化工进展 ›› 2024, Vol. 43 ›› Issue (9): 5262-5274.DOI: 10.16085/j.issn.1000-6613.2023-1393
• 资源与环境化工 • 上一篇
锂铝层状双金属氢氧化物的制备及其锂脱嵌过程
杨新衡1,2( ), 纪志永1,2(
), 纪志永1,2( ), 郭志远1,2, 刘萁1,2, 张盼盼1,2, 汪婧1,2, 刘杰1,2, 毕京涛1,2, 赵颖颖1,2, 袁俊生1,2
), 郭志远1,2, 刘萁1,2, 张盼盼1,2, 汪婧1,2, 刘杰1,2, 毕京涛1,2, 赵颖颖1,2, 袁俊生1,2
- 1.河北工业大学化工学院,海水资源高效利用化工技术教育部工程研究中心,天津 300401
2.河北省现代海洋化工技术协同创新中心,天津 300401
-
收稿日期:2023-08-11修回日期:2023-10-26出版日期:2024-09-15发布日期:2024-09-30 -
通讯作者:纪志永 -
作者简介:杨新衡(1998—),男,硕士研究生,研究方向为化学工程。E-mail:yxh508761762@163.com。 -
基金资助:国家自然科学基金(21978064);河北省自然科学基金(B2022202024)
Preparation of lithium aluminum layered double hydroxides and their lithium deintercalation performance
YANG Xinheng1,2( ), JI Zhiyong1,2(
), JI Zhiyong1,2( ), GUO Zhiyuan1,2, LIU Qi1,2, ZHANG Panpan1,2, WANG Jing1,2, LIU Jie1,2, BI Jingtao1,2, ZHAO Yingying1,2, YUAN Junsheng1,2
), GUO Zhiyuan1,2, LIU Qi1,2, ZHANG Panpan1,2, WANG Jing1,2, LIU Jie1,2, BI Jingtao1,2, ZHAO Yingying1,2, YUAN Junsheng1,2
- 1.Engineering Research Center of Seawater Utilization Technology of Ministry of Education, School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300401, China
2.Hebei Collaborative Innovation Center of Modern Marine Chemical Technology, Tianjin 300401, China
-
Received:2023-08-11Revised:2023-10-26Online:2024-09-15Published:2024-09-30 -
Contact:JI Zhiyong
摘要:
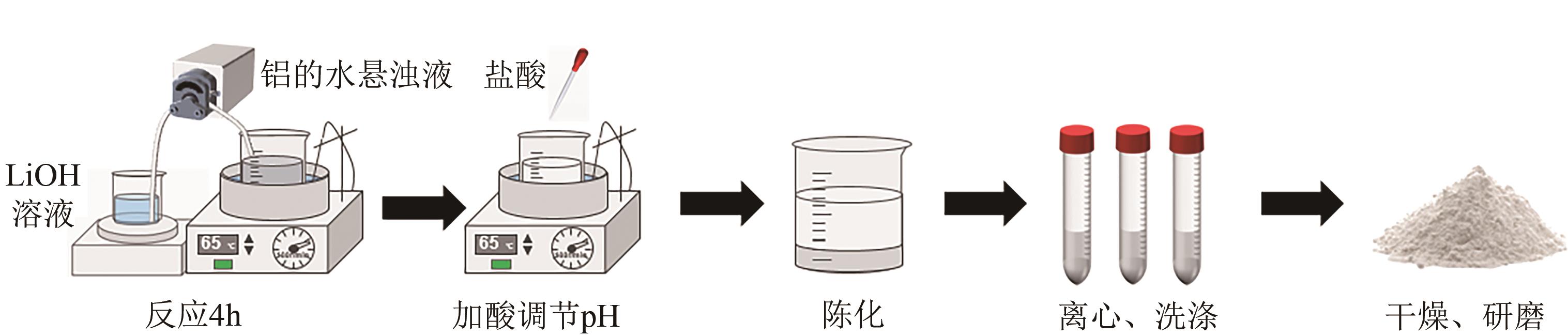
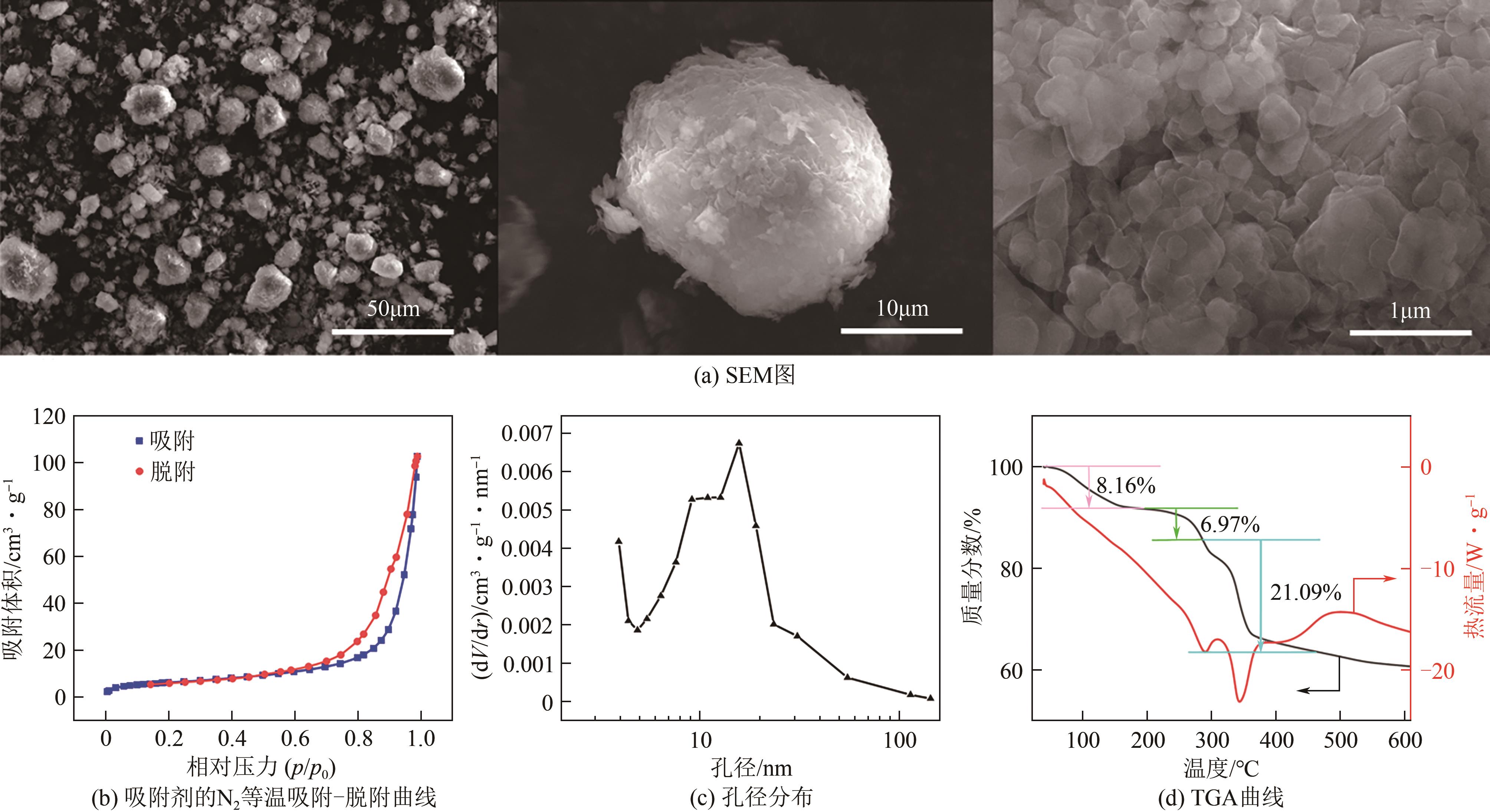
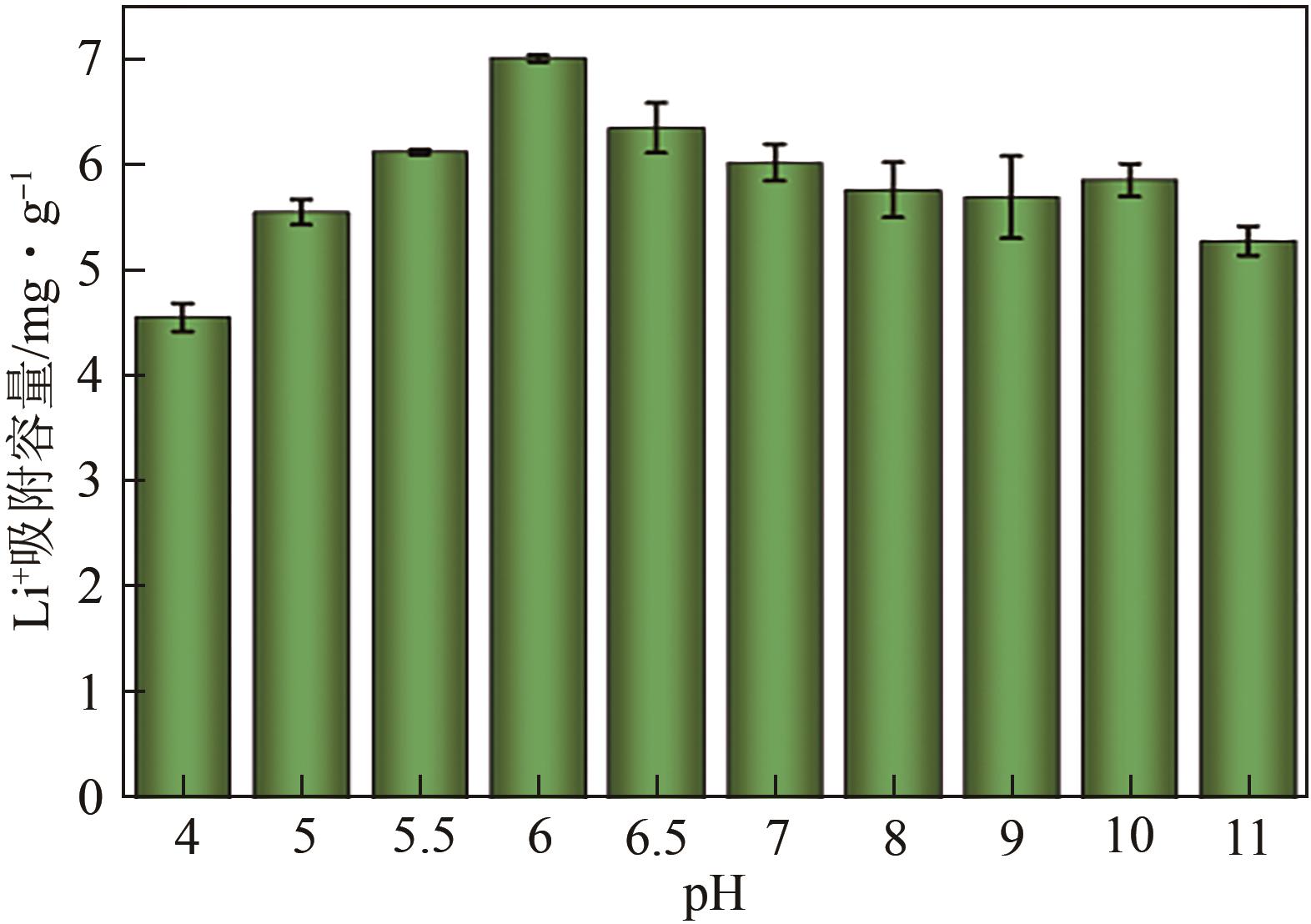
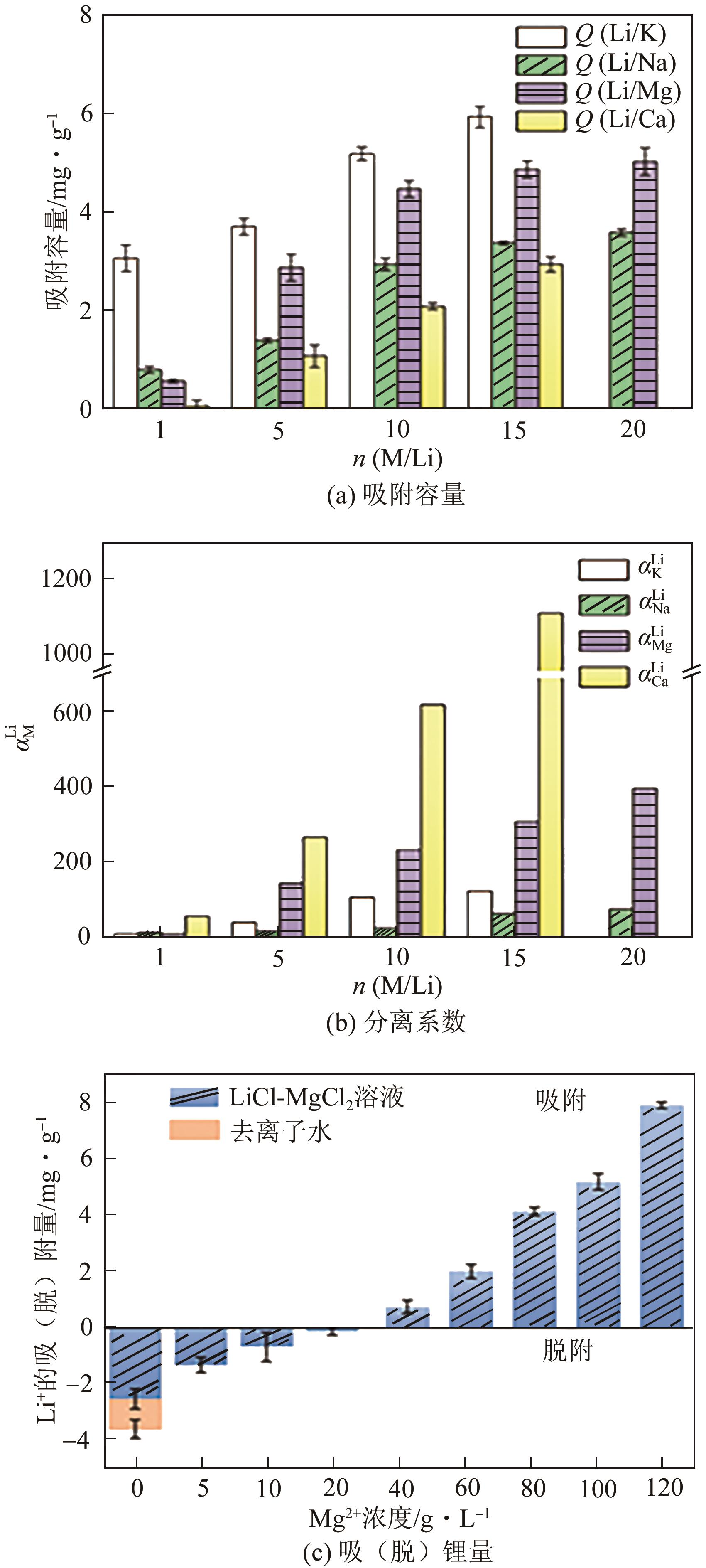
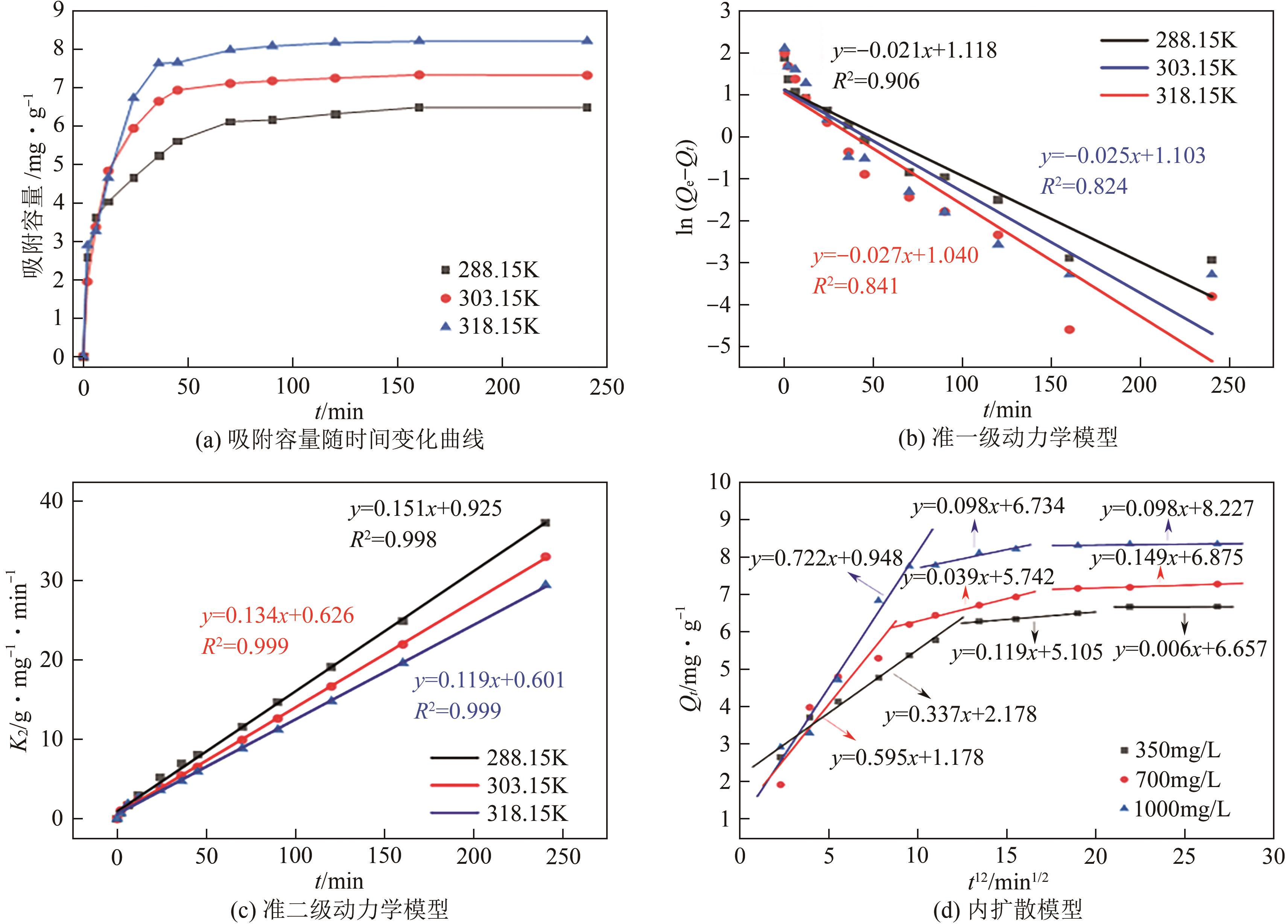
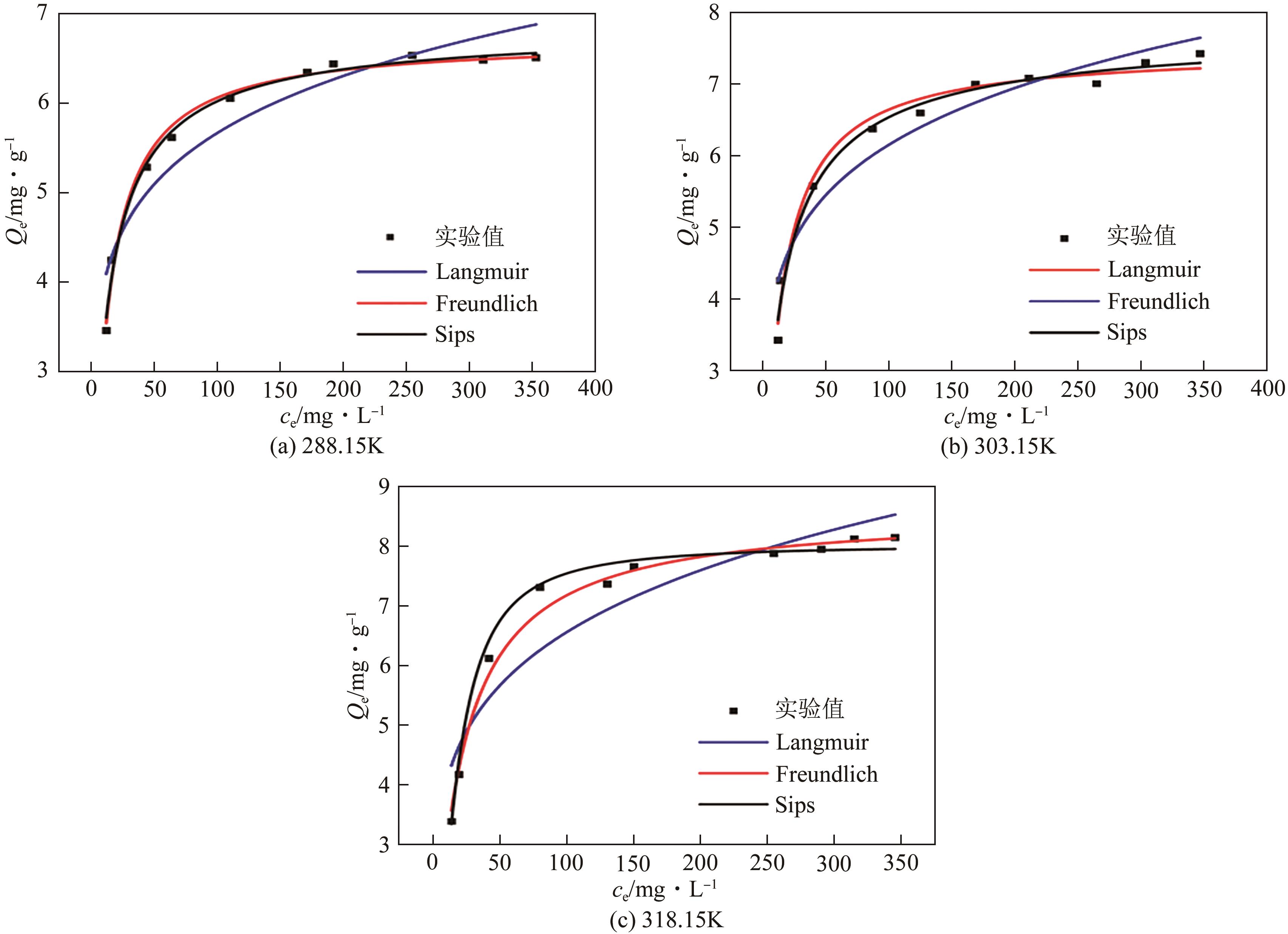
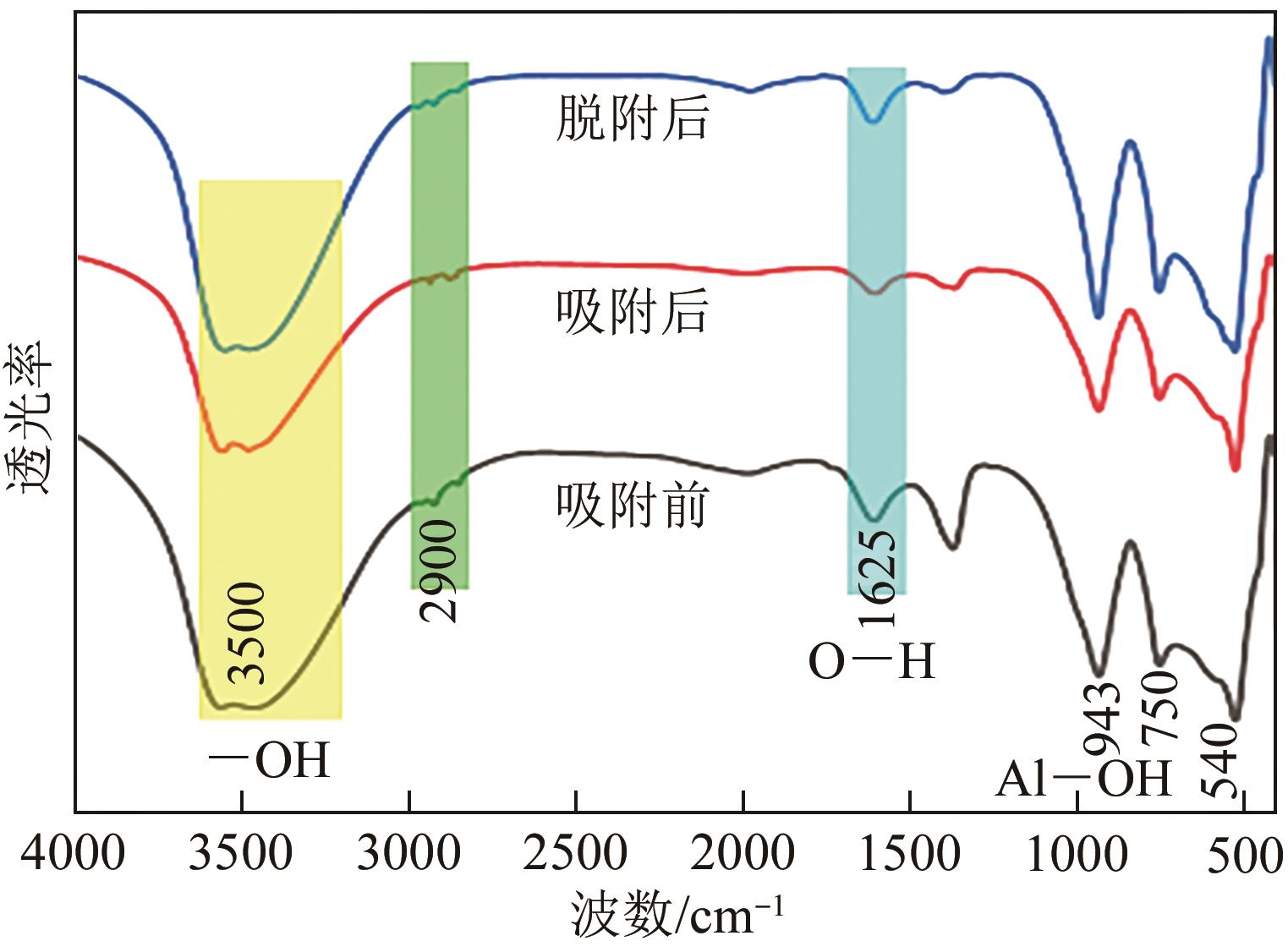
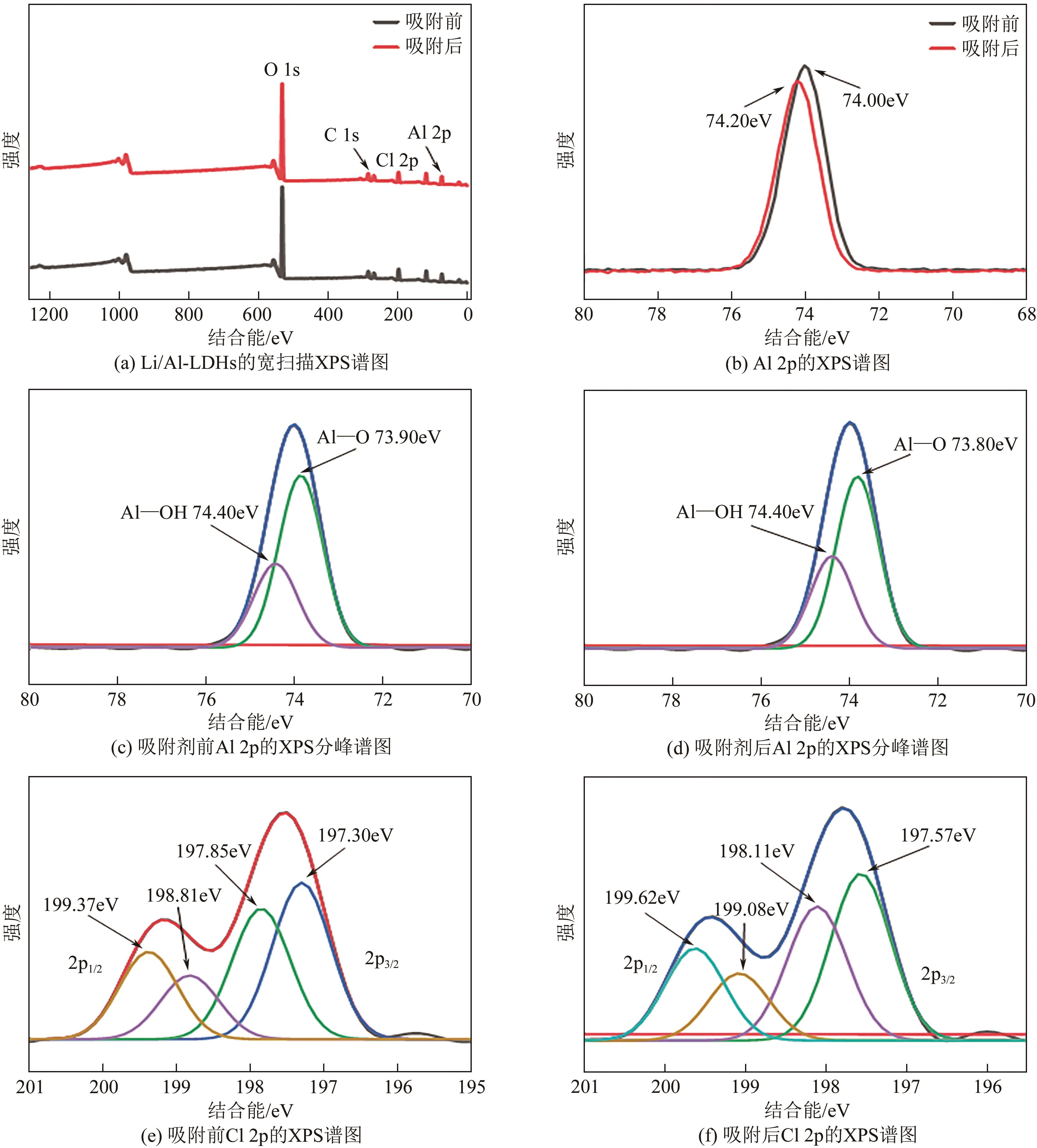
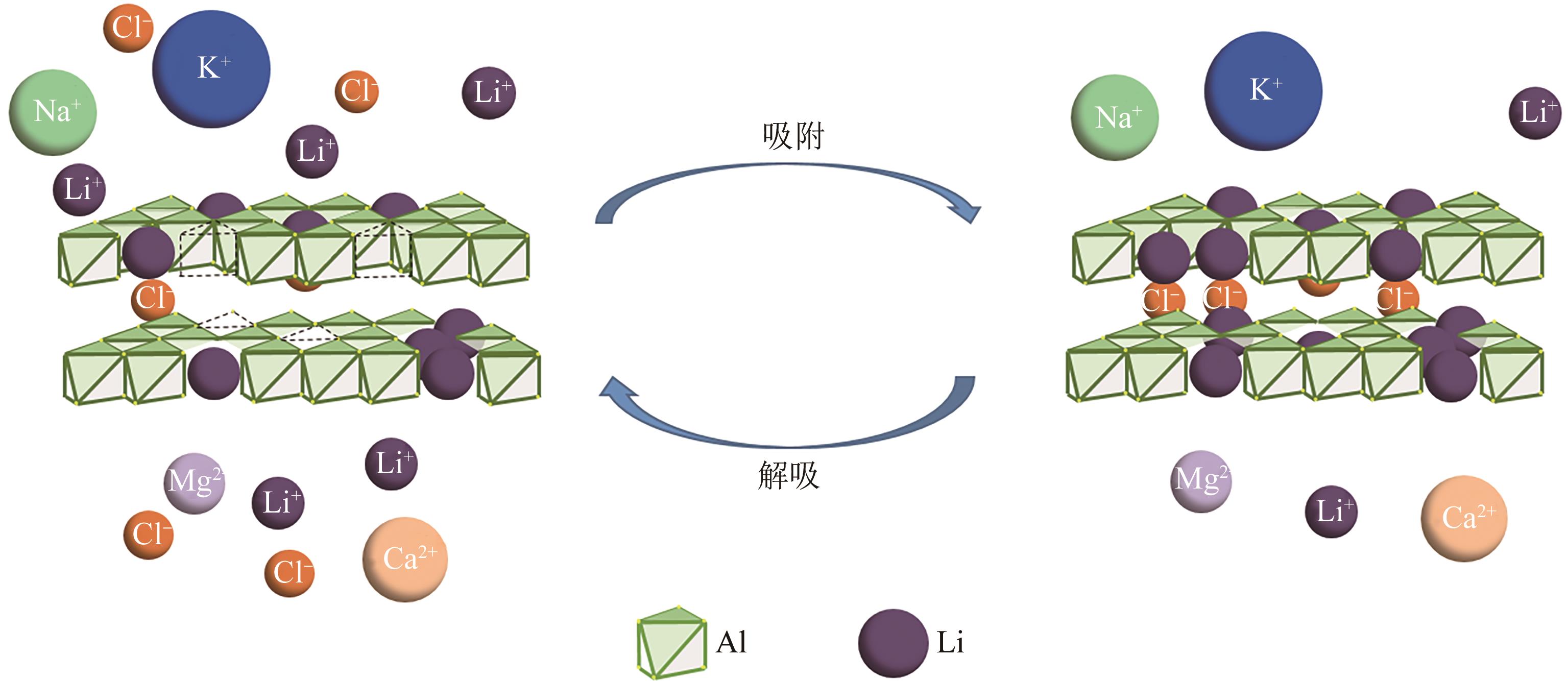
锂铝层状双金属氢氧化物(Li/Al-LDHs)是一种适用于高镁锂比卤水中提锂的吸附剂,但其存在吸附容量偏低、吸附机理与适用条件不够清晰等问题。本工作以铝粉、氢氧化锂为原料制备Li/Al-LDHs,考察了适用条件下溶液中阴阳离子对吸附过程的影响并进行了不同溶液组成下的提锂性能测试,结合红外光谱测试和X射线光电子能谱等表征明晰了锂脱嵌机理。结果表明,Li/Al-LDHs在卤水中的最大吸附容量为8.350mg/g,阳离子选择性顺序为Li+>Na+>Mg2+>Ca2+>K+。Li/Al-LDHs对Li+的吸附符合Langmuir等温吸附模型和准二级吸附动力学模型,吸附过程主要是基于尺寸筛分效应进行的,Li+进入吸附剂层间的空位后需要等量的阴离子(Cl-、SO42-等)进行电荷平衡,其吸锂容量随溶液体系总离子浓度的增加而变大,因此更适用于从高含盐量卤水中提锂。研究结果可为后续废旧金属铝制取Li/Al-LDHs用于盐湖卤水提锂提供技术参考。
