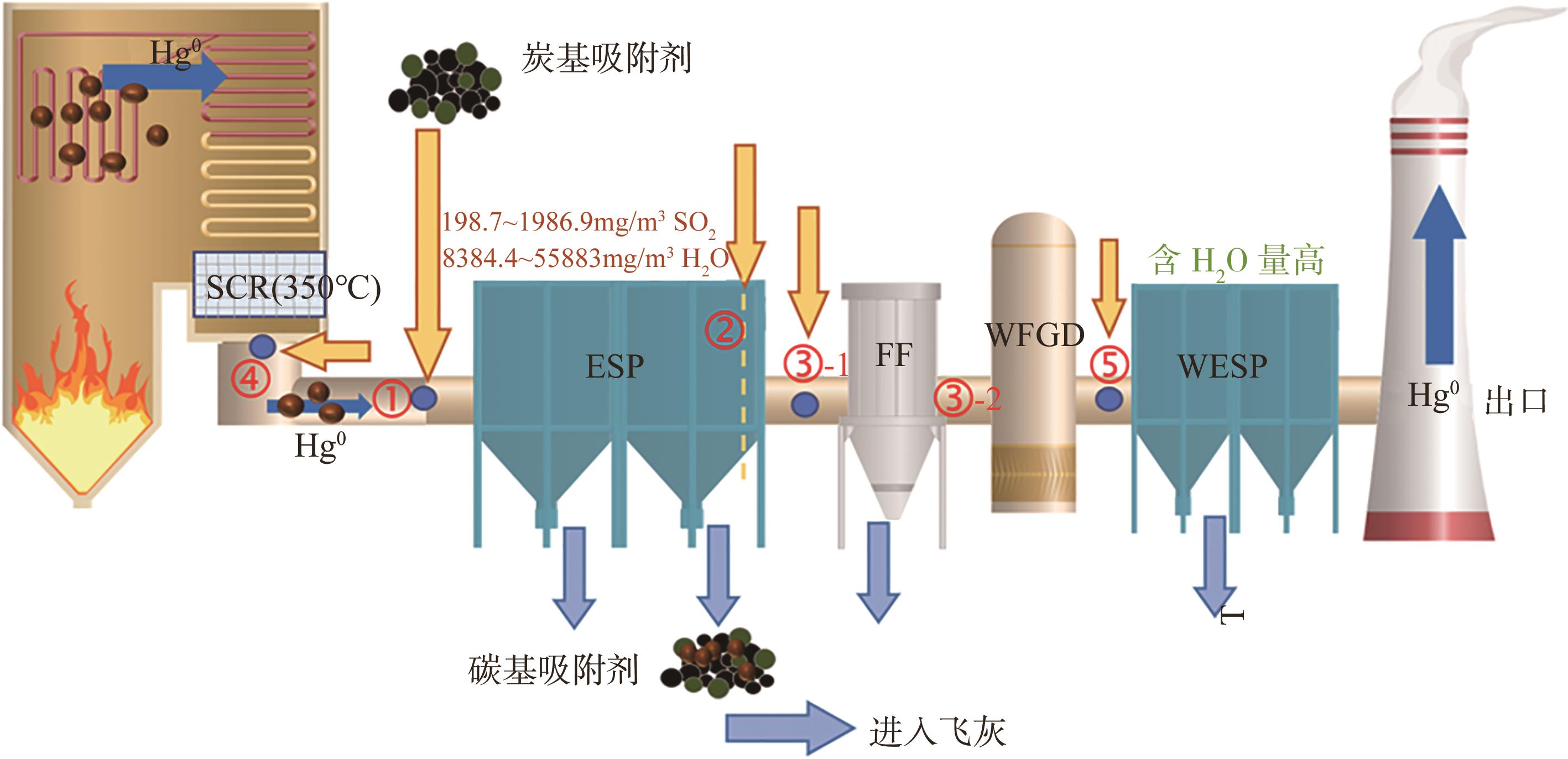
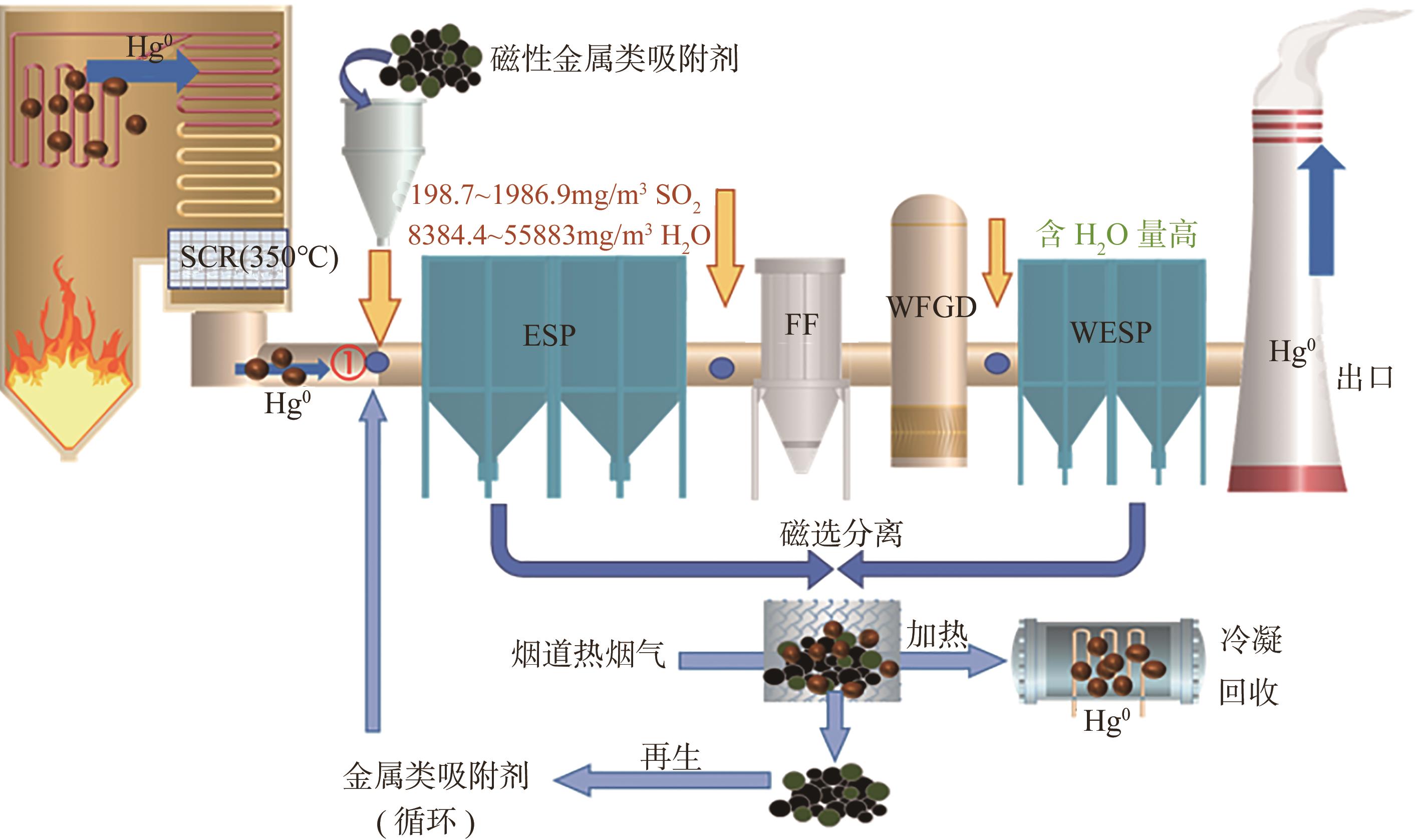
化工进展 ›› 2025, Vol. 44 ›› Issue (1): 513-524.DOI: 10.16085/j.issn.1000-6613.2024-0103
活性炭类和磁性金属类吸附剂喷射脱汞技术应用对比及最新进展
倪鹏1( ), 王先泓2, 黄钰涵2, 马晓彤1, 马子轸2, 谈琰2, 张华伟2, 刘亭2(
), 王先泓2, 黄钰涵2, 马晓彤1, 马子轸2, 谈琰2, 张华伟2, 刘亭2( )
)
- 1.山东科技大学储能技术学院,山东 青岛 266590
2.青岛理工大学环境与市政工程学院,山东 青岛 266520
-
收稿日期:2024-01-14修回日期:2024-05-22出版日期:2025-01-15发布日期:2025-02-13 -
通讯作者:刘亭 -
作者简介:倪鹏(1988-),男,讲师,博士,研究方向为燃煤烟气中重金属控制和垃圾焚烧烟气污染物控制。E-mail: nipeng198802@163.com。 -
基金资助:山东省自然科学基金(ZR2022QB084);国家自然科学基金(22078168);山东省泰山学者专家人才工程(taqn202211178)
Latest progress and comparison of the injection demercuration application of activated carbon and magnetic metals adsorbents
NI Peng1( ), WANG Xianhong2, HUANG Yuhan2, MA Xiaotong1, MA Zizhen2, TAN Yan2, ZHANG Huawei2, LIU Ting2(
), WANG Xianhong2, HUANG Yuhan2, MA Xiaotong1, MA Zizhen2, TAN Yan2, ZHANG Huawei2, LIU Ting2( )
)
- 1.College of Energy Storage Technology, Shandong University of Science and Technology, Qingdao 266590, Shandong, China
2.School of Environmental and Municipal Engineering, Qingdao University of Technology, Qingdao 266520, Shandong, China
-
Received:2024-01-14Revised:2024-05-22Online:2025-01-15Published:2025-02-13 -
Contact:LIU Ting
摘要:
吸附剂喷射脱汞技术是一项除现有设备协同脱汞外的单项脱汞技术,本文主要回顾了现有的活性炭类和磁性金属类两类吸附剂在喷射脱汞应用研究中的问题和优点,介绍了两类材料的性能特征,阐述了喷射位置应用差异,总结了脱汞后样品的性能和回收处置差异。重点讲述了活性炭类吸附剂脱汞的理论和实验研究发展,以及脱汞后汞的化合物稳定性分析;详细介绍了磁性金属类吸附剂的发展、实际应用和磁选回收汞的流程及参数。结果表明,硫化物和硒化物类的吸附剂展现出了优越的能力和低的浸出性,是一项非常有前景的吸附剂。最后对单项脱汞技术在应用中需要解决的问题、不足和技术应用前景进行了展望。
中图分类号:
引用本文
倪鹏, 王先泓, 黄钰涵, 马晓彤, 马子轸, 谈琰, 张华伟, 刘亭. 活性炭类和磁性金属类吸附剂喷射脱汞技术应用对比及最新进展[J]. 化工进展, 2025, 44(1): 513-524.
NI Peng, WANG Xianhong, HUANG Yuhan, MA Xiaotong, MA Zizhen, TAN Yan, ZHANG Huawei, LIU Ting. Latest progress and comparison of the injection demercuration application of activated carbon and magnetic metals adsorbents[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 513-524.
| 样品 | 反应气氛 | 初始Hg0浓度 /μg·m-3 | 最优反应 温度/℃ | 样品量 | 流量 /L·min-1 | Hg0脱除效率 /% | 吸附容量 /μg·g-1 | 吸附速率 /μg·g-1·min-1 |
|---|---|---|---|---|---|---|---|---|
| NH4Br-AC[ | 216.1mg/m3 NO,51319mg/m3 O2,1845.0mg/m3 SO2,0~152130mg/m3 CO2,0~51919.3mg/m3 H2O | 18~76.8 | 150 | 50mg 吸附剂+ 500mg SiO2 | 2.0 | 80 | 11491 | — |
| E8Fe3[ | 752.8mg/m3 NO,48387.1~135161.3mg/m3 O2,1162.5mg/m3 SO2,41536.8mg/m3 H2O | 50 | 90~150 (130) | 300mg 吸附剂+ 4g QZ | 0.85 | 76.9 | — | — |
| S8KI3[ | 337.7mg/m3 NO,54041.6mg/m3 O2,1082.2mg/m3 SO2,7608.0mg/m3 H2O | 60 | 80~160 (160) | 80mg+920mg QZ | 0.85 | 96 | — | — |
| SAC-800[ | 253.3mg/m3 NO,45035mg/m3 O2,901.7mg/m3 SO2,15216mg/m3 H2O | 80 | 80~160 (160) | 50mg+950mg QZ | 1 | 90.8 | 2382.6 | — |
| EAC-800 | 253.3mg/m3 NO,45035mg/m3 O2,901.7mg/m3 SO2,15216mg/m3 H2O | 80 | 80~160 (160) | 50mg+950mg QZ | 1 | 92.8 | 2909.5 | — |
Co0.4-Fe12 /RSWU(500)[ | 362.9mg/m3 NO,58065mg/m3 O2,1162.6mg/m3 SO2,8174.4mg/m3 H2O | 65 | 70~160 (160) | 60mg+500mg QZ | 0.8 | 89 | 4833.3 | — |
| g-C3N4[ | 93.0/186.1mg/m3 NO,49618mg/m3 O2,596.0/1192.1mg/m3 SO2, 55883mg/m3 H2O | 55 | 20~1000 (160) | — | 1.2 | 50 | >116 | 1.29 |
| B1C2[ | 36459mg/m3 O2,N2 | 33±1 | 80~180(80) | 30mg+200mg QZ | 1.5 | 60 | 584.2 | 0.18 |
| Fe-5%Ce-1%Mn/BC[ | N2 | 100 | 50 | 1000mg | 1.4 | 97 | 24 | 0.144 |
| BC/MBC[ | 39694.7/59542.0mg/m3 O2,163750.7/955212.5mg/m3 CO2,22353.0/67059.2mg/m3 H2O | 85 | 120 | 50mg | 1.2 | 90 | — | — |
| MSC[ | N2 | 65/700 | 50~125(100) | 10mg | 0.3 | 90 | 7828.39 | 28.67 |
| E8B5[ | 7608mg/m3 H2O,45035mg/m3 O2,1082mg/m3 SO2,337. mg/m3 NO | 50 | 130 | 300mg+4g QZ | 0.8 | 94.6 | 326.46 | — |
表1 不同活性炭类吸附剂的性能对比
| 样品 | 反应气氛 | 初始Hg0浓度 /μg·m-3 | 最优反应 温度/℃ | 样品量 | 流量 /L·min-1 | Hg0脱除效率 /% | 吸附容量 /μg·g-1 | 吸附速率 /μg·g-1·min-1 |
|---|---|---|---|---|---|---|---|---|
| NH4Br-AC[ | 216.1mg/m3 NO,51319mg/m3 O2,1845.0mg/m3 SO2,0~152130mg/m3 CO2,0~51919.3mg/m3 H2O | 18~76.8 | 150 | 50mg 吸附剂+ 500mg SiO2 | 2.0 | 80 | 11491 | — |
