化工进展 ›› 2025, Vol. 44 ›› Issue (1): 445-464.DOI: 10.16085/j.issn.1000-6613.2023-2255
胺吸收体系中CO2催化解吸再生技术的研究进展
王宁1,2,3( ), 陆诗建1,2,3(
), 陆诗建1,2,3( ), 刘玲1,2,3(
), 刘玲1,2,3( ), 梁静3, 刘苗苗1,2,3, 孙梦圆1,2,3, 康国俊1,2,3
), 梁静3, 刘苗苗1,2,3, 孙梦圆1,2,3, 康国俊1,2,3
- 1.中国矿业大学江苏省煤基温室气体减排与资源化利用重点实验室,江苏 徐州 221008
2.中国矿业大学碳中和研究院,江苏 徐州 221008
3.中国矿业大学化工学院,江苏 徐州 221116
-
收稿日期:2023-12-23修回日期:2024-03-01出版日期:2025-01-15发布日期:2025-02-13 -
通讯作者:陆诗建,刘玲 -
作者简介:王宁(1997—),女,硕士研究生,研究方向为CCUS技术。E-mail:ts21040014a31@cumt.edu.cn。 -
基金资助:中央高校基本科研业务费项目-科研基地建设专项(2023KYJD1004);江苏省煤基温室气体减排与资源化利用重点实验室创新能力建设专项(220ZDZZ01A);烟气二氧化碳直接矿化与催化转化利用重大科技示范(BE2022613)
Research progress of catalytic regeneration for energy-efficient CO2 capture in amine absorption system