中图分类号:
引用本文
杨新衡, 纪志永, 郭志远, 刘萁, 张盼盼, 汪婧, 刘杰, 毕京涛, 赵颖颖, 袁俊生. 锂铝层状双金属氢氧化物的制备及其锂脱嵌过程[J]. 化工进展, 2024, 43(9): 5262-5274.
YANG Xinheng, JI Zhiyong, GUO Zhiyuan, LIU Qi, ZHANG Panpan, WANG Jing, LIU Jie, BI Jingtao, ZHAO Yingying, YUAN Junsheng. Preparation of lithium aluminum layered double hydroxides and their lithium deintercalation performance[J]. Chemical Industry and Engineering Progress, 2024, 43(9): 5262-5274.
| 元素 | 前体中各离子质量分数/% | Li/Al-LDHs各离子质量分数/% |
|---|---|---|
| Li | 13.39 | 2.61 |
| Al | 22.84 | 23.12 |
| Cl | 17.19 | 13.22 |
表1 Li/Al-LDHs的元素分析结果
| 元素 | 前体中各离子质量分数/% | Li/Al-LDHs各离子质量分数/% |
|---|---|---|
| Li | 13.39 | 2.61 |
| Al | 22.84 | 23.12 |
| Cl | 17.19 | 13.22 |
| T/K | Qe,exp/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| K1/min-1 | Qe,cal/mg·g-1 | R2 | K2/g·mg-1·min-1 | Qe,cal/mg·g-1 | R2 | ||
| 288.15 | 6.674 | -0.021 | 3.057 | 0.906 | 0.143 | 6.805 | 0.998 |
| 303.15 | 7.278 | -0.025 | 3.013 | 0.824 | 0.137 | 7.377 | 0.999 |
| 318.15 | 8.350 | -0.027 | 3.016 | 0.841 | 0.116 | 8.490 | 0.999 |
表2 Li/Al-LDHs的相关动力学模型参数
| T/K | Qe,exp/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|---|
| K1/min-1 | Qe,cal/mg·g-1 | R2 | K2/g·mg-1·min-1 | Qe,cal/mg·g-1 | R2 | ||
| 288.15 | 6.674 | -0.021 | 3.057 | 0.906 | 0.143 | 6.805 | 0.998 |
| 303.15 | 7.278 | -0.025 | 3.013 | 0.824 | 0.137 | 7.377 | 0.999 |
| 318.15 | 8.350 | -0.027 | 3.016 | 0.841 | 0.116 | 8.490 | 0.999 |
| T/K | Langmuir | Freundlich | Sips | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KL/L·mg-1 | qmax/mg·g-1 | RL | R2 | KF/mg1-n ·L n ·g-1 | 1/n | R2 | KS/mg1-n ·L n ·g-1 | qmax/mg·g-1 | R2 | |
| 288.15 | 0.093 | 6.701 | 0.027 | 0.989 | 2.781 | 0.154 | 0.896 | 0.122 | 6.857 | 0.985 |
| 303.15 | 0.080 | 7.495 | 0.032 | 0.985 | 2.771 | 0.174 | 0.885 | 0.118 | 0.782 | 0.981 |
| 318.15 | 0.050 | 8.504 | 0.050 | 0.990 | 2.468 | 0.212 | 0.911 | 0.019 | 8.033 | 0.983 |
