化工进展 ›› 2023, Vol. 42 ›› Issue (11): 5981-5992.DOI: 10.16085/j.issn.1000-6613.2022-2259
• 资源与环境化工 • 上一篇
废白土衍生吸附剂协同去除水中的四环素和铜
柯玉鑫1,2( ), 朱晓丽1,2(
), 朱晓丽1,2( ), 司绍诚1,2, 张婷1,2, 王军强1,3, 张子夜3
), 司绍诚1,2, 张婷1,2, 王军强1,3, 张子夜3
- 1.西北大学城市与环境学院,陕西 西安 710127
2.陕西省地表系统与环境承载力重点实验室,陕西 西安 710127
3.西安金博瑞生态科技有限公司,陕西 西安 710065
-
收稿日期:2022-12-04修回日期:2023-04-23出版日期:2023-11-20发布日期:2023-12-15 -
通讯作者:朱晓丽 -
作者简介:柯玉鑫(1997—),男,博士研究生,研究方向为多介质污染物修复技术。E-mail:keyuxin_ak@163.com。 -
基金资助:国家重点研发计划(2021YFC1808902);陕西省重点研发计划(2019NY-200);西安市科技计划(2019-GXYD18.9);陕西省农业科技创新项目(NYJK-2022-XA-02)
Adsorbent derived from spent bleaching earth for the synergistic removal of tetracycline and copper in wastewater
KE Yuxin1,2( ), ZHU Xiaoli1,2(
), ZHU Xiaoli1,2( ), SI Shaocheng1,2, ZHANG Ting1,2, WANG Junqiang1,3, ZHANG Ziye3
), SI Shaocheng1,2, ZHANG Ting1,2, WANG Junqiang1,3, ZHANG Ziye3
- 1.College of Urban and Environmental Science, Northwest University, Xi’an 710127, Shaanxi, China
2.Shaanxi Key Laboratory of Earth Surface System and Environment Carrying Capacity, Xi’an 710127, Shaanxi, China
3.Xi’an Jinborui Ecological Tech. Co. , Ltd. , Xi’an 710065, Shaanxi, China
-
Received:2022-12-04Revised:2023-04-23Online:2023-11-20Published:2023-12-15 -
Contact:ZHU Xiaoli
摘要:
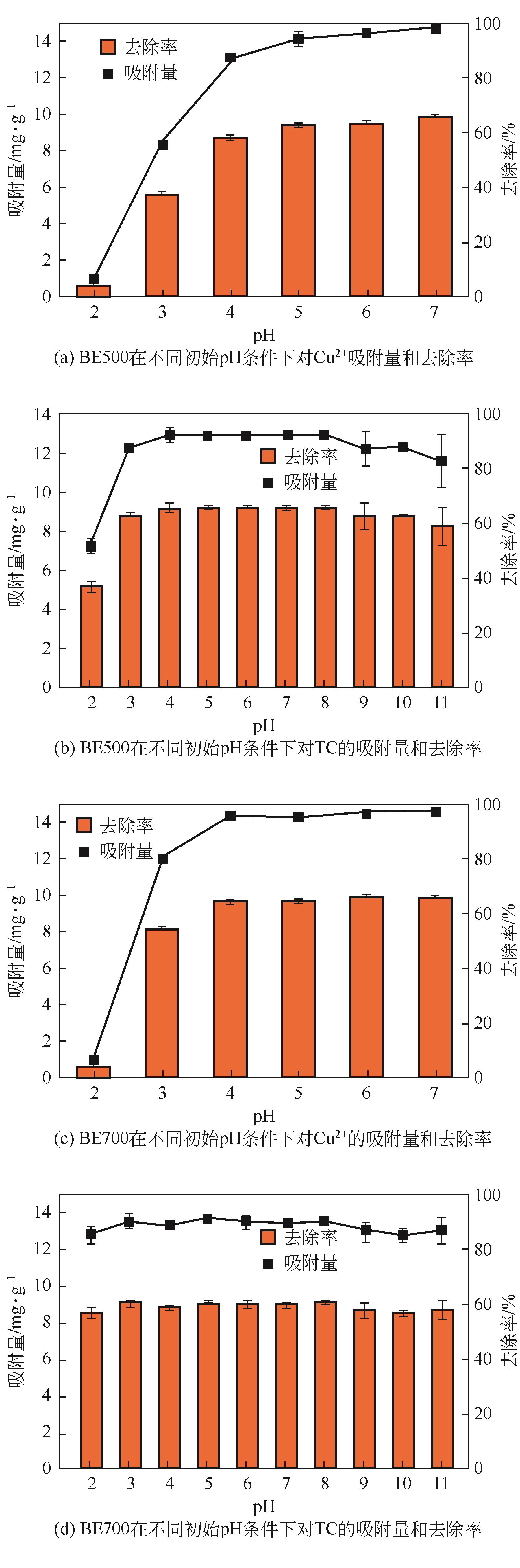
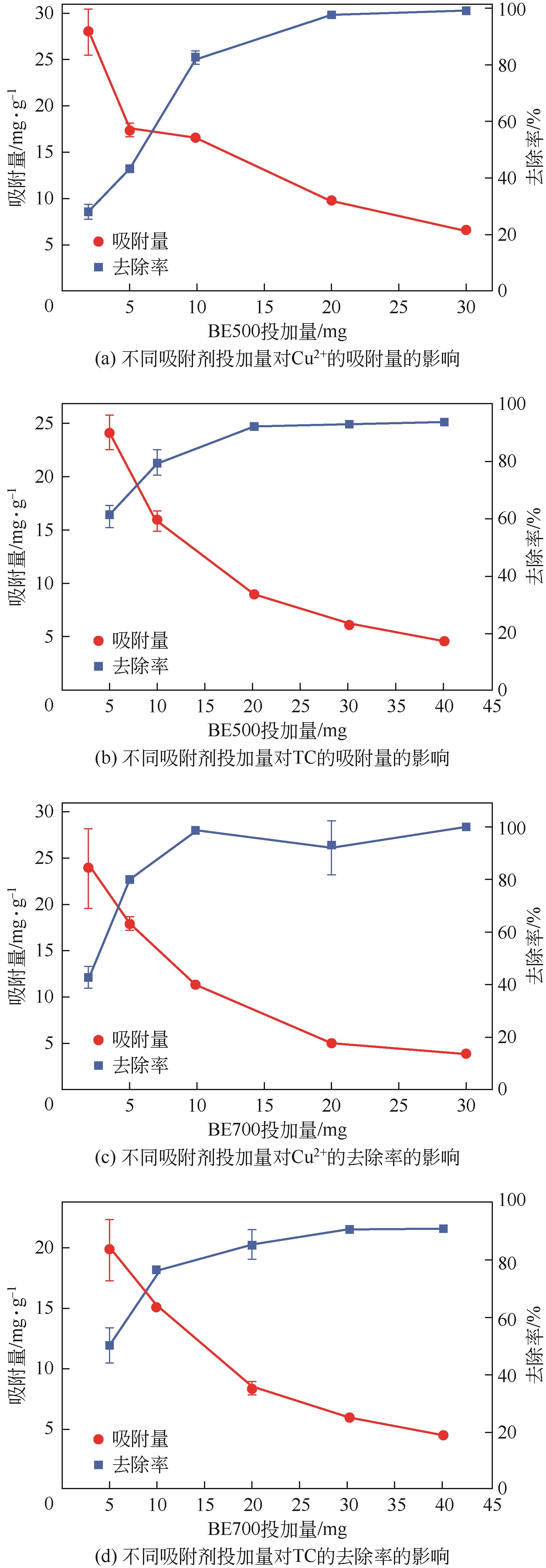
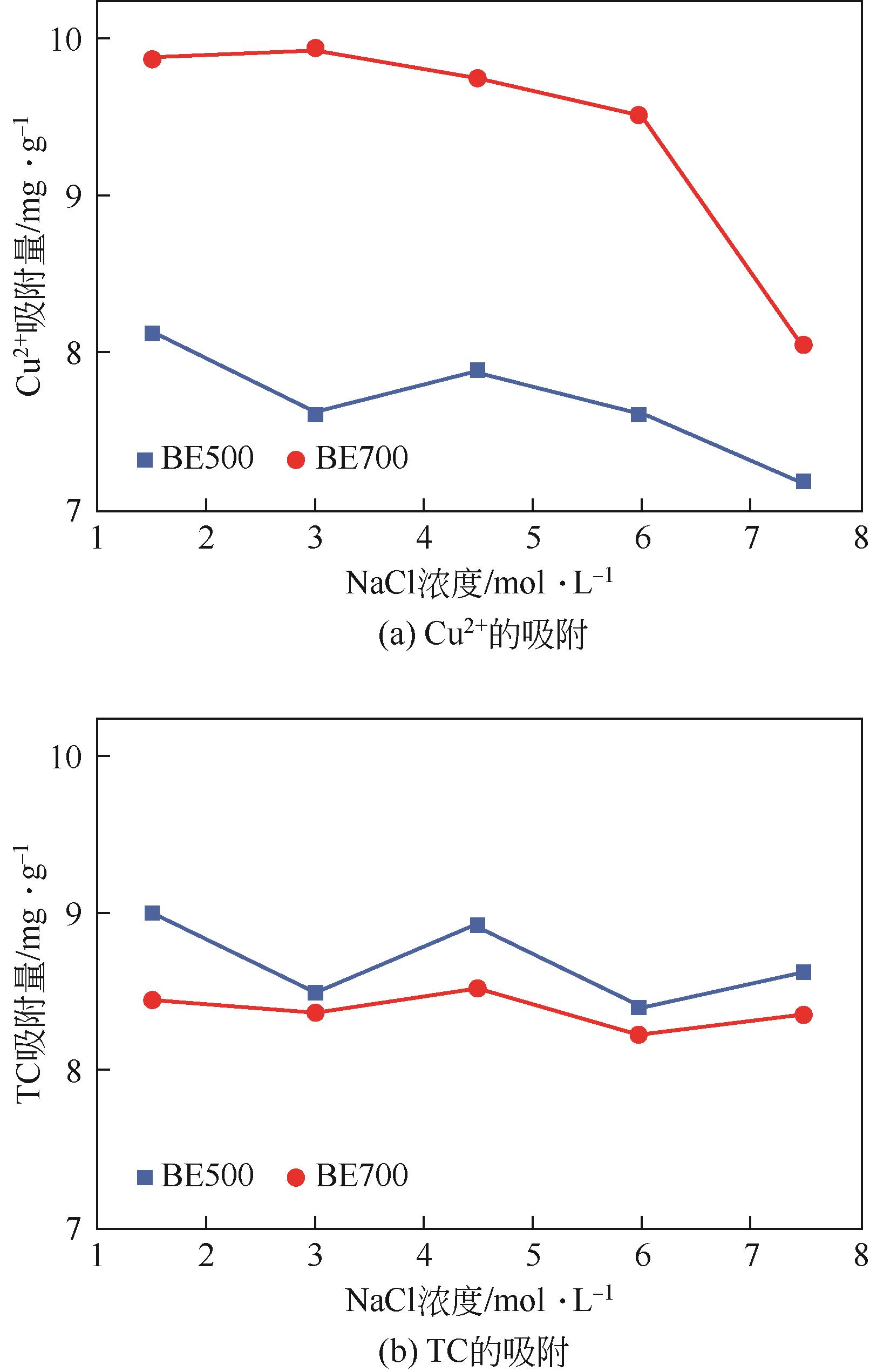
为有效利用食用油行业产生的白土废弃物,以及解决畜禽养殖废水中的重金属及抗生素残留问题。本研究通过热解的方式将废白土转化为新型吸附剂BE500和BE700,用于水中铜离子(Cu2+)和四环素(TC)的去除,并考察了吸附剂投加量、反应pH、离子干扰和竞争条件下的吸附效果。结果显示,BE700更适合于Cu2+的吸附,而BE500对TC的去除效率更高。相比于Cu2+,TC吸附量对溶液pH变化的敏感度更低。动力学模型拟合结果显示,BE500和BE700对Cu2+和TC的吸附主要是发生了化学反应,且吸附速率主要受到液膜扩散的控制。等温吸附和热力学模型拟合结果则表明BE500和BE700与Cu2+和TC之间主要发生了多层吸附,且吸附是自发的吸热过程。此外,离子干扰实验显示,Cl-和Na+对吸附效果的影响较小。而竞争吸附实验显示了在二元体系中BE500和BE700对Cu2+和TC的吸附量较一元体系显著增加,即Cu2+和TC之间的反应促进了Cu2+和TC的协同去除。表征结果显示孔隙填充、离子交换、络合反应、氢键和π-π相互作用均参与了对Cu2+和TC的去除机制。
中图分类号:
引用本文
柯玉鑫, 朱晓丽, 司绍诚, 张婷, 王军强, 张子夜. 废白土衍生吸附剂协同去除水中的四环素和铜[J]. 化工进展, 2023, 42(11): 5981-5992.
KE Yuxin, ZHU Xiaoli, SI Shaocheng, ZHANG Ting, WANG Junqiang, ZHANG Ziye. Adsorbent derived from spent bleaching earth for the synergistic removal of tetracycline and copper in wastewater[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5981-5992.
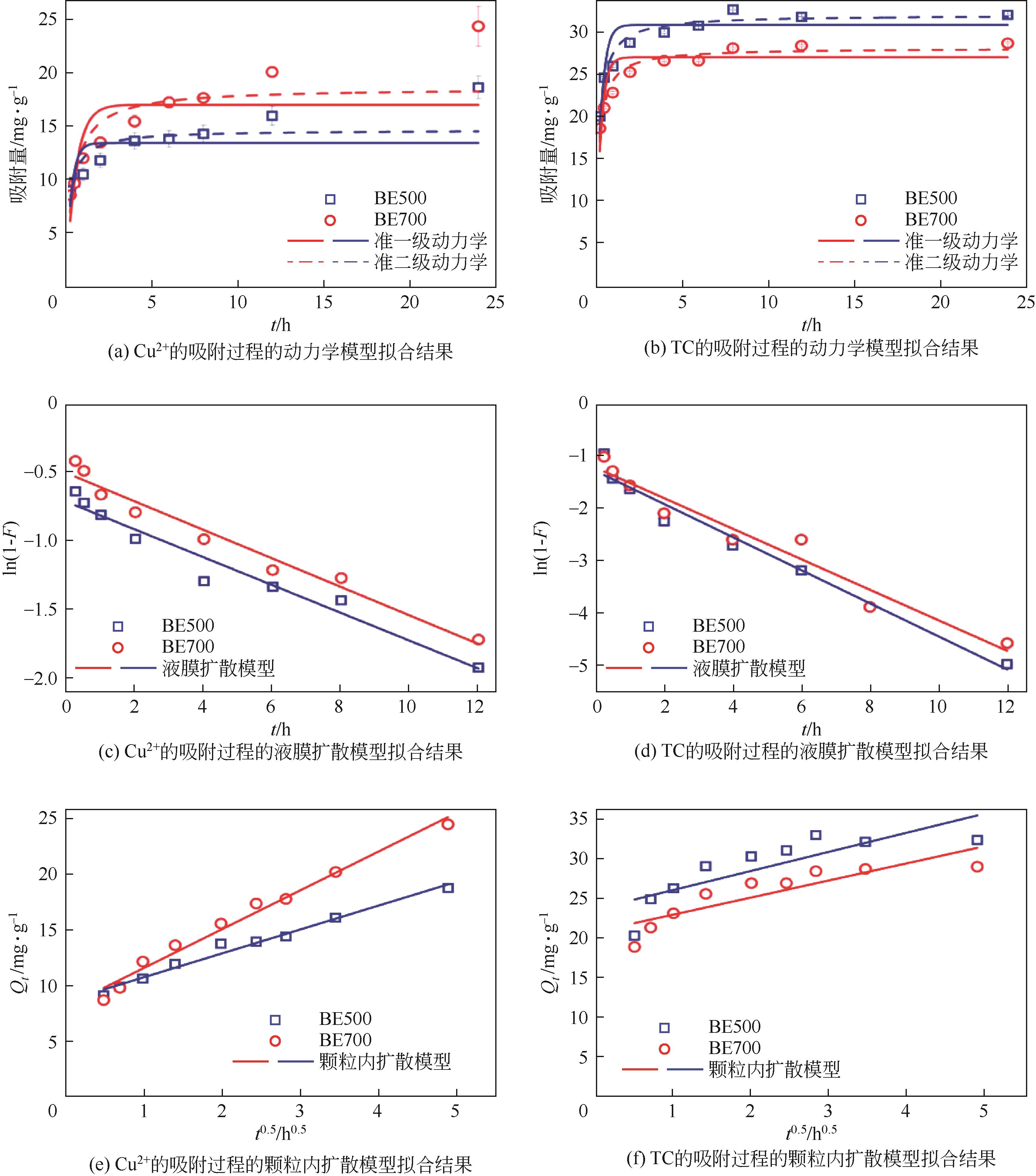
| 扩散模型 | Cu2+ | TC | ||
|---|---|---|---|---|
| BE500 | BE700 | BE500 | BE700 | |
| 准一级动力学 | ||||
| Qe/mg·g-1 | 13.4 | 17.0 | 30.7 | 26.9 |
| k1/h-1 | 3.270 | 1.770 | 3.327 | 3.538 |