| E8Fe3[ | 752.8mg/m3 NO,48387.1~135161.3mg/m3 O2,1162.5mg/m3 SO2,41536.8mg/m3 H2O | 50 | 90~150 (130) | 300mg 吸附剂+ 4g QZ | 0.85 | 76.9 | — | — |
| S8KI3[ | 337.7mg/m3 NO,54041.6mg/m3 O2,1082.2mg/m3 SO2,7608.0mg/m3 H2O | 60 | 80~160 (160) | 80mg+920mg QZ | 0.85 | 96 | — | — |
| SAC-800[ | 253.3mg/m3 NO,45035mg/m3 O2,901.7mg/m3 SO2,15216mg/m3 H2O | 80 | 80~160 (160) | 50mg+950mg QZ | 1 | 90.8 | 2382.6 | — |
| EAC-800 | 253.3mg/m3 NO,45035mg/m3 O2,901.7mg/m3 SO2,15216mg/m3 H2O | 80 | 80~160 (160) | 50mg+950mg QZ | 1 | 92.8 | 2909.5 | — |
Co0.4-Fe12 /RSWU(500)[ | 362.9mg/m3 NO,58065mg/m3 O2,1162.6mg/m3 SO2,8174.4mg/m3 H2O | 65 | 70~160 (160) | 60mg+500mg QZ | 0.8 | 89 | 4833.3 | — |
| g-C3N4[ | 93.0/186.1mg/m3 NO,49618mg/m3 O2,596.0/1192.1mg/m3 SO2, 55883mg/m3 H2O | 55 | 20~1000 (160) | — | 1.2 | 50 | >116 | 1.29 |
| B1C2[ | 36459mg/m3 O2,N2 | 33±1 | 80~180(80) | 30mg+200mg QZ | 1.5 | 60 | 584.2 | 0.18 |
| Fe-5%Ce-1%Mn/BC[ | N2 | 100 | 50 | 1000mg | 1.4 | 97 | 24 | 0.144 |
| BC/MBC[ | 39694.7/59542.0mg/m3 O2,163750.7/955212.5mg/m3 CO2,22353.0/67059.2mg/m3 H2O | 85 | 120 | 50mg | 1.2 | 90 | — | — |
| MSC[ | N2 | 65/700 | 50~125(100) | 10mg | 0.3 | 90 | 7828.39 | 28.67 |
| E8B5[ | 7608mg/m3 H2O,45035mg/m3 O2,1082mg/m3 SO2,337. mg/m3 NO | 50 | 130 | 300mg+4g QZ | 0.8 | 94.6 | 326.46 | — |
| 名称 | 机组 | 效率/% | 喷射量/g·h-1 | 用过的吸附剂处理 | 形貌 |
|---|---|---|---|---|---|
| 商用活性炭[ | 480MW | 90① | 25000 | 允许灰分销售,C/Hg比②未知,成本包括喷射装置和飞灰无害化处理等 |  |
| CuCl2/磁珠[ | 570MW | 89.8① | 560000 | 磁选分离吸附剂,吸附剂回收比例未知,成本包括喷射装置、磁选组合机构和加热脱附汞装置等 |  |
| 实验室喷射台架 | 80.6 | 3.79 | |||
| 溴化稻壳活性炭[ | 实验室喷射台架 | 70.6 | 7.3 | C/Hg比:未知 | |
| 循环流化床 | 82.9 | 未知 | C/Hg比:20000 |
表2 燃煤烟气中不同喷射吸附剂的对比
| 名称 | 机组 | 效率/% | 喷射量/g·h-1 | 用过的吸附剂处理 | 形貌 |
|---|---|---|---|---|---|
| 商用活性炭[ | 480MW | 90① | 25000 | 允许灰分销售,C/Hg比②未知,成本包括喷射装置和飞灰无害化处理等 |  |
| CuCl2/磁珠[ | 570MW | 89.8① | 560000 | 磁选分离吸附剂,吸附剂回收比例未知,成本包括喷射装置、磁选组合机构和加热脱附汞装置等 |  |
| 实验室喷射台架 | 80.6 | 3.79 | |||
| 溴化稻壳活性炭[ | 实验室喷射台架 | 70.6 | 7.3 | C/Hg比:未知 | |
| 循环流化床 | 82.9 | 未知 | C/Hg比:20000 |
| 名称 | 初始汞浓度/μg·m-3 | 反应温度/℃ | 脱汞产物 | 花费 | 产物稳定性/℃ |
|---|---|---|---|---|---|
| 商用活性炭 | 5.8 | 154 | HgO | 500USD/h | 400~600 |
| CuCl2/磁珠[ | 10 | 150 | HgCl2 | 第一次循环花费117USD/h;随着循环次数增加,费用增加 | 100~150 |
| 溴化稻壳活性炭Br-RHC | 12.3 | 120 | HgO | 未知 | 400~600 |
表3 不同吸附剂的喷射应用对比
| 名称 | 初始汞浓度/μg·m-3 | 反应温度/℃ | 脱汞产物 | 花费 | 产物稳定性/℃ |
|---|---|---|---|---|---|
| 商用活性炭 | 5.8 | 154 | HgO | 500USD/h | 400~600 |
| CuCl2/磁珠[ | 10 | 150 | HgCl2 | 第一次循环花费117USD/h;随着循环次数增加,费用增加 | 100~150 |
| 溴化稻壳活性炭Br-RHC | 12.3 | 120 | HgO | 未知 | 400~600 |
| 文献中样品 | 温度/℃ | 脱汞效率/% | 吸附容量/mg·g-1 | 吸附速率/μg·g-1·min-1 | 产物 |
|---|---|---|---|---|---|
| MoSe3[ | 50 | 100 | 1000 | 240 | HgSe |
| RT-CuSe[ | 60 | 99.2 | 345 | 42.5 | HgO/HgSe |
| xSe-FeS[ | 60 | 90 | 2.45 | 0.43 | HgS/HgSe |
| 改性Fe-Ti尖晶石[ | 60 | 100 | 0.69 | 1.92 | HgO/HgSO4/HgS |
| CuFeS2[ | 80 | 100 | 37.24 | 63.40 | β-HgS |
| (Fe2.3Ti0.7)1-δ O4[ | 250 | 87~99 | 3.94 | 6.57 | HgO |
| g-C3N4[ | 160 | 50 | >0.12 | 1.29 | π-/σ-汞-三嗪化合物 |
| MoS3/TiO2[ | 80 | 100 | 28.1 | 34.8 | HgS |
| Co9S8-PC[ | 100 | 100 | 43.18 | 11.4 | HgS |
| Fe x Co1-x S y @CF[ | 150 | 99 | 45.6 | >2.5 | HgS |
| ZnO@CuS[ | 75 | 89 | 60.53 | 16.81 | Hg-OM/HgS |
| 载硫V2O5-MoO3/TiO2[ | 60 | 100 | 20.7 | 35.4 | HgS |
| 载硫MoO3/Fe-Ti尖晶石[ | 60 | 100 | 66.3 | 93.3 | HgS |
| FeWS x /TiO2[ | 100 | 100 | 9.0 | 25.0 | HgS |
表4 不同脱汞材料的吸附容量及汞产物的对比
| 文献中样品 | 温度/℃ | 脱汞效率/% | 吸附容量/mg·g-1 | 吸附速率/μg·g-1·min-1 | 产物 |
|---|---|---|---|---|---|
| MoSe3[ | 50 | 100 | 1000 | 240 | HgSe |
| RT-CuSe[ | 60 | 99.2 | 345 | 42.5 | HgO/HgSe |
| xSe-FeS[ | 60 | 90 | 2.45 | 0.43 | HgS/HgSe |
| 改性Fe-Ti尖晶石[ | 60 | 100 | 0.69 | 1.92 | HgO/HgSO4/HgS |
| CuFeS2[ | 80 | 100 | 37.24 | 63.40 | β-HgS |
| (Fe2.3Ti0.7)1-δ O4[ | 250 | 87~99 | 3.94 | 6.57 | HgO |