表3 Li/Al-LDHs对锂的吸附等温线拟合参数
| T/K | Langmuir | Freundlich | Sips | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KL/L·mg-1 | qmax/mg·g-1 | RL | R2 | KF/mg1-n ·L n ·g-1 | 1/n | R2 | KS/mg1-n ·L n ·g-1 | qmax/mg·g-1 | R2 | |
| 288.15 | 0.093 | 6.701 | 0.027 | 0.989 | 2.781 | 0.154 | 0.896 | 0.122 | 6.857 | 0.985 |
| 303.15 | 0.080 | 7.495 | 0.032 | 0.985 | 2.771 | 0.174 | 0.885 | 0.118 | 0.782 | 0.981 |
| 318.15 | 0.050 | 8.504 | 0.050 | 0.990 | 2.468 | 0.212 | 0.911 | 0.019 | 8.033 | 0.983 |
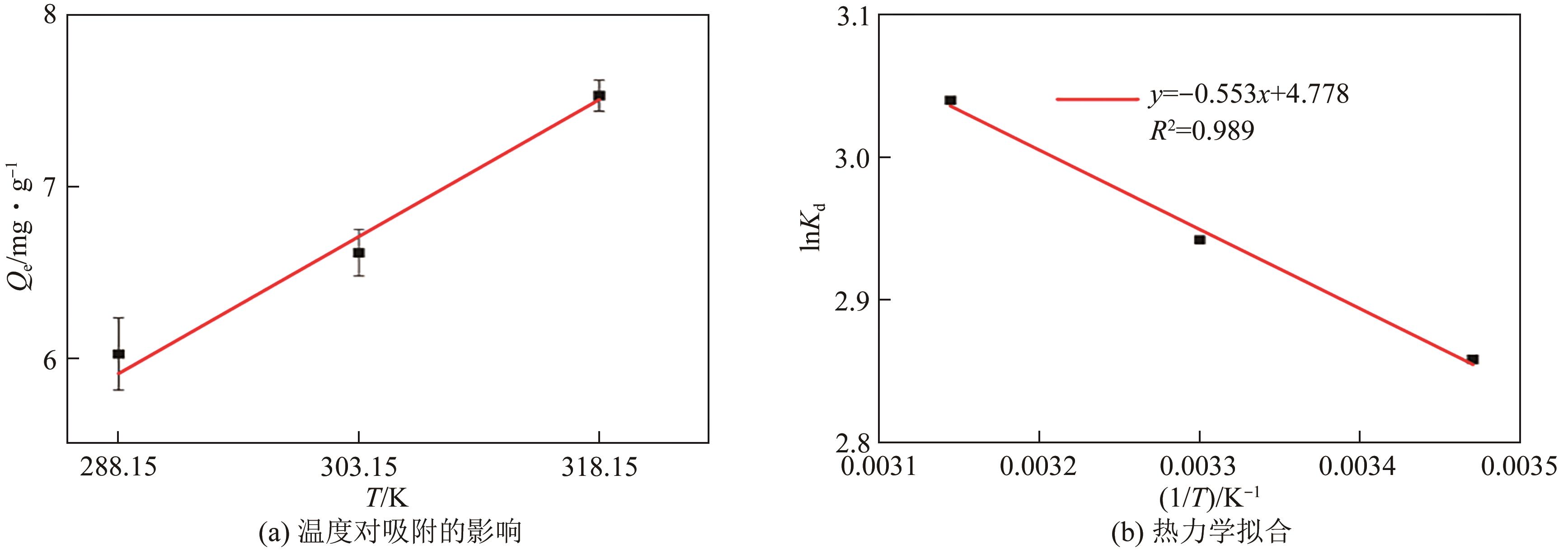
| T/K | ΔG | ΔH | ΔS |
|---|---|---|---|
| 288.15 | -12.18 | 10.58 | 79.03 |
| 303.15 | -12.29 | 10.58 | 79.03 |
| 318.15 | -12.47 | 10.58 | 79.03 |
表4 Li/Al-LDHs的相关热力学模型参数
| T/K | ΔG | ΔH | ΔS |
|---|---|---|---|
| 288.15 | -12.18 | 10.58 | 79.03 |
| 303.15 | -12.29 | 10.58 | 79.03 |
| 318.15 | -12.47 | 10.58 | 79.03 |
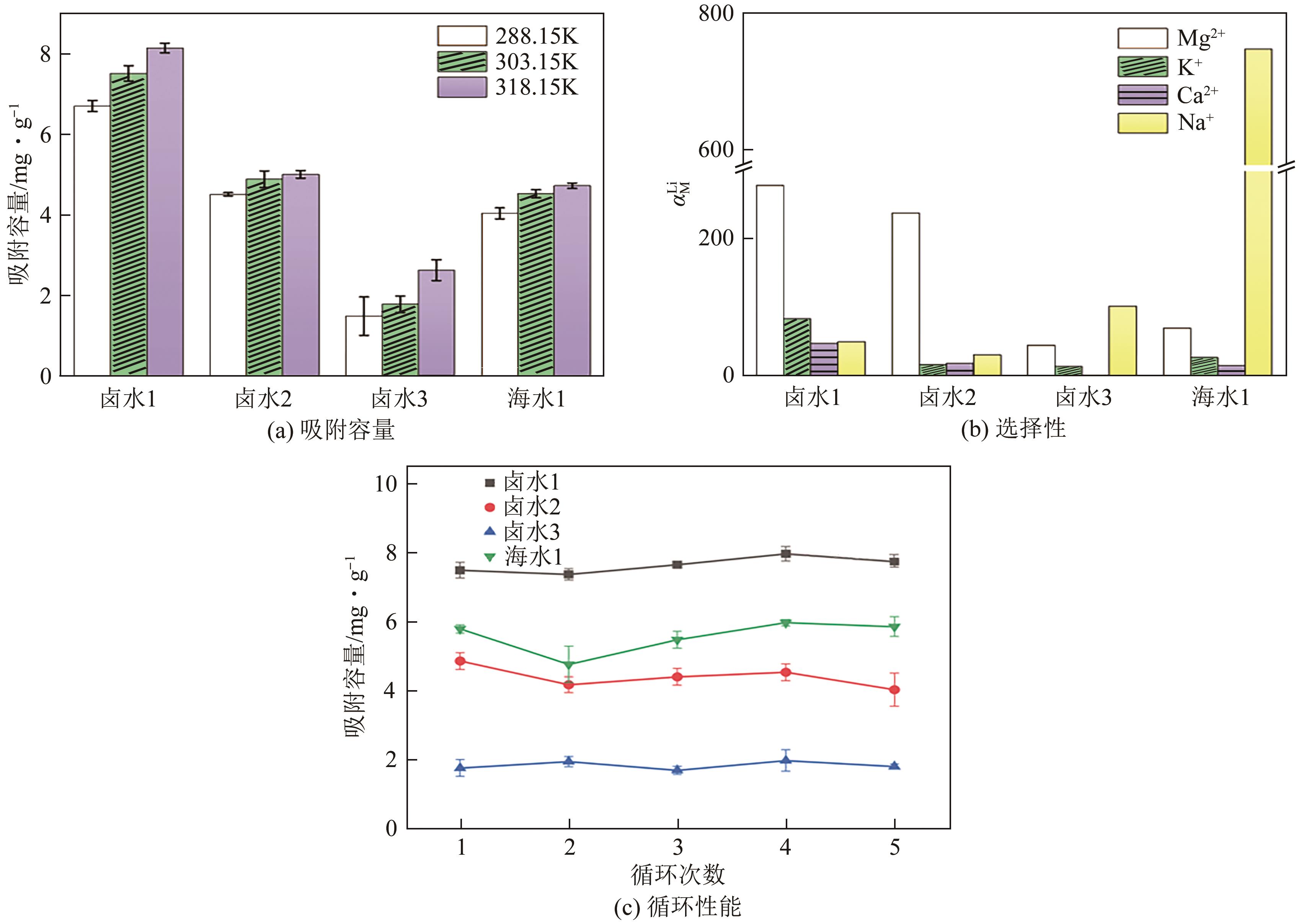
| 原料液 | Li+ | Mg2+ | Ca+ | Na+ | K+ | Cl- | |
|---|---|---|---|---|---|---|---|
| 卤水1 | 0.39 | 120.33 | 0.05 | 1.39 | 0.48 | 182.62 | — |
| 卤水2 | 0.25 | 89.10 | 0.23 | 1.81 | 0.73 | 83.27 | 28.10 |
| 卤水3 | 0.25 | 8.42 | 0.04 | 16.73 | 2.43 | 31.71 | 23.99 |
| 海水1 | 0.60 | 3.60 | 0.06 | 130.78 | 3.79 | 210.31 | — |
表5 原料液主要阴阳离子成分分析 (g/L)
| 原料液 | Li+ | Mg2+ | Ca+ | Na+ | K+ | Cl- | |
|---|---|---|---|---|---|---|---|
| 卤水1 | 0.39 | 120.33 | 0.05 | 1.39 | 0.48 | 182.62 | — |
| 卤水2 | 0.25 | 89.10 | 0.23 | 1.81 | 0.73 | 83.27 | 28.10 |
| 卤水3 | 0.25 | 8.42 | 0.04 | 16.73 | 2.43 | 31.71 | 23.99 |
| 海水1 | 0.60 | 3.60 | 0.06 | 130.78 | 3.79 | 210.31 | — |
| 吸附材料 | 原料液种类 | 参考文献 | |
|---|---|---|---|
| Li/Al-LDHs | 7.27 | 含0.4g/L Li+、120g/L Mg2+的察尔汗盐湖老卤 | [ |
| L-LDHs | 3.40 | 含0.14g/L Li+、92g/L Na+的地下卤水 | [ |
| 粒状Li/Al-LDHs | 6.40 | 含1.1g/L Li+、76.78g/L Na+的东台吉乃尔卤水 | [ |
| Li/Al-LDHs | 5.48 | 乌鲁米耶湖天然卤水 | [ |
| LDHs | <7.00 | c(Li+)=330mg/L的盐湖卤水 | [ |
| Li/Al-LDHs | 5.69 | c(Li+)=350mg/L的Li-Na-MgCl2体系 | [ |
| Li/Al-LDHs | 8.350 | c(Li+)=350mg/L、c(Mg2+)=120g/L的卤水 | 本工作 |
表6 与其他吸附剂的吸附容量对比