| R2 | 0.423 | 0.719 | 0.698 | 0.640 |
| 准二级动力学 | ||||
| Qe/mg·g-1 | 14.6 | 18.6 | 31.9 | 27.995 |
| k2/g·mg-1·h-1 | 0.306 | 0.126 | 0.184 | 0.220 |
| R2 | 0.700 | 0.873 | 0.930 | 0.917 |
| 液膜扩散模型 | ||||
| Kfd/mg·g-1 | 0.102 | 0.104 | 0.317 | 0.292 |
| R2 | 0.951 | 0.963 | 0.965 | 0.949 |
| C/mg·L-1 | -0.729 | -0.519 | -1.312 | -1.249 |
| 颗粒内扩散模型 | ||||
| Ki/mg·g-1·h0.5 | 2.158 | 3.506 | 2.398 | 2.147 |
| R2 | 0.979 | 0.978 | 0.627 | 0.719 |
| C/mg·L-1 | 8.396 | 7.866 | 23.245 | 20.407 |
表1 动力学、液膜扩散和颗粒内扩散模型的拟合结果
| 扩散模型 | Cu2+ | TC | ||
|---|---|---|---|---|
| BE500 | BE700 | BE500 | BE700 | |
| 准一级动力学 | ||||
| Qe/mg·g-1 | 13.4 | 17.0 | 30.7 | 26.9 |
| k1/h-1 | 3.270 | 1.770 | 3.327 | 3.538 |
| R2 | 0.423 | 0.719 | 0.698 | 0.640 |
| 准二级动力学 | ||||
| Qe/mg·g-1 | 14.6 | 18.6 | 31.9 | 27.995 |
| k2/g·mg-1·h-1 | 0.306 | 0.126 | 0.184 | 0.220 |
| R2 | 0.700 | 0.873 | 0.930 | 0.917 |
| 液膜扩散模型 | ||||
| Kfd/mg·g-1 | 0.102 | 0.104 | 0.317 | 0.292 |
| R2 | 0.951 | 0.963 | 0.965 | 0.949 |
| C/mg·L-1 | -0.729 | -0.519 | -1.312 | -1.249 |
| 颗粒内扩散模型 | ||||
| Ki/mg·g-1·h0.5 | 2.158 | 3.506 | 2.398 | 2.147 |
| R2 | 0.979 | 0.978 | 0.627 | 0.719 |
| C/mg·L-1 | 8.396 | 7.866 | 23.245 | 20.407 |
| 参数 | Cu2+ | TC | ||||
|---|---|---|---|---|---|---|
| BE500 | BE700 | BE500 | BE700 | |||
| Langmuir | ||||||
| 15℃ | Qm | 12.999 | 15.248 | 36.124 | 36.091 | |
| KL | 0.996 | 30.313 | 0.154 | 0.098 | ||
| R2 | 0.469 | 0.577 | 0.933 | 0.950 | ||
| 25℃ | Qm | 14.098 | 17.930 | 41.090 | 36.821 | |
| KL | 2.790 | 206.743 | 0.176 | 0.202 | ||
| R2 | 0.534 | 0.942 | 0.942 | 0.943 | ||
| 35℃ | Qm | 15.790 | 20.643 | 41.950 | 46.705 | |
| KL | 9.516 | 9.039 | 0.310 | 0.121 | ||
| R2 | 0.832 | 0.897 | 0.908 | 0.899 | ||
| Freundlich | ||||||
| 15℃ | KF | 7.050 | 11.959 | 8.831 | 6.348 | |
| n | 21.358 | 11.485 | 2.938 | 2.529 | ||
| R2 | 0.818 | 0.856 | 0.997 | 0.981 | ||
| 25℃ | KF | 9.314 | 14.849 | 10.442 | 9.559 | |
| n | 7.463 | 13.947 | 3.037 | 3.070 | ||
| R2 | 0.848 | 0.970 | 0.993 | 0.972 | ||
| 35℃ | KF | 11.786 | 14.645 | 12.212 | 8.519 | |
| n | 9.901 | 7.680 | 3.173 | 2.456 | ||
| R2 | 0.972 | 0.958 | 0.979 | 0.959 | ||
表2 等温吸附模型拟合参数
| 参数 | Cu2+ | TC | ||||
|---|---|---|---|---|---|---|
| BE500 | BE700 | BE500 | BE700 | |||
| Langmuir | ||||||
| 15℃ | Qm | 12.999 | 15.248 | 36.124 | 36.091 | |
| KL | 0.996 | 30.313 | 0.154 | 0.098 | ||
| R2 | 0.469 | 0.577 | 0.933 | 0.950 | ||
| 25℃ | Qm | 14.098 | 17.930 | 41.090 | 36.821 | |
| KL | 2.790 | 206.743 | 0.176 | 0.202 | ||
| R2 | 0.534 | 0.942 | 0.942 | 0.943 | ||
| 35℃ | Qm | 15.790 | 20.643 | 41.950 | 46.705 | |
| KL | 9.516 | 9.039 | 0.310 | 0.121 | ||
| R2 | 0.832 | 0.897 | 0.908 | 0.899 | ||
| Freundlich | ||||||
| 15℃ | KF | 7.050 | 11.959 | 8.831 | 6.348 | |
| n | 21.358 | 11.485 | 2.938 | 2.529 | ||
| R2 | 0.818 | 0.856 | 0.997 | 0.981 | ||
| 25℃ | KF | 9.314 | 14.849 | 10.442 | 9.559 | |
| n | 7.463 | 13.947 | 3.037 | 3.070 | ||
| R2 | 0.848 | 0.970 | 0.993 | 0.972 | ||
| 35℃ | KF | 11.786 | 14.645 | 12.212 | 8.519 | |
| n | 9.901 | 7.680 | 3.173 | 2.456 | ||
| R2 | 0.972 | 0.958 | 0.979 | 0.959 | ||
| 吸附剂 | 理论最大吸附量(Qm) | 参考文献 | |
|---|---|---|---|
| TC | Cu2+ | ||
| BE500 | 41.090mg/g | 本研究 | |
| BE700 | 17.930mg/g | 本研究 | |
| 茶渣生物炭 | 8.081mg/g | [ | |
| 壳聚糖 | 4.24mg/g | [ | |
| 改性石英砂 | 25.60mg/g | [ | |
| 多基团秸秆纤维 | 4.71mg/g | [ | |
| 自养硝化颗粒污泥 | 15.02mg/g | [ | |
| 苹果树枝生物炭 | 15.85mg/g | [ | |
表3 废白土衍生吸附剂与其他吸附剂材料的性能比较
| 吸附剂 | 理论最大吸附量(Qm) | 参考文献 | |
|---|---|---|---|
| TC | Cu2+ | ||
| BE500 | 41.090mg/g | 本研究 | |
| BE700 | 17.930mg/g | 本研究 | |
| 茶渣生物炭 | 8.081mg/g | [ | |
| 壳聚糖 | 4.24mg/g | [ | |
| 改性石英砂 | 25.60mg/g | [ | |
| 多基团秸秆纤维 | 4.71mg/g | [ | |
| 自养硝化颗粒污泥 | 15.02mg/g | [ | |
| 苹果树枝生物炭 | 15.85mg/g | [ | |
| 吸附剂 | 温度/K | Cu2+ | TC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| lnKd | ΔG/kJ∙mol-1 | ΔH/kJ∙mol-1 | ΔS/J∙mol-1∙K-1 | lnKd | ΔG/kJ∙mol-1 | ΔH/kJ∙mol-1 | ΔS/J∙mol-1∙K-1 | ||
| BE500 | 288 | 2.215 | -5.307 | 0.665 | 0.030 | 2.023 | -4.846 | 2.494 | 0.034 |
| 298 | 3.329 | -8.251 | 2.449 | -6.070 | |||||
| 308 | 4.903 | -12.561 | 2.712 | -6.949 | |||||
| BE700 | 288 | 5.793 | -13.878 | 0.083 | 0.027 | 1.324 | -3.173 | 1.663 | 0.032 |
| 298 | 8.178 | -20.273 | 2.034 | -5.043 | |||||
| 308 | 5.208 | -13.342 | 1.903 | -4.874 | |||||
表4 热力学模型拟合参数
| 吸附剂 | 温度/K | Cu2+ | TC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| lnKd | ΔG/kJ∙mol-1 | ΔH/kJ∙mol-1 | ΔS/J∙mol-1∙K-1 | lnKd | ΔG/kJ∙mol-1 | ΔH/kJ∙mol-1 | ΔS/J∙mol-1∙K-1 | ||
| BE500 | 288 | 2.215 | -5.307 | 0.665 | 0.030 | 2.023 | -4.846 | 2.494 | 0.034 |
| 298 | 3.329 | -8.251 | 2.449 | -6.070 | |||||
| 308 | 4.903 | -12.561 | 2.712 | -6.949 | |||||
| BE700 | 288 | 5.793 | -13.878 | 0.083 | 0.027 | 1.324 | -3.173 | 1.663 | 0.032 |
| 298 | 8.178 | -20.273 | 2.034 | -5.043 | |||||
| 308 | 5.208 | -13.342 | 1.903 | -4.874 | |||||
| 1 | KANG Jin, LIU Huijuan, ZHENG Yuming, et al. Systematic study of synergistic and antagonistic effects on adsorption of tetracycline and copper onto a chitosan[J]. Journal of Colloid and Interface Science, 2010, 344(1): 117-125. |
| 2 | HU Hao, ZHOU Qi, LI Xiang, et al. Phytoremediation of anaerobically digested swine wastewater contaminated by oxytetracycline via Lemna aequinoctialis: Nutrient removal, growth characteristics and degradation pathways[J]. Bioresource Technology, 2019, 291: 121853. |
| 3 | PRICE Michael S, CLASSEN John J, PAYNE Gary A. Aspergillus niger absorbs copper and zinc from swine wastewater[J]. Bioresource Technology, 2001, 77(1): 41-49. |
| 4 | 冯爱玲, 王海北, 赵磊, 等. 粉煤基沸石对Cu2+吸附行为的实验研究[J]. 有色金属工程, 2017, 7(5): 60-64. |
| FENG Ailing, WANG Haibei, ZHAO Lei, et al. Experimental study on copper adsorption behavior of zeolitized fly ash[J]. Nonferrous Metals Engineering, 2017, 7(5): 60-64. | |
| 5 | CHANG Yii Shiuan, Pek Ing AU, MUBARAK Nabisab Mujawar, et al. Adsorption of Cu(Ⅱ) and Ni(Ⅱ) ions from wastewater onto bentonite and bentonite/GO composite[J]. Environmental Science and Pollution Research, 2020, 27(26): 33270-33296. |
| 6 | LIU Yan, CHEN Qin, SINGH Rajendra Prasad. Low-cost RSAC and adsorption characteristics in the removal of copper ions from wastewater[J]. Applied Sciences, 2022, 12(11): 5612. |
| 7 | 柴琴琴, 呼世斌, 刘建伟, 等. 有机改性对凹凸棒黏土吸附四环素类抗生素的影响[J]. 中国环境监测, 2018, 34(5): 95-103. |
| CHAI Qinqin, HU Shibin, LIU Jianwei, et al. Effects of the organic modification on the attapulgite adsorption for tetracycline antibiotics[J]. Environmental Monitoring in China, 2018, 34(5): 95-103. | |
| 8 | 刘总堂, 邵江, 李艳, 等. 碱改性小麦秸秆生物炭对水中四环素的吸附性能[J]. 中国环境科学, 2022, 42(8): 3736-3743. |
| LIU Zongtang, SHAO Jiang, LI Yan, et al. Adsorption performance of tetracycline in water by alkali-modified wheat straw biochars[J]. China Environmental Science, 2022, 42(8): 3736-3743. | |
| 9 | ZHAO Rui, MA Tingting, ZHAO Shuai, et al. Uniform and stable immobilization of metal-organic frameworks into chitosan matrix for enhanced tetracycline removal from water[J]. Chemical Engineering Journal, 2020, 382: 122893. |
| 10 | 武占省. 食用油脂脱色用高效活性白土的研制及其吸附性能研究[D]. 石河子: 石河子大学, 2006. |
| WU Zhansheng. The development of acid activated bentonite for edible oil bleaching and investigation of its adsorption property[D]. Shihezi: Shihezi University, 2006. | |
| 11 | BACHMANN Suyanne Angie Lunelli, DE CÁSSIA SIQUEIRA CURTO VALLE Rita, VEGINI Atilano Antonio, et al. Determination of optimum conditions for thermal regeneration and characterization of a spent bleaching earth[J]. Journal of Environmental Chemical Engineering, 2020, 8(2): 103503. |
| 12 | BESHARA Abdelhamid, CHEESEMAN Christopher R. Reuse of spent bleaching earth by polymerisation of residual organics[J]. Waste Management, 2014, 34(10): 1770-1774. |
| 13 | 乔宝权, 周丹, 李琪, 等. 以废白土为原料制备生物柴油及其资源化利用[J]. 化工进展, 2016, 35(8): 2398-2405. |
| QIAO Baoquan, ZHOU Dan, LI Qi, et al. Biodiesel production from spent bleaching clay and its resource utilization[J]. Chemical Industry and Engineering Progress, 2016, 35(8): 2398-2405. | |
| 14 | 陈兆辉. 基于废白土的炭材料制备及其去除水中Pb(Ⅱ)的研究[D]. 郑州: 河南工业大学, 2020. |
| CHEN Zhaohui. Preparation of carbon material based on spent bleaching earth and study of Pb(Ⅱ) removal in water[D]. Zhengzhou: Henan University of Technology, 2020. | |
| 15 | CUI Song, KE Yuxin, FU Qiang, et al. Optimization preparation of biochar from garden waste and quantitative analysis for Cd2+ adsorption mechanism in aqueous solution[J]. Biomass Conversion and Biorefinery, 2022: 1-13. |
| 16 | SUI Long, TANG Chunyu, DU Qing, et al. Preparation and characterization of boron-doped corn straw biochar: Fe(Ⅱ) removal equilibrium and kinetics[J]. Journal of Environmental Sciences, 2021, 106: 116-123. |
| 17 | 余剑, 丁恒, 张智霖, 等. 改性菱角壳生物炭吸附水中土霉素性能与机理[J]. 中国环境科学, 2021, 41(12): 5688-5700. |
| YU Jian, DING Heng, ZHANG Zhilin, et al. Sorption characteristics and mechanism of oxytetracycline in water by modified biochar derived from chestnut shell[J]. China Environmental Science, 2021, 41(12): 5688-5700. | |
| 18 | KANG Xirui, GENG Na, LI Yaping, et al. Treatment of cadmium and zinc-contaminated water systems using modified biochar: contaminant uptake, adsorption ability, and mechanism[J]. Bioresource Technology, 2022, 363: 127817. |
| 19 | ZHOU Yaoyu, LIU Xiaocheng, XIANG Yujia, et al. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: adsorption mechanism and modelling[J]. Bioresource Technology, 2017, 245: 266-273. |
| 20 | KE Yuxin, CUI Song, FU Qiang, et al. Effects of pyrolysis temperature and aging treatment on the adsorption of Cd2+ and Zn2+ by coffee grounds biochar[J]. Chemosphere, 2022, 296: 134051. |
| 21 | 朱晓丽, 张婷, 王军强, 等. 改性废白土炭复合材料对恩诺沙星的吸附效应分析[J]. 农业环境科学学报, 2022, 41(2): 346-356. |
