化工进展 ›› 2025, Vol. 44 ›› Issue (1): 253-265.DOI: 10.16085/j.issn.1000-6613.2024-0066
二氧化碳加氢制高级醇Fe基催化剂研究进展
- 内蒙古鄂尔多斯电力冶金集团股份有限公司,内蒙古 鄂尔多斯 016064
-
收稿日期:2024-01-09修回日期:2024-06-11出版日期:2025-01-15发布日期:2025-02-13 -
通讯作者:牛强 -
作者简介:秦婷婷(1993—),女,博士,工程师,研究方向为C1催化转化、水处理技术开发。E-mail:qintingting1@chinaerdos.com。 -
基金资助:内蒙古自治区科技重大专项(2021ZD0042);内蒙古自治区科技计划(2023YFHH0043)
Research progress on Fe-based catalysts for CO2 hydrogenation to higher alcohols
- Inner Mongolia Erdos Electric Power and Metallurgy Group Co. , Ltd. , Ordos 016064, Inner Mongolia, China
-
Received:2024-01-09Revised:2024-06-11Online:2025-01-15Published:2025-02-13 -
Contact:NIU Qiang
摘要:
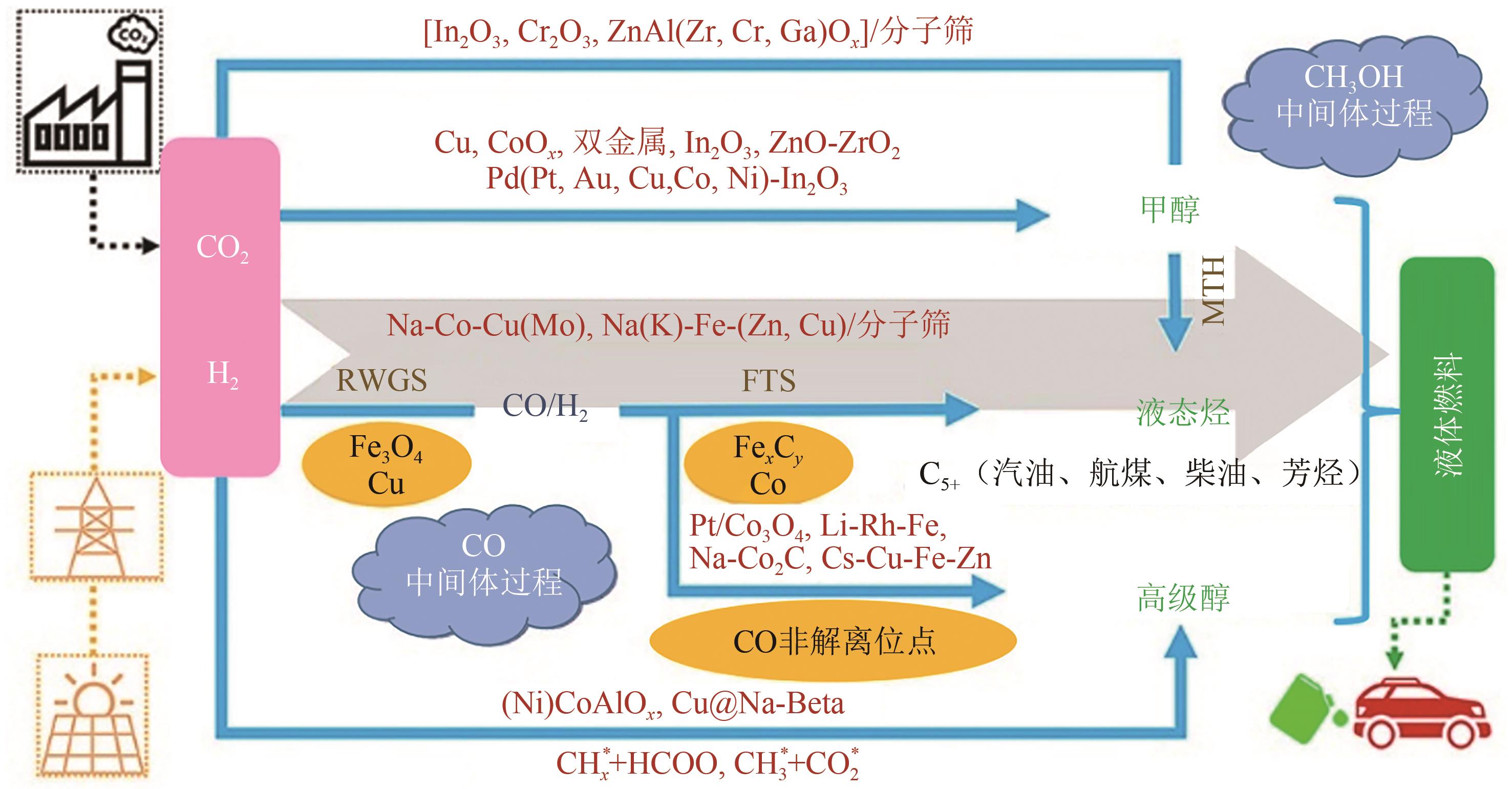
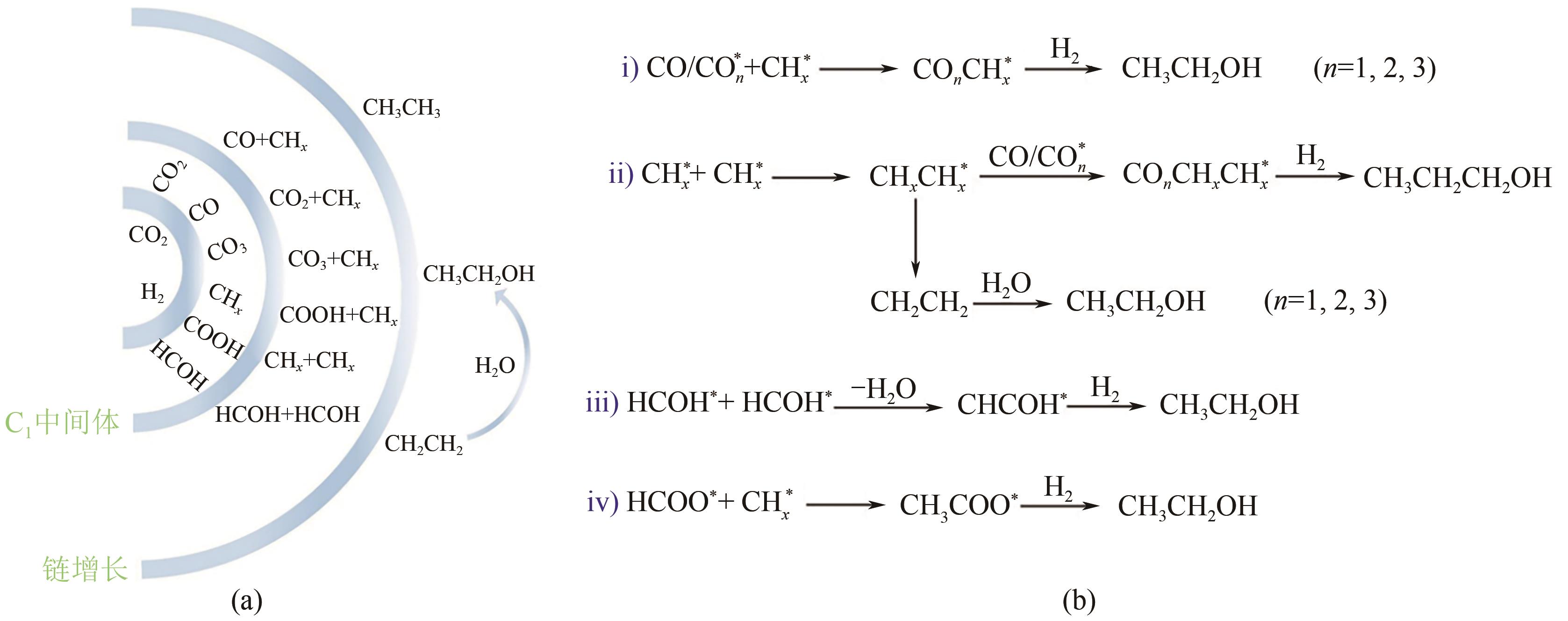
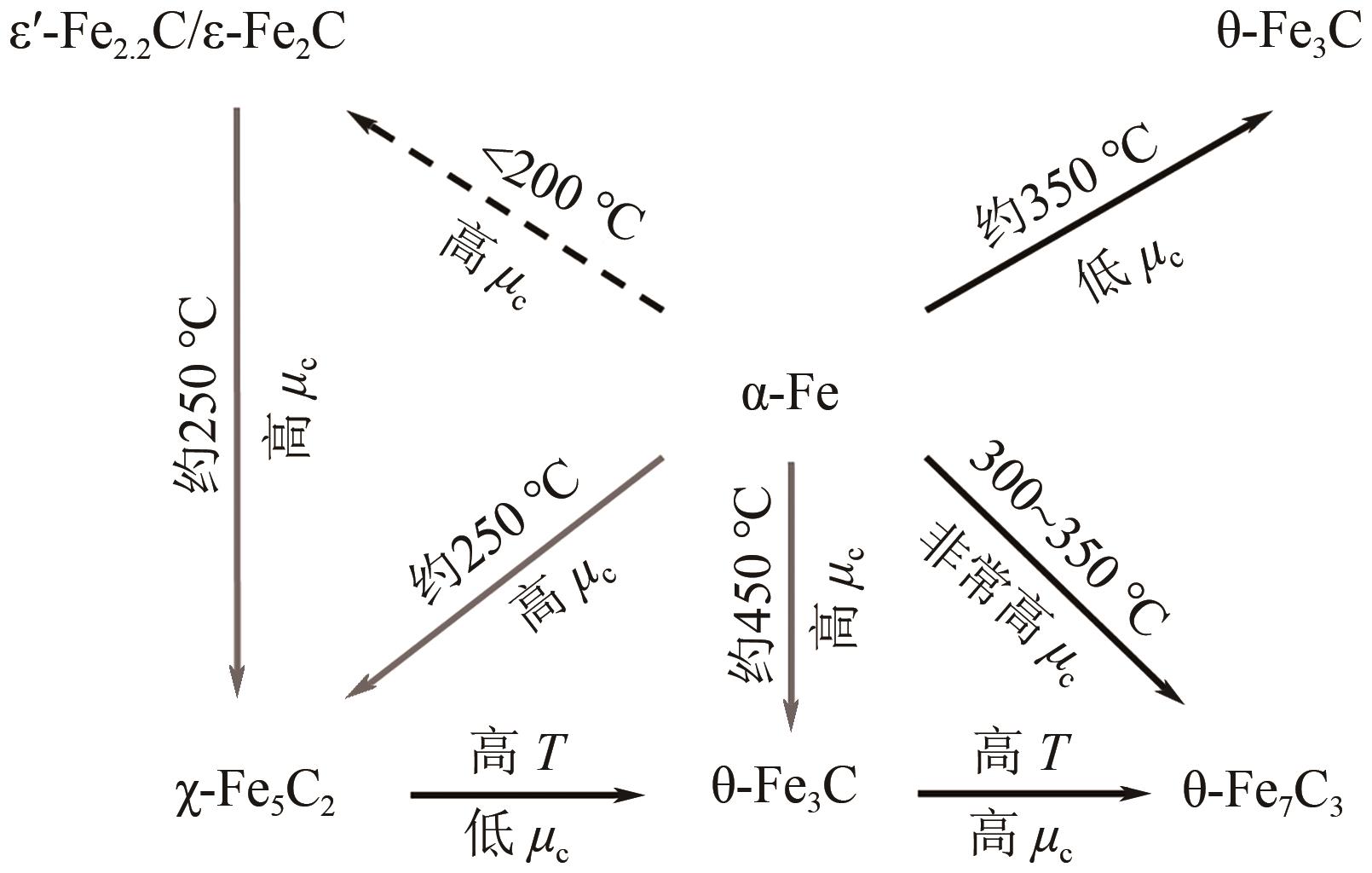
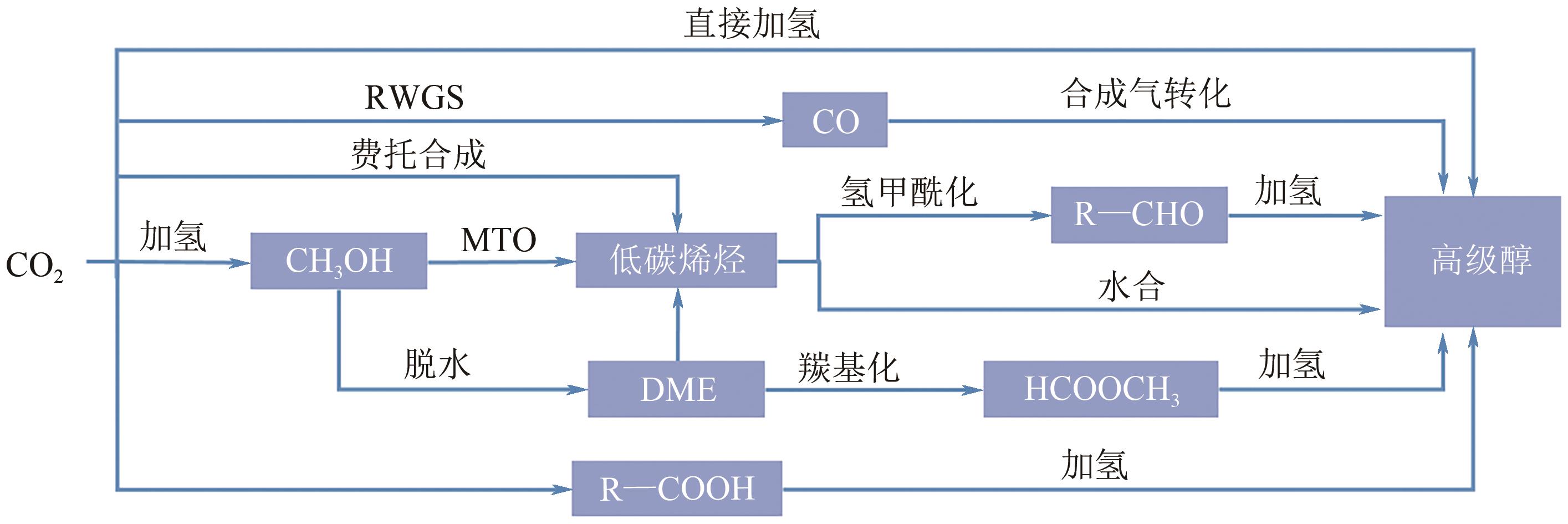
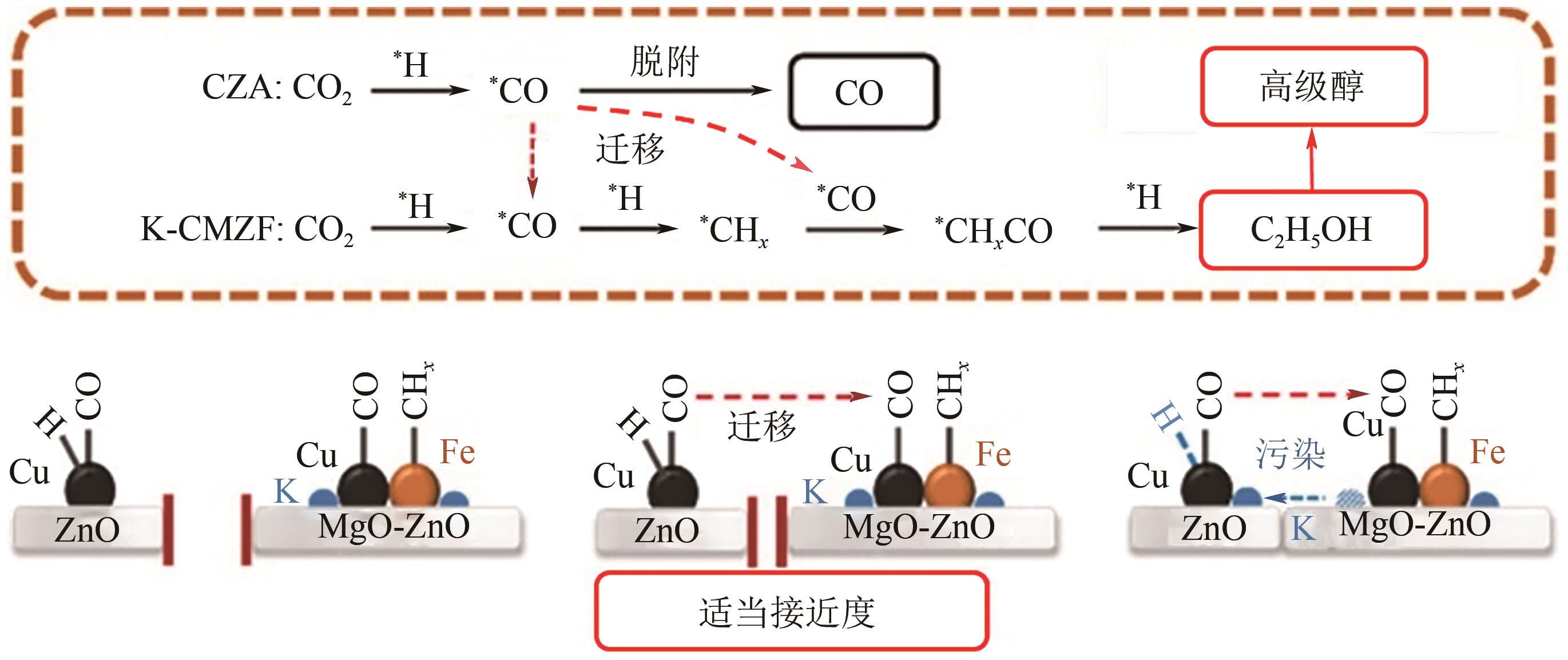
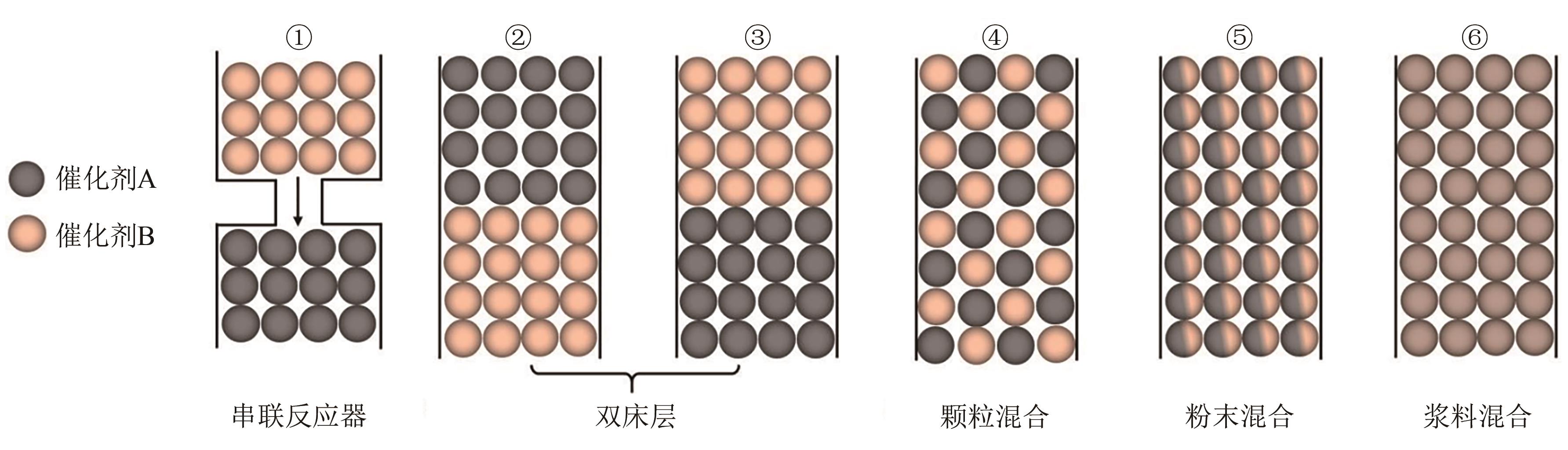
高级醇(HA)可作为重要的能源化工基础原料。CO2加氢制备高级醇(CO2-HAS)具有流程短、效率高、操作简便等特点,该技术兼具碳减排和高附加值利用的双重意义。Fe基催化剂的低成本和高活性优势使其具备工业应用潜力,但仍存在反应网络复杂、C—C键生成难控制、高级醇收率不理想等问题。本文从热力学角度分析了CO2-HAS的固有限制和适宜的工艺条件,简述了高级醇生成的转化路径和Fe物种演变。进一步阐述了反应条件、助剂、制备方法和载体等因素对Fe基催化剂反应性能的影响,并揭示了Fe基多功能催化反应偶联的构建策略以及对性能的促进机制。基于此,指出中间物种的定向转化和C—C偶联的精准调控是Fe基催化剂设计的关键考量。预测3D打印自催化技术将助力Fe基催化剂的大规模制备,多元化的技术整合可能是CO2-HAS产业化开发的可行方式之一。廉价的绿氢制备技术与CO2捕集技术的突破与耦合将推动CO2-HAS的高质量发展并成为主流趋势。
中图分类号:
引用本文
秦婷婷, 牛强. 二氧化碳加氢制高级醇Fe基催化剂研究进展[J]. 化工进展, 2025, 44(1): 253-265.
QIN Tingting, NIU Qiang. Research progress on Fe-based catalysts for CO2 hydrogenation to higher alcohols[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 253-265.
| 序号 | 反应方程 | ΔG298K/kJ·mol-1 | ΔH298K/kJ·mol-1 | K298K |
|---|---|---|---|---|
| (1) | CO2+H2 | 28.6 | 41.1 | 9.67×10-6 |
| (2) | CO+3H2 | -141.9 | -206.0 | — |
| (3) | CO2+4H2 | -113.5 | -165.0 | 7.79×1019 |
| (4) | CO+2H2 | — | -90.4 | — |
| (5) | CO2+3H2 | 3.5 | -49.3 | 2.45×10-1 |
| (6) | 2CH3OH | — | -24.52 | — |
| (7) | CH3OCH3+CO | — | — | — |
| (8) | CH3COOCH3+2H2 | — | — | — |
| (9) | CH3OH+CO+2H2 | -97.0 | -165.1 | — |
| (10) | 2CH4+H2O | — | — | — |
| (11) | C2H5OH→CH3CHO+H2 | — | — | — |
| (12) | 2CO+4H2 | -221.1 | -253.6 | — |
| (13) | 2CO2+6H2 | -32.4 | -86.7 | 4.70×105 |
| (14) | C2H5OH+3H2O | — | — | — |
| (15) | 2CO2+7H2 | -78.7 | -132.1 | 6.26×1013 |
| (16) | 3CO2+10H2 | -70.9 | -125.0 | 2.64×1012 |
| (17) | CH4 | — | 74.9 | — |
| (18) | 2CO | — | -172.5 | — |
表1 CO2加氢过程的主要反应
| 序号 | 反应方程 | ΔG298K/kJ·mol-1 | ΔH298K/kJ·mol-1 | K298K |
|---|---|---|---|---|
| (1) | CO2+H2 | 28.6 | 41.1 | 9.67×10-6 |
| (2) | CO+3H2 | -141.9 | -206.0 | — |
| (3) | CO2+4H2 | -113.5 | -165.0 | 7.79×1019 |
| (4) | CO+2H2 | — | -90.4 | — |
| (5) | CO2+3H2 | 3.5 | -49.3 | 2.45×10-1 |
| (6) | 2CH3OH | — | -24.52 | — |
| (7) | CH3OCH3+CO | — | — | — |
| (8) | CH3COOCH3+2H2 | — | — | — |
| (9) | CH3OH+CO+2H2 | -97.0 | -165.1 | — |
| (10) | 2CH4+H2O | — | — | — |
| (11) | C2H5OH→CH3CHO+H2 | — | — | — |
| (12) | 2CO+4H2 | -221.1 | -253.6 | — |
| (13) | 2CO2+6H2 | -32.4 | -86.7 | 4.70×105 |
| (14) | C2H5OH+3H2O | — | — | — |
| (15) | 2CO2+7H2 | -78.7 | -132.1 | 6.26×1013 |
| (16) | 3CO2+10H2 | -70.9 | -125.0 | 2.64×1012 |
| (17) | CH4 | — | 74.9 | — |
| (18) | 2CO | — | -172.5 | — |
| 序号 | 催化剂 | 制备/混合方法 | 温度/℃ | 压力/MPa | H2/CO2 | GHSV | CO2转化率/% | C2+OH选择性/% | C2+OH收率 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 30.4 | 15.8 | 70.6mg/(g·h) | [ |
| 2 | K/S-CuFeZn | 共沉淀+浸渍 | 320 | 5 | 3 | 3000mL/(g·h) | 36.1 | 20.2 | 50.7mg/(g·h) | [ |
| 3 | 10Mn1K-FeC | 热分解 | 300 | 3 | 3 | 6000mL/(g·h) | 40.5 | 10.7 | 4.3%① | [ |
| 4 | MnCuK-FeC | 热分解 | 300 | 3 | 3 | 6000mL/(g·h) | 40.8 | 13.5 | 5.6%① | [ |
