化工进展 ›› 2024, Vol. 43 ›› Issue (1): 87-99.DOI: 10.16085/j.issn.1000-6613.2023-1146
溶液结晶过程强化
冯瑶光1( ), 陈奎1, 赵佳伟1, 王娜1, 王霆1, 黄欣1, 周丽娜1, 郝红勋1,2(
), 陈奎1, 赵佳伟1, 王娜1, 王霆1, 黄欣1, 周丽娜1, 郝红勋1,2( )
)
- 1.天津大学化工学院,国家工业结晶工程技术研究中心 天津 300072
2.天津化学化工协同创新中心,天津 300072
-
收稿日期:2023-07-09修回日期:2023-08-17出版日期:2024-01-20发布日期:2024-02-05 -
通讯作者:郝红勋 -
作者简介:冯瑶光(1997—),男,博士研究生,研究方向为工业结晶。E-mail:2019207028@tju.edu.cn。 -
基金资助:国家自然科学基金(21978201)
Process intensification of solution crystallization
FENG Yaoguang1( ), CHEN Kui1, ZHAO Jiawei1, WANG Na1, WANG Ting1, HUANG Xin1, ZHOU Lina1, HAO Hongxun1,2(
), CHEN Kui1, ZHAO Jiawei1, WANG Na1, WANG Ting1, HUANG Xin1, ZHOU Lina1, HAO Hongxun1,2( )
)
- 1.National Engineering Research Center of Industrial Crystallization Technology, School of Chemical Engineering and Technology, Tianjin University, Tianjin, China
2.Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin, 300072, China
-
Received:2023-07-09Revised:2023-08-17Online:2024-01-20Published:2024-02-05 -
Contact:HAO Hongxun
摘要:
溶液结晶是化学工业中最重要的产品分离、纯化和功能化技术之一,广泛应用于医药、食品、精细化工等领域。溶液结晶中晶体的成核和生长过程将决定最终晶体产品的晶型、晶习、粒度、纯度等关键质量指标。因此,对溶液结晶过程,尤其是晶体成核和生长过程进行强化既有利于提高过程效率,也有助于满足晶体产品不同的性能需求。本文围绕晶体成核和生长强化这一关键问题,从受限空间、物理场、添加剂和模板剂等方面系统综述了溶液结晶中的过程强化策略。探讨了各种过程强化策略的优点和局限性,并总结了溶液结晶过程强化策略的主要研究重点和发展前景。
中图分类号:
引用本文
冯瑶光, 陈奎, 赵佳伟, 王娜, 王霆, 黄欣, 周丽娜, 郝红勋. 溶液结晶过程强化[J]. 化工进展, 2024, 43(1): 87-99.
FENG Yaoguang, CHEN Kui, ZHAO Jiawei, WANG Na, WANG Ting, HUANG Xin, ZHOU Lina, HAO Hongxun. Process intensification of solution crystallization[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 87-99.
| 溶液结晶物系 | 存在问题 | 强化方法 | 强化效果 | 文献来源 |
|---|---|---|---|---|
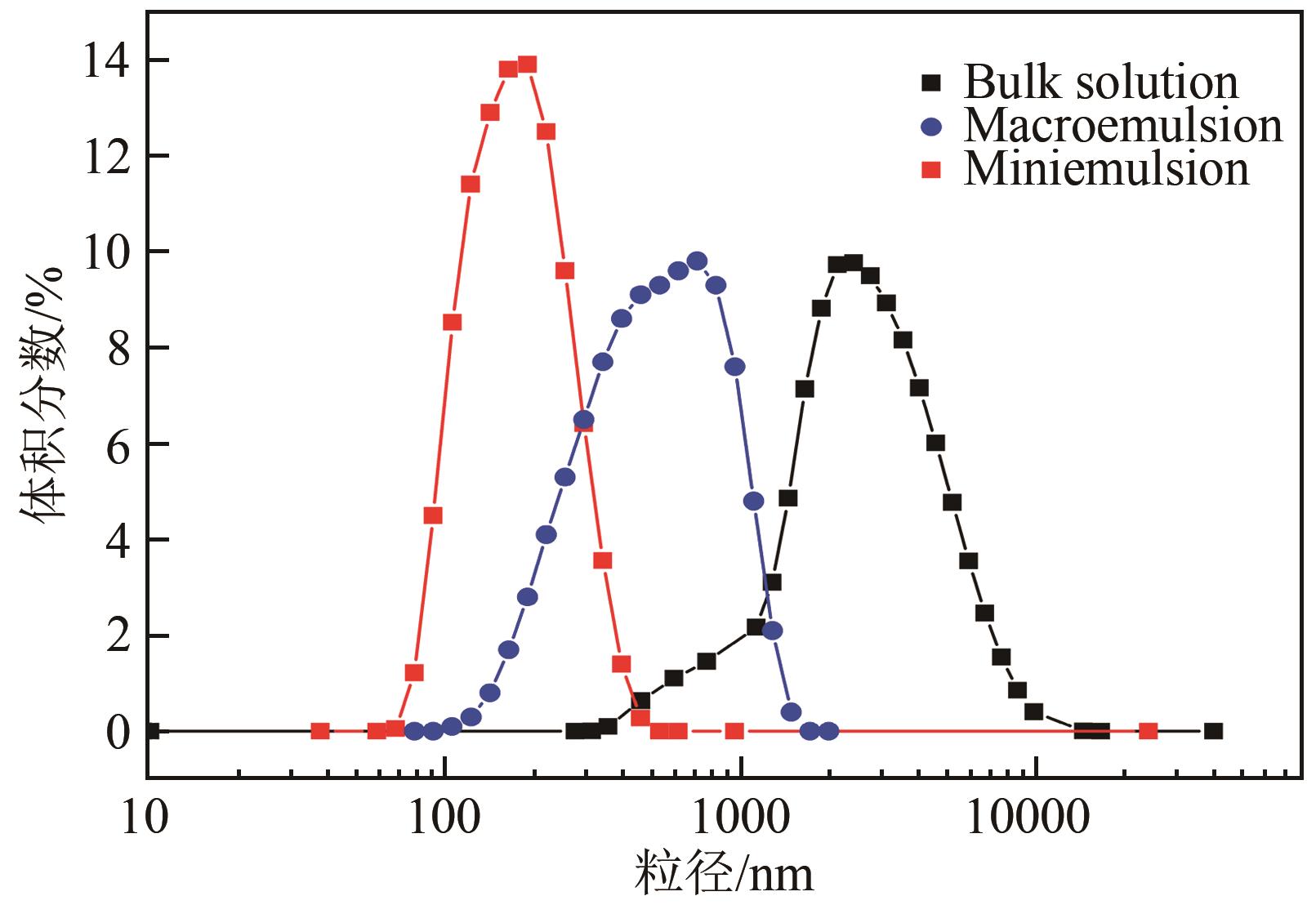
| 甘氨酸 | 传统结晶的时间长达数小时 | 微乳液 | 结晶时间缩短至几分钟,实现对形状、尺寸和尺寸分布的控制 | [ |
| 颜料红146 | 传统结晶的晶体尺寸较大 | 微乳液 | 粒径小一个数量级且更均匀,满足喷墨印刷要求 | [ |
| 度鲁特韦钠 | 管式结晶的晶体尺寸过大和晶体聚集导致堵塞 | 微流体耦合超声 | 与市售产品相比,中值粒径和粒径分布分别减少了30%和60% | [ |
| 溶菌酶 | 晶体成核和生长速率较慢 | 微流体耦合超声 | 成核速率增加三倍,晶体尺寸更均匀 | [ |
表1 部分溶液结晶的限域空间强化策略及强化效果
| 溶液结晶物系 | 存在问题 | 强化方法 | 强化效果 | 文献来源 |
|---|---|---|---|---|
| 甘氨酸 | 传统结晶的时间长达数小时 | 微乳液 | 结晶时间缩短至几分钟,实现对形状、尺寸和尺寸分布的控制 | [ |
| 颜料红146 | 传统结晶的晶体尺寸较大 | 微乳液 | 粒径小一个数量级且更均匀,满足喷墨印刷要求 | [ |
| 度鲁特韦钠 | 管式结晶的晶体尺寸过大和晶体聚集导致堵塞 | 微流体耦合超声 | 与市售产品相比,中值粒径和粒径分布分别减少了30%和60% | [ |
| 溶菌酶 | 晶体成核和生长速率较慢 | 微流体耦合超声 | 成核速率增加三倍,晶体尺寸更均匀 | [ |
| 溶液结晶物系 | 存在问题 | 强化方法 | 强化效果 | 文献来源 |
|---|---|---|---|---|
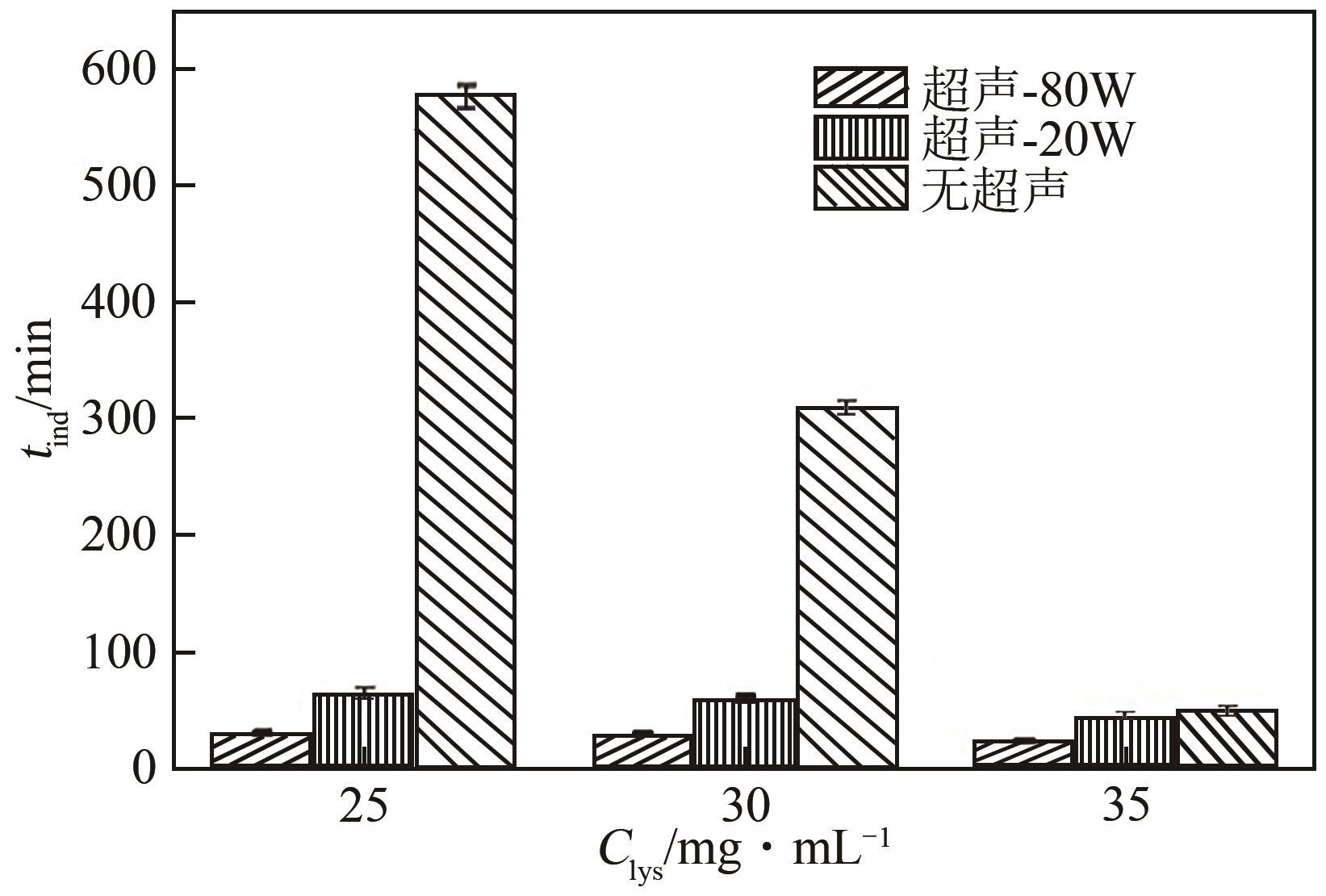
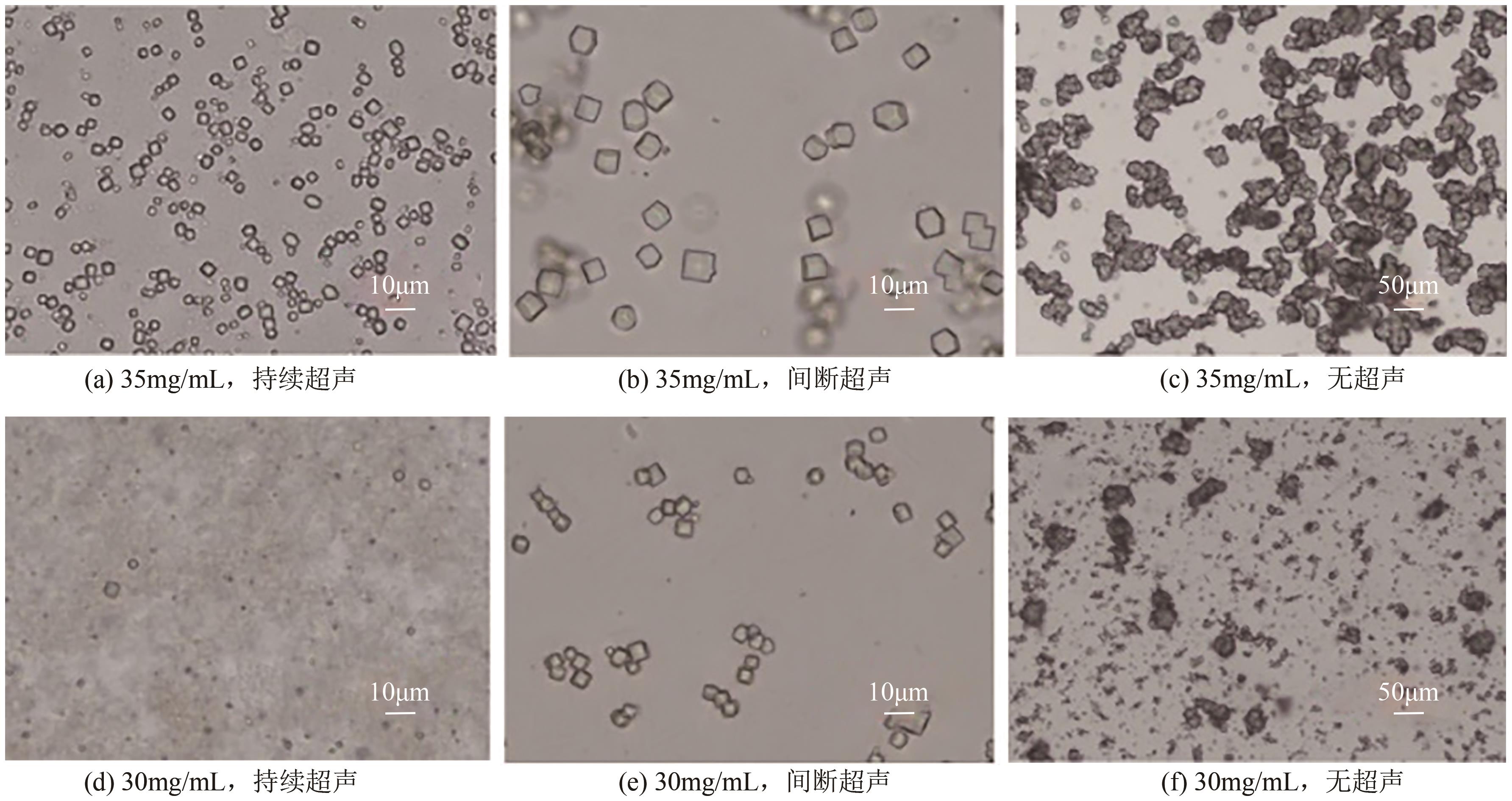
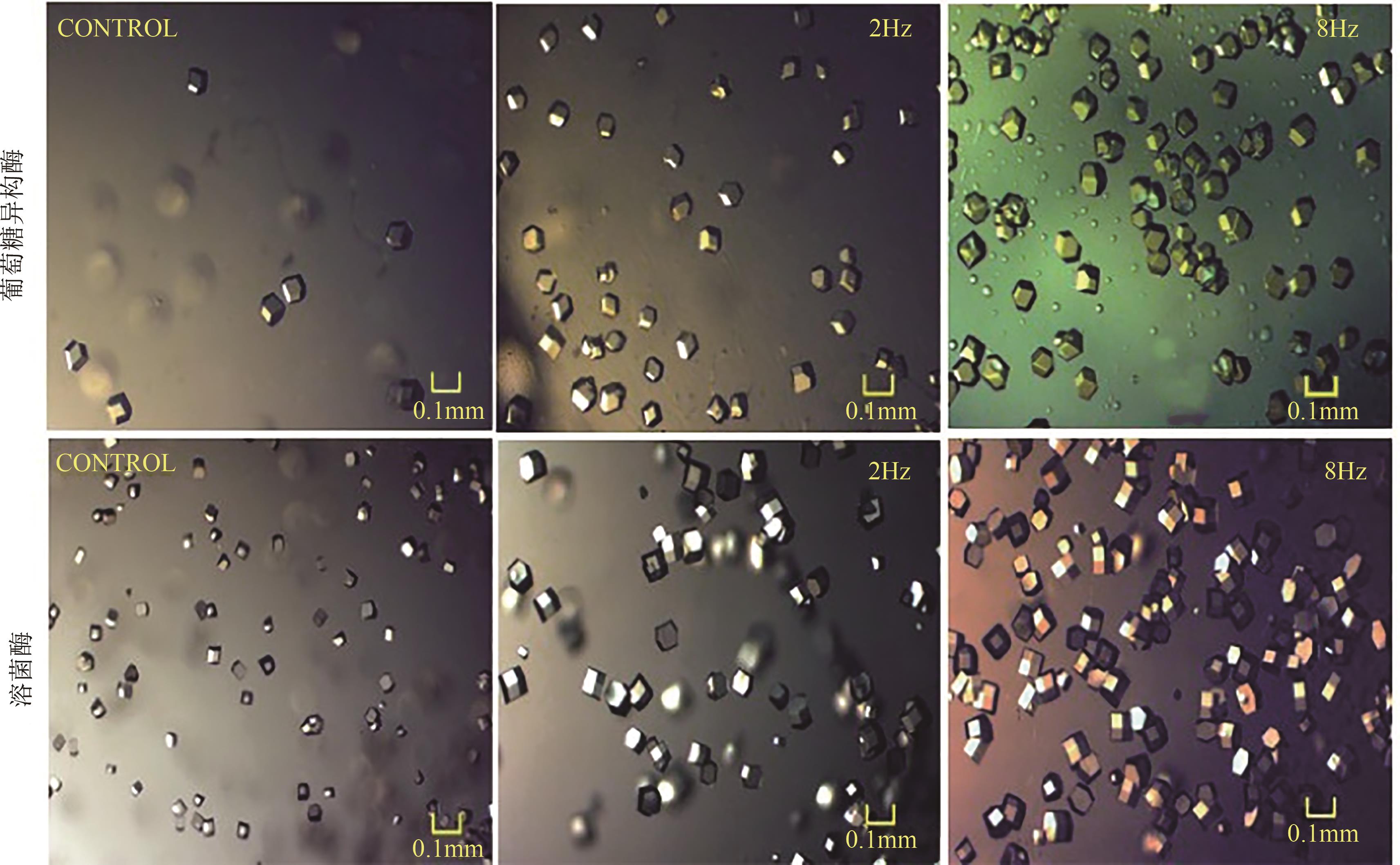
| 溶菌酶 | 晶体成核和生长速率较慢,传统结晶的 晶体粒径分布较宽 | 超声场 | 在较低浓度下的成核诱导期缩短到1/10,晶体粒径分布更窄,形貌更均匀 | [ |
| 氨苄西林 | 传统结晶形成针状晶体,导致压片困难 | 超声场 | 形成片状晶体,成核诱导时间缩短到1/8,产率提高约4.75倍 | [ |
| 溶菌酶 | 晶体成核和生长速率较慢 | 电场 | 成核速率增加,结晶产率提高约2.6倍 | [ |
| 溶菌酶 | 晶体成核和生长速率较慢 | 激光 | 结晶时间缩短到1/20 | [ |
表2 部分溶液结晶的物理场强化策略及强化效果
| 溶液结晶物系 | 存在问题 | 强化方法 | 强化效果 | 文献来源 |
|---|---|---|---|---|
| 溶菌酶 | 晶体成核和生长速率较慢,传统结晶的 晶体粒径分布较宽 | 超声场 | 在较低浓度下的成核诱导期缩短到1/10,晶体粒径分布更窄,形貌更均匀 | [ |
| 氨苄西林 | 传统结晶形成针状晶体,导致压片困难 | 超声场 | 形成片状晶体,成核诱导时间缩短到1/8,产率提高约4.75倍 | [ |
| 溶菌酶 | 晶体成核和生长速率较慢 | 电场 | 成核速率增加,结晶产率提高约2.6倍 | [ |
| 溶菌酶 | 晶体成核和生长速率较慢 | 激光 | 结晶时间缩短到1/20 | [ |

图11 有、无DNA添加下溶菌酶结晶的晶体图像[98](a)不加小牛DNA;(b)加入1.0mg/mL小牛DNA;(c)加入5.0mg/mL小牛DNA;(d)不加鲑鱼DNA;(e)加入10mg/mL鲑鱼DNA;(f)加入20mg/mL小牛DNA;(g)不加鲱鱼DNA;(h)加入10mg/mL鲱鱼DNA;(i)加入20mg/mL鲱鱼DNA
| 溶液结晶物系 | 存在问题 | 添加剂 | 强化效果 | 文献来源 |
|---|---|---|---|---|
| 胰岛素 | 晶体成核和生长缓慢,结晶控制困难 | 氨基酸 | 晶体成核概率提高4~7倍 | [ |
| 溶菌酶 | 晶体成核和生长速率较慢 | 离子液体 | 成核速率提高1~3倍 | [ |
| 溶菌酶 | 晶体成核和生长速率较慢 | 动物DNA | 晶体成核概率提高2.8~33倍 | [ |
表3 部分溶液结晶的添加剂强化策略及强化效果
| 溶液结晶物系 | 存在问题 | 添加剂 | 强化效果 | 文献来源 |
|---|---|---|---|---|
| 胰岛素 | 晶体成核和生长缓慢,结晶控制困难 | 氨基酸 | 晶体成核概率提高4~7倍 | [ |
| 溶菌酶 | 晶体成核和生长速率较慢 | 离子液体 | 成核速率提高1~3倍 | [ |
| 溶菌酶 | 晶体成核和生长速率较慢 | 动物DNA | 晶体成核概率提高2.8~33倍 | [ |
| 溶液结晶物系 | 存在问题 | 模板剂 | 强化效果 | 文献来源 |
|---|---|---|---|---|
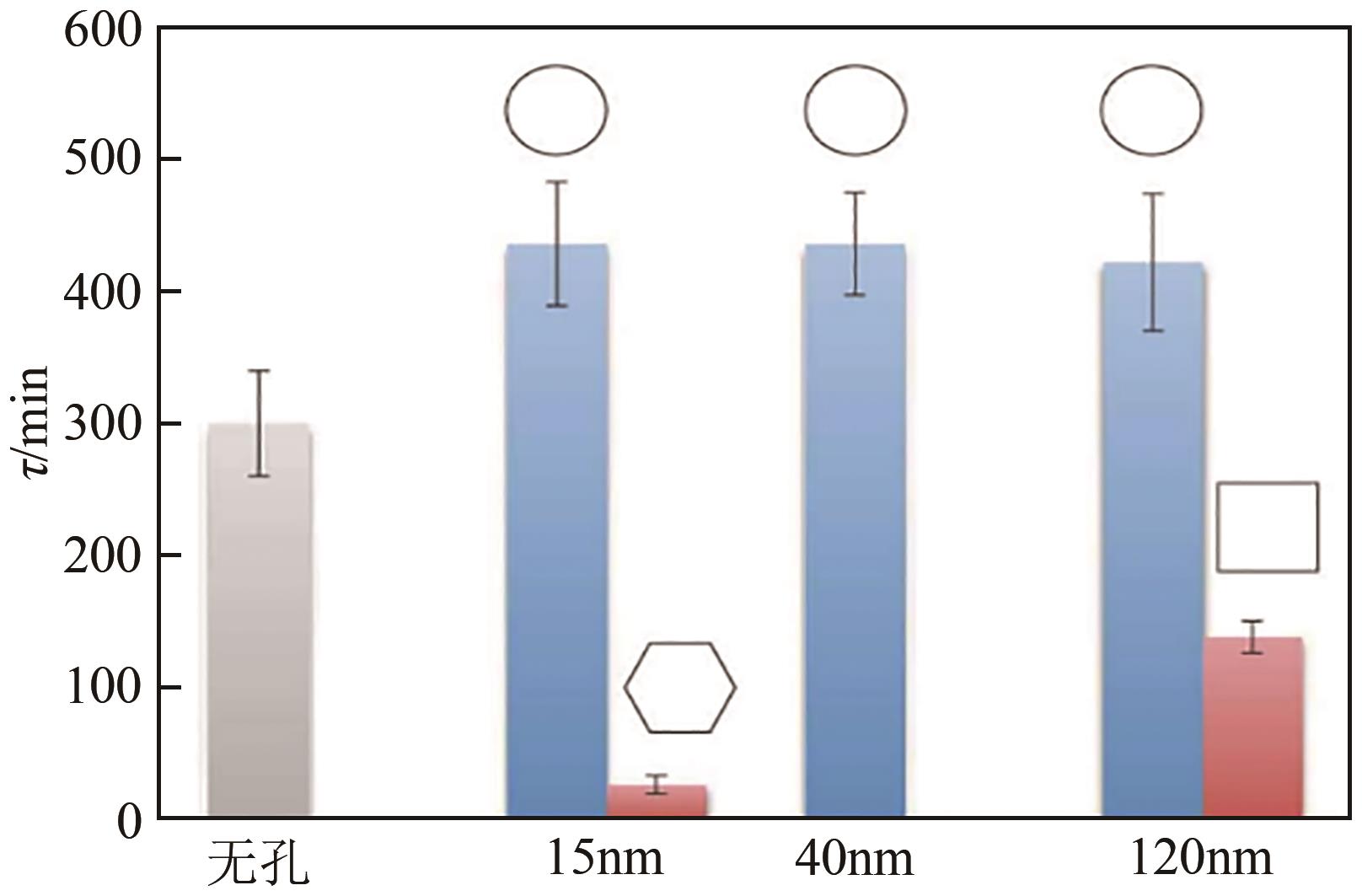
| 非诺贝特 | 结晶诱导期较长 | 甘露醇、二氧化硅、微晶纤维素、羧甲基纤维素、乳糖和聚己内酯 | 最佳条件下结晶诱导期从22h缩短至15min | [ |
| 非诺贝特 | 结晶诱导期较长 | 微晶纤维素 | 结晶诱导期从460min缩短至38min | [ |
| 香兰素 | 结晶诱导期较长 | 功能化二氧化硅 | 诱导期从4h缩短到20min | [ |
表4 部分溶液结晶的模板剂强化策略及强化效果
| 溶液结晶物系 | 存在问题 | 模板剂 | 强化效果 | 文献来源 |
|---|---|---|---|---|
| 非诺贝特 | 结晶诱导期较长 | 甘露醇、二氧化硅、微晶纤维素、羧甲基纤维素、乳糖和聚己内酯 | 最佳条件下结晶诱导期从22h缩短至15min | [ |
| 非诺贝特 | 结晶诱导期较长 | 微晶纤维素 | 结晶诱导期从460min缩短至38min | [ |
| 香兰素 | 结晶诱导期较长 | 功能化二氧化硅 | 诱导期从4h缩短到20min | [ |
| 1 | 文婷, 王海蓉, 黄唯, 等. 结晶过程晶体粒度分布控制研究进展[J]. 化学工业与工程, 2021, 38(4): 44-55. |
| WEN Ting, WANG Hairong, HUANG Wei, et al. Research progress on controlling of crystal size distribution (CSD) in crystallization process[J]. Chemical Industry and Engineering, 2021, 38(4): 44-55. | |
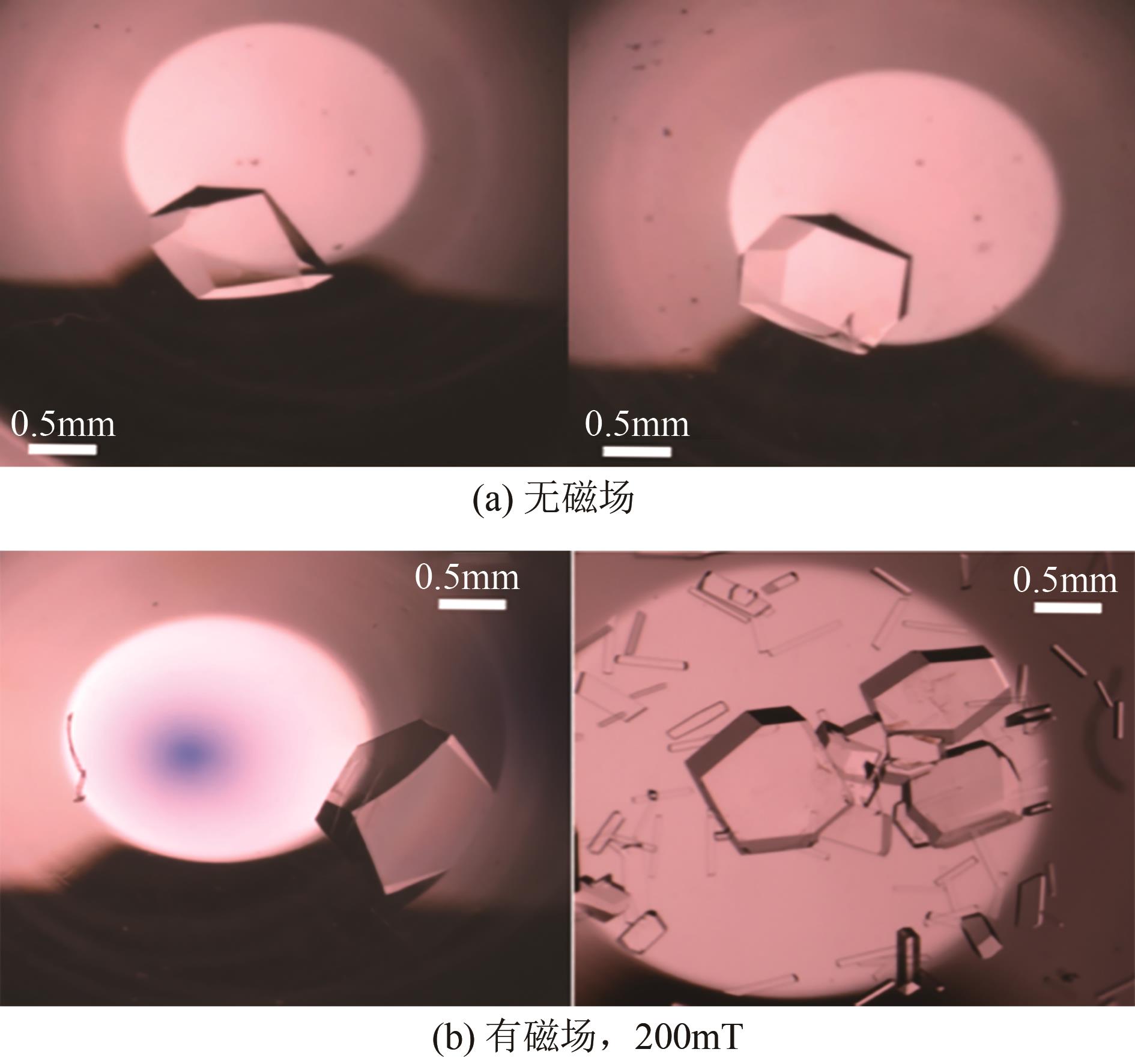
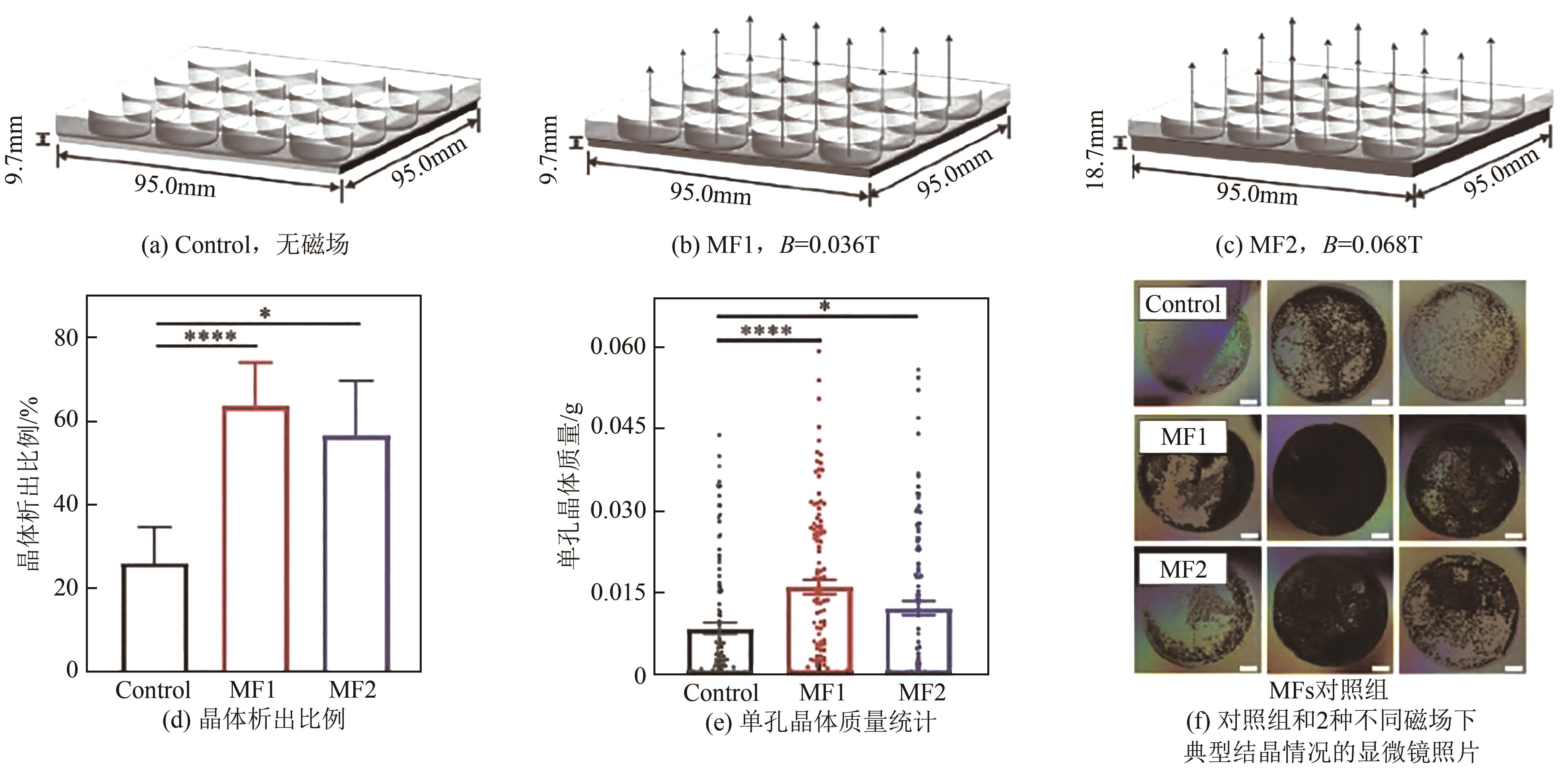
| 2 | 邢晓红, 欧阳金波, 周利民, 等. 限域空间内的结晶研究进展[J]. 化学工业与工程, 2022, 39(5): 39-48. |
| XING Xiaohong, OUYANG Jinbo, ZHOU Limin, et al. Research progress of crystallization in confined space[J]. Chemical Industry and Engineering, 2022, 39(5): 39-48. | |
| 3 | LIU Fan, BAGI S D, SU Qinglin, et al. Targeting particle size specification in pharmaceutical crystallization: A review on recent process design and development strategies and particle size measurements[J]. Organic Process Research & Development, 2022, 26(12): 3190-3203. |
| 4 | ILA M, LOUHI-KULTANEN M. Purification of monoethylene glycol by melt crystallization[J]. Chemical Engineering Science, 2023, 272: 118601. |
| 5 | LI Junjie, DEEPAK F L. In situ kinetic observations on crystal nucleation and growth[J]. Chemical Reviews, 2022, 122(23): 16911-16982. |
| 6 | LI Xin, WANG Jingkang, WANG Ting, et al. Molecular mechanism of crystal nucleation from solution[J]. Science China Chemistry, 2021, 64(9): 1460-1481. |
| 7 | XIAO Yan, WANG Jingkang, HUANG Xin, et al. Determination methods for crystal nucleation kinetics in solutions[J]. Crystal Growth & Design, 2018, 18(1): 540-551. |
| 8 | LYNCH A, JIA Lijun, SVÄRD M, et al. Crystal growth of salicylamide in organic solvents[J]. Crystal Growth & Design, 2018, 18(12): 7305-7315. |
| 9 | MING Hui, ZHU Mingfu, LI Lu, et al. A review of solvent freeze-out technology for protein crystallization[J]. CrystEngComm, 2021, 23(14): 2723-2732. |
| 10 | ZHANG Chenyan, LIU Jie, WANG Mengying, et al. Protein crystallization irradiated by audible sound: The effect of varying sound frequency[J]. Crystal Growth & Design, 2019, 19(1): 258-267. |
| 11 | WANG Qianjin, ZHAO Gang, ZHANG Chenyan. Cyclodextrin and its derivatives enhance protein crystallization by grafted on crystallization plates[J]. Journal of Crystal Growth, 2020, 536: 125591. |
| 12 | YAN Erkai, ZHAO Fengzhu, ZHANG Chenyan, et al. Seeding protein crystallization with cross-linked protein crystals[J]. Crystal Growth & Design, 2018, 18(2): 1090-1100. |
| 13 | CHENG Xiaowei, HUANG Xin, TIAN Beiqian, et al. Behaviors and physical mechanism of ceftezole sodium de-agglomeration driven by ultrasound[J]. Ultrasonics Sonochemistry, 2021, 74: 105570. |
| 14 | SHANG Zeren, LI Mingchen, HOU Baohong, et al. Ultrasound assisted crystallization of cephalexin monohydrate: Nucleation mechanism and crystal habit control[J]. Chinese Journal of Chemical Engineering, 2022, 41: 430-440. |
| 15 | HU Xueyan, ZHAO Yiting, XIAO Wu, et al. Improved spherical particle preparation of ceftriaxone sodium via membrane-assisted spherical crystallization[J]. Industrial & Engineering Chemistry Research, 2023, 62(10): 4444-4454. |
| 16 | YIN Yongheng, GAO Zhenguo, BAO Ying, et al. Gelation phenomenon during antisolvent crystallization of cefotaxime sodium[J]. Industrial & Engineering Chemistry Research, 2014, 53(3): 1286-1292. |
| 17 | CHENG Xiaowei, LI Fei, LUO Liang, et al. On the selection of wetting liquid for spherical agglomeration of cefotaxime sodium[J]. Powder Technology, 2020, 363: 593-601. |
| 18 | 盛磊, 李培钰, 牛宇超, 等. 微尺度过程强化的结晶颗粒制备研究进展[J]. 化工学报, 2021, 72(1): 143-157. |
| SHENG Lei, LI Peiyu, NIU Yuchao, et al. Progresses in the preparation of micro-scale process-enhanced crystalline particles[J]. CIESC Journal, 2021, 72(1): 143-157. | |
| 19 | WU Mengyuan, YUAN Zhijie, NIU Yuchao, et al. Interfacial induction and regulation for microscale crystallization process: A critical review[J]. Frontiers of Chemical Science and Engineering, 2022, 16(6): 838-853. |
| 20 | MELDRUM F C, O'SHAUGHNESSY C. Crystallization in confinement[J]. Advanced Materials, 2020, 32(31): 2001068. |
| 21 | SANDER J R G, ZEIGER B W, SUSLICK K S. Sonocrystallization and sonofragmentation[J]. Ultrasonics Sonochemistry, 2014, 21(6): 1908-1915. |
| 22 | FENG Yaoguang, HAO Hongxun, CHEN Yiqing, et al. Enhancement of crystallization process of the organic pharmaceutical molecules through high pressure[J]. Crystals, 2022, 12(3): 432. |
| 23 | WANG Lingyu, TANG Weiwei, DU Shichao, et al. Additive-induced selective crystallization of the elusive form- Ⅱ of γ-aminobutyric acid[J]. Chemical Engineering & Technology, 2020, 43(6): 1137-1143. |
| 24 | NAHI O, KULAK A N, BROAD A, et al. Solvent-mediated enhancement of additive-controlled crystallization[J]. Crystal Growth & Design, 2021, 21(12): 7104-7115. |
| 25 | SAVCHENKO M, SEBASTIAN V, LOPEZ-LOPEZ M T, et al. Magnetite mineralization inside cross-linked protein crystals[J]. Crystal Growth & Design, 2023, 23(6): 4032-4040. |
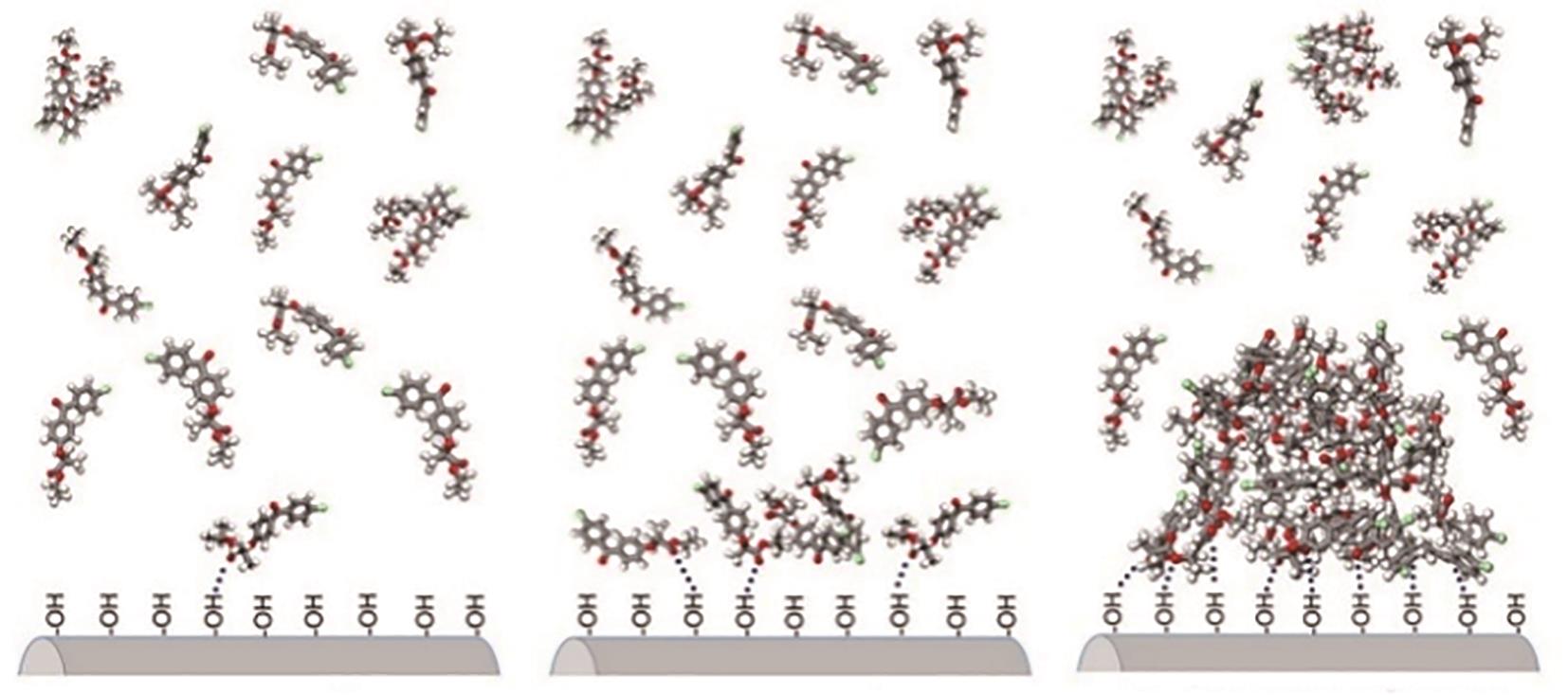
| 26 | KATSMAN A, POLISHCHUK I, POKROY B. On the mechanism of calcium carbonate polymorph selection via confinement[J]. Faraday Discussions, 2022, 235: 433-445. |
| 27 | BRASCHINSKY A, STEED J W. Molecular clusters in confined spaces[J]. Coordination Chemistry Reviews, 2022, 473: 214840. |
| 28 | PRILESZKY T A, FURST E M. Crystallization kinetics of partially crystalline emulsion droplets in a microfluidic device[J]. Langmuir, 2016, 32(20): 5141-5146. |
| 29 | CHEN Cen, COOK Oliver, NICHOLSON C E, et al. Leapfrogging Ostwald’s rule of stages: Crystallization of stable γ-glycine directly from microemulsions[J]. Crystal Growth & Design, 2011, 11(6): 2228-2237. |
| 30 | NICHOLSON C E, CHEN Cen, MENDIS B, et al. Stable polymorphs crystallized directly under thermodynamic control in three-dimensional nanoconfinement: A generic methodology[J]. Crystal Growth & Design, 2011, 11(2): 363-366. |
| 31 | LIU Qi, WANG Jingkang, WU Hao, et al. Manipulating of crystal morphology and polymorph by crystallization in microemulsions[J]. Industrial & Engineering Chemistry Research, 2020, 59(29): 13024-13032. |
| 32 | LEE Sooheyong, Haeng Sub WI, Wonhyuk JO, et al. Multiple pathways of crystal nucleation in an extremely supersaturated aqueous potassium dihydrogen phosphate (KDP) solution droplet[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(48): 13618-13623. |
| 33 | TOLDY A I, BADRUDDOZA A Z M, ZHENG Lu, et al. Spherical crystallization of glycine from monodisperse microfluidic emulsions[J]. Crystal Growth & Design, 2012, 12(8): 3977-3982. |
| 34 | MENG Xianze, WANG Yongli, LI Xin, et al. Confined crystallization of pigment red 146 in emulsion droplets and its mechanism[J]. Nanomaterials, 2019, 9(3): 379. |
| 35 | YANG Mei, GAO Yuan, LIU Yun, et al. Integration of microfluidic systems with external fields for multiphase process intensification[J]. Chemical Engineering Science, 2021, 234: 116450. |
| 36 | SHI Huanhuan, XIAO Yan, FERGUSON S, et al. Progress of crystallization in microfluidic devices[J]. Lab on a Chip, 2017, 17(13): 2167-2185. |
| 37 | NISHIGAKI A, MARUYAMA M, NUMATA M, et al. Microflow system promotes acetaminophen crystal nucleation[J]. Engineering in Life Sciences, 2020, 20(9/10): 395-401. |
| 38 | FERNANDEZ RIVAS D, KUHN S. Synergy of microfluidics and ultrasound[J]. Topics in Current Chemistry, 2016, 374(5): 1-30. |
| 39 | LIU Fen, LUO Wei, QIU Junjie, et al. Continuous antisolvent crystallization of dolutegravir sodium using microfluidics[J]. Industrial & Engineering Chemistry Research, 2022, 61(19): 6693-6702. |
| 40 | FERREIRA J, OPSTEYN J, ROCHA F, et al. Ultrasonic protein crystallization: Promoting nucleation in microdroplets through pulsed sonication[J]. Chemical Engineering Research and Design, 2020, 162: 249-257. |
| 41 | GERARD C J J, FERRY G, VUILLARD L M, et al. A chemical library to screen protein and protein-ligand crystallization using a versatile microfluidic platform[J]. Crystal Growth & Design, 2018, 18(9): 5130-5137. |
| 42 | ZHU Deyong, ZHOU Xiaohu, ZHENG Bo. A double emulsion-based, plastic-glass hybrid microfluidic platform for protein crystallization[J]. Micromachines, 2015, 6(11): 1629-1644. |
| 43 | BHATTACHARYA S, KUNDU P, LIU J S, et al. Feedback-system-control integrated microfluidic system for fast screening of protein crystallization conditions[J]. Crystal Growth & Design, 2020, 20(7): 4325-4334. |
| 44 | COLIAIE P, KELKAR M S, LANGSTON M, et al. Advanced continuous-flow microfluidic device for parallel screening of crystal polymorphs, morphology, and kinetics at controlled supersaturation[J]. Lab on a Chip, 2021, 21(12): 2333-2342. |
| 45 | KHURSHID S, SARIDAKIS E, GOVADA L, et al. Porous nucleating agents for protein crystallization[J]. Nature Protocols, 2014, 9(7): 1621-1633. |
| 46 | SARIDAKIS E, CHAYEN N E. Imprinted polymers assisting protein crystallization[J]. Trends in Biotechnology, 2013, 31(9): 515-520. |
| 47 | KERTIS F, KHURSHID S, OKMAN O, et al. Heterogeneous nucleation of protein crystals using nanoporous gold nucleants[J]. Journal of Materials Chemistry, 2012, 22(41): 21928. |
| 48 | JIANG Qi, WARD M D. Crystallization under nanoscale confinement[J]. Chemical Society Reviews, 2014, 43(7): 2066-2079. |
| 49 | BRADY A B, WEBER J, YUAN Ke, et al. In situ observations of barium sulfate nucleation in nanopores[J]. Crystal Growth & Design, 2022, 22(12): 6941-6951. |
| 50 | KHODAPARAST S, MARCOS J, SHARRATT W N, et al. Surface-induced crystallization of sodium dodecyl sulfate (SDS) micellar solutions in confinement[J]. Langmuir, 2021, 37(1): 230-239. |
| 51 | DIAO Ying, HARADA T, MYERSON A S, et al. The role of nanopore shape in surface-induced crystallization[J]. Nature Materials, 2011, 10(11): 867-871. |
| 52 | PAGE A J, SEAR R P. Heterogeneous nucleation in and out of pores[J]. Physical Review Letters, 2006, 97(6): 065701. |
| 53 | NANEV C N, SARIDAKIS E, CHAYEN N E. Protein crystal nucleation in pores[J]. Scientific Reports, 2017, 7: 35821. |
| 54 | ANDUIX-CANTO C, LEVENSTEIN M A, KIM Yi-Yeoun, et al. Exploiting confinement to study the crystallization pathway of calcium sulfate[J]. Advanced Functional Materials, 2021, 31(50): 2107312. |
| 55 | BEINER M, RENGARAJAN, PANKAJ S, et al. Manipulating the crystalline state of pharmaceuticals by nanoconfinement[J]. Nano Letters, 2007, 7(5): 1381-1385. |
| 56 | ANDUIX-CANTO C, KIM Yi-Yeoun, WANG Yunwei, et al. Effect of nanoscale confinement on the crystallization of potassium ferrocyanide[J]. Crystal Growth & Design, 2016, 16(9): 5403-5411. |
| 57 | DWYER L M, MICHAELIS V K, O’MAHONY M, et al. Confined crystallization of fenofibrate in nanoporous silica[J]. CrystEngComm, 2015, 17(41): 7922-7929. |
| 58 | PRASAD R, DALVI S V. Sonocrystallization: Monitoring and controlling crystallization using ultrasound[J]. Chemical Engineering Science, 2020, 226: 115911. |
| 59 | JORDENS J, GIELEN B, XIOURAS C, et al. Sonocrystallisation: Observations, theories and guidelines[J]. Chemical Engineering and Processing: Process Intensification, 2019, 139: 130-154. |
| 60 | NALESSO S, BUSSEMAKER M J, SEAR R P, et al. A review on possible mechanisms of sonocrystallisation in solution[J]. Ultrasonics Sonochemistry, 2019, 57: 125-138. |
| 61 | SABNIS S S, SINGH S D, GOGATE P R. Improvements in azithromycin recrystallization using ultrasound for size reduction[J]. Ultrasonics Sonochemistry, 2022, 83: 105922. |
| 62 | MAO Yafei, LI Fei, WANG Ting, et al. Enhancement of lysozyme crystallization under ultrasound field[J]. Ultrasonics Sonochemistry, 2020, 63: 104975. |
| 63 | YU Fei, MAO Yafei, ZHAO Hongtu, et al. Enhancement of continuous crystallization of lysozyme through ultrasound[J]. Organic Process Research & Development, 2021, 25(11): 2508-2515. |
| 64 | WANG Jiayuan, LI Fei, LAKERVELD R. Process intensification for pharmaceutical crystallization[J]. Chemical Engineering and Processing: Process Intensification, 2018, 127: 111-126. |
| 65 | AWARI H D, SABNIS S S, GOGATE P R. Improved crystallization of ampicillin trihydrate based on the use of ultrasound[J]. Industrial & Engineering Chemistry Research, 2022, 61(6): 2538-2547. |
| 66 | HUSSAIN M N, JORDENS J, JOHN J J, et al. Enhancing pharmaceutical crystallization in a flow crystallizer with ultrasound: Anti-solvent crystallization[J]. Ultrasonics Sonochemistry, 2019, 59: 104743. |
| 67 | ALEXANDER L F, RADACSI N. Application of electric fields for controlling crystallization[J]. CrystEngComm, 2019, 21(34): 5014-5031. |
| 68 | YUAN Zhijie, WU Mengyuan, MENG Yingshuang, et al. Protein crystal regulation and harvest via electric field-based method[J]. Current Opinion in Chemical Engineering, 2022, 36: 100744. |
| 69 | KOIZUMI H, UDA S. Theoretical and practical studies on effects of external electrostatic electric field on nucleation and growth kinetics of protein crystals[J]. Progress in Crystal Growth and Characterization of Materials, 2022, 68(3): 100568. |
| 70 | PAREJA-RIVERA C, CUÉLLAR-CRUZ M, ESTURAU-ESCOFET N, et al. Recent advances in the understanding of the influence of electric and magnetic fields on protein crystal growth[J]. Crystal Growth & Design, 2017, 17(1): 135-145. |
| 71 | RUBIN E, OWEN C, STOJANOFF V. Crystallization under an external electric field: A case study of glucose isomerase[J]. Crystals, 2017, 7(7): 206. |
| 72 | LI Fei, LAKERVELD R. Electric-field-assisted protein crystallization in continuous flow[J]. Crystal Growth & Design, 2018, 18(5): 2964-2971. |
| 73 | LI W W, RADACSI N, KRAMER H J M, et al. Solid separation from a mixed suspension through electric-field-enhanced crystallization[J]. Angewandte Chemie International Edition, 2016, 55(52): 16088-16091. |
| 74 | AZMI N S M, ANUAR N, OTHMAN M F, et al. Electric-potential-assisted crystallisation of L-isoleucine: A study of nucleation kinetics and its associated parameters[J]. Crystals, 2021, 11(6): 620. |
| 75 | YIN Dachuan. Protein crystallization in a magnetic field[J]. Progress in Crystal Growth and Characterization of Materials, 2015, 61(1): 1-26. |
| 76 | SURADE S, OCHI T, NIETLISPACH D, et al. Investigations into protein crystallization in the presence of a strong magnetic field[J]. Crystal Growth & Design, 2010, 10(2): 691-699. |
| 77 | Sun RYU, In OH, CHO Sang, et al. Enhancing protein crystallization under a magnetic field[J]. Crystals, 2020, 10(9): 821. |
| 78 | 张翔飞, 周数, 谢灿, 等. 中等强度稳态磁场影响小分子结晶的初步探索[J]. 激光生物学报, 2022, 31(3): 243-249. |
| ZHANG Xiangfei, ZHOU Shu, XIE Can, et al. A preliminary study of the effects of moderate intensity static magnetic fields on small molecule crystallization[J]. Acta Laser Biology Sinica, 2022, 31(3): 243-249. | |
| 79 | ZHAO Yihan, HOU Baohong, LIU Chunhao, et al. Mechanistic study on the effect of magnetic field on the crystallization of organic small molecules[J]. Industrial & Engineering Chemistry Research, 2021, 60(43): 15741-15751. |
| 80 | TAI C Y, WU Chikao, CHANG Mengchun. Effects of magnetic field on the crystallization of CaCO3 using permanent magnets[J]. Chemical Engineering Science, 2008, 63(23): 5606-5612. |
| 81 | ASAKUMA Y, MIURA M. Effect of microwave radiation on diffusion behavior of anti-solvent during crystallization[J]. Journal of Crystal Growth, 2014, 402: 32-36. |
| 82 | OGUNNIRAN O, BINNER E R, SKLAVOUNOS A H, et al. Enhancing evaporative mass transfer and steam stripping using microwave heating[J]. Chemical Engineering Science, 2017, 165: 147-153. |
| 83 | RADACSI N, HORST J H TER, STEFANIDIS G D. Microwave-assisted evaporative crystallization of niflumic acid for particle size reduction[J]. Crystal Growth & Design, 2013, 13(10): 4186-4189. |
| 84 | LI Liye, GUO Zhichao, HAN Wenxiang, et al. The effect of microwave on the primary nucleation of CaSO4 from aqueous solutions[J]. Powder Technology, 2017, 317: 189-196. |
| 85 | CONSTANCE E N, ZAAKAN A, ALSHARARI F, et al. Effect of microwave heating on the crystallization of glutathione tripeptide on silver nanoparticle films[J]. The Journal of Physical Chemistry C, 2017, 121(10): 5585-5593. |
| 86 | YUYAMA K, CHANG Kaidi, TU Jingru, et al. Rapid localized crystallization of lysozyme by laser trapping[J]. Physical Chemistry Chemical Physics, 2018, 20(9): 6034-6039. |
| 87 | LIAO Zhiyu, WYNNE K. A metastable amorphous intermediate is responsible for laser-induced nucleation of glycine[J]. Journal of the American Chemical Society, 2022, 144(15): 6727-6733. |
| 88 | KOREDE V, NAGALINGAM N, PENHA F M, et al. A review of laser-induced crystallization from solution[J]. Crystal Growth & Design, 2023, 23(5): 3873-3916. |
| 89 | YU Jiachen, YAN Jianfeng, JIANG Lan. Crystallization of polymorphic sulfathiazole controlled by femtosecond laser-induced cavitation bubbles[J]. Crystal Growth & Design, 2021, 21(6): 3202-3210. |
| 90 | XU Shijie, CAO Di, LIU Yixuan, et al. Role of additives in crystal nucleation from solutions: A review[J]. Crystal Growth & Design, 2022, 22(3): 2001-2022. |
| 91 | 王子豪, 马源昌, 李梓铭, 等. 添加剂在药物结晶中的应用研究进展[J]. 山东化工, 2022, 51(22): 78-80. |
| WANG Zihao, MA Yuanchang, LI Ziming, et al. Research progress on the application of additives in drug crystallization[J]. Shandong Chemical Industry, 2022, 51(22): 78-80. | |
| 92 | 尚泽仁, 胡卫国, 汤伟伟, 等. 离子液体在药物晶体工程中的应用[J]. 化工进展, 2019, 38(5): 2389-2401. |
| SHANG Zeren, HU Weiguo, TANG Weiwei, et al. Application of ionic liquids in pharmaceutical crystal engineering[J]. Chemical Industry and Engineering Progress, 2019, 38(5): 2389-2401. | |
| 93 | KASKIEWICZ P L, ROSBOTTOM I, HAMMOND R B, et al. Understanding and designing tailor-made additives for controlling nucleation: Case study of p-aminobenzoic acid crystallizing from ethanolic solutions[J]. Crystal Growth & Design, 2021, 21(4): 1946-1958. |
| 94 | WU Hao, WANG Jingkang, LIU Qi, et al. Influences and the mechanism of additives on intensifying nucleation and growth of p-methylacetanilide[J]. Crystal Growth & Design, 2020, 20(2): 973-983. |
| 95 | BODNÁR K, HUDSON S P, RASMUSON Å C. Promotion of mefenamic acid nucleation by a surfactant additive, docusate sodium[J]. Crystal Growth & Design, 2019, 19(2): 591-603. |
| 96 | LINK F J, HENG J Y Y. Enhancing the crystallisation of insulin using amino acids as soft-templates to control nucleation[J]. CrystEngComm, 2021, 23(22): 3951-3960. |
| 97 | YU Xiaoxi, TIAN Ningning, HUANG Fang, et al. Evaluating the role of ionic liquids (ILs) in the crystallization of lysozyme[J]. Journal of Molecular Liquids, 2019, 296: 112018. |
| 98 | ZHANG Bo, WANG Yao, Shiki THI, et al. Enhancement of lysozyme crystallization using DNA as a polymeric additive[J]. Crystals, 2019, 9(4): 186. |
| 99 | HAN Dandan, WANG Yan, YANG Yang, et al. Revealing the role of a surfactant in the nucleation and crystal growth of thiamine nitrate: Experiments and simulation studies[J]. CrystEngComm, 2019, 21(23): 3576-3585. |
| 100 | SU Nannan, WANG Yongli, XIAO Yan, et al. Mechanism of influence of organic impurity on crystallization of sodium sulfate[J]. Industrial & Engineering Chemistry Research, 2018, 57(5): 1705-1713. |
| 101 | POORNACHARY S K, HAN Guangjun, KWEK Jin Wang, et al. Crystallizing micronized particles of a poorly water-soluble active pharmaceutical ingredient: Nucleation enhancement by polymeric additives[J]. Crystal Growth & Design, 2016, 16(2): 749-758. |
| 102 | WU Hao, WANG Jingkang, HUANG Xin, et al. Enlarging crystal size of zoxamide by polymeric additives that modulate burst nucleation[J]. Journal of Molecular Liquids, 2022, 357: 119088. |
| 103 | CHENG Xiaowei, HUANG Xin, HAO Yunhui, et al. Unveiling the role of additives in modifying crystallization behaviors of 4-(hydroxymethyl) benzoic acid[J]. Industrial & Engineering Chemistry Research, 2022, 61(20): 7193-7203. |
| 104 | PARAMBIL J V, POORNACHARY S K, HINDER S J, et al. Establishing template-induced polymorphic domains for API crystallisation: The case of carbamazepine[J]. CrystEngComm, 2015, 17(33): 6384-6392. |