| ZHU Xiaoli, ZHANG Ting, WANG Junqiang, et al. Adsorption effects of enrofloxacin by modified spent bleaching earth carbon composites[J]. Journal of Agro-Environment Science, 2022, 41(2): 346-356. | |
| 22 | YANG Jiwen, JI Guozhao, GAO Yuan, et al. High-yield and high-performance porous biochar produced from pyrolysis of peanut shell with low-dose ammonium polyphosphate for chloramphenicol adsorption[J]. Journal of Cleaner Production, 2020, 264: 121516. |
| 23 | 李燕, 陈梅芹, 乔艳辉, 等. 废白土-花生壳生物炭吸附剂的制备及对Pb(Ⅱ)的吸附[J]. 材料导报, 2022, 36(6): 33-38. |
| LI Yan, CHEN Meiqin, QIAO Yanhui, et al. Preparation of spent bleaching earth-peanut shell biochar adsorbent for removal of Pb(Ⅱ) in water[J]. Materials Reports, 2022, 36(6): 33-38. | |
| 24 | QIN Wenxiu, SUN Na, WANG Guozhong, et al. Seaweed-derived hierarchically porous carbon for highly efficient removal of tetracycline[J]. Chinese Journal of Chemical Physics, 2022, 35(3): 578-588. |
| 25 | 徐龙凤, 魏群山, 吕强, 等. 水体模拟颗粒物对四环素的吸附特性及基本规律[J]. 环境科学, 2018, 39(4): 1668-1676. |
| XU Longfeng, WEI Qunshan, LU Qiang, et al. Adsorption of tetracycline on simulated suspended particles in water[J]. Environmental Science, 2018, 39(4): 1668-1676. | |
| 26 | TAN Yuehui, WAN Xirui, ZHOU Ting, et al. Novel Zn-Fe engineered kiwi branch biochar for the removal of Pb(Ⅱ) from aqueous solution[J]. Journal of Hazardous Materials, 2022, 424: 127349. |
| 27 | CUI Limei, WANG Yaoguang, HU Lihua, et al. Mechanism of Pb(Ⅱ) and methylene blue adsorption onto magnetic carbonate hydroxyapatite/graphene oxide[J]. RSC Advances, 2015, 5(13): 9759-9770. |
| 28 | ZHOU Yaoyu, LIU Xiaocheng, TANG Lin, et al. Insight into highly efficient co-removal of p-nitrophenol and lead by nitrogen-functionalized magnetic ordered mesoporous carbon: Performance and modelling[J]. Journal of Hazardous Materials, 2017, 333: 80-87. |
| 29 | 杨育红, 寇丽栋, 范庆峰, 等. 镁改性污泥基生物炭去除水中磷和抗生素[J]. 中国环境科学, 2022, 42(9): 4137-4144. |
| YANG Yuhong, KOU Lidong, FAN Qingfeng, et al. Removal of phosphate and antibiotics by magnesium modified sludge-derived biochar[J]. China Environmental Science, 2022, 42(9): 4137-4144. | |
| 30 | 杜恩菊, 李杨, 冯伟, 等. 丝瓜络固定颤藻吸附Pb2+的动力学及机理[J]. 中国环境科学, 2021, 41(12): 5701-5709. |
| DU Enju, LI Yang, FENG Wei, et al. Kinetics and mechanism of adsorption Pb2+ by immobilized oscillatoria lutea with loofah[J]. China Environmental Science, 2021, 41(12): 5701-5709. | |
| 31 | 刘秀芸, 王刚, 雷雨昕, 等. 巯基改性玉米秸秆对水中Cu(Ⅱ)的吸附特性[J]. 中国环境科学, 2022, 42(3): 1220-1229. |