| g-C3N4[ | 160 | 50 | >0.12 | 1.29 | π-/σ-汞-三嗪化合物 |
| MoS3/TiO2[ | 80 | 100 | 28.1 | 34.8 | HgS |
| Co9S8-PC[ | 100 | 100 | 43.18 | 11.4 | HgS |
| Fe x Co1-x S y @CF[ | 150 | 99 | 45.6 | >2.5 | HgS |
| ZnO@CuS[ | 75 | 89 | 60.53 | 16.81 | Hg-OM/HgS |
| 载硫V2O5-MoO3/TiO2[ | 60 | 100 | 20.7 | 35.4 | HgS |
| 载硫MoO3/Fe-Ti尖晶石[ | 60 | 100 | 66.3 | 93.3 | HgS |
| FeWS x /TiO2[ | 100 | 100 | 9.0 | 25.0 | HgS |
| 1 | KOULOCHERIS Vassilis, PLAKIA Anthoula, LOULI Vasiliki, et al. Calculating the chemical and phase equilibria of mercury in natural gas[J]. Fluid Phase Equilibria, 2021, 544: 113089. |
| 2 | OUTRIDGE Peter, MASON R P, WANG Feiyue, et al. Updated global and oceanic mercury budgets for the United Nations Global Mercury Assessment 2018[J]. Environmental Science & Technology, 2018, 52(20): 11466-11477. |
| 3 | ZHAO Shilin, PUDASAINEE Deepak, DUAN Yufeng, et al. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies[J]. Progress in Energy and Combustion Science, 2019, 73: 26-64. |
| 4 | 冯新斌, 史建波, 李平, 等. 我国汞污染研究与履约进展[J]. 中国科学院院刊, 2020, 35(11): 1344-1350. |
| FENG Xinbin, SHI Jianbo, LI Ping, et al. Progress of mercury pollution research and implementation of minamata convention in China[j]. Bulletin of Chinese Academy of Sciences[J], 2020, 35(11): 1344-1350. | |
| 5 | LI Ying, YU Jianglong, LIU Yangxian, et al. A review on removal of mercury from flue gas utilizing existing air pollutant control devices (APCDs)[J]. Journal of Hazardous Materials, 2022, 427: 128132. |
| 6 | XIAO Rihong, GAO Tian, CUI Xiangzheng, et al. Removal of elemental mercury from flue gas by recyclable CuCl2 modified magnetospheres catalyst from fly ash: Part 6. Commercial scale demonstration at a 1000MWth coal-fired power plant[J]. Fuel, 2022, 310: 122219. |
| 7 | SHI Mengting, LUO Guangqian, XU Yang, et al. Using H2S plasma to modify activated carbon for elemental mercury removal[J]. Fuel, 2019, 254: 115549. |
| 8 | CAO Limei, YANG Jie, XU Yi, et al. The coupling use of electro-chemical and advanced oxidation to enhance the gaseous elemental mercury removal in flue gas[J]. Separation and Purification Technology, 2021, 257: 117883. |
| 9 | 高天, 张伊黎, 熊卓, 等. 改性氧化钛光催化氧化单质汞性能及其影响因素研究进展[J]. 化工进展, 2022, 41(2): 690-700. |
| GAO Tian, ZHANG Yili, XIONG Zhuo, et al. Research progress of modified titanium oxide photocatalytic oxidation of elemental mercury and its influencing factors[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 690-700. | |
| 10 | WANG Yan, WANG Zhuliang, PAN Jianfeng, et al. Removal of gaseous hydrogen sulfide using Fenton reagent in a spraying reactor[J]. Fuel, 2019, 239: 70-75. |
| 11 | LI Hailong, ZHANG Wei, QU Wenqi, et al. Facile pathway towards crystallinity adjustment and performance enhancement of copper selenide for vapor-phase elemental mercury sequestration[J]. Chemical Engineering Journal, 2022, 430: 132811. |
| 12 | 杜雯, 殷立宝, 禚玉群, 等. 100MW燃煤电厂非碳基吸附剂喷射脱汞实验研究[J]. 化工学报, 2014, 65(11): 4413-4419. |
| DU Wen, YIN Libao, ZHUO Yuqun, et al. Experimental study on mercury capture using non-carbon sorbents in 100MW coal-fired power plant[J]. CIESC Journal, 2014, 65(11): 4413-4419. | |
| 13 | HONG Qinyuan, XU Haomiao, LI Jiaxing, et al. Shell-thickness-induced spontaneous inward migration of mercury in porous ZnO@CuS for gaseous mercury immobilization[J]. Chemical Engineering Journal, 2021, 420: 127592. |
| 14 | 石其其, 王玉亭, 沈伯雄, 等. 负载氯的分级多孔炭制备及其脱汞性能的研究[J]. 燃料化学学报, 2019, 47(8): 1000-1007. |
| SHI Qiqi, WANG Yuting, SHEN Boxiong, et al. Synthesis of hierarchical porous carbon loaded with chlorine and its mercury removal performance[J]. Journal of Fuel Chemistry and Technology, 2019, 47(8): 1000-1007. | |
| 15 | ABBAS Tauqeer, GONFA Girma, LETHESH Kallidanthiyil Chellappan, et al. Mercury capture from natural gas by carbon supported ionic liquids: Synthesis, evaluation and molecular mechanism[J]. Fuel, 2016, 177: 296-303. |