| 5 | K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 28.1 | 约15.7 | 69.6mg/(g·h) | [ |
| 6 | CuZnFe0.5K0.15 | 共沉淀+浸渍 | 300 | 6 | 3 | 5000mL/(g·h) | 42.3 | 36.7 | 170mg/(mL·h) | [ |
| 7 | K/Cu-Zn-Fe | 共沉淀 | 300 | 7 | 3 | 5000h-1 | 44.2 | 26.9 | 11.9%① | [ |
| 8 | K/Cu-Zn-Fe | 共沉淀+浸渍 | 300 | 7 | 3 | 5000mL/(g·h) | 45.0 | 19.0 | 8.6%① | [ |
| 9 | PDA/CuFeZn-N450 | 共沉淀 | 320 | 4 | 3 | 7200mL/(g·h) | 约7.0 | 41.5 | 55.9mg/(mL·h) | [ |
| 10 | CuFeZn-0.7PDA | 共沉淀 | 310 | 4 | 3 | 7200mL/(g·h) | 10.6 | 33.1 | 58.2mg/(mL·h) | [ |
| 11 | KFeCu/a-ZrO2 | 分步沉淀+浸渍 | 320 | 4 | 3 | 12000mL/(g·h) | 25.7 | 26.1 | 125.0mg/(g·h) | [ |
| 12 | K-0.82-FeIn/Ce-ZrO2 | 浸渍 | 300 | 10 | 3 | 4500mL/(g·h) | 29.6 | 28.7 | 8.5%① | [ |
| 13 | (K2O)5%/CuZnFeZrO2 | 共沉淀+浸渍 | 320 | 3 | 3 | 3600mL/(g·h) | 25.5 | 11.0 | 2.55mmol/(mL·h) | [ |
| 14 | Fe-Cu-Al-K | 均匀凝胶法 | 330 | 8 | 3 | 20000h-1 | 41.4 | 11.4 | 4.7%① | [ |
| 15 | sp-CuNaFe | 物理溅射 | 310 | 3 | 3 | 28800mL/(g·h) | 32.3 | 约10.0 | 153.0mg/(g·h) | [ |
| 16 | Na-Fe@C | 水热+浸渍+碳化 | 320 | 5 | 3 | 9000mL/(g·h) | 25.5 | 12.0(乙醇) | 52.3mg/(g·h) | [ |
| 17 | Na-ZnFe@C | 水热+浸渍+碳化 | 320 | 5 | 3 | 9000mL/(g·h) | 38.4 | 20.3(乙醇) | 158.1mg/(g·h) | [ |
| 18 | 2%Na-Fe@C | 水热+热解+浸渍 | 320 | 5 | 3 | 9000mL/(g·h) | 32.8 | 16.3 | 5.3%① | [ |
| 19 | Cr(1%)-CuFe | 溶胶-凝胶法 | 320 | 4 | 3 | 6000mL/(g·h) | 38.4 | 29.2 | 104.1mg/(g·h) | [ |
| 20 | 1%Na-50Co50Fe | 草酸盐分解 | 270 | 0.92 | 2.5 | 2.0nL/(g·h) | 22.7 | 5.3 | 1.2%① | [ |
| 21 | 90Fe10Co1.0K | 草酸盐分解 | 240 | 1.2 | 3 | 1.5nL/(g·h) | 14.5 | 5.9 | 0.85%① | [ |
| 22 | Cs-Cu0.8Fe1.0Zn1.0 | 共沉淀 | 330 | 5 | 3 | 4500h-1 | 36.6 | 19.8 | 73.4mg/(g·h) | [ |
| 23 | FeNaS-0.6 | 沉淀 | 320 | 3 | 3 | 8000mL/(g·h) | 32.0 | 16.1 | 78.5mg/(g·h) | [ |
| 24 | NaS-Fe | 沉淀/洗涤 | 320 | 3 | 3 | 8000mL/(g·h) | 32.0 | 15.9 | 78.5mg/(g·h) | [ |
| 25 | Fe/Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 22.6 | 3.3 | 0.7%① | [ |
| 26 | Fe/K-Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 34.4 | 27.3 | 9.4%① | [ |
| 27 | In_2_Fe/K-Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 36.7 | 42.0 | 15.4%① | [ |
| 28 | CuZnFe1.0K/ATP-CZO | 浸渍 | 320 | 6 | 3 | 5000mL/(g·h) | 14.3 | 35.1(混合醇ROH) | 5.0%① | [ |
| 29 | Rh-Fe/SiO2 | 浸渍 | 260 | 5 | 3 | 6000mL/(g·h) | 26.7 | 16.0 | 4.3%① | [ |
| 30 | 2.5%Fe/TiO2 | 浸渍 | 270 | 2 | 1 | 8000mL/(g·h) | 2.7 | 2.8 | 0.07%① | [ |
| 31 | 2%Rh-2.5%Fe/TiO2 | 浸渍 | 270 | 2 | 1 | 8000mL/(g·h) | 9.2 | 6.4 | 0.6%① | [ |
| 32 | 2.5%RhFeLi/TiO2 | 浸渍 | 250 | 3 | 3 | 6000h-1 | 15.7 | 31.4 | 4.9%① | [ |
| 33 | 2.0K20Fe5Rh-SiO2 | 浸渍 | 250 | 5 | 3 | 7000mL/(g·h) | 18.4 | 15.9 | 2.9%① | [ |
| 34 | 0.1Pd/Fe3O4 | 浸渍 | 300 | 0.1 | 4 | 60000mL/(g·h) | 0.3 | 97.5 | 0.41mmol/(g·h) | [ |
| 35 | PdFe | 水热+浸渍 | 300 | 5 | 3 | 6000mL/(g·h) | 33.3 | 19.1 | 86.5mg/(g·h) | [ |
| 36 | 0.3K-1Pd/Fe2O3 | 浸渍 | 320 | 4 | 3 | 6000mL/(g·h) | 30.0 | 13.4 | 52.2mg/(g·h) | [ |
| 37 | Cu25Fe22Co3K3 | 浸渍 | 350 | 6 | 3 | 5000h-1 | 20.0 | 6.4(混合醇ROH) | 1.3%① | [ |
| 38 | CuZn1.0K0.15/Cu25Fe22Co3K3 | 共沉淀+浸渍 | 350 | 6 | 3 | 5000h-1 | 32.4 | 11.8(混合醇ROH) | 3.8%① | [ |
| 39 | CuZnAl/K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 42.3 | 17.4 | 106.5mg/(g·h) | [ |
| 40 | 4.7KCuFeZn/CuZnAlZr | 共沉淀+粉末混合 | 300 | 5 | 3 | 3000mL/(g·h) | 27.0 | 24.6 | 42.0mg/(g·h) | [ |
| 41 | 0.6S-KCFZ+CuZnAlZr | 共沉淀+浸渍+粉末混合 | 320 | 5 | 3 | 12000mL/(g·h) | 36.6 | 18.2 | 173.9mg/(g·h) | [ |
| 42 | Na-Fe@C/K-CuZnAl | 水热+热解+ 浸渍+共沉淀 | 320 | 5 | 3 | 4500mL/(g·h) | 39.2 | 35.0 | 12.4%① | [ |
| 43 | FeCuAlK+CuZnAlK | 均匀凝胶法+物理混合 | 330 | 8 | 3 | 20000h-1 | 39.5 | 15.8 | 6.2%① | [ |
| 44 | MnCuK-FeC(1)/CuZnAlZr(1) | 热分解+共沉淀 | 300 | 3 | 3 | 6000mL/(g·h) | 42.1 | 15.5 | 6.5%① | [ |
