化工进展 ›› 2023, Vol. 42 ›› Issue (11): 5861-5870.DOI: 10.16085/j.issn.1000-6613.2022-2351
• 材料科学与技术 • 上一篇
机械活化重质碳酸钙制备复合碳酸钙及其对溶液中的Cu2+吸附性能
郭亚宁1( ), 季军荣2, 焦妍惠1, 张庆年2, 周洲2, 韦德恩2, 童张法1, 李立硕1(
), 季军荣2, 焦妍惠1, 张庆年2, 周洲2, 韦德恩2, 童张法1, 李立硕1( )
)
- 1.广西大学化学化工学院,广西 南宁 530004
2.崇左南方水泥有限公司广西钙基材料协同创新中心,广西 南宁 530004
-
收稿日期:2022-12-23修回日期:2023-03-12出版日期:2023-11-20发布日期:2023-12-15 -
通讯作者:李立硕 -
作者简介:郭亚宁(1999—),女,硕士研究生,研究方向为无机材料。E-mail:gyn426@126.com。 -
基金资助:广西钙基材料协同创新中心项目(CZNF-JSZX20-03);广西碳酸钙产业化工程院公司级科研项目(GJZX2022-4)
Preparation of calcium carbonate composite by mechanically activated of ground calcium carbonate and its adsorption properties on Cu2+ ions in solution
GUO Yaning1( ), JI Junrong2, JIAO Yanhui1, ZHANG Qingnian2, ZHOU Zhou2, WEI Deen2, TONG Zhangfa1, LI Lishuo1(
), JI Junrong2, JIAO Yanhui1, ZHANG Qingnian2, ZHOU Zhou2, WEI Deen2, TONG Zhangfa1, LI Lishuo1( )
)
- 1.School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, Guangxi, China
2.Chongzuo South Cement Co. , Ltd. , Guangxi Collaborative Innovation Center of Calcium-based Materials, Nanning 530004, Guangxi, China
-
Received:2022-12-23Revised:2023-03-12Online:2023-11-20Published:2023-12-15 -
Contact:LI Lishuo
摘要:
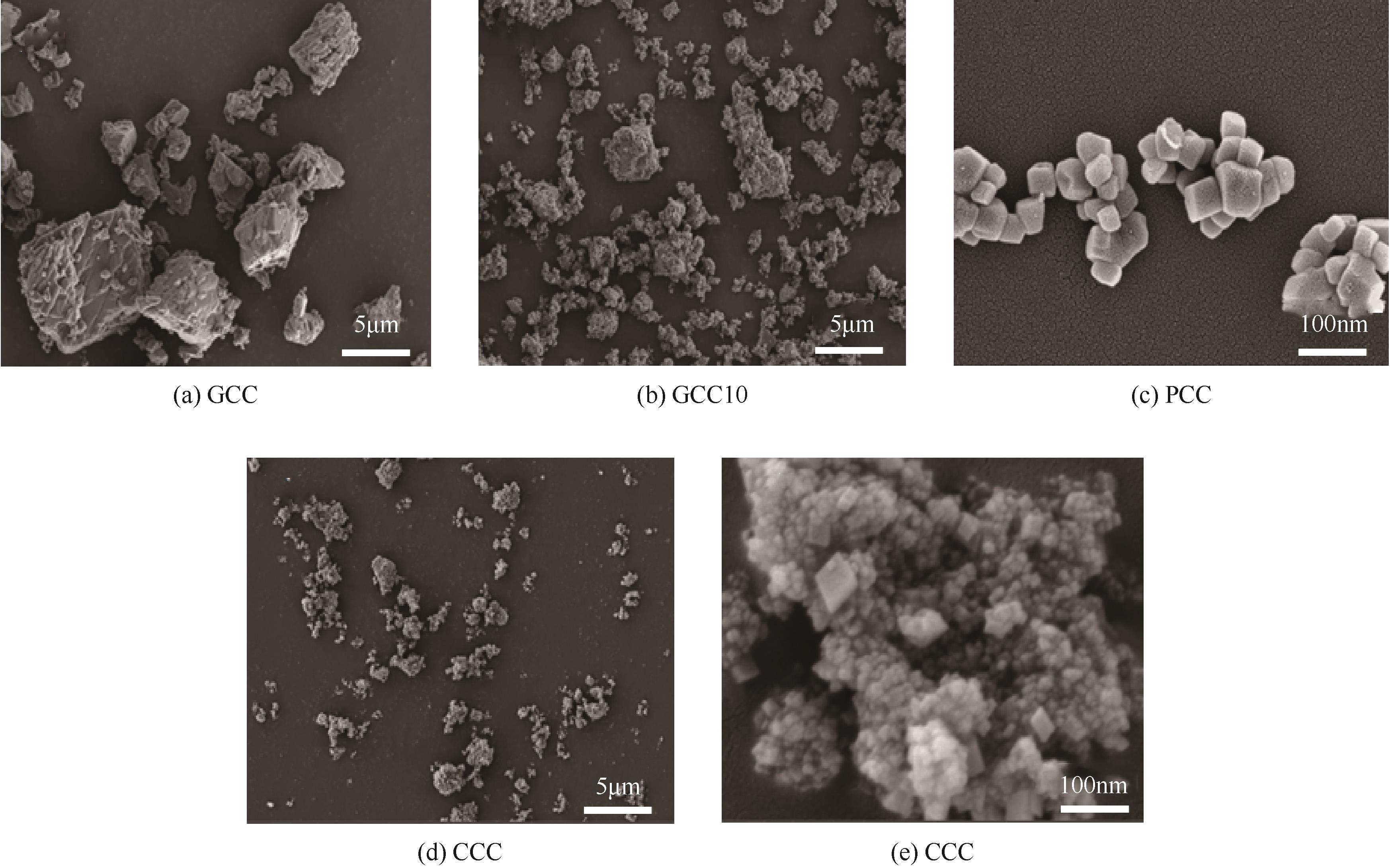
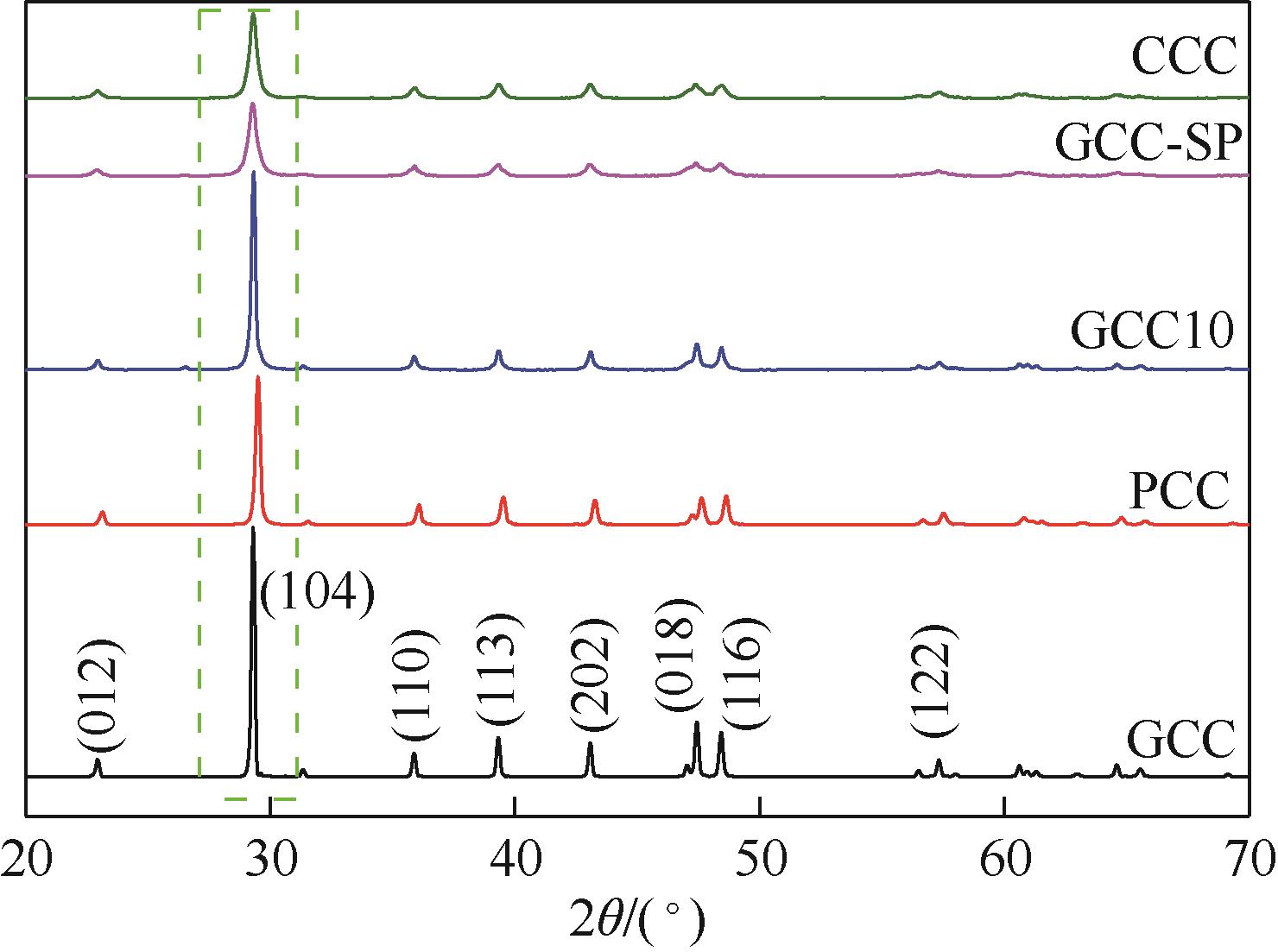
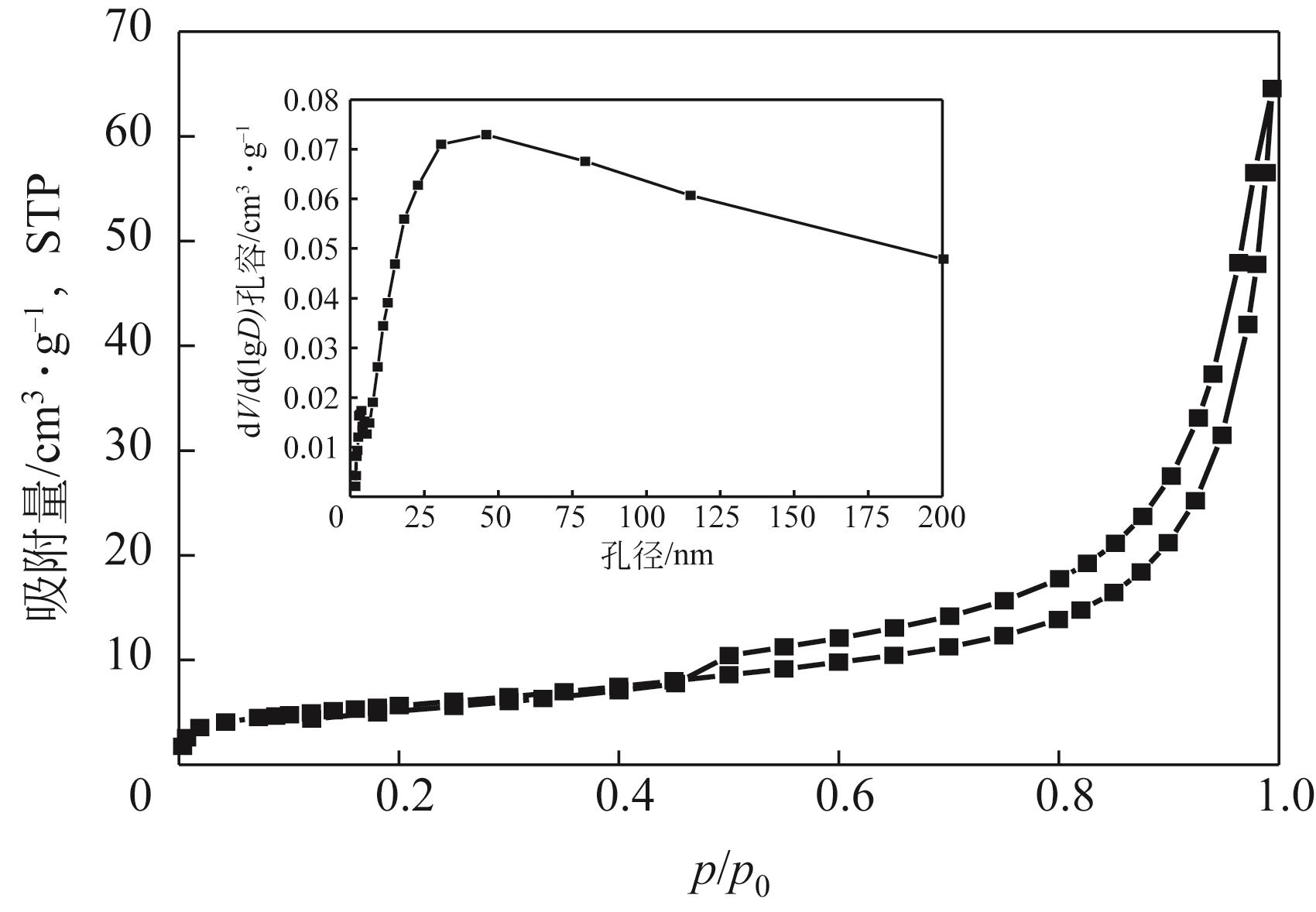
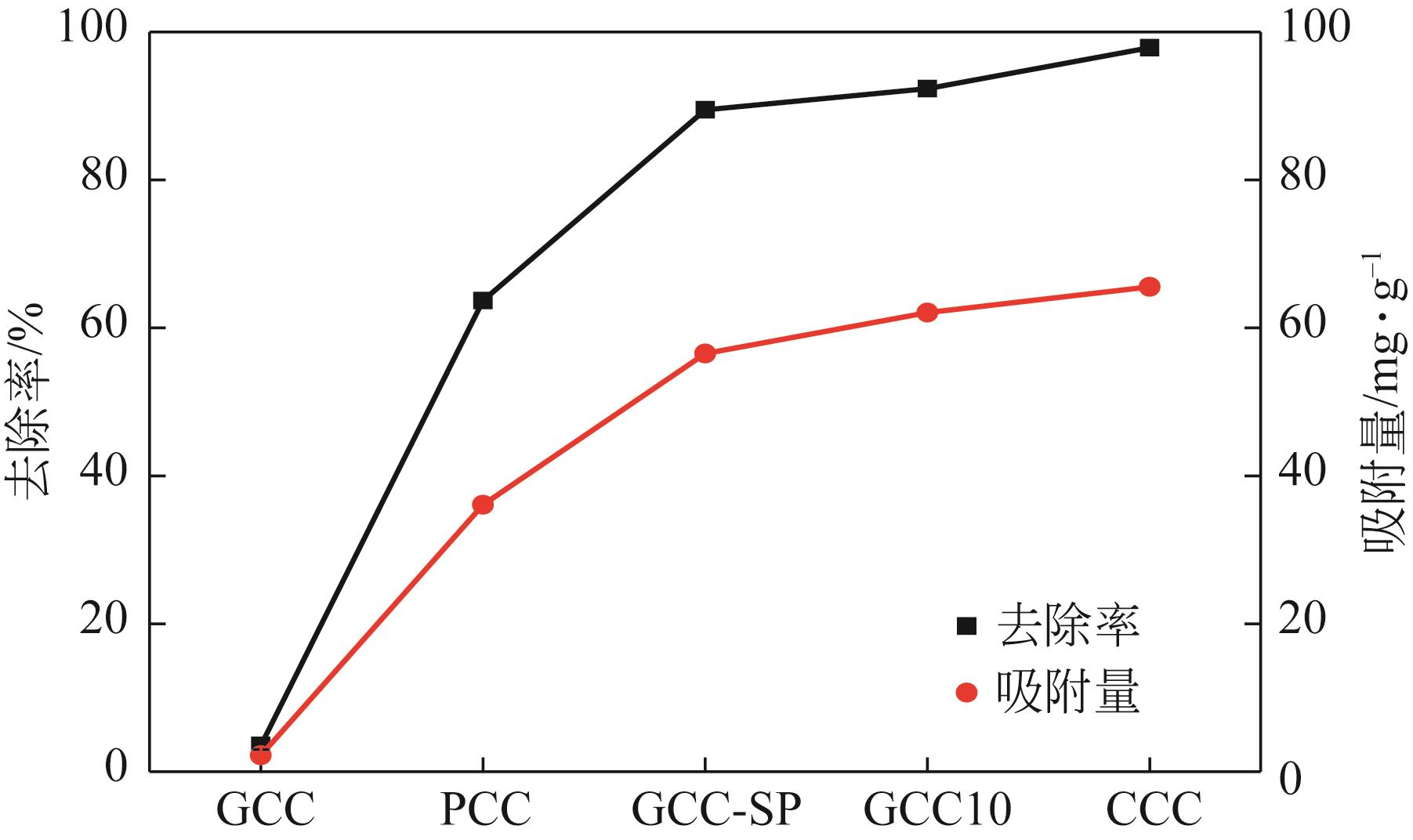
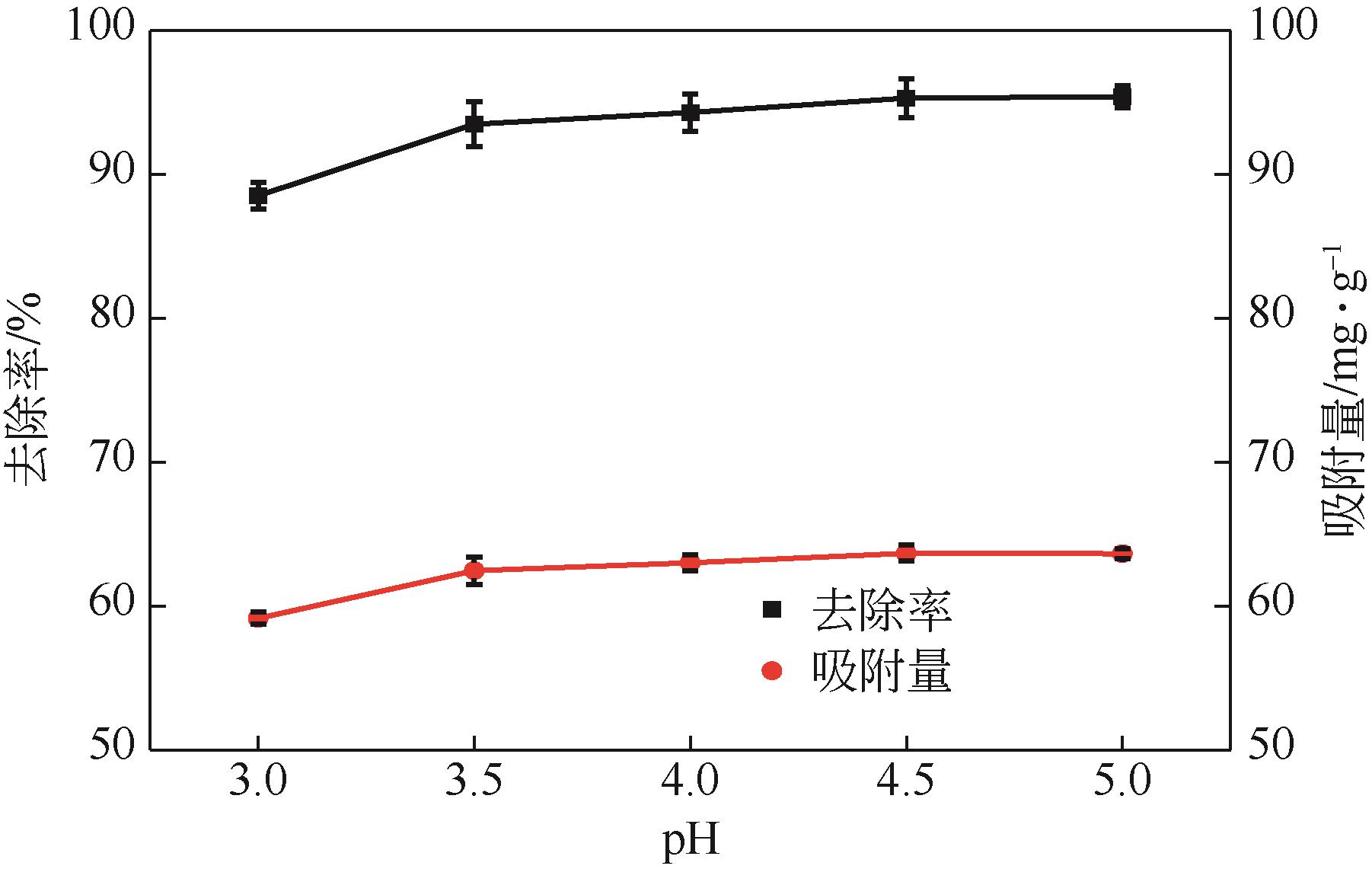
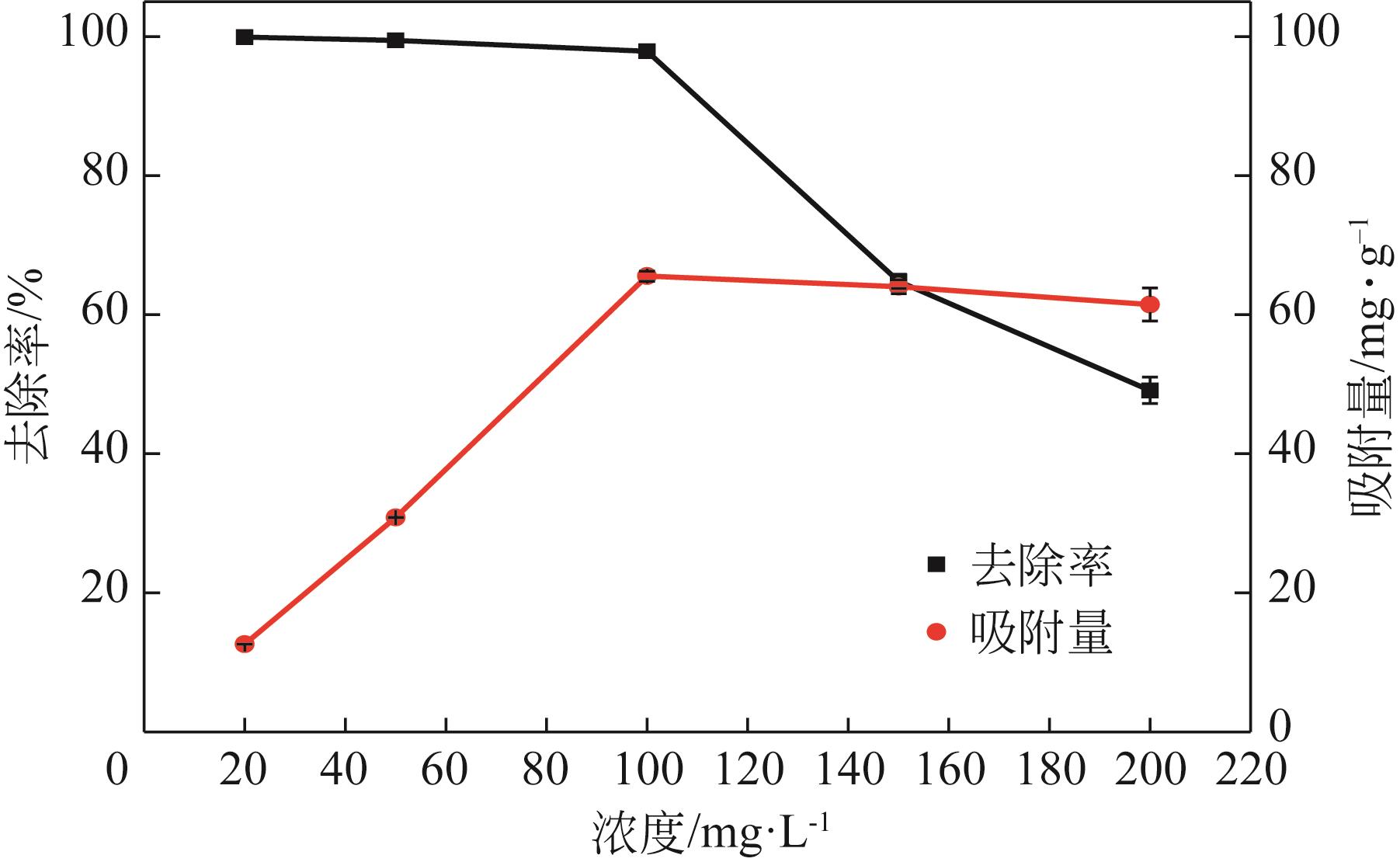
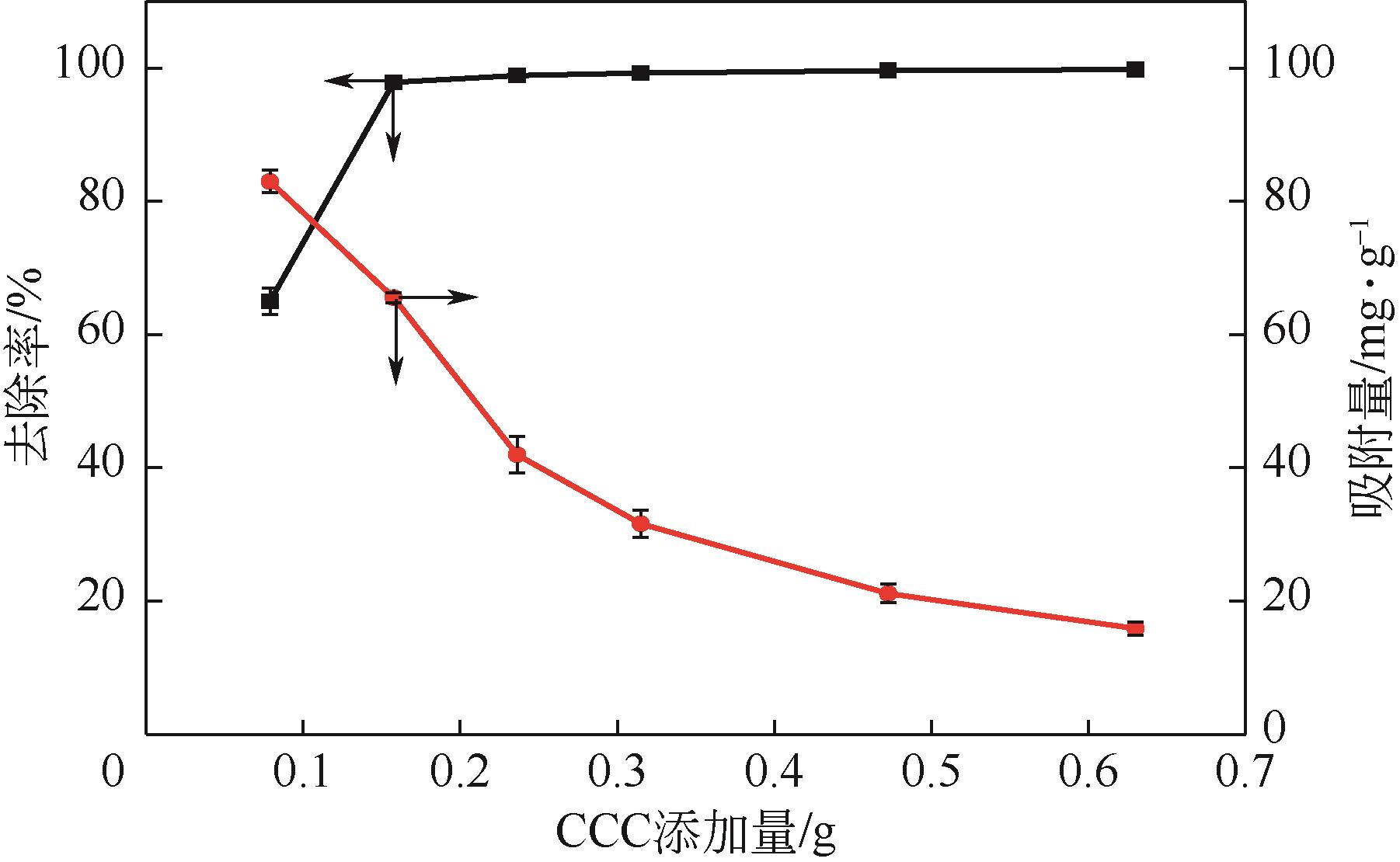
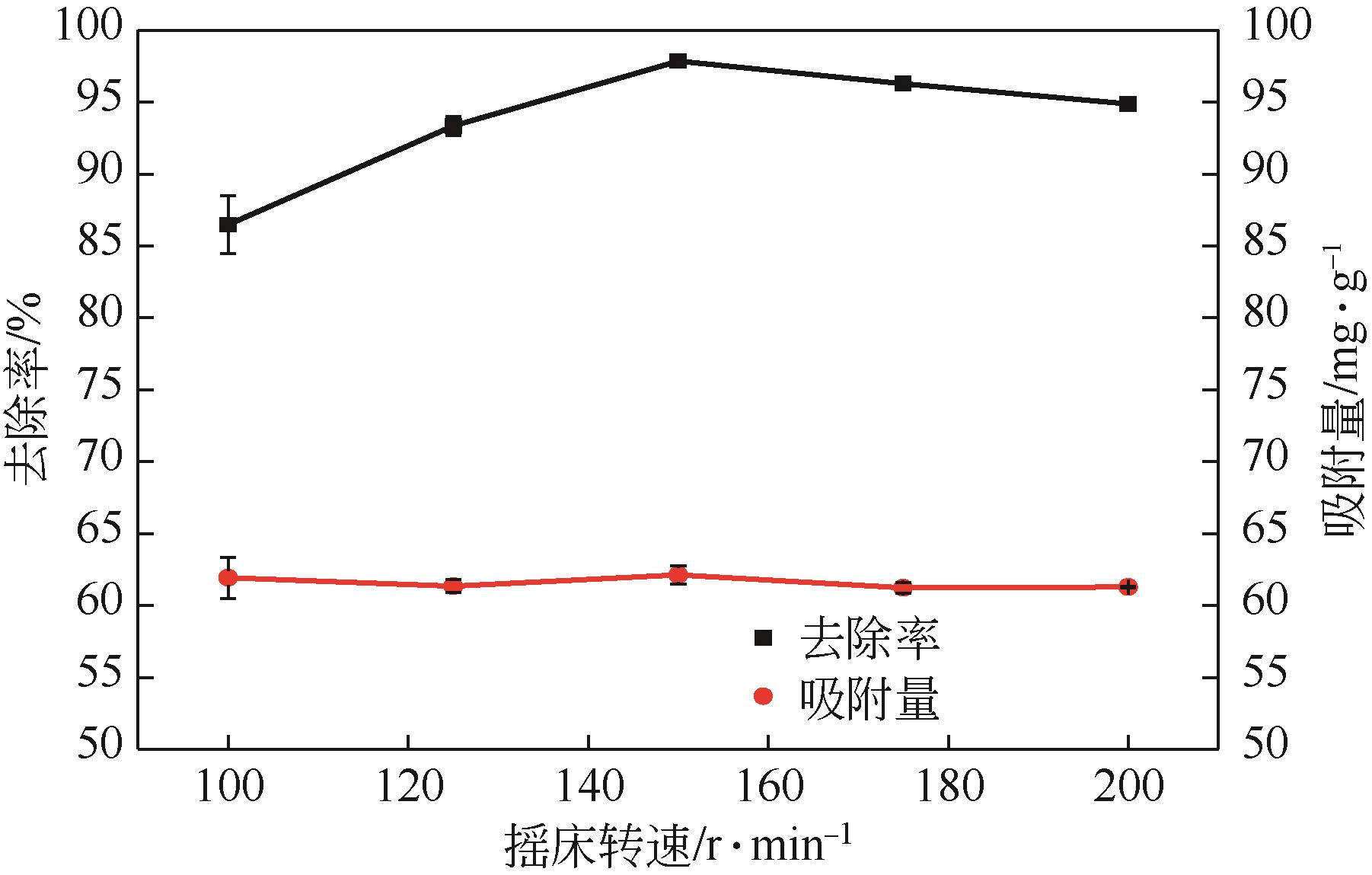
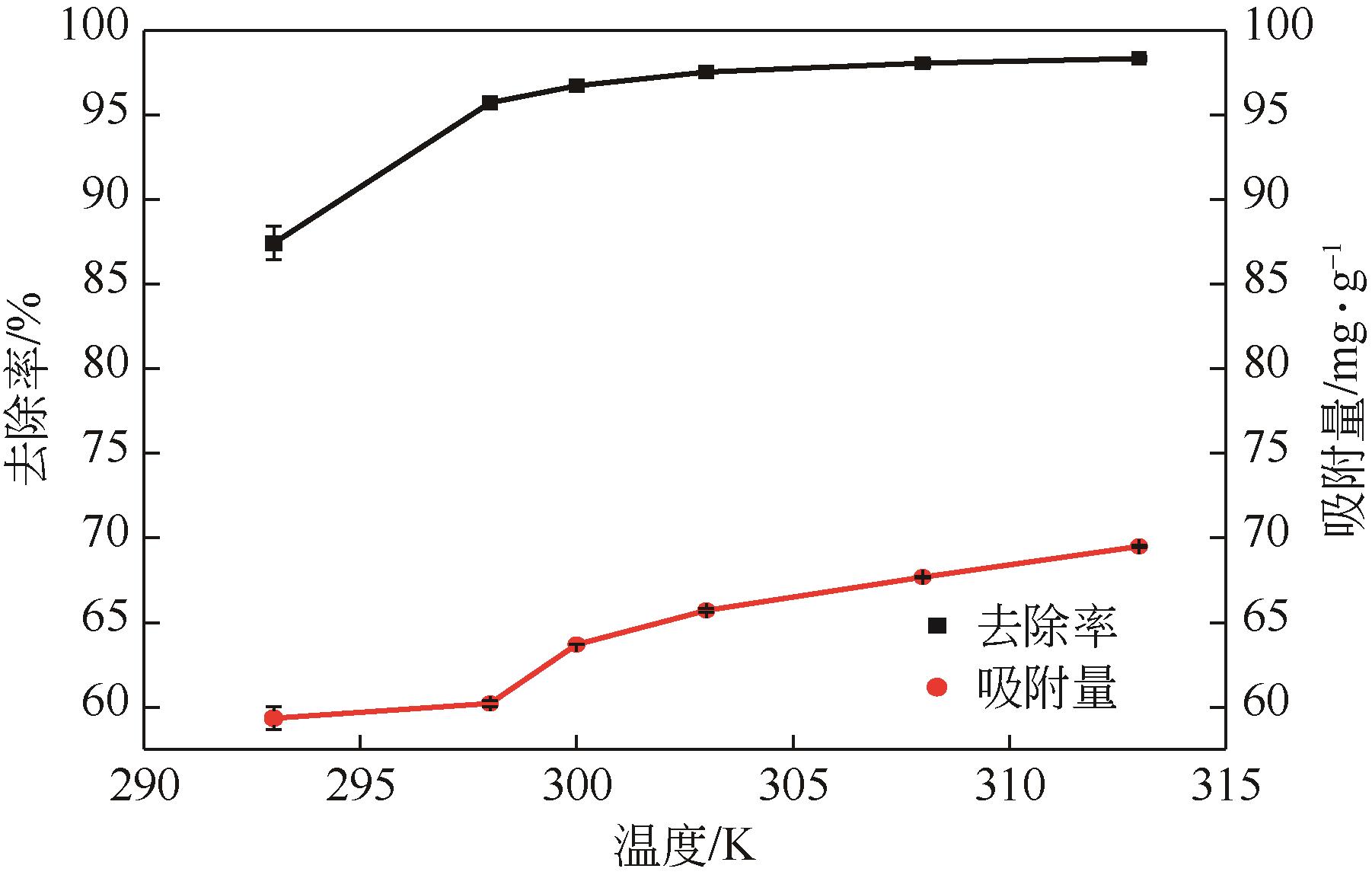
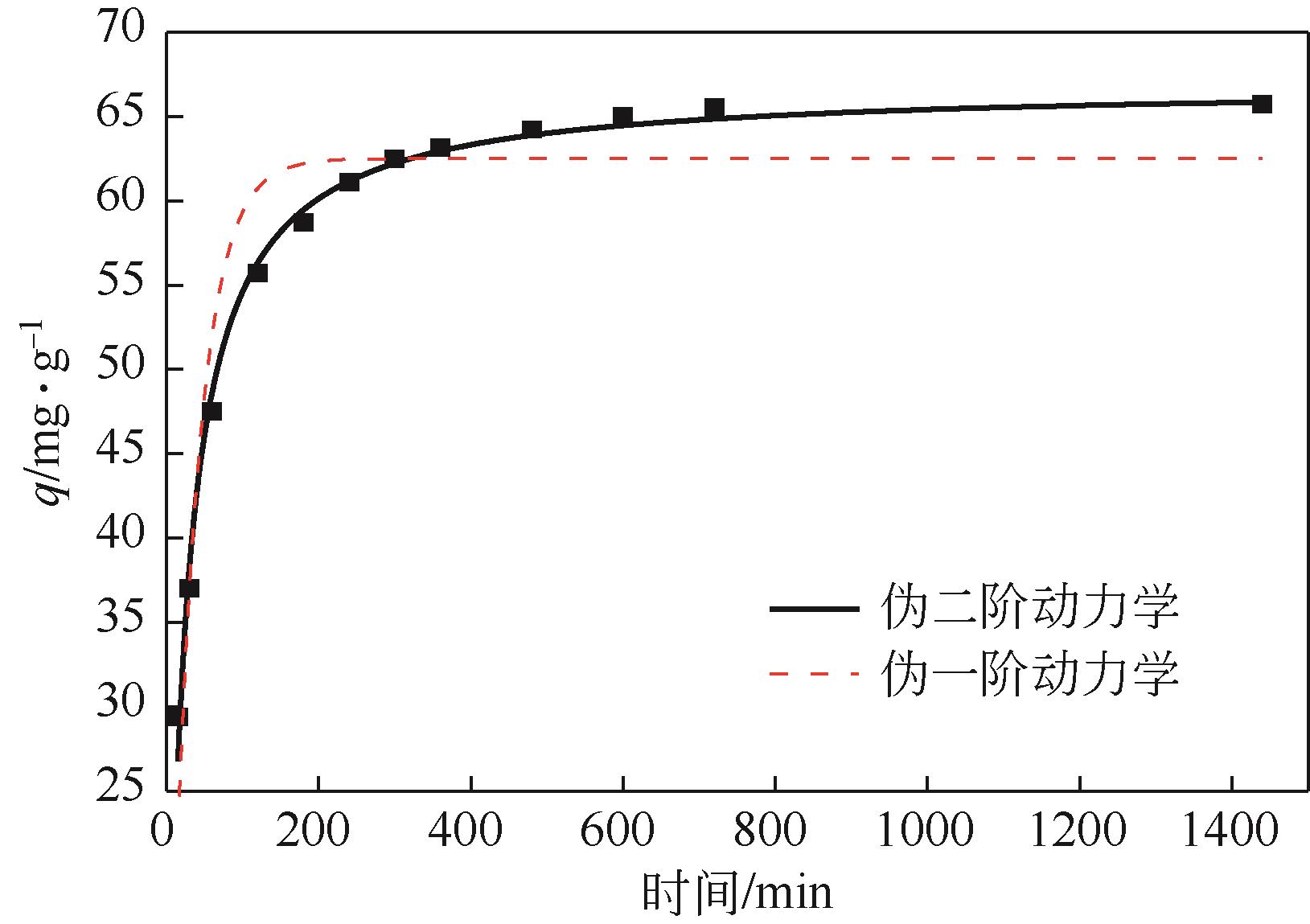
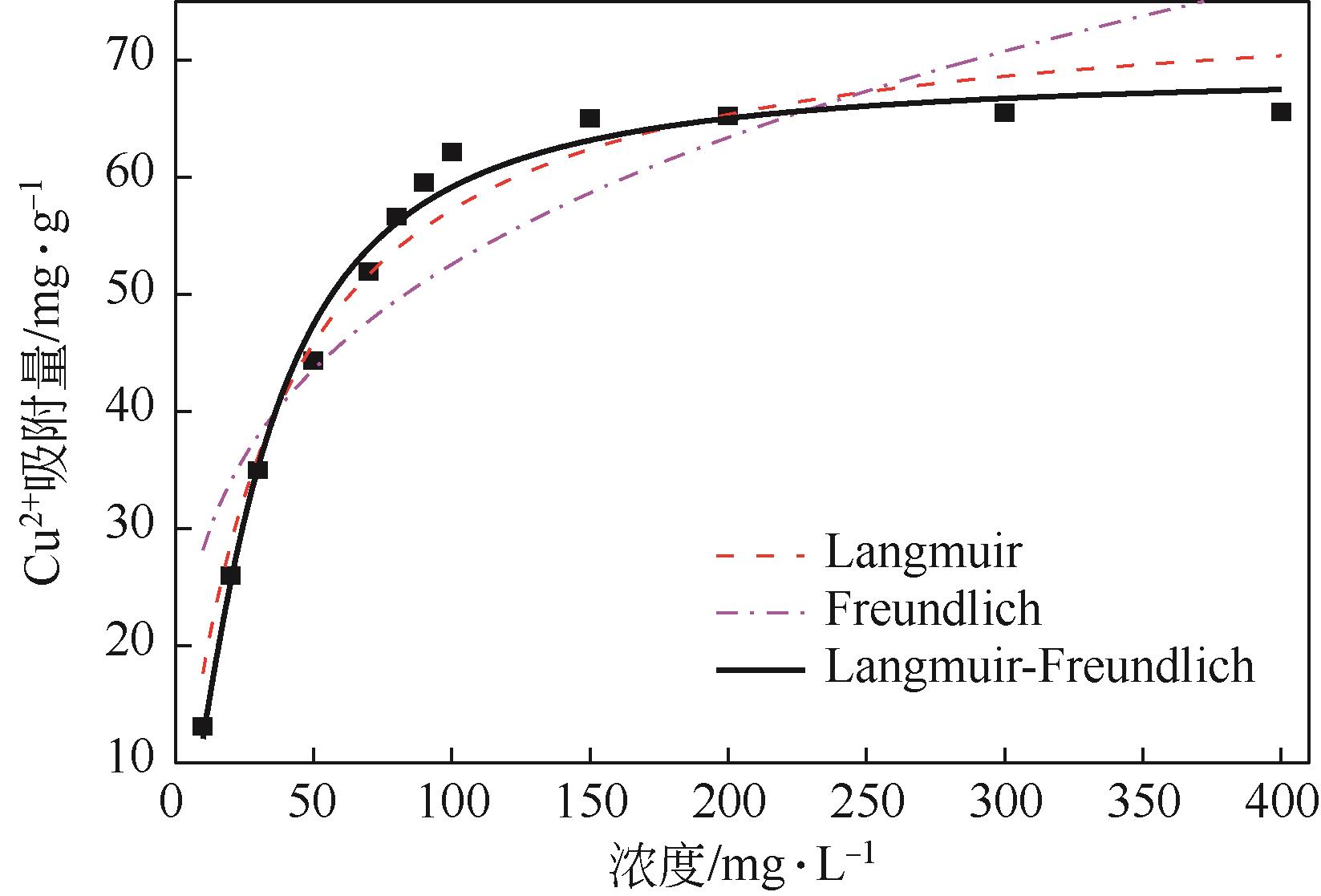
利用机械活化法对重质碳酸钙进行了纳米化表面改性,以聚丙烯酸钠和纳米碳酸钙为改性原料与重质碳酸钙进行混合研磨,成功制备了纳米碳酸钙包覆重质碳酸钙的复合钙,并考察了复合钙对溶液中的Cu2+的吸附性能。研究结果如下:扫描电子显微镜和X射线衍射仪分析证实了纳米碳酸钙包覆在重质碳酸钙表面,傅里叶变换红外光谱分析表明复合碳酸钙表面羟基明显增加,比表面积分析表明复合碳酸钙的比表面积为20.5m2/g,具有介孔结构。复合碳酸钙对Cu2+的最大吸附量为65.5mg/g,去除率可达98%。吸附性能研究表明,复合碳酸钙对Cu2+吸附动力学符合伪二阶动力学模型,等温吸附过程符合Langmuir-Freundich模型,证明吸附过程以离子交换反应为主,该过程为吸热反应。
中图分类号:
引用本文
郭亚宁, 季军荣, 焦妍惠, 张庆年, 周洲, 韦德恩, 童张法, 李立硕. 机械活化重质碳酸钙制备复合碳酸钙及其对溶液中的Cu2+吸附性能[J]. 化工进展, 2023, 42(11): 5861-5870.
GUO Yaning, JI Junrong, JIAO Yanhui, ZHANG Qingnian, ZHOU Zhou, WEI Deen, TONG Zhangfa, LI Lishuo. Preparation of calcium carbonate composite by mechanically activated of ground calcium carbonate and its adsorption properties on Cu2+ ions in solution[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5861-5870.
| 项目 | 不同碳酸钙 | ||||
|---|---|---|---|---|---|
| GCC | GCC10 | GCC-SP | CCC | PCC | |
| 比表面积/m2·g-1 | 4.25 | 8.40 | 6.92 | 20.50 | 35.20 |
表1 不同碳酸钙的比表面积
| 项目 | 不同碳酸钙 | ||||
|---|---|---|---|---|---|
| GCC | GCC10 | GCC-SP | CCC | PCC | |
| 比表面积/m2·g-1 | 4.25 | 8.40 | 6.92 | 20.50 | 35.20 |
| 项目 | 不同碳酸钙 | ||||
|---|---|---|---|---|---|
| GCC | PCC | GCC-SP | GCC10 | CCC | |
| 吸附量/m2·g-1 | 2.23 | 36.10 | 56.54 | 62.05 | 65.53 |
表2 不同碳酸钙的吸附量
| 项目 | 不同碳酸钙 | ||||
|---|---|---|---|---|---|
| GCC | PCC | GCC-SP | GCC10 | CCC | |
| 吸附量/m2·g-1 | 2.23 | 36.10 | 56.54 | 62.05 | 65.53 |
| 模型 | k/min-1 | q/mg·g-1 | R2 |
|---|---|---|---|
| 伪一阶动力学模型 | 0.02987 | 62.50497 | 0.90587 |
| 伪二阶动力学模型 | 0.00067 | 66.87839 | 0.99190 |
表3 使用伪一阶和伪二阶模型对复合碳酸钙吸附Cu2+的拟合结果
| 模型 | k/min-1 | q/mg·g-1 | R2 |
|---|---|---|---|
| 伪一阶动力学模型 | 0.02987 | 62.50497 | 0.90587 |
| 伪二阶动力学模型 | 0.00067 | 66.87839 | 0.99190 |
| 模型 | n | R2 | ||
|---|---|---|---|---|
| Langmuir模型 | 0.03012 | 76.21394 | — | 0.96225 |
| Freundich模型 | 15.11485 | — | 3.69497 | 0.76707 |
Langmuir- Freundich 模型 | 0.03441 | 68.97473 | 0.68857 | 0.98725 |
表4 使用Langmuir模型、Freundlich模型和Langmuir-Freundich模型对复合碳酸钙吸附Cu2+的拟合结果
| 模型 | n | R2 | ||
|---|---|---|---|---|
| Langmuir模型 | 0.03012 | 76.21394 | — | 0.96225 |
| Freundich模型 | 15.11485 | — | 3.69497 | 0.76707 |
Langmuir- Freundich 模型 | 0.03441 | 68.97473 | 0.68857 | 0.98725 |
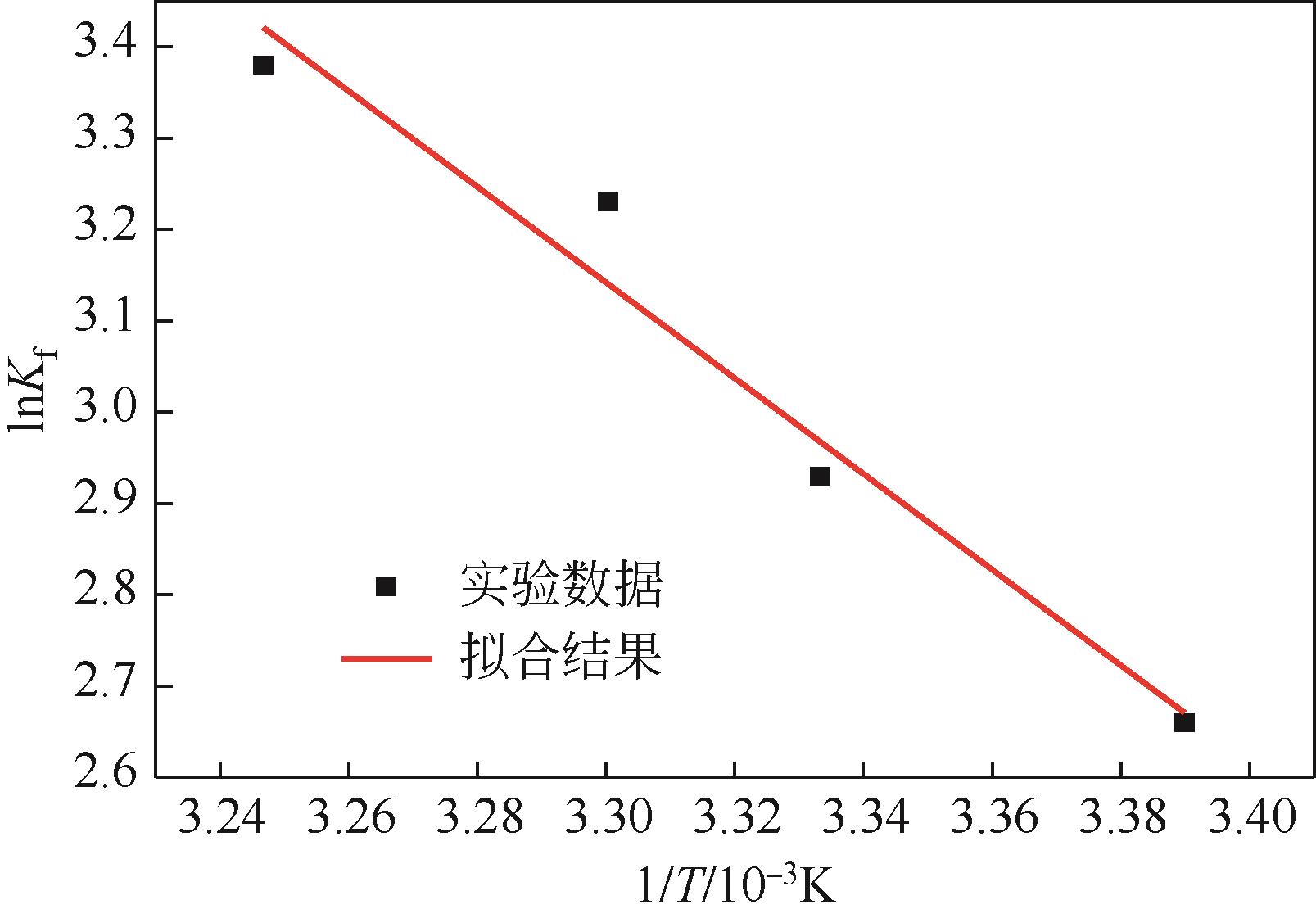
| 温度/K | ΔG/kJ·mol-1 | ΔHθ/kJ·mol-1 | ΔSθ/J·mol-1·K-1 | |
|---|---|---|---|---|
| 298 | 2.66 | -6.58 | 43.60 | 170.01 |
| 300 | 2.93 | -7.31 | ||
| 303 | 3.23 | -8.13 | ||
| 308 | 3.38 | -8.66 |
表5 复合碳酸钙热力学参数
| 温度/K | ΔG/kJ·mol-1 | ΔHθ/kJ·mol-1 | ΔSθ/J·mol-1·K-1 | |
|---|---|---|---|---|
| 298 | 2.66 | -6.58 | 43.60 | 170.01 |
| 300 | 2.93 | -7.31 | ||
| 303 | 3.23 | -8.13 | ||
| 308 | 3.38 | -8.66 |
| 1 | HE Yang, GOU Shaohua, ZHOU Lihua, et al. Amidoxime-functionalized polyacrylamide-modified chitosan containing imidazoline groups for effective removal of Cu2+ and Ni2+ [J]. Carbohydrate Polymers, 2021, 252: 117160. |
| 2 | ZOU Yidong, WANG Xiangxue, Khan Ayub, et al. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review[J]. Environmental Science & Technology, 2016, 50(14): 7290-7304. |
| 3 | Thomas Maciej, Kozik Violetta, Bąk Andrzej, et al. Removal of heavy metal ions from wastewaters: An application of sodium trithiocarbonate and wastewater toxicity assessment[J]. Materials, 2021, 14(3): 655. |
| 4 | ISLAM Md Shahinul, CHOI Won San, Bora NAM, et al. Needle-like iron oxide@CaCO3 adsorbents for ultrafast removal of anionic and cationic heavy metal ions[J]. Chemical Engineering Journal, 2017, 307: 208-219. |
| 5 | CHADA Venkata Gopal Reddy, HAUSNER Douglas B, STRONGIN Daniel R, et al. Divalent Cd and Pb uptake on calcite{1014}cleavage faces: An XPS and AFM study[J]. Journal of Colloid and Interface Science, 2005, 288(2): 350-360. |
| 6 | HU Huimin, LI Xuewei, HUANG Pengwu, et al. Efficient removal of copper from wastewater by using mechanically activated calcium carbonate[J]. Journal of Environmental Management, 2017, 203: 1-7. |
| 7 | PENG Weijun, CHANG Luping, LI Peiya, et al. An overview on the surfactants used in ion flotation[J]. Journal of Molecular Liquids, 2019, 286: 110955. |
| 8 | ZHAO Yunliang, KANG Shichang, QIN Lei, et al. Self-assembled gels of Fe-chitosan/montmorillonite nanosheets: Dye degradation by the synergistic effect of adsorption and photo-Fenton reaction[J]. Chemical Engineering Journal, 2020, 379: 122322. |
| 9 | PARK Seong-Jik, KANG Ku, LEE Changgu, et al. Remediation of metal-contaminated marine sediments using active capping with limestone, steel slag, and activated carbon: A laboratory experiment[J]. Environmental Technology, 2019, 40(26): 3479-3491. |