WANG Ning1,2,3( ), LU Shijian1,2,3(
), LU Shijian1,2,3( ), LIU Ling1,2,3(
), LIU Ling1,2,3( ), LIANG Jing3, LIU Miaomiao1,2,3, SUN Mengyuan1,2,3, KANG Guojun1,2,3
), LIANG Jing3, LIU Miaomiao1,2,3, SUN Mengyuan1,2,3, KANG Guojun1,2,3
- 1.Jiangsu Key Laboratory of Coal-based Greenhouse Gas Control and Utilization, China University of Mining and Technology, Xuzhou 221008, Jiangsu, China
2.Carbon Neutrality Institute, China University of Mining and Technology, Xuzhou 221008, Jiangsu, China
3.School of Chemical Engineering and Technology, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
-
Received:2023-12-23Revised:2024-03-01Online:2025-01-15Published:2025-02-13 -
Contact:LU Shijian, LIU Ling
摘要:
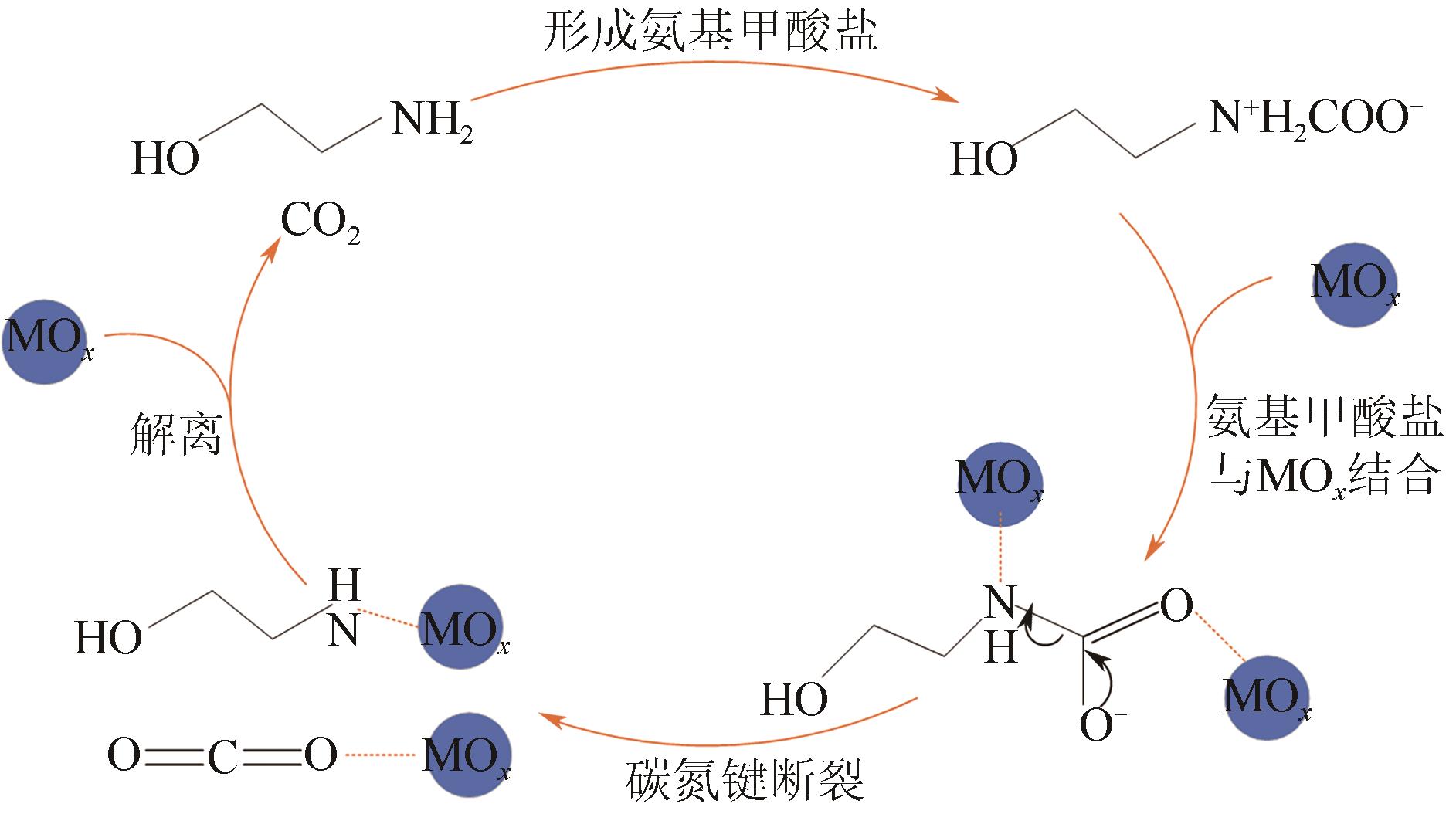
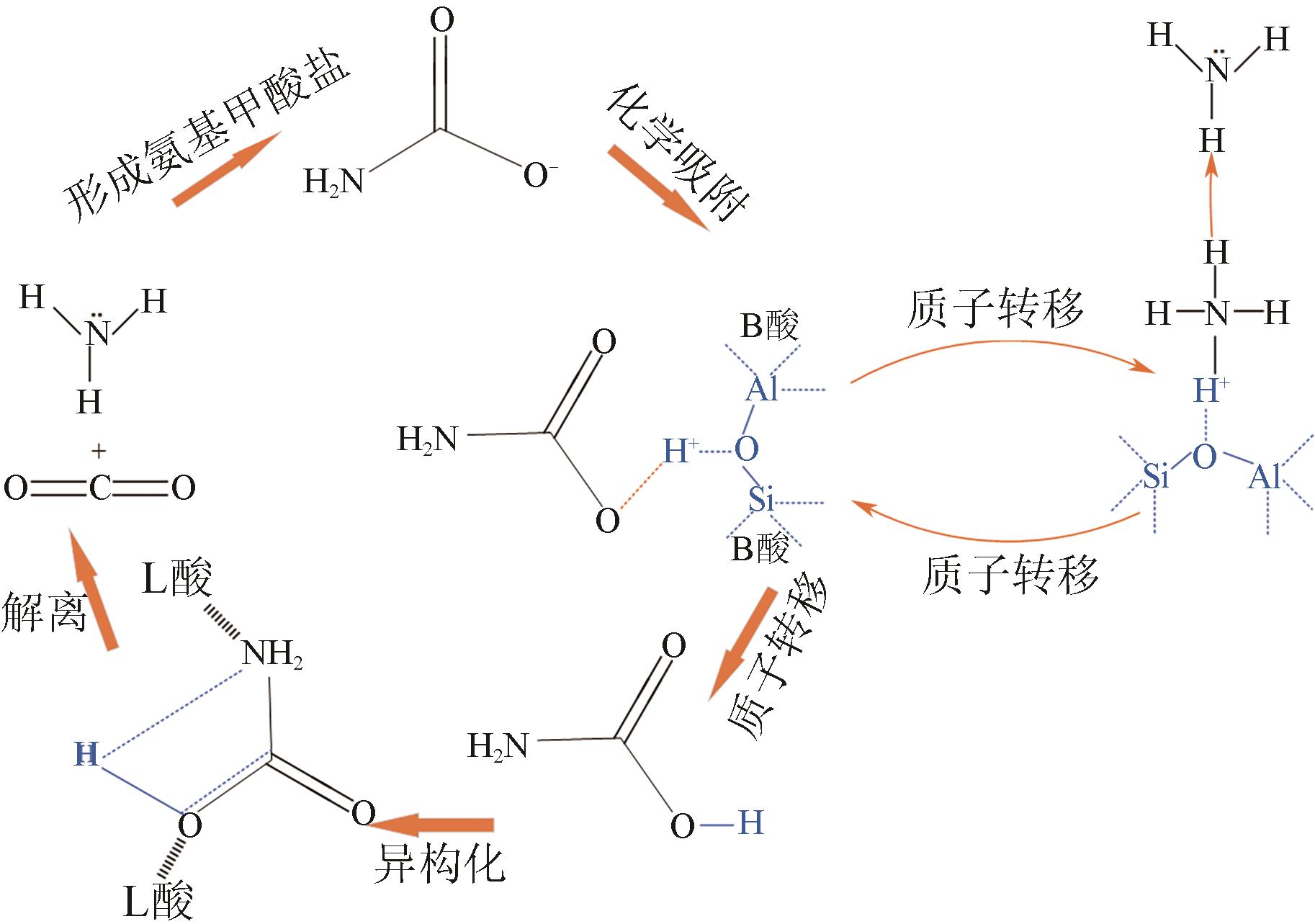
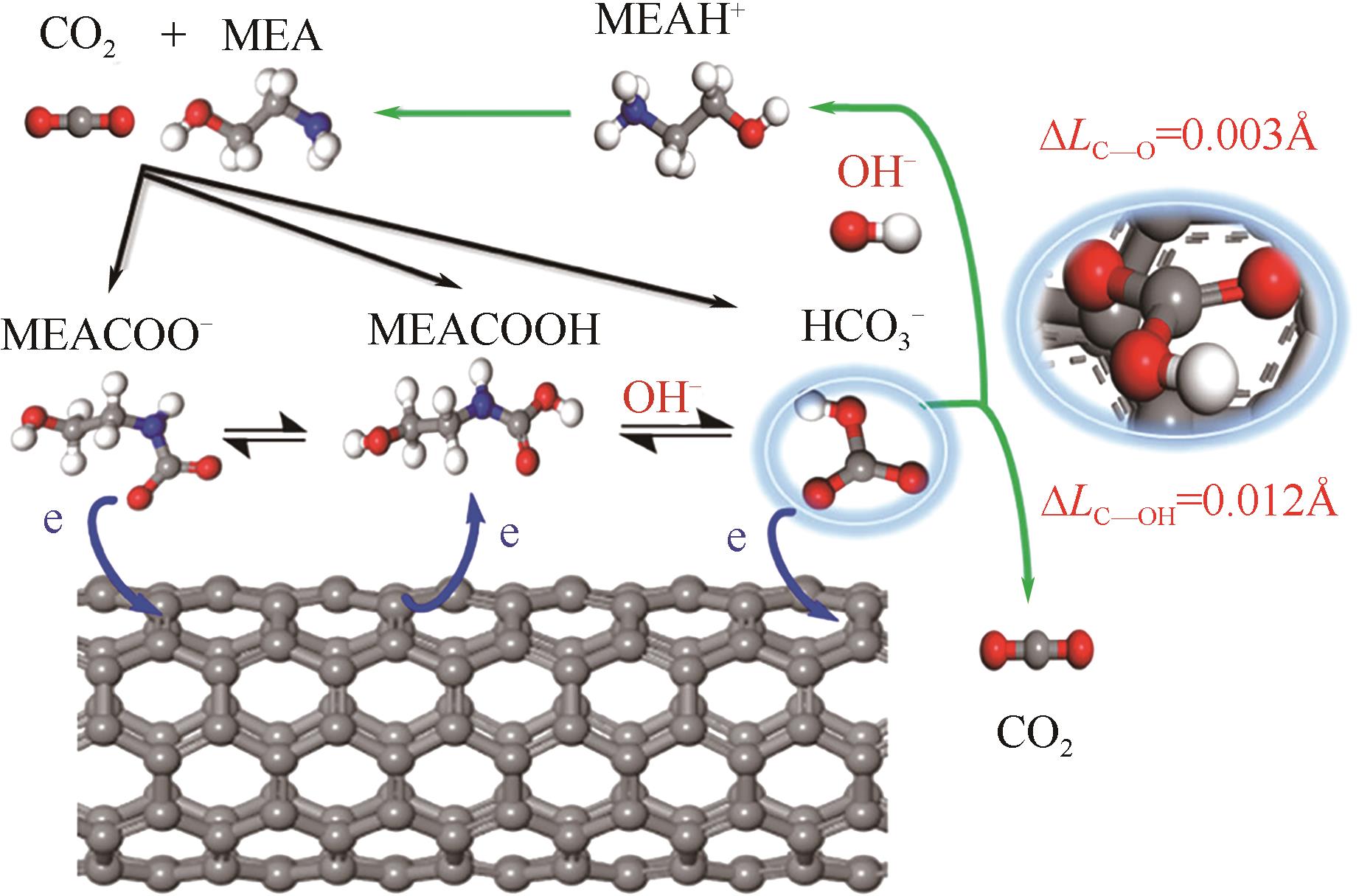
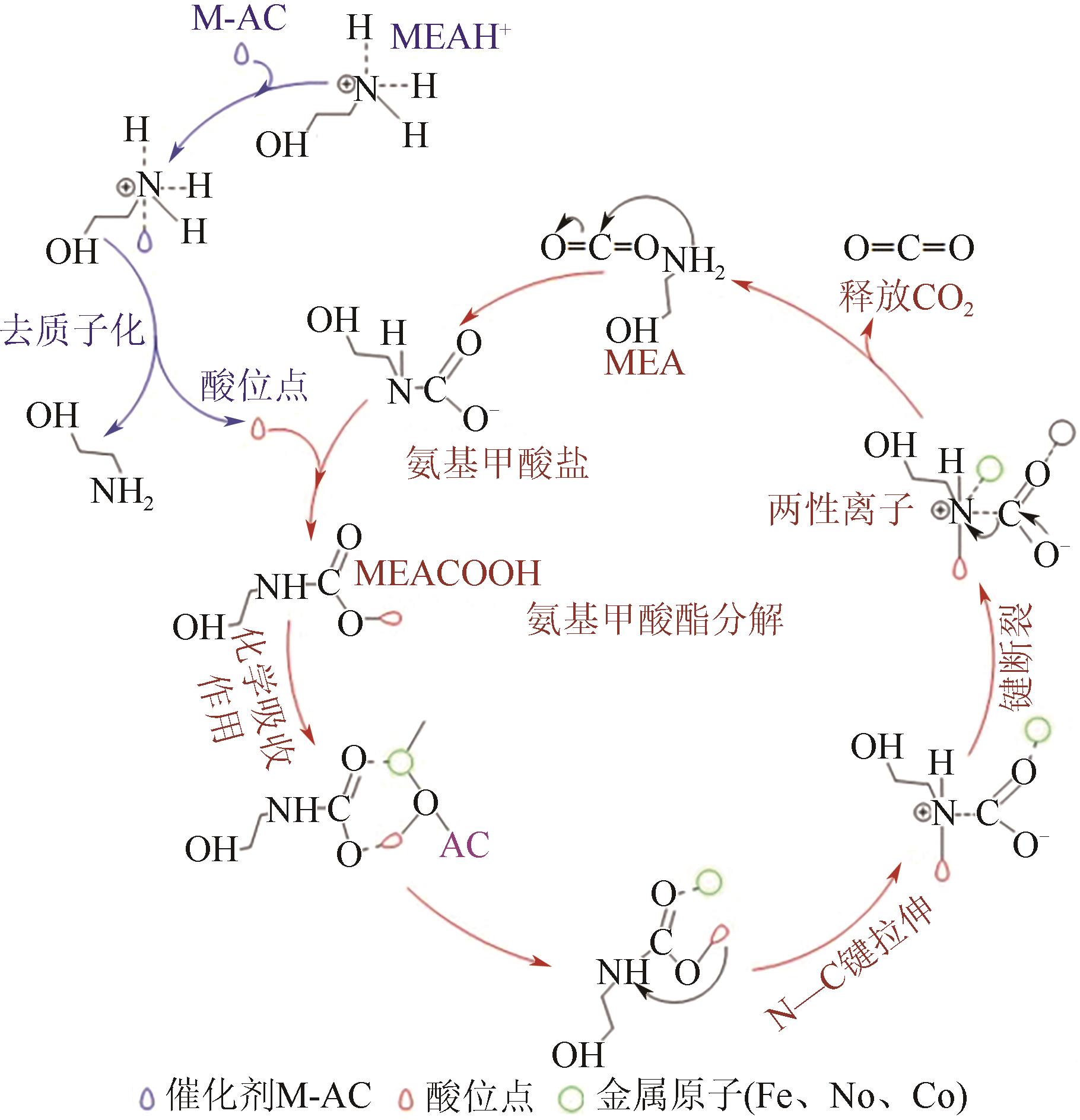
人类工业活动造成大气中CO2含量逐渐增加,形成温室效应,导致全球气候异常。碳捕集、利用与封存(CCUS)技术,尤其是CO2化学吸收过程,是实现大规模CO2减排和遏制全球气候变化的最有效的方法之一。然而,由于CO2捕集技术的高能耗高成本是导致CCUS技术无法大规模推广和商业化应用的瓶颈之一。近年来,胺吸收剂催化再生技术作为一种具有大规模应用潜力的CO2捕集节能新技术引起了国内外研究者的广泛关注。本文综述了胺吸收体系中CO2催化解吸再生技术的研究现状,详细介绍了非均相催化剂的种类、特点、优缺点和面临的挑战,阐述了胺溶液中CO2催化解吸反应机理以及Lewis酸、Brønsted酸和碱性活性位点等在催化反应过程中的作用机制,总结了影响催化剂解吸再生性能的主要因素。最后,全面分析了催化解吸再生技术用于燃烧后CO2捕集的现状,并对未来的研究趋势进行了展望。
中图分类号:
引用本文
王宁, 陆诗建, 刘玲, 梁静, 刘苗苗, 孙梦圆, 康国俊. 胺吸收体系中CO2催化解吸再生技术的研究进展[J]. 化工进展, 2025, 44(1): 445-464.
