化工进展 ›› 2022, Vol. 41 ›› Issue (3): 1654-1666.DOI: 10.16085/j.issn.1000-6613.2021-2147
煤制乙二醇关键单元技术与低碳集成工艺的研究进展
储根云1( ), 范英杰1, 张大伟1, 高明林2, 梅树美2, 杨庆春1,2(
), 范英杰1, 张大伟1, 高明林2, 梅树美2, 杨庆春1,2( )
)
- 1.合肥工业大学化学与化工学院,安徽 合肥 230009
2.安徽昊源化工集团有限公司,安徽 阜阳 236023
-
收稿日期:2021-10-18修回日期:2021-11-17出版日期:2022-03-23发布日期:2022-03-28 -
通讯作者:杨庆春 -
作者简介:储根云(1998—),女,硕士研究生,研究方向为化工过程系统工程。E-mail:3171803243@qq.com 。 -
基金资助:国家自然科学基金(22108052);安徽省自然科学基金(1908085QB69)
Progress in key unit technologies and low-carbon integrated processes of coal to ethylene glycol process
CHU Genyun1( ), FAN Yingjie1, ZHANG Dawei1, GAO Minglin2, MEI Shumei2, YANG Qingchun1,2(
), FAN Yingjie1, ZHANG Dawei1, GAO Minglin2, MEI Shumei2, YANG Qingchun1,2( )
)
- 1.School of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei 230009, Anhui, China
2.Anhui Haoyuan Chemical Group Co. , Ltd. , Fuyang 236023, Anhui, China
-
Received:2021-10-18Revised:2021-11-17Online:2022-03-23Published:2022-03-28 -
Contact:YANG Qingchun
摘要:
