量分数/% | 41.5 | 8.3 | 2.8 | 0.9 | 38 | 8.5 |
| 原子分数/% | 68.8 | 7.8 | 2.4 | 0.5 | 18.1 | 2.4 |
| 样品 | 比表面积 /m2·g-1 | 孔体积 /m3·g-1 | 平均孔径 /nm | 平均粒度 |
|---|---|---|---|---|
| BC | 18.3 | 0.05 | 28.6 | 68.5μm |
| FBC | 42.2 | 0.06 | 14.4 | 418.5nm |
| PBC | 40.2 | 0.14 | 19.7 | 111.9μm |
| PFBC | 57.2 | 0.15 | 14.6 | 440.6nm |
表2 生物炭的比表面积、孔结构及粒度参数
| 样品 | 比表面积 /m2·g-1 | 孔体积 /m3·g-1 | 平均孔径 /nm | 平均粒度 |
|---|---|---|---|---|
| BC | 18.3 | 0.05 | 28.6 | 68.5μm |
| FBC | 42.2 | 0.06 | 14.4 | 418.5nm |
| PBC | 40.2 | 0.14 | 19.7 | 111.9μm |
| PFBC | 57.2 | 0.15 | 14.6 | 440.6nm |
| 样品 | 元素质量分数/% | 元素比 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | Mo | Fe | H/C | O/C | (O+N)/C | |
| BC | 14.8 | 2.5 | 25.6 | 1.4 | 0.0 | 2.4 | 0.17 | 1.73 | 1.82 |
PBC PFBC | 13.4 10.1 | 2.0 2.2 | 24.8 19.7 | 1.2 1.8 | 5.9 2.6 | 1.6 17.5 | 0.15 0.22 | 1.85 1.95 | 1.94 2.13 |
表3 生物炭的元素组成分析
| 样品 | 元素质量分数/% | 元素比 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | Mo | Fe | H/C | O/C | (O+N)/C | |
| BC | 14.8 | 2.5 | 25.6 | 1.4 | 0.0 | 2.4 | 0.17 | 1.73 | 1.82 |
PBC PFBC | 13.4 10.1 | 2.0 2.2 | 24.8 19.7 | 1.2 1.8 | 5.9 2.6 | 1.6 17.5 | 0.15 0.22 | 1.85 1.95 | 1.94 2.13 |
| 结合能/eV | 特征峰 | 占比/% | ||
|---|---|---|---|---|
| BC | PBC | PFBC | ||
| 284.8 | C—C | 60.6 | 58.5 | 43.5 |
| 286.1 | C—OH | 19.2 | 19.5 | 19.5 |
| 286.8 | C—O—C | 7.2 | 8.8 | 14.2 |
287.8 288.7 | C | 6.0 | 6.4 | 7.9 |
| O—C | 7.0 | 6.8 | 14.9 | |
| 530.9 | Fe—O | 42.3 | 40.1 | 44.0 |
| 531.8 | C—O | 32.2 | 35.3 | 26.8 |
| 533.1 | C | 25.5 | 24.6 | 29.2 |
| 711.1/724.1 | Fe(Ⅱ) | 59.6 | 60.2 | 63.6 |
| 713.5/726.5 | Fe(Ⅲ) | 40.4 | 39.8 | 36.4 |
表4 生物炭的XPS谱图分析
| 结合能/eV | 特征峰 | 占比/% | ||
|---|---|---|---|---|
| BC | PBC | PFBC | ||
| 284.8 | C—C | 60.6 | 58.5 | 43.5 |
| 286.1 | C—OH | 19.2 | 19.5 | 19.5 |
| 286.8 | C—O—C | 7.2 | 8.8 | 14.2 |
287.8 288.7 | C | 6.0 | 6.4 | 7.9 |
| O—C | 7.0 | 6.8 | 14.9 | |
| 530.9 | Fe—O | 42.3 | 40.1 | 44.0 |
| 531.8 | C—O | 32.2 | 35.3 | 26.8 |
| 533.1 | C | 25.5 | 24.6 | 29.2 |
| 711.1/724.1 | Fe(Ⅱ) | 59.6 | 60.2 | 63.6 |
| 713.5/726.5 | Fe(Ⅲ) | 40.4 | 39.8 | 36.4 |
| 拟合模型 | 参数 | 10mg/L | 30mg/L | 50mg/L |
|---|---|---|---|---|
| 实验吸附量 | qe/mg·g-1 | 38.2 | 112.4 | 167.7 |
| 准一级动力学模型 | qe/mg·g-1 | 23.3 | 88.9 | 121.5 |
| k1/h-1 | 0.279 | 0.220 | 0.149 | |
| R2 | 0.973 | 0.985 | 0.960 | |
| 准二级动力学模型 | qm/mg·g-1 | 38.5 | 116.3 | 166.7 |
| k2/g·mg-1·h-1 | 0.047 | 0.008 | 0.003 | |
| R2 | 0.999 | 0.995 | 0.990 |
表5 吸附动力学拟合参数
| 拟合模型 | 参数 | 10mg/L | 30mg/L | 50mg/L |
|---|---|---|---|---|
| 实验吸附量 | qe/mg·g-1 | 38.2 | 112.4 | 167.7 |
| 准一级动力学模型 | qe/mg·g-1 | 23.3 | 88.9 | 121.5 |
| k1/h-1 | 0.279 | 0.220 | 0.149 | |
| R2 | 0.973 | 0.985 | 0.960 | |
| 准二级动力学模型 | qm/mg·g-1 | 38.5 | 116.3 | 166.7 |
| k2/g·mg-1·h-1 | 0.047 | 0.008 | 0.003 | |
| R2 | 0.999 | 0.995 | 0.990 |
| 拟合模型 | 参数 | 25℃ | 35℃ | 45℃ |
|---|---|---|---|---|
| 实验吸附量 | qmax/mg·g-1 | 491.3 | 548.5 | 644.2 |
| Langmuir等温线 | qmax/mg·g-1 | 500.0 | 555.6 | 666.7 |
| KL/L·mg-1 | 0.035 | 0.035 | 0.021 | |
| R2 | 0.999 | 0.999 | 0.996 | |
| Freundlich等温线 | KF/mg·g-1·(L·mg-1)1/n | 86.05 | 73.19 | 80.39 |
| 1/n | 0.307 | 0.316 | 0.340 | |
| R2 | 0.923 | 0.952 | 0.974 |
表6 吸附等温线拟合参数
| 拟合模型 | 参数 | 25℃ | 35℃ | 45℃ |
|---|---|---|---|---|
| 实验吸附量 | qmax/mg·g-1 | 491.3 | 548.5 | 644.2 |
| Langmuir等温线 | qmax/mg·g-1 | 500.0 | 555.6 | 666.7 |
| KL/L·mg-1 | 0.035 | 0.035 | 0.021 | |
| R2 | 0.999 | 0.999 | 0.996 | |
| Freundlich等温线 | KF/mg·g-1·(L·mg-1)1/n | 86.05 | 73.19 | 80.39 |
| 1/n | 0.307 | 0.316 | 0.340 | |
| R2 | 0.923 | 0.952 | 0.974 |
| 原料 | 改性方法 | 饱和吸附量/mg·g-1 | 参考文献 | ||
|---|---|---|---|---|---|
| 25℃ | 35℃ | 45℃ | |||
| 污泥 | H3PO4·12MoO3+球磨 | 500.0 | 555.6 | 666.7 | 本文 |
| 污泥 | NaOH+壳聚糖+NH4Fe(SO4)2·12H2O+(NH4)2Fe(SO4)2·6H2O | 184.3 | 199.3 | 240.3 | [ |
| 污泥 | KOH+FeCl2+FeCl3 | 140.7 | 141.6 | 139.8 | [ |
| 污泥 | ZnCl2+壳聚糖+FeSO4·7H2O+Na2S·9H2O | 183.0 | 199.4 | 223.9 | [ |
| 污泥 | ZnCl2+FeSO4·7H2O+Na2S·9H2O | 174.1 | 154.6 | — | [ |
| 造纸污泥 | 未改性 | 125.2 | — | — | [ |
| 花生壳 | CaCl2 | 72.3 | — | — | [ |
| 橘子皮 | MnCl2+FeCl3·6H2O+FeCl2·4H2O | 167.5 | — | — | [ |
| 秸秆 | KOH | 307.8 | 379.5 | 491.2 | [ |
| 秸秆 | MnCl2 | 736.0 | 876.0 | — | [ |
| 木质纤维素 | HCl | 1078.9 | 1901.4 | — | [ |
表7 不同生物炭对TC的饱和吸附量
| 原料 | 改性方法 | 饱和吸附量/mg·g-1 | 参考文献 | ||
|---|---|---|---|---|---|
| 25℃ | 35℃ | 45℃ | |||
| 污泥 | H3PO4·12MoO3+球磨 | 500.0 | 555.6 | 666.7 | 本文 |
| 污泥 | NaOH+壳聚糖+NH4Fe(SO4)2·12H2O+(NH4)2Fe(SO4)2·6H2O | 184.3 | 199.3 | 240.3 | [ |
| 污泥 | KOH+FeCl2+FeCl3 | 140.7 | 141.6 | 139.8 | [ |
| 污泥 | ZnCl2+壳聚糖+FeSO4·7H2O+Na2S·9H2O | 183.0 | 199.4 | 223.9 | [ |
| 污泥 | ZnCl2+FeSO4·7H2O+Na2S·9H2O | 174.1 | 154.6 | — | [ |
| 造纸污泥 | 未改性 | 125.2 | — | — | [ |
| 花生壳 | CaCl2 | 72.3 | — | — | [ |
| 橘子皮 | MnCl2+FeCl3·6H2O+FeCl2·4H2O | 167.5 | — | — | [ |
| 秸秆 | KOH | 307.8 | 379.5 | 491.2 | [ |
| 秸秆 | MnCl2 | 736.0 | 876.0 | — | [ |
| 木质纤维素 | HCl | 1078.9 | 1901.4 | — | [ |
| 样品 | 比表面积/m2·g-1 | 总孔容/m3·g-1 | 平均孔径/nm |
|---|---|---|---|
| PFBC | 57.2 | 0.15 | 14.6 |
| PFBC-TC | 6.0 | 0.04 | 17.5 |
表8 PFBC吸附TC前后的比表面积和孔结构参数
| 样品 | 比表面积/m2·g-1 | 总孔容/m3·g-1 | 平均孔径/nm |
|---|---|---|---|
| PFBC | 57.2 | 0.15 | 14.6 |
| PFBC-TC | 6.0 | 0.04 | 17.5 |
| 1 | 郑惠东. 水环境中抗生素来源及对健康的影响[J]. 环境卫生学杂志, 2018, 8(1): 73-77. |
| ZHENG Huidong. The source of antibiotics in aquatic environment and its impact on human health[J]. Journal of Environmental Hygiene, 2018, 8(1): 73-77. | |
| 2 | LIU Qing, ZHONG Lubin, ZHAO Quanbao, et al. Synthesis of Fe3O4/polyacrylonitrile composite electrospun nanofiber mat for effective adsorption of tetracycline[J]. ACS Applied Materials & Interfaces, 2015, 7(27): 14573-14583. |
| 3 | ZENG Zhuotong, YE Shujing, WU Haipeng, et al. Research on the sustainable efficacy of g-MoS2 decorated biochar nanocomposites for removing tetracycline hydrochloride from antibiotic-polluted aqueous solution[J]. The Science of the Total Environment, 2019, 648: 206-217. |
| 4 | QIAO Disi, LI Zehao, DUAN Jinyou, et al. Adsorption and photocatalytic degradation mechanism of magnetic graphene oxide/ZnO nanocomposites for tetracycline contaminants[J]. Chemical Engineering Journal, 2020, 400: 125952. |
| 5 | SUN Hongwei, YANG Jingjie, WANG Yue, et al. Study on the removal efficiency and mechanism of tetracycline in water[J]. Coatings, 2021, 11(11): 1354. |
| 6 | ZHENG Shimei, WANG Yandong, CHEN Cuihong, et al. Current progress in natural degradation and enhanced removal techniques of antibiotics in the environment: A review[J]. International Journal of Environmental Research and Public Health, 2022, 19(17): 10919. |
| 7 | ZHANG Peizhen, LI Yanfei, CAO Yaoyao, et al. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures[J]. Bioresource Technology, 2019, 285: 121348. |
| 8 | MA Yongfei, LI Ming, LI Ping, et al. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal[J]. Bioresource Technology, 2021, 319: 124199. |
| 9 | LIANG Huagen, ZHU Chenxi, JI Shan, et al. Magnetic Fe2O3/biochar composite prepared in a molten salt medium for antibiotic removal in water[J]. Biochar, 2022, 4(1): 3. |
| 10 | ZHANG Qingfa, CAI Hongzhen, YI Weiming, et al. Biocomposites from organic solid wastes derived biochars: A review[J]. Materials, 2020, 13(18): 3923. |
| 11 | LIANG Meina, LU Lin, HE Huijun, et al. Applications of biochar and modified biochar in heavy metal contaminated soil: A descriptive review[J]. Sustainability, 2021, 13(24): 14041. |
| 12 | 戴晓虎. 我国污泥处理处置现状及发展趋势[J]. 科学, 2020, 72(6): 30-34. |
| DAI Xiaohu. Applications and perspectives of sludge treatment and disposal in China[J]. Science, 2020, 72(6): 30-34. | |
| 13 | 周善磊, 徐雅兵, 张宇锡, 等. 污泥基生物炭制备及其对活性黑5染料的吸附研究[J]. 山东化工, 2023, 52(17): 239-241. |
| ZHOU Shanlei, XU Yabing, ZHANG Yuxi, et al. Preparation of sludge-based biochar and its adsorption to reactive black 5 dye[J]. Shandong Chemical Industry, 2023, 52(17): 239-241. | |
| 14 | 贺丹丹, 张泽宇, 刘娟丽, 等. 市政污泥生物炭在废水吸附处理中的应用[J]. 精细化工, 2024(7): 1447-1457. |
| HE Dandan, ZHANG Zeyu, LIU Juanli, et al. Application of municipal sludge biochar in wastewater adsorption treatment[J]. Fine Chemicals, 2024(7): 1447-1457. | |
| 15 | DEVI Parmila, SAROHA Anil K. Utilization of sludge based adsorbents for the removal of various pollutants: A review[J]. The Science of the Total Environment, 2017, 578: 16-33. |
| 16 | 费永鑫, 马会强, 李爽. 改性活性污泥生物炭对水中苯酚吸附性能研究[J]. 辽宁石油化工大学学报, 2022, 42(3): 19-24. |
| FEI Yongxin, MA Huiqiang, LI Shuang. Study on adsorption performance of modified activated sludge biochar for phenol in water[J]. Journal of Liaoning Petrochemical University, 2022, 42(3): 19-24. | |
| 17 | ZHANG Xu, SHU Xin, ZHOU Xiaolin, et al. Magnetic reed biochar materials as adsorbents for aqueous copper and phenol removal[J]. Environmental Science and Pollution Research International, 2023, 30(2): 3659-3667. |
| 18 | KASERA Nitesh, AUGOUSTIDES Victoria, KOLAR Praveen, et al. Effect of surface modification by oxygen-enriched chemicals on the surface properties of pine bark biochars[J]. Processes, 2022, 10(10): 2136. |
| 19 | HE Xian, HONG Zhineng, JIANG Jun, et al. Enhancement of Cd(Ⅱ) adsorption by rice straw biochar through oxidant and acid modifications[J]. Environmental Science and Pollution Research International, 2021, 28(31): 42787-42797. |
| 20 | GUO Zijing, CHEN Xin, HANG Jiacheng, et al. Oxidative magnetization of biochar at relatively low pyrolysis temperature for efficient removal of different types of pollutants[J]. Bioresource Technology, 2023, 387: 129572. |
| 21 | LIU Sen, LI Jihui, XU Shuang, et al. A modified method for enhancing adsorption capability of banana pseudostem biochar towards methylene blue at low temperature[J]. Bioresource Technology, 2019, 282: 48-55. |
| 22 | LIN Shenglun, ZHANG Hongjie, CHEN Wei-Hsin, et al. Low-temperature biochar production from torrefaction for wastewater treatment: A review[J]. Bioresource Technology, 2023, 387: 129588. |
| 23 | WU Jingqi, WANG Tongshuai, LIU Yuyan, et al. Norfloxacin adsorption and subsequent degradation on ball-milling tailored N-doped biochar[J]. Chemosphere, 2022, 303(Pt 3): 135264. |
| 24 | HUANG Zhexi, YI Yunqiang, ZHANG Nuanqin, et al. Removal of fluconazole from aqueous solution by magnetic biochar treated by ball milling: Adsorption performance and mechanism[J]. Environmental Science and Pollution Research International, 2022, 29(22): 33335-33344. |
| 25 | SHAN Danna, DENG Shubo, ZHAO Tianning, et al. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling[J]. Journal of Hazardous Materials, 2016, 305: 156-163. |
| 26 | HE Juan, TANG Jingchun, ZHANG Zheng, et al. Magnetic ball-milled FeS@biochar as persulfate activator for degradation of tetracycline[J]. Chemical Engineering Journal, 2021, 404: 126997. |
| 27 | WEI Zehua, LI Haihong, JIA Miaomiao, et al. NaOH-ball-milled co-modified magnetic biochar and its oil adsorption properties[J]. Particuology, 2023, 83: 40-49. |
| 28 | GUO Tianxiang, ZHANG Yonghe, GENG Yuhan, et al. Surface oxidation modification of nitrogen doping biochar for enhancing CO2 adsorption[J]. Industrial Crops and Products, 2023, 206: 117582. |
| 29 | ZHANG Dawei, HE Qianqian, HU Xiaolan, et al. Enhanced adsorption for the removal of tetracycline hydrochloride (TC) using ball-milled biochar derived from crayfish shell[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 615: 126254. |
| 30 | 蔡思颖, 张伟军, 陈康, 等. 中药渣生物炭的制备及其对水中四环素的吸附特性研究[J]. 安全与环境工程, 2022, 29(3): 178-186. |
| CAI Siying, ZHANG Weijun, CHEN Kang, et al. Preparation of Chinese medicine wastes biochar and its adsorption characteristics to tetracycline in water[J]. Safety and Environmental Engineering, 2022, 29(3): 178-186. | |
| 31 | MEI Yanglu, XU Jin, ZHANG Yin, et al. Effect of Fe-N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution[J]. Bioresource Technology, 2021, 325: 124732. |
| 32 | ZHAO Zhendong, WU Qianqian, NIE Tiantian, et al. Quantitative evaluation of relationships between adsorption and partition of atrazine in biochar-amended soils with biochar characteristics[J]. RSC Advances, 2019, 9(8): 4162-4171. |
| 33 | YANG Hucheng, YU Hao, WANG Jiahao, et al. Magnetic porous biochar as a renewable and highly effective adsorbent for the removal of tetracycline hydrochloride in water[J]. Environmental Science and Pollution Research, 2021, 28(43): 61513-61525. |
| 34 | ZOU Chenglong, WU Qin, NIE Fahui, et al. Application of magnetic porous graphite biochar prepared through one-step modification in the adsorption of tetracycline and ciprofloxacin from aqueous solutions[J]. Waste and Biomass Valorization, 2024, 15(3): 1477-1494. |
| 35 | 张庆乐, 王忠, 李瑞. 丹参残渣生物炭对四环素的吸附特征[J]. 山东化工, 2022, 51(19): 47-50. |
| ZHANG Qingle, WANG Zhong, LI Rui. Adsorption characteristics of tetracycline on biochar from salvia miltiorrhiza[J]. Shandong Chemical Industry, 2022, 51(19): 47-50. | |
| 36 | DHIBAR Subhendu, Suchetana PAL, KARMAKAR Kripasindhu, et al. Two novel low molecular weight gelator-driven supramolecular metallogels efficient in antimicrobial activity applications[J]. RSC Advances, 2023, 13(47): 32842-32849. |
| 37 | OULD M’HAMED Mohamed. Ball milling for heterocyclic compounds synthesis in green chemistry: A review[J]. Synthetic Communications, 2015, 45(22): 2511-2528. |
| 38 | SHI Qiyu, WANG Wangbo, ZHANG Hongmin, et al. Porous biochar derived from walnut shell as an efficient adsorbent for tetracycline removal[J]. Bioresource Technology, 2023, 383: 129213. |
| 39 | FAN Shisuo, FAN Xinru, WANG Shuo, et al. Effect of chitosan modification on the properties of magnetic porous biochar and its adsorption performance towards tetracycline and Cu2+ [J]. Sustainable Chemistry and Pharmacy, 2023, 33: 101057. |
| 40 | KIM Ji Eun, BHATIA Shashi Kant, SONG Hak Jin, et al. Adsorptive removal of tetracycline from aqueous solution by maple leaf-derived biochar[J]. Bioresource Technology, 2020, 306: 123092. |
| 41 | FAN Shisuo, TANG Jie, WANG Yi, et al. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism[J]. Journal of Molecular Liquids, 2016, 220: 432-441. |
| 42 | QI Liqiang, TENG Fei, DENG Xin, et al. Experimental study on adsorption of Hg(Ⅱ) with microwave-assisted alkali-modified fly ash[J]. Powder Technology, 2019, 351: 153-158. |
| 43 | 张娟香, 赵保卫, 马锋锋, 等. 造纸污泥生物炭对四环素的吸附特性及机理[J]. 中国环境科学, 2020, 40(9): 3821-3828. |
| ZHANG Juanxiang, ZHAO Baowei, MA Fengfeng, et al. Adsorption characteristics and mechanism of tetracycline by biochars derived from paper industry sludge[J]. China Environmental Science, 2020, 40(9): 3821-3828. | |
| 44 | 黄慧, 管映兵, 孙旭伟, 等. 生物炭-壳聚糖制备凝胶颗粒对水中四环素的高效去除[J]. 水处理技术, 2023, 49(7): 32-37. |
| HUANG Hui, GUAN Yingbing, SUN Xuwei, et al. Adsorption behavior of chlortetracycline on the biochar-based magnetic gel balloon[J]. Technology of Water Treatment, 2023, 49(7): 32-37. | |
| 45 | LIU Juanli, ZHOU Baiqin, ZHANG Hong, et al. A novel biochar modified by chitosan-Fe/S for tetracycline adsorption and studies on site energy distribution[J]. Bioresource Technology, 2019, 294: 122152. |
| 46 | MA Juan, ZHOU Baiqin, ZHANG Hong, et al. Fe/S modified sludge-based biochar for tetracycline removal from water[J]. Powder Technology, 2020, 364: 889-900. |
| 47 | 范方方, 仝仲凯, 左卫元. 钙改性花生壳生物炭对废水中四环素的吸附研究[J]. 无机盐工业, 2023, 55(6): 109-115. |
| FAN Fangfang, TONG Zhongkai, ZUO Weiyuan. Study on adsorption of tetracycline from wastewater by calcium modified peanut shell biochar[J]. Inorganic Chemicals Industry, 2023, 55(6): 109-115. | |
| 48 | 林冰峰, 陈志豪, 杨芳俐, 等. 锰铁氧体改性生物炭对四环素的吸附性能研究[J]. 农业环境科学学报, 2023, 42(7): 1585-1596. |
| LIN Bingfeng, CHEN Zhihao, YANG Fangli, et al. Adsorption performance of tetracycline by manganese ferrite-modified biochar[J]. Journal of Agro-Environment Science, 2023, 42(7): 1585-1596. | |
| 49 | 徐晋, 马一凡, 姚国庆, 等. KOH活化小麦秸秆生物炭对废水中四环素的高效去除[J]. 环境科学, 2022, 43(12): 5635-5646. |
| XU Jin, MA Yifan, YAO Guoqing, et al. Effect of KOH activation on the properties of biochar and its adsorption behavior on tetracycline removal from an aqueous solution[J]. Environmental Science, 2022, 43(12): 5635-5646. | |
| 50 | 赵志伟, 陈晨, 梁志杰, 等. 锰氧化物改性生物炭对水中四环素的强化吸附[J]. 农业环境科学学报, 2021, 40(1): 194-201. |
| ZHAO Zhiwei, CHEN Chen, LIANG Zhijie, et al. Enhanced adsorption activity of manganese oxide-modified biochar for the removal of tetracycline from aqueous solution[J]. Journal of Agro-Environment Science, 2021, 40(1): 194-201. | |
| 51 | 李国亭, 李康丽, 张帅阳, 等. 木质纤维素生物炭对亚甲基蓝和四环素的吸附对比研究[J]. 江苏农业科学, 2021, 49(18): 234-240. |
| LI Guoting, LI Kangli, ZHANG Shuaiyang, et al. Comparative study on adsorption of methylene blue and tetracycline by lignocellulosic biochar[J]. Jiangsu Agricultural Sciences, 2021, 49(18): 234-240. | |
| 52 | LI Bin, ZHANG Yin, XU Jin, et al. Effect of carbonization methods on the properties of tea waste biochars and their application in tetracycline removal from aqueous solutions[J]. Chemosphere, 2021, 267: 129283. |
| 53 | QU Jianhua, ZHANG Bo, TONG Hua, et al. High-efficiency decontamination of Pb(Ⅱ) and tetracycline in contaminated water using ball-milled magnetic bone derived biochar[J]. Journal of Cleaner Production, 2023, 385: 135683. |
| 54 | WANG Kaifeng, YAO Runlin, ZHANG Dongqing, et al. Tetracycline adsorption performance and mechanism using calcium hydroxide-modified biochars[J]. Toxics, 2023, 11(10): 841. |
| 55 | 刘森. 香蕉假茎生物炭的改性及其对亚甲基蓝的吸附性能研究[D]. 海口: 海南大学, 2020. |
| LIU Sen. Study on modification of banana pseudostem biochar and its adsorption properties for methylene blue[D]. Haikou: Hainan University, 2020. |
| [1] | 李琢宇, 余美琪, 陈孝彦, 胡若晖, 王庆宏, 陈春茂, 詹亚力. 炼油废催化剂吸附去除水中硝基苯的特性与机制[J]. 化工进展, 2025, 44(2): 1076-1087. |
| [2] | 游小银, 汪楚乔, 刘才华, 彭小明. Z型CN/NGBO/BV催化剂体系的构筑及光类芬顿降解四环素性能[J]. 化工进展, 2025, 44(1): 286-296. |
| [3] | 杨润农, 白帆飞, 林梓荣, 孙永明, 尹祥. 分子筛吸附脱除有机硫的研究进展[J]. 化工进展, 2025, 44(1): 329-340. |
| [4] | 倪鹏, 王先泓, 黄钰涵, 马晓彤, 马子轸, 谈琰, 张华伟, 刘亭. 活性炭类和磁性金属类吸附剂喷射脱汞技术应用对比及最新进展[J]. 化工进展, 2025, 44(1): 513-524. |
| [5] | 刘新维, 高珊, 王红涛, 王建成. 气化细渣、铝灰的活化及其吸附性能[J]. 化工进展, 2025, 44(1): 558-571. |
| [6] | 张炜, 黄赳, 朱晓芳, 李鹏. 凹凸棒石基钴钨水滑石吸附铅的性能及机理[J]. 化工进展, 2025, 44(1): 596-606. |
| [7] | 石磊, 王倩, 赵晓胜, 刘宏臣, 车远军, 段玉, 李庆. 油页岩灰基分子筛的制备及对亚甲基蓝的吸附[J]. 化工进展, 2024, 43(S1): 650-661. |
| [8] | 刘丽, 冯博, 文洋, 古启雄. 硅基介孔材料的合成、功能化及对金属的吸附研究进展[J]. 化工进展, 2024, 43(9): 5063-5078. |
| [9] | 吴宇琦, 李江涛, 丁建智, 宋秀兰, 苏冰琴. 焙烧镁铝水滑石脱除厌氧消化沼气中CO2的效果及机制[J]. 化工进展, 2024, 43(9): 5250-5261. |
| [10] | 杨新衡, 纪志永, 郭志远, 刘萁, 张盼盼, 汪婧, 刘杰, 毕京涛, 赵颖颖, 袁俊生. 锂铝层状双金属氢氧化物的制备及其锂脱嵌过程[J]. 化工进展, 2024, 43(9): 5262-5274. |
| [11] | 卞维柏, 张睿轩, 潘建明. 无机金属锂离子筛材料制备方法研究进展[J]. 化工进展, 2024, 43(8): 4173-4186. |
| [12] | 王嘉, 李文翠, 吴凡, 高新芊, 陆安慧. NiMo/Al2O3催化剂活性组分分布调控及其加氢脱硫应用[J]. 化工进展, 2024, 43(8): 4393-4402. |
| [13] | 郑云香, 高艺伦, 李宴汝, 刘青霖, 张浩腾, 王向鹏. 氨基三乙酸酐改性多孔双网络水凝胶的制备及吸附性能[J]. 化工进展, 2024, 43(8): 4542-4549. |
| [14] | 刘玉灿, 高中鲁, 徐心怡, 纪现国, 张岩, 孙洪伟, 王港. 钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理[J]. 化工进展, 2024, 43(8): 4630-4641. |
| [15] | 胡君杰, 黄兴俊, 雷成, 杨敏, 兰元宵, 罗建洪. 页岩气采出水中小分子有机物的深度处理[J]. 化工进展, 2024, 43(8): 4674-4680. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||