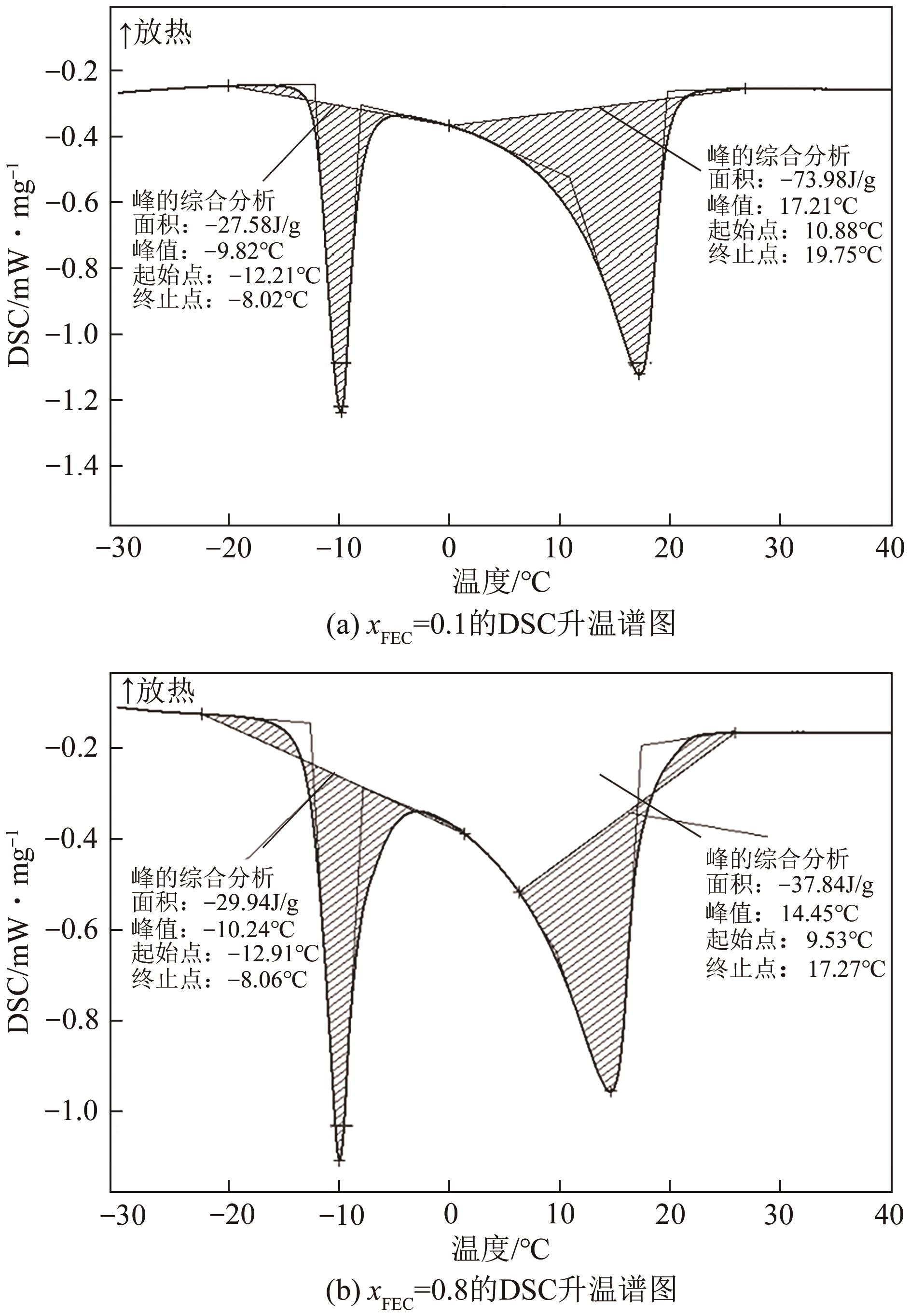
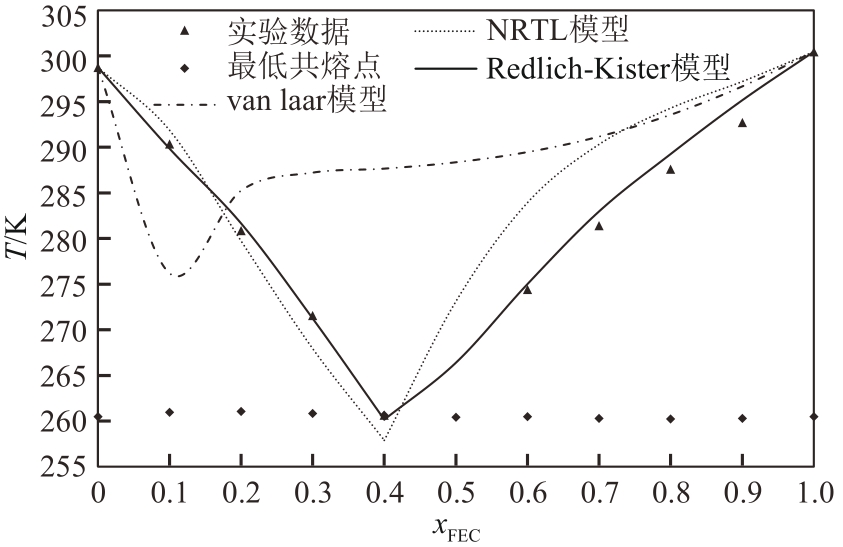
| 1 |
许杰, 姚万浩, 姚宜稳, 等. 添加剂氟代碳酸乙烯酯对锂离子电池性能的影响[J]. 物理化学学报, 2009, 25(2): 201-206.
|
|
XU Jie, YAO Wanhao, YAO Yiwen, et al. Effect of fluoroethylene carbonate additive on the performance of lithium ion battery[J]. Acta Physico-Chimica Sinica, 2009, 25(2): 201-206.
|
| 2 |
姚桂. 氟代碳酸乙烯酯的合成[D]. 湘潭: 湘潭大学, 2012.
|
|
YAO Gui. Synthesis of fluoroethylene carbonate[D]. Xiangtan: Xiangtan University, 2012.
|
| 3 |
沈锦良, 张先林, 杨志勇, 等. 氟代碳酸乙烯酯的除酸除水方法: CN101717389A[P]. 2010-06-02.
|
|
SHEN Jinliang, ZHANG Xianlin, YANG Zhiyong, et al. Disacidifying and dewatering method of fluoroethylene carbonate: CN101717389A[P]. 2010-06-02.
|
| 4 |
吴茂祥, 方桂煌, 潘荧, 等. 一种高纯度氟代碳酸乙烯酯的精制方法: CN101870652A[P]. 2010-10-27.
|
|
WU Maoxiang, FANG Guihuang, PAN Ying, et al. Refining method of fluoro ethylene carbonate with high purity: CN101870652A[P]. 2010-10-27.
|