| 16 | GUO Yongfu, YAN Naiqiang, YANG Shijian, et al. Conversion of elemental mercury with a novel membrane delivery catalytic oxidation system (MDCOs)[J]. Environmental Science & Technology, 2011, 45(2): 706-711. |
| 17 | WANG Ben, SONG Zijian, SUN Lushi. A review: Comparison of multi-air-pollutant removal by advanced oxidation processes—Industrial implementation for catalytic oxidation processes[J]. Chemical Engineering Journal, 2021, 409: 128136. |
| 18 | 王闰林, 段钰锋, 耿新泽, 等. 75t/h CFB燃煤锅炉烟道喷射吸附剂脱汞数值模拟[J]. 东南大学学报(自然科学版), 2020, 50(2): 342-350. |
| WANG Runlin, DUAN Yufeng, GENG Xinze, et al. Numerical simulation on sorbent injection into flue gas for mercury capture in 75t/h CFB coal-fired boiler[J]. Journal of Southeast University (Natural Science Edition), 2020, 50(2): 342-350. | |
| 19 | SHI Qiqi, WANG Yuting, ZHANG Xiao, et al. Hierarchically porous biochar synthesized with CaCO3 template for efficient Hg0 adsorption from flue gas[J]. Fuel Processing Technology, 2020, 199: 106247. |
| 20 | YANG Wei, CHEN Hui, HAN Xuan, et al. Preparation of magnetic Co-Fe modified porous carbon from agricultural wastes by microwave and steam activation for mercury removal[J]. Journal of hazardous materials, 2020, 381: 120981. |
| 21 | WANG Chang, ZHANG Xufan, MEI Jian, et al. Outstanding performance of magnetically separable sulfureted MoO3/Fe-Ti spinel for gaseous Hg0 recovery from smelting flue gas: mechanism and adsorption kinetics[J]. Environmental Science & Technology, 2020, 54(12): 7659-7668. |
| 22 | YANG Jianping, LI Qin, ZHU Wenbing, et al. Recyclable chalcopyrite sorbent for mercury removal from coal combustion flue gas[J]. Fuel, 2021, 290: 120049. |
| 23 | LI Hailong, HUANG Guohai, YANG Qin, et al. Numerical simulation of sorbent injection for mercury removal within an electrostatic precipitator: In-flight plus wall-bounded mechanism[J]. Fuel, 2022, 309: 122142. |
| 24 | ZHAO Weimeng, GENG Xinze, LU Jincheng, et al. Mercury removal performance of brominated biomass activated carbon injection in simulated and coal-fired flue gas[J]. Fuel, 2021, 285: 119131. |
| 25 | BROWN Thomas D, SMITH Dennis N, O'DOWD William J, et al. Control of mercury emissions from coal-fired power plants: A preliminary cost assessment and the next steps for accurately assessing control costs[J]. Fuel Processing Technology, 2000, 65: 311-341. |
| 26 | LIU Ziyang, ADEWUYI Yusuf G, SHI Shuo, et al. Removal of gaseous Hg0 using novel seaweed biomass-based activated carbon[J]. Chemical Engineering Journal, 2019, 366: 41-49. |
| 27 | 睢辉, 张梦泽, 董勇, 等. 燃煤烟气中单质汞吸附与氧化机理研究进展[J]. 化工进展, 2014, 33(6): 1582-1588. |
| SUI Hui, ZHANG Mengze, DONG Yong, et al. Research progress of adsorption and oxidation mechanism of elemental mercury from coal-fired flue gas[J]. Chemical Industry and Engineering Progress, 2014, 33(6): 1582-1588. | |
| 28 | LIU Dongjing, ZHANG Zhen, LUO Fei, et al. Elemental mercury capture from simulated flue gas by graphite-phase carbon nitride[J]. Energy & Fuels, 2020, 34 (6): 6851-6861. |
| 29 | LUO Zifeng, JIA Tao, LIU Qianyan, et al. Development of CuInS2/g-C3N4 nanolayer for efficient adsorption of elemental mercury from coal combustion flue gas[J]. Chemical Engineering Journal, 2021, 426: 131905. |
| 30 | YANG Qin, YANG Zequn, LI Hailong, et al. Selenide functionalized natural mineral sulfides as efficient sorbents for elemental mercury capture from coal combustion flue gas[J]. Chemical Engineering Journal, 2020, 398: 125611. |
| 31 | LIAO Yong, CHEN Dong, ZOU Sijie, et al. Recyclable naturally derived magnetic pyrrhotite for elemental mercury recovery from flue gas[J]. Environmental Science & Technology, 2016, 50(19): 10562-10569. |