| 吸附材料 | 原料液种类 | 参考文献 | |
|---|---|---|---|
| Li/Al-LDHs | 7.27 | 含0.4g/L Li+、120g/L Mg2+的察尔汗盐湖老卤 | [ |
| L-LDHs | 3.40 | 含0.14g/L Li+、92g/L Na+的地下卤水 | [ |
| 粒状Li/Al-LDHs | 6.40 | 含1.1g/L Li+、76.78g/L Na+的东台吉乃尔卤水 | [ |
| Li/Al-LDHs | 5.48 | 乌鲁米耶湖天然卤水 | [ |
| LDHs | <7.00 | c(Li+)=330mg/L的盐湖卤水 | [ |
| Li/Al-LDHs | 5.69 | c(Li+)=350mg/L的Li-Na-MgCl2体系 | [ |
| Li/Al-LDHs | 8.350 | c(Li+)=350mg/L、c(Mg2+)=120g/L的卤水 | 本工作 |
| 1 | ZHANG Xueqian, DONG Mengfei, XIONG Yali, et al. Aqueous rechargeable Li+/Na+ hybrid ion battery with high energy density and long cycle life[J]. Small, 2020, 16(41): 2003585. |
| 2 | AUDI G, BERSILLON O, BLACHOT J, et al. The Nubase evaluation of nuclear and decay properties[J]. Nuclear Physics A, 2003, 729(1): 3-128. |
| 3 | SWAIN Basudev. Recovery and recycling of lithium: A review[J]. Separation and Purification Technology, 2017, 172: 388-403. |
| 4 | CHEN Shangqing, CHEN Zishen, WEI Zhenwei, et al. Titanium-based ion sieve with enhanced post-separation ability for high performance lithium recovery from geothermal water[J]. Chemical Engineering Journal, 2021, 410: 128320. |
| 5 | 韩佳欢, 乜贞, 方朝合, 等. 中国锂资源供需现状分析[J]. 无机盐工业, 2021, 53(12): 61-66. |
| HAN Jiahuan, NIE Zhen, FANG Chaohe, et al. Analysis of existing circumstance of supply and demand on China’s lithium resources[J]. Inorganic Chemicals Industry, 2021, 53(12): 61-66. | |
| 6 | 王核, 黄亮, 白洪阳, 等. 中国锂资源的主要类型、分布和开发利用现状: 评述和展望[J]. 大地构造与成矿学, 2022, 46(5): 848-866. |
| WANG He, HUANG Liang, BAI Hongyang, et al. Types, distribution, development and utilization of lithium mineral resources in China: Review and perspective[J]. Geotectonica et Metallogenia, 2022, 46(5): 848-866. | |
| 7 | 康锦, 卫丽娜, 成怀刚. 离子液体用于盐湖卤水萃取提锂的研究进展[J]. 无机盐工业, 2022, 54(1): 1-6. |
| KANG Jin, WEI Lina, CHENG Huaigang. Research progress on application of ionic liquids in extracting lithium from salt lakes[J]. Inorganic Chemicals Industry, 2022, 54(1): 1-6. | |
