化工进展 ›› 2023, Vol. 42 ›› Issue (1): 94-106.DOI: 10.16085/j.issn.1000-6613.2022-1320
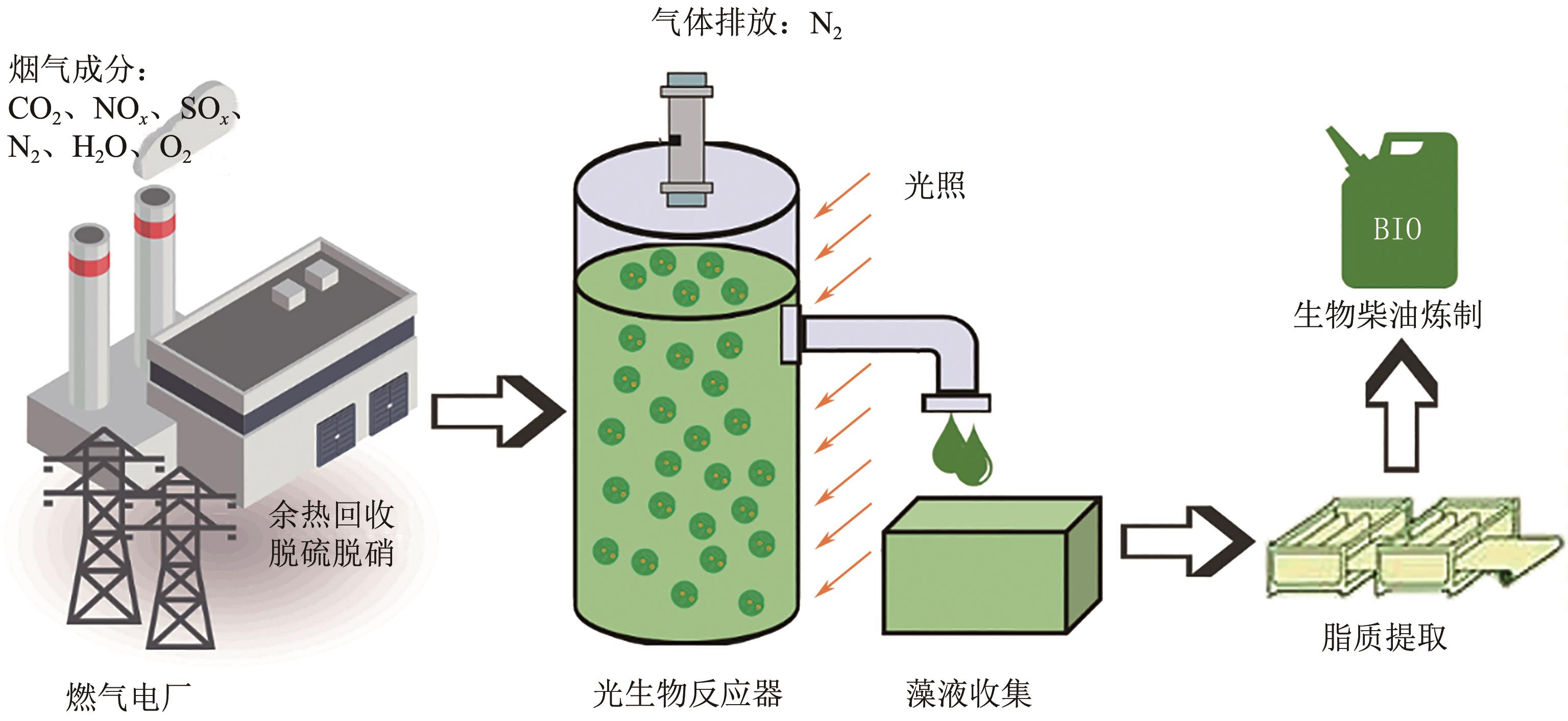
吸收-微藻法固定燃气电厂低浓度CO2同步产油技术研究进展
- 1.北京林业大学环境科学与工程学院,北京 100083
2.北京市南水北调环线管理处,北京 100176
-
收稿日期:2022-07-14修回日期:2022-10-17出版日期:2023-01-25发布日期:2023-02-20 -
通讯作者:马伟芳 -
作者简介:秦振芳(1999—),女,硕士研究生,研究方向为碳捕集及利用。E-mail:1748938063@qq.com。 -
基金资助:北京市科技计划(Z211100004321001)
Research progress on absorption-microalgae fixation of low concentration CO2 and synchronous oil production in gas power plant
QIN Zhenfang1( ), LIAO Rihong2, MA Weifang1(
), LIAO Rihong2, MA Weifang1( )
)
- 1.College of Environmental Science and Engineering, Beijing Forestry University, Beijing 100083, China
2.Administrative Office of Beijing South-to-North Water Diversion Project, Beijing 100176, China
-
Received:2022-07-14Revised:2022-10-17Online:2023-01-25Published:2023-02-20 -
Contact:MA Weifang
摘要:
燃气电厂利用稳定、清洁的化石能源发电,在“双碳”背景下发电过程产生的低浓度CO2的捕集和资源化利用,对于实现碳中和至关重要。针对低浓度CO2捕集难度大、脱附费用高的问题,利用CO2吸收液同步培养微藻产油提供了一种实现低浓度CO2捕集与资源化利用于一体的新途径。具有高CO2捕集能力和同时快速培养微藻能力的吸收液是溶液设计和配制的决定性因素。本文总结了现有吸收液的应用现状,梳理出复合吸收液耦合微藻营养调控的碳捕集发展前景,其中吸收液的碱度和盐度对微藻同化CO2具有显著影响。讨论了在不同温度和光照的工艺条件对微藻生物转化CO2的影响,阐述了CO2气体以微孔鼓泡和气升导流的方式通入反应器对CO2捕集和微藻生长的不同效果。从促进微藻吸收CO2同步产油的角度,介绍了藻种诱变驯化和基因改造以提升环境适应性同时增强脂质生产的研究进展,最后通过经济分析展望了规模化应用吸收-微藻法的经济可行性。与传统的CO2吸收和微藻固定方法相比,吸收-微藻法一体化复合工艺可以成为从燃气电厂中捕获 CO2具有竞争力的替代方案。
中图分类号:
引用本文
秦振芳, 廖日红, 马伟芳. 吸收-微藻法固定燃气电厂低浓度CO2同步产油技术研究进展[J]. 化工进展, 2023, 42(1): 94-106.
QIN Zhenfang, LIAO Rihong, MA Weifang. Research progress on absorption-microalgae fixation of low concentration CO2 and synchronous oil production in gas power plant[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 94-106.
| 项目 | 超低排放燃煤电厂 | 燃气电厂 |
|---|---|---|
| CO2体积分数/% | 10~16 | 3~5 |
| H2O体积分数/% | 5~9 | 7~9 |
| N2体积分数/% | 73~77 | 73~75 |
| O2体积分数/% | 3~7 | 11~13 |
| NO x 浓度/mg·m-3 | 22~44 | 15~30 |
| SO x 浓度/mg·m-3 | 8~24 | 0~5 |
表1 超低排放燃煤电厂和燃气电厂的烟气成分[8-10]
| 项目 | 超低排放燃煤电厂 | 燃气电厂 |
|---|---|---|
| CO2体积分数/% | 10~16 | 3~5 |
| H2O体积分数/% | 5~9 | 7~9 |
| N2体积分数/% | 73~77 | 73~75 |
| O2体积分数/% | 3~7 | 11~13 |
| NO x 浓度/mg·m-3 | 22~44 | 15~30 |
| SO x 浓度/mg·m-3 | 8~24 | 0~5 |
| 项目 | Heynigia riparia SX01 | Dunaliella salina | Chlorella vulgaris P12 | Scenedesmus obtusiusculus | Chlorella vulgaris (ISC-23) |
|---|---|---|---|---|---|
| CO2体积分数/% | 5(混合空气) | 6(混合空气) | 6(混合空气) | 4~5(烟气成分) | 6(混合空气) |
| CO2固定效率/g·L-1·d-1 | 0.370 | 0.067 | 2.290 | 0.111 | 3.222 |
| 微藻生物量/g·L-1 | 2.37 | 0.26 | 10 | 1.09 | 14.30 |
表2 低浓度CO2下微藻的固碳效果[21-25]
| 项目 | Heynigia riparia SX01 | Dunaliella salina | Chlorella vulgaris P12 | Scenedesmus obtusiusculus | Chlorella vulgaris (ISC-23) |
|---|---|---|---|---|---|
| CO2体积分数/% | 5(混合空气) | 6(混合空气) | 6(混合空气) | 4~5(烟气成分) | 6(混合空气) |
| CO2固定效率/g·L-1·d-1 | 0.370 | 0.067 | 2.290 | 0.111 | 3.222 |
| 微藻生物量/g·L-1 | 2.37 | 0.26 | 10 | 1.09 | 14.30 |
| 吸收液类型 | 成分化学式 | 常用吸收液 | 吸收反应式 | 优缺点 | 参考文献 |
|---|---|---|---|---|---|
| 胺基溶液 | RNH2 | 伯胺:MEA 仲胺:DEA 叔胺:MDEA | (1) RNH2+CO2+H2O (2) 2RNH2+CO2 | 优点:低CO2下有高水溶性,相当大的吸收动力学速率;稳定性好 缺点:吸收效率差;易被O2降解,产生腐蚀性;CO2负载量低 | [ |
| 碳酸盐溶液 | RCO3 | K2CO3、Na2CO3 | RCO3+CO2+H2O | 优点:成本低;CO2在溶剂中溶解度高;溶剂毒性低;不易降解,不易被腐蚀; 缺点:反应速度慢;强腐蚀性 | [ |
| 氨溶液 | NH3 | NH3 | (1) CO2+NH3 (2) NH2COONH4+H2O (3) NH4HCO3+NH3 (4) NH2COONH4+CO2+H2O (5) CO2+NH3+H2O | 优点:在烟气环境中降解率低;腐蚀程度低;易再生,吸收能力高;可与污染性气体NO x 反应 缺点:NH3逃逸率高 | [ |
| 氨基酸盐溶液 | RR'NH | 脯氨酸钾/钠、赖氨酸钾/钠、甘氨酸钾、肌氨酸钾、氨基酸钠 | (1) CO2+RR'NH (2) RR'N+HCOO-+B (R代表CHCOO-,R'代表氨基酸盐离子, B是溶液中的任何碱) | 优点:低挥发性;低毒性;良好的生物降解潜力;不易被烟气中O2降解 缺点:再生能耗高;易沉淀和堵塞装置 | [ |
表3 化学吸收法固定CO2常用的吸收液类型
| 吸收液类型 | 成分化学式 | 常用吸收液 | 吸收反应式 | 优缺点 | 参考文献 |
|---|---|---|---|---|---|
| 胺基溶液 | RNH2 | 伯胺:MEA 仲胺:DEA 叔胺:MDEA | (1) RNH2+CO2+H2O (2) 2RNH2+CO2 | 优点:低CO2下有高水溶性,相当大的吸收动力学速率;稳定性好 缺点:吸收效率差;易被O2降解,产生腐蚀性;CO2负载量低 | [ |
| 碳酸盐溶液 | RCO3 | K2CO3、Na2CO3 | RCO3+CO2+H2O | 优点:成本低;CO2在溶剂中溶解度高;溶剂毒性低;不易降解,不易被腐蚀; 缺点:反应速度慢;强腐蚀性 | [ |
| 氨溶液 | NH3 | NH3 | (1) CO2+NH3 (2) NH2COONH4+H2O (3) NH4HCO3+NH3 (4) NH2COONH4+CO2+H2O (5) CO2+NH3+H2O | 优点:在烟气环境中降解率低;腐蚀程度低;易再生,吸收能力高;可与污染性气体NO x 反应 缺点:NH3逃逸率高 | [ |
| 氨基酸盐溶液 | RR'NH | 脯氨酸钾/钠、赖氨酸钾/钠、甘氨酸钾、肌氨酸钾、氨基酸钠 | (1) CO2+RR'NH (2) RR'N+HCOO-+B (R代表CHCOO-,R'代表氨基酸盐离子, B是溶液中的任何碱) | 优点:低挥发性;低毒性;良好的生物降解潜力;不易被烟气中O2降解 缺点:再生能耗高;易沉淀和堵塞装置 | [ |
| 吸收液类型 | 吸收液 成分 | 微藻种类 | CO2占比 | 反应器 | 培养基 | CO2固定率 | 微藻生物量/ 生长速率 | 脂质 产量 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
| 碳酸盐溶液 | K2CO3 | Chlorella sp. | 0.5和0.7的CO2负载 | 锥形瓶 | 改良F 培养基 | — | 最大体积生产率:0.380g·L-1·d-1 生物质浓度:1.80g·L-1 | — | [ |
| Na2CO3 | Euhalothece ZM001 | — | T形瓶反应器(含剪切力) | 标准M 培养基 | 59% | 4.79g·L-1 最大生物质产率:1.210g·L-1·d-1 | — | [ | |
| Na2CO3 | Scenedesmus sp. | 10% | 玻璃瓶 持续通气 | BG-11 培养基 | 6.6% | 259mg·L-1 | 22.43~ 90.85mg·L-1 | [ | |
| Na2CO3 | Euhalothece sp. | 35mmol·LPBR-1·d-1 | 锥形瓶 | M培养基 | (0.422± 0.057)g·L-1·d-1 | 最大比生长率:1.670d-1 生物质产率:(0.845± 0.113)g·L-1·d-1 | — | [ | |
| Na2CO3 | T.elongatus BP-1 | 6.3% | 静态混合-平板气升式反应器 | BG-11 培养基 | — | 最大生物质产率:2.900gDW·L-1·d-1 | — | [ | |
| 胺基 溶液 | MEA | Spirulina | 半连续管式 生物器 | Zarrouk 培养基 | 16% | 生物质产率:62.100mg·L-1·d-1 | 8.3%±1.4% | [ | |
| TEA | Scenedesmus sp. | 4% | 管式玻璃鼓泡反应器 | BG-11 培养基 | 增加了30.5% | 生物质产率:664.600mg·L-1·d-1 | — | [ | |
| AMP | Scenedesmusaccuminatus | 0~5% | 管式玻璃鼓泡反应器 | BG-11 培养基 | 619mg·L-1·d-1 | 细胞密度:1780mg·L-1 | — | [ | |
| DEA | Coccomyxasubellipsoidea C-169 | 2% | 管式生物器 | Basal 培养基 | 0.97g·L-1 | 生物质产率:225.980mg·L-1·d-1 | 产量:64.33%; 产率: 59.900mg·L-1·d-1 | [ | |
| DEA | Chlamydomonas sp.; Chlorella sp.; Pseudochlorococcum sp. | 纯CO2 | 烧瓶 (螺旋管通气) | 营养 培养基 | 0.012mol·mL-1·d-1;0.135mol·mL-1·d-1; 0.012mol·mL-1·d-1; | 比生长率: 0.365d-1; 0.352d-1; 0.669d-1 | — | [ | |
| 混合 吸收液 | DEA+ K2CO3 | Spirulina | 0.36mL | 管式生物器 | Zarrouk 培养基 | 43.7% | 生物质产率:174.200mg·L-1·d-1 | — | [ |
| NH3+ K2CO3 | Chlorella sp. | 5% | 锥形烧瓶 | BG-11 培养基 | >60% | 生物质产率:49mg·L-1·d-1 | 59mg·L-1·d-1 | [ | |
| MDEA+ PZ | Chlorella sorokiniana BTA 9031 | 15% | 锥形烧瓶 | BG-11 培养基 | 72.4%~84.8% | 最大生物质浓度:(1.20±0.028)g·L-1 | 最高:23%±0.013% | [ |
表4 吸收-微藻法中不同类型吸收液的固碳效果
| 吸收液类型 | 吸收液 成分 | 微藻种类 | CO2占比 | 反应器 | 培养基 | CO2固定率 | 微藻生物量/ 生长速率 | 脂质 产量 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
| 碳酸盐溶液 | K2CO3 | Chlorella sp. | 0.5和0.7的CO2负载 | 锥形瓶 | 改良F 培养基 | — | 最大体积生产率:0.380g·L-1·d-1 生物质浓度:1.80g·L-1 | — | [ |
| Na2CO3 | Euhalothece ZM001 | — | T形瓶反应器(含剪切力) | 标准M 培养基 | 59% | 4.79g·L-1 最大生物质产率:1.210g·L-1·d-1 | — | [ | |
| Na2CO3 | Scenedesmus sp. | 10% | 玻璃瓶 持续通气 | BG-11 培养基 | 6.6% | 259mg·L-1 | 22.43~ 90.85mg·L-1 | [ | |
| Na2CO3 | Euhalothece sp. | 35mmol·LPBR-1·d-1 | 锥形瓶 | M培养基 | (0.422± 0.057)g·L-1·d-1 | 最大比生长率:1.670d-1 生物质产率:(0.845± 0.113)g·L-1·d-1 | — | [ | |
| Na2CO3 | T.elongatus BP-1 | 6.3% | 静态混合-平板气升式反应器 | BG-11 培养基 | — | 最大生物质产率:2.900gDW·L-1·d-1 | — | [ | |
| 胺基 溶液 | MEA | Spirulina | 半连续管式 生物器 | Zarrouk 培养基 | 16% | 生物质产率:62.100mg·L-1·d-1 | 8.3%±1.4% | [ | |
| TEA | Scenedesmus sp. | 4% | 管式玻璃鼓泡反应器 | BG-11 培养基 | 增加了30.5% | 生物质产率:664.600mg·L-1·d-1 | — | [ | |
| AMP | Scenedesmusaccuminatus | 0~5% | 管式玻璃鼓泡反应器 | BG-11 培养基 | 619mg·L-1·d-1 | 细胞密度:1780mg·L-1 | — | [ | |
| DEA | Coccomyxasubellipsoidea C-169 | 2% | 管式生物器 | Basal 培养基 | 0.97g·L-1 | 生物质产率:225.980mg·L-1·d-1 | 产量:64.33%; 产率: 59.900mg·L-1·d-1 | [ | |
| DEA | Chlamydomonas sp.; Chlorella sp.; Pseudochlorococcum sp. | 纯CO2 | 烧瓶 (螺旋管通气) | 营养 培养基 | 0.012mol·mL-1·d-1;0.135mol·mL-1·d-1; 0.012mol·mL-1·d-1; | 比生长率: 0.365d-1; 0.352d-1; 0.669d-1 | — | [ | |
| 混合 吸收液 | DEA+ K2CO3 | Spirulina | 0.36mL | 管式生物器 | Zarrouk 培养基 | 43.7% | 生物质产率:174.200mg·L-1·d-1 | — | [ |
| NH3+ K2CO3 | Chlorella sp. | 5% | 锥形烧瓶 | BG-11 培养基 | >60% | 生物质产率:49mg·L-1·d-1 | 59mg·L-1·d-1 | [ | |
| MDEA+ PZ | Chlorella sorokiniana BTA 9031 | 15% | 锥形烧瓶 | BG-11 培养基 | 72.4%~84.8% | 最大生物质浓度:(1.20±0.028)g·L-1 | 最高:23%±0.013% | [ |
| 性状 | 微藻 | 诱变方法 | 改进后性状 | 参考文献 |
|---|---|---|---|---|
| 高生长速率 | Chlorella sp. | Se-137-伽马射线照射突变 | 生物质产量提高25% | [ |
| Chlorella vulgaris | 紫外线照射突变 | 比增长率提高29.4% | [ | |
| 对环境的高耐受性 | Chlorella sp. S30 | ALE | 得到耐受30g·L-1盐的淡水菌株 | [ |
| 脂质的高产量 | Desmodesmus sp. | 大气和室温等离子体的突变 | 脂质生产增加>100% | [ |
| Chlorella L166 | 低温等离子体(LTP) | 油脂含量提高>12% | [ | |
| Tetraselmis sp. | EMS | 产脂率最高达到48%±0.9% | [ |
表5 微藻诱变获得特殊性状
| 性状 | 微藻 | 诱变方法 | 改进后性状 | 参考文献 |
|---|---|---|---|---|
| 高生长速率 | Chlorella sp. | Se-137-伽马射线照射突变 | 生物质产量提高25% | [ |
| Chlorella vulgaris | 紫外线照射突变 | 比增长率提高29.4% | [ | |
| 对环境的高耐受性 | Chlorella sp. S30 | ALE | 得到耐受30g·L-1盐的淡水菌株 | [ |
| 脂质的高产量 | Desmodesmus sp. | 大气和室温等离子体的突变 | 脂质生产增加>100% | [ |
| Chlorella L166 | 低温等离子体(LTP) | 油脂含量提高>12% | [ | |
| Tetraselmis sp. | EMS | 产脂率最高达到48%±0.9% | [ |
| 基因改造方法 | 微藻 | 目标基因 | 对基因表达的影响 | 改进结果 | 参考文献 |
|---|---|---|---|---|---|
| 参与脂质生物合成的酶的过度表达 | Fistuliferasolaris | G6PD和PGD的过度表达 | 5.5倍和4.8倍增长 | 脂质产量增加1.5倍 | [ |
Neochloris oleoabundans | DGAT2的过度 表达 | 2倍增长 | 总脂质含量增加1.6~2.3倍,总脂质生产力增加1.6~3.2倍;而TAG含量增加1.8~3.2倍,TAG生产力增加1.6~4.3倍 | [ | |
| 阻断竞争途径 | Chlamydomonasreinhardtii | ADP-葡萄糖 焦磷酸化酶 (AGPase) | 钝化AGPase钝化 | TAG含量增加10倍 | [ |
| Thalassiosirapseudonana | 脂肪酶/磷脂酶/脂酰基转移酶 | RNAi方法 | 脂质含量增加3.5倍 | [ | |
| 改变脂肪酸链长度 | Phaeodactylumtricornutum | Δ 5去饱和酶 PtD5b的过表达 | 3.2倍增长 | 单不饱和脂肪酸(MUFA)和多不饱和脂肪酸 (PUFA)分别增加了75%和64% | [ |
Chlamydomonas reinhardtii | Cracs1/Cracs2的敲除 | 减少55% | 分泌的FAs可以达到8.19mg·(109细胞)-1和9.66mg·(109细胞)-1 | [ |
表6 基因改造促进微藻脂质积累和脂质质量
| 基因改造方法 | 微藻 | 目标基因 | 对基因表达的影响 | 改进结果 | 参考文献 |
|---|---|---|---|---|---|
| 参与脂质生物合成的酶的过度表达 | Fistuliferasolaris | G6PD和PGD的过度表达 | 5.5倍和4.8倍增长 | 脂质产量增加1.5倍 | [ |
Neochloris oleoabundans | DGAT2的过度 表达 | 2倍增长 | 总脂质含量增加1.6~2.3倍,总脂质生产力增加1.6~3.2倍;而TAG含量增加1.8~3.2倍,TAG生产力增加1.6~4.3倍 | [ | |
| 阻断竞争途径 | Chlamydomonasreinhardtii | ADP-葡萄糖 焦磷酸化酶 (AGPase) | 钝化AGPase钝化 | TAG含量增加10倍 | [ |
| Thalassiosirapseudonana | 脂肪酶/磷脂酶/脂酰基转移酶 | RNAi方法 | 脂质含量增加3.5倍 | [ | |
| 改变脂肪酸链长度 | Phaeodactylumtricornutum | Δ 5去饱和酶 PtD5b的过表达 | 3.2倍增长 | 单不饱和脂肪酸(MUFA)和多不饱和脂肪酸 (PUFA)分别增加了75%和64% | [ |
Chlamydomonas reinhardtii | Cracs1/Cracs2的敲除 | 减少55% | 分泌的FAs可以达到8.19mg·(109细胞)-1和9.66mg·(109细胞)-1 | [ |
| 因素 | 假设参数 |
|---|---|
| S-V比/m-1 | 4 |
| 太阳辐射/MJ·m-2·d-1 | 20 |
| 光合效率/% | 2~6 |
| CO2固定率/% | 0、10、25、40 |
| 特定种植成本/CNY·m-3 | 1080~2160 |
| 特定运营成本/CNY·dt-1 | 720~1224 |
| 特定能耗/W·m-3 | 4 |
| (意外开支+所有者成本)/% | 30 |
| 贴现现金流/% | 8 |
表7 用于CO2生物固定技术经济分析的假设
| 因素 | 假设参数 |
|---|---|
| S-V比/m-1 | 4 |
| 太阳辐射/MJ·m-2·d-1 | 20 |
| 光合效率/% | 2~6 |
| CO2固定率/% | 0、10、25、40 |
| 特定种植成本/CNY·m-3 | 1080~2160 |
| 特定运营成本/CNY·dt-1 | 720~1224 |
| 特定能耗/W·m-3 | 4 |
| (意外开支+所有者成本)/% | 30 |
| 贴现现金流/% | 8 |
| 财务参数 | 价值 |
|---|---|
| 总收益/CNY·a-1 | 33582953 |
| 总运营成本/CNY·a-1 | 29511332 |
| EBITDA/CNY·a-1 | 4071627 |
| 折旧、利息、维修费/CNY·a-1 | 3572957 |
| 净收益/CNY·a-1 | 498663 |
| ROI/% | 10 |
| 回报时间/a | 10 |
表8 生物柴油生产规模扩大方案项目的经济分析
| 财务参数 | 价值 |
|---|---|
| 总收益/CNY·a-1 | 33582953 |
| 总运营成本/CNY·a-1 | 29511332 |
| EBITDA/CNY·a-1 | 4071627 |
| 折旧、利息、维修费/CNY·a-1 | 3572957 |
| 净收益/CNY·a-1 | 498663 |
| ROI/% | 10 |
| 回报时间/a | 10 |
| 项目 | 传统化学吸收法 | 吸收-微藻法 |
|---|---|---|
| 固定资本/106CNY | 552 | 357 |
| 运行费用/106CNY·a-1 | 44 | 113 |
| 预期收入/106CNY·a-1 | — | 100 |
表9 不同CO2捕集工艺的经济对比
| 项目 | 传统化学吸收法 | 吸收-微藻法 |
|---|---|---|
| 固定资本/106CNY | 552 | 357 |
| 运行费用/106CNY·a-1 | 44 | 113 |
| 预期收入/106CNY·a-1 | — | 100 |
| 1 | 蒲波. 浅析碳达峰碳中和背景下的天然气发电对稳定电力系统建设的意义[J]. 清洗世界, 2021, 37(11): 95-96. |
| PU Bo. Analysis on the significance of natural gas power generation in stable power system construction under the background of carbon peaking and carbon neutralization[J]. Cleaning World, 2021, 37(11): 95-96. | |
| 2 | 练玉华, 简伟奇, 解婷婷, 等. 燃气-蒸汽联合循环电厂烟气CO2捕集与干冰联产技术的研究[J]. 山东化工, 2021, 50(10): 48-50. |
| LIAN Yuhua, JIAN Weiqi, XIE Tingting, et al. Study on combinative production of power generation with CO2 collection and dry ice in gas-steam combined cyele power plant[J]. Shandong Chemical Industry, 2021, 50(10): 48-50. | |
| 3 | 王照成, 晏双华. 碳捕集技术在燃气电厂中的应用[J]. 现代化工, 2018, 38(9): 195-197. |
| WANG Zhaocheng, YAN Shuanghua. Application of carbon capture technology in gas power plant[J]. Modern Chemical Industry, 2018, 38(9): 195-197. | |
| 4 | SONG Chunfeng, LIU Qingling, DENG Shuai, et al. Cryogenic-based CO2 capture technologies: state-of-the-art developments and current challenges[J]. Renewable and sustainable Energy Reviews, 2019, 101: 265-278. |
| 5 | MANDIK Yohanis Irenius, CHEIRSILP Benjamas, SRINUANPAN Sirasit, et al. Zero-waste biorefinery of oleaginous microalgae as promising sources of biofuels and biochemicals through direct transesterification and acid hydrolysis[J]. Process Biochemistry, 2020, 95: 214-222. |
| 6 | Preeti PAL, CHEW Kit Wayne, YEN Hong Wei, et al. Cultivation of oily microalgae for the production of third-generation biofuels[J]. Sustainability, 2019, 11(19): 5424. |
| 7 | WANG Shumin. Near-zero air pollutant emission technologies and applications for clean coal-fired power[J]. Engineering, 2020, 6(12): 1408-1422. |
| 8 | 徐静馨, 朱法华, 王圣, 等. 超低排放燃煤电厂和燃气电厂综合对比[J]. 中国电力, 2020, 53(2): 164-172, 179. |
| XU Jingxin, ZHU Fahua, WANG Sheng, et al. Comprehensive comparison of ultra-low emission coal-fired power plants and gas-fired power plants[J]. Electric Power, 2020, 53(2): 164-172, 179. | |
| 9 | 樊慧, 段天宇, 朱博骐, 等. 燃气电厂与超低排放燃煤电厂环境及生态效应对比[J]. 天然气工业, 2020, 40(7): 146-153. |
| FAN Hui, DUAN Tianyu, ZHU Boqi, et al. Comparison of environmental and ecological effects between gas-fired and ultra-low emission coal-fired power generation plants[J]. Natural Gas Industry, 2020, 40(7): 146-153. | |
| 10 | 刘志坦, 李玉刚, 王凯. 中国燃气电厂烟气排放现状及政策趋势[J]. 中国电力, 2018, 51(1): 147-153. |
| LIU Zhitan, LI Yugang, WANG Kai. The environmental protection status quo of China’s gas power plants and the trending in policies[J]. Electric Power, 2018, 51(1): 147-153. | |
| 11 | SINGH Shailendra Kumar, SUNDARAM Shanthy, SINHA Sukrat, et al. Recent advances in CO2 uptake and fixation mechanism of cyanobacteria and microalgae[J]. Critical Reviews in Environmental Science and Technology, 2016, 46(16): 1297-1323. |
| 12 | SINGH Shailendra Kumar, RAHMAN Akhlaqur, DIXIT Kritika, et al. Evaluation of promising algal strains for sustainable exploitation coupled with CO2 fixation[J]. Environmental Technology, 2016, 37(5): 613-622. |
| 13 | CHENG Dujia, LI Xuyang, YUAN Yizhong, et al. Adaptive evolution and carbon dioxide fixation of Chlorella sp. in simulated flue gas[J]. Science of the Total Environment, 2019, 650: 2931-2938. |
| 14 | 郝宗娣, 杨勋, 时杰, 等. 微藻生物柴油的研究进展[J]. 上海海洋大学学报, 2013, 22(2): 282-288. |
| HAO Zongdi, YANG Xun, SHI Jie, et al. Research progress of microalgal biodiesel[J]. Journal of Shanghai Ocean University, 2013, 22(2): 282-288. | |
| 15 | THAWECHAI Tipawan, CHEIRSILP Benjamas, LOUHASAKUL Yasmi, et al. Mitigation of carbon dioxide by oleaginous microalgae for lipids and pigments production: effect of light illumination and carbon dioxide feeding strategies[J]. Bioresource Technology, 2016, 219: 139-149. |
| 16 | 马国杰, 常春, 孙绍辉. 能源微藻规模化培养影响因素的研究进展[J]. 化工进展, 2019, 38(12): 5323-5329. |
| MA Guojie, CHANG Chun, SUN Shaohui. Research progress on influencing factors of large scale cultivation of microalgae for energy production[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5323-5329. | |
| 17 | ZHANG Xing. Microalgae removal of CO2 from flue gas[C]//IEA Clean Coal Centre, 2015 |
| 18 | PAVLIK David, ZHONG Yingkui, DAIEK Carly, et al. Microalgae cultivation for carbon dioxide sequestration and protein production using a high-efficiency photobioreactor system[J]. Algal Research, 2017, 25: 413-420. |
| 19 | GOUVEIA Luisa, OLIVEIRA Ana Cristina. Microalgae as a raw material for biofuels production[J]. Journal of Industrial Microbiology & Biotechnology, 2009, 36(2): 269-274. |
| 20 | CHISTI Yusuf. Biodiesel from microalgae[J]. Biotechnology Advances, 2007, 25(3): 294-306. |
| 21 | JIN Xuejie, GONG Sanqiang, CHEN Zishuo, et al. Potential microalgal strains for converting flue gas CO2 into biomass[J]. Journal of Applied Phycology, 2021, 33(1): 47-55. |
| 22 | CHEN Yimin, XU Changan. How to narrow the CO2 gap from growth-optimal to flue gas levels by using microalgae for carbon capture and sustainable biomass production[J]. Journal of Cleaner Production, 2021, 280: 124448. |
| 23 | ANJOS Mariana, FERNANDES Bruno D, VICENTE António A, et al. Optimization of CO2 bio-mitigation by chlorella vulgaris[J]. Bioresource Technology, 2013, 139: 149-154. |
| 24 | Adrián ESTRADA-GRAF, Sergio HERNÁNDEZ, MORALES Marcia. Biomitigation of CO2 from flue gas by Scenedesmus obtusiusculus AT-UAM using a hybrid photobioreactor coupled to a biomass recovery stage by electro-coagulation-flotation[J]. Environmental Science and Pollution Research, 2020, 27(23): 28561-28574. |
| 25 | AZARI Aryandokht, TAVAKOLI Hossein, BARKDOLL Brian D, et al. Predictive model of algal biofuel production based on experimental data[J]. Algal Research, 2020, 47: 101843. |
| 26 | 李煦, 荣峻峰, 宗保宁. 微藻碳减排与生物质利用技术研究进展[J]. 石油炼制与化工, 2021, 52(10): 62-71. |
| LI Xu, RONG Junfeng, ZONG Baoning. Research progress of carbon emmision reduction by microalgae and biomass utilization[J]. Petroleum Processing and Petrochemicals, 2021, 52(10): 62-71. | |
| 27 | MOHEIMANI N R. Tetraselmis suecica culture for CO2 bioremediation of untreated flue gas from a coal-fired power station[J]. Journal of Applied Phycology, 2016, 28(4): 2139-2146. |
| 28 | WEN Xiaobin, DU Kui, WANG Zhongjie, et al. Effective cultivation of microalgae for biofuel production: a pilot-scale evaluation of a novel oleaginous microalga Graesiella sp. WBG-1[J]. Biotechnology for Biofuels, 2016, 9(1): 1-12. |
| 29 | HUNTLEY Mark E, REDALJE Donald G. CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal[J]. Mitigation and Adaptation Strategies for Global Change, 2007, 12(4): 573-608. |
| 30 | GARZON-SANABRIA Andrea J, DAVIS Ryan T, NIKOLOV Zivko L. Harvesting Nannochloris oculata by inorganic electrolyte flocculation: effect of initial cell density, ionic strength, coagulant dosage, and media pH[J]. Bioresource Technology, 2012, 118: 418-424. |
| 31 | 张晋阳, 王慧岭, 滕杰, 等. 微藻规模化采收工艺设计[J]. 现代农业科技, 2018(8): 218-219. |
| ZHANG Jinyang, WANG Huiling, TENG Jie, et al. Process design for scale harvesting of microalgae[J]. Modern Agricultural Science and Technology, 2018(8): 218-219. | |
| 32 | 才金玲, 冯辰辰, 倪国倩, 等. 微藻采收技术的研究进展[J]. 微生物学通报, 2020, 47(8): 2571-2581. |
| CAI Jinling, FENG Chenchen, NI Guoqian, et al. Progress in microalgae harvesting technology[J]. Microbiology China, 2020, 47(8): 2571-2581. | |
| 33 | YU Jingwen, WANG Shujuan, YU Hai. Experimental studies and rate-based simulations of CO2 absorption with aqueous ammonia and piperazine blended solutions[J]. International Journal of Greenhouse Gas Control, 2016, 50: 135-146. |
| 34 | JIANG Kaiqi, LI Kangkang, YU Hai, et al. Piperazine-promoted aqueous-ammonia-based CO2 capture: process optimisation and modification[J]. Chemical Engineering Journal, 2018, 347: 334-342. |
| 35 | LILLIA Stefano, BONALUMI Davide, FOSBØL Philip L, et al. Thermodynamic and kinetic properties of NH3-K2CO3-CO2-H2O system for carbon capture applications[J]. International Journal of Greenhouse Gas Control, 2019, 85: 121-131. |
| 36 | LU Ruize, LI Kangkang, CHEN Jian, et al. CO2 capture using piperazine-promoted, aqueous ammonia solution: rate-based modelling and process simulation[J]. International Journal of Greenhouse Gas Control, 2017, 65: 65-75. |
| 37 | LI Yifu, WANG Li’ao, TAN Zhongchao, et al. Experimental studies on carbon dioxide absorption using potassium carbonate solutions with amino acid salts[J]. Separation and Purification Technology, 2019, 219: 47-54. |
| 38 | GUO Hui, LI Hui, SHEN Shufeng. CO2 capture by water-lean amino acid salts: absorption performance and mechanism[J]. Energy & Fuels, 2018, 32(6): 6943-6954. |
| 39 | LI Yifu, WANG Liao, ZHANG Zhien, et al. Carbon dioxide absorption from biogas by amino acid salt promoted potassium carbonate solutions in a hollow fiber membrane contactor: a numerical study[J]. Energy & Fuels, 2018, 32(3): 3637-3646. |
| 40 | FENG Qi, SUN Baochang, WANG Lei, et al. Enhancement of CO2 absorption into K2CO3 solution by cyclohexane in a high-shear reactor[J]. Energy & Fuels, 2019, 33(7): 6628-6633. |
| 41 | SHARMA Anjana, BHATTACHARYA Abhishek. Enhanced biomimetic sequestration of CO2 into CaCO3 using purified carbonic anhydrase from indigenous bacterial strains[J]. Journal of Molecular Catalysis B: Enzymatic, 2010, 67(1/2): 122-128. |
| 42 | HORNBOSTEL K, NGUYEN D, BOURCIER W, et al. Packed and fluidized bed absorber modeling for carbon capture with micro-encapsulated sodium carbonate solution[J]. Applied Energy, 2019, 235: 1192-1204. |
| 43 | OCHEDI Friday O, YU Jianglong, YU Hai, et al. Carbon dioxide capture using liquid absorption methods: a review[J]. Environmental Chemistry Letters, 2021, 19(1): 77-109. |
| 44 | HUERTAS José I, GOMEZ Martin D, GIRALDO Nicolas, et al. CO2 absorbing capacity of MEA[J]. Journal of Chemistry, 2015, 2015: 965015. |
| 45 | GRIMEKIS Dimitrios, GIANNOULIDIS Sotirios, MANOU Konstantina, et al. Experimental investigation of CO2 solubility and its absorption rate into promoted aqueous potassium carbonate solutions at elevated temperatures[J]. International Journal of Greenhouse Gas Control, 2019, 81: 83-92. |
| 46 | ZHANG Zhien, LI Yifu, ZHANG Wenxiang, et al. Effectiveness of amino acid salt solutions in capturing CO2: a review[J]. Renewable and Sustainable Energy Reviews, 2018, 98: 179-188. |
| 47 | ZHENG Qi, MARTIN Gregory J O, KENTISH Sandra E. Energy efficient transfer of carbon dioxide from flue gases to microalgal systems[J]. Energy & Environmental Science, 2016, 9(3): 1074-1082. |
| 48 | CHI Zhanyou, XIE Yuxiao, ELLOY Farah, et al. Bicarbonate-based integrated carbon capture and algae production system with alkalihalophilic cyanobacterium[J]. Bioresource Technology, 2013, 133: 513-521. |
| 49 | RODAS-ZULUAGA Laura Isabel, Lizbeth CASTAÑEDA-HERNÁNDEZ, CASTILLO-VACAS Eduardo Israel, et al. Bio-capture and influence of CO2 on the growth rate and biomass composition of the microalgae Botryococcus braunii and Scenedesmus sp.[J]. Journal of CO2 Utilization, 2021, 43: 101371. |
| 50 | KISHI Masatoshi, TODA Tatsuki. Carbon fixation properties of three alkalihalophilic microalgal strains under high alkalinity[J]. Journal of Applied Phycology, 2018, 30(1): 401-410. |
| 51 | BERGMANN Peter, Walter TRÖSCH. Repeated fed-batch cultivation of Thermosynechococcus elongatus BP-1 in flat-panel airlift photobioreactors with static mixers for improved light utilization: influence of nitrate, carbon supply and photobioreactor design[J]. Algal Research, 2016, 17: 79-86. |
| 52 | ROSA Gabriel Martins da, MORAES Luiza, CARDIAS Bruna Barcelos, et al. Chemical absorption and CO2 biofixation via the cultivation of Spirulina in semicontinuous mode with nutrient recycle[J]. Bioresource Technology, 2015, 192: 321-327. |
| 53 | KIM Garam, CHOI Wookjin, LEE Chang Hee, et al. Enhancement of dissolved inorganic carbon and carbon fixation by green alga Scenedesmus sp. in the presence of alkanolamine CO2 absorbents[J]. Biochemical Engineering Journal, 2013, 78: 18-23. |
| 54 | 邹帅, 李玉芹, 马怡然, 等. 二乙醇胺强化胶球藻Coccomyxa subellipsoidea C-169固定CO2和积累油脂[J]. 化工进展, 2021, 40(9): 5222-5230. |
| ZOU Shuai, LI Yuqin, MA Yiran, et al. Diethanolamine strengthening CO2 fixation and lipid accumulation in Coccomyxa subellipsoidea C-169[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5222-5230. | |
| 55 | Sulaiman AL-ZUHAIR, ALKETBI Sara, Mohamed AL-MARZOUQI. Regenerating diethanolamine aqueous solution for CO2 absorption using microalgae[J]. Industrial Biotechnology, 2016, 12(2): 105-108. |
| 56 | CARDIAS Bruna Barcelos, DE MORAIS Michele Greque, COSTA Jorge Alberto Vieira. CO2 conversion by the integration of biological and chemical methods: Spirulina sp. LEB 18 cultivation with diethanolamine and potassium carbonate addition[J]. Bioresource Technology, 2018, 267: 77-83. |
| 57 | SONG Chunfeng, QIU Yiting, XIE Meilian, et al. Novel regeneration and utilization concept using rich chemical absorption solvent as a carbon source for microalgae biomass production[J]. Industrial & Engineering Chemistry Research, 2019, 58(27): 11720-11727. |
| 58 | KHAN Anoar Ali, MONDAL Madhumanti, HALDER G N, et al. Bioconversion of chemically captured carbon dioxide into microalgal lipids, a potential source of biodiesel: an integrated technique[J]. Fuel, 2022, 311: 122549. |
| 59 | BHATTACHARYA Sourish, SOUNDARYA Ramya, MISHRA Sandhya. Ammonium bicarbonate as nutrient substitute for improving biomass productivity ofChlorella variabilis [J]. Chemical Engineering & Technology, 2016, 39(9): 1738-1742. |
| 60 | Paul KÖNST, MIRELES Ileana Hernandez, VAN DER STEL Rob, et al. Integrated system for capturing CO2 as feedstock for algae production[J]. Energy Procedia, 2017, 114: 7126-7132. |
| 61 | DEVGOSWAMI Cr, KALITA M, TALUKDAR Jayanta, et al. Studies on the growth behavior of Chlorella, Haematococcus and Scenedesmus sp. in culture media with different concentrations of sodium bicarbonate and carbon dioxide gas[J]. African Journal of Biotechnology, 2011, 10: 13128-13138. |
| 62 | YIN Dacong, WANG Zhongjie, WEN Xiaobin, et al. Effects of carbon concentration, pH, and bubbling depth on carbon dioxide absorption ratio in microalgae medium[J]. Environmental Science and Pollution Research International, 2019, 26(32): 32902-32910. |
| 63 | SHEKH Ajam, SHARMA Aditi, SCHENK Peer M, et al. Microalgae cultivation: photobioreactors, CO2 utilization, and value-added products of industrial importance[J]. Journal of Chemical Technology & Biotechnology, 2022, 97(5): 1064-1085. |
| SHEKH Ajam, SHARMA Aditi, SCHENK Peer M, et al. Microalgae cultivation: photobioreactors, CO2 utilization, and value-added products of industrial importance[J]. Journal of Chemical Technology & Biotechnology, 2022, 97(5): 1064-1085. | |
| 64 | ZHAO Bingtao, SU Yaxin. Process effect of microalgal-carbon dioxide fixation and biomass production: a review[J]. Renewable and Sustainable Energy Reviews, 2014, 31: 121-132. |
| 65 | SEYED HOSSEINI Nekoo, SHANG Helen, SCOTT John Ashley. Biosequestration of industrial off-gas CO2 for enhanced lipid productivity in open microalgae cultivation systems[J]. Renewable and Sustainable Energy Reviews, 2018, 92: 458-469. |
| 66 | OGBONDA Kemka H, AMINIGO Rebecca E, Gideon O ABU. Influence of temperature and pH on biomass production and protein biosynthesis in a putative Spirulina sp.[J]. Bioresource Technology, 2007, 98(11): 2207-2211. |
| 67 | SINGH Har Mohan, KOTHARI Richa, GUPTA Rakesh, et al. Bio-fixation of flue gas from thermal power plants with algal biomass: overview and research perspectives[J]. Journal of Environmental Management, 2019, 245: 519-539. |
| 68 | KIM Jinsoo, LEE Joo Youp, LU Ting. A model for autotrophic growth of Chlorella vulgaris under photolimitation and photoinhibition in cylindrical photobioreactor[J]. Biochemical Engineering Journal, 2015, 99: 55-60. |
| 69 | AMINI KHOEYI Zahra, SEYFABADI Jafar, RAMEZANPOUR Zohreh. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris[J]. Aquaculture International, 2012, 20(1): 41-49. |
| 70 | ATTA Madiha, IDRIS Ani, BUKHARI Ataullah, et al. Intensity of blue LED light: a potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris [J]. Bioresource Technology, 2013, 148: 373-378. |
| 71 | PIRES J C M, ALVIM-FERRAZ M C M, MARTINS F G, et al. Carbon dioxide capture from flue gases using microalgae: engineering aspects and biorefinery concept[J]. Renewable and Sustainable Energy Reviews, 2012, 16(5): 3043-3053. |
| 72 | VASUMATHI K K, PREMALATHA M, SUBRAMANIAN P. Parameters influencing the design of photobioreactor for the growth of microalgae[J]. Renewable and Sustainable Energy Reviews, 2012, 16(7): 5443-5450. |
| 73 | HUANG Yun, ZHAO Sha, DING Yudong, et al. Optimizing the gas distributor based on CO2 bubble dynamic behaviors to improve microalgal biomass production in an air-lift photo-bioreactor[J]. Bioresource Technology, 2017, 233: 84-91. |
| 74 | DING Yudong, ZHAO Sha, LIAO Qiang, et al. Effect of CO2 bubbles behaviors on microalgal cells distribution and growth in bubble column photobioreactor[J]. International Journal of Hydrogen Energy, 2016, 41(8): 4879-4887. |
| 75 | FAN L h, ZHANG Y t, CHENG L h, et al. Optimization of carbon dioxide fixation by Chlorella vulgaris cultivated in a membrane-photobioreactor[J]. Chemical Engineering & Technology, 2007, 30(8): 1094-1099. |
| 76 | KIM Hyun Woo, CHENG Jing, RITTMANN Bruce E. Direct membrane-carbonation photobioreactor producing photoautotrophic biomass via carbon dioxide transfer and nutrient removal[J]. Bioresource Technology, 2016, 204: 32-37. |
| 77 | CHENG Wenchao, HUANG Jianke, CHEN Jianpei. Computational fluid dynamics simulation of mixing characteristics and light regime in tubular photobioreactors with novel static mixers[J]. Journal of Chemical Technology & Biotechnology, 2016, 91: 327-335. |
| 78 | OJAH Aastha, SABRI Laith S, ALDAHHAN Muthanna H. Local volumetric mass transfer coefficient estimation for Scenedesmus microalgae culture in a cylindrical airlift photobioreactor[J]. Journal of Chemical Technology & Biotechnology, 2021, 96(3): 764-774. |
| 79 | LUO Huping, AL-DAHHAN Muthanna H. Airlift column photobioreactors for Porphyridium sp. culturing: Part I. Effects of hydrodynamics and reactor geometry[J]. Biotechnology and Bioengineering, 2012, 109(4): 932-941. |
| 80 | 杨端鹏, 李仲先, 王胜男, 等. 微藻基因诱变研究及应用进展[J]. 海洋科学, 2021, 45(11): 165-178. |
| YANG Duanpeng, LI Zhongxian, WANG Shengnan, et al. Advances in the research and application of mutagenesis in microalgae[J]. Marine Sciences, 2021, 45(11): 165-178. | |
| 81 | CHENG Jun, ZHU Yanxia, ZHANG Ze, et al. Modification and improvement of microalgae strains for strengthening CO2 fixation from coal-fired flue gas in power plants[J]. Bioresource Technology, 2019, 291: 121850. |
| 82 | SHIN Sung Eun, Jong Min LIM, Hyun Gi KOH, et al. CRISPR/Cas9-induced knockout and knock-in mutations in chlamydomonas reinhardtii[J]. Scientific Reports, 2016, 6: 27810. |
| 83 | SUN Han, WU Tao, CHEN Stephenie Hiu Yuet, et al. Powerful tools for productivity improvements in microalgal production[J]. Renewable and Sustainable Energy Reviews, 2021, 152: 111609. |
| 84 | RAJEEV Anjaly, SIBY Aiswarya, KOOTTUNGAL Merin James, et al. Knocking down barriers: advances in siRNA delivery[J]. ChemistrySelect, 2021, 6(46): 13350-13362. |
| 85 | WEI Li, XIN Yi, WANG Qintao, et al. RNAi-based targeted gene knockdown in the model oleaginous microalgaeNannochloropsis oceanica [J]. The Plant Journal, 2017, 89(6): 1236-1250. |
| 86 | Iben SØRENSEN, FEI Zhangjun, ANDREAS Amanda, et al. Stable transformation and reverse genetic analysis of Penium margaritaceum: a platform for studies of charophyte green algae, the immediate ancestors of land plants[J]. The Plant Journal, 2014, 77(3): 339-351. |
| 87 | CHENG Jun, LU Hongxiang, HUANG Yun, et al. Enhancing growth rate and lipid yield of Chlorella with nuclear irradiation under high salt and CO2 stress[J]. Bioresource Technology, 2016, 203: 220-227. |
| 88 | SMALLEY Tressa, FIELDS Francis J, BERNDT Anthony J E, et al. Improving biomass and lipid yields of Desmodesmus armatus and Chlorella vulgaris through mutagenesis and high-throughput screening[J]. Biomass and Bioenergy, 2020, 142: 105755. |
| 89 | LI Xuyang, YUAN Yizhong, CHENG Dujia, et al. Exploring stress tolerance mechanism of evolved freshwater strain Chlorella sp. S30 under 30g/L salt[J]. Bioresource Technology, 2018, 250: 495-504. |
| 90 | LI Pengfei, SUN Xin, SUN Zhe, et al. Biochemical and genetic changes revealing the enhanced lipid accumulation in Desmodesmus sp. mutated by atmospheric and room temperature plasma[J]. Renewable Energy, 2021, 172: 368-381. |
| 91 | SONG Chunfeng, HAN Xiaoxuan, YIN Qingrong, et al. Performance intensification of CO2 absorption and microalgae conversion (CAMC) hybrid system via low temperature plasma (LTP) treatment[J]. The Science of the Total Environment, 2021, 801: 149791. |
| 92 | Dinesh KUMAR S, SOJIN Kang, SANTHANAM P, et al. Triggering of fatty acids on Tetraselmis sp. by ethyl methanesulfonate mutagenic treatment[J]. Bioresource Technology Reports, 2018, 2: 21-28. |
| 93 | OSADA Kyoko, MAEDA Yoshiaki, YOSHINO Tomoko, et al. Enhanced NADPH production in the pentose phosphate pathway accelerates lipid accumulation in the oleaginous diatom Fistulifera solaris [J]. Algal Research, 2017, 23: 126-134. |
| 94 | KLAITONG Paeka, Sirirat FA-AROONSAWAT, CHUNGJATUPORNCHAI Wipa. Accelerated triacylglycerol production and altered fatty acid composition in oleaginous microalga Neochloris oleoabundans by overexpression of diacylglycerol acyltransferase 2[J]. Microbial Cell Factories, 2017, 16(1): 61. |
| 95 | LI Yantao, HAN Danxiang, HU Guangrong, et al. Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii [J]. Biotechnology and Bioengineering, 2010, 107(2): 258-268. |
| 96 | TRENTACOSTE Emily M, SHRESTHA Roshan P, SMITH Sarah R, et al. Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(49): 19748-19753. |
| 97 | PENG Kuntao, ZHENG Cunni, XUE Jiao, et al. Delta 5 fatty acid desaturase upregulates the synthesis of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum [J]. Journal of Agricultural and Food Chemistry, 2014, 62(35): 8773-8776. |
| 98 | JIA Bin, SONG Yanzi, WU Min, et al. Characterization of long-chain acyl-CoA synthetases which stimulate secretion of fatty acids in green algae Chlamydomonas reinhardtii [J]. Biotechnology for Biofuels, 2016, 9(1): 184. |
| 99 | REZVANI S, MOHEIMANI N R, BAHRI P A. Techno-economic assessment of CO2 bio-fixation using microalgae in connection with three different state-of-the-art power plants[J]. Computers & Chemical Engineering, 2016, 84: 290-301. |
| 100 | BRANCO-VIEIRA M, MATA T M, MARTINS A A, et al. Economic analysis of microalgae biodiesel production in a small-scale facility[J]. Energy Reports, 2020, 6: 325-332. |
| 101 | MORES Patricia L, MANASSALDI Juan I, SCENNA Nicolás J, et al. Optimization of the design, operating conditions, and coupling configuration of combined cycle power plants and CO2 capture processes by minimizing the mitigation cost[J]. Chemical Engineering Journal, 2018, 331: 870-894. |
| 102 | OLIVEIRA Gisela M, Nídia CAETANO, MATA Teresa M, et al. Biofixation of CO2 emissions from natural gas combined cycle power plant[J]. Energy Reports, 2020, 6: 140-146. |
| 103 | 万伟华, 程军, 郭王彪. 我国微藻固定烟气CO2潜力时空格局分析[J]. 煤炭科学技术, 2022, 50(6): 107-116. |
| WAN Weihua, CHENG Jun, GUO Wangbiao. Analysis on temporal and spatial pattern of CO2 fixation ability from coal-fired flue gas fixed by microalgae in China[J]. Coal Science and Technology, 2022, 50(6): 107-116. |
| [1] | 金鑫, 李玉姗, 解青青, 王梦雨, 夏星帆, 杨朝合. 多孔材料催化丙酮缩甘油合成研究进展[J]. 化工进展, 2023, 42(2): 731-743. |
| [2] | 赵建兵, 杨丹, 舒原草, 朱俊波, 普仕萍, 宋晓丹, 刘守庆, 柴希娟, 李雪梅. Na2CO3/CF固体碱对菜籽油酯交换反应的催化性能[J]. 化工进展, 2022, 41(7): 3608-3614. |
| [3] | 邹鹏程, 金光远, 李臻峰, 宋春芳, 韩太柏, 祝玉莲. 一种具有模式搅拌的微波反应釜内多物理场特性分析[J]. 化工进展, 2022, 41(5): 2301-2310. |
| [4] | 唐文秀, 王学明, 郭亮, 季立豪, 高聪, 陈修来, 刘立明. 代谢工程改造大肠杆菌生产琥珀酸[J]. 化工进展, 2022, 41(2): 938-950. |
| [5] | 马鑫, 王霜, 李法社, 张逸水, 蒋上. 生物柴油雾化特性仿真模拟及实验研究[J]. 化工进展, 2022, 41(2): 655-665. |
| [6] | 朱长辉, 朱文超, 罗嘉, 田保河, 孙佳琳, 邹志云. 微波强化酯交换反应制备生物柴油研究进展[J]. 化工进展, 2022, 41(10): 5145-5154. |
| [7] | 纪淑兰, 李迅, 王飞. 丝瓜络固定化米根霉催化光皮树油制备生物柴油[J]. 化工进展, 2022, 41(10): 5381-5389. |
| [8] | 岳倩倩, 高李璟, 肖国民, 魏瑞平, 雷严. 生物柴油连续化生产设备及工艺进展[J]. 化工进展, 2021, 40(S2): 81-88. |
| [9] | 邹帅, 李玉芹, 马怡然, 齐振华, 贾权威. 二乙醇胺强化胶球藻Coccomyxa subellipsoidea C-169固定CO2和积累油脂[J]. 化工进展, 2021, 40(9): 5222-5230. |
| [10] | 包文君, 李子富, 王雪梅, 高瑞岭, 程世昆, 门玉. 产油酵母利用廉价原料合成油脂的研究进展[J]. 化工进展, 2021, 40(5): 2484-2495. |
| [11] | 孟心宇, 徐杰, 万杰, 刘雁军, 王晓丽, 张君, 郑锋, 阚建飞, 吴功德. 碳酸甘油酯的合成研究及产业化进展[J]. 化工进展, 2020, 39(9): 3739-3749. |
| [12] | 邢海亮,董训赞,韩本勇,耿树香,宁德鲁,马婷,余旭亚. 二氧化碳联合核桃壳提取液促进单针藻Monoraphidium sp. QLZ-3的生长和油脂积累[J]. 化工进展, 2020, 39(4): 1575-1582. |
| [13] | 王霜,王友昊,李法社,王文超,隋猛. 基于紫外吸光度的生物柴油氧化降解程度分析[J]. 化工进展, 2020, 39(2): 506-512. |
| [14] | 滕雯, 陈勇, 隋猛, 李法社. TEPA与[MI][C6H2(OH)3COO]复配对小桐子生物柴油抗氧化性的影响[J]. 化工进展, 2020, 39(11): 4427-4434. |
| [15] | 黄泽健,罗祎青,袁希钢. 水处理集成微藻生物柴油生命周期系统环境影响评价[J]. 化工进展, 2020, 39(1): 34-41. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||