| 45 | ZnFe2O4/Fe-Zn-Na# | 有机物燃烧+共沉淀+浸渍 | 320 | 5 | 3 | 2000mL/(g·h) | 38.8 | 30.0 | 55.5mg/(g·h) | [ |
表2 Fe基CO2加氢制备高级醇性能汇总
| 序号 | 催化剂 | 制备/混合方法 | 温度/℃ | 压力/MPa | H2/CO2 | GHSV | CO2转化率/% | C2+OH选择性/% | C2+OH收率 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 30.4 | 15.8 | 70.6mg/(g·h) | [ |
| 2 | K/S-CuFeZn | 共沉淀+浸渍 | 320 | 5 | 3 | 3000mL/(g·h) | 36.1 | 20.2 | 50.7mg/(g·h) | [ |
| 3 | 10Mn1K-FeC | 热分解 | 300 | 3 | 3 | 6000mL/(g·h) | 40.5 | 10.7 | 4.3%① | [ |
| 4 | MnCuK-FeC | 热分解 | 300 | 3 | 3 | 6000mL/(g·h) | 40.8 | 13.5 | 5.6%① | [ |
| 5 | K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 28.1 | 约15.7 | 69.6mg/(g·h) | [ |
| 6 | CuZnFe0.5K0.15 | 共沉淀+浸渍 | 300 | 6 | 3 | 5000mL/(g·h) | 42.3 | 36.7 | 170mg/(mL·h) | [ |
| 7 | K/Cu-Zn-Fe | 共沉淀 | 300 | 7 | 3 | 5000h-1 | 44.2 | 26.9 | 11.9%① | [ |
| 8 | K/Cu-Zn-Fe | 共沉淀+浸渍 | 300 | 7 | 3 | 5000mL/(g·h) | 45.0 | 19.0 | 8.6%① | [ |
| 9 | PDA/CuFeZn-N450 | 共沉淀 | 320 | 4 | 3 | 7200mL/(g·h) | 约7.0 | 41.5 | 55.9mg/(mL·h) | [ |
| 10 | CuFeZn-0.7PDA | 共沉淀 | 310 | 4 | 3 | 7200mL/(g·h) | 10.6 | 33.1 | 58.2mg/(mL·h) | [ |
| 11 | KFeCu/a-ZrO2 | 分步沉淀+浸渍 | 320 | 4 | 3 | 12000mL/(g·h) | 25.7 | 26.1 | 125.0mg/(g·h) | [ |
| 12 | K-0.82-FeIn/Ce-ZrO2 | 浸渍 | 300 | 10 | 3 | 4500mL/(g·h) | 29.6 | 28.7 | 8.5%① | [ |
| 13 | (K2O)5%/CuZnFeZrO2 | 共沉淀+浸渍 | 320 | 3 | 3 | 3600mL/(g·h) | 25.5 | 11.0 | 2.55mmol/(mL·h) | [ |
| 14 | Fe-Cu-Al-K | 均匀凝胶法 | 330 | 8 | 3 | 20000h-1 | 41.4 | 11.4 | 4.7%① | [ |
| 15 | sp-CuNaFe | 物理溅射 | 310 | 3 | 3 | 28800mL/(g·h) | 32.3 | 约10.0 | 153.0mg/(g·h) | [ |
| 16 | Na-Fe@C | 水热+浸渍+碳化 | 320 | 5 | 3 | 9000mL/(g·h) | 25.5 | 12.0(乙醇) | 52.3mg/(g·h) | [ |
| 17 | Na-ZnFe@C | 水热+浸渍+碳化 | 320 | 5 | 3 | 9000mL/(g·h) | 38.4 | 20.3(乙醇) | 158.1mg/(g·h) | [ |
| 18 | 2%Na-Fe@C | 水热+热解+浸渍 | 320 | 5 | 3 | 9000mL/(g·h) | 32.8 | 16.3 | 5.3%① | [ |
| 19 | Cr(1%)-CuFe | 溶胶-凝胶法 | 320 | 4 | 3 | 6000mL/(g·h) | 38.4 | 29.2 | 104.1mg/(g·h) | [ |
| 20 | 1%Na-50Co50Fe | 草酸盐分解 | 270 | 0.92 | 2.5 | 2.0nL/(g·h) | 22.7 | 5.3 | 1.2%① | [ |
| 21 | 90Fe10Co1.0K | 草酸盐分解 | 240 | 1.2 | 3 | 1.5nL/(g·h) | 14.5 | 5.9 | 0.85%① | [ |
| 22 | Cs-Cu0.8Fe1.0Zn1.0 | 共沉淀 | 330 | 5 | 3 | 4500h-1 | 36.6 | 19.8 | 73.4mg/(g·h) | [ |
| 23 | FeNaS-0.6 | 沉淀 | 320 | 3 | 3 | 8000mL/(g·h) | 32.0 | 16.1 | 78.5mg/(g·h) | [ |
| 24 | NaS-Fe | 沉淀/洗涤 | 320 | 3 | 3 | 8000mL/(g·h) | 32.0 | 15.9 | 78.5mg/(g·h) | [ |
| 25 | Fe/Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 22.6 | 3.3 | 0.7%① | [ |
| 26 | Fe/K-Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 34.4 | 27.3 | 9.4%① | [ |
| 27 | In_2_Fe/K-Al2O3 | 浸渍 | 300 | 2 | 3 | 4000mL/(g·h) | 36.7 | 42.0 | 15.4%① | [ |
| 28 | CuZnFe1.0K/ATP-CZO | 浸渍 | 320 | 6 | 3 | 5000mL/(g·h) | 14.3 | 35.1(混合醇ROH) | 5.0%① | [ |
| 29 | Rh-Fe/SiO2 | 浸渍 | 260 | 5 | 3 | 6000mL/(g·h) | 26.7 | 16.0 | 4.3%① | [ |
| 30 | 2.5%Fe/TiO2 | 浸渍 | 270 | 2 | 1 | 8000mL/(g·h) | 2.7 | 2.8 | 0.07%① | [ |
| 31 | 2%Rh-2.5%Fe/TiO2 | 浸渍 | 270 | 2 | 1 | 8000mL/(g·h) | 9.2 | 6.4 | 0.6%① | [ |
| 32 | 2.5%RhFeLi/TiO2 | 浸渍 | 250 | 3 | 3 | 6000h-1 | 15.7 | 31.4 | 4.9%① | [ |
| 33 | 2.0K20Fe5Rh-SiO2 | 浸渍 | 250 | 5 | 3 | 7000mL/(g·h) | 18.4 | 15.9 | 2.9%① | [ |
| 34 | 0.1Pd/Fe3O4 | 浸渍 | 300 | 0.1 | 4 | 60000mL/(g·h) | 0.3 | 97.5 | 0.41mmol/(g·h) | [ |
| 35 | PdFe | 水热+浸渍 | 300 | 5 | 3 | 6000mL/(g·h) | 33.3 | 19.1 | 86.5mg/(g·h) | [ |
| 36 | 0.3K-1Pd/Fe2O3 | 浸渍 | 320 | 4 | 3 | 6000mL/(g·h) | 30.0 | 13.4 | 52.2mg/(g·h) | [ |
| 37 | Cu25Fe22Co3K3 | 浸渍 | 350 | 6 | 3 | 5000h-1 | 20.0 | 6.4(混合醇ROH) | 1.3%① | [ |
| 38 | CuZn1.0K0.15/Cu25Fe22Co3K3 | 共沉淀+浸渍 | 350 | 6 | 3 | 5000h-1 | 32.4 | 11.8(混合醇ROH) | 3.8%① | [ |