| 8 | LI Xiaowei, CHEN Wang, CHEN Linlin, et al. Temperature-responsive liquid-liquid extraction of Li+ from high Mg/Li ratio brine[J]. Separation and Purification Technology, 2023, 322: 124309. |
| 9 | HUA Junyuan, MA Xiaohua, JI Wenhui, et al. Unveiling the mechanism of liquid-liquid extraction separation of Li+/Mg2+ using tributyl phosphate/ionic liquid mixed solvents[J]. Journal of Molecular Liquids, 2022, 365: 120080. |
| 10 | JI Pengyuan, JI Zhiyong, CHEN Qingbai, et al. Effect of coexisting ions on recovering lithium from high Mg2+/Li+ ratio brines by selective-electrodialysis[J]. Separation and Purification Technology, 2018, 207: 1-11. |
| 11 | HUA Junyuan, HE Jintao, PEI Hongchang, et al. Supported ionic liquid membrane contactor with crown ether functionalized polyimide membrane for high-efficient Li+/Mg2+ selective separation[J]. Journal of Membrane Science, 2023, 687: 122038. |
| 12 | GUO Zhiyuan, JI Zhiyong, WANG Jing, et al. Electrochemical lithium extraction based on “rocking-chair” electrode system with high energy-efficient: The driving mode of constant current-constant voltage[J]. Desalination, 2022, 533: 115767. |
| 13 | ZOU Fan, XU Tao, WU Zhensheng, et al. Lithium and sodium adsorption on monolayer tellurene[J]. The Journal of Physical Chemistry C, 2020, 124(51): 28074-28082. |
| 14 | MARTHI R, YANG P, OWUSU-FORDJOUR E Y, et al. Role of stacking faults and hydroxyl groups on the lithium adsorption/desorption properties of layered H2TiO3 [J]. Materials Today Advances, 2022, 14: 100237. |
| 15 | LIU Gui, ZHAO Zhongwei, HE Lihua. Highly selective lithium recovery from high Mg/Li ratio brines[J]. Desalination, 2020, 474: 114185. |
| 16 | ZHOU Guolang, CHEN Linlin, LI Xiaowei, et al. Construction of truncated-octahedral LiMn2O4 for battery-like electrochemical lithium recovery from brine[J]. Green Energy & Environment, 2023, 8(4): 1081-1090. |
| 17 | GUO Zhiyuan, JI Zhiyong, WANG Jing, et al. Development of electrochemical lithium extraction based on a rocking chair system of LiMn2O4/Li1- x Mn2O4: Self-driven plus external voltage driven[J]. Separation and Purification Technology, 2021, 259: 118154. |