| 5 |
吴巍, 刘红光, 叶学海, 等. 一种氟代碳酸乙烯酯粗产品的连续提纯方法: CN102887883A[P]. 2013-01-23.
|
|
WU Wei, LIU Hongguang, YE Xuehai, et al. Continuous purifying method of crude product of fluoroethylene carbonate: CN102887883A[P]. 2013-01-23.
|
| 6 |
邵俊华, 孔东波, 张利娟, 等. 高纯度氟代碳酸乙烯酯的提纯方法: CN105801554A[P]. 2016-07-27.
|
|
SHAO Junhua, KONG Dongbo, ZHANG Lijuan, et al. Purification method of high-purity fluoroethylene carbonate: CN105801554A[P]. 2016-07-27.
|
| 7 |
PERRY R H, GREEN D W. Perry’s chemical engineer’ handsbook[M]. 5th ed. New York: McGraw-Hill Book Company, 1997: 18-35.
|
| 8 |
CONG Shan, LIU Ying, LI Hong, et al. Purification and separation of durene by static melt crystallization[J]. Chinese Journal of Chemical Engineering, 2015, 23(3): 505-509.
|
| 9 |
顾鸣海. 用熔融结晶法分离有机物[J]. 上海化工, 2003, 28(12): 26-29.
|
|
GU Minghai. Refining organic compounds with melt crystallization[J]. Shanghai Chemical Industry, 2003, 28(12): 26-29.
|
| 10 |
BEIERLING Thorsten, OSIANDER Jan, SADOWSKI Gabriele. Melt crystallization of isomeric long-chain aldehydes from hydroformylation[J]. Separation and Purification Technology, 2013, 118: 13-24.
|
| 11 |
舒伟锋, 贾雪枫, 邱敏芳, 等. 一种分级结晶制备电子级氟代碳酸乙烯酯的方法: CN110878078A[P]. 2020-03-13.
|
|
SHU Weifeng, JIA Xuefeng, QIU Minfang, et al. Method for preparing electronic-grade fluoroethylene carbonate through fractional crystallization: CN110878078A[P]. 2020-03-13.
|
| 12 |
王圣贤, 朱振涛, 贾国文, 等. 一种氟代碳酸乙烯酯的纯化方法及其所得产品: CN113563300A[P]. 2021-10-29.
|
|
WANG Shengxian, ZHU Zhentao, JIA Guowen, et al. Purification method of fluoroethylene carbonate and a product obtained by the purification method: CN113563300A[P]. 2021-10-29.
|
| 13 |
祁长青, 董茂, 张啸峰, 等. 生产氟代碳酸乙烯酯的熔融结晶系统: CN217828946U[P]. 2022-11-18.
|
|
QI Changqing, DONG Mao, ZHANG Xiaofeng, et al. Melt crystallization system for producing fluoroethylene carbonate: CN217828946U[P]. 2022-11-18.
|
| 14 |
李智勇. 6-叔丁基间甲酚熔融结晶过程研究[D]. 天津: 天津大学, 2012.
|
|
LI Zhiyong. A study on the melt crystallization process of 6-tert-buty-3-methylphenol[D]. Tianjin: Tianjin University, 2012.
|
| 15 |
胡琳珊. 碳酸亚乙烯酯熔融结晶工艺研究[D]. 天津: 天津科技大学, 2015.
|
|
HU Linshan. Study on the melt crystallization technology of vinylene carbonate[D]. Tianjin: Tianjin University of Science and Technology, 2015.
|
| 16 |
沈澍. 熔融结晶法纯化对二甲苯研究[D]. 天津: 天津大学, 2017.
|
|
SHEN Shu. Study on the purification of para-xylene by melt crystallization[D]. Tianjin: Tianjin University, 2017.
|
| 17 |
金克新, 陈勇强, 谢朝钢. 硝基氯苯体系固液平衡研究[J]. 化学工业与工程, 1993, 10(2): 1-8, 15.
|
|
JIN Kexin, CHEN Yongqiang, XIE Chaogang. The study of solid-liquid equilibrium for nitrochlorobenzene system[J]. Chemical Industry and Engineering, 1993, 10(2): 1-8, 15.
|
| 18 |
刘振海. 热分析导论[M]. 北京: 化学工业出版社, 1991: 210-220.
|
|
LIU Zhenhai. Introduction to thermal analysis[M]. Beijing: Chemical Industry Press, 1991: 210-220.
|
| 19 |
GMEHLING Jürgen G, ANDERSON Thomas F, PRAUSNITZ John M. Solid-liquid equilibria using UNIFAC[J]. Industrial and Engineering Chemical Fundamentals, 1978, 17(4): 269-273.
|
| 20 |
朱自强, 吴有庭. 化工热力学[M]. 3版. 北京: 化学工业出版社, 2010.
|
|
ZHU Ziqiang, WU Youting. Chemical engineering thermodynamics[M]. 3rd ed. Beijing: Chemical Industry Press, 2010.
|
| 21 |
卢咏琰. TDI熔融结晶过程研究[D]. 天津: 天津大学, 2006.
|
|
LU Yongyan. A study on the melt crystallization process of TDI[D]. Tianjin: Tianjin University, 2006.
|
 ), 艾波1, 吴高胜1, 李瑜哲1, 宗睿1, 许保云1(
), 艾波1, 吴高胜1, 李瑜哲1, 宗睿1, 许保云1( ), 杜丽君2
), 杜丽君2
 ), AI Bo1, WU Gaosheng1, LI Yuzhe1, ZONG Rui1, XU Baoyun1(
), AI Bo1, WU Gaosheng1, LI Yuzhe1, ZONG Rui1, XU Baoyun1( ), DU Lijun2
), DU Lijun2