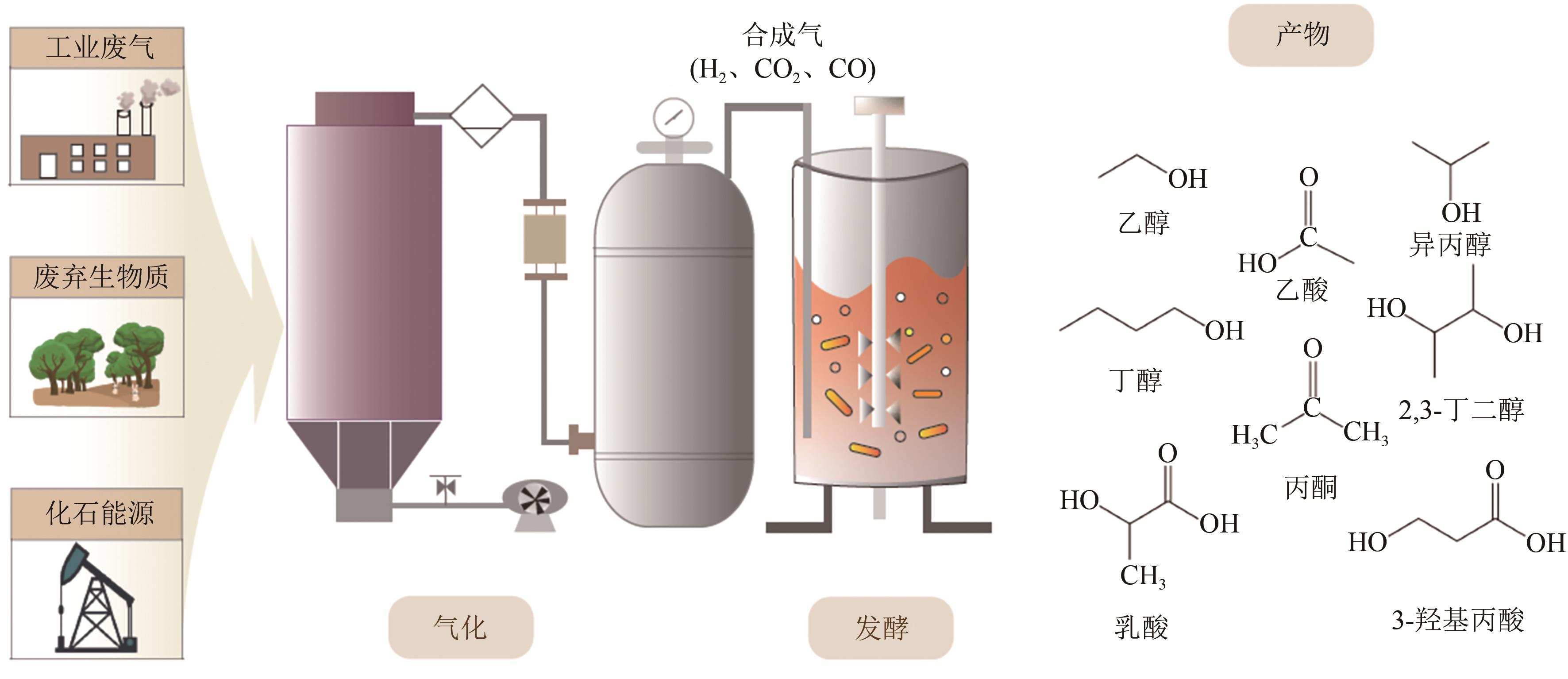
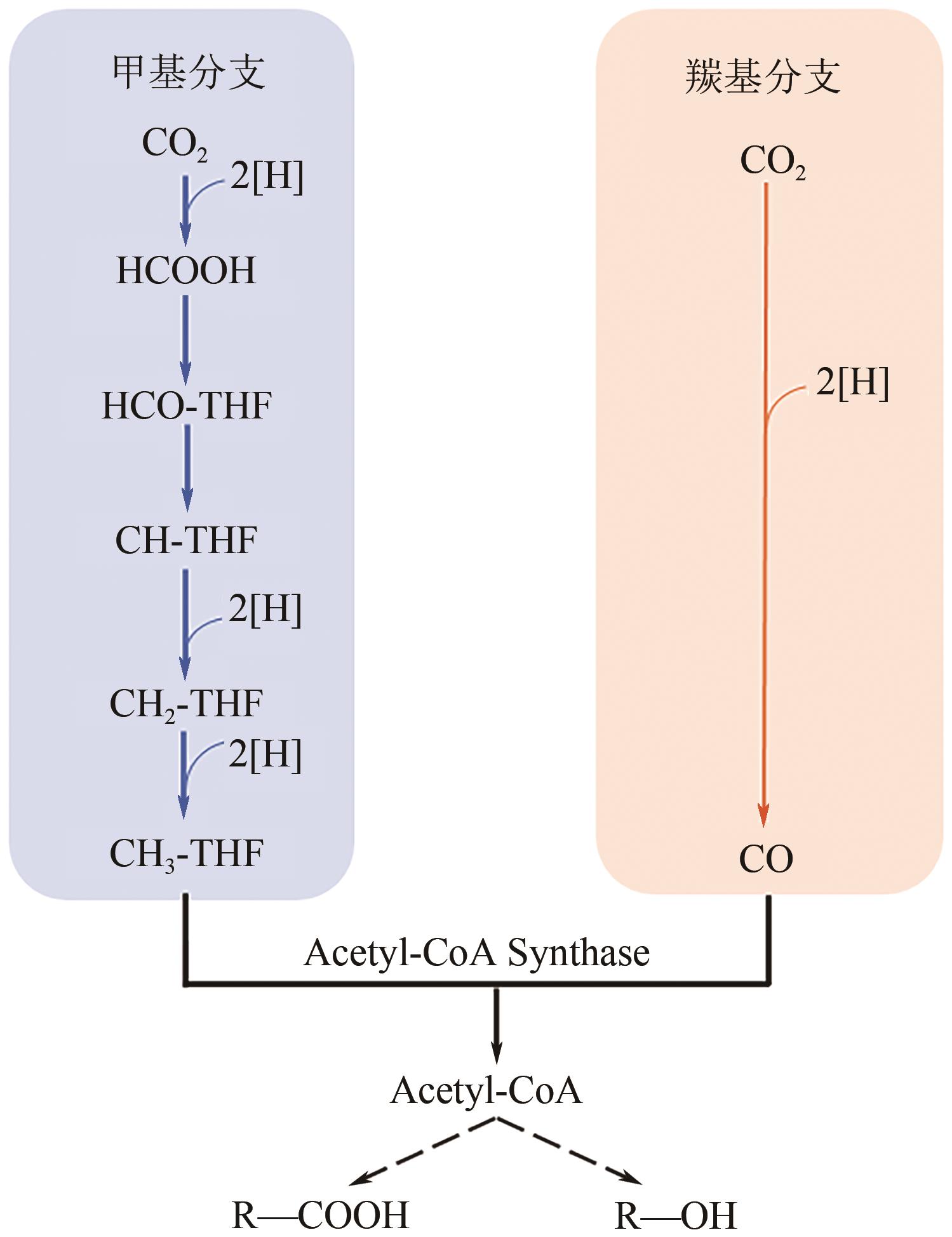
化工进展 ›› 2023, Vol. 42 ›› Issue (1): 73-85.DOI: 10.16085/j.issn.1000-6613.2022-1543
合成气的生物利用与定向转化
李婉麒1,2( ), 杨凤娟3(
), 杨凤娟3( ), 贾德臣1,2, 姜卫红1(
), 贾德臣1,2, 姜卫红1( ), 顾阳1(
), 顾阳1( )
)
- 1.中国科学院分子植物科学卓越创新中心,中国科学院合成生物学重点实验室,上海 200032
2.中国科学院大学,北京 100039
3.上海师范大学生命科学学院,上海 200234
-
收稿日期:2022-08-19修回日期:2022-10-07出版日期:2023-01-25发布日期:2023-02-20 -
通讯作者:姜卫红,顾阳 -
作者简介:李婉麒(1999—),女,硕士研究生,研究方向微生物代谢工程。E-mail:liwanqi@cemps.ac.cn
杨凤娟(1999—),女,硕士研究生,研究方向微生物代谢工程。E-mail:1000511033@smail.shnu.edu.cn。 -
基金资助:国家重点研发计划(2021YFC2103500);上海市科学技术委员会科研计划(21DZ1209100);中国科学院洁净能源创新研究院合作基金(DNL202013)
Biological utilization and conversion of syngas
LI Wanqi1,2( ), YANG Fengjuan3(
), YANG Fengjuan3( ), JIA Dechen1,2, JIANG Weihong1(
), JIA Dechen1,2, JIANG Weihong1( ), GU Yang1(
), GU Yang1( )
)
- 1.CAS Center for Excellence in Molecular Plant Sciences, CAS-Key Laboratory of Synthetic Biology, Chinese Academy of Sciences, Shanghai 200032, China
2.University of Chinese Academy of Sciences, Beijing 100039, China
3.College of Life Sciences, Shanghai Normal University, Shanghai 200234, China
-
Received:2022-08-19Revised:2022-10-07Online:2023-01-25Published:2023-02-20 -
Contact:JIANG Weihong, GU Yang
摘要:
合成气是来源于石化、煤化工以及生物质加工行业的一类重要原料气体。现有的化学催化路线可将合成气转化为氨、烯烃、甲醇等大宗化工产品,但尚无法实现选择性地合成具有较高附加值的长碳链化合物,而发展合成气的生物转化路线是克服上述难题、拓展产业链的有效策略。本文综述了随着分子遗传操作工具以及合成生物学的快速发展,合成气生物利用相关的菌株代谢工程设计、改造以及发酵工艺优化等方面的研究进展和产业化进程,并指出目前该技术路线在固碳效率、产物合成种类及产量方面还存在不足,亟待优化以满足大规模工业化推广应用的要求。本文还对合成气生物利用与转化的研究现状进行了梳理和总结,并探讨了未来的发展方向,以期为建立具有经济竞争力的合成气生物利用技术和工艺提供参考。
中图分类号:
引用本文
李婉麒, 杨凤娟, 贾德臣, 姜卫红, 顾阳. 合成气的生物利用与定向转化[J]. 化工进展, 2023, 42(1): 73-85.
LI Wanqi, YANG Fengjuan, JIA Dechen, JIANG Weihong, GU Yang. Biological utilization and conversion of syngas[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 73-85.
| 来源 | 气体组成及体积分数/% | |||||
|---|---|---|---|---|---|---|
| 氢气 | 一氧化碳 | 二氧化碳 | 氮气+氩气 | 氧气 | 甲烷 | |
| 天然气蒸气转化 | 72.5 | 15.0 | 8.5 | 1.0 | — | 3.0 |
| 水煤浆加压气化 | 33.8 | 48.4 | 17.6 | 0.05 | — | 0.15 |
| Shell粉煤 气化 | 27.4 | 63.5 | 1.48 | 7.6 | — | 0.02 |
| 灰熔聚流化床粉煤气化 | 34.2 | 35.6 | 27.5 | 1.1 | 0.1~0.2 | 1.5 |
| 地下煤气化(一段法) | 46.5 | 15.5 | 29.0 | 1.3 | 0.2 | 7.5 |
| 地下煤气化(两段法) | 64.0 | 11.3 | 11.0 | 3.5 | — | 9.9 |
| 焦炉气 | 54.6 | 6~7 | 1~3 | 3~5 | 0.3~0.7 | 24~28 |
| 电石炉气 | 5.0 | 70~90 | 1~3 | 2~5 | 0.2~1.0 | — |
| 渣油部分 氧化 | 49.3 | 50.1 | — | 0.2~0.3 | — | 0.3 |
| 生物质气化 | 37~43 | 31~34 | 3~10 | 10~20 | — | 2~3 |
表1 不同原料来源的合成气组成[5]
| 来源 | 气体组成及体积分数/% | |||||
|---|---|---|---|---|---|---|
| 氢气 | 一氧化碳 | 二氧化碳 | 氮气+氩气 | 氧气 | 甲烷 | |
| 天然气蒸气转化 | 72.5 | 15.0 | 8.5 | 1.0 | — | 3.0 |
| 水煤浆加压气化 | 33.8 | 48.4 | 17.6 | 0.05 | — | 0.15 |
| Shell粉煤 气化 | 27.4 | 63.5 | 1.48 | 7.6 | — | 0.02 |
| 灰熔聚流化床粉煤气化 | 34.2 | 35.6 | 27.5 | 1.1 | 0.1~0.2 | 1.5 |
| 地下煤气化(一段法) | 46.5 | 15.5 | 29.0 | 1.3 | 0.2 | 7.5 |
| 地下煤气化(两段法) | 64.0 | 11.3 | 11.0 | 3.5 | — | 9.9 |
| 焦炉气 | 54.6 | 6~7 | 1~3 | 3~5 | 0.3~0.7 | 24~28 |
| 电石炉气 | 5.0 | 70~90 | 1~3 | 2~5 | 0.2~1.0 | — |
| 渣油部分 氧化 | 49.3 | 50.1 | — | 0.2~0.3 | — | 0.3 |
| 生物质气化 | 37~43 | 31~34 | 3~10 | 10~20 | — | 2~3 |
| 原料 | 成分及体积分数/% | |||||
|---|---|---|---|---|---|---|
| 一氧化碳 | 二氧化碳 | 氢气 | 氮气 | 甲烷 | 其他 | |
| 柳枝稷 | 14.7 | 16.5 | 4.4 | 56.8 | 4.2 | 3.4 |
| 松木片 | 16.1 | 13.6 | 16.6 | 37.6 | 2.7 | 13.4 |
| 柳树 | 9.4 | 17.1 | 7.2 | 60.5 | 3.3 | 2.5 |
| 可可壳 | 8 | 16 | 9 | 61.5 | 2.3 | 3.2 |
| 乳制品生物质 | 8.7 | 15.7 | 18.6 | 56 | 0.6 | 0.4 |
| 草地早熟禾秸秆 | 12.9 | 17.4 | 2.6 | 64.2 | 2.1 | 0.8 |
| 拆除木材/纸张残余物 | 9.2 | 16.1 | 6.1 | 63.2 | 2.8 | 2.6 |
表2 来自各种木质纤维素生物质来源的合成气组成[7]
| 原料 | 成分及体积分数/% | |||||
|---|---|---|---|---|---|---|
| 一氧化碳 | 二氧化碳 | 氢气 | 氮气 | 甲烷 | 其他 | |
| 柳枝稷 | 14.7 | 16.5 | 4.4 | 56.8 | 4.2 | 3.4 |
| 松木片 | 16.1 | 13.6 | 16.6 | 37.6 | 2.7 | 13.4 |
| 柳树 | 9.4 | 17.1 | 7.2 | 60.5 | 3.3 | 2.5 |
| 可可壳 | 8 | 16 | 9 | 61.5 | 2.3 | 3.2 |
| 乳制品生物质 | 8.7 | 15.7 | 18.6 | 56 | 0.6 | 0.4 |
| 草地早熟禾秸秆 | 12.9 | 17.4 | 2.6 | 64.2 | 2.1 | 0.8 |
| 拆除木材/纸张残余物 | 9.2 | 16.1 | 6.1 | 63.2 | 2.8 | 2.6 |
| 微生物 | 底物 | 产品 | 最佳生长温度/℃ | 最适pH | 参考文献 |
|---|---|---|---|---|---|
| Acetobacterium bakii | 氢气/二氧化碳;一氧化碳;甲醇 | 乙酸 | 20 | 6.5 | [ |
| Acetobacterium woodii | 氢气/二氧化碳;甲醇;甲酸 | 乙酸 | 30 | 7.0 | [ |
| Acetohalobium arabaticum | 氢气/二氧化碳;一氧化碳 | 乙酸 | 38~40 | 7.6~8.0 | [ |
| Blautia producta | 氢气/二氧化碳;一氧化碳 | 乙酸 | 37 | 7.0 | [ |