| 18 | ZHAO Muhua, ZHAO Chong, ZHANG Yang, et al. One-pot granulation of cross-linked PVA/LMO for efficient lithium recovery from gas field brine[J]. Journal of Environmental Chemical Engineering, 2023, 11(5): 110859. |
| 19 | CHEN Fangjie, LIN Yi, ZHANG Xun, et al. Preparation and evaluation of H2TiO3@attapulgite with high adsorption and selectivity for lithium ions[J]. Materials Letters, 2023, 345: 134471. |
| 20 | RUI Jicheng, DENG Ning, ZHAO Yiying, et al. Activation of persulfate via Mn doped Mg/Al layered double hydroxide for effective degradation of organics: Insights from chemical and structural variability of catalyst[J]. Chemosphere, 2022, 302: 134849. |
| 21 | SHENG Bingchun, SU Haiping, YU Jianguo, et al. Lithium extraction process from low grade Na+/K+ brines dependent on high layer charge layered double hydroxides[J]. Desalination, 2023, 565: 116856. |
| 22 | ZHONG Jing, LIN Sen, YU Jianguo. Li+ adsorption performance and mechanism using lithium/aluminum layered double hydroxides in low grade brines[J]. Desalination, 2021, 505: 114983. |
| 23 | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| SHENG Bingchun, YU Jianguo, LIN Sen. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent[J]. CIESC Journal, 2023, 74(8): 3375-3385. | |
| 24 | 张文丁, 邓小川, 朱朝梁, 等. 以PAC为原料制备锂吸附剂及其吸附性能的研究[J]. 无机盐工业, 2020, 52(2): 12-16. |
| ZHANG Wending, DENG Xiaochuan, ZHU Chaoliang, et al. Preparation of lithium adsorbent from PAC and its adsorption properties[J]. Inorganic Chemicals Industry, 2020, 52(2): 12-16. | |
| 25 | DONG Mingzhe, LUO Qinglong, LI Jun, et al. Reconstruction of MgAl-layered double hydroxides to LiAl-layered double hydroxides for scalable lithium extraction from salt lake brine[J]. Minerals Engineering, 2023, 202: 108293. |
| 26 | SING K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619. |
| 27 | 奚干卿. 离子半径的研究[J]. 海南师范学院学报(自然科学版), 2001, 14(3): 68-75. |
