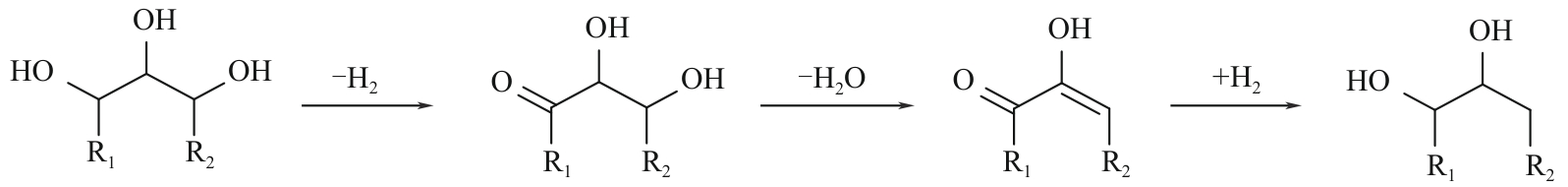化工进展 ›› 2025, Vol. 44 ›› Issue (1): 228-252.DOI: 10.16085/j.issn.1000-6613.2023-2286
生物质基多元醇催化转化制备二醇
宋顺明1( ), 张敬雯1, 张良清1(
), 张敬雯1, 张良清1( ), 邱佳容1, 陈剑锋1, 曾宪海2
), 邱佳容1, 陈剑锋1, 曾宪海2
- 1.福州大学先进制造学院,福建 晋江 362251
2.厦门大学能源学院,福建 厦门 361102
-
收稿日期:2023-12-27修回日期:2024-02-05出版日期:2025-01-15发布日期:2025-02-13 -
通讯作者:张良清 -
作者简介:宋顺明(1998—),男,硕士研究生,研究方向为生物质催化。E-mail:473256871@qq.com。 -
基金资助:国家自然科学基金(22108038);齐鲁工业大学(山东省科学院)生物基材料与绿色造纸国家重点实验室开放基金(GZKF202308);泉州市科技计划(2022N030);福建省自然科学基金(2022J01573);国家重点研发计划(2021YFC2101604);广东省重点领域研发计划(020B0101070001)
Catalytic transformation of biomass-derived polyols to diols
SONG Shunming1( ), ZHANG Jingwen1, ZHANG Liangqing1(
), ZHANG Jingwen1, ZHANG Liangqing1( ), QIU Jiarong1, CHEN Jianfeng1, ZENG Xianhai2
), QIU Jiarong1, CHEN Jianfeng1, ZENG Xianhai2
- 1.School of Advanced Manufacturing, Fuzhou University, Jinjiang 362251, Fujian, China
2.College of Energy, Xiamen University, Xiamen 361102, Fujian, China
-
Received:2023-12-27Revised:2024-02-05Online:2025-01-15Published:2025-02-13 -
Contact:ZHANG Liangqing
摘要:
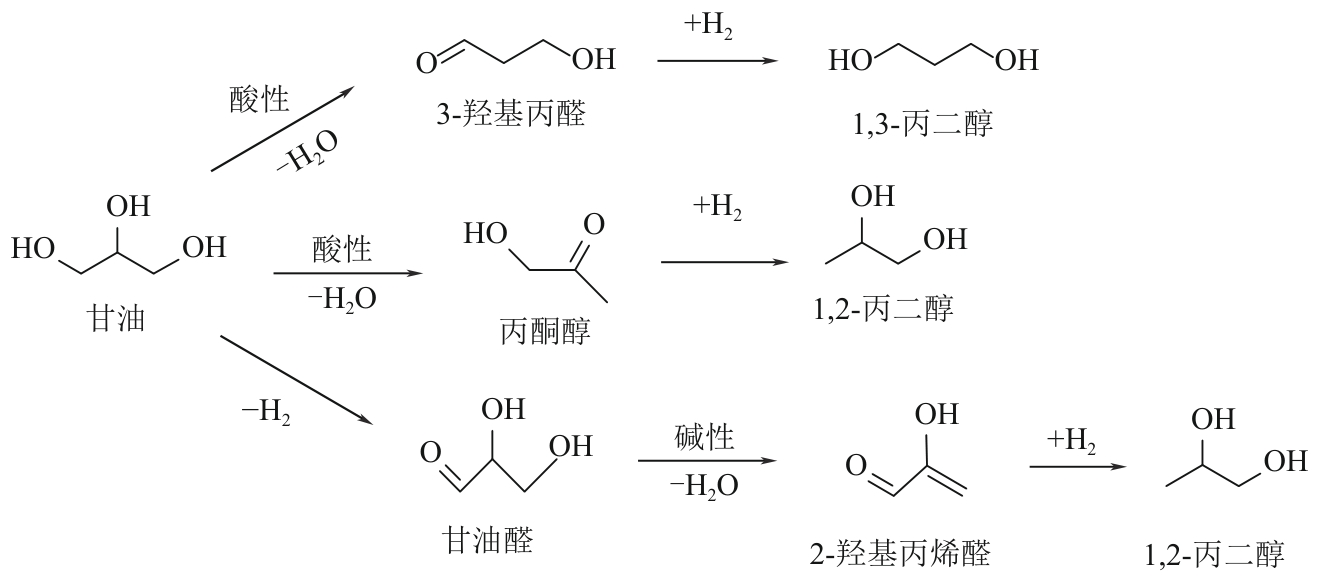
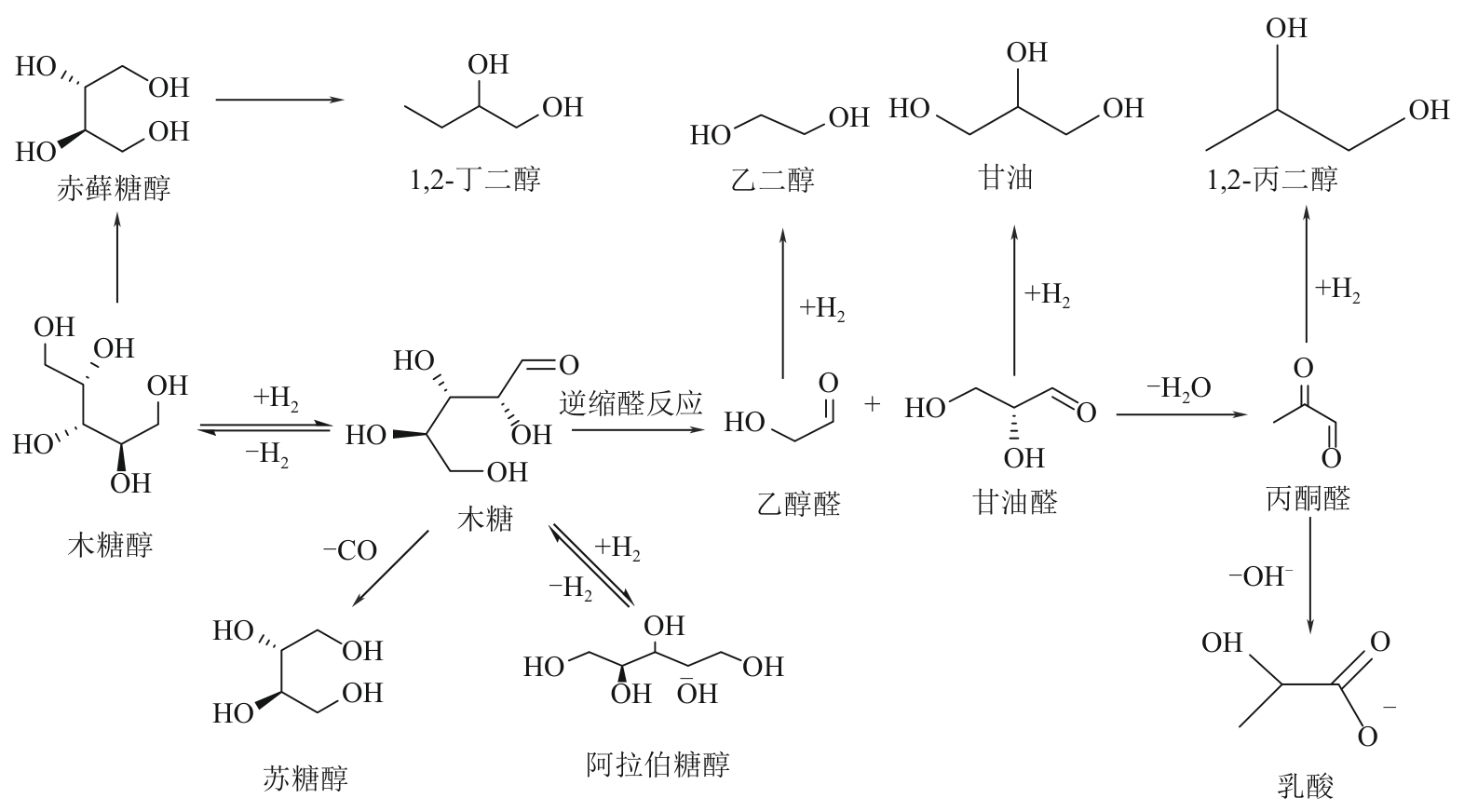
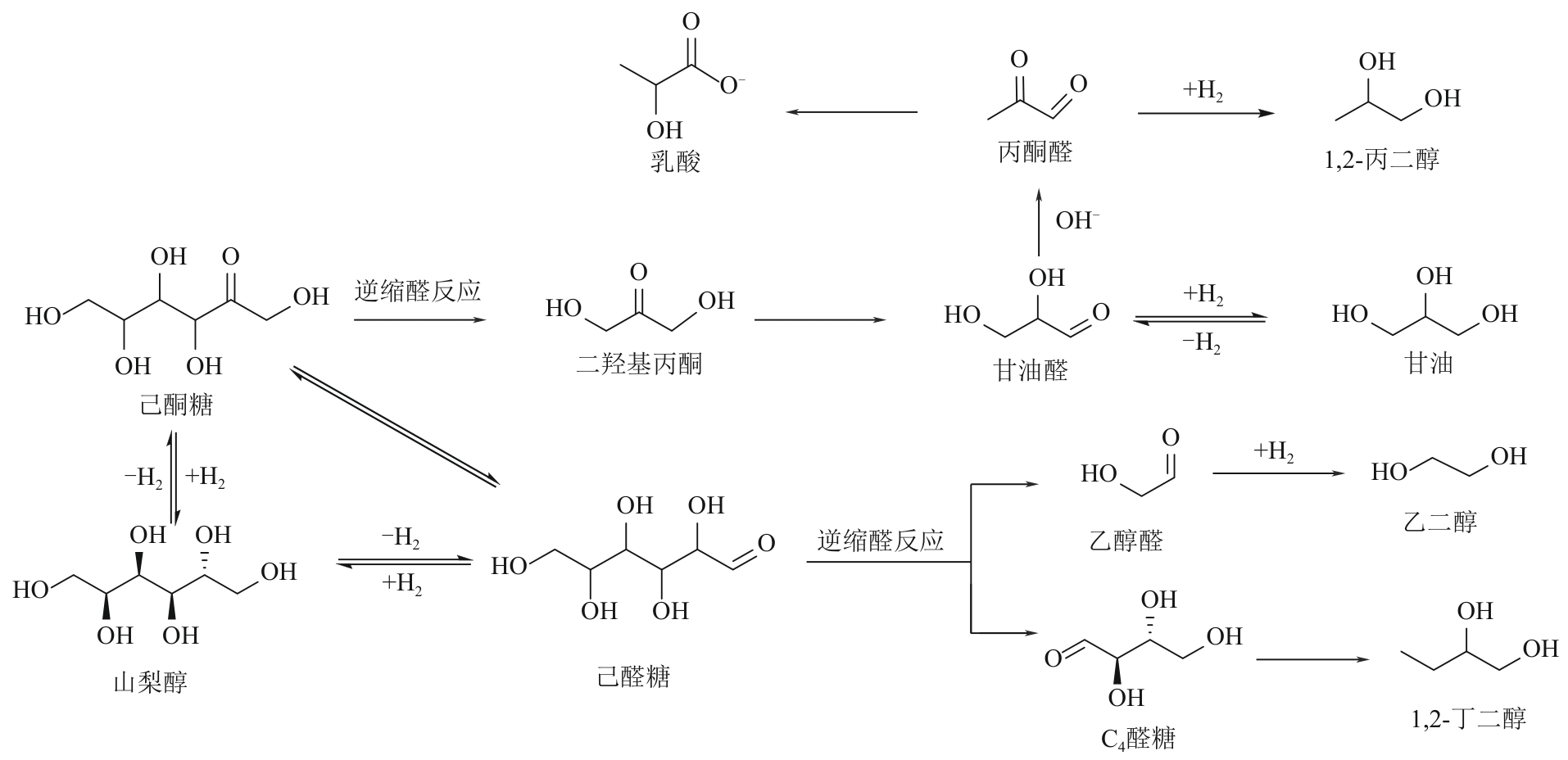
二元醇(包括乙二醇、丙二醇和丁二醇等)在化工、医疗、生物以及农业等许多领域都有广泛的应用,市场需求量大。目前,二元醇主要以化石资源为原料制备,但是化石资源有限的储量和使用过程中存在环境污染的问题,寻找一条可持续的、绿色的二元醇生产途径受到了越来越多的关注。生物质资源是自然界中唯一可再生的有机碳资源,以生物质基多元醇为原料制备二元醇有望克服化石资源的短缺并实现绿色可持续性发展。本文综述了国内外生物质基多元醇(甘油、赤藓糖醇、木糖醇和山梨醇)催化转化制备二醇催化剂的最新进展,总结了近年来多元醇催化氢解制备二醇的催化类型(外源氢体系和原位氢源体系)、催化效率、反应溶剂、反应途径、催化机制和催化稳定性,并对其未来的发展进行了展望,以期为生物质基多元醇高效催化转化为二元醇提供参考。
中图分类号:
引用本文
宋顺明, 张敬雯, 张良清, 邱佳容, 陈剑锋, 曾宪海. 生物质基多元醇催化转化制备二醇[J]. 化工进展, 2025, 44(1): 228-252.
SONG Shunming, ZHANG Jingwen, ZHANG Liangqing, QIU Jiarong, CHEN Jianfeng, ZENG Xianhai. Catalytic transformation of biomass-derived polyols to diols[J]. Chemical Industry and Engineering Progress, 2025, 44(1): 228-252.
| 条目 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Ru/TiO2 | 水 | 12h,5MPa H2,443K | 66.3 | 乙二醇,26.0;1,2-丙二醇,47.7 | — | [ |
| 2 | Ru/TiO2 | 水 | 12h,5MPa H2,453K | 90.1 | 乙二醇,41.3;1,2-丙二醇,20.6 | — | [ |
| 3 | Ru/m-ZrO2 | 水 | 3h,6MPa H2,473K | 22.9 | 乙二醇,21.0;1,2-丙二醇,45.7 | — | [ |
| 4 | Pt/m-ZrO2 | 水 | 3h,6MPa H2,473K | 20.4 | 乙二醇,7.4;1,2-丙二醇,85.3 | — | [ |
| 5 | 5%Ru/SiO2(质量分数) | 水 | 5h,8MPa H2,513K | 21.7 | 乙二醇,60.5;1,2-丙二醇,28.7 | — | [ |
| 6 | Ru-Cu/m-ZrO2 | 水 | 12h,2.5MPa H2,453K | 14.0 | 乙二醇,6.0;1,2-丙二醇,89.0; 1,3-丙二醇,2.0 | 2次(稳定) | [ |
| 7 | Ru2Fe1/CNT | 水 | 12h,4MPa H2,473K | 86.0 | 乙二醇,23.5;1,2-丙二醇,52.3 | — | [ |
| 8 | Ru/MCM-41 | 水 | 4h,6MPa H2,503K | 62.0 | 乙二醇,9.0;1,2-丙二醇,38.0; 1,3-丙二醇,20.0 | 5次(稳定) | [ |
| 9 | Pd/Co | 异丙醇 | 24h,5MPa N2,453K | 100 | 乙二醇,8.2;1,2-丙二醇,64 | — | [ |
| 10 | Pd/Fe | 异丙醇 | 24h,5MPa N2,453K | 100 | 乙二醇,1.8;1,2-丙二醇,55.9 | — | [ |
| 11 | Pt/m-WO3 | 水 | 12h,5.5MPa H2,453K | 18.0 | 1,2-丙二醇,4.1;1,3-丙二醇,39.3 | — | [ |
| 12 | Pt/WO x /Al2O3 | 水 | 6h,9MPa H2,443K | 21.2 | 1,2-丙二醇,21.0;1,3-丙二醇,58.9 | — | [ |
| 13 | Pt/WO3/TiO2 | 水 | 8h,4MPa H2,393K | 100 | 1,3-丙二醇,36.0; | 4次(稳定) | [ |
| 14 | Pt/Al-WO x | 水 | 12h,3MPa H2,433K | 79.0 | 1,2-丙二醇,1.4;1,3-丙二醇,40.6 | 4次(稳定) | [ |
表1 贵金属催化剂催化甘油制备二醇
| 条目 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Ru/TiO2 | 水 | 12h,5MPa H2,443K | 66.3 | 乙二醇,26.0;1,2-丙二醇,47.7 | — | [ |
| 2 | Ru/TiO2 | 水 | 12h,5MPa H2,453K | 90.1 | 乙二醇,41.3;1,2-丙二醇,20.6 | — | [ |
| 3 | Ru/m-ZrO2 | 水 | 3h,6MPa H2,473K | 22.9 | 乙二醇,21.0;1,2-丙二醇,45.7 | — | [ |
| 4 | Pt/m-ZrO2 | 水 | 3h,6MPa H2,473K | 20.4 | 乙二醇,7.4;1,2-丙二醇,85.3 | — | [ |
| 5 | 5%Ru/SiO2(质量分数) | 水 | 5h,8MPa H2,513K | 21.7 | 乙二醇,60.5;1,2-丙二醇,28.7 | — | [ |
| 6 | Ru-Cu/m-ZrO2 | 水 | 12h,2.5MPa H2,453K | 14.0 | 乙二醇,6.0;1,2-丙二醇,89.0; 1,3-丙二醇,2.0 | 2次(稳定) | [ |
| 7 | Ru2Fe1/CNT | 水 | 12h,4MPa H2,473K | 86.0 | 乙二醇,23.5;1,2-丙二醇,52.3 | — | [ |
| 8 | Ru/MCM-41 | 水 | 4h,6MPa H2,503K | 62.0 | 乙二醇,9.0;1,2-丙二醇,38.0; 1,3-丙二醇,20.0 | 5次(稳定) | [ |
| 9 | Pd/Co | 异丙醇 | 24h,5MPa N2,453K | 100 | 乙二醇,8.2;1,2-丙二醇,64 | — | [ |
| 10 | Pd/Fe | 异丙醇 | 24h,5MPa N2,453K | 100 | 乙二醇,1.8;1,2-丙二醇,55.9 | — | [ |
| 11 | Pt/m-WO3 | 水 | 12h,5.5MPa H2,453K | 18.0 | 1,2-丙二醇,4.1;1,3-丙二醇,39.3 | — | [ |
| 12 | Pt/WO x /Al2O3 | 水 | 6h,9MPa H2,443K | 21.2 | 1,2-丙二醇,21.0;1,3-丙二醇,58.9 | — | [ |
| 13 | Pt/WO3/TiO2 | 水 | 8h,4MPa H2,393K | 100 | 1,3-丙二醇,36.0; | 4次(稳定) | [ |
| 14 | Pt/Al-WO x | 水 | 12h,3MPa H2,433K | 79.0 | 1,2-丙二醇,1.4;1,3-丙二醇,40.6 | 4次(稳定) | [ |
| 序号 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Cu/SBA-15 | 水 | 6h,4MPa H2,503K | 90.3 | 1,2-丙二醇,97.3 | 3次(稳定) | [ |
| 2 | Cu/Zn/Al | 水 | 20h,1.7MPa H2,473K | 72.6 | 乙二醇,7.6;1,2-丙二醇,76.4 | — | [ |
| 3 | Cu/Al2O3 | 水 | 6h,5MPa H2,493K | 61.0 | 乙二醇,1.1;1,2-丙二醇,56.9 | — | [ |
| 4 | Cu/B/Al2O3 | 水 | 12h,5MPa H2,513K | 93.8 | 1,2-丙二醇,97.70 | 300h | [ |
| 5 | CuAl2O4 | 水 | 12h,5MPa H2,513K | 90.0 | 1,2-丙二醇,90.0 | — | [ |
| 6 | Cu/TiO2 | NaOH和水 | 24h,5MPa H2,473K | 52.0 | 1,2-丙二醇,92 | — | [ |
| 7 | Raney Ni | 水 | 1h,0.1MPa N2,453K | 100 | 乙二醇,25.0;1,2-丙二醇,32.0 | 3次(稳定) | [ |
| 8 | Ni/AC | 水 | 6h,5MPa H2,473K | 44.3 | 乙二醇,7.9;1,2-丙二醇,76.1 | — | [ |
| 9 | Ni-Ce/AC | 水 | 6h,5MPa H2,473K | 90.4 | 乙二醇,10.7;1,2-丙二醇,65.7 | — | [ |
| 10 | NiCuY | 水 | 30h,4.6MPa N2,533K | 96.4 | 1,2-丙二醇,43.9 | 30h | [ |
| 11 | Cu-Mg-Al | 乙醇 | 10h,3MPa N2,483K | 95.1 | 1,2-丙二醇,92.2 | — | [ |
| 12 | Ni-Cu/Al2O3 | 甲酸 | 10h,4.5MPa N2,473K | 33.5 | 1,2-丙二醇,85.9 | — | [ |
| 13 | Cu/Zn/Al | 甲醇 | 1h,2MPa H2,523K | 95.8 | 1,2-丙二醇,77.2 | — | [ |
| 14 | Cu-Ni/AC | 水 | 3h,0.75MPa H2523K | 95.7 | 1,2-丙二醇,87.3 | — | [ |
表2 非贵金属催化剂催化甘油制备二醇
| 序号 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Cu/SBA-15 | 水 | 6h,4MPa H2,503K | 90.3 | 1,2-丙二醇,97.3 | 3次(稳定) | [ |
| 2 | Cu/Zn/Al | 水 | 20h,1.7MPa H2,473K | 72.6 | 乙二醇,7.6;1,2-丙二醇,76.4 | — | [ |
| 3 | Cu/Al2O3 | 水 | 6h,5MPa H2,493K | 61.0 | 乙二醇,1.1;1,2-丙二醇,56.9 | — | [ |
| 4 | Cu/B/Al2O3 | 水 | 12h,5MPa H2,513K | 93.8 | 1,2-丙二醇,97.70 | 300h | [ |
| 5 | CuAl2O4 | 水 | 12h,5MPa H2,513K | 90.0 | 1,2-丙二醇,90.0 | — | [ |
| 6 | Cu/TiO2 | NaOH和水 | 24h,5MPa H2,473K | 52.0 | 1,2-丙二醇,92 | — | [ |