| 10 | BOCK Sergej, KIJATKIN Christian, BERBEN Dirk, et al. Absorption and remission characterization of pure, dielectric (nano-) powders using diffuse reflectance spectroscopy: An end-to-end instruction[J]. Applied Sciences, 2019, 9(22): 4933. |
| 11 | KIM Yu-mi, ROH Y. Environmental application of biogenic magnetite nanoparticles to remediate chromium(Ⅲ/Ⅵ)-contaminated water[J]. Minerals, 2019, 9(5): 260. |
| 12 | PUNG A, GOLDFLAM M, BURCKEL D, et al. Enhancing absorption bandwidth through vertically oriented metamaterials[J]. Applied Sciences, 2019, 9(11): 2223. |
| 13 | KUMAR Vijay, SINGH Vinay Kumar, SRIVASTAVA Abhinav, et al. Mechanochemically synthesized high alumina cement and their implementation as low cement castables with some micro-fine additives[J]. Journal of Asian Ceramic Societies, 2015, 3(1): 92-102. |
| 14 | ROMEO H E, FANOVICH M A. Synthesis of tetracalcium phosphate from mechanochemically activated reactants and assessment as a component of bone cements[J]. Journal of Materials Science: Materials in Medicine, 2008, 19(7): 2751-2760. |
| 15 | HUANG Xiang, DONG Kai, LIU Lan, et al. Physicochemical and structural characteristics of nano eggshell calcium prepared by wet ball milling[J]. LWT, 2020, 131: 109721. |
| 16 | 李永利, 乔冠军, 金志浩. 纳米BN包覆的Al2O3复合粉的制备及其烧结性能研究[J]. 硅酸盐学报, 2002, 30(4): 491-495. |
| LI Yongli, QIAO Guanjun, JIN Zhihao. Study on fabrication and sintering of Al2O3 composite powder coated with nano-sized BN[J]. Journal of the Chinese Ceramic Society, 2002, 30(4): 491-495. | |
| 17 | 梁朝, 李春全, 孙志明, 等. 新型有机改性剂对重质碳酸钙的表面改性效果及机理[J]. 无机盐工业, 2022, 54(7): 70-77. |
| LIANG Chao, LI Chunquan, SUN Zhiming, et al. Surface modification effect and mechanism of new organic modifiers on ground calcium carbonate[J]. Inorganic Chemicals Industry, 2022, 54(7): 70-77. | |