| 39 | CuZnAl/K-CuMgZnFe | 共沉淀 | 320 | 5 | 3 | 6000mL/(g·h) | 42.3 | 17.4 | 106.5mg/(g·h) | [ |
| 40 | 4.7KCuFeZn/CuZnAlZr | 共沉淀+粉末混合 | 300 | 5 | 3 | 3000mL/(g·h) | 27.0 | 24.6 | 42.0mg/(g·h) | [ |
| 41 | 0.6S-KCFZ+CuZnAlZr | 共沉淀+浸渍+粉末混合 | 320 | 5 | 3 | 12000mL/(g·h) | 36.6 | 18.2 | 173.9mg/(g·h) | [ |
| 42 | Na-Fe@C/K-CuZnAl | 水热+热解+ 浸渍+共沉淀 | 320 | 5 | 3 | 4500mL/(g·h) | 39.2 | 35.0 | 12.4%① | [ |
| 43 | FeCuAlK+CuZnAlK | 均匀凝胶法+物理混合 | 330 | 8 | 3 | 20000h-1 | 39.5 | 15.8 | 6.2%① | [ |
| 44 | MnCuK-FeC(1)/CuZnAlZr(1) | 热分解+共沉淀 | 300 | 3 | 3 | 6000mL/(g·h) | 42.1 | 15.5 | 6.5%① | [ |
| 45 | ZnFe2O4/Fe-Zn-Na# | 有机物燃烧+共沉淀+浸渍 | 320 | 5 | 3 | 2000mL/(g·h) | 38.8 | 30.0 | 55.5mg/(g·h) | [ |
| 1 | PETER Sebastian C. Reduction of CO2 to chemicals and fuels: A solution to global warming and energy crisis[J]. ACS Energy Letters, 2018, 3(7): 1557-1561. |
| 2 | GAO Peng, ZHANG Lina, LI Shenggang, et al. Novel heterogeneous catalysts for CO2 hydrogenation to liquid fuels[J]. ACS Central Science, 2020, 6(10): 1657-1670. |
| 3 | JIANG Xiao, NIE Xiaowa, GUO Xinwen, et al. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis[J]. Chemical Reviews, 2020, 120(15): 7984-8034. |
| 4 | YE Runping, DING Jie, GONG Weibo, et al. CO2 hydrogenation to high-value products via heterogeneous catalysis[J]. Nature Communications, 2019, 10(1): 5698. |
| 5 | ZHANG Shunan, WU Zhaoxuan, LIU Xiufang, et al. A short review of recent advances in direct CO2 hydrogenation to alcohols[J]. Topics in Catalysis, 2021, 64(5): 371-394. |
| 6 | MURTHY Pradeep S, LIANG Weibin, JIANG Yijiao, et al. Cu-based nanocatalysts for CO2 hydrogenation to methanol[J]. Energy & Fuels, 2021, 35(10): 8558-8584. |
| 7 | BOWKER Michael. Methanol synthesis from CO2 hydrogenation[J]. ChemCatChem, 2019, 11(17): 4238-4246. |
| 8 | LI Zelong, WANG Jijie, QU Yuanzhi, et al. Highly selective conversion of carbon dioxide to lower olefins[J]. ACS Catalysis, 2017, 7(12): 8544-8548. |
| 9 | LI Zelong, QU Yuanzhi, WANG Jijie, et al. Highly selective conversion of carbon dioxide to aromatics over tandem catalysts[J]. Joule, 2019, 3(2): 570-583. |
| 10 | CUI Xu, GAO Peng, LI Shenggang, et al. Selective production of aromatics directly from carbon dioxide hydrogenation[J]. ACS Catalysis, 2019, 9(5): 3866-3876. |
| 11 | NI Youming, CHEN Zhiyang, FU Yi, et al. Selective conversion of CO2 and H2 into aromatics[J]. Nature Communications, 2018, 9(1): 3457. |
| 12 | WEI Jian, GE Qingjie, YAO Ruwei, et al. Directly converting CO2 into a gasoline fuel[J]. Nature Communications, 2017, 8: 15174. |
| 13 | PRIETO Gonzalo. Carbon dioxide hydrogenation into higher hydrocarbons and oxygenates: Thermodynamic and kinetic bounds and progress with heterogeneous and homogeneous catalysis[J]. ChemSusChem, 2017, 10(6): 1056-1070. |
| 14 | GAO Peng, LI Shenggang, BU Xianni, et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst[J]. Nature Chemistry, 2017, 9(10): 1019-1024. |
| 15 | ZENG Feng, MEBRAHTU Chalachew, XI Xiaoying, et al. Catalysts design for higher alcohols synthesis by CO2 hydrogenation: Trends and future perspectives[J]. Applied Catalysis B: Environmental, 2021, 291: 120073. |
| 16 | LATSIOU Angeliki I, CHARISIOU Nikolaos D, FRONTISTIS Zacharias, et al. CO2 hydrogenation for the production of higher alcohols: Trends in catalyst developments, challenges and opportunities[J]. Catalysis Today, 2023, 420: 114179. |
| 17 | XU Di, WANG Yanqiu, DING Mingyue, et al. Advances in higher alcohol synthesis from CO2 hydrogenation[J]. Chem, 2021, 7(4): 849-881. |
| 18 | LI Jiachen, WANG Liguo, CAO Yan, et al. Recent advances on the reduction of CO2 to important C2+ oxygenated chemicals and fuels[J]. Chinese Journal of Chemical Engineering, 2018, 26(11): 2266-2279. |
| 19 | DELUGA G A, SALGE J R, SCHMIDT L D, et al. Renewable hydrogen from ethanol by autothermal reforming[J]. Science, 2004, 303(5660): 993-997. |
| 20 | LATIF Mohd Nor, WAN ISAHAK Wan Nor Roslam, SAMSURI Alinda, et al. Recent advances in the technologies and catalytic processes of ethanol production[J]. Catalysts, 2023, 13(7): 1093. |
| 21 | LAN Ethan I, LIAO James C. Microbial synthesis of n-butanol, isobutanol, and other higher alcohols from diverse resources[J]. Bioresource Technology, 2013, 135: 339-349. |
| 22 | BAI F W, ANDERSON W A, MOO-YOUNG M. Ethanol fermentation technologies from sugar and starch feedstocks[J]. Biotechnology Advances, 2008, 26(1): 89-105. |
| 23 | HE Xinyi. CO2 hydrogenation for ethanol production: A thermodynamic analysis[J]. International Journal of Oil, Gas and Coal Engineering, 2017, 5(6): 145-152. |
| 24 | AZIZ M A A, JALIL A A, TRIWAHYONO S, et al. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects[J]. Green Chemistry, 2015, 17(5): 2647-2663. |
| 25 | JIANG Yongjun, WANG Kangzhou, WANG Yuan, et al. Recent advances in thermocatalytic hydrogenation of carbon dioxide to light olefins and liquid fuels via modified Fischer-Tropsch pathway[J]. Journal of CO2 Utilization, 2023, 67: 102321. |
| 26 | NIE Xiaowa, LI Wenhui, JIANG Xiao, et al. Recent advances in catalytic CO2 hydrogenation to alcohols and hydrocarbons[M]// Advances in Catalysis. Amsterdam: Elsevier, 2019, 65: 121-233. |
| 27 | LI Xiaopeng, KE Jucang, LI Rui, et al. Research progress of hydrogenation of carbon dioxide to ethanol[J]. Chemical Engineering Science, 2023, 282: 119226. |
| 28 | STANGELAND Kristian, LI Hailong, YU Zhixin. Thermodynamic analysis of chemical and phase equilibria in CO2 hydrogenation to methanol, dimethyl ether, and higher alcohols[J]. Industrial & Engineering Chemistry Research, 2018, 57(11): 4081-4094. |
| 29 | KANGVANSURA Praewpilin, CHEW Ly May, SAENGSUI Worasarit, et al. Product distribution of CO2 hydrogenation by K- and Mn-promoted Fe catalysts supported on N-functionalized carbon nanotubes[J]. Catalysis Today, 2016, 275: 59-65. |
| 30 | TAKAGAWA Makoto, OKAMOTO Atsushi, FUJIMURA Hiromitsu, et al. Ethanol synthesis from carbon dioxide and hydrogen[J]. Studies in Surface Science and Catalysis, 1998, 114: 525-528. |
| 31 | INUI Tomoyuki, YAMAMOTO Tetsuo, INOUE Masahito, et al. Highly effective synthesis of ethanol by CO2-hydrogenation on well balanced multi-functional FT-type composite catalysts[J]. Applied Catalysis A: General, 1999, 186(1/2): 395-406. |
| 32 | HE Yiming, MÜLLER Fabian H, PALKOVITS Regina, et al. Tandem catalysis for CO2 conversion to higher alcohols: A review[J]. Applied Catalysis B: Environment and Energy, 2024, 345: 123663. |
| 33 | KUSAMA Hitoshi, OKABE Kiyomi, SAYAMA Kazuhiro, et al. Ethanol synthesis by catalytic hydrogenation of CO2 over Rh-Fe/SiO2 catalysts[J]. Energy, 1997, 22(2/3): 343-348. |
| 34 | YANG Chengsheng, MU Rentao, WANG Guishuo, et al. Hydroxyl-mediated ethanol selectivity of CO2 hydrogenation[J]. Chemical Science, 2019, 10(11): 3161-3167. |
| 35 | GORYACHEV Andrey, PUSTOVARENKO Alexey, SHTERK Genrikh, et al. A multi-parametric catalyst screening for CO2 hydrogenation to ethanol[J]. ChemCatChem, 2021, 13(14): 3324-3332. |
| 36 | GOGATE Makarand R, DAVIS Robert J. Comparative study of CO and CO2 hydrogenation over supported Rh-Fe catalysts[J]. Catalysis Communications, 2010, 11(10): 901-906. |