| 7 | Raney Ni | 水 | 1h,0.1MPa N2,453K | 100 | 乙二醇,25.0;1,2-丙二醇,32.0 | 3次(稳定) | [ |
| 8 | Ni/AC | 水 | 6h,5MPa H2,473K | 44.3 | 乙二醇,7.9;1,2-丙二醇,76.1 | — | [ |
| 9 | Ni-Ce/AC | 水 | 6h,5MPa H2,473K | 90.4 | 乙二醇,10.7;1,2-丙二醇,65.7 | — | [ |
| 10 | NiCuY | 水 | 30h,4.6MPa N2,533K | 96.4 | 1,2-丙二醇,43.9 | 30h | [ |
| 11 | Cu-Mg-Al | 乙醇 | 10h,3MPa N2,483K | 95.1 | 1,2-丙二醇,92.2 | — | [ |
| 12 | Ni-Cu/Al2O3 | 甲酸 | 10h,4.5MPa N2,473K | 33.5 | 1,2-丙二醇,85.9 | — | [ |
| 13 | Cu/Zn/Al | 甲醇 | 1h,2MPa H2,523K | 95.8 | 1,2-丙二醇,77.2 | — | [ |
| 14 | Cu-Ni/AC | 水 | 3h,0.75MPa H2523K | 95.7 | 1,2-丙二醇,87.3 | — | [ |
| 序号 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Ir-ReO x /SiO2 | 水 | 24h,8MPa H2,373K | 74.0 | 1,3-丁二醇,12.0;1,4-丁二醇,33.0 | 4次 | [ |
| 2 | Ir-ReO x /TiO2 | 水 | 30h,25bar H2,473K | 99.6 | 1,2-丁二醇,17.5;1,3-丁二醇,11.1; 1,4-丁二醇,2.1;2,3-丁二醇,22.3 | — | [ |
| 3 | Ir-ReO x /TiO2 | 水 | 4h,8MPa H2,373K | 36 | 1,4-丁二醇,36.0 | — | [ |
| 4 | Rh-ReO x /TiO2 | 水 | 14h,25bar H2,473K | — | 丁二醇,34.0 | — | [ |
| 5 | Ru-ReO x /TiO2 | 水 | 14h,25bar H2,473K | — | 丁二醇,55.0 | — | [ |
| 6 | ReO x-Pd/CeO2 | 1,4-二氧六环 | 24h,8MPa H2,433K | 98 | 1,2-丁二醇,79.0 | — | [ |
| 7 | Pt/W/SBA-15 | 水 | 12h,5MPa H2,463K | 82.2 | 1,4-丁二醇,37.6 | — | [ |
| 8 | Pt/W/Ti-SBA-15 | 水 | 12h,5MPa H2,463K | 94.0 | 1,3-丁二醇,20.3;1,4-丁二醇,34.7 | — | [ |
| 9 | Cu/CaO-Al2O3 | 水 | 10h,2.8MPa H2,507K | 85.4 | 1,2-丁二醇,38.6 1,2-丙二醇,15.7 乙二醇,10.9 | — | [ |
表3 不同催化剂催化赤藓糖醇制备二醇
| 序号 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Ir-ReO x /SiO2 | 水 | 24h,8MPa H2,373K | 74.0 | 1,3-丁二醇,12.0;1,4-丁二醇,33.0 | 4次 | [ |
| 2 | Ir-ReO x /TiO2 | 水 | 30h,25bar H2,473K | 99.6 | 1,2-丁二醇,17.5;1,3-丁二醇,11.1; 1,4-丁二醇,2.1;2,3-丁二醇,22.3 | — | [ |
| 3 | Ir-ReO x /TiO2 | 水 | 4h,8MPa H2,373K | 36 | 1,4-丁二醇,36.0 | — | [ |
| 4 | Rh-ReO x /TiO2 | 水 | 14h,25bar H2,473K | — | 丁二醇,34.0 | — | [ |
| 5 | Ru-ReO x /TiO2 | 水 | 14h,25bar H2,473K | — | 丁二醇,55.0 | — | [ |
| 6 | ReO x-Pd/CeO2 | 1,4-二氧六环 | 24h,8MPa H2,433K | 98 | 1,2-丁二醇,79.0 | — | [ |
| 7 | Pt/W/SBA-15 | 水 | 12h,5MPa H2,463K | 82.2 | 1,4-丁二醇,37.6 | — | [ |
| 8 | Pt/W/Ti-SBA-15 | 水 | 12h,5MPa H2,463K | 94.0 | 1,3-丁二醇,20.3;1,4-丁二醇,34.7 | — | [ |
| 9 | Cu/CaO-Al2O3 | 水 | 10h,2.8MPa H2,507K | 85.4 | 1,2-丁二醇,38.6 1,2-丙二醇,15.7 乙二醇,10.9 | — | [ |
| 序号 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Ru/C | Ca(OH)2和水 | 1h,4MPa H2,473K | 100 | 乙二醇,32.4;丙二醇,24.9 | — | [ |
| 2 | Pt/C | Ca(OH)2和水 | 4h,8MPa H2,473K | 97 | 乙二醇,37.0;丙二醇,30.0 | — | [ |
| 3 | Ru-CTF | Ca(OH)2和水 | 4h,8MPa H2,473K | 100 | 乙二醇,36.0;丙二醇,44.0 | 5次(稳定) | [ |
| 4 | Ru-(Mn-Al)O x | 水 | 3h,6MPa H2,473K | 80 | 乙二醇,21.0;丙二醇,28.0 | — | [ |
| 5 | Ru/MnO/C | 10%异丙醇 | 3h,6MPa H2,473K | 100 | 乙二醇,25.0;丙二醇,34.0 | — | [ |
| 6 | Pd-Pt/TiO2 | BaO和水 | 6h,1MPa N2,493K | 79.3 | 乙二醇和丙二醇,44.1 | — | [ |
| 7 | Cu-SiO2 | Ca(OH)2和水 | 2h,6MPa H2,473K | 26.8 | 乙二醇,27.9;丙二醇,28.5 | 3次(稳定) | [ |
| 8 | CuCeSBA | 水 | 3h,5MPa H2,493K | 100 | 乙二醇,24.5;丙二醇,41.5 | — | [ |
| 9 | Ni-CaO/C | 水 | 2h,4MPa H2,473K | 100 | 乙二醇,28.4;丙二醇,26.0 | 5次(稳定) | [ |
| 10 | 纳米-Ni/meso-Ce-TiO2 | 水 | 4h,5MPa H2,513K | 87.9 | 乙二醇,50.2;丙二醇,33.0 | 8次(稳定) | [ |
| 11 | NI2P/AC | Ca(OH)2和水 | 0.75h,4MPa H2,473K | 99.0 | 乙二醇,28.5;丙二醇,42.9 | — | [ |
| 12 | NiCu-SiO2 | 水 | 2h,8MPa H2,473K | 94.4 | 乙二醇,28.8;丙二醇,31.6 | 5次 | [ |
| 13 | Cu-Ni-ZrO2 | 水 | 3h,4MPa H2,518K | 97.0 | 乙二醇,21.6;丙二醇,35.0 | 6次(稳定) | [ |
| 14 | Cu-Ni-ZrO2(CTH) | 异丙醇 | 3h,4MPa H2,518K | 96.4 | 乙二醇,15.9;丙二醇,27.7 | — | [ |
| 15 | Ni-W/CeO2 | 水 | 2h,4MPa H2,453K | 100 | 乙二醇,11.9;丙二醇,32.5 | 3次 | [ |
表4 不同催化剂催化木糖醇制备二醇
| 序号 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Ru/C | Ca(OH)2和水 | 1h,4MPa H2,473K | 100 | 乙二醇,32.4;丙二醇,24.9 | — | [ |
| 2 | Pt/C | Ca(OH)2和水 | 4h,8MPa H2,473K | 97 | 乙二醇,37.0;丙二醇,30.0 | — | [ |
| 3 | Ru-CTF | Ca(OH)2和水 | 4h,8MPa H2,473K | 100 | 乙二醇,36.0;丙二醇,44.0 | 5次(稳定) | [ |
| 4 | Ru-(Mn-Al)O x | 水 | 3h,6MPa H2,473K | 80 | 乙二醇,21.0;丙二醇,28.0 | — | [ |
| 5 | Ru/MnO/C | 10%异丙醇 | 3h,6MPa H2,473K | 100 | 乙二醇,25.0;丙二醇,34.0 | — | [ |
| 6 | Pd-Pt/TiO2 | BaO和水 | 6h,1MPa N2,493K | 79.3 | 乙二醇和丙二醇,44.1 | — | [ |
| 7 | Cu-SiO2 | Ca(OH)2和水 | 2h,6MPa H2,473K | 26.8 | 乙二醇,27.9;丙二醇,28.5 | 3次(稳定) | [ |
| 8 | CuCeSBA | 水 | 3h,5MPa H2,493K | 100 | 乙二醇,24.5;丙二醇,41.5 | — | [ |
| 9 | Ni-CaO/C | 水 | 2h,4MPa H2,473K | 100 | 乙二醇,28.4;丙二醇,26.0 | 5次(稳定) | [ |
| 10 | 纳米-Ni/meso-Ce-TiO2 | 水 | 4h,5MPa H2,513K | 87.9 | 乙二醇,50.2;丙二醇,33.0 | 8次(稳定) | [ |
| 11 | NI2P/AC | Ca(OH)2和水 | 0.75h,4MPa H2,473K | 99.0 | 乙二醇,28.5;丙二醇,42.9 | — | [ |
| 12 | NiCu-SiO2 | 水 | 2h,8MPa H2,473K | 94.4 | 乙二醇,28.8;丙二醇,31.6 | 5次 | [ |
| 13 | Cu-Ni-ZrO2 | 水 | 3h,4MPa H2,518K | 97.0 | 乙二醇,21.6;丙二醇,35.0 | 6次(稳定) | [ |
| 14 | Cu-Ni-ZrO2(CTH) | 异丙醇 | 3h,4MPa H2,518K | 96.4 | 乙二醇,15.9;丙二醇,27.7 | — | [ |
| 15 | Ni-W/CeO2 | 水 | 2h,4MPa H2,453K | 100 | 乙二醇,11.9;丙二醇,32.5 | 3次 | [ |
| 序号 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Ru/CNFs | Ca(OH)2和水 | 2h,8MPa H2,493K | 85.7 | 乙二醇,19.3;1,2-丙二醇,32.0 | — | [ |
| 2 | Ru/NMCN | Ca(OH)2和水 | 2h,6MPa H2,483K | 97.3 | 乙二醇,23.2;1,2-丙二醇,39.1 | 5次 | [ |
| 3 | Ru/Al2O3 | 水 | 2h,4MPa H2,493K | 73.9 | 乙二醇和1,2-丙二醇,19.3 | — | [ |
| 4 | SSRu | Ca(OH)2和水 | 4h,10MPa H2,523K | 88.7 | 乙二醇,23.0;丙二醇,12.4 | — | [ |
| 5 | Ru/WO x /CNFs | 水 | 2h,5MPa H2,478K | 99.6 | 乙二醇,25.6;丙二醇,34.6 | 5次(稳定) | [ |
| 6 | RuRe/C | Ca(OH)2和水 | 2h,8MPa H2,473K | 84.0 | 乙二醇和1,2-丙二醇,52.0 | — | [ |
| 7 | Pd/Fe2O3 | 水 | 24h,5MPa H2,453K | 91.0 | 乙二醇,13.4;1,2-丙二醇,27.9 | — | [ |
| 8 | Pd/ZrO2 | 水 | 1.5h,5MPa H2,453K | 100 | 乙二醇和1,2-丙二醇,56.9 | — | [ |
| 9 | PtPd/C | 水 | 1h,1MPa N2,523K | 93.4 | 乙二醇,8.4;1,2-丙二醇,32.4 | 3次(稳定) | [ |
| 10 | Ru/AMCN | 水 | 2h,5MPa H2,478K | 99.5 | 乙二醇,18.3;1,2-丙二醇,29.4 | 5次(稳定) | [ |
表5 贵金属催化剂催化山梨醇制备二醇
| 序号 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Ru/CNFs | Ca(OH)2和水 | 2h,8MPa H2,493K | 85.7 | 乙二醇,19.3;1,2-丙二醇,32.0 | — | [ |
| 2 | Ru/NMCN | Ca(OH)2和水 | 2h,6MPa H2,483K | 97.3 | 乙二醇,23.2;1,2-丙二醇,39.1 | 5次 | [ |
| 3 | Ru/Al2O3 | 水 | 2h,4MPa H2,493K | 73.9 | 乙二醇和1,2-丙二醇,19.3 | — | [ |
| 4 | SSRu | Ca(OH)2和水 | 4h,10MPa H2,523K | 88.7 | 乙二醇,23.0;丙二醇,12.4 | — | [ |
| 5 | Ru/WO x /CNFs | 水 | 2h,5MPa H2,478K | 99.6 | 乙二醇,25.6;丙二醇,34.6 | 5次(稳定) | [ |
| 6 | RuRe/C | Ca(OH)2和水 | 2h,8MPa H2,473K | 84.0 | 乙二醇和1,2-丙二醇,52.0 | — | [ |
| 7 | Pd/Fe2O3 | 水 | 24h,5MPa H2,453K | 91.0 | 乙二醇,13.4;1,2-丙二醇,27.9 | — | [ |
| 8 | Pd/ZrO2 | 水 | 1.5h,5MPa H2,453K | 100 | 乙二醇和1,2-丙二醇,56.9 | — | [ |
| 9 | PtPd/C | 水 | 1h,1MPa N2,523K | 93.4 | 乙二醇,8.4;1,2-丙二醇,32.4 | 3次(稳定) | [ |
| 10 | Ru/AMCN | 水 | 2h,5MPa H2,478K | 99.5 | 乙二醇,18.3;1,2-丙二醇,29.4 | 5次(稳定) | [ |
| 序号 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Ni/Mg1.29Al0.06O1.38 | 水 | 2h,2MPa H2,473K | 97.7 | 乙二醇,11.9;1,2-丙二醇,33.5 | 5次(稳定) | [ |
| 2 | 6%Ni-NaY | Ca(OH)2和水 | 6h,6MPa H2,493K | 75.0 | 乙二醇,7.0;1,2-丙二醇,69.0 | — | [ |
| 3 | Ce-Ni/Al2O3 | Ca(OH)2和水 | 8h,7MPa H2,493K | 95.6 | 乙二醇,17.7;1,2-丙二醇,35.6 | 10h | [ |
| 4 | M-10Ni2CeAl | 水 | 8h,5MPa H2,493K | 62.3 | 乙二醇,21.2;1,2-丙二醇,37.7 | 12次(稳定) | [ |
| 5 | 2%Ni2P/AC | 水 | 1h,4MPa H2,473K | 98.6 | 乙二醇,17.0;1,2-丙二醇,27.7 | 3次(稳定) | [ |
| 6 | Ni-Mg-AlLDH | 水 | 2h,2MPa H2,473K | 99.3 | 乙二醇,18.8;1,2-丙二醇,28.2 | 5次(稳定) | [ |
| 7 | 10%Ni/5% La2O3/ZrO2 | 水 | 24h,5MPa H2,453K | 96.8 | 乙二醇,20.3;1,2-丙二醇,26.8 | — | [ |
| 8 | Ni(6%)/FA | Ca(OH)2和水 | 6h,6MPa H2,473K | 48.0 | 乙二醇,12.0;1,2-丙二醇,34.0 | 3次(稳定) | [ |
| 9 | Ni/SrHAP-R | 水 | 6h,6MPa H2,473K | 68.0 | 乙二醇,21.8;1,2-丙二醇,27.2 | 4次(稳定) | [ |
| 10 | Ni-MgO | Ca(OH)2和水 | 4h,6MPa H2,473K | 85.6 | 乙二醇,20.2;1,2-丙二醇,31.9 | — | [ |
| 11 | Co-MgO | Ca(OH)2和水 | 4h,6MPa H2,473K | 80.3 | 乙二醇,16.4;1,2-丙二醇,20.8 | — | [ |
| 12 | Cu-MgO | Ca(OH)2和水 | 4h,6MPa H2,473K | 78.7 | 乙二醇,23.7;1,2-丙二醇,32.6 | — | [ |
| 13 | Pd-Cu/ZrO2 | 水 | 4h,5MPa H2,493K | 约100 | 乙二醇,16.3;1,2-丙二醇,36.8 | 5次(稳定) | [ |
| 14 | Cu/CaO-Al2O3 | 水 | 6h,7MPa H2,453K | 98.1 | 乙二醇,15.4;1,2-丙二醇,46.1 | 3次(稳定) | [ |
表6 非贵金属催化剂催化山梨醇制备二醇
| 序号 | 催化剂 | 溶剂 | 条件 | 转化率/% | 选择性/% | 循环 | 参考文献 |
|---|---|---|---|---|---|---|---|
| 1 | Ni/Mg1.29Al0.06O1.38 | 水 | 2h,2MPa H2,473K | 97.7 | 乙二醇,11.9;1,2-丙二醇,33.5 | 5次(稳定) | [ |
| 2 | 6%Ni-NaY | Ca(OH)2和水 | 6h,6MPa H2,493K | 75.0 | 乙二醇,7.0;1,2-丙二醇,69.0 | — | [ |
| 3 | Ce-Ni/Al2O3 | Ca(OH)2和水 | 8h,7MPa H2,493K | 95.6 | 乙二醇,17.7;1,2-丙二醇,35.6 | 10h | [ |
| 4 | M-10Ni2CeAl | 水 | 8h,5MPa H2,493K | 62.3 | 乙二醇,21.2;1,2-丙二醇,37.7 | 12次(稳定) | [ |
| 5 | 2%Ni2P/AC | 水 | 1h,4MPa H2,473K | 98.6 | 乙二醇,17.0;1,2-丙二醇,27.7 | 3次(稳定) | [ |
| 6 | Ni-Mg-AlLDH | 水 | 2h,2MPa H2,473K | 99.3 | 乙二醇,18.8;1,2-丙二醇,28.2 | 5次(稳定) | [ |
| 7 | 10%Ni/5% La2O3/ZrO2 | 水 | 24h,5MPa H2,453K | 96.8 | 乙二醇,20.3;1,2-丙二醇,26.8 | — | [ |
| 8 | Ni(6%)/FA | Ca(OH)2和水 | 6h,6MPa H2,473K | 48.0 | 乙二醇,12.0;1,2-丙二醇,34.0 | 3次(稳定) | [ |
| 9 | Ni/SrHAP-R | 水 | 6h,6MPa H2,473K | 68.0 | 乙二醇,21.8;1,2-丙二醇,27.2 | 4次(稳定) | [ |
| 10 | Ni-MgO | Ca(OH)2和水 | 4h,6MPa H2,473K | 85.6 | 乙二醇,20.2;1,2-丙二醇,31.9 | — | [ |
| 11 | Co-MgO | Ca(OH)2和水 | 4h,6MPa H2,473K | 80.3 | 乙二醇,16.4;1,2-丙二醇,20.8 | — | [ |
| 12 | Cu-MgO | Ca(OH)2和水 | 4h,6MPa H2,473K | 78.7 | 乙二醇,23.7;1,2-丙二醇,32.6 | — | [ |
| 13 | Pd-Cu/ZrO2 | 水 | 4h,5MPa H2,493K | 约100 | 乙二醇,16.3;1,2-丙二醇,36.8 | 5次(稳定) | [ |
| 14 | Cu/CaO-Al2O3 | 水 | 6h,7MPa H2,453K | 98.1 | 乙二醇,15.4;1,2-丙二醇,46.1 | 3次(稳定) | [ |
| 1 | DENG Weiping, FENG Yunchao, FU Jie, et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels[J]. Green Energy & Environment, 2023, 8(1): 10-114. |
| 2 | TAMURA Masazumi, NAKAGAWA Yoshinao, TOMISHIGE Keiichi. Reduction of sugar derivatives to valuable chemicals: Utilization of asymmetric carbons[J]. Catalysis Science & Technology, 2020, 10(12): 3805-3824. |
| 3 | COLLARD François-Xavier, BLIN Joël. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin[J]. Renewable and Sustainable Energy Reviews, 2014, 38: 594-608. |
| 4 | SCHUTYSER W, RENDERS T, VAN DEN BOSCH S, et al. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading[J]. Chemical Society Reviews, 2018, 47(3): 852-908. |
| 5 | SUN Zhuohua, Bálint FRIDRICH, DE SANTI Alessandra, et al. Bright side of lignin depolymerization: Toward new platform chemicals[J]. Chemical Reviews, 2018, 118(2): 614-678. |
| 6 | 杨启悦, 吴巧妹, 邱佳容, 等. 生物基平台化合物催化转化制备糠醇[J]. 化学进展, 2022, 34(8): 1748-1759. |
| YANG Qiyue, WU Qiaomei, QIU Jiarong, et al. Catalytic conversion of bio-based platform compounds to fufuryl alcohol[J]. Progress in Chemistry, 2022, 34(8): 1748-1759. | |
| 7 | RICHARD Ahorsu, FRANCESC Medina, MAGDA Constantí. Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: A review[J]. Energies, 2018, 11(12): 3366. |
| 8 | ANNUAR N H R, ALEXZMAN Z A, DAUD A R M, et al. A review on hydrogenolysis of sorbitol over heterogeneous catalysts[J]. Journal of Environmental Chemical Engineering, 2022, 10(2): 107229. |
| 9 | CARVALHO Santulla Leide Bernardes Vasconcelos, DE MORAES MEDEIROS Eliane Bezerra, DE SOUZA WANDERLEY Ayrton, et al. Production of xylitol from acidic hydrolysates of lignocellulosic biomass by catalytic hydrogenation over a Ni-Ru/C catalyst[J]. Chemical Engineering Research and Design, 2021, 174: 11-18. |
| 10 | 卢怡, 郑志锋, 黄元波, 等. 半纤维素选择性催化制备糠醛及其衍生物的研究进展[J]. 林产化学与工业, 2018, 38(3): 1-16. |
| LU Yi, ZHENG Zhifeng, HUANG Yuanbo, et al. Research advances in preparation of furfural and its derivatives by selective catalytic conversion of hemicellulose[J]. Chemistry and Industry of Forest Products, 2018, 38(3): 1-16. | |
| 11 | WANG Yantao, ZHAO Deyang, Daily RODRÍGUEZ-PADRÓN, et al. Recent advances in catalytic hydrogenation of furfural[J]. Catalysts, 2019, 9(10): 796. |
| 12 | FENG Yunchao, LONG Sishi, TANG Xing, et al. Earth-abundant 3d-transition-metal catalysts for lignocellulosic biomass conversion[J]. Chemical Society Reviews, 2021, 50(10): 6042-6093. |
| 13 | MANAENKOV Oleg V, KISLITSA Olga V, MATVEEVA Valentina G, et al. Cellulose conversion into hexitols and glycols in water: Recent advances in catalyst development[J]. Frontiers in Chemistry, 2019, 7: 834. |
| 14 | CAI Chiliu, ZHU Changhui, WANG Haiyong, et al. Catalytic hydrogenolysis of biomass-derived polyhydric compounds to C2-C3 small-molecule polyols: A review[J]. Current Organic Chemistry, 2019, 23(20): 2180-2189. |
| 15 | YANG Qingchun, CHU Genyun, ZHANG Lihao, et al. Pathways toward carbon-neutral coal to ethylene glycol processes by integrating with different renewable energy-based hydrogen production technologies[J]. Energy Conversion and Management, 2022, 258: 115529. |
| 16 | TAO Yuanming, BU Chongyang, ZOU Lihua, et al. A comprehensive review on microbial production of 1,2-propanediol: Micro-organisms, metabolic pathways, and metabolic engineering[J]. Biotechnology for Biofuels, 2021, 14(1): 216. |
| 17 | GREISH A A, KUSTOV L M. Catalytic conversion of glycerol in the presence of Ni/F-Al2O3 catalyst[J]. Russian Journal of Physical Chemistry A, 2018, 92(11): 2351-2353. |
| 18 | FAHMY Tamer Y A, FAHMY Yehia, MOBARAK Fardous, et al. Biomass pyrolysis: Past, present, and future[J]. Environment, Development and Sustainability, 2020, 22(1): 17-32. |
| 19 | Daniel TINÔCO, BORSCHIVER Suzana, COUTINHO Paulo L, et al. Technological development of the bio-based 2,3-butanediol process[J]. Biofuels, Bioproducts and Biorefining, 2021, 15(2): 357-376. |
| 20 | XIE Shaoqu, LI Zhuoxi, ZHU Guodian, et al. Cleaner production and downstream processing of bio-based 2,3-butanediol: A review[J]. Journal of Cleaner Production, 2022, 343: 131033. |
| 21 | LI Suiyi, WU Yingji, My Uyen DAO, et al. Spotlighting of the role of catalysis for biomass conversion to green fuels towards a sustainable environment: Latest innovation avenues, insights, challenges, and future perspectives[J]. Chemosphere, 2023, 318: 137954. |
| 22 | GAO Ge, FENG Shanshan, JIANG Zhicheng, et al. Efficient hydrogenation of glucose to polyols over hydrotalcite-derived PtNi alloy catalyst under mild conditions[J]. Industrial & Engineering Chemistry Research, 2023, 62(7): 3140-3150. |
| 23 | SATARI Behzad, KARIMI Keikhosro, KUMAR Rajeev. Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: A review[J]. Sustainable Energy & Fuels, 2019, 3(1): 11-62. |
| 24 | DAI Leilei, WANG Yunpu, LIU Yuhuan, et al. A review on selective production of value-added chemicals via catalytic pyrolysis of lignocellulosic biomass[J]. Science of the Total Environment, 2020, 749: 142386. |
| 25 | NANDA Malaya R, YUAN Zhongshun, QIN Wensheng, et al. Recent advancements in catalytic conversion of glycerol into propylene glycol: A review[J]. Catalysis Reviews, 2016, 58(3): 309-336. |
| 26 | MARINOIU Adriana, COBZARU Claudia, CARCADEA Elena, et al. Hydrogenolysis of glycerol to propylene glycol using heterogeneous catalysts in basic aqueous solutions[J]. Reaction Kinetics, Mechanisms and Catalysis, 2013, 110(1): 63-73. |
| 27 | MANE Rasika, JEON Yukwon, RODE Chandrashekhar. A review on non-noble metal catalysts for glycerol hydrodeoxygenation to 1,2-propanediol with and without external hydrogen[J]. Green Chemistry, 2022, 24(18): 6751-6781. |
| 28 | CHUN MINH Adrian Loy, SAMUDRALA Shanthi Priya, BHATTACHARYA Sankar. Valorisation of glycerol through catalytic hydrogenolysis routes for sustainable production of value-added C3 chemicals: Current and future trends[J]. Sustainable Energy & Fuels, 2022, 6(3): 596-639. |
| 29 | ZHAO Huaiyuan, ZHENG Liping, LI Xuewen, et al. Hydrogenolysis of glycerol to 1,2-propanediol over Cu-based catalysts: A short review[J]. Catalysis Today, 2020, 355: 84-95. |
| 30 | WANG Shuai, YIN Kehua, ZHANG Yichi, et al. Glycerol hydrogenolysis to propylene glycol and ethylene glycol on zirconia supported noble metal catalysts[J]. ACS Catalysis, 2013, 3(9): 2112-2121. |
| 31 | FENG Jian, FU Haiyan, WANG Jinbo, et al. Hydrogenolysis of glycerol to glycols over ruthenium catalysts: Effect of support and catalyst reduction temperature[J]. Catalysis Communications, 2008, 9(6): 1458-1464. |
| 32 | BALARAJU M, REKHA V, B L A Prabhavathi DEVI,et al. Surface and structural properties of titania-supported Ru catalysts for hydrogenolysis of glycerol[J]. Applied Catalysis A: General, 2010, 384(1/2): 107-114. |
| 33 | LUO Zhicheng, ZHU Zhiguo, XIAO Rui, et al. Selective production of 1,2-propanediol or 1,3-propanediol from glycerol hydrogenolysis over transition metal doped Pt/TiO2 [J]. Chemistry, an Asian Journal, 2023, 18(3): e202201046. |
| 34 | MANE Rasika B, PATIL Shivanand T, GURAV Hanmant, et al. Effect of Ru precursors and reduction conditions on catalyst performance in glycerol hydrogenolysis[J]. ChemistrySelect, 2017, 2(4): 1734-1745. |
| 35 | VASILIADOU E S, LEMONIDOU A A. Investigating the performance and deactivation behaviour of silica supported copper catalysts in glycerol hydrogenolysis[J]. Applied Catalysis A: General, 2011, 396(1/2): 177-185. |
| 36 | VASILIADOU Efterpi S, LEMONIDOU Angeliki A. Parameters affecting the formation of 1,2-propanediol from glycerol over Ru/SiO2 catalyst[J]. Organic Process Research & Development, 2011, 15(4): 925-931. |
| 37 | SALGADO Ana Luiza P, ARAÚJO Felipe C, SOARES André V H, et al. Glycerol hydrogenolysis over Ru-Cu bimetallic catalysts supported on modified zirconias[J]. Applied Catalysis A: General, 2021, 626: 118359. |
| 38 | LI Bodong, WANG Juan, YUAN Youzhu, et al. Carbon nanotube-supported RuFe bimetallic nanoparticles as efficient and robust catalysts for aqueous-phase selective hydrogenolysis of glycerol to glycols[J]. ACS Catalysis, 2011, 1(11): 1521-1528. |
| 39 | SUN Qianhui, WANG Shuai, LIU Haichao. Selective hydrogenolysis of glycerol to propylene glycol on supported Pd catalysts: Promoting effects of ZnO and mechanistic assessment of active PdZn alloy surfaces[J]. ACS Catalysis, 2017, 7(7): 4265-4275. |
| 40 | JIANG Tao, HUAI Qiang, GENG Tong, et al. Catalytic performance of Pd-Ni bimetallic catalyst for glycerol hydrogenolysis[J]. Biomass and Bioenergy, 2015, 78: 71-79. |
| 41 | 陈鸿哲. 铜基催化剂催化甘油氢解制备1,2-丙二醇研究[D]. 合肥: 合肥工业大学, 2018. |
| CHEN Hongzhe. Study of Cu-based catalysts on glycerol hydrogenolysis to 1,2-propanediol[D]. Hefei: Hefei University of Technology, 2018. | |
| 42 | BHOWMIK Susmita, ENJAMURI Nagasuresh, DARBHA Srinivas. Hydrogenolysis of glycerol in an aqueous medium over Pt/WO3/zirconium phosphate catalysts studied by 1H NMR spectroscopy[J]. New Journal of Chemistry, 2021, 45(11): 5013-5022. |
| 43 | 温英林, 刘世钰, 沈卫华, 等. Pt/WO x /Al2O3及其改性催化剂氢解甘油制备1,3-丙二醇[J]. 低碳化学与化工, 2021, 46(5): 68-73. |
| WEN Yinglin, LIU Shiyu, SHENG Weihua, et al. Preparation of 1,3-propanediol by hydrogenolysis of glycerol over Pt/WO x /Al2O3 and its modified catalysts[J]. Low-Carbon Chemistry and Chemical Engineering, 2021, 46(05): 68-73. | |
| 44 | KUROSAKA T, SASAKI Y, NARIBAYASHI I. Manufacture of propanediol such as 1,3-propanediol involves hydrogenating glycerin in presence of solid catalyst containing primary metallic element and secondary metallic element chosen from molybdenum, tantalum and/or niobium: JP2008143798(A)[P]. 2008-01-04. |
| 45 | ZHOU Zhiming, JIA Hongyan, GUO Yong, et al. The promotional effect of sulfates on TiO2 supported Pt-WO x catalyst for hydrogenolysis of glycerol[J]. ChemCatChem, 2021, 13(18): 3953-3959. |
| 46 | 仝庆, 高强, 许波连, 等. Pt/WO3/ZrO2催化甘油选择性氢解制备1,3-丙二醇[J]. 有机化学, 2017, 37(3): 753-758. |
| TONG Qing, GAO Qiang, XU Bolian, et al. Pt/WO3/ZrO2-catalyzed selective hydrogenolysis of glycerol to produce 1,3-propanediol[J]. Chinese Journal of Organic Chemistry, 2017, 37(3): 753-758. | |
| 47 | VANAMA Pavan Kumar, KUMAR Ashish, GINJUPALLI Srinivasa Rao, et al. Vapor-phase hydrogenolysis of glycerol over nanostructured Ru/MCM-41 catalysts[J]. Catalysis Today, 2015, 250: 226-238. |
| 48 | MAURIELLO F, ARIGA H, MUSOLINO M G, et al. Exploring the catalytic properties of supported palladium catalysts in the transfer hydrogenolysis of glycerol[J]. Applied Catalysis B: Environmental, 2015, 166/167: 121-131. |
| 49 | LIU Longjie, ZHANG Yanhua, WANG Aiqin, et al. Mesoporous WO3 supported Pt catalyst for hydrogenolysis of glycerol to 1,3-propanediol[J]. Chinese Journal of Catalysis, 2012, 33(7/8): 1257-1261. |
| 50 | YANG Man, WU Keying, SUN Shaodong, et al. Regulating oxygen defects via atomically dispersed alumina on Pt/WO x catalyst for enhanced hydrogenolysis of glycerol to 1,3-propanediol[J]. Applied Catalysis B: Environment and Energy, 2022, 307: 121207. |
| 51 | SHAN Jianfeng, LIU Huan, LU Kuan, et al. Identification of the dehydration active sites in glycerol hydrogenolysis to 1,2-propanediol over Cu/SiO2 catalysts[J]. Journal of Catalysis, 2020, 383: 13-23. |
| 52 | GAO Qiang, XU Bolian, TONG Qing, et al. Selective hydrogenolysis of raw glycerol to 1,2-propanediol over Cu-ZnO catalysts in fixed-bed reactor[J]. Bioscience, Biotechnology, and Biochemistry, 2016, 80(2): 215-220. |
| 53 | FENG Y, YIN H, SHEN L, et al. Gas-phase hydrogenolysis of glycerol catalyzed by Cu/MO x catalysts[J]. Chemical Engineering & Technology, 2013, 36(1): 73-82. |
| 54 | KIM D Dong Won, Sang Ho HA, MOON Myung Jun, et al. Hydrogenolysis of glycerol to propylene glycol on nanosized Cu-Zn-Al catalysts prepared using microwave process[J]. Journal of Nanoscience and Nanotechnology, 2015, 15(1): 656-659. |
| 55 | HIRUNSIT Pussana, LUADTHONG Chuleeporn, FAUNGNAWAKIJ Kajornsak. Effect of alumina hydroxylation on glycerol hydrogenolysis to 1,2-propanediol over Cu/Al2O3: Combined experiment and DFT investigation[J]. RSC Advances, 2015, 5(15): 11188-11197. |
| 56 | 马松. Cu/B/Ca/Al2O3催化甘油氢解制备1,2-丙二醇的性能研究[D]. 北京: 北京化工大学, 2017. |
| MA Song. Catalytic hydrogenolysis of glycerol to 1,2-propanediol over Cu/B/Ca/Al2O3 Catalysts[D]. Beijing: Beijing University of Chemical Technology, 2017. | |
| 57 | KWAK Byoung Kyu, PARK Dae Sung, YUN Yang Sik, et al. Preparation and characterization of nanocrystalline CuAl2O4 spinel catalysts by sol-gel method for the hydrogenolysis of glycerol[J]. Catalysis Communications, 2012, 24:90-95. |
| 58 | KHADZHIEV V I, DMITRIEV G S, MEL’CHAKOV I S, et al. Kinetics of hydrogenolysis of glycerol into 1,2-propylene glycol on a copper catalyst[J]. Kinetics and catalysis, 2019, 60(6): 802-807. |
| 59 | ŻELAZNY A, SAMSON K, GRABOWSKI R, et al. Hydrogenolysis of glycerol to propylene glycol over Cu/oxide catalysts: Influence of the support and reaction conditions[J]. Reaction Kinetics, Mechanisms and Catalysis, 2017, 121(1): 329-343. |
| 60 | YIN Anyuan, GUO Xiuying, DAI Weilin, et al. The synthesis of propylene glycol and ethylene glycol from glycerol using Raney Ni as a versatile catalyst[J]. Green Chemistry, 2009, 11(10): 1514-1516. |
| 61 | YU Weiqiang, XU Jie, MA Hong, et al. A remarkable enhancement of catalytic activity for KBH4 treating the carbothermal reduced Ni/AC catalyst in glycerol hydrogenolysis[J]. Catalysis Communications, 2010, 11(5): 493-497. |
| 62 | YU Weiqiang, ZHAO Jing, MA Hong, et al. Aqueous hydrogenolysis of glycerol over Ni-Ce/AC catalyst: Promoting effect of Ce on catalytic performance[J]. Applied Catalysis A: General, 2010, 383(1/2): 73-78. |
| 63 | XIA Shuixin, ZHENG Liping, WANG Lina, et al. Hydrogen-free synthesis of 1,2-propanediol from glycerol over Cu-Mg-Al catalysts[J]. RSC Advances, 2013, 3(37): 16569-16576. |
| 64 | GANDARIAS Inaki, ARIAS Pedro Luis, FERNÁNDEZ Sara G, et al. Hydrogenolysis through catalytic transfer hydrogenation: Glycerol conversion to 1,2-propanediol[J]. Catalysis Today, 2012, 195(1): 22-31. |
| 65 | YFANTI V L, LEMONIDOU A A. Effect of hydrogen donor on glycerol hydrodeoxygenation to 1,2-propanediol[J]. Catalysis Today, 2020, 355: 727-736. |
| 66 | GANDARIAS I, ARIAS P L, REQUIES J, et al. Liquid-phase glycerol hydrogenolysis to 1,2-propanediol under nitrogen pressure using 2-propanol as hydrogen source[J]. Journal of Catalysis, 2011, 282(1): 237-247. |
| 67 | XU Wenfeng, NIU Pengyu, GUO Heqin, et al. Study on the performance of platinum and tungsten bifunctional catalyst supported on Al2O3 in the hydrogenolysis of glycerol to 1,3-propanediol[J]. Journal of Fuel Chemistry and Technology, 2021, 49(9): 1270-1280. |
| 68 | DE ANDRADE Taynara S, SOUZA Mariana M V M, MANFRO Robinson L. Hydrogenolysis of glycerol to 1,2-propanediol without external H2 addition in alkaline medium using Ni-Cu catalysts supported on Y zeolite[J]. Renewable Energy, 2020, 160: 919-930. |
| 69 | KASHIF Mohammad, THANGARASU Sadhasivam, Tae Hwan OH, et al. Vapor-phase hydrogenolysis of glycerol to value-added 1,2-propanediol over copper-nickel bimetallic catalysts supported on activated carbon[J]. Korean Journal of Chemical Engineering, 2022, 39(10): 2652-2663. |
| 70 | RZECHONEK Dorota A, DOBROWOLSKI Adam, RYMOWICZ Waldemar, et al. Recent advances in biological production of erythritol[J]. Critical Reviews in Biotechnology, 2018, 38(4): 620-633. |
| 71 | AMARASEKARA Ananda S, Shahrukh R ALI, FERNANDO Harshica, et al. A comparison of homogeneous and heterogeneous Brønsted acid catalysts in the reactions of meso-erythritol with aldehyde/ketones[J]. SN Applied Sciences, 2019, 1(3): 212. |
| 72 | NAKAGAWA Yoshinao, KASUMI Takafumi, OGIHARA Jun, et al. Erythritol: Another C4 platform chemical in biomass refinery[J]. ACS Omega, 2020, 5(6): 2520-2530. |
| 73 | ARAI Takahiro, TAMURA Masazumi, NAKAGAWA Yoshinao, et al. Synthesis of 2-butanol by selective hydrogenolysis of 1,4-anhydroerythritol over molybdenum oxide-modified rhodium-supported silica[J]. ChemSusChem, 2016, 9(13): 1680-1688. |
| 74 | AMADA Yasushi, WATANABE Hideo, HIRAI Yuichirou, et al. Production of biobutanediols by the hydrogenolysis of erythritol[J]. ChemSusChem, 2012, 5(10): 1991-1999. |
| 75 | VIRGILIO Emanuel M, PADRÓ Cristina L, María E SAD. Effect of support properties on selective butanediols production from erythritol using Ir/ReO x catalysts[J]. ChemCatChem, 2021, 13(17): 3889-3906. |
| 76 | GU Minyan, LIU Lujie, NAKAGAWA Yoshinao, et al. Selective hydrogenolysis of erythritol over Ir-ReO x /rutile-TiO2 catalyst[J]. ChemSusChem, 2021, 14(2): 642-654. |
| 77 | VIRGILIO Emanuel Martín, PADRÓ Cristina Liliana, Maria Eugenia SAD. Butenediols production from erythritol on Rh promoted catalyst[J]. Latin American Applied Research, 2020, 50(2): 89-94. |
| 78 | VIRGILIO Emanuel M, PADRÓ Cristina L, María E SAD. Ru/ReO x /TiO2 selective and reusable catalyst for C-O hydrogenolysis of C4 polyols[J]. ChemCatChem, 2023, 15: e202201618. |
| 79 | Nobuhiko OTA, TAMURA Masazumi, NAKAGAWA Yoshinao, et al. Hydrodeoxygenation of vicinal OH groups over heterogeneous rhenium catalyst promoted by palladium and ceria support[J]. Angewandte Chemie-International Edition, 2015, 54(6): 1897-1900. |
| 80 | BHOWMIK Susmita, AKULA Venugopal, SETHIA Govind, et al. Promoting effect of titanium on C—O hydrogenolysis of erythritol to 1,4-butanediol over Pt/W/Ti-SBA-15 catalysts[J]. Applied Catalysis A: General, 2023, 666: 119425. |
| 81 | BHOWMIK Susmita, ENJAMURI Nagasuresh, MARIMUTHU Banu, et al. C—O hydrogenolysis of C3—C4 polyols selectively to terminal diols over Pt/W/SBA-15 catalysts[J]. Catalysts, 2022, 12(9) : 1070. |
| 82 | JIN Xin, SHEN Jian, YAN Wenjuan, et al. Sorbitol hydrogenolysis over hybrid Cu/CaO-Al2O3 catalysts: Tunable activity and selectivity with solid base incorporation[J]. ACS Catalysis, 2015, 5(11): 6545-6558. |
| 83 | 刘露杰, 张建, 王亮, 等. 生物质基多元醇的多相催化选择性氢解[J]. 化学学报, 2023, 81(5): 533-547. |
| LIU Lujie, ZHANG Jian, WANG Liang, et al. Heterogeneous catalysts for selective hydrogenolysis of biomass-derived polyols[J]. Acta Chimica Sinica, 2023, 81(5): 533-547. | |
| 84 | HEISIG Carina, GLOTZBACH Christoph, SCHIRRMEISTER Steffen, et al. Selective hydrogenolysis of biomass-derived xylitol to glycols: Reaction network and kinetics[J]. Chemical Engineering & Technology, 2021, 44(4): 761-772. |
| 85 | XIA Qi, JIN Xin, ZHANG Guangyu, et al. Catalytic deoxygenation of xylitol to renewable chemicals: Advances on catalyst design and mechanistic studies[J]. The Chemical Record, 2021, 21(1): 133-148. |
| 86 | LI Shenglin, ZAN Yifan, SUN Yuanyuan, et al. Efficient one-pot hydrogenolysis of biomass-derived xylitol into ethylene glycol and 1,2-propylene glycol over Cu-Ni-ZrO2 catalyst without solid bases[J]. Journal of Energy Chemistry, 2019, 28: 101-106. |
| 87 | ZHANG Liangqing, HUANG Suchang, QIU Jiarong, et al. Selective transformation of biomass-derived substrates to 1,2-butanediol: A comprehensive review and new insights[J]. Industrial Crops and Products, 2023, 202: 116984. |
| 88 | MODAK Arindam, GILL Deepika, SHARMA Komal, et al. Facile hydrogenolysis of sugars to 1,2-glycols by Ru@PPh3/OPPh3 confined large-pore mesoporous silica[J]. The Journal of Physical Chemistry Letters, 2023, 14(48): 10832-10846. |
| 89 | BEINE Anna Katharina, LUDOVICY Jil, CHAI Jiachun, et al. Ru on N-doped carbon for the selective hydrogenolysis of sugars and sugar alcohols[J]. ChemCatChem, 2022, 14(11): 202101908. |
| 90 | Zuzana MAGYAROVÁ, Milan KRÁLIK, Tomš SOTÁK. Utilization of zeolite catalysts in biomass exploitation: A minireview[J]. Monatshefte Für Chemie, 2023, 154(8): 815-835. |
| 91 | SUN Jiying, LIU Haichao. Selective hydrogenolysis of biomass-derived xylitol to ethylene glycol and propylene glycol on supported Ru catalysts[J]. Green Chemistry, 2011, 13(1): 135-142. |
| 92 | Marek GŁÓWKA, KRAWCZYK Tomasz. New trends and perspectives in production of 1,2-propanediol[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(19): 7274-7287. |
| 93 | AUNEAU Florian, BERCHU Maeva, AUBERT Guillaume, et al. Exploring the reaction conditions for Ru/C catalyzed selective hydrogenolysis of xylitol alkaline aqueous solutions to glycols in a trickle-bed reactor[J]. Catalysis Today, 2014, 234: 100-106. |
| 94 | LIU Chengwei, ZHANG Chenghua, LIU Kangkai, et al. Aqueous-phase hydrogenolysis of glucose to value-added chemicals and biofuels: A comparative study of active metals[J]. Biomass and Bioenergy, 2015, 72: 189-199. |
| 95 | BEINE Anna Katharina, KRÜGER Andreas J D, ARTZ Jens, et al. Selective production of glycols from xylitol over Ru on covalent triazine frameworks-suppressing decarbonylation reactions[J]. Green Chemistry, 2018, 20(6): 1316-1322. |