| LIU Xiuyun, WANG Gang, LEI Yuxin, et al. Adsorption performance and mechanism of mercaptoacetyl corn straw for Cu(Ⅱ) in aqueous solution[J]. China Environmental Science, 2022, 42(3): 1220-1229. | |
| 32 | 范世锁, 刘文浦, 王锦涛, 等. 茶渣生物炭制备及其对溶液中四环素的去除特性[J]. 环境科学, 2020, 41(3): 1308-1318. |
| FAN Shisuo, LIU Wenpu, WANG Jingtao, et al. Preparation of tea waste biochar and tts application in tetracycline removal from aqueous solution[J]. Environmental Science, 2020, 41(3): 1308-1318. | |
| 33 | TURAN Busra, SARIGOL Gulhan, DEMIRCIVI Pelin. Adsorption of tetracycline antibiotics using metal and clay embedded cross-linked chitosan[J]. Materials Chemistry and Physics, 2022, 279: 125781. |
| 34 | 李川, 刘元慧, 王让, 等. 改性石英砂吸附水中四环素的机制研究[J]. 中国给水排水, 2019, 35(3): 71-77. |
| LI Chuan, LIU Yuanhui, WANG Rang, et al. Adsorption mechanism of tetracycline by modified quartz sand[J]. China Water and Wastewater, 2019, 35(3): 71-77. | |
| 35 | 孟佩佩, 张丹凤, 伦乐豪, 等. 多基团秸秆纤维对水体中铜/磺胺甲恶唑复合污染物的定量吸附性能[J]. 环境工程学报,2023, 17(2): 390-403. |
| MENG Peipei, ZHANG Danfeng, Lehao LUN, et al. Quantitative adsorption behavior of copper/sulfonamides compound pollutants in aqueous solution by multi-groups straw fibers[J]. Chinese Journal of Environmental Engineering, 2023, 17(2): 390-403. | |
| 36 | 张斌超, 曾敏静, 张立楠, 等. 自养硝化颗粒污泥吸附铜离子性能及吸附等温线[J]. 化工进展, 2020, 39(4): 1583-1590. |
| ZHANG Binchao, ZENG Minjing, ZHANG Linan, et al. Adsorption of Cu2+ by autotrophic nitrifying granular sludge and its adsorption isotherm[J] Chemical Industry and Engineering Progress, 2020, 39(4): 1583-1590. | |
| 37 | 王彤彤, 马江波, 曲东, 等. 两种木材生物炭对铜离子的吸附特性及其机制[J]. 环境科学, 2017, 38(5): 2161-2171. |
| WANG Tongtong, MA Jiangbo, QU Dong, et al. Characteristics and mechanism of copper adsorption from aqueous solutions on biochar produced from sawdust and apple branch[J]. Environmental Science, 2017, 38(5): 2161-2171. | |
| 38 | 唐玉婷, 丁思淳, 韩承霖. 腐殖酸负载对凹凸棒土吸附Zn(Ⅱ)的影响[J]. 华南理工大学学报(自然科学版), 2022, 50(4): 110-118. |
| TANG Yuting, DING Sichun, HAN Chenglin. Effect of humic acid loading on palygorskite’s adsorption of Zn(Ⅱ)[J]. Journal of South China University of Technology (Natural Science Edition), 2022, 50(4): 110-118. | |
| 39 | 孙宁妍, 邱海燕, 兰贵红, 等. 磁性羟基磷灰石改性氮化硼对Pb2+的吸附特性[J]. 精细化工, 2022, 39(4): 725-733. |
| SUN Ningyan, QIU Haiyan, LAN Guihong, et al. Adsorption performance of Pb2+ by magnetic hydroxyapatite modified boron nitride[J]. Fine Chemicals, 2022, 39(4): 725-733. | |