| 37 | CHEN Yuan, CHOI Saemin, THOMPSON Levi T. Low temperature CO2 hydrogenation to alcohols and hydrocarbons over Mo2C supported metal catalysts[J]. Journal of Catalysis, 2016, 343: 147-156. |
| 38 | CAPARRÓS Francisco J, SOLER Lluís, ROSSELL Marta D, et al. Remarkable carbon dioxide hydrogenation to ethanol on a palladium/iron oxide single-atom catalyst[J]. ChemCatChem, 2018, 10(11): 2365-2369. |
| 39 | LI Shanggui, GUO Haijun, LUO Cairong, et al. Effect of iron promoter on structure and performance of K/Cu-Zn catalyst for higher alcohols synthesis from CO2 hydrogenation[J]. Catalysis Letters, 2013, 143(4): 345-355. |
| 40 | GNANAMANI Muthu Kumaran, JACOBS Gary, HAMDEH Hussein H, et al. Hydrogenation of carbon dioxide over Co-Fe bimetallic catalysts[J]. ACS Catalysis, 2016, 6(2): 913-927. |
| 41 | GNANAMANI Muthu Kumaran, HAMDEH Hussein H, JACOBS Gary, et al. Hydrogenation of carbon dioxide over K-promoted FeCo bimetallic catalysts prepared from mixed metal oxalates[J]. ChemCatChem, 2017, 9(7): 1303-1312. |
| 42 | DING Fanshu, ZHANG Anfeng, LIU Min, et al. CO2 hydrogenation to hydrocarbons over iron-based catalyst: Effects of physicochemical properties of Al2O3 supports[J]. Industrial & Engineering Chemistry Research, 2014, 53(45): 17563-17569. |
| 43 | WANG Yang, WANG Wenhang, HE Ruosong, et al. Carbon-based electron buffer layer on ZnO x -Fe5C2-Fe3O4 boosts ethanol synthesis from CO2 hydrogenation[J]. Angewandte Chemie International Edition, 2023, 62(46): e202311786. |
| 44 | LU Fangxu, CHEN Xin, WANG Wen, et al. Adjusting the CO2 hydrogenation pathway via the synergic effects of iron carbides and iron oxides[J]. Catalysis Science & Technology, 2021, 11(23): 7694-7703. |
| 45 | VISCONTI Carlo Giorgio, MARTINELLI Michela, FALBO Leonardo, et al. CO2 hydrogenation to lower olefins on a high surface area K-promoted bulk Fe-catalyst[J]. Applied Catalysis B: Environmental, 2017, 200: 530-542. |
| 46 | LIU Junhui, ZHANG Guanghui, JIANG Xiao, et al. Insight into the role of Fe5C2 in CO2 catalytic hydrogenation to hydrocarbons[J]. Catalysis Today, 2021, 371: 162-170. |
| 47 | DE SMIT Emiel, CINQUINI Fabrizio, BEALE Andrew M., et al. Stability and reactivity of ε-χ-θ iron carbide catalyst phases in Fischer-Tropsch synthesis: Controlling μC [J]. Journal of the American Chemical Society, 2010, 132(42): 14928-14941. |
| 48 | XU Di, YANG Hengquan, HONG Xinlin, et al. Tandem catalysis of direct CO2 hydrogenation to higher alcohols[J]. ACS Catalysis, 2021, 11(15): 8978-8984. |
| 49 | WANG Yanqiu, ZHANG Xinxin, HONG Xinlin, et al. Sulfate-promoted higher alcohol synthesis from CO2 hydrogenation[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(27): 8980-8987. |
| 50 | HUANG Jiamin, ZHANG Guanghui, ZHU Jie, et al. Boosting the production of higher alcohols from CO2 and H2 over Mn- and K-modified iron carbide[J]. Industrial & Engineering Chemistry Research, 2022, 61(21): 7266-7274. |
| 51 | HUANG Jiamin, ZHANG Guanghui, WANG Mingrui, et al. The synthesis of higher alcohols from CO2 hydrogenation over Mn-Cu-K modified Fe5C2 and CuZnAlZr tandem catalysts[J]. Frontiers in Energy Research, 2023, 10: 995800. |
| 52 | XU Di, DING Mingyue, HONG Xinlin, et al. Mechanistic aspects of the role of K promotion on Cu-Fe-based catalysts for higher alcohol synthesis from CO2 hydrogenation[J]. ACS Catalysis, 2020, 10(24): 14516-14526. |
| 53 | HIGUCHI Katsumi, HANEDA Yoko, TABATA Kenji, et al. A study for the durability of catalysts in ethanol synthesis by hydrogenation of carbon dioxide[J]. Studies in Surface Science and Catalysis, 1998, 114: 517-520. |
