化工进展 ›› 2025, Vol. 44 ›› Issue (1): 158-168.DOI: 10.16085/j.issn.1000-6613.2024-0014
PEM水电解制氢低铱催化剂发展现状及展望
- 中石化石油化工科学研究院有限公司,北京 100083
-
收稿日期:2024-01-04修回日期:2024-02-27出版日期:2025-01-15发布日期:2025-02-13 -
通讯作者:郑金玉 -
作者简介:洪思琦(1998—),男,硕士研究生,主要从事水电解制氢催化剂制备工作。E-mail:hongsiqi.ripp@sinopec.com。 -
基金资助:国家重点研究计划(2023YFA1507400)
Development status and prospect of low iridium catalysts for hydrogen production by PEM electrolysis
HONG Siqi( ), GU Fangwei, ZHENG Jinyu(
), GU Fangwei, ZHENG Jinyu( )
)
- SINOPEC Research Institute of Petroleum Processing, Beijing 100083, China
-
Received:2024-01-04Revised:2024-02-27Online:2025-01-15Published:2025-02-13 -
Contact:ZHENG Jinyu
摘要:
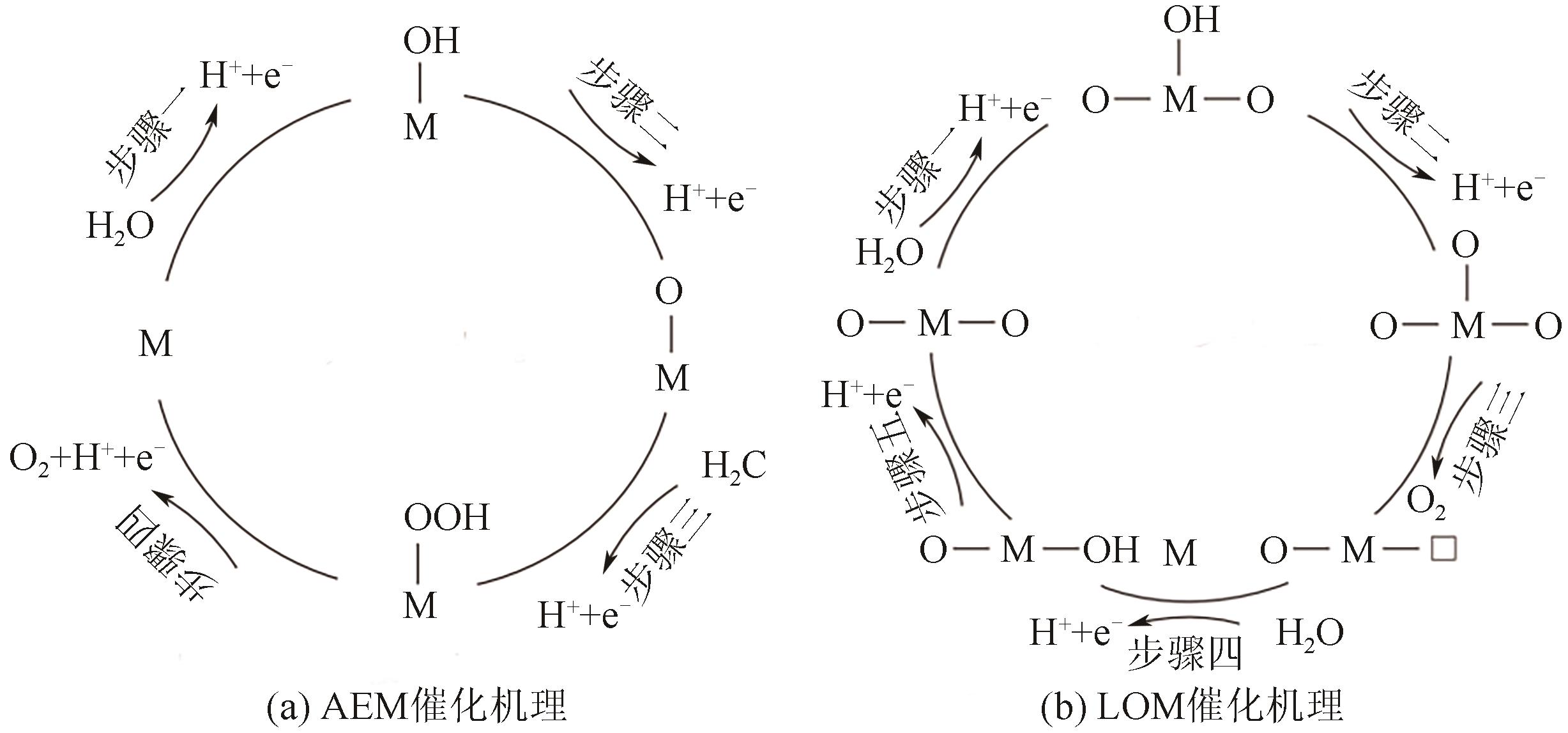
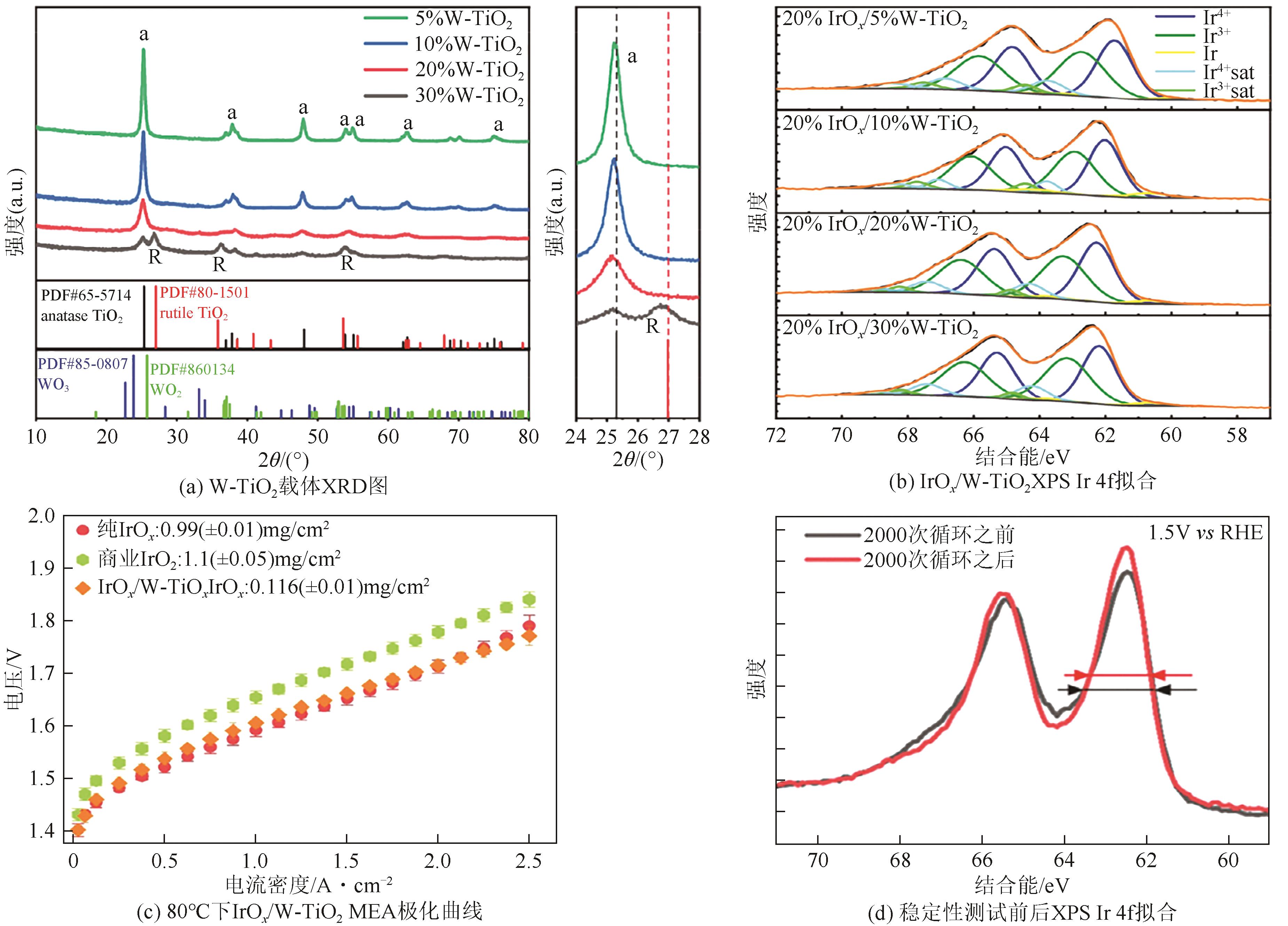
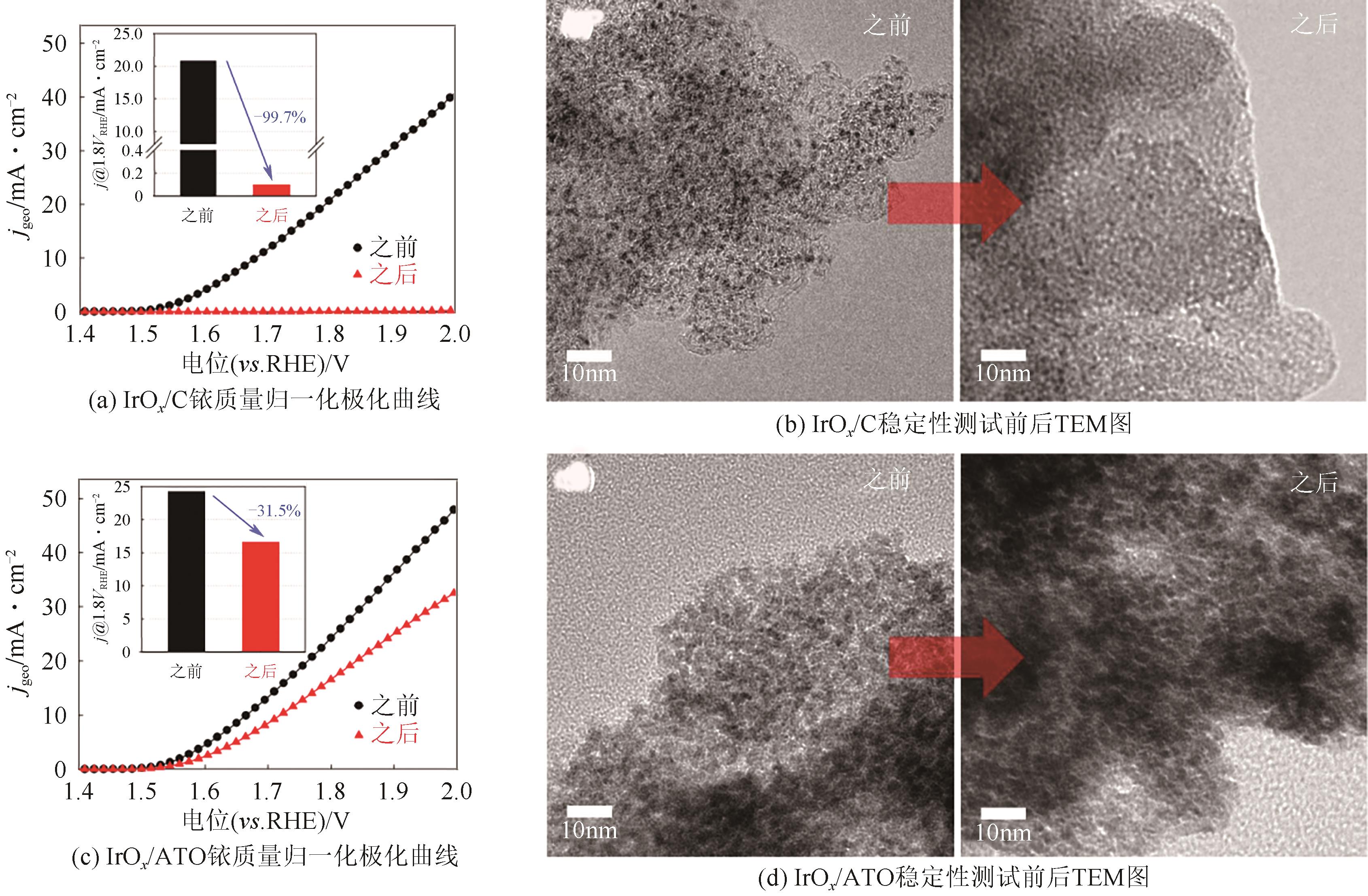
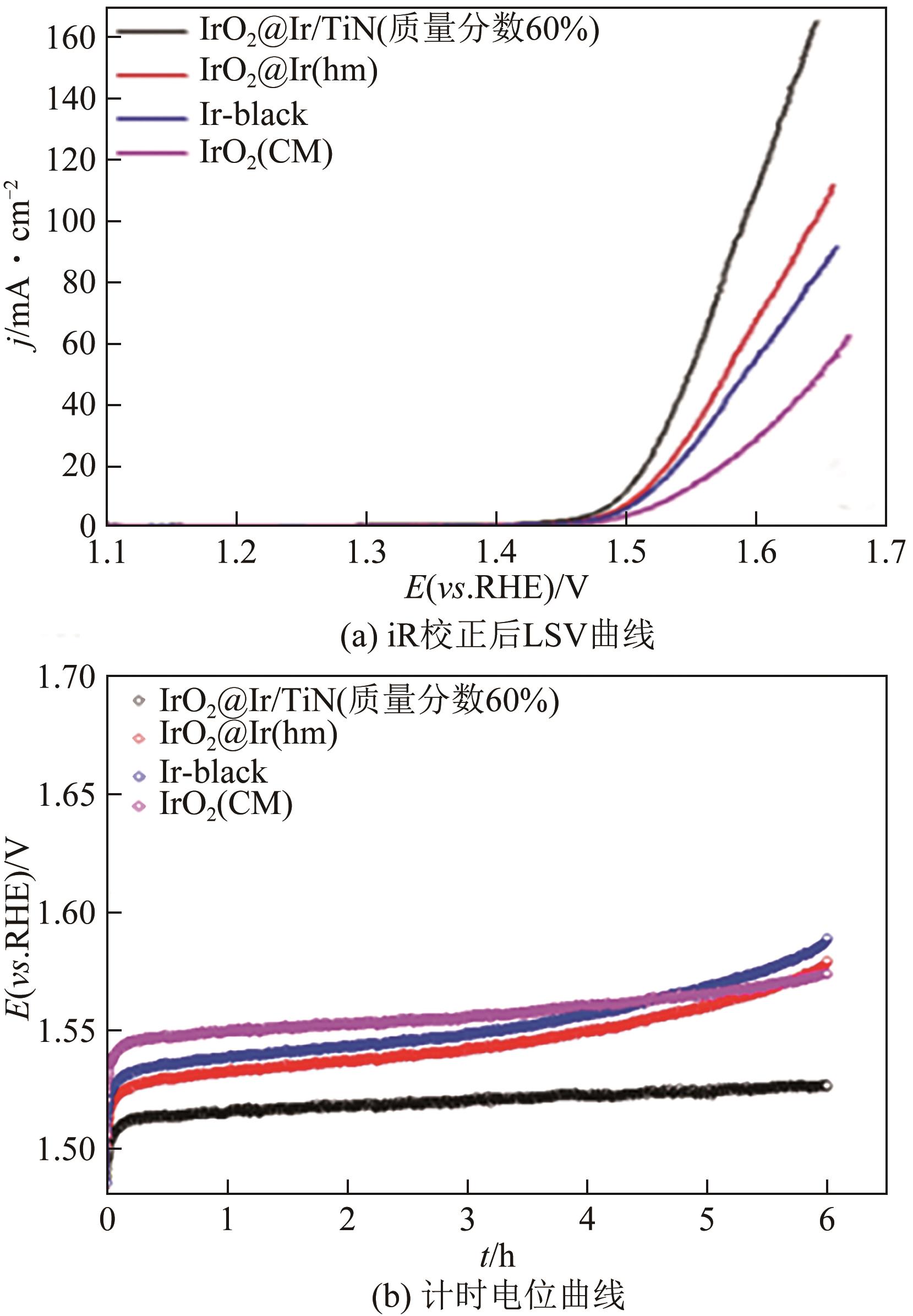
随着绿色可再生能源的快速发展,质子交换膜(PEM)水电解制氢技术作为连接可再生能源和氢能的关键环节而备受关注。本文综述了PEM电解槽阳极的工作原理、技术现状及低铱催化剂的研究进展。PEM电解技术前景广阔,但铱资源稀缺且价格高,制约了PEM电解技术的推广。开发低铱、高活性且稳定的析氧反应(OER)催化剂成为了PEM电解技术发展的关键。目前低铱催化剂主要集中在掺杂型和负载型两类催化剂。这两类低铱催化剂相较铱氧化物表现出更高催化活性。本文阐述了最近报道的几种低铱催化剂,如双钙钛矿、Gd掺杂IrO2、IrNiO x 核壳结构、IrO x /ATO等。并总结了现阶段低铱催化剂在活性和稳定性方面的主要挑战,如掺杂型催化剂存在晶相不稳定的问题,负载型催化剂的Ir利用率有待进一步提高等。最后,本文总结并展望如何进一步推动高活性、高稳定性的低铱催化剂研发,以期为PEM水电解技术的未来发展提供强有力的支持。
中图分类号:
引用本文
洪思琦, 顾方伟, 郑金玉. PEM水电解制氢低铱催化剂发展现状及展望[J]. 化工进展, 2025, 44(1): 158-168.
HONG Siqi, GU Fangwei, ZHENG Jinyu. Development status and prospect of low iridium catalysts for hydrogen production by PEM electrolysis[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 158-168.
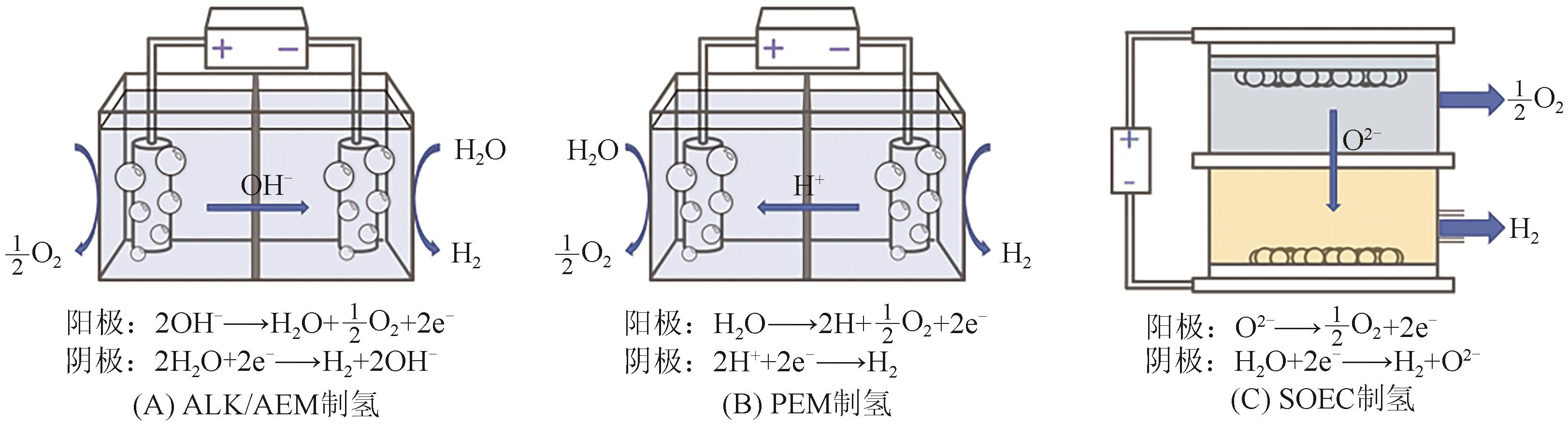
| 制氢技术 | 阴阳极隔膜 | 工作温度/℃ | 电流密度/A·cm-2 | 能耗/kW·h·m-3 | 优点 | 缺点 | 商业化程度 |
|---|---|---|---|---|---|---|---|
| ALK | PPS | 70~100 | 0.2~0.5 | 4.5~5.5 | 不使用贵金属催化剂、成本低 | 效率较低、碱液腐蚀设备、压力-液位控制困难 | 充分商业化 |
| PEM | 质子交换膜 | 60~90 | 1~6 | 3.6~4.4 | 负荷波动可调节幅度大、安全性高、启停速度快 | 价格高、单槽规模小 | 商业化初期 |
| AEM | 阴离子交换膜 | 60~90 | 0.3~0.8 | 4.8 | 体积小、安全性高,不使用贵金属催化剂 | 规模小、阴离子交换膜技术不成熟 | 实验室研发阶段 |
| SOEC | 氧化物导电体 | 600~1000 | 0.3 | 2.23~2.27 | 无需使用贵金属催化剂、效率高 | 启停速度慢,材料衰减快 | 实验室研发阶段 |
表1 不同水电解制氢技术的性能对比
| 制氢技术 | 阴阳极隔膜 | 工作温度/℃ | 电流密度/A·cm-2 | 能耗/kW·h·m-3 | 优点 | 缺点 | 商业化程度 |
|---|---|---|---|---|---|---|---|
| ALK | PPS | 70~100 | 0.2~0.5 | 4.5~5.5 | 不使用贵金属催化剂、成本低 | 效率较低、碱液腐蚀设备、压力-液位控制困难 | 充分商业化 |
| PEM | 质子交换膜 | 60~90 | 1~6 | 3.6~4.4 | 负荷波动可调节幅度大、安全性高、启停速度快 | 价格高、单槽规模小 | 商业化初期 |
| AEM | 阴离子交换膜 | 60~90 | 0.3~0.8 | 4.8 | 体积小、安全性高,不使用贵金属催化剂 | 规模小、阴离子交换膜技术不成熟 | 实验室研发阶段 |
| SOEC | 氧化物导电体 | 600~1000 | 0.3 | 2.23~2.27 | 无需使用贵金属催化剂、效率高 | 启停速度慢,材料衰减快 | 实验室研发阶段 |
| 样品 | Ir³+ | Ir⁴+ | ||
|---|---|---|---|---|
| 位置/eV | 强度/% | 位置/eV | 强度/% | |
| plrO₂ | 62.3 | 53.5 | 63.9 | 46.5 |
| 3%-Gd-pIrO₂ | 62.1 | 44.2 | 63.5 | 55.8 |
| 5%-Gd-pIrO₂ | 62.2 | 43.5 | 63.6 | 56.5 |
| 7%-Gd-pIrO₂ | 62.2 | 52.6 | 63.8 | 47.4 |
| 10%-Gd-pIrO₂ | 62.2 | 58.4 | 63.7 | 41.6 |
表2 Gd-pIrO2催化剂XPS Ir 4f光谱拟合结果[29]
| 样品 | Ir³+ | Ir⁴+ | ||
|---|---|---|---|---|
| 位置/eV | 强度/% | 位置/eV | 强度/% | |
| plrO₂ | 62.3 | 53.5 | 63.9 | 46.5 |
| 3%-Gd-pIrO₂ | 62.1 | 44.2 | 63.5 | 55.8 |
| 5%-Gd-pIrO₂ | 62.2 | 43.5 | 63.6 | 56.5 |
| 7%-Gd-pIrO₂ | 62.2 | 52.6 | 63.8 | 47.4 |
| 10%-Gd-pIrO₂ | 62.2 | 58.4 | 63.7 | 41.6 |
| 1 | 何青,孟照鑫,沈轶 等. “双碳”目标下我国氢能政策分析与思考[J]. 热力发电, 2021, 50(11): 27-36. |
| HE Qing, MENG Zhaoxin, SHEN Yi, et al. Analysis and thinking of hydrogen energy policies in China under "double carbon" target[J]. Thermal Power Generation, 2021, 50(11): 27-36. | |
| 2 | International Energy Agency. 2050年净零排放: 全球能源部门路线图[R/OL]. (2021). |
| 3 | 米万良, 荣峻峰. 质子交换膜(PEM)水电解制氢技术进展及应用前景[J]. 石油炼制与化工, 2021, 52(10): 78-87. |
| MI Wanliang, RONG Junfeng. Progress and application prospects of pem water electrolysis technology for hydrogen production[J]. Petroleum Processing and Petrochemicals, 2021, 52(10): 78-87. | |
| 4 | 牟树君, 林今, 邢学韬, 等. 高温固体氧化物电解水制氢储能技术及应用展望[J]. 电网技术, 2017, 41(10): 3385-3391. |
| MU Shujun, LIN Jin, XING Xuetao, et al. Technology and application prospect of high-temperature solid oxide electrolysis cell[J]. Power System Technology, 2017, 41(10): 3385-3391. | |
| 5 | 陈彬, 谢和平, 刘涛, 等. 碳中和背景下先进制氢原理与技术研究进展[J]. 工程科学与技术, 2022, 54(1): 106-116. |
| CHEN Bin, XIE Heping, LIU Tao, et al. Principles and progress of advanced hydrogen production technologies in the context of carbon neutrality[J]. Advanced Engineering Sciences, 2022, 54(1): 106-116. | |
| 6 | MINKE Christine, SUERMANN Michel, BENSMANN Boris, et al. Is iridium demand a potential bottleneck in the realization of large-scale PEM water electrolysis?[J]. International Journal of Hydrogen Energy, 2021, 46(46): 23581-23590. |
| 7 | 梁宵. 低铱钙钛矿水氧化催化剂微结构调控与性能研究[D]. 长春:吉林大学, 2021. |
| LIANG Xiao.Microstructural modulation and electrocatalytic performance of low-iridium perovskite for water oxidation[D]. Changchun: Jilin University, 2021 | |
| 8 | ZHANG Lulu, ZHU Shangqian, DONG Shuyu, et al. Co nanoparticles encapsulated in porous N-doped carbon nanofibers as an efficient electrocatalyst for hydrogen evolution reaction[J]. Journal of the Electrochemical Society, 2018, 165(15): J3271-J3275. |
| 9 | VESBORG Peter C K, JARAMILLO Thomas F. Addressing the terawatt challenge: Scalability in the supply of chemical elements for renewable energy[J]. RSC Advances, 2012, 2(21): 7933-7947. |
| 10 | BROOKS D, 曲艺, 倪慧峰. 铱资源是否足以支撑未来氢能经济的发展[J]. 贵金属, 2022, 43(S1): 93-100. |
| BROOKS D. Will there be enough iridium to meet demand from the hydrogen economy[J]. Precious Metals, 2022, 43(S1): 93-100. | |
| 11 | HUANG Zhenfeng, SONG Jiajia, DOU Shuo, et al. Strategies to break the scaling relation toward enhanced oxygen electrocatalysis[J]. Matter, 2019, 1(6): 1494-1518. |
| 12 | WOHLFAHRT-MEHRENS M, HEITBAUM J. Oxygen evolution on Ru and RuO2 electrodes studied using isotope labelling and on-line mass spectrometry[J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1987, 237(2): 251-260. |
| 13 | FIERRO Stéphane, NAGEL Tina, BALTRUSCHAT Helmut, et al. Investigation of the oxygen evolution reaction on Ti/IrO2 electrodes using isotope labelling and on-line mass spectrometry[J]. Electrochemistry Communications, 2007, 9(8): 1969-1974. |
| 14 | Tyler MEFFORD J, RONG Xi, ABAKUMOV Artem M, et al. Water electrolysis on La1- x Sr x CoO3- δ perovskite electrocatalysts[J]. Nature Communications, 2016, 7: 11053. |
| 15 | KASIAN Olga, GROTE Jan-Philipp, GEIGER Simon, et al. The common intermediates of oxygen evolution and dissolution reactions during water electrolysis on iridium[J]. Angewandte Chemie International Edition, 2018, 57(9): 2488-2491. |
| 16 | 何泽兴, 史成香, 陈志超, 等. 质子交换膜电解水制氢技术的发展现状及展望[J]. 化工进展, 2021, 40(9): 4762-4773. |
| HE Zexing, SHI Chengxiang, CHEN Zhichao, et al. Development status and prospects of proton exchange membrane water electrolysis[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4762-4773. | |
| 17 | RASTEN Egil, HAGEN Georg, TUNOLD Reidar. Electrocatalysis in water electrolysis with solid polymer electrolyte[J]. Electrochimica Acta, 2003, 48(25/26): 3945-3952. |
| 18 | SONG Shidong, ZHANG Huamin, MA Xiaoping, et al. Electrochemical investigation of electrocatalysts for the oxygen evolution reaction in PEM water electrolyzers[J]. International Journal of Hydrogen Energy, 2008, 33(19): 4955-4961. |
| 19 | SIRACUSANO S, BAGLIO V, STASSI A, et al. Investigation of IrO2 electrocatalysts prepared by a sulfite-couplex route for the O2 evolution reaction in solid polymer electrolyte water electrolyzers[J]. International Journal of Hydrogen Energy, 2011, 36(13): 7822-7831. |
| 20 | SLAVCHEVA E, RADEV I, BLIZNAKOV S, et al. Sputtered iridium oxide films as electrocatalysts for water splitting via PEM electrolysis[J]. Electrochimica Acta, 2007, 52(12): 3889-3894. |
| 21 | OUATTARA Lassiné, FIERRO Stéphane, FREY Olivier, et al. Electrochemical comparison of IrO2 prepared by anodic oxidation of pure iridium and IrO2 prepared by thermal decomposition of H2IrCl6 precursor solution[J]. Journal of Applied Electrochemistry, 2009, 39(8): 1361-1367. |
| 22 | Peter KÚŠ, OSTROVERKH Anna, Klára ŠEVČÍKOVÁ, et al. Magnetron sputtered Ir thin film on TiC-based support sublayer as low-loading anode catalyst for proton exchange membrane water electrolysis[J]. International Journal of Hydrogen Energy, 2016, 41(34): 15124-15132. |
| 23 | LETTENMEIER P, MAJCHEL J, WANG L, et al. Highly active nano-sized iridium catalysts: Synthesis and operando spectroscopy in a proton exchange membrane electrolyzer[J]. Chemical Science, 2018, 9(14): 3570-3579. |
| 24 | LI Guoqiang, LI Songtao, XIAO Meiling, et al. Nanoporous IrO2 catalyst with enhanced activity and durability for water oxidation owing to its micro/mesoporous structure[J]. Nanoscale, 2017, 9(27): 9291-9298 |
| 25 | ORTEL Erik, REIER Tobias, STRASSER Peter, et al. Mesoporous IrO2 films templated by PEO-PB-PEO block-copolymers: Self-assembly, crystallization behavior, and electrocatalytic performance[J]. Chem Mater, 2011, 23(13): 3201-3209. |
| 26 | 李佳坤. 质子交换膜(PEM)水电解制氢用新型析氧电极研究[D]. 长沙: 湖南大学, 2019. |
| LI Jiakun. Research on novel oxygen evolution electrode for proton exchangemembrane water electrolysis [D]. Changsha: Hunan University, 2019 | |
| 27 | MURAKAMI Y, TSUCHIYA S, YAHIKOZAWA K, et al. Preparation of ultrafine RuO2 and IrO2 particles by a sol-gel process[J]. Journal of Materials Science Letters, 1994, 13(24): 1773-1774. |
| 28 | WANG Yahui, HAO Shaoyun, LIU Xiangnan, et al. Ce-doped IrO2 electrocatalysts with enhanced performance for water oxidation in acidic media[J]. ACS Applied Materials & Interfaces, 2020, 12(33): 37006-37012. |
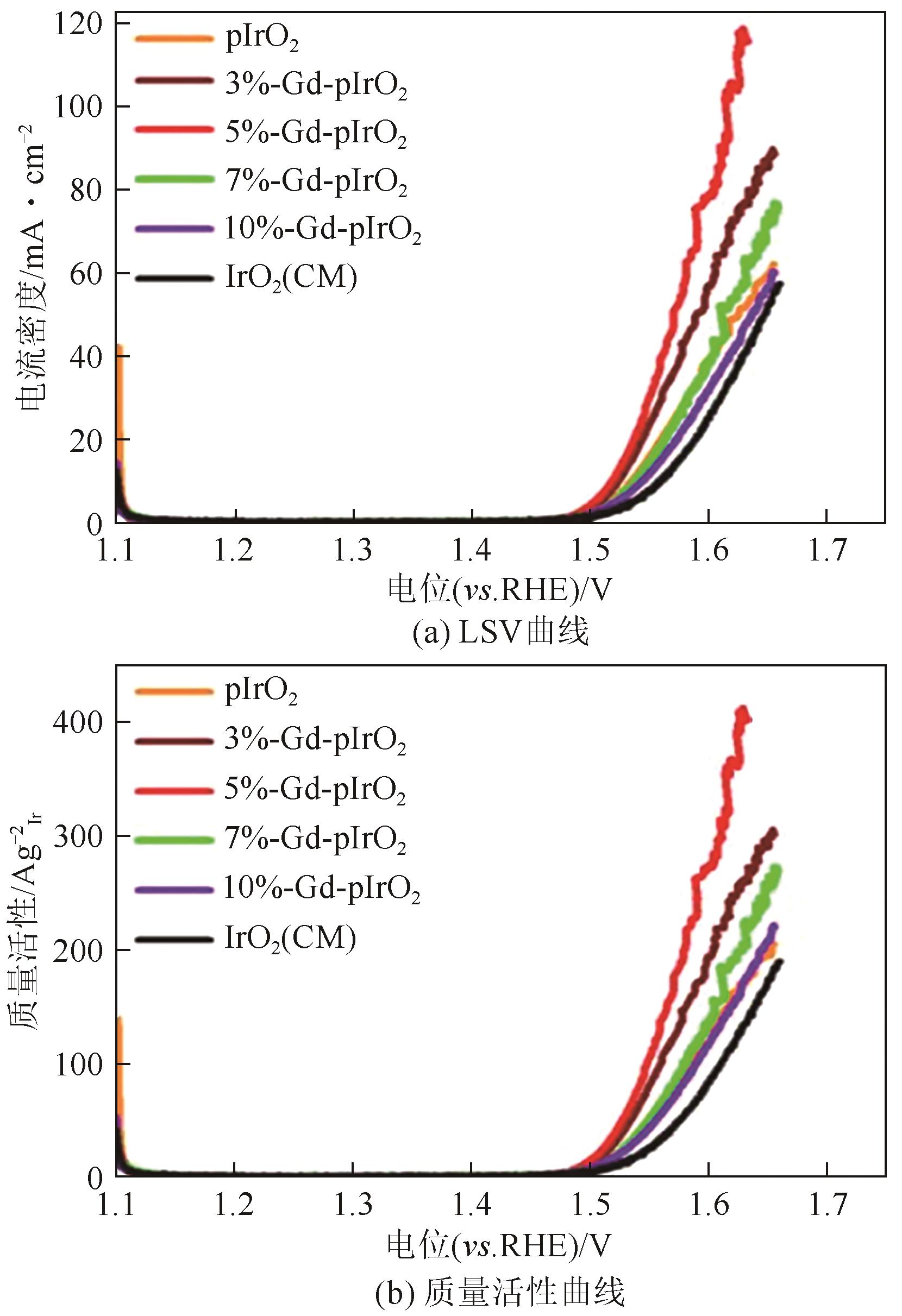
| 29 | WANG Yibo, HOU Shuai, MA Rongpeng, et al. Modulating crystallinity and surface electronic structure of IrO2 via gadolinium doping to promote acidic oxygen evolution[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(32): 10710-10716. |
| 30 | NONG Hong Nhan, REIER Tobias, Hyung-Suk OH, et al. A unique oxygen ligand environment facilitates water oxidation in hole-doped IrNiO x core-shell electrocatalysts[J]. Nature Catalysis, 2018, 1: 841-851. |
| 31 | SUN Wei, SONG Ya, GONG Xueqing, et al. An efficiently tuned d-orbital occupation of IrO2 by doping with Cu for enhancing the oxygen evolution reaction activity[J]. Chemical Science, 2015, 6(8): 4993-4999. |
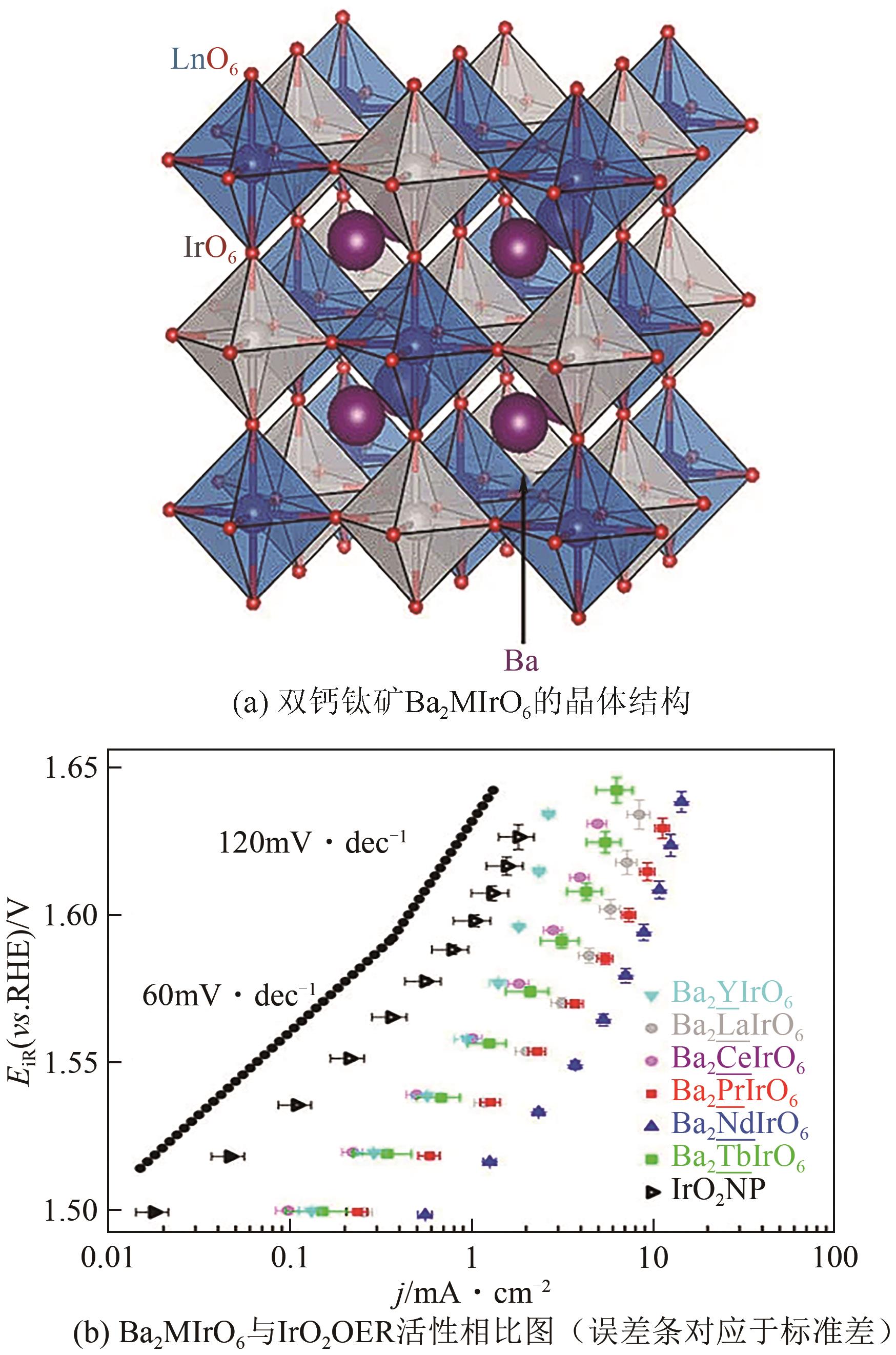
| 32 | Oscar DIAZ-MORALES, RAAIJMAN Stefan, KORTLEVER Ruud, et al. Iridium-based double perovskites for efficient water oxidation in acid media[J]. Nature Communications, 2016, 7: 12363. |
| 33 | LEBEDEV Dmitry, POVIA Mauro, WALTAR Kay, et al. Highly active and stable iridium pyrochlores for oxygen evolution reaction[J]. Chemistry of Materials, 2017, 29(12): 5182-5191. |
| 34 | ZHANG Qi, CHEN Hui, YANG Lan, et al. Non-catalytic, instant iridium (Ir) leaching: A non-negligible aspect in identifying Ir-based perovskite oxygen-evolving electrocatalysts[J]. Chinese Journal of Catalysis, 2022, 43(3): 885-893. |
| 35 | MIN Xiangping, SHI Yan, LU Zhuoxin, et al. High performance and cost-effective supported IrO x catalyst for proton exchange membrane water electrolysis[J]. Electrochimica Acta, 2021, 385: 138391. |
| 36 | Hong LYU, ZUO Jian, ZHOU Wei, et al. Synthesis and activities of IrO2/Ti1- x W x O2 electrocatalyst for oxygen evolution in solid polymer electrolyte water electrolyzer[J]. Journal of Electroanalytical Chemistry, 2019, 833: 471-479. |
| 37 | HAO Chuanpu, Hong LYU, MI Cangen, et al. Investigation of mesoporous niobium-doped TiO2 as an oxygen evolution catalyst support in an SPE water electrolyzer[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 746-756. |
| 38 | REGMI Yagya N, TZANETOPOULOS Eden, ZENG Guosong, et al. Supported oxygen evolution catalysts by design: Toward lower precious metal loading and improved conductivity in proton exchange membrane water electrolyzers[J]. ACS Catalysis, 2020, 10(21): 13125-13135. |
| 39 | SMITH J R, WALSH F C, CLARKE R L. Electrodes based on Magnéli phase titanium oxides: The properties and applications of Ebonex® materials[J]. Journal of Applied Electrochemistry, 1998, 28(10): 1021-1033. |
| 40 | WALSH F C, WILLS R G A. The continuing development of Magnéli phase titanium sub-oxides and Ebonex® electrodes[J]. Electrochimica Acta, 2010, 55(22): 6342-6351. |
| 41 | XU Junyuan, LIU Gaoyang, LI Jianling, et al. The electrocatalytic properties of an IrO2/SnO2 catalyst using SnO2 as a support and an assisting reagent for the oxygen evolution reaction[J]. Electrochimica Acta, 2012, 59: 105-112. |
| 42 | ZHENG Min, WANG Bao. One-step synthesis of antimony-doped tin dioxide nanocrystallites and their property[J]. Transactions of Nonferrous Metals Society of China, 2009, 19(2): 404-409. |
| 43 | WU Xu, SCOTT Keith. RuO2 supported on Sb-doped SnO2 nanoparticles for polymer electrolyte membrane water electrolysers[J]. International Journal of Hydrogen Energy, 2011, 36(10): 5806-5810. |
| 44 | Hyung-Suk OH, NONG Hong Nhan, REIER Tobias, et al. Oxide-supported Ir nanodendrites with high activity and durability for the oxygen evolution reaction in acid PEM water electrolyzers[J]. Chemical Science, 2015, 6(6): 3321-3328. |
| 45 | Hyung-Suk OH, NONG Hong Nhan, REIER Tobias, et al. Electrochemical catalyst-support effects and their stabilizing role for IrO x nanoparticle catalysts during the oxygen evolution reaction[J]. Journal of the American Chemical Society, 2016, 138(38): 12552-12563. |
| 46 | NONG Hong Nhan, Hyung-Suk OH, REIER Tobias, et al. Oxide‐supported IrNiO x core-shell particles as efficient, cost‐effective, and stable catalysts for electrochemical water splitting[J]. Angewandte Chemie International Edition, 2015, 54(10): 2975-2979. |
| 47 | DATTA Moni Kanchan, KADAKIA Karan, VELIKOKHATNYI Oleg I, et al. High performance robust F-doped tin oxide based oxygen evolution electro-catalysts for PEM based water electrolysis[J]. Journal of Materials Chemistry A, 2013, 1(12): 4026-4037. |
| 48 | KADAKIA Karan Sandeep, JAMPANI Prashanth, VELIKOKHATNYI Oleg I, et al. Nanostructured (Ir, Sn)O2: F-oxygen evolution reaction anode electro-catalyst powders for PEM based water electrolysis[J]. Journal of the Electrochemical Society, 2014, 161(9): F868-F875. |
| 49 | BANERJEE A N, KUNDOO S, SAHA P, et al. Synthesis and characterization of nano-crystalline fluorine-doped tin oxide thin films by sol-gel method[J]. Journal of Sol-Gel Science and Technology, 2003, 28(1): 105-110. |
| 50 | ABBOU Sofyane, CHATTOT Raphaël, MARTIN Vincent, et al. Manipulating the corrosion resistance of SnO2 aerogels through doping for efficient and durable oxygen evolution reaction electrocatalysis in acidic media[J]. ACS Catalysis, 2020, 10(13): 7283-7294. |
| 51 | SUI Sheng, MA Lirong, ZHAI Yuchun. Investigation on the proton exchange membrane water electrolyzer using supported anode catalyst[J]. Asia-Pacific Journal of Chemical Engineering, 2009, 4(1): 8-11. |
| 52 | MA Lirong, SUI Sheng, ZHAI Yuchun. Preparation and characterization of Ir/TiC catalyst for oxygen evolution[J]. Journal of Power Sources, 2008, 177(2): 470-477. |
| 53 | ISLAM Jahowa, KIM Sang-Kyung, THIEN Phan Thanh, et al. Enhancing the activity and durability of iridium electrocatalyst supported on boron carbide by tuning the chemical state of iridium for oxygen evolution reaction[J]. Journal of Power Sources, 2021, 512: 230506. |
| 54 | NIKIFOROV A V, TOMÁS GARCÍA A L, PETRUSHINA I M, et al. Preparation and study of IrO2/SiC-Si supported anode catalyst for high temperature PEM steam electrolysers[J]. International Journal of Hydrogen Energy, 2011, 36(10): 5797-5805. |
| 55 | POLONSKÝ J, MAZÚR P, PAIDAR M, et al. Performance of a PEM water electrolyser using a TaC-supported iridium oxide electrocatalyst[J]. International Journal of Hydrogen Energy, 2014, 39(7): 3072-3078. |
| 56 | IGNASZAK Anna, SONG Chaojie, ZHU Weimin, et al. Titanium carbide and its core-shelled derivative TiC@TiO2 as catalyst supports for proton exchange membrane fuel cells[J]. Electrochimica Acta, 2012, 69: 397-405. |
| 57 | KAKINUMA Katsuyoshi, WAKASUGI Yuko, UCHIDA Makoto, et al. Electrochemical activity and durability of platinum catalysts supported on nanometer-size titanium nitride particles for polymer electrolyte fuel cells[J]. Electrochemistry, 2011, 79(5): 399-403. |
| 58 | LI Guoqiang, LI Kai, YANG Long, et al. Boosted performance of Ir species by employing TiN as the support toward oxygen evolution reaction[J]. ACS Applied Materials & Interfaces, 2018, 10(44): 38117-38124. |
| 59 | ZHANG Kaikai, Wanshan MAI, LI Jin, et al. Highly scattered Ir oxides on TiN as an efficient oxygen evolution reaction electrocatalyst in acidic media[J]. Journal of Materials Science, 2020, 55(8): 3507-3520. |
| [1] | 靳钰阳, 牛传峰, 刘英硕, 丁石. 石墨粉/Nafion-铅复合电极电还原草酸制乙醇酸[J]. 化工进展, 2025, 44(2): 1003-1013. |
| [2] | 李佳优, 张雨涵, 姜楠, 蒋博龙. 过渡金属硫化物NiS(x)@NF催化剂水热法制备及其析氢性能[J]. 化工进展, 2025, 44(1): 297-304. |
| [3] | 李乐天, 陆诗建, 刘含笑, 吴黎明, 刘玲, 康国俊. 有机胺富液解吸再生研究进展[J]. 化工进展, 2025, 44(1): 490-499. |
| [4] | 何然, 梁宏, 黄洪, 羊宥郦, 郑强, 李琋. 乙炔黑/Fe3O4阴极制备及电Fenton氧化降解2,4,6-三氯苯酚[J]. 化工进展, 2025, 44(1): 572-582. |
| [5] | 林梅洁, 米烁东, 包成. 金属-掺杂氧化铈体系H2/CO电化学反应机理研究进展[J]. 化工进展, 2024, 43(S1): 209-224. |
| [6] | 马桂璇, 徐子桐, 肖志华, 宁国庆, 魏强, 徐春明. 氧硫双掺杂CNTs水系导电剂辅助构筑高性能石墨/SiO负极[J]. 化工进展, 2024, 43(S1): 443-456. |
| [7] | 朱昊, 刘汉飞, 高源, 黄益平, 费孝诚, 韩卫清. 盐分对电催化降解性能与机理的影响[J]. 化工进展, 2024, 43(S1): 571-580. |
| [8] | 王正峰, 谢雨杭, 李伟科, 范永春, 康钟尹, 付乾. 多孔炭修饰的吸附催化一体化电极高效电解碳酸氢盐[J]. 化工进展, 2024, 43(9): 4892-4899. |
| [9] | 梁宏成, 赵冬妮, 权银, 李敬妮, 胡欣怡. SEI膜形貌与结构对锂离子电池性能的影响[J]. 化工进展, 2024, 43(9): 5049-5062. |
| [10] | 吴剑扬, 王汝娜, 陈耀, 申兰耀, 于永利, 蒋宁, 邱景义, 周恒辉. 锂离子电池高镍正极材料前体的制备工艺[J]. 化工进展, 2024, 43(9): 5079-5085. |
| [11] | 李美萱, 成建凤, 黄国勇, 徐盛明, 郁丰善, 翁雅青, 曹才放, 温嘉玮, 王俊莲, 王春霞, 顾斌涛, 张袁华, 刘斌, 王才平, 潘剑明, 徐泽良, 王翀, 王珂. 高电压镍锰酸锂正极材料的合成与电化学机理[J]. 化工进展, 2024, 43(9): 5086-5094. |
| [12] | 屈芸, 成丽媛, 代国亮, 王刚, 郭羽晴, 孙洁. PAN/MXene同轴纤维电极的制备及性能[J]. 化工进展, 2024, 43(9): 5113-5122. |
| [13] | 杨光, 姜瑞婷, 张玥, 符子剑, 刘伟. 五氧化二钒/碳纳米复合材料在超级电容器中的应用[J]. 化工进展, 2024, 43(7): 3857-3871. |
| [14] | 罗臻, 王庆吉, 王占生, 杨雪莹, 谢加才, 王浩. 炼化污染场地抽出水强氧化短程处理工艺[J]. 化工进展, 2024, 43(7): 4155-4163. |
| [15] | 李莹莹, 刘安, 姜乐妍, 李晖, 陈春钰, 居殿春. 过渡金属硫化物Co9S8的制备及电化学性能研究进展[J]. 化工进展, 2024, 43(6): 3114-3127. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||