| 96 | Maxime RIVIÈRE, PERRET Noémie, DELCROIX Damien, et al. Solvent effect in hydrogenolysis of xylitol over bifunctional Ru/MnO/C catalysts under alkaline-free conditions[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(3): 4076-4085. |
| 97 | XIA Qi, ZHANG Guangyu, WANG Jinyao, et al. Synergistic bimetallic Pd-Pt/TiO2 catalysts for hydrogenolysis of xylitol with in situ-formed H2 [J]. Industrial & Engineering Chemistry Research, 2020, 59(31): 13879-13891. |
| 98 | HUANG Zhiwei, CHEN Jing, JIA Yuqing, et al. Selective hydrogenolysis of xylitol to ethylene glycol and propylene glycol over copper catalysts[J]. Applied Catalysis B: Environmental, 2014, 147: 377-386. |
| 99 | ZHU Shanhui, GAO Xiaoqing, ZHU Yulei, et al. A highly efficient and robust Cu/SiO2 catalyst prepared by the ammonia evaporation hydrothermal method for glycerol hydrogenolysis to 1,2-propanediol[J]. Catalysis Science & Technology, 2015, 5(2): 1169-1180. |
| 100 | ZHU Shanhui, GAO Xiaoqing, ZHU Yulei, et al. Tailored mesoporous copper/ceria catalysts for the selective hydrogenolysis of biomass-derived glycerol and sugar alcohols[J]. Green Chemistry, 2016, 18(3): 782-791. |
| 101 | Maxime RIVIÈRE, PERRET Noémie, DELCROIX Damien, et al. Ru-(Mn-M)O x solid base catalysts for the upgrading of xylitol to glycols in water[J]. Catalysts, 2018, 8(8): 331. |
| 102 | SUN Jiying, LIU Haichao. Selective hydrogenolysis of biomass-derived xylitol to ethylene glycol and propylene glycol on Ni/C and basic oxide-promoted Ni/C catalysts[J]. Catalysis Today, 2014, 234: 75-82. |
| 103 | ZHOU Zhiwei, DAI Songshan, QIN Juan, et al. Preparation of nano-Ni/meso-Ce-TiO2 by one-step in a sol-gel system and its catalytic performance for hydrogenolysis of xylitol[J]. RSC Advances, 2015, 5(86): 70410-70416. |
| 104 | Tomáš SOTÁK, SCHMIDT Tomáš, HRONEC Milan. Hydrogenolysis of polyalcohols in the presence of metal phosphide catalysts[J]. Applied Catalysis A: General, 2013, 459: 26-33. |
| 105 | LIU Hailong, HUANG Zhiwei, KANG Haixiao, et al. Efficient bimetallic NiCu-SiO2 catalysts for selective hydrogenolysis of xylitol to ethylene glycol and propylene glycol[J]. Applied Catalysis B: Environmental, 2018, 220: 251-263. |
| 106 | ZHU Peng, HAN Chunyang, XIA Haian. High-efficient hydrogenolysis of xylose to polyols over Ni-W/CeO2 catalysts[J]. Catalysis Letters, 2024,154(3): 1219-1231. |
| 107 | JIA Yuqing, SUN Qianhui, LIU Haichao. Selective hydrogenolysis of biomass-derived sorbitol to propylene glycol and ethylene glycol on in-situ formed PdZn alloy catalysts[J]. Applied Catalysis A: General, 2020, 603: 117770. |
| 108 | CAI Chiliu, WANG Haiyong, XIN Haosheng, et al. Hydrogenolysis of biomass-derived sorbitol over La-promoted Ni/ZrO2 catalysts[J]. RSC Advances, 2020, 10(7): 3993-4001. |
| 109 | KANDASAMY Thirunavukkarasu, BANU Marimuthu, VIJAYA SHANTHI R, et al. Suitability of different supported Ru, Pt and Ni catalysts for the hydrogenolysis of sorbitol[J]. Results in Engineering, 2022, 15: 100594. |
| 110 | JIA Yuqing, LIU Haichao. Mechanistic insight into the selective hydrogenolysis of sorbitol to propylene glycol and ethylene glycol on supported Ru catalysts[J]. Catalysis Science & Technology, 2016, 6(19): 7042-7052. |
| 111 | XU Chunping, PAONE Emilia, Daily RODRÍGUEZ-PADRÓN, et al. Reductive catalytic routes towards sustainable production of hydrogen, fuels and chemicals from biomass derived polyols[J]. Renewable and Sustainable Energy Reviews, 2020, 127: 109852. |
| 112 | ZHANG Jun, LI Jibiao, WU Shubin, et al. Advances in the catalytic production and utilization of sorbitol[J]. Industrial & Engineering Chemistry Research, 2013, 52(34): 11799-11815. |
| 113 | DU Weichen, ZHENG Liping, SHI Juanjuan, et al. Production of C2 and C3 polyols from d-sorbitol over hydrotalcite-like compounds mediated bi-functional Ni-Mg-Al-O x catalysts[J]. Fuel Processing Technology, 2015, 139: 86-90. |
| 114 | ZHAO L, ZHOU J H, SUI Z J, et al. Hydrogenolysis of sorbitol to glycols over carbon nanofiber supported ruthenium catalyst[J]. Chemical Engineering Science, 2010, 65(1): 30-35. |
| 115 | ZHOU Jinghong, LIU Guocai, SUI Zhijun, et al. Hydrogenolysis of sorbitol to glycols over carbon nanofibers-supported ruthenium catalyst: The role of base promoter[J]. Chinese Journal of Catalysis, 2014, 35(5): 692-702. |
| 116 | 董慧焕, 郭星翠, 秦张峰, 等. 官能化碳纳米管负载Ru催化山梨醇氢解制备低碳二元醇[J]. 燃料化学学报, 2015, 43(12): 1454-1460. |
| DONG Huihuan, GUO Xingcui, QIN Zhangfeng, et al. Effect of modified groups of carbon nanotubes on catalytic properties of Ru/CNTs catalysts for hydrogenolysis of sorbitol[J]. Journal of Fuel Chemistry and Technology, 2015, 43(12): 1454-1460. | |
| 117 | GUO Xingcui, DONG Huihuan, LI Bin, et al. Influence of the functional groups of multiwalled carbon nanotubes on performance of Ru catalysts in sorbitol hydrogenolysis to glycols[J]. Journal of Molecular Catalysis A: Chemical, 2017, 426: 79-87. |
| 118 | GUO Xingcui, GUAN Jing, LI Bin, et al. Conversion of biomass-derived sorbitol to glycols over carbon-materials supported Ru-based catalysts[J]. Scientific Reports, 2015, 5: 16451. |
| 119 | JIN Xin, THAPA Prem S, SUBRAMANIAM Bala, et al. Kinetic modeling of sorbitol hydrogenolysis over bimetallic RuRe/C catalyst[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(11): 6037-6047. |
| 120 | MURILLO LEO I, LÓPEZ GRANADOS M, FIERRO J L G, et al. Selective conversion of sorbitol to glycols and stability of nickel-ruthenium supported on calcium hydroxide catalysts[J]. Applied Catalysis B: Environmental, 2016, 185: 141-149. |
| 121 | LEO Inmaculada Murillo, GRANADOS Manuel Lopez, FIERRO Jose Luis Garcia, et al. Sorbitol hydrogenolysis to glycols by supported ruthenium catalysts[J]. Chinese Journal of Catalysis, 2014, 35(5): 614-621. |
| 122 | TRONCI Stefania, PITTAU Barbara. Conversion of glucose and sorbitol in the presence of Ru/C and Pt/C catalysts[J]. RSC Advances, 2015, 5(29): 23086-23093. |
| 123 | GUMINA Bianca, MAURIELLO Francesco, PIETROPAOLO Rosario, et al. Hydrogenolysis of sorbitol into valuable C3-C2 alcohols at low H2 pressure promoted by the heterogeneous Pd/Fe3O4 catalyst[J]. Molecular Catalysis, 2018, 446: 152-160. |
| 124 | YIN Bin, JIN Xin, ZHANG Guangyu, et al. Catalytic transfer hydrogenolysis of bio-polyols to renewable chemicals over bimetallic PtPd/C catalysts: Size-dependent activity and selectivity[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(13): 5305-5316. |
| 125 | WANG Xicheng, LIU Xiaoran, XU Yue, et al. Sorbitol hydrogenolysis to glycerol and glycols over M-MgO (M=Ni, Co, Cu) nanocomposite: A comparative study of active metals[J]. Chinese Journal of Catalysis, 2015, 36(9): 1614-1622. |
| 126 | BANU M, SIVASANKER S, SANKARANARAYANAN T M, et al. Hydrogenolysis of sorbitol over Ni and Pt loaded on NaY[J]. Catalysis Communications, 2011, 12(7): 673-677. |
| 127 | YE Linmin, DUAN Xinping, LIN Haiqiang, et al. Improved performance of magnetically recoverable Ce-promoted Ni/Al2O3 catalysts for aqueous-phase hydrogenolysis of sorbitol to glycols[J]. Catalysis Today, 2012, 183(1): 65-71. |
| 128 | ZHOU Zhiwei, ZHANG Jiaqi, QIN Juan, et al. Ordered mesoporous NiCeAl containing catalysts for hydrogenolysis of sorbitol to glycols[J]. Russian Journal of Physical Chemistry A, 2018, 92(3): 456-465. |
| 129 | VIJAYA SHANTHI R, MAHALAKSHMY R, THIRUNAVUKKARASU K, et al. Hydrogenolysis of sorbitol over Ni supported on Ca- and Ca(Sr)-hydroxyapatites[J]. Molecular Catalysis, 2018, 451: 170-177. |
| 130 | DU Weichen, ZHENG Liping, LI Xuewen, et al. Plate-like Ni-Mg-Al layered double hydroxide synthesized via a solvent-free approach and its application in hydrogenolysis of D-sorbitol[J]. Applied Clay Science, 2016, 123: 166-172. |
| 131 | VIJAYA SHANTHI R, SANKARANARAYANAN T M, MAHALAKSHMY R, et al. Fly ash based Ni catalyst for conversion of sorbitol into glycols[J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 1752-1757. |
| 132 | JIA Yuqing, LIU Haichao. Selective hydrogenolysis of sorbitol to ethylene glycol and propylene glycol on ZrO2-supported bimetallic Pd-Cu catalysts[J]. Chinese Journal of Catalysis, 2015, 36(9): 1552-1559. |
| 133 | WANG Xinde, BEINE Anna Katharina, HAUSOUL Peter J C, et al. Cu/C‐catalyzed hydrogenolysis of sorbitol to glycols-on the influence of particle size and base[J]. ChemCatChem, 2019, 11(16): 4123-4129. |
| 134 | NAKAGAWA Yoshinao, TAMURA Masazumi, TOMISHIGE Keiichi. Catalytic conversions of furfural to pentanediols[J]. Catalysis Surveys from Asia, 2015, 19(4): 249-256. |
| 135 | LIU Sibao, OKUYAMA Yasuyo, TAMURA Masazumi, et al. Selective transformation of hemicellulose (xylan) into n-pentane, pentanols or xylitol over a rhenium-modified iridium catalyst combined with acids[J]. Green Chemistry, 2016, 18(1): 165-175. |
| 136 | YUAN Zhengqiu, DAI Wei, ZHANG Shenghong, et al. Heterogeneous strategies for selective conversion of lignocellulosic polysaccharides[J]. Cellulose, 2022, 29(6): 3059-3077. |
| 137 | YAMAGUCHI Kosuke, NAKAGAWA Yoshinao, LI Congcong, et al. Utilization of Ni as a non-noble-metal Co-catalyst for ceria-supported rhenium oxide in combination of deoxydehydration and hydrogenation of vicinal diols[J]. ACS Catalysis, 2022, 12(20): 12582-12595. |
| 138 | SAAVEDRA Johnny, DOAN Hieu A, PURSELL Christopher J, et al. The critical role of water at the gold-titania interface in catalytic CO oxidation[J]. Science, 2014, 345(6204): 1599-1602. |
| 139 | PIRES Mattheus H M, PASSOS Fabio B, XING Yutao. Hydrogenolysis of glycerol over ZSM-5 supported ruthenium and copper catalysts: Structural study and effects in reaction[J]. Catalysis Today, 2023, 419: 114161. |
| 140 | FAN Yiqiu, CHENG Shijie, WANG Hao, et al. Pt-WO x on monoclinic or tetrahedral ZrO2: Crystal phase effect of zirconia on glycerol hydrogenolysis to 1,3-propanediol[J]. Applied Catalysis B: Environmental, 2017, 217: 331-341. |
| 141 | WANG Zhihui, ZHANG Wei, LI Cuiqing, et al. Recent progress of hydrogenation and hydrogenolysis catalysts derived from layered double hydroxides[J]. Catalysts, 2022, 12(11) : 1484. |
| 142 | AUBRECHT Jaroslav, POSPELOVA Violetta, KIKHTYANIN Oleg, et al. Do metal-oxide promoters of Cu hydrogenolysis catalysts affect the Cu intrinsic activity?[J]. Applied Catalysis A: General, 2020, 608: 117889. |
| [1] | 李琢宇, 余美琪, 陈孝彦, 胡若晖, 王庆宏, 陈春茂, 詹亚力. 炼油废催化剂吸附去除水中硝基苯的特性与机制[J]. 化工进展, 2025, 44(2): 1076-1087. |
| [2] | 祁帅杰, 黄亚继, 徐鹏程, 齐景伟, 李志远, 时浩, 赵佳琪, 高嘉炜, 刘俊, 张煜尧. 废弃木质建筑模板与典型生物质热解产物分布及特性对比[J]. 化工进展, 2025, 44(2): 1120-1128. |
| [3] | 刘法志, 张鹏威, 刘涛, 谢玉仙, 何建乐, 苏胜, 徐俊, 向军. Sb改性钒钛SCR脱硝催化剂抗CO中毒性能[J]. 化工进展, 2025, 44(2): 1129-1137. |
| [4] | 洪思琦, 顾方伟, 郑金玉. PEM水电解制氢低铱催化剂发展现状及展望[J]. 化工进展, 2025, 44(1): 158-168. |
| [5] | 李雪莲, 曹志会, 雷普瑛, 白冰, 王璇, 张金鑫, 侯凯, 刘爱芳, 齐凯, 高丽丽. 珊瑚状Mo2C/Mo3P@NC异质结电极高效催化Li-CO2电池[J]. 化工进展, 2025, 44(1): 202-211. |
| [6] | 秦婷婷, 牛强. 二氧化碳加氢制高级醇Fe基催化剂研究进展[J]. 化工进展, 2025, 44(1): 253-265. |
| [7] | 庄柯, 陈宏, 许芸, 仲兆平, 周峻伍, 周凯, 董月红. SiO2改性Ce-V-W/Ti催化剂载体的抗碱(土)金属中毒性能[J]. 化工进展, 2025, 44(1): 266-276. |
| [8] | 董家彤, 单梦晴, 王华. Au-CuO/Cu2O串联催化增强电催化CO2还原制乙醇[J]. 化工进展, 2025, 44(1): 277-285. |
| [9] | 游小银, 汪楚乔, 刘才华, 彭小明. Z型CN/NGBO/BV催化剂体系的构筑及光类芬顿降解四环素性能[J]. 化工进展, 2025, 44(1): 286-296. |
| [10] | 李佳优, 张雨涵, 姜楠, 蒋博龙. 过渡金属硫化物NiS(x)@NF催化剂水热法制备及其析氢性能[J]. 化工进展, 2025, 44(1): 297-304. |
| [11] | 王宁, 陆诗建, 刘玲, 梁静, 刘苗苗, 孙梦圆, 康国俊. 胺吸收体系中CO2催化解吸再生技术的研究进展[J]. 化工进展, 2025, 44(1): 445-464. |
| [12] | 倪鹏, 王先泓, 黄钰涵, 马晓彤, 马子轸, 谈琰, 张华伟, 刘亭. 活性炭类和磁性金属类吸附剂喷射脱汞技术应用对比及最新进展[J]. 化工进展, 2025, 44(1): 513-524. |
| [13] | 李新月, 李振京, 韩沂杭, 郭永强, 闫瑜, 哈力米热·卡热木拉提, 赵会吉, 柴永明, 刘东, 殷长龙. 油脂加氢脱氧生产绿色柴油催化剂的研究进展[J]. 化工进展, 2024, 43(S1): 351-364. |
| [14] | 李帅哲, 聂懿宸, PHIDSAVARD Keomeesay, 顾雯, 张伟, 刘娜, 徐高翔, 刘莹, 李兴勇, 陈玉保. 非贵金属催化生物质加氢脱氧制备烃基生物燃料的研究进展[J]. 化工进展, 2024, 43(S1): 225-242. |
| [15] | 熊磊, 丁飞燕, 李聪, 王群乐, 吕起, 翟晓娜, 刘峰. 金属Pt负载型非均相催化剂研究进展[J]. 化工进展, 2024, 43(S1): 295-304. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||