| 54 | JIA Yazhen, WANG Bin, WEN Yueli, et al. Mechanism of stability and deactivation of N-doped CuFeZn catalysts for C2+ alcohols synthesis by hydrogenation of CO2 [J]. Fuel Processing Technology, 2023, 250: 107901. |
| 55 | YANG Chen, WANG Bin, WEN Yueli, et al. Composition control of CuFeZn catalyst derived by PDA and its effect on synthesis of C2+ alcohols from CO2 [J]. Fuel, 2022, 327: 125055. |
| 56 | LIU Tangkang, XU Di, SONG Mengyang, et al. K-ZrO2 interfaces boost CO2 hydrogenation to higher alcohols[J]. ACS Catalysis, 2023, 13(7): 4667-4674. |
| 57 | XI Xiaoying, ZENG Feng, ZHANG Heng, et al. CO2 hydrogenation to higher alcohols over K-promoted bimetallic Fe-In catalysts on a Ce-ZrO2 support[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(18): 6235-6249. |
| 58 | GUO Wei, GAO Wengui, WANG Hua, et al. Higher alcohols synthesis from CO2 hydrogenation over K2O-modified CuZnFeZrO2 catalysts[J]. Advanced Materials Research, 2013, 827: 20-24. |
| 59 | SI Zhiyan, WANG Linkai, HAN Yu, et al. Synthesis of alkene and ethanol in CO2 hydrogenation on a highly active sputtering CuNaFe catalyst[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(45): 14972-14979. |
| 60 | WANG Yang, WANG Kangzhou, ZHANG Baizhang, et al. Direct conversion of CO2 to ethanol boosted by intimacy-sensitive multifunctional catalysts[J]. ACS Catalysis, 2021, 11(18): 11742-11753. |
| 61 | ZHANG Qian, WANG Sen, GENG Rui, et al. Hydrogenation of CO2 to higher alcohols on an efficient Cr-modified CuFe catalyst[J]. Applied Catalysis B: Environmental, 2023, 337: 123013. |
| 62 | XU Di, DING Mingyue, HONG Xinlin, et al. Selective C2+ alcohol synthesis from direct CO2 hydrogenation over a Cs-promoted Cu-Fe-Zn catalyst[J]. ACS Catalysis, 2020, 10(9): 5250-5260. |
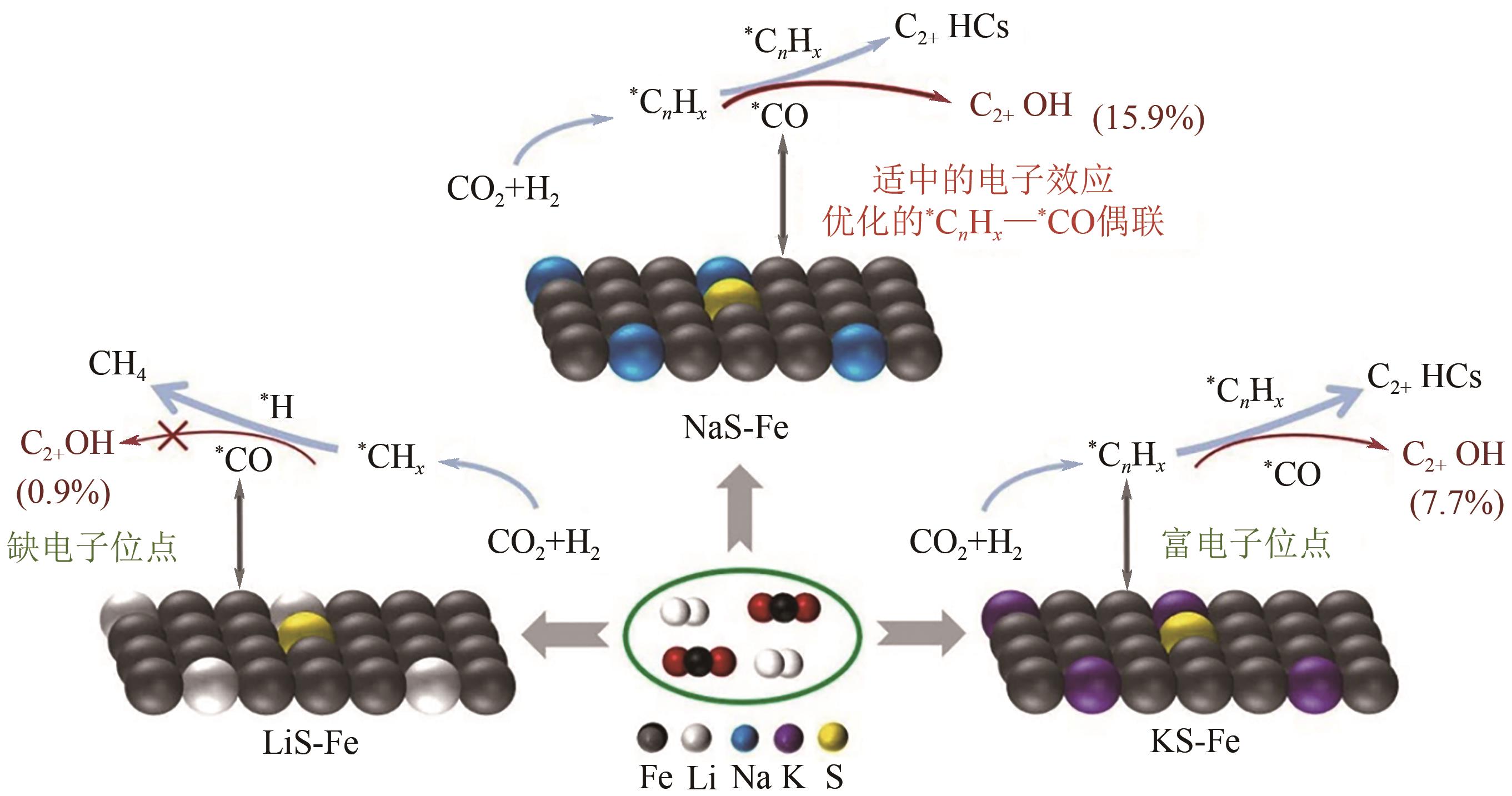
| 63 | YAO Ruwei, WEI Jian, GE Qingjie, et al. Monometallic iron catalysts with synergistic Na and S for higher alcohols synthesis via CO2 hydrogenation[J]. Applied Catalysis B: Environmental, 2021, 298: 120556. |
| 64 | YAO Ruwei, WU Bin, YU Yang, et al. Regulating the electronic property of iron catalysts for higher alcohols synthesis from CO2 hydrogenation[J]. Applied Catalysis B: Environment and Energy, 2024, 355: 124159. |
| 65 | GOUD Devender, CHURIPARD Sathyapal R, BAGCHI Debabrata, et al. Strain-enhanced phase transformation of iron oxide for higher alcohol production from CO2 [J]. ACS Catalysis, 2022, 12(18): 11118-11128. |
| 66 | GUO Haijun, DING Shuai, ZHANG Hairong, et al. Promotion effect of iron addition on the structure and CO2 hydrogenation performance of attapulgite/Ce0.75Zr0.25O2 nanocomposite supported Cu-ZnO based catalyst[J]. Molecular Catalysis, 2021, 513: 111820. |
| 67 | WANG Yanqiu, ZHOU Ying, ZHANG Xinxin, et al. PdFe alloy-Fe5C2 interfaces for efficient CO2 hydrogenation to higher alcohols[J]. Applied Catalysis B: Environmental and Energy, 2024, 345: 123691. |
| 68 | ZHOU Ying, WANG Yanqiu, LU Hanjun, et al. Highly dispersed K- and Pd-comodified Fe catalyst for CO2 hydrogenation to higher alcohols[J]. ACS Sustainable Chemistry & Engineering, 2024, 12(8): 3322-3330. |
| 69 | GUO Haijun, LI Shanggui, PENG Fen, et al. Roles investigation of promoters in K/Cu-Zn catalyst and higher alcohols synthesis from CO2 hydrogenation over a novel two-stage bed catalyst combination system[J]. Catalysis Letters, 2014, 145(2): 620-630. |
| 70 | WANG Yanqiu, XU Di, ZHANG Xinxin, et al. Selective C2+ alcohol synthesis by CO2 hydrogenation via a reaction-coupling strategy[J]. Catalysis Science & Technology, 2022, 12(5): 1539-1550. |