| 32 | ZHANG Huawei, LI Zishun, LIU Ting, et al. Satisfactory anti-interference and high performance of the 1Co-1Ce/Mn@ZSM-5 catalyst for simultaneous removal of NO and Hg0 in abominable flue gas [J]. Environmental Science & Technology, 2022, 56(6): 3596-3603. |
| 33 | ZHOU Mengli, XU Yang, LUO Guangqian, et al. Facile synthesis of phosphorus-doped porous biochars for efficient removal of elemental mercury from coal combustion flue gas[J]. Chemical Engineering Journal, 2022, 432: 134440. |
| 34 | JIA Li, YU Yue, GUO Jinrong, et al. Study of the molecular structure and elemental mercury adsorption mechanism of biomass char[J]. Energy & Fuels, 2020, 34(10): 12743-12756. |
| 35 | XU Wen, HUSSAIN Arshad, LIU Yangxian. A review on modification methods of adsorbents for elemental mercury from flue gas[J]. Chemical Engineering Journal, 2018, 346: 692-711. |
| 36 | GENG Xinze, DUAN Yufeng, ZHAO Shilin, et al. Mechanism study of mechanochemical bromination on fly ash mercury removal adsorbent [J]. Chemosphere, 2021, 274: 129637. |
| 37 | GENG Xinze, LIU Xiaoshuo, DING Xunlei, et al. Mechanochemical bromination of unburned carbon in fly ash and its mercury removal mechanism: DFT study[J]. Journal of Hazardous Materials, 2022, 423: 127198. |
| 38 | JIA Li, YU Yue, LI Zepeng, et al. Study on the Hg0 removal characteristics and synergistic mechanism of iron-based modified biochar doped with multiple metals[J]. Bioresource Technology, 2021, 332: 125086. |
| 39 | ZHOU Qiang, DUAN Yufeng, CHEN Mingming, et al. Studies on mercury adsorption species and equilibrium on activated carbon surface[J]. Energy & Fuels, 2017, 31(12): 14211-14218. |
| 40 | YANG Jianping, XU Hong, CHEN Hong, et al. Removal of flue gas mercury by porous carbons derived from one-pot carbonization and activation of wood sawdust in a molten salt medium[J]. Journal of Hazardous Materials, 2022, 424: 127336. |
| 41 | GENG Xinze, ZHAO Weimeng, ZHOU Qiang, et al. effect of the mechanochemical process on the stability of mercury in simulated fly ash. Part 2: Sulfur additive[J]. Industrial & Engineering Chemistry Research, 2021, 60(42): 15115-15124. |
| 42 | GENG Xinze, ZHAO Weimeng, ZHOU Qiang, et al. Effect of the mechanochemical process on the stability of mercury in simulated fly ash. Part 1: Ball milling[J]. Industrial & Engineering Chemistry Research, 2021, 60(41): 14737-14746. |
| 43 | LIU Dongjing, XU Wen, LIU Yangxian. Seaweed bio-chars modified with metal chloride for elemental mercury capture from simulated flue gas[J]. Atmospheric Pollution Research, 2020, 11(6): 122-130. |
| 44 | LIU Ziyang, YANG Wei, XU Wen, et al. Removal of elemental mercury by bio-chars derived from seaweed impregnated with potassium iodine[J]. Chemical Engineering Journal, 2018, 339: 468-478. |
| 45 | ZHOU Yuming, ZHAO Yongchun, ZHANG Junying, et al. Mercury adsorption and oxidation over magnetic biochar in oxyfuel combustion atmosphere: Impact of enriched CO2 and H2O[J]. Fuel, 2019, 251: 458-465. |
| 46 | XU Wen, PAN Jianfeng, FAN Baowei, et al. Removal of gaseous elemental mercury using seaweed chars impregnated by NH4Cl and NH4Br[J]. Journal of Cleaner Production, 2019, 216: 277-287. |
| 47 | 陶君, 谷小兵, 王鸿宇, 等. 模拟烟气卤素改性稻壳焦喷射脱汞试验[J]. 洁净煤技术, 2021, 27(6): 186-192. |
| TAO Jun, GU Xiaobing, WANG Hongyu, et al. Experiment on mercury removal in simulated flue gas by injecting halogen modified rice husk coke[J]. Clean Coal Technology, 2021, 27(6): 186-192. | |
| 48 | 佘敏, 段钰锋, 朱纯, 等. 改性生物质活性焦碳管吸附剂汞吸附性能实验研究[J]. 工程热物理学报, 2015, 36(9): 2060-2064. |
| SHE Min, DUAN Yufeng, ZHU Chun, et al. Experimental study on mercury adsorption of modified biomass activated carbon for sorbents traps[J]. Journal of Engineering Thermophysics, 2015, (9): 36(9): 2060-2064. | |
| 49 | 周强, 段钰锋, 洪亚光, 等. 模拟烟气活性炭喷射脱汞实验研究[J]. 中国电机工程学报, 2013, 33(35): 36-43. |