| 18 | HE Guandi, ZHANG Zhenming, WU Xianliang, et al. Adsorption of heavy metals on soil collected from lixisol of typical Karst areas in the presence of CaCO3 and soil clay and their competition behavior[J]. Sustainability, 2020, 12(18): 7315. |
| 19 | ZHANG Yu, ZHANG Liang, GAO Ruohui, et al. CaCO3-coated PVA/BC-based composite for the simultaneous adsorption of Cu( Ⅱ ), Cd( Ⅱ ), Pb( Ⅱ ) in aqueous solution[J]. Carbohydrate Polymers, 2021, 267: 118227. |
| 20 | WEN Tong, ZHAO Yunliang, ZHANG Tingting, et al. Effect of anions species on copper removal from wastewater by using mechanically activated calcium carbonate[J]. Chemosphere, 2019, 230: 127-135. |
| 21 | WEN Tong, ZHAO Yunliang, ZHANG Tingting, et al. Selective recovery of heavy metals from wastewater by mechanically activated calcium carbonate: Inspiration from nature[J]. Chemosphere, 2020, 246: 125842. |
| 22 | ZHANG Zhen, HE Shuran, ZHANG Yulong, et al. Spectroscopic investigation of Cu2+, Pb2+ and Cd2+ adsorption behaviors by chitosan-coated argillaceous limestone: Competition and mechanisms[J]. Environmental Pollution, 2019, 254: 112938. |
| 23 | Said Ahmed, HU Huimin, LIU Yanchu, et al. Mechanochemical activation of phlogopite to enhance its capacity as absorbent for the removal of heavy metal ions[J]. Water, Air & Soil Pollution, 2021, 232(1): 1-10. |
| 24 | 熊博文, 赵云良, 张婷婷, 等. 机械力活化某铜尾矿处理模拟含铜废水试验[J]. 金属矿山, 2019(7): 199-203. |
| XIONG Bowen, ZHAO Yunliang, ZHANG Tingting, et al. Removal of Cu(Ⅱ) from copper-bearing wastewater by using mechanically-activated copper tailings[J]. Metal Mine, 2019(7): 199-203. | |
| 25 | MALLAKPOUR Shadpour, KHADEM Elham. Facile and cost-effective preparation of PVA/modified calcium carbonate nanocomposites via ultrasonic irradiation: Application in adsorption of heavy metal and oxygen permeation property[J]. Ultrasonics Sonochemistry, 2017, 39: 430-438. |
| 26 | BABATUNDE Kawthar Adewumi, NEGASH Berihun Mamo, JUFAR Shiferaw Regassa, et al. Adsorption of gases on heterogeneous shale surfaces: A review[J]. Journal of Petroleum Science and Engineering, 2022, 208: 109466. |
| 27 | 邓晓阳, 郑强, 曲笑原, 等. 白云石制备似立方体状方解石型碳酸钙晶体及其机理研究[J]. 人工晶体学报, 2022, 51(4): 704-715. |
| DENG Xiaoyang, ZHENG Qiang, QU Xiaoyuan, et al. Preparation of near cube form calcite type calcium carbonate crystal from dolomite and its mechanism[J]. Journal of Synthetic Crystals, 2022, 51(4): 704-715. | |
| 28 | Annane Kahina, Lemlikchi Wahiba, Tingry Sophie. Efficiency of eggshell as a low-cost adsorbent for removal of cadmium: Kinetic and isotherm studies[J]. Biomass Conversion and Biorefinery, 2021: 1-12. |
| 29 | Wahid Fazli, WANG Fengping, XIE Yanyan, et al. Reusable ternary PVA films containing bacterial cellulose fibers and ε-polylysine with improved mechanical and antibacterial properties[J]. Colloids and Surfaces B, Biointerfaces, 2019, 183: 110486. |
| 30 | CHONG Kai Yin, CHIA Chin Hua, ZAKARIA Sarani, et al. Vaterite calcium carbonate for the adsorption of Congo red from aqueous solutions[J]. Journal of Environmental Chemical Engineering, 2014, 2(4): 2156-2161. |
| 31 | 胡盛, 袁晓慧, 高雪, 等. 两种偶联剂对咸丰重质碳酸钙干法改性的影响[J]. 非金属矿, 2020, 43(2): 73-76. |
| HU Sheng, YUAN Xiaohui, GAO Xue, et al. Effect of two coupling agents on dry modification of heavy calcium carbonate from Xianfeng[J]. Non-Metallic Mines, 2020, 43(2): 73-76. | |
| 32 | 胡盛, 高雪, 洪颖, 等. 咸丰重质碳酸钙的干法改性研究[J]. 矿产综合利用, 2021(3): 176-179, 175. |
| HU Sheng, GAO Xue, HONG Ying, et al. Study on dry modification of heavy calcium carbonate from Xianfeng[J]. Multipurpose Utilization of Mineral Resources, 2021(3): 176-179, 175. | |
| 33 | Yaozhong LYU, ZHANG Liming, LI Mengnan, et al. Physicochemical properties and digestibility of potato starch treated by ball milling with tea polyphenols[J]. International Journal of Biological Macromolecules, 2019, 129: 207-213. |
| 34 | 杨明镜, 孙建恒, 王军林, 等. 铁尾矿粉用作混凝土矿物掺合料的机械活化细度优化研究[J/OL].(2022-05-26)[2023-05-18]. . |
| YANG Mingjing, SUN Jianheng, WANG Junlin, et al. Activity Optimization of Fineness of Mechanical Activation for Iron Tailing Powder as Concrete Mineral Admixtures[J/OL]. (2022-05-26)[2023-05-18]. . | |
| 35 | ZHANG Jiupeng, ZUO Jing, AI Weidong, et al. Preparation of mesoporous coal-gasification fine slag adsorbent via amine modification and applications in CO2 capture[J]. Applied Surface Science, 2021, 537: 147938. |
| 36 | MONIER M, AYAD D M, ABDEL-LATIF D A. Adsorption of Cu(Ⅱ), Cd( Ⅱ ) and Ni( Ⅱ ) ions by cross-linked magnetic chitosan-2-aminopyridine glyoxal Schiff’s base[J]. Colloids and Surfaces B: Biointerfaces, 2012, 94: 250-258. |
| 37 | MA Jianqing, SHEN Yu, SHEN Chensi, et al. Al-doping chitosan- Fe(Ⅲ) hydrogel for the removal of fluoride from aqueous solutions[J]. Chemical Engineering Journal, 2014, 248: 98-106. |
| 38 | LU Wei, DAI Zhongran, LI Le, et al. Preparation of composite hydrogel (PCG) and its adsorption performance for uranium(Ⅵ)[J]. Journal of Molecular Liquids, 2020, 303: 112604. |
| 39 | 司承运, 胡浩, 程婧柔, 等. D-101大孔吸附树脂对中药根皮苷的吸附行为[J]. 离子交换与吸附, 2020, 36(6): 541-553. |
| SI Chengyun, HU Hao, CHENG Jingrou, et al. Adsorption behavior of D-101 macroporous resin for phloridzin[J]. Ion Exchange and Adsorption, 2020, 36(6): 541-553. | |
| 40 | PLUNKETT Kyle N, BERKOWSKI Kimberly L, MOORE Jeffrey S. Chymotrypsin responsive hydrogel: Application of a disulfide exchange protocol for the preparation of methacrylamide containing peptides[J]. Biomacromolecules, 2005, 6(2): 632-637. |
| 41 | Jonathan SUAZO-HERNÁNDEZ, Karen MANQUIÁN-CERDA, DE LA LUZ MORA María, et al. Efficient and selective removal of SeVI and AsV mixed contaminants from aqueous media by montmorillonite-nanoscale zero valent iron nanocomposite[J]. Journal of Hazardous Materials, 2021, 403: 123639. |
| 42 | ZHANG Jing, YAO Bin, PING Hang, et al. Template-free synthesis of hierarchical porous calcium carbonate microspheres for efficient water treatment[J]. RSC Advances, 2016, 6(1): 472-480. |
| 43 | JILAL Issam, BARKANY Soufian EL, BAHARI Zahra, et al. New quaternized cellulose based on hydroxyethyl cellulose (HEC) grafted EDTA: Synthesis, characterization and application for Pb( Ⅱ ) and Cu(Ⅱ) removal[J]. Carbohydrate Polymers, 2018, 180: 156-167. |