| Butyribacterium methylotrophicum | 氢气/二氧化碳;一氧化碳 | 乙酸、乙醇、丁酸、丁醇 | 37~40 | 7.5 | [ |
| Clostridium aceticum | 氢气/二氧化碳;一氧化碳 | 乙酸 | 30 | 8.3 | [ |
| Clostridium autoethanogenum | 氢气/二氧化碳;一氧化碳 | 2,3-丁二醇、乙酸、乙醇 | 37 | 6.0 | [ |
| Clostridium carboxidivorans | 氢气/二氧化碳;一氧化碳 | 乙酸、乙醇、丁酸、丁醇 | 38 | 5.0~7.0 | [ |
| Clostridium coskatii | 氢气/二氧化碳;一氧化碳 | 乙酸、乙醇 | 37 | 6.0 | [ |
| Clostridium drakei | 氢气/二氧化碳;一氧化碳 | 乙酸、乙醇、丁酸 | 30 | 5.4~7.5 | [ |
| Clostridium formicaceticum | CO, CH3OH | 乙酸、甲酸 | 37 | 8.1 | [ |
| Clostridium ljungdahlii | 氢气/二氧化碳;一氧化碳;甲酸 | 2,3-丁二醇、乙酸、乙醇 | 37 | 6.0 | [ |
| Clostridium magnum | 氢气/二氧化碳;甲醇 | 乙酸 | 30 | 7.2 | [ |
| Clostridium mayombei | 氢气/二氧化碳 | 乙酸 | 33 | 7.3 | [ |
| Clostridium ragsdalei | 氢气/二氧化碳;一氧化碳 | 2,3-丁二醇、乙酸、乙醇 | 37 | 6.3 | [ |
| Clostridium scatologenes | 氢气/二氧化碳;一氧化碳;甲酸 | 乙酸、乙醇、丁酸 | 37 | 5.4~7.0 | [ |
| Eubacterium limosum | 氢气/二氧化碳;一氧化碳;甲醇;甲酸 | 乙酸、丁酸 | 37 | 7.0 | [ |
| Eubacterium callanderi | 氢气/二氧化碳;一氧化碳;甲醇 | 乙酸、丁酸 | 37 | 7.0 | [ |
| Sporomusa ovata | 氢气/二氧化碳;甲醇;甲酸 | 乙酸 | 34 | 6.3 | [ |
| Oxobacter pfennigii | 氢气/二氧化碳;一氧化碳 | 乙酸、丁酸 | 36~38 | 7.3 | [ |
| Thermoacetogenium phaeum | 氢气/二氧化碳;甲醇;甲酸 | 乙酸 | 60 | 6.8 | [ |
| Thermoanaerobacter kivui | 氢气/二氧化碳;一氧化碳;甲酸 | 乙酸 | 66 | 6.4 | [ |
| Treponema primitia | 氢气/二氧化碳 | 乙酸 | 30 | 7.2 | [ |
| Moorella thermoacetica | 氢气/二氧化碳;一氧化碳;甲醇;甲酸 | 乙酸 | 55 | 7.0 | [ |
表3 不同产乙酸菌的特征
| 微生物 | 底物 | 产品 | 最佳生长温度/℃ | 最适pH | 参考文献 |
|---|---|---|---|---|---|
| Acetobacterium bakii | 氢气/二氧化碳;一氧化碳;甲醇 | 乙酸 | 20 | 6.5 | [ |
| Acetobacterium woodii | 氢气/二氧化碳;甲醇;甲酸 | 乙酸 | 30 | 7.0 | [ |
| Acetohalobium arabaticum | 氢气/二氧化碳;一氧化碳 | 乙酸 | 38~40 | 7.6~8.0 | [ |
| Blautia producta | 氢气/二氧化碳;一氧化碳 | 乙酸 | 37 | 7.0 | [ |
| Butyribacterium methylotrophicum | 氢气/二氧化碳;一氧化碳 | 乙酸、乙醇、丁酸、丁醇 | 37~40 | 7.5 | [ |
| Clostridium aceticum | 氢气/二氧化碳;一氧化碳 | 乙酸 | 30 | 8.3 | [ |
| Clostridium autoethanogenum | 氢气/二氧化碳;一氧化碳 | 2,3-丁二醇、乙酸、乙醇 | 37 | 6.0 | [ |
| Clostridium carboxidivorans | 氢气/二氧化碳;一氧化碳 | 乙酸、乙醇、丁酸、丁醇 | 38 | 5.0~7.0 | [ |
| Clostridium coskatii | 氢气/二氧化碳;一氧化碳 | 乙酸、乙醇 | 37 | 6.0 | [ |
| Clostridium drakei | 氢气/二氧化碳;一氧化碳 | 乙酸、乙醇、丁酸 | 30 | 5.4~7.5 | [ |
| Clostridium formicaceticum | CO, CH3OH | 乙酸、甲酸 | 37 | 8.1 | [ |
| Clostridium ljungdahlii | 氢气/二氧化碳;一氧化碳;甲酸 | 2,3-丁二醇、乙酸、乙醇 | 37 | 6.0 | [ |
| Clostridium magnum | 氢气/二氧化碳;甲醇 | 乙酸 | 30 | 7.2 | [ |
| Clostridium mayombei | 氢气/二氧化碳 | 乙酸 | 33 | 7.3 | [ |
| Clostridium ragsdalei | 氢气/二氧化碳;一氧化碳 | 2,3-丁二醇、乙酸、乙醇 | 37 | 6.3 | [ |
| Clostridium scatologenes | 氢气/二氧化碳;一氧化碳;甲酸 | 乙酸、乙醇、丁酸 | 37 | 5.4~7.0 | [ |
| Eubacterium limosum | 氢气/二氧化碳;一氧化碳;甲醇;甲酸 | 乙酸、丁酸 | 37 | 7.0 | [ |
| Eubacterium callanderi | 氢气/二氧化碳;一氧化碳;甲醇 | 乙酸、丁酸 | 37 | 7.0 | [ |
| Sporomusa ovata | 氢气/二氧化碳;甲醇;甲酸 | 乙酸 | 34 | 6.3 | [ |
| Oxobacter pfennigii | 氢气/二氧化碳;一氧化碳 | 乙酸、丁酸 | 36~38 | 7.3 | [ |
| Thermoacetogenium phaeum | 氢气/二氧化碳;甲醇;甲酸 | 乙酸 | 60 | 6.8 | [ |
| Thermoanaerobacter kivui | 氢气/二氧化碳;一氧化碳;甲酸 | 乙酸 | 66 | 6.4 | [ |
| Treponema primitia | 氢气/二氧化碳 | 乙酸 | 30 | 7.2 | [ |
| Moorella thermoacetica | 氢气/二氧化碳;一氧化碳;甲醇;甲酸 | 乙酸 | 55 | 7.0 | [ |
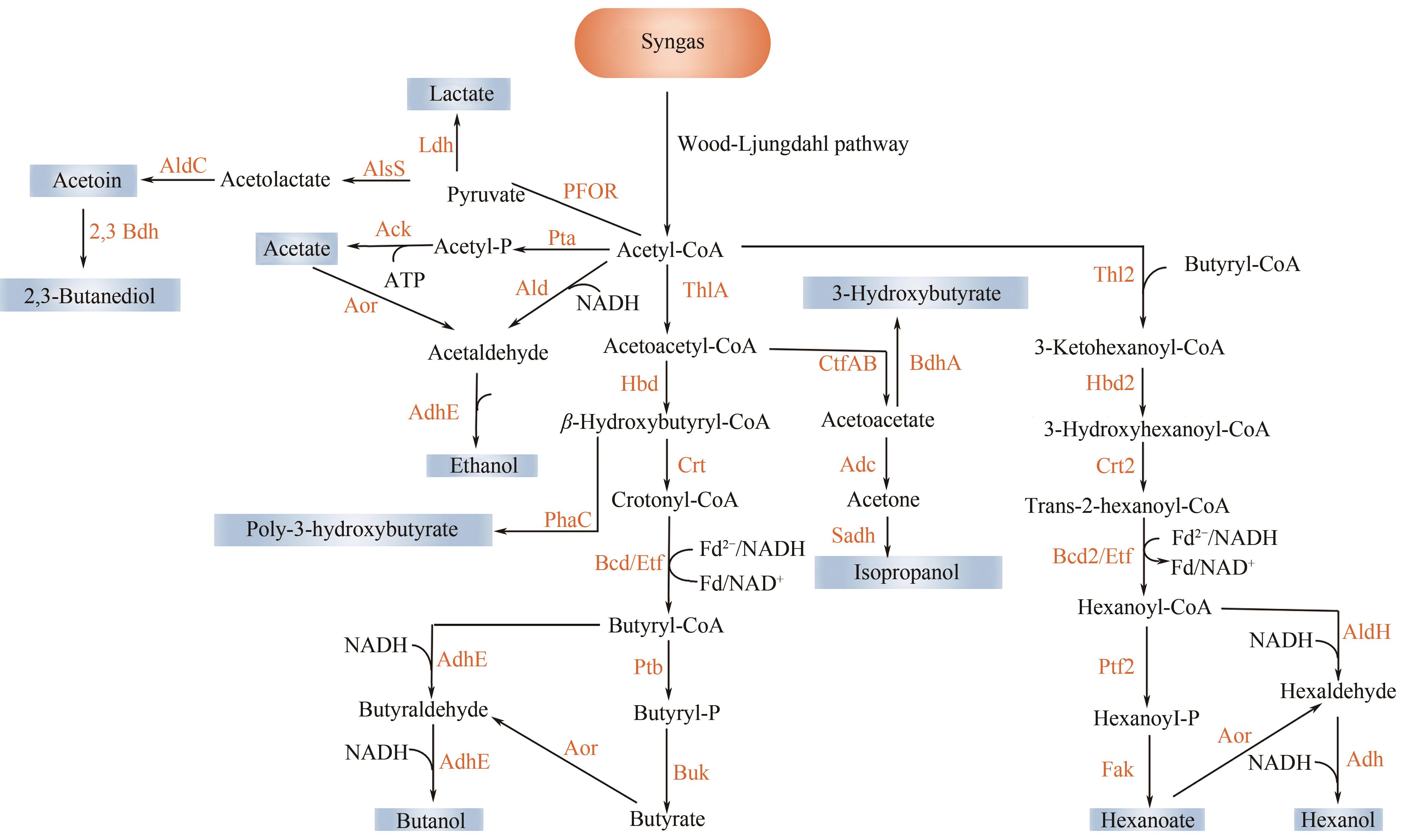
图3 食气梭菌合成产物的代谢途径ThlA(Thl2)—硫解酶;Hbd(Hbd2)—3-羟基丁酸辅酶 A 脱氢酶;Crt(Crt2)—巴豆酸酶;Bcd(Bcd2)—丁酰辅酶 A 脱氢酶;Ptb(Ptf2)—磷酸乙酰转移酶;Buk—丁酸激酶;CtfAB—辅酶A亚基A和B转移酶;Adc—乙酰乙酸脱羧酶;Sadh—伯/仲醇脱氢酶;BdhA—3-羟基丁酸脱氢酶;Aor—醛氧化还原酶;AdhE—醇醛脱氢酶;Ald—乙醛脱氢酶;PFOR—丙酮酸铁氧还蛋白氧化还原酶,AlsS—乙酰乳酸合酶;AldC—乙偶姻脱羧酶;2,3 Bdh—2,3-丁二醇脱氢酶;Ldh—乳酸脱氢酶;Pta—磷酸乙酰转移酶;Ack—乙酸激酶;Fak—脂肪酸激酶;Ptb(Ptf2)—磷酸乙酰转移酶;Adh—醇脱氢酶;AldH—己醛脱氢酶;AldH—己醛脱氢酶; PhaC—多羟基烷酸合酶;Syngas—合成气;Acetyl-CoA—乙酰辅酶A; Acetoacetyl-CoA—乙酰乙酰辅酶A;Crotonyl-CoA—巴豆酰辅酶A;Butyryl-P—丁酰辅酶A;Butyryl-P—丁酰磷酸;Butyrate—丁酸;Acetoacetate—乙酰乙酸;Acetone—丙酮;Isopropanol—异丙醇;3-Hydroxybutyrate—3-羟基丁酸;Butyraldehyde—丁醛;Butanol—丁醇;Acetaldehyde—乙醛;Ethanol—乙醇;Pyruvate—丙酮;Acetolactate—乙酰乳酸;Acetoin—乙偶姻;2,3-Butanediol—2,3-丁二醇;Lactate—乳酸;Acetyl-P—乙酰磷酸; Acetate—乙酸;3-Ketohexanoyl-CoA—3-氧己基酰辅酶A;3-Hydroxyhexanoyl-CoA—3-羟基己基辅酶A; Trans-2-hexanoyl-CoA—反-2-己酰基辅酶A;Hexanoyl-CoA—己烷基辅酶A;Hexanoyl-P—乙酰磷酸;Hexanoate—己酸;Hexaldehyde—己醛;Hexanol—己醇
| 1 | HUMPHREYS Christopher M, MINTON Nigel P. Advances in metabolic engineering in the microbial production of fuels and chemicals from C1 gas[J]. Current Opinion in Biotechnology, 2018, 50: 174-181. |
| 2 | SCHUCHMANN Kai, Volker MÜLLER. Energetics and application of heterotrophy in acetogenic bacteria[J]. Applied and Environmental Microbiology, 2016, 82(14): 4056-4069. |