| XI Ganqing. A study of ionic radius[J]. Journal of Hainan Normal University (Natural Science), 2001, 14(3): 68-75. | |
| 28 | VOLKOV A G, PAULA S, DEAMER D W. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers[J]. Bioelectrochemistry and Bioenergetics, 1997, 42(2): 153-160. |
| 29 | QIAN Hongbo, HUANG Shaodong, BA Zhichen, et al. HTO/cellulose aerogel for rapid and highly selective Li+ recovery from seawater[J]. Molecules, 2021, 26(13): 4054. |
| 30 | JIANG Huixiong, YANG Ying, SUN Shuying, et al. Adsorption of lithium ions on lithium-aluminum hydroxides: Equilibrium and kinetics[J]. The Canadian Journal of Chemical Engineering, 2020, 98(2): 544-555. |
| 31 | MA Bin, Sanghwa OH, SHIN Won Sik, et al. Removal of Co2+, Sr2+ and Cs+ from aqueous solution by phosphate-modified montmorillonite (PMM)[J]. Desalination, 2011, 276(1/2/3): 336-346. |
| 32 | ISUPOV V P, KOTSUPALO N P, NEMUDRY A P, et al. Aluminium hydroxide as selective sorbent of lithium salts from brines and technical solutions[M]//Studies in Surface Science and Catalysis. Amsterdam: Elsevier 1999: 621-652. |
| 33 | HU Fangping, LIN Sen, LI Ping, et al. Quantitative effects of desorption intensity on structural stability and readsorption performance of lithium/aluminum layered double hydroxides in cyclic Li+ extraction from brines with ultrahigh Mg/Li ratio[J]. Industrial & Engineering Chemistry Research, 2020, 59(30): 13539-13548. |
| 34 | HEIDARI N, MOMENI P. Selective adsorption of lithium ions from Urmia Lake onto aluminum hydroxide[J]. Environmental Earth Sciences, 2017, 76(16): 551. |
| 35 | RYABTSEV A D. Benefication technology for lithium-containing hydrogenic mineral products[J]. Journal of Mining Science, 2005, 41(6): 573-582. |
| 36 | JIANG Huixiong, ZHANG Shuiyi, YANG Ying, et al. Synergic and competitive adsorption of Li-Na-MgCl2 onto lithium-aluminum hydroxides[J]. Adsorption, 2020, 26(7): 1039-1049. |