WANG Ning, LU Shijian, LIU Ling, LIANG Jing, LIU Miaomiao, SUN Mengyuan, KANG Guojun. Research progress of catalytic regeneration for energy-efficient CO2 capture in amine absorption system[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 445-464.
| 1 | Se-Young OH, YUN Seokwon, KIM Jin-Kuk. Process integration and design for maximizing energy efficiency of a coal-fired power plant integrated with amine-based CO2 capture process[J]. Applied Energy, 2018, 216: 311-322. |
| 2 | WANG Peng, GUO Yafei, ZHAO Chuanwen, et al. Biomass derived wood ash with amine modification for post-combustion CO2 capture[J]. Applied Energy, 2017, 201: 34-44. |
| 3 | LIANG Zhiwu, FU Kaiyun, IDEM Raphael, et al. Review on current advances, future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents[J]. Chinese Journal of Chemical Engineering, 2016, 24(2): 278-288. |
| 4 | CHENG Chin-hung, LI Kangkang, YU Hai, et al. Amine-based post-combustion CO2 capture mediated by metal ions: Advancement of CO2 desorption using copper ions[J]. Applied Energy, 2018, 211: 1030-1038. |
| 5 | BARZAGLI Francesco, GIORGI Claudia, MANI Fabrizio, et al. Reversible carbon dioxide capture by aqueous and non-aqueous amine-based absorbents: A comparative analysis carried out by 13C NMR spectroscopy[J]. Applied Energy, 2018, 220: 208-219. |
| 6 | 李晓静. 固体材料对AEE水溶液解吸CO2过程的强化[D]. 大连: 大连理工大学, 2020. |
| LI Xiaojing. Enhancement of CO2 desorption from 2-(2-aminoethylamine) ethanol aqueous solution by solid materials[D]. Dalian: Dalian University of Technology, 2020. | |
| 7 | ZHANG Weidong, JIN Xianhang, TU Weiwei, et al. Development of MEA-based CO2 phase change absorbent[J]. Applied Energy, 2017, 195: 316-323. |
| 8 | Se-Young OH, BINNS Michael, CHO Habin, et al. Energy minimization of MEA-based CO2 capture process[J]. Applied Energy, 2016, 169: 353-362. |
| 9 | ZHANG Zhien, BORHANI Tohid N, OLABI Abdul G. Status and perspective of CO2 absorption process[J]. Energy, 2020, 205: 118057. |
| 10 | SACHDE Darshan, ROCHELLE Gary T. Absorber intercooling configurations using aqueous piperazine for capture from sources with 4% to 27% CO2 [J]. Energy Procedia, 2014, 63: 1637-1656. |
| 11 | LIN Yu-Jeng, MADAN Tarun, ROCHELLE Gary T. Regeneration with rich bypass of aqueous piperazine and monoethanolamine for CO2 capture[J]. Industrial & Engineering Chemistry Research, 2014, 53(10): 4067-4074. |
| 12 | JIANG Kaiqi, LI Kangkang, YU Hai, et al. Piperazine-promoted aqueous-ammonia-based CO2 capture: Process optimisation and modification[J]. Chemical Engineering Journal, 2018, 347: 334-342. |
| 13 | LIN Yu-Jeng, ROCHELLE Gary T. Optimum heat of absorption for CO2 capture using the advanced flash stripper[J]. International Journal of Greenhouse Gas Control, 2016, 53: 169-177. |
| 14 | ZHANG Xiaowen, HUANG Yufei, YANG Jian, et al. Amine-based CO2 capture aided by acid-basic bifunctional catalyst: Advancement of amine regeneration using metal modified MCM-41[J]. Chemical Engineering Journal, 2020, 383: 123077. |
| 15 | WASEEM Muhammad, Mohamed AL-MARZOUQI, GHASEM Nayef. A review of catalytically enhanced CO2-rich amine solutions regeneration[J]. Journal of Environmental Chemical Engineering, 2023, 11(4): 110188. |
| 16 | 张芯. 固体酸型催化剂降低醇胺溶液解吸能耗的实验研究[D]. 长沙: 湖南大学, 2017. |
| ZHANG Xin. Experimental study on the single and blended solvents regeneration of a CO2-loaded solution using different solid acid catalysts[D]. Changsha: Hunan University, 2017. | |
| 17 | ALIVAND Masood S, MAZAHERI Omid, WU Yue, et al. Catalytic solvent regeneration for energy-efficient CO2 capture[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(51): 18755-18788. |
| 18 | IDEM Raphael, SHI Huancong, GELOWITZ Don, et al. Catalytic method and apparatus for separating a gaseous component from an incoming gas stream: US20130108532[P]. 2017-03-07. |
| 19 | BHATTI Umair H, SHAH Abdul K, KIM Jeong Nam, et al. Effects of transition metal oxide catalysts on MEA solvent regeneration for the post-combustion carbon capture process[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(7): 5862-5868. |
| 20 | BHATTI Umair H, SIVANESAN Dharmalingam, Dae Ho LIM, et al. Metal oxide catalyst-aided solvent regeneration: A promising method to economize post-combustion CO2 capture process[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 93: 150-157. |
| 21 | 崔梦星. 固体材料对非水吸收液捕集CO2的强化研究[D]. 大连: 大连理工大学, 2018. |
| CUI Mengxing. The reinforcement of CO2 capture in non-aqueous absorbent with solid materials[D]. Dalian: Dalian University of Technology, 2018. | |
| 22 | AKACHUKU Ananda, OSEI Priscilla Anima, Benjamin DECARDI-NELSON, et al. Experimental and kinetic study of the catalytic desorption of CO2 from CO2-loaded monoethanolamine (MEA) and blended monoethanolamine—Methyl-diethanolamine (MEA-MDEA) solutions[J]. Energy, 2019, 179: 475-489. |
| 23 | 李晨旭. 新型CO2捕集吸收体系构建及其再生过程的研究[D]. 石家庄: 河北科技大学, 2020. |
| LI Chenxu. Study on novel absorbent systems for CO2 capture and its regeneration characteristics[D]. Shijiazhuang: Hebei University of Science and Technology, 2020. | |