| 40 | 王超, 王迎亚, 陈宁华, 等. 磁性膨润土对四环素的吸附特性[J]. 精细化工, 2017, 34(10): 1185-1193. |
| WANG Chao, WANG Yingya, CHEN Ninghua, et al. Adsorption of tetracycline by magnetic bentonite[J]. Fine Chemicals, 2017, 34(10): 1185-1193. | |
| 41 | WANG Rongzhong, HUANG Danlian, LIU Yunguo, et al. Synergistic removal of copper and tetracycline from aqueous solution by steam-activated bamboo-derived biochar[J]. Journal of Hazardous Materials, 2020, 384: 121470. |
| 42 | WANG Ke, WANG Yue, ZHANG Shiyu, et al. Tailoring a novel hierarchical cheese-like porous biochar from algae residue to boost sulfathiazole removal[J]. Environmental Science and Ecotechnology, 2022, 10: 100168. |
| 43 | SHI Jindou, GUO Caili, LEI Changyang, et al. High-performance biochar derived from the residue of Chaga mushroom (Inonotus obliquus) for pollutants removal[J]. Bioresource Technology, 2022, 344: 126268. |
| 44 | YAO Guangyuan, LIU Yuqiang, ZHENG Shuilin, et al. High removal efficiency of diatomite-based X zeolite for Cu2+ and Zn2+ [J]. Materials, 2021, 14(21): 6525. |
| 45 | 郭志伟, 赵宝龙, 郑志宏, 等. 碳化改性硅藻土对四环素的吸附[J]. 环境工程, 2022, 40(5): 44-52. |
| GUO Zhiwei, ZHAO Baolong, ZHENG Zhihong, et al. Preparation of modified diatomite via carbonization and its adsorption performance on tetracycline[J]. Environmental Engineering, 2022, 40(5): 44-52. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [4] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [5] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [6] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [7] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [8] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| [9] | 赵重阳, 赵磊, 石详文, 黄俊, 李治尧, 沈凯, 张亚平. O2/H2O/SO2 对改性富铁凹凸棒石高温吸附PbCl2 的影响[J]. 化工进展, 2023, 42(4): 2190-2200. |
| [10] | 郭帅帅, 陈锦路, 金梁程龙, 陶醉, 陈小丽, 彭国文. 基于海水提铀的多孔芳香框架材料研究进展[J]. 化工进展, 2023, 42(3): 1426-1436. |
| [11] | 郭亚宁, 季军荣, 焦妍惠, 张庆年, 周洲, 韦德恩, 童张法, 李立硕. 机械活化重质碳酸钙制备复合碳酸钙及其对溶液中的Cu2+吸附性能[J]. 化工进展, 2023, 42(11): 5861-5870. |
| [12] | 王胜楠, 郑旭. 空气取水用活性炭纤维复合吸附剂的研究[J]. 化工进展, 2023, 42(10): 5567-5573. |
| [13] | 吴中杰, 谢连科, 王晶辉, 黄仁亮. 多级结构羟基硝酸铜纳米酶的制备及其对酚类污染物的降解[J]. 化工进展, 2023, 42(1): 497-505. |
| [14] | 曹正凯, 米晓斌, 吴子明, 孙士可, 曹均丰, 彭德强, 梁相程. 煤合成气净化除尘装置压降问题分析及应用优化[J]. 化工进展, 2022, 41(S1): 15-21. |
| [15] | 王子航, 梁瑞升, 邓超和, 王佳韵. 离子凝胶复合吸附剂的制备及空气取水性能[J]. 化工进展, 2022, 41(S1): 389-396. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||