| 71 | YANG Haiyan, WEI Zhangqian, ZHANG Jian, et al. Tuning the selectivity of CO2 hydrogenation to alcohols by crystal structure engineering[J]. Chem, 2024, 10: 2245-2265. |
| 72 | KIM Jun-Sik, LEE Sunmook, LEE Sang-Bong, et al. Performance of catalytic reactors for the hydrogenation of CO2 to hydrocarbons[J]. Catalysis Today, 2006, 115(1/2/3/4): 228-234. |
| 73 | DING Mingyue, TU Junling, QIU Minghuang, et al. Impact of potassium promoter on Cu-Fe based mixed alcohols synthesis catalyst[J]. Applied Energy, 2015, 138: 584-589. |
| 74 | SHOLEHA Novia Amalia, HOLILAH Holilah, BAHRUJI Hasliza, et al. Recent trend of metal promoter role for CO2 hydrogenation to C1 and C2+ products[J]. South African Journal of Chemical Engineering, 2023, 44: 14-30. |
| 75 | GNANAMANI Muthu Kumaran, JACOBS Gary, KEOGH Robert A, et al. Fischer-Tropsch synthesis: Effect of pretreatment conditions of cobalt on activity and selectivity for hydrogenation of carbon dioxide[J]. Applied Catalysis A: General, 2015, 499: 39-46. |
| 76 | INUI Tomoyuki, YAMAMOTO Tetsuo. Effective synthesis of ethanol from CO2 on polyfunctional composite catalysts[J]. Catalysis Today, 1998, 45(1/2/3/4): 209-214. |
| 77 | ZHANG Shunan, HUANG Chaojie, SHAO Zilong, et al. Revealing and regulating the complex reaction mechanism of CO2 hydrogenation to higher alcohols on multifunctional tandem catalysts[J]. ACS Catalysis, 2023, 13(5): 3055-3065. |
| 78 | BEDIAKO Bernard Baffour Asare, QIAN Qingli, HAN Buxin. Synthesis of C2+ chemicals from CO2 and H2 via C—C bond formation[J]. Accounts of Chemical Research, 2021, 54(10): 2467-2476. |
| 79 | 潞安集团CO2重整利用工业尾气生产20万吨乙醇项目预计 9月试车[EB/OL]. . |
| 80 | “ 5万吨/年乙烯多相氢甲酰化及其加氢制正丙醇工业化技术”通过科技成果鉴定[EB/OL]. . |
| 81 | JI Lei, LI Lei, JI Xuqiang, et al. Highly selective electrochemical reduction of CO2 to alcohols on an FeP nanoarray[J]. Angewandte Chemie International Edition, 2020, 59(2): 758-762. |
| 82 | JI Lei, CHANG Le, ZHANG Ya, et al. Electrocatalytic CO2 reduction to alcohols with high selectivity over a two-dimensional Fe2P2S6 nanosheet[J]. ACS Catalysis, 2019, 9(11): 9721-9725. |
| 83 | AMPELLI C, GENOVESE C, PASSALACQUA R, et al. The use of a solar photoelectrochemical reactor for sustainable production of energy[J]. Theoretical Foundations of Chemical Engineering, 2012, 46(6): 651-657. |
| 84 | ARRIGO Rosa, SCHUSTER Manfred E, WRABETZ Sabine, et al. New insights from microcalorimetry on the FeO x /CNT-based electrocatalysts active in the conversion of CO2 to fuels[J]. ChemSusChem, 2012, 5(3): 577-586. |
| 85 | KALIYAPERUMAL Alamelu, GUPTA Pooja, PRASAD Yadavalli Satya Sivaram, et al. Recent progress and perspective of the electrochemical conversion of carbon dioxide to alcohols[J]. ACS Engineering Au, 2023, 3(6): 403-425. |
| 86 | LU Peng, CHEN Qingjun, YANG Guohui, et al. Space-confined self-regulation mechanism from a capsule catalyst to realize an ethanol direct synthesis strategy[J]. ACS Catalysis, 2020, 10(2): 1366-1374. |
| 87 | CHEN Jie, ZHA Yajun, LIU Bing, et al. Rationally designed water enriched nano reactor for stable CO2 hydrogenation with near 100% ethanol selectivity over diatomic palladium active sites[J]. ACS Catalysis, 2023, 13(10): 7110-7121. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 王集杰, 韩哲, 陈思宇, 汤驰洲, 沙峰, 唐珊, 姚婷婷, 李灿. 太阳燃料甲醇合成[J]. 化工进展, 2022, 41(3): 1309-1317. |
| [3] | 刘畅, 刘忠文. CO2加氢一步制二甲醚展望[J]. 化工进展, 2022, 41(3): 1115-1120. |
| [4] | 刘瑞琴, 孟凡会, 王立言, 张鹏, 张俊峰, 谭猗生, 李忠. 有序介孔CuCoZr催化剂的制备及其催化合成气制乙醇及高级醇性能[J]. 化工进展, 2022, 41(11): 5870-5878. |
| [5] | 贾晨喜, 邵敬爱, 白小薇, 肖建军, 杨海平, 陈汉平. 二氧化碳加氢制甲醇铜基催化剂性能的研究进展[J]. 化工进展, 2020, 39(9): 3658-3668. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||