| 105 | FRANK D S, MATZGER A J. Influence of chemical functionality on the rate of polymer-induced heteronucleation[J]. Crystal Growth & Design, 2017, 17(8): 4056-4059. |
| 106 | PARAMBIL J V, POORNACHARY S K, HENG J Y Y, et al. Template-induced nucleation for controlling crystal polymorphism: From molecular mechanisms to applications in pharmaceutical processing[J]. CrystEngComm, 2019, 21(28): 4122-4135. |
| 107 | CARIDI A, KULKARNI S A, DI PROFIO G, et al. Template-induced nucleation of isonicotinamide polymorphs[J]. Crystal Growth & Design, 2014, 14(3): 1135-1141. |
| 108 | CHADWICK K, CHEN Jie, MYERSON A S, et al. Toward the rational design of crystalline surfaces for heteroepitaxy: Role of molecular functionality[J]. Crystal Growth & Design, 2012, 12(3): 1159-1166. |
| 109 | ARRIBAS BUENO R, CROWLEY C M, DAVERN P, et al. Heterogeneous crystallization of fenofibrate onto pharmaceutical excipients[J]. Crystal Growth & Design, 2018, 18(4): 2151-2164. |
| 110 | VERMA V, ZEGLINSKI J, HUDSON S, et al. Dependence of heterogeneous nucleation on hydrogen bonding lifetime and complementarity[J]. Crystal Growth & Design, 2018, 18(11): 7158-7172. |
| 111 | OUYANG Jinbo, XING Xiaohong, CHEN Jian, et al. Effects of solvent, supersaturation ratio and silica template on morphology and polymorph evolution of vanillin during swift cooling crystallization[J]. Particuology, 2022, 65: 93-104. |
| 112 | YAZDANPANAH N, TESTA C J, PERALA S R K, et al. Continuous heterogeneous crystallization on excipient surfaces[J]. Crystal Growth & Design, 2017, 17(6): 3321-3330. |
| 113 | LING Jing, CHADWICK K. Heterogeneous crystallization inside microporous polymer particles as a process intensification technology for the manufacture of drug formulations[J]. Organic Process Research & Development, 2017, 21(6): 827-834. |
| 114 | ERAL H B, LÓPEZ-MEJÍAS V, O’MAHONY M, et al. Biocompatible alginate microgel particles as heteronucleants and encapsulating vehicles for hydrophilic and hydrophobic drugs[J]. Crystal Growth & Design, 2014, 14(4): 2073-2082. |
| [1] | 苏梦军, 刘剑, 辛靖, 陈禹霏, 张海洪, 韩龙年, 朱元宝, 李洪宝. 气液混合强化在固定床加氢过程中的应用进展[J]. 化工进展, 2024, 43(1): 100-110. |
| [2] | 翟霖晓, 崔怡洲, 李成祥, 石孝刚, 高金森, 蓝兴英. 微气泡发生器的研究与应用进展[J]. 化工进展, 2024, 43(1): 111-123. |
| [3] | 田时泓, 郭磊, 李娜, 宇文超, 许磊, 郭胜惠, 巨少华. 微波加热强化闪蒸工艺的科学基础及发展趋势[J]. 化工进展, 2024, 43(1): 135-144. |
| [4] | 王立华, 蔡苏杭, 江文涛, 罗倩, 罗勇, 陈建峰. 微纳尺度气液传质强化油品催化加氢反应[J]. 化工进展, 2024, 43(1): 19-33. |
| [5] | 张梁, 马骥, 贺高红, 姜晓滨, 肖武. 膜调控的头孢呋辛钠溶析-冷却耦合结晶成核介稳区测定及分析[J]. 化工进展, 2024, 43(1): 260-268. |
| [6] | 刘锋, 褚阳, 李会峰, 李明丰, 朱玫, 张润强. 汽油中大分子硫醇催化转化反应过程强化[J]. 化工进展, 2024, 43(1): 279-284. |
| [7] | 王一棪, 王达锐, 沈震浩, 何俊琳, 孙洪敏, 杨为民. 全结晶MCM-22分子筛催化剂的制备及其催化性能[J]. 化工进展, 2024, 43(1): 285-291. |
| [8] | 袁谅, 从海峰, 李鑫钢. 微通道内气液流动与传质特性的研究进展[J]. 化工进展, 2024, 43(1): 34-48. |
| [9] | 张祚群, 高扬, 白超杰, 薛立新. 二次界面聚合同步反扩散原位生长ZIF-8纳米粒子制备聚酰胺混合基质反渗透(RO)膜[J]. 化工进展, 2023, 42(S1): 364-373. |
| [10] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [11] | 董佳宇, 王斯民. 超声强化对二甲苯结晶特性及调控机理实验[J]. 化工进展, 2023, 42(9): 4504-4513. |
| [12] | 常印龙, 周启民, 王青月, 王文俊, 李伯耿, 刘平伟. 废弃聚烯烃的高值化学回收研究进展[J]. 化工进展, 2023, 42(8): 3965-3978. |
| [13] | 尹新宇, 皮丕辉, 文秀芳, 钱宇. 特殊浸润性材料在防治油气管道中水合物成核与聚集的应用[J]. 化工进展, 2023, 42(8): 4076-4092. |
| [14] | 王兰江, 梁瑜, 汤琼, 唐明兴, 李学宽, 刘雷, 董晋湘. 快速热解铂前体合成高分散的Pt/HY催化剂及其萘深度加氢性能[J]. 化工进展, 2023, 42(8): 4159-4166. |
| [15] | 王达锐, 孙洪敏, 薛明伟, 王一棪, 刘威, 杨为民. 微波法高效合成全结晶ZSM-5分子筛催化剂及其催化性能[J]. 化工进展, 2023, 42(7): 3582-3588. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||