| 24 | JI Long, LI Jiabi, ZHAI Rongrong, et al. Metal oxyhydroxide catalysts promoted CO2 absorption and desorption in amine-based carbon capture: A feasibility study[J]. ACS Omega, 2022, 7(49): 44620-44630. |
| 25 | YU Yanan, SHEN Yao, WANG Ke, et al. A facile synthesized robust catalyst for efficient regeneration of biphasic solvent in CO2 capture: Characterization, performance, and mechanism[J]. Separation and Purification Technology, 2023, 319: 124057. |
| 26 | LIU Shenghua, MAO Xudong, CHEN Hao, et al. Catalytic-CO2-desorption studies of BZA-AEP mixed absorbent by the Lewis acid catalyst CeO2-γ-Al2O3 [J]. Molecules, 2023, 28(11): 4438. |
| 27 | LAI Qinghua, TOAN Sam, ASSIRI Mohammed. A,et al. Catalyst-TiO(OH)2 could drastically reduce the energy consumption of CO2 capture[J]. Nature Communications, 2018, 9(1): 2672. |
| 28 | 李晓静, 张永春, 陈绍云. 改性氧化钛对羟乙基乙二胺水溶液解吸CO2的强化[J]. 化工进展, 2020, 39(5): 2026-2032. |
| LI Xiaojing, ZHANG Yongchun, CHEN Shaoyun. Enhancement of CO2 desorption from 2-(2-aminoethylamino) ethanol aqueous solution by modified titanium oxide[J]. Chemical Industry and Engineering Progress, 2020, 39(5): 2026-2032. | |
| 29 | JIANG Cong, FAN Maohong, GAO Ge, et al. Nanostructured AlOOH—A promising catalyst to reduce energy consumption for amine-based CO2 capture[J]. Separation and Purification Technology, 2022, 303: 122232. |
| 30 | ZHANG Xiaowen, ZHANG Xin, LIU Helei, et al. Reduction of energy requirement of CO2 desorption from a rich CO2-loaded MEA solution by using solid acid catalysts[J]. Applied Energy, 2017, 202: 673-684. |
| 31 | ZHANG Xin, LIU Helei, LIANG Zhiwu. CO2 desorption in single and blended amine solvents with and without catalyst[J]. Energy Procedia, 2017, 114: 1862-1868. |
| 32 | PRASONGTHUM Natthawan, NATEWONG Paweesuda, REUBROY CHAROEN Prasert, et al. Solvent regeneration of a CO2-loaded BEA—AMP Bi-blend amine solvent with the aid of a solid Brønsted Ce(SO4)2/ZrO2 superacid catalyst[J]. Energy & Fuels, 2019, 33(2): 1334-1343. |
| 33 | LI Lijun, LIU Yingying, WU Kejing, et al. Catalytic solvent regeneration of a CO2-loaded MEA solution using an acidic catalyst from industrial rough metatitanic acid[J]. Greenhouse Gases: Science and Technology, 2020, 10(2): 449-460. |
| 34 | GENG Zanbu, YANG Yang, WANG Yixi, et al. Catalytic regeneration of amine-based absorbents for CO2 capture: The effect of acidic sites and accessibility[J]. Separation and Purification Technology, 2023, 327: 124889. |
| 35 | WEI Ying, PARMENTIER Tanja E, DE JONG Krijn P, et al. Tailoring and visualizing the pore architecture of hierarchical zeolites[J]. Chemical Society Reviews, 2015, 44(20): 7234-7261. |
| 36 | JAVAD KALBASI Roozbeh, MANSOURI Sanaz, MAZAHERI Omid. In situ polymerization of poly(vinylimidazole) into the pores of hierarchical MFI zeolite as an acid–base bifunctional catalyst for one-pot C—C bond cascade reactions[J]. Research on Chemical Intermediates, 2018, 44(5): 3279-3291. |
| 37 | ZHOU Cheng, KHALIL Ibrahim, RAMMAL Fatima, et al. A critical revisit of zeolites for CO2 desorption in primary amine solution argues its genuine catalytic function[J]. ACS Catalysis, 2022, 12(18): 11485-11493. |
| 38 | ZHANG Xiaowen, HUANG Yufei, GAO Hongxia, et al. Zeolite catalyst-aided tri-solvent blend amine regeneration: An alternative pathway to reduce the energy consumption in amine-based CO2 capture process[J]. Applied Energy, 2019, 240: 827-841. |
| 39 | KIM Yong Tae, JUNG Kwang-Deog, PARK Eun Duck. A comparative study for gas-phase dehydration of glycerol over H-zeolites[J]. Applied Catalysis A: General, 2011, 393(1/2): 275-287. |
| 40 | LI Jiyang, CORMA Avelino, YU Jihong. Synthesis of new zeolite structures[J]. Chemical Society Reviews, 2015, 44(20): 7112-7127. |
| 41 | Karin MÖLLER, BEIN Thomas. Mesoporosity—A new dimension for zeolites[J]. Chemical Society Reviews, 2013, 42(9): 3689-3707. |
| 42 | ZHANG Ke, OSTRAAT Michele L. Innovations in hierarchical zeolite synthesis[J]. Catalysis Today, 2016, 264: 3-15. |
| 43 | SCHREIBER Moritz W, PLAISANCE Craig P, Martin BAUMGÄRTL, et al. Lewis-Brønsted acid pairs in Ga/H-ZSM-5 to catalyze dehydrogenation of light alkanes[J]. Journal of the American Chemical Society, 2018, 140(14): 4849-4859. |
| 44 | WANG Chuanfu, ZHANG Lei, HUANG Xin, et al. Maximizing sinusoidal channels of HZSM-5 for high shape-selectivity to p-xylene[J]. Nature Communications, 2019, 10(1): 4348. |
| 45 | SHI Huancong, NAAMI Abdulaziz, IDEM Raphael, et al. Catalytic and non catalytic solvent regeneration during absorption-based CO2 capture with single and blended reactive amine solvents[J]. International Journal of Greenhouse Gas Control, 2014, 26: 39-50. |
| 46 | AFARI Daniel B, COKER James, Jessica NARKU-TETTEH, et al. Comparative kinetic studies of solid absorber catalyst (K/MgO) and solid desorber catalyst (HZSM-5)-aided CO2 absorption and desorption from aqueous solutions of MEA and blended solutions of BEA-AMP and MEA-MDEA[J]. Industrial & Engineering Chemistry Research, 2018, 57(46): 15824-15839. |
| 47 | COKER James, AFARI Daniel Boafo, Jessica NARKU-TETTEH, et al. Mass-transfer studies of solid-base catalyst-aided CO2 absorption and solid-acid catalyst-aided CO2 desorption for CO2 capture in a pilot plant using aqueous solutions of MEA and blends of MEA-MDEA and BEA-AMP[J]. Clean Energy, 2019, 3(4): 263-277. |