| 37 | SALEM Mansour A S, KHAN Amjad Mumtaz, MANEA Yahiya Kadaf. A novel nano-hybrid carbon architecture as chemo sensor for natural hazards: Active adsorption of Rose Bengal dye and detection of hazard pollutants at ppb level[J]. Journal of Environmental Chemical Engineering, 2022, 10(1): 107032. |
| 38 | LIU Yuting, WANG Mingkuang, CHEN Tsan Yao, et al. Arsenate sorption on lithium/aluminum layered double hydroxide intercalated by chloride and on gibbsite: sorption isotherms, envelopes, and spectroscopic studies[J]. Environmental Science & Technology, 2006, 40(24): 7784-7789. |
| [1] | 刘丽, 冯博, 文洋, 古启雄. 硅基介孔材料的合成、功能化及对金属的吸附研究进展[J]. 化工进展, 2024, 43(9): 5063-5078. |
| [2] | 吴宇琦, 李江涛, 丁建智, 宋秀兰, 苏冰琴. 焙烧镁铝水滑石脱除厌氧消化沼气中CO2的效果及机制[J]. 化工进展, 2024, 43(9): 5250-5261. |
| [3] | 王嘉, 李文翠, 吴凡, 高新芊, 陆安慧. NiMo/Al2O3催化剂活性组分分布调控及其加氢脱硫应用[J]. 化工进展, 2024, 43(8): 4393-4402. |
| [4] | 郑云香, 高艺伦, 李宴汝, 刘青霖, 张浩腾, 王向鹏. 氨基三乙酸酐改性多孔双网络水凝胶的制备及吸附性能[J]. 化工进展, 2024, 43(8): 4542-4549. |
| [5] | 刘玉灿, 高中鲁, 徐心怡, 纪现国, 张岩, 孙洪伟, 王港. 钙改性水葫芦基生物炭吸附水中敌草隆的效能与机理[J]. 化工进展, 2024, 43(8): 4630-4641. |
| [6] | 胡君杰, 黄兴俊, 雷成, 杨敏, 兰元宵, 罗建洪. 页岩气采出水中小分子有机物的深度处理[J]. 化工进展, 2024, 43(8): 4674-4680. |
| [7] | 武哲, 曲树光, 冯练享, 曾湘楚. 海藻酸钠/微晶纤维素复合水凝胶对水中甲基橙和亚甲基蓝的吸附性能与机理[J]. 化工进展, 2024, 43(8): 4681-4693. |
| [8] | 黄鸿, 欧阳浩民, 杨依静, 李昌霖, 陈烁娜. 硫化零价铁-微生物复合吸附剂对磷酸三(2-氯乙基)酯的吸附-降解机制[J]. 化工进展, 2024, 43(8): 4704-4713. |
| [9] | 郭长滨, 李蒙蒙, 冯梦晗, 原田, 张克强, 罗艳丽, 王风. 铈掺杂镧基钙钛矿制备及对水体磷酸盐和植酸的吸附性能[J]. 化工进展, 2024, 43(8): 4748-4756. |
| [10] | 毛华恺, 余洋, 张悦, 夏广坤, 吴赟韬, 楼乐瑶, 牛文娟, 刘念. 生物炭光催化氧化-吸附协同降解亚硝酸盐[J]. 化工进展, 2024, 43(8): 4757-4765. |
| [11] | 卞维柏, 张睿轩, 潘建明. 无机金属锂离子筛材料制备方法研究进展[J]. 化工进展, 2024, 43(8): 4173-4186. |
| [12] | 黄军, 张应娟, 林茵童, 韦雪纯, 吴雨桐, 毋高博, 莫钧麟, 赵祯霞, 赵钟兴. 蚕沙基生物多孔炭的制备及对杀虫单/呋虫胺的协同吸附与缓释性能[J]. 化工进展, 2024, 43(7): 3964-3971. |
| [13] | 张世蕊, 范朕连, 宋慧平, 张丽娜, 高宏宇, 程淑艳, 程芳琴. 粉煤灰负载光催化材料的研究进展[J]. 化工进展, 2024, 43(7): 4043-4058. |
| [14] | 刘克峰, 刘陶然, 蔡勇, 胡雪生, 董卫刚, 周华群, 高飞. 二氧化碳捕集技术研究和工程示范进展[J]. 化工进展, 2024, 43(6): 2901-2914. |
| [15] | 智远, 马吉亮, 陈晓平, 刘道银, 梁财. 流化床喷雾浸渍制备负载型钠基CO2吸附剂脱碳性能[J]. 化工进展, 2024, 43(6): 2961-2967. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||