| ZHOU Qiang, DUAN Yufeng, HONG Yaguang, et al. Experimental study on mercury capture using activated carbon injection in simulated flue gas[J]. Proceedings of the CSEE, 2013, 33(35): 36-43. | |
| 50 | ZHOU Yuming, YANG Jianping, DONG Liangchen, et al. Removal of elemental mercury from flue gas by recyclable CuCl2 modified magnetospheres from fly ash: Part 5. Industrial scale studies at a 50 MWth coal-fired power plant[J]. Fuel, 2020, 266: 117052. |
| 51 | LIU Huan, CHEN Yu, GAO Tian, et al. Elemental mercury removal from flue gas using modified tonstein: Performance of adsorbent injection at an entrained flow reactor system and 50MW coal-fired power plant in China[J]. Journal of Cleaner Production, 2021, 287: 124998. |
| 52 | ZHU Mingqing, YAN Qituan, DUAN Yufeng, et al. Study on preparation and mercury adsorption characteristics of columnar sulfur-impregnated activated petroleum coke[J]. Energy & Fuels, 2020, 34(9): 10740-10751. |
| 53 | ZHOU Qiang, TAO Xin, DI Guancheng, et al. Elemental mercury capture from flue gas by magnetic recyclable Fe6Mn1- x Ce x O y sorbent. Part 1. Performance evaluation and regeneration[J]. Fuel, 2021, 304: 120723. |
| 54 | YANG Shu, LIU Cao, WANG Pingshan, et al. Co9S8 nanoparticles-embedded porous carbon: A highly efficient sorbent for mercury capture from nonferrous smelting flue gas[J]. Journal of Hazardous Materials, 2021, 412: 124970. |
| 55 | XU Haomiao, HONG Qinyuan, LI Jiaxing, et al. Heterogeneous reaction mechanisms and functional materials for elemental mercury removal from industrial flue gas[J]. ACS ES&T Engineering, 2021, 1(10): 1383-1400. |
| 56 | ZHANG Shibo, Mercedes DÍAZ-SOMOANO, ZHAO Yongchun, et al. Research on the mechanism of elemental mercury removal over Mn-based SCR catalysts by a developed Hg-TPD method[J]. Energy & fuels, 2019, 33(3): 2467-2476. |
| 57 | QU Zan, YAN Naiqiang, LIU Ping, et al. Oxidation and stabilization of elemental mercury from coal-fired flue gas by sulfur monobromide[J]. Environmental Science & Technology, 2010, 44(10): 3889-3894. |
| 58 | YANG Zequn, LI Hailong, YANG Jianping, et al. Nanosized copper selenide functionalized zeolitic imidazolate framework-8 (CuSe/ZIF-8) for efficient immobilization of gas-phase elemental mercury[J]. Advanced Functional Materials, 2019, 29(17): 1807191. |
| 59 | HU Qixing, WANG Chang, GENG Yang, et al. Remarkable differences between copper-based sulfides and iron-based sulfides for the adsorption of high concentrations of gaseous elemental mercury: Mechanisms, kinetics, and significance [J]. Journal of Colloid and Interface Science, 2021, 582: 581-590. |
| 60 | LIU Ting, XIONG Zhuo, NI Peng, et al. Review on adsorbents in elemental mercury removal in coal combustion flue gas, smelting flue gas and natural gas[J]. Chemical Engineering Journal, 2023, 454: 140095. |
| 61 | LÓPEZ-ANTÓN M A, DÍAZ-SOMOANO M, MARTÍNEZ-TARAZONA M R. Retention of elemental mercury in fly ashes in different atmospheres[J]. Energy & Fuels, 2007, 21(1): 99-103. |
| 62 | Behrooz GHORISHI S, LEE Chun W, JOZEWICZ Wojciech S, et al. Effects of fly ash transition metal content and flue gas HCl/SO2 ratio on mercury speciation in waste combustion[J]. Environmental Engineering Science, 2005, 22(2): 221-231. |
| 63 | WU Shengji, AZHAR UDDIN Md, SASAOKA Eiji. Characteristics of the removal of mercury vapor in coal derived fuel gas over iron oxide sorbents[J]. Fuel, 2006, 85(2): 213-218. |
| 64 | GUO Pan, GUO Xin, ZHENG Chuguang. Roles of γ-Fe2O3 in fly ash for mercury removal: Results of density functional theory study[J]. Applied Surface Science, 2010, 256(23): 6991-6996. |
| 65 | GUO Pan, GUO Xin, ZHENG Chuguang. Computational insights into interactions between Hg species and α-Fe2O3(001)[J]. Fuel, 2011, 90(5): 1840-1846. |