| 44 | NONGBE Medy C, GUILLAUME Bretel, TCHIRIOUA Ekou, et al. Cellulose paper grafted with polyamines as powerful adsorbent for heavy metals[J]. Cellulose, 2018, 25(7): 4043-4055. |
| 45 | DAI Hongjie, HUANG Yue, HUANG Huihua. Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue[J]. Carbohydrate Polymers, 2018, 185: 1-11. |
| 46 | YAN Yi, YANG Shuai, JIANG Feng, et al. Efficient removal of lead ions from aqueous solutions using ZnSe/ZnO/Bio-CaCO3 [J]. Water Science and Technology, 2020, 81(1): 91-101. |
| 47 | ZHANG Tingting, WEN Tong, ZHAO Yunliang, et al. Antibacterial activity of the sediment of copper removal from wastewater by using mechanically activated calcium carbonate[J]. Journal of Cleaner Production, 2018, 203: 1019-1027. |
| 48 | YOU Nan, WANG Xiaofeng, LI Jiyu, et al. Synergistic removal of arsanilic acid using adsorption and magnetic separation technique based on Fe3O4@ graphene nanocomposite[J]. Journal of Industrial and Engineering Chemistry, 2019, 70: 346-354. |
| 49 | SONG Yuexian, CHEN Su, YOU Nan, et al. Nanocomposites of zero-valent iron@activated carbon derived from corn stalk for adsorptive removal of tetracycline antibiotics[J]. Chemosphere, 2020, 255: 126917. |
| 50 | WANG Shuai, WANG Hao, WANG Shixing, et al. Highly effective and selective adsorption of Au(Ⅲ) from aqueous solution by poly(ethylene sulfide) functionalized chitosan: Kinetics, isothermal adsorption and thermodynamics[J]. Microporous and Mesoporous Materials, 2022, 341: 112074. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [9] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [10] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [11] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [12] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [13] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [14] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [15] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||