| 3 | HEIJSTRA Björn D, LEANG Ching, JUMINAGA Alex. Gas fermentation: cellular engineering possibilities and scale up[J]. Microbial Cell Factories, 2017, 16(1): 60. |
| 4 | 瑙莫汗, 李维俊, 丽丽. 谈合成气的开发与利用[J]. 环境与发展, 2019, 31(4): 3, 5. |
| NAOMOHAN, LI Weijun, LI Li. Development and utilization of syngas[J]. Environment and Development, 2019, 31(4): 3, 5. | |
| 5 | 葛庆杰. 第六章 合成气化学[J]. 工业催化, 2016, 24(3): 82-104. |
| GE Qingjie. Chapter Ⅵ: Synthesis gas chemistry [J]. Industrial Catalysis, 2016, 24(3): 82-104. | |
| 6 | DATAR Rohit P, SHENKMAN Rustin M, CATENI Bruno G, et al. Fermentation of biomass-generated producer gas to ethanol[J]. Biotechnology and Bioengineering, 2004, 86(5): 587-594. |
| 7 | Oscar TIRADO-ACEVEDO, CHINN Mari S, GRUNDEN Amy M. Production of biofuels from synthesis gas using microbial catalysts[J]. Advances in Applied Microbiology, 2010, 70: 57-92. |
| 8 | BENGELSDORF Frank R, BECK Matthias H, Catarina ERZ, et al. Bacterial anaerobic synthesis gas (syngas) and CO2 + H2 fermentation[J]. Advances in Applied Microbiology, 2018, 103: 143-221. |
| 9 | SCHUCHMANN Kai, Volker MÜLLER. Autotrophy at the thermodynamic limit of life: a model for energy conservation in acetogenic bacteria[J]. Nature Reviews Microbiology, 2014, 12(12): 809-821. |
| 10 | LIEW FungMin, MARTIN Michael E, TAPPEL Ryan C, et al. Gas fermentation—A flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks[J]. Frontiers in Microbiology, 2016, 7: 694. |
| 11 | KOTSYURBENKO O R, SIMANKOVA M V, NOZHEVNIKOVA A N, et al. New species of psychrophilic acetogens: Acetobacterium bakii sp. nov., A. paludosum sp. nov., A. fimetarium sp. nov[J]. Archives of Microbiology, 1995, 163(1): 29-34. |
| 12 | HWANG Soonkyu, SONG Yoseb, CHO Byung Kwan. Draft genome sequence of Acetobacterium bakii DSM 8239, a potential psychrophilic chemical producer through syngas fermentation[J]. Genome Announcements, 2015, 3(5): e01070-e01015. |
| 13 | BALCH W E, SCHOBERTH S, TANNER R S, et al. Acetobacterium, a new genus of hydrogen-oxidizing, carbon dioxide-reducing, anaerobic bacteria[J]. International Journal of Systematic Bacteriology, 1977, 27(4): 355-361. |
| 14 | BACHE Regina, PFENNIG Norbert. Selective isolation of Acetobacterium woodii on methoxylated aromatic acids and determination of growth yields[J]. Archives of Microbiology, 1981, 130(3): 255-261. |
| 15 | SIKORSKI Johannes, LAPIDUS Alla, CHERTKOV Olga, et al. Complete genome sequence of Acetohalobium arabaticum type strain (Z-7288 T)[J]. Standards in Genomic Sciences, 2010, 3(1): 57-65. |
| 16 | GEERLIGS G, ALDRICH H C, HARDER W, et al. Isolation and characterization of a carbon monoxide utilizing strain of the acetogen Peptostreptococcus productus [J]. Archives of Microbiology, 1987, 148(4): 305-313. |
| 17 | LIU Chengxu, FINEGOLD Sydney M, SONG Yuli, et al. Reclassification of Clostridium coccoides, Ruminococcus hansenii, Ruminococcus hydrogenotrophicus, Ruminococcus luti, Ruminococcus productus and Ruminococcus schinkii as Blautia coccoides Gen. nov., comb. nov., Blautia hansenii comb. nov., Blautia hydrogenotrophica comb. nov., Blautia luti comb. nov., Blautia producta comb. nov., Blautia schinkii comb. nov. and description of Blautia wexlerae sp. nov., isolated from human faeces[J]. International Journal of Systematic and Evolutionary Microbiology, 2008, 58(8): 1896-1902. |
| 18 | LYND L, KERBY R, ZEIKUS J G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum [J]. Journal of Bacteriology, 1982, 149(1): 255-263. |
| 19 | ZEIKUS J G, LYND Lee H, THOMPSON T E, et al. Isolation and characterization of a new, methylotrophic, acidogenic anaerobe, the marburg strain[J]. Current Microbiology, 1980, 3(6): 381-386. |
| 20 | BRAUN M, MAYER F, GOTTSCHALK G. Clostridium aceticum (Wieringa), a microorganism producing acetic acid from molecular hydrogen and carbon dioxide[J]. Archives of Microbiology, 1981, 128(3): 288-293. |
| 21 | MAYER Alexander, Torben SCHÄDLER, TRUNZ Sascha, et al. Carbon monoxide conversion with Clostridium aceticum [J]. Biotechnology and Bioengineering, 2018, 115(11): 2740-2750. |
| 22 | ABRINI Jamal, NAVEAU Henry, NYNS Edmond Jacques. Clostridium autoethanogenum, sp. nov., an anaerobic bacterium that produces ethanol from carbon monoxide[J]. Archives of Microbiology, 1994, 161(4): 345-351. |
| 23 | Michael KÖPKE, MIHALCEA Christophe, LIEW Fungmin, et al. 2, 3-Butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas[J]. Applied and Environmental Microbiology, 2011, 77(15): 5467-5475. |
| 24 | LIOU Jack S C, BALKWILL David L, DRAKE Gwendolyn R, et al. Clostridium carboxidivorans sp. nov., a solvent-producing clostridium isolated from an agricultural settling lagoon, and reclassification of the acetogen Clostridium scatologenes strain SL1 as Clostridium drakei sp. nov[J]. International Journal of Systematic and Evolutionary Microbiology, 2005, 55(5): 2085-2091. |
| 25 | LI Ning, YANG Junjie, CHAI Changsheng, et al. Complete genome sequence of Clostridium carboxidivorans P7T, a syngas-fermenting bacterium capable of producing long-chain alcohols[J]. Journal of Biotechnology, 2015, 211: 44-45. |
| 26 | BENGELSDORF Frank R, POEHLEIN Anja, LINDER Sonja, et al. Industrial acetogenic biocatalysts: a comparative metabolic and genomic analysis[J]. Frontiers in Microbiology, 2016, 7: 1036. |
| 27 | GÖßNER Anita S, PICARDAL Flynn, TANNER Ralph S, et al. Carbon metabolism of the moderately acid-tolerant acetogen Clostridium drakei isolated from peat[J]. FEMS Microbiology Letters, 2008, 287(2): 236-242. |
| 28 | JEONG Yujin, SONG Yoseb, SHIN Hyeon Seok, et al. Draft genome sequence of acid-tolerant Clostridium drakei SL1T, a potential chemical producer through syngas fermentation[J]. Genome Announcements, 2014, 2(3): e00387. |
| 29 | ANDREESEN J R, GOTTSCHALK G, SCHLEGEL H G. Clostridium formicoaceticum nov. spec. isolation, description and distinction from C. aceticum and C. thermoaceticum [J]. Archiv Fur Mikrobiologie, 1970, 72(2): 154-174. |
| 30 | KARL Michael M, POEHLEIN Anja, BENGELSDORF Frank R, et al. Complete genome sequence of the autotrophic acetogen Clostridium formicaceticum DSM 92T using nanopore and illumina sequencing data[J]. Genome Announcements, 2017, 5(21):e00423. |
| 31 | TANNER R S, MILLER L M, YANG D. Clostridium ljungdahlii sp. nov., an acetogenic species in clostridial rRNA homology group I[J]. International Journal of Systematic Bacteriology, 1993, 43(2): 232-236. |
| 32 | Michael KÖPKE, HELD Claudia, HUJER Sandra, et al. Clostridium ljungdahlii represents a microbial production platform based on syngas[J]. PNAS, 2010, 107(29): 13087-13092. |
| 33 | SCHINK Bernhard. Clostridium magnum sp. nov., a non-autotrophic homoacetogenic bacterium[J]. Archives of Microbiology, 1984, 137(3): 250-255. |
| 34 | UHLIG Ronny, POEHLEIN Anja, FISCHER Ralf-Jörg, et al. Genome sequence of the autotrophic acetogen Clostridium magnum DSM 2767[J]. Genome Announcements, 2016, 4(3): e00464-e00416. |
| 35 | KANE Matthew D, BRAUMAN Alain, BREZNAK John A. Clostridium mayombei sp. nov., an H2/CO2 acetogenic bacterium from the gut of the African soil-feeding termite, Cubitermes speciosus[J]. Archives of Microbiology, 1991, 156(2): 99-104. |
| 36 | ZHU Zhengang, GUO Ting, ZHENG Huajun, et al. Complete genome sequence of a malodorant-producing acetogen, Clostridium scatologenes ATCC 25775T[J]. Journal of Biotechnology, 2015, 212: 19-20. |
| 37 | GENTHNER B R, DAVIS C L, BRYANT M P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species[J]. The Journal of Surgical Research, 1981, 42(1): 12-19. |
| 38 | SONG Yoseb, CHO Byung Kwan. Draft genome sequence of chemolithoautotrophic acetogenic butanol-producing Eubacterium limosum ATCC 8486[J]. Genome Announcements, 2015, 3(1): e01564-e01514. |
| 39 | CHANG In Seop, KIM Byung Hong, LOVITT Robert W, et al. Effect of CO partial pressure on cell-recycled continuous CO fermentation by Eubacterium limosum KIST612[J]. Process Biochemistry, 2001, 37(4): 411-421. |
| 40 | Hanseong ROH, Hyeok Jin KO, KIM Daehee, et al. Complete genome sequence of a carbon monoxide-utilizing acetogen, Eubacterium limosum KIST612[J]. Journal of Bacteriology, 2011, 193(1): 307-308. |
| 41 | Bernhard MÖLLER, Rolf OßMER, HOWARD Bernard H, et al. Sporomusa, a new genus of gram-negative anaerobic bacteria including Sporomusa sphaeroides spec. nov. and Sporomusa ovata spec. nov[J]. Archives of Microbiology, 1984, 139(4): 388-396. |
| 42 | POEHLEIN Anja, GOTTSCHALK Gerhard, DANIEL Rolf. First insights into the genome of the gram-negative, endospore-forming organism Sporomusa ovata strain H1 DSM 2662[J]. Genome Announcements, 2013, 1(5): e00734-e00713. |
| 43 | KRUMHOLZ L R, BRYANT M P. Clostridium pfennigii sp. nov. uses methoxyl groups of monobenzenoids and produces butyrate[J]. International Journal of Systematic Bacteriology, 1985, 35(4): 454-456. |
| 44 | HATTORI S, KAMAGATA Y, HANADA S, et al. Thermacetogenium phaeum Gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium[J]. International Journal of Systematic and Evolutionary Microbiology, 2000, 50(4): 1601-1609. |
| 45 | OEHLER Dirk, POEHLEIN Anja, LEIMBACH Andreas, et al. Genome-guided analysis of physiological and morphological traits of the fermentative acetate oxidizer Thermacetogenium phaeum [J]. BMC Genomics, 2012, 13: 723. |
| 46 | DANIEL S L, HSU T, DEAN S I, et al. Characterization of the H2- and CO-dependent chemolithotrophic potentials of the acetogens Clostridium thermoaceticum and Acetogenium kivui [J]. Journal of Bacteriology, 1990, 172(8): 4464-4471. |
| 47 | HESS Verena, POEHLEIN Anja, WEGHOFF Marie Charlotte, et al. A genome-guided analysis of energy conservation in the thermophilic, cytochrome-free acetogenic bacterium Thermoanaerobacter kivui [J]. BMC Genomics, 2014, 15(1): 1139. |
| 48 | GRABER Joseph R, LEADBETTER Jared R, BREZNAK John A. Description of Treponema azotonutricium sp. nov. and Treponema primitia sp. nov., the first spirochetes isolated from termite guts[J]. Applied and Environmental Microbiology, 2004, 70(3): 1315-1320. |
| 49 | ROSENTHAL Adam Z, MATSON Eric G, ELDAR Avigdor, et al. RNA-seq reveals cooperative metabolic interactions between two termite-gut spirochete species in co-culture[J]. The ISME Journal, 2011, 5(7): 1133-1142. |
| 50 | FONTAINE F E, PETERSON W H, MCCOY E, et al. A new type of glucose fermentation by Clostridium thermoaceticum [J]. Journal of Bacteriology, 1942, 43(6): 701-715. |
| 51 | BENGELSDORF Frank R, POEHLEIN Anja, ESSER Carola, et al. Complete genome sequence of the acetogenic bacterium Moorella thermoacetica DSM 2955T[J]. Genome Announcements, 2015, 3(5): e01157-e01115. |
| 52 | Amaresh DAS, LJUNGDAHL Lars G. Electron-transport system in acetogens[M]//Biochemistry and physiology of Anaerobic bacteria. New York: Springer-Verlag, 2006: 191-204. |
| 53 | GOTTWALD M, ANDREESEN J R, LEGALL J, et al. Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum [J]. Journal of Bacteriology, 1975, 122(1): 325-328. |
| 54 | Volker MÜLLER. Energy conservation in acetogenic bacteria[J]. Applied and Environmental Microbiology, 2003, 69(11): 6345-6353. |
| 55 | POEHLEIN Anja, CEBULLA Martin, Marcus M ILG, et al. The complete genome sequence of Clostridium aceticum: a missing link between rnf- and cytochrome-containing autotrophic acetogens[J]. mBio, 2015, 6(5): e01168-e01115. |
| 56 | Bettina SCHIEL-BENGELSDORF, Peter DÜRRE. Pathway engineering and synthetic biology using acetogens[J]. FEBS Letters, 2012, 586(15): 2191-2198. |
| 57 | PIERCE Elizabeth, XIE Gary, BARABOTE Ravi D, et al. The complete genome sequence of Moorella thermoacetica (f. Clostridium thermoaceticum)[J]. Environmental Microbiology, 2008, 10(10): 2550-2573. |
| 58 | 贾德臣, 姜卫红, 顾阳. 食气梭菌的研究进展[J]. 微生物学通报, 2019, 46(2): 374-387. |
| JIA Dechen, JIANG Weihong, GU Yang. Research progresses in gas-fermenting clostridia[J]. Microbiology China, 2019, 46(2): 374-387. | |
| 59 | YANG Yunpeng, LANG Nannan, YANG Gaohua, et al. Improving the performance of solventogenic clostridia by reinforcing the biotin synthetic pathway[J]. Metabolic Engineering, 2016, 35: 121-128. |
| 60 | MISOPH M, DRAKE H L. Effect of CO2 on the fermentation capacities of the acetogen peptostreptococcus productus U-1[J]. Journal of Bacteriology, 1996, 178(11): 3140-3145. |
| 61 | HEISKANEN Harri, Ilkka VIRKAJÄRVI, VIIKARI Liisa. The effect of syngas composition on the growth and product formation of Butyribacterium methylotrophicum [J]. Enzyme and Microbial Technology, 2007, 41(3): 362-367. |
| 62 | KANAUCHI Osamu, FUKUDA Masanobu, MATSUMOTO Yoshiaki, et al. Eubacterium limosum ameliorates experimental colitis and metabolite of microbe attenuates colonic inflammatory action with increase of mucosal integrity[J]. World Journal of Gastroenterology, 2006, 12(7): 1071-1077. |
| 63 | LIU Ziyong, JIA Dechen, ZHANG Kundi, et al. Ethanol metabolism dynamics in Clostridium ljungdahlii grown on carbon monoxide[J]. Applied and Environmental Microbiology, 2020, 86(14): e00730-e00720. |
| 64 | ZHU Haifeng, LIU Ziyong, ZHOU Xia, et al. Energy conservation and carbon flux distribution during fermentation of CO or H2/CO2 by Clostridium ljungdahlii [J]. Frontiers in Microbiology, 2020, 11: 416. |
| 65 | JIN Sangrak, Jiyun BAE, SONG Yoseb, et al. Synthetic biology on acetogenic bacteria for highly efficient conversion of C1 gases to biochemicals[J]. International Journal of Molecular Sciences, 2020, 21(20): 7639. |
| 66 | UTTURKAR Sagar M, KLINGEMAN Dawn M, BRUNO-BARCENA José M, et al. Sequence data for Clostridium autoethanogenum using three generations of sequencing technologies[J]. Scientific Data, 2015, 2: 150014. |
| 67 | HEAP John T, PENNINGTON Oliver J, CARTMAN Stephen T, et al. The ClosTron: a universal gene knock-out system for the genus Clostridium [J]. Journal of Microbiological Methods, 2007, 70(3): 452-464. |
| 68 | LIEW Fungmin, HENSTRA Anne M, Michael KӦPKE, et al. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production[J]. Metabolic Engineering, 2017, 40: 104-114. |
| 69 | MOCK Johanna, ZHENG Yanning, MUELLER Alexander P, et al. Energy conservation associated with ethanol formation from H2 and CO2 in Clostridium autoethanogenum involving electron bifurcation[J]. Journal of Bacteriology, 2015, 197(18): 2965-2980. |
| 70 | LEANG Ching, UEKI Toshiyuki, NEVIN Kelly P, et al. A genetic system for Clostridium ljungdahlii: a chassis for autotrophic production of biocommodities and a model homoacetogen[J]. Applied and Environmental Microbiology, 2013, 79(4): 1102-1109. |
| 71 | UEKI Toshiyuki, NEVIN Kelly P, WOODARD Trevor L, et al. Converting carbon dioxide to butyrate with an engineered strain of Clostridium ljungdahlii [J]. mBio, 2014, 5(5): e01636-e01614. |
| 72 | HUANG He, CHAI Changsheng, LI Ning, et al. CRISPR/Cas9-based efficient genome editing in Clostridium ljungdahlii, an autotrophic gas-fermenting bacterium[J]. ACS Synthetic Biology, 2016, 5(12): 1355-1361. |
| 73 | ZHAO Ran, LIU Yanqiang, ZHANG Huan, et al. CRISPR-Cas12a-mediated gene deletion and regulation in Clostridium ljungdahlii and its application in carbon flux redirection in synthesis gas fermentation[J]. ACS Synthetic Biology, 2019, 8(10): 2270-2279. |
| 74 | HUANG He, CHAI Changsheng, YANG Sheng, et al. Phage serine integrase-mediated genome engineering for efficient expression of chemical biosynthetic pathway in gas-fermenting Clostridium ljungdahlii [J]. Metabolic Engineering, 2019, 52: 293-302. |
| 75 | BANERJEE Areen, LEANG Ching, UEKI Toshiyuki, et al. Lactose-inducible system for metabolic engineering of Clostridium ljungdahlii [J]. Applied and Environmental Microbiology, 2014, 80(8): 2410-2416. |
| 76 | BECK Zachary Q, CERVIN Marguerite A, CHOTANI Gopal K, et al. Recombinant anaerobic acetogenic bacteria for production of isoprene and/or industrial bio-products using synthesis gas: US20140234926[P]. 2014-08-21. |
| 77 | ZHANG Lu, ZHAO Ran, JIA Dechen, et al. Engineering Clostridium ljungdahlii as the gas-fermenting cell factory for the production of biofuels and biochemicals[J]. Current Opinion in Chemical Biology, 2020, 59: 54-61. |
| 78 | PHILIPPS Gabriele, DE VRIES Sebastian, JENNEWEIN Stefan. Development of a metabolic pathway transfer and genomic integration system for the syngas-fermenting bacterium Clostridium ljungdahlii [J]. Biotechnology for Biofuels, 2019, 12: 112. |
| 79 | VAN OPIJNEN Tim, BODI Kip L, CAMILLI Andrew. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms[J]. Nature Methods, 2009, 6(10): 767-772. |
| 80 | WOODS Craig, HUMPHREYS Christopher M, Claudio TOMI-ANDRINO, et al. Required gene set for autotrophic growth of Clostridium autoethanogenum [J]. Applied and Environmental Microbiology, 2022, 88(7): e0247921. |
| 81 | STRAUB Melanie, DEMLER Martin, Dirk WEUSTER-BOTZ, et al. Selective enhancement of autotrophic acetate production with genetically modified Acetobacterium woodii [J]. Journal of Biotechnology, 2014, 178: 67-72. |
| 82 | JONES Shawn W, FAST Alan G, CARLSON Ellinor D, et al. CO2 fixation by anaerobic non-photosynthetic mixotrophy for improved carbon conversion[J]. Nature Communications, 2016, 7: 12800. |
| 83 | YANG Gaohua, JIA Dechen, JIN Lin, et al. Rapid generation of universal synthetic promoters for controlled gene expression in both gas-fermenting and Saccharolytic Clostridium species [J]. ACS Synthetic Biology, 2017, 6(9): 1672-1678. |
| 84 | SOHN Yu Jung, Jina SON, Seo Young JO, et al. Chemoautotroph Cupriavidus necator as a potential game-changer for global warming and plastic waste problem: a review[J]. Bioresource Technology, 2021, 340: 125693. |
| 85 | BOMAR Martin, HIPPE Hans, SCHINK Bernhard. Lithotrophic growth and hydrogen metabolism by Clostridium magnum [J]. FEMS Microbiology Letters, 1991, 83(3): 347-349. |
| 86 | KOEPKE Michael, SIMPSON Sean Dennis, LIEW Fungmin. Recombinant microorganism and methods of production thereof: US20140186928[P]. 2014-07-03. |
| 87 | LIEW Fungmin Eric, NOGLE Robert, ABDALLA Tanus, et al. Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale[J]. Nature Biotechnology, 2022, 40(3): 335-344. |
| 88 | SREEKUMAR Sanil, BAER Zachary C, GROSS Elad, et al. Chemocatalytic upgrading of tailored fermentation products toward biodiesel[J]. ChemSusChem, 2014, 7(9): 2445-2448. |
| 89 | DE SOUZA PINTO LEMGRUBER Renato, VALGEPEA Kaspar, TAPPEL Ryan, et al. Systems-level engineering and characterisation of Clostridium autoethanogenum through heterologous production of poly-3-hydroxybutyrate (PHB)[J]. Metabolic Engineering, 2019, 53: 14-23. |
| 90 | Bastian VÖGELI, SCHULZ Luca, GARG Shivani, et al. Cell-free prototyping enables implementation of optimized reverse β-oxidation pathways in heterotrophic and autotrophic bacteria[J]. Nature Communications, 2022, 13(1): 3058. |
| 91 | MINTON Nigel P, EHSAAN Muhammad, HUMPHREYS Christopher M, et al. A roadmap for gene system development in Clostridium [J]. Anaerobe, 2016, 41: 104-112. |
| 92 | BAKER Jonathan P, Javier SÁEZ-SÁEZ, JENSEN Sheila I, et al. A clean in-frame knockout system for gene deletion in Acetobacterium woodii [J]. Journal of Biotechnology, 2022, 353: 9-18. |
| 93 | HOFFMEISTER Sabrina, GERDOM Marzena, BENGELSDORF Frank R, et al. Acetone production with metabolically engineered strains of Acetobacterium woodii [J]. Metabolic Engineering, 2016, 36: 37-47. |
| 94 | Kübra ARSLAN, SCHOCH Teresa, Franziska HÖFELE, et al. Engineering Acetobacterium woodii for the production of isopropanol and acetone from carbon dioxide and hydrogen[J]. Biotechnology Journal, 2022, 17(5): 2100515. |
| 95 | KANG Seulgi, SONG Yoseb, JIN Sangrak, et al. Adaptive laboratory evolution of Eubacterium limosum ATCC 8486 on carbon monoxide[J]. Frontiers in Microbiology, 2020, 11: 402. |
| 96 | LEE Seong Hyuk, LEE Sung Mok, LEE Jung Hyun, et al. Biological process for coproduction of hydrogen and thermophilic enzymes during CO fermentation[J]. Bioresource Technology, 2020, 305: 123067. |
| 97 | SCHWARZ Fabian M, Volker MÜLLER. Whole-cell biocatalysis for hydrogen storage and syngas conversion to formate using a thermophilic acetogen[J]. Biotechnology for Biofuels, 2020, 13: 32. |
| 98 | Anton RÜCKEL, HANNEMANN Jens, MAIERHOFER Carolin, et al. Studies on syngas fermentation with Clostridium carboxidivorans in stirred-tank reactors with defined gas impurities[J]. Frontiers in Microbiology, 2021, 12: 655390. |
| 99 | MONIR Minhaj Uddin, YOUSUF Abu, AZIZ Azrina Abd. Syngas fermentation to bioethanol[M]//Lignocellulosic biomass to liquid biofuels. Amsterdam: Elsevier, 2020: 195-216. |
| 100 | BREDWELL M D, WORDEN R M. Mass-transfer properties of microbubbles. 1. Experimental studies[J]. Biotechnology Progress, 1998, 14(1): 31-38. |
| 101 | ZHANG Lijuan, HU Peng, PAN Jiang, et al. Immobilization of trophic anaerobic acetogen for semi-continuous syngas fermentation[J]. Chinese Journal of Chemical Engineering, 2021, 29: 311-316. |
| 102 | KASTER Jeffrey A, MICHELSEN Donald L, VELANDER William H. Increased oxygen transfer in a yeast fermentation using a microbubble dispersion[J]. Applied Biochemistry and Biotechnology, 1990, 24/25(1): 469-484. |
| 103 | PHILLIPS J R, KLASSON K T, CLAUSEN E C, et al. Biological production of ethanol from coal synthesis gas[J]. Applied Biochemistry and Biotechnology, 1993, 39/40(1): 559-571. |
| 104 | 张存胜, 刘岩, 杨莉, 等. 工业废弃合成气厌氧发酵产己醇研究进展[J]. 化工进展, 2021, 40(3): 1604-1610. |
| ZHANG Cunsheng, LIU Yan, YANG Li, et al. Research progress of hexanol production through anaerobic fermentation of wasted industrial syngas[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1604-1610. | |
| 105 | 陈铭, 刘超, 王雯, 等. 温度和pH对厌氧微生物转化合成气定向产乙酸的影响[J]. 环境工程, 2019, 37(12): 183-187, 206. |
| CHEN Ming, LIU Chao, WANG Wen, et al. Effect of temperature and pH on acetate production by syngas bio-transformation in fermentation[J]. Environmental Engineering, 2019, 37(12): 183-187, 206. | |
| 106 | 邓立康, 刘佳昕, 刘晓峰, 等. 利用合成气生产生物基醇类化学品的研究进展[J]. 微生物学杂志, 2022, 42(3): 110-119. |
| DENG Likang, LIU Jiaxin, LIU Xiaofeng, et al. Advances in production of bio-based alcoholic chemicals from syngas[J]. Journal of Microbiology, 2022, 42(3): 110-119. | |
| 107 | KUNDIYANA Dimple K, HUHNKE Raymond L, MADDIPATI Prasanth, et al. Feasibility of incorporating cotton seed extract in Clostridium strain P11 fermentation medium during synthesis gas fermentation[J]. Bioresource Technology, 2010, 101(24): 9673-9680. |
| 108 | SAXENA Jyotisna, TANNER Ralph S. Effect of trace metals on ethanol production from synthesis gas by the ethanologenic acetogen, Clostridium ragsdalei [J]. Journal of Industrial Microbiology & Biotechnology, 2011, 38(4): 513-521. |
| 109 | SIMPSON S D, FORSTER R L S, TRAN P L, et al. Processes of producing alcohols: WO2009022925A1[P]. 2008-08-15. |
| 110 | MIHALCEA Christophe Daniel, FUNG Jennifer Mon Yee, Bakir AL-SINAWI, et al. Optimised fermentation media: US20130230894[P]. 2013-09-05. |
| 111 | SUN Xiao, ATIYEH Hasan K, ZHANG Hailin, et al. Enhanced ethanol production from syngas by Clostridium ragsdalei in continuous stirred tank reactor using medium with poultry litter biochar[J]. Applied Energy, 2019, 236: 1269-1279. |
| 112 | KIM Ji Yeon, PARK Sehoon, JEONG Jiyeong, et al. Methanol supply speeds up synthesis gas fermentation by methylotrophic-acetogenic bacterium, Eubacterium limosum KIST612[J]. Bioresource Technology, 2021, 321: 124521. |
| 113 | JACK Joshua, Jonathan LO, MANESS Pin Ching, et al. Directing Clostridium ljungdahlii fermentation products via hydrogen to carbon monoxide ratio in syngas[J]. Biomass and Bioenergy, 2019, 124: 95-101. |
| 114 | COTTER Jacqueline L, CHINN Mari S, GRUNDEN Amy M. Influence of process parameters on growth of Clostridium ljungdahlii and Clostridium autoethanogenum on synthesis gas[J]. Enzyme and Microbial Technology, 2009, 44(5): 281-288. |
| 115 | SHEN Shaohuang, GU Yang, CHAI Changsheng, et al. Enhanced alcohol titre and ratio in carbon monoxide-rich off-gas fermentation of Clostridium carboxidivorans through combination of trace metals optimization with variable-temperature cultivation[J]. Bioresource Technology, 2017, 239: 236-243. |
| 116 | JIA Dechen, HE Meiyu, TIAN Yi, et al. Metabolic engineering of gas-fermenting Clostridium ljungdahlii for efficient co-production of isopropanol, 3-hydroxybutyrate, and ethanol[J]. ACS Synthetic Biology, 2021, 10(10): 2628-2638. |
| 117 | Hyun Ju OH, Ja Kyong KO, GONG Gyeongtaek, et al. Production of hexanol as the main product through syngas fermentation by Clostridium carboxidivorans P7[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 850370. |
| [1] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [2] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| [3] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [4] | 田园, 娄舒洁, 孟闪茹, 闫敬如, 肖海成. 合成气制高碳醇钴基催化剂研究进展[J]. 化工进展, 2023, 42(4): 1869-1876. |
| [5] | 阮鹏, 杨润农, 林梓荣, 孙永明. 甲烷催化部分氧化制合成气催化剂的研究进展[J]. 化工进展, 2023, 42(4): 1832-1846. |
| [6] | 周晨, 傅杰, 张国俊. 2022年度国家自然科学基金委员会化学工程与工业化学领域科学基金项目申请和评审工作综述[J]. 化工进展, 2023, 42(1): 553-558. |
| [7] | 邓少碧, 边洲峰. 核壳结构在甲烷干重整中的应用[J]. 化工进展, 2023, 42(1): 247-254. |
| [8] | 张大洲, 卢文新, 商宽祥, 胡媛, 朱凡, 张宗飞. 草酸二甲酯加氢制乙醇酸甲酯反应网络分析及其多相加氢催化剂研究进展[J]. 化工进展, 2023, 42(1): 204-214. |
| [9] | 石轩, 杨东元, 胡浩斌, 王焦飞, 张壮壮, 贺建勋, 代成义, 马晓迅. 苯与合成气在ZnAlCrO x &HZSM-5双功能催化剂上一步法制甲苯/二甲苯[J]. 化工进展, 2022, 41(S1): 247-259. |
| [10] | 曹正凯, 米晓斌, 吴子明, 孙士可, 曹均丰, 彭德强, 梁相程. 煤合成气净化除尘装置压降问题分析及应用优化[J]. 化工进展, 2022, 41(S1): 15-21. |
| [11] | 胡文德, 王仰东, 王传明. 合成气直接催化转化制低碳烯烃研究进展[J]. 化工进展, 2022, 41(9): 4754-4766. |
| [12] | 张鹏, 孟凡会, 杨贵楠, 李忠. 金属氧化物在OX-ZEO催化剂中催化CO x 加氢制低碳烯烃研究进展[J]. 化工进展, 2022, 41(8): 4159-4172. |
| [13] | 周红军, 周颖, 徐春明. 中国碳中和目标下CO2转化的思考与实践[J]. 化工进展, 2022, 41(6): 3381-3385. |
| [14] | 储根云, 范英杰, 张大伟, 高明林, 梅树美, 杨庆春. 煤制乙二醇关键单元技术与低碳集成工艺的研究进展[J]. 化工进展, 2022, 41(3): 1654-1666. |
| [15] | 吴涵竹, 司志豪, 秦培勇. 生物乙醇原位分离技术的研究进展[J]. 化工进展, 2022, 41(3): 1318-1329. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||