| 66 | LIU Ting, XUE Lucheng, GUO Xin, et al. Mechanisms of elemental mercury transformation on α-Fe2O3(001) surface from experimental and theoretical study: Influences of HCl, O2 and SO2 [J]. Environmental Science & Technology, 2016, 50 (24): 13585-13591. |
| 67 | LIU Tao, XUE Lucheng, GUO Xin, et al. DFT study of mercury adsorption on α-Fe2O3 surface: Role of oxygen[J]. Fuel, 2014, 115: 179-185. |
| 68 | LIU Tao, GUO Xin, ZHENG Chuguang. Density functional study of Hg adsorption mechanism on α-Fe2O3 with H2S[J]. Proceedings of the Combustion Institute, 2013, 34(2): 2803-2810. |
| 69 | XUE Lucheng, LIU Ting, GUO Xin, et al. Hg oxidation reaction mechanism on Fe2O3 with H2S: Comparison between theory and experiments[J]. Proceedings of the Combustion Institute, 2015, 35(3): 2867-2874. |
| 70 | MA Lingjun, HAN Lina, CHEN Shuai, et al. Rapid synthesis of magnetic zeolite materials from fly ash and iron-containing wastes using supercritical water for elemental mercury removal from flue gas[J]. Fuel Processing Technology, 2019, 189: 39-48. |
| 71 | CAO Tiantian, ZHOU Zijian, CHEN Qian, et al. Magnetically responsive catalytic sorbent for removal of Hg0 and NO[J]. Fuel Processing Technology, 2017, 160: 158-169. |
| 72 | YANG Shijian, GUO Yongfu, YAN Naiqiang, et al. Nanosized cation-deficient Fe-Ti spinel: A novel magnetic sorbent for elemental mercury capture from flue gas[J]. ACS Applied Materials & Interfaces, 2011, 3(2): 209-217. |
| 73 | YANG Shijian, GUO Yongfu, YAN Naiqiang, et al. Elemental mercury capture from flue gas by magnetic Mn-Fe spinel: Effect of chemical heterogeneity[J]. Industrial & Engineering Chemistry Research, 2011, 50(16): 9650-9656. |
| 74 | JIA Li, FAN Baoguo, YAO Yuxing, et al. Study on the elemental mercury adsorption characteristics and mechanism of iron-based modified biochar materials[J]. Energy & Fuels, 2018, 32(12): 12554-12566. |
| 75 | DONG Lu, WANG Hai, HUANG Yaji, et al. Elemental mercury removal from coal-fired flue gas using recyclable magnetic Mn-Fe based attapulgite sorbent[J]. Chemical Engineering Journal, 2021, 407: 127182. |
| 76 | YANG Shijian, YAN Naiqiang, GUO Yongfu, et al. Gaseous elemental mercury capture from flue gas using magnetic nanosized (Fe3- x Mn x )1- δ O4 [J]. Environmental Science & Technology, 2011, 45(4): 1540-1546. |
| 77 | YANG Jianping, ZHAO Yongchun, GUO Xin, et al. Removal of elemental mercury from flue gas by recyclable CuCl2 modified magnetospheres from fly ash. Part 4. Performance of sorbent injection in an entrained flow reactor system[J]. Fuel, 2018, 220: 403-411. |
| 78 | YANG Jianping, ZHAO Yongchun, CHANG Lin, et al. Mercury adsorption and oxidation over cobalt oxide loaded magnetospheres catalyst from fly ash in oxyfuel combustion flue gas[J]. Environmental Science & Technology, 2015, 49(13): 8210-8218. |
| 79 | YANG Jianping, ZHAO Yongchun, ZHANG Junying, et al. Regenerable cobalt oxide loaded magnetosphere catalyst from fly ash for mercury removal in coal combustion flue gas[J]. Environmental Science & Technology, 2014, 48(24): 14837-14843. |
| 80 | DANG Hao, LIAO Yong, Tsz Wai NG, et al. The simultaneous centralized control of elemental mercury emission and deep desulfurization from the flue gas using magnetic Mn-Fe spinel as a co-benefit of the wet electrostatic precipitator[J]. Fuel Processing Technology, 2016, 142: 345-351. |
| 81 | ZHANG Bowen, PETCHER Samuel, GAO Hui, et al. Magnetic sulfur-doped carbons for mercury adsorption[J]. Journal of Colloid and Interface Science, 2021, 603: 728-737. |
| 82 | INC. Alstom Power. Demonstration of Mer-Cure™ technology for enhanced mercury control[R]. United States, Office of Scientific and Technical Information (OSTI), 2008. |
| 83 | PAVLISH John H, SONDREAL Everett A, MANN Michael D, et al. Status review of mercury control options for coal-fired power plants[J]. Fuel Processing Technology, 2003, 82(2/3): 89-165. |
| 84 | YANG Jianping, ZHAO Yongchun, ZHANG Junying, et al. Removal of elemental mercury from flue gas by recyclable CuCl2 modified magnetospheres catalyst from fly ash. Part 1. Catalyst characterization and performance evaluation[J]. Fuel, 2016, 164: 419-428. |
| 85 | XIN Feng, XIAO Rihong, ZHAO Yongchun, et al. Surface sulfidation modification of magnetospheres from fly ash for elemental mercury removal from coal combustion flue gas[J]. Chemical Engineering Journal, 2022, 436: 135212. |
| 86 | LIU Zhilou, ZHANG Zhiheng, LI Ziliang, et al. 3D hierarchical iron-cobalt sulfide anchored on carbon fiber with abundant active short chain sulfur for high-efficiency capture of elemental mercury[J]. Chemical Engineering Journal, 2021, 418: 129442. |
| 87 | YANG Zequn, LI Hailong, YANG Junwei, et al. Amorphous molybdenum selenide nanosheet as an efficient trap for the permanent sequestration of vapor-phase elemental mercury[J]. Advanced Science, 2019, 6(20): 1901410. |
| 88 | AHMED Snober, BROCKGREITENS John, XU Ke, et al. Mercury removal: A nanoselenium sponge for instantaneous mercury removal to undetectable levels [J]. Advanced Functional Materials, 2017, 27(17): 1606572. |
| 89 | ZOU Sijie, LIAO Yong, XIONG Shangchao, et al. H2S-modified Fe-Ti spinel: A recyclable magnetic sorbent for recovering gaseous elemental mercury from flue gas as a co-benefit of wet electrostatic precipitators[J]. Environmental Science & Technology, 2017, 51(6): 3426-3434. |
| 90 | MEI Jian, WANG Chang, KONG Lingnan, et al. Outstanding performance of recyclable amorphous MoS3 supported on TiO2 for capturing high concentrations of gaseous elemental mercury: Mechanism, kinetics, and application[J]. Environmental Science & Technology, 2019, 53(8): 4480-4489. |
| 91 | WANG Chang, QIN Ruiyang, ZHANG Xufan, et al. Safe disposal of deactivated commercial selective catalytic reduction catalyst (V2O5-MoO3/TiO2) as a low-cost and regenerable sorbent to recover gaseous elemental mercury in smelting flue gas[J]. Journal of Hazardous Materials, 2021, 406: 124744. |
| 92 | WANG Chang, HU Qixing, ZHANG Qi, et al. Novel synergetic effect of Fe and W in FeWS x /TiO2 on Capturing high concentrations of gaseous Hg0 from smelting flue gas: adsorption kinetics and structure-activity relationship[J]. Industrial & Engineering Chemistry Research, 2020, 59(7): 2745-2753. |
| [1] | 李琢宇, 余美琪, 陈孝彦, 胡若晖, 王庆宏, 陈春茂, 詹亚力. 炼油废催化剂吸附去除水中硝基苯的特性与机制[J]. 化工进展, 2025, 44(2): 1076-1087. |
| [2] | 刘法志, 张鹏威, 刘涛, 谢玉仙, 何建乐, 苏胜, 徐俊, 向军. Sb改性钒钛SCR脱硝催化剂抗CO中毒性能[J]. 化工进展, 2025, 44(2): 1129-1137. |
| [3] | 洪思琦, 顾方伟, 郑金玉. PEM水电解制氢低铱催化剂发展现状及展望[J]. 化工进展, 2025, 44(1): 158-168. |
| [4] | 李雪莲, 曹志会, 雷普瑛, 白冰, 王璇, 张金鑫, 侯凯, 刘爱芳, 齐凯, 高丽丽. 珊瑚状Mo2C/Mo3P@NC异质结电极高效催化Li-CO2电池[J]. 化工进展, 2025, 44(1): 202-211. |
| [5] | 宋顺明, 张敬雯, 张良清, 邱佳容, 陈剑锋, 曾宪海. 生物质基多元醇催化转化制备二醇[J]. 化工进展, 2025, 44(1): 228-252. |
| [6] | 秦婷婷, 牛强. 二氧化碳加氢制高级醇Fe基催化剂研究进展[J]. 化工进展, 2025, 44(1): 253-265. |
| [7] | 庄柯, 陈宏, 许芸, 仲兆平, 周峻伍, 周凯, 董月红. SiO2改性Ce-V-W/Ti催化剂载体的抗碱(土)金属中毒性能[J]. 化工进展, 2025, 44(1): 266-276. |
| [8] | 董家彤, 单梦晴, 王华. Au-CuO/Cu2O串联催化增强电催化CO2还原制乙醇[J]. 化工进展, 2025, 44(1): 277-285. |
| [9] | 游小银, 汪楚乔, 刘才华, 彭小明. Z型CN/NGBO/BV催化剂体系的构筑及光类芬顿降解四环素性能[J]. 化工进展, 2025, 44(1): 286-296. |
| [10] | 李佳优, 张雨涵, 姜楠, 蒋博龙. 过渡金属硫化物NiS(x)@NF催化剂水热法制备及其析氢性能[J]. 化工进展, 2025, 44(1): 297-304. |
| [11] | 王宁, 陆诗建, 刘玲, 梁静, 刘苗苗, 孙梦圆, 康国俊. 胺吸收体系中CO2催化解吸再生技术的研究进展[J]. 化工进展, 2025, 44(1): 445-464. |
| [12] | 李书鹏, 独学渊, 李霏, 郭丽莉, 李广贺. 还原材料修复卤代溶剂污染地下水的研究进展[J]. 化工进展, 2025, 44(1): 500-512. |
| [13] | 张炜, 黄赳, 朱晓芳, 李鹏. 凹凸棒石基钴钨水滑石吸附铅的性能及机理[J]. 化工进展, 2025, 44(1): 596-606. |
| [14] | 李新月, 李振京, 韩沂杭, 郭永强, 闫瑜, 哈力米热·卡热木拉提, 赵会吉, 柴永明, 刘东, 殷长龙. 油脂加氢脱氧生产绿色柴油催化剂的研究进展[J]. 化工进展, 2024, 43(S1): 351-364. |
| [15] | 林梅洁, 米烁东, 包成. 金属-掺杂氧化铈体系H2/CO电化学反应机理研究进展[J]. 化工进展, 2024, 43(S1): 209-224. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||