| 48 | 王皓, 唐思扬, 钟山, 等. MEA吸收CO2富液解吸过程中固体颗粒表面的强化作用分析[J]. 化工学报, 2023, 74(4): 1539-1548. |
| WANG Hao, Tang Siyang, Zhong Shan, et al. An investigation of the enhancing effect of solid particle surface on the CO2 desorption behavior in chemical sorption process with MEA solution[J]. CIESC Journal, 2023, 74(4): 1539-1548. | |
| 49 | SUN Qiang, GAO Hongxia, SEMA Teerawat, et al. Enhanced CO2 desorption rate for rich amine solution regeneration over hierarchical HZSM-5 catalyst[J]. Chemical Engineering Journal, 2023, 469: 143871. |
| 50 | LIANG Zhiwu, IDEM Raphael, TONTIWACHWUTHIKUL Paitoon, et al. Experimental study on the solvent regeneration of a CO2-loaded MEA solution using single and hybrid solid acid catalysts[J]. AIChE Journal, 2016, 62(3): 753-765. |
| 51 | MOLINER Manuel, Cristina MARTíNEZ, CORMA Avelino. Synthesis strategies for preparing useful small pore zeolites and zeotypes for gas separations and catalysis[J]. Chemistry of Materials, 2014, 26(1): 246-258. |
| 52 | YANG Yannan, YU Chengzhong. Advances in silica based nanoparticles for targeted cancer therapy[J]. Nanomedicine, 2016, 12(2): 317-332. |
| 53 | GIRALDO L F, LÓPEZ B L, PÉREZ L, et al. Mesoporous silica applications[J]. Macromolecular Symposia, 2007, 258(1): 129-141. |
| 54 | LIU Helei, ZHANG Xin, GAO Hongxia, et al. Investigation of CO2 regeneration in single and blended amine solvents with and without catalyst[J]. Industrial & Engineering Chemistry Research, 2017, 56(27): 7656-7664. |
| 55 | CUI Mengxing, CHEN Siming, QI Tianqinji, et al. Investigation of CO2 capture in nonaqueous ethylethanolamine solution mixed with porous solids[J]. Journal of Chemical & Engineering Data, 2018, 63(5): 1198-1205. |
| 56 | GAO Hongxia, HUANG Yufei, ZHANG Xiaowen, et al. Catalytic performance and mechanism of SO4 2-/ZrO2/SBA-15 catalyst for CO2 desorption in CO2-loaded monoethanolamine solution[J]. Applied Energy, 2020, 259. |
| 57 | 王玉龙. 酸性催化剂强化单乙醇胺CO2解吸的动力学与热力学研究[D]. 北京: 华北电力大学, 2021. |
| WANG Yulong. Study on kinetics and thermodynamics of CO2 desorption of monoethanolamine enhanced by acid catalysts[D]. Beijing: North China Electric Power University, 2021. | |
| 58 | SHI Huancong, PENG Jiacheng, CHENG Xiaofang, et al. The CO2 desorption analysis of tri-solvent MEA+BEA+DEEA with several commercial solid acid catalysts[J]. International Journal of Greenhouse Gas Control, 2022, 116: 103647. |
| 59 | SHI Huancong, FU Junxing, WU Qiming, et al. Studies of the coordination effect of DEA-MEA blended amines (within 1+4 to 2+3M) under heterogeneous catalysis by means of absorption and desorption parameters[J]. Separation and Purification Technology, 2020, 236: 116179. |
| 60 | XING Lei, WEI Kexin, LI Qiangwei, et al. One-step synthesized SO4 2-/ZrO2-HZSM-5 solid acid catalyst for carbamate decomposition in CO2 capture[J]. Environmental Science & Technology, 2020, 54(21): 13944-13952. |
| 61 | SUN Qiang, GAO Hongxia, MAO Yu, et al. Efficient nickel-based catalysts for amine regeneration of CO2 capture: From experimental to calculations verifications[J]. AIChE Journal, 2022, 68(8): e17706. |
| 62 | LI Mingyue, XING Lei, XU Zhongfei, et al. Embedded Mo/Mn atomic regulation for durable acidity-reinforced HZSM-5 catalyst toward energy-efficient amine regeneration[J]. Environmental Science and Technology, 2023, 57(41): 15465-15474. |
| 63 | KAWI S, LAI M W. Supercritical fluid extraction of surfactant from Si-MCM-41[J]. AIChE Journal, 2002, 48(7): 1572-1580. |
| 64 | ATANGA Marktus A, REZAEI Fateme, JAWAD Abbas, et al. Oxidative dehydrogenation of propane to propylene with carbon dioxide[J]. Applied Catalysis B: Environmental, 2018, 220: 429-445. |
| 65 | DE MORAIS BATISTA Andressa H, DE SOUSA Francisco F, HONORATO Sara B, et al. Ethylbenzene to chemicals: Catalytic conversion of ethylbenzene into styrene over metal-containing MCM-41[J]. Journal of Molecular Catalysis A: Chemical, 2010, 315(1): 86-98. |
| 66 | ZHANG Xiaowen, ZHU Zhiqing, SUN Xiaoyu, et al. Reducing energy penalty of CO2 capture using Fe promoted SO4 2-/ZrO2/MCM-41 catalyst[J]. Environmental Science & Technology, 2019, 53(10): 6094-6102. |
| 67 | SUN Qiang, LI Tianhao, MAO Yu, et al. Reducing heat duty of MEA regeneration using a sulfonic acid-functionalized mesoporous MCM-41 catalyst[J]. Industrial & Engineering Chemistry Research, 2021, 60(50): 18304-18315. |
| 68 | MORALES Gabriel, OSATIASHTIANI Amin, Blanca HERNÁNDEZ, et al. Conformal sulfated zirconia monolayer catalysts for the one-pot synthesis of ethyl levulinate from glucose[J]. Chemical Communications, 2014, 50(79): 11742-11745. |
| 69 | OSATIASHTIANI Amin, LEE Adam F, GRANOLLERS Marta, et al. Hydrothermally stable, conformal, sulfated zirconia monolayer catalysts for glucose conversion to 5-HMF[J]. ACS Catalysis, 2015, 5(7): 4345-4352. |
| 70 | HUANG Yufei, ZHANG Xiaowen, LUO Xiao, et al. Catalytic performance and mechanism of meso-microporous material β-SBA-15-supported FeZr catalysts for CO2 desorption in CO2-loaded aqueous amine solution[J]. Industrial & Engineering Chemistry Research, 2021, 60(6): 2698-2709. |
| 71 | LIU Yamin, YE Qing, SHEN Mei, et al. Carbon dioxide capture by functionalized solid amine sorbents with simulated flue gas conditions[J]. Environmental Science & Technology, 2011, 45(13): 5710-5716. |
| 72 | KISHOR Rupak, GHOSHAL Aloke Kumar. Understanding the hydrothermal, thermal, mechanical and hydrolytic stability of mesoporous KIT-6: A comprehensive study[J]. Microporous and Mesoporous Materials, 2017, 242: 127-135. |
| 73 | ZHANG Rui, LI Ting, ZHANG Yiming, et al. CuO modified KIT-6 as a high-efficiency catalyst for energy-efficient amine solvent regeneration[J]. Separation and Purification Technology, 2022, 300: 121702. |
| 74 | WANG Qi, ASTRUC Didier. State of the art and prospects in metal-organic framework (MOF)-based and MOF-derived nanocatalysis[J]. Chemical Reviews, 2020, 120(2): 1438-1511. |
| 75 | WEI Yong sheng, ZHANG Mei, ZOU Ruqiang, et al. Metal-organic framework-based catalysts with single metal sites[J]. Chemical Reviews, 2020, 120(21): 12089-12174. |
| 76 | LI Yuchen, CHEN Zhen, ZHAN Guoxiong, et al. Inducing efficient proton transfer through Fe/Ni@COF to promote amine-based solvent regeneration for achieving low-cost capture of CO2 from industrial flue gas[J]. Separation and Purification Technology, 2022, 298: 121676. |
| 77 | DING Huimin, LI Jian, XIE Guohua, et al. An AIEgen-based 3D covalent organic framework for white light-emitting diodes[J]. Nature Communications, 2018, 9(1): 5234. |
| 78 | AN Shanlong, XU Teng, XING Lei, et al. Recent progress and prospects in solid acid-catalyzed CO2 desorption from amine-rich liquid[J]. Gas Science and Engineering, 2023, 120: 205152. |
| 79 | GAO Feiyue, HU Shaojin, ZHANG Xiaolong, et al. High-curvature transition-metal chalcogenide nanostructures with a pronounced proximity effect enable fast and selective CO2 electroreduction[J]. Angewandte Chemie International Edition, 2020, 59(22): 8706-8712. |
| 80 | TRICKETT Christopher A, OSBORN POPP Thomas M, SU Ji, et al. Identification of the strong Brønsted acid site in a metal-organic framework solid acid catalyst[J]. Nature Chemistry, 2019, 11(2): 170-176. |
| 81 | JIANG Juncong, Felipe GÁNDARA, ZHANG Yuebiao, et al. Superacidity in sulfated metal-organic framework-808[J]. Journal of the American Chemical Society, 2014, 136(37): 12844-12847. |
| 82 | ALIVAND Masood S, MAZAHERI Omid, WU Yue, et al. Engineered assembly of water-dispersible nanocatalysts enables low-cost and green CO2 capture[J]. Nature Communications, 2022, 13(1): 1249. |
| 83 | XING Lei, WEI Kexin, LI Yuchen, et al. TiO2 coating strategy for robust catalysis of the metal-organic framework toward energy-efficient CO2 capture[J]. Environmental Science & Technology, 2021, 55(16): 11216-11224. |
| 84 | WEI Kexin, XING Lei, LI Yuchen, et al. Heteropolyacid modified cerium-based MOFs catalyst for amine solution regeneration in CO2 capture[J]. Separation and Purification Technology, 2022, 293: 121144. |
| 85 | XING Lei, LI Meng, LI Mingyue, et al. MOF-derived robust and synergetic acid sites inducing C—N bond disruption for energy-efficient CO2 desorption[J]. Environmental Science & Technology, 2022, 56(24): 17936-17945. |
| 86 | LI Xiaojing, ZHANG Yongchun, CHEN Shaoyun. Enhancement of CO2 desorption from reinforced 2-(2-aminoethylamine) ethanol aqueous solution by multi-walled carbon nanotubes[J]. Energy & Fuels, 2019, 33(7): 6577-6584. |
| 87 | GAO Yangyan, HE Xin, MAO Keke, et al. Catalytic CO2 capture via ultrasonically activating dually functionalized carbon nanotubes[J]. ACS Nano, 2023, 17(9): 8345-8354. |
| 88 | LI Xiaojing, XU Qian, LIU Zhishan, et al. Nonacid carbon materials as catalysts for monoethanolamine energy-efficient regeneration[J]. Environmental Science & Technology, 2023, 57(27): 9975-9983. |
| 89 | BHATTI Ali Hassan, WARIS Mamoona, KAZMI Wajahat W, et al. Metal impregnated activated carbon as cost-effective and scalable catalysts for amine-based CO2 capture[J]. Journal of Environmental Chemical Engineering, 2023, 11(1): 109231. |
| 90 | BHATTI Umair H, ALIVAND Masood S, BARZAGLI Francesco, et al. Functionalized carbon spheres for energy-efficient CO2 capture: Synthesis, application, and reaction mechanism[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(32): 11955-11964. |
| 91 | 赵月. 赖氨酸钾水溶液捕集电厂烟气中CO2的应用基础研究[D]. 石家庄: 河北科技大学, 2018. |
| ZHAO Yue. Applied fundamental research on CO2 capture using aqueous potassium lysinate solutions from power plant flue gas[D]. Shijiazhuang: Hebei University of Science and Technology, 2018. | |
| 92 | SUN Qiang, XIONG Jia, GAO Hongxia, et al. Evaluation of hybrid amines and alcohol solvent with ion-exchange resin catalysts for energy-efficient CO2 capture[J]. Green Chemistry, 2023, 25(12): 4647-4655. |
| 93 | RASHWAN M A, EL-SHAKOUR Zeinab A ABD. Low-cost, highly-performance fired clay bodies incorporating natural stone sludge: Microstructure and engineering properties[J]. Cleaner Waste Systems, 2022, 3: 100041. |
| 94 | TAO Huayu, QIAN Xi, ZHOU Yi, et al. Research progress of clay minerals in carbon dioxide capture[J]. Renewable and Sustainable Energy Reviews, 2022, 164: 112536. |
| 95 | LU Manjing, WANG Jiaqi, WANG Yuzhong, et al. Heterogeneous photo-Fenton catalytic degradation of practical pharmaceutical wastewater by modified attapulgite supported multi-metal oxides[J]. Water, 2021, 13(2): 156. |
| 96 | TAN Zhan, ZHANG Shangshang, YUE Xinwei, et al. Attapulgite as a cost-effective catalyst for low-energy consumption amine-based CO2 capture[J]. Separation and Purification Technology, 2022, 298: 121577. |
| 97 | BHATTI Umair H, KAZMI Wajahat W, MUHAMMAD Hafiz A, et al. Practical and inexpensive acid-activated montmorillonite catalysts for energy-efficient CO2 capture[J]. Green Chemistry, 2020, 22(19): 6328-6333. |
| 98 | BHATTI Umair H, KAZMI Wajahat W, MIN Gwan Hong, et al. Facilely synthesized M-montmorillonite (M=Cr, Fe, and Co) as efficient catalysts for enhancing CO2 desorption from amine solution[J]. Industrial & Engineering Chemistry Research, 2021, 60(36): 13318-13325. |
| 99 | ZHANG Rui, LI Yufan, ZHANG Yiming, et al. Energy-saving effect of low-cost and environmentally friendly sepiolite as an efficient catalyst carrier for CO2 capture[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(11): 4353-4363. |
| 100 | TAN Zhan, ZHANG Shangshang, ZHAO Fangfang, et al. SnO2/ATP catalyst enabling energy-efficient and green amine-based CO2 capture[J]. Chemical Engineering Journal, 2023, 453: 139801. |
| 101 | QIN Ling, GAO Xiaojian, LI Qiyan. Influences of coal fly ash containing ammonium salts on properties of cement paste[J]. Journal of Environmental Management, 2019, 249: 109374. |
| 102 | DINDI Abdallah, QUANG Dang Viet, VEGA Lourdes F, et al. Applications of fly ash for CO2 capture, utilization, and storage[J]. Journal of CO2 Utilization, 2019, 29: 82-102. |
| 103 | HEMALATHA T, RAMASWAMY Ananth. A review on fly ash characteristics—Towards promoting high volume utilization in developing sustainable concrete[J]. Journal of Cleaner Production, 2017, 147: 546-559. |
| 104 | CHEN Linlin, LU Shijian, ZHANG Lei, et al. Solid waste of fly ash toward energy-efficient CO2 capture[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(22): 8281-8293. |
| 105 | Zening Lyu, QIAO Kun, CHU Fengming, et al. Experimental study of divalent metal ion effects on ammonia escape and carbon dioxide desorption in regeneration process of ammonia decarbonization[J]. Chemical Engineering Journal, 2022, 435: 134841. |
| 106 | SRISANG Wayuta, POURYOUSEFI Fatemeh, OSEI Priscilla Anima, et al. Evaluation of the heat duty of catalyst-aided amine-based post combustion CO2 capture[J]. Chemical Engineering Science, 2017, 170: 48-57. |
| 107 | HU Xiayi, YU Qian, CUI Yuanyuan, et al. Toward solvent development for industrial CO2 capture by optimizing the catalyst-amine formulation for lower energy consumption in the solvent regeneration process[J]. Energy & Fuels, 2019, 33(11): 11507-11515. |
| 108 | ZHANG Xiaowen, LIU Helei, LIANG Zhiwu, et al. Reducing energy consumption of CO2 desorption in CO2-loaded aqueous amine solution using Al2O3/HZSM-5 bifunctional catalysts[J]. Applied Energy, 2018, 229: 562-576. |
| 109 | SALEH BAIRQ Zain ALI, GAO Hongxia, HUANG Yufei, et al. Enhancing CO2 desorption performance in rich MEA solution by addition of SO4 2-/ZrO2/SiO2 bifunctional catalyst[J]. Applied Energy, 2019, 252: 113440. |
| 110 | ZHANG Xiaowen, HONG Jieling, LIU Helei, et al. SO4 2-/ZrO2 supported on γ-Al2O3 as a catalyst for CO2 desorption from CO2-loaded monoethanolamine solutions[J]. AIChE Journal, 2018, 64(11): 3988-4001. |
| 111 | NATEWONG Paweesuda, PRASONGTHUM Natthawan, REUBROYC HAROEN Prasert, et al. Evaluating the CO2 capture performance using a BEA-AMP biblend amine solvent with novel high-performing absorber and desorber catalysts in a bench-scale CO2 capture pilot plant[J]. Energy & Fuels, 2019, 33(4): 3390-3402. |
| 112 | SALEH BAIRQ Zain ALI, GAO Hongxia, MURSHED Fatima Abduh Mohammed, et al. Modified heterogeneous catalyst-aided regeneration of CO2 capture amines: A promising perspective for a drastic reduction in energy consumption[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(25): 9526-9536. |
| 113 | XU Yin, JIN Baosheng, JIANG Hejia, et al. Investigation of the regeneration of a CO2-loaded ammonia solution with solid acid catalysts: A promising alternative for reducing regeneration energy[J]. Fuel Processing Technology, 2020, 205: 106452. |
| 114 | BHATTI Umair, Sungchan NAM, PARK Sungyoul, et al. Performance and mechanism of metal oxide catalyst-aided amine solvent regeneration[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 1207 |
| [1] | 陆诗建, 张娟娟, 杨菲, 刘玲, 陈思铭, 康国俊, 房芹芹. 化学吸收法胺液逃逸控制技术研究进展[J]. 化工进展, 2024, 43(8): 4562-4570. |
| [2] | 唐思扬, 李星宇, 鲁厚芳, 钟山, 梁斌. 低能耗化学吸收碳捕集技术展望[J]. 化工进展, 2022, 41(3): 1102-1106. |
| [3] | 张静, 马慧玲, 曾得福, 姚潇毅. 水热催化制备绿色柴油工艺中催化剂的失活与再生[J]. 化工进展, 2022, 41(2): 682-689. |
| [4] | 张卫风, 许元龙, 王秋华. CO2醇胺富液低能耗再生研究进展[J]. 化工进展, 2021, 40(8): 4497-4507. |
| [5] | 张克舫1,2,刘中良1,王远亚1,李艳霞1. 化学吸收法CO2捕集解吸能耗的分析计算[J]. 化工进展, 2013, 32(12): 3008-3014. |
| [6] | 蒋 波,张晓东,孙 立,许 敏. 微波促进生物柴油制备的研究进展 [J]. 化工进展, 2010, 29(11): 2057-. |
| [7] | 晏水平,方梦祥,张卫风,骆仲泱,岑可法. 烟气中CO2化学吸收法脱除技术分析与进展 [J]. 化工进展, 2006, 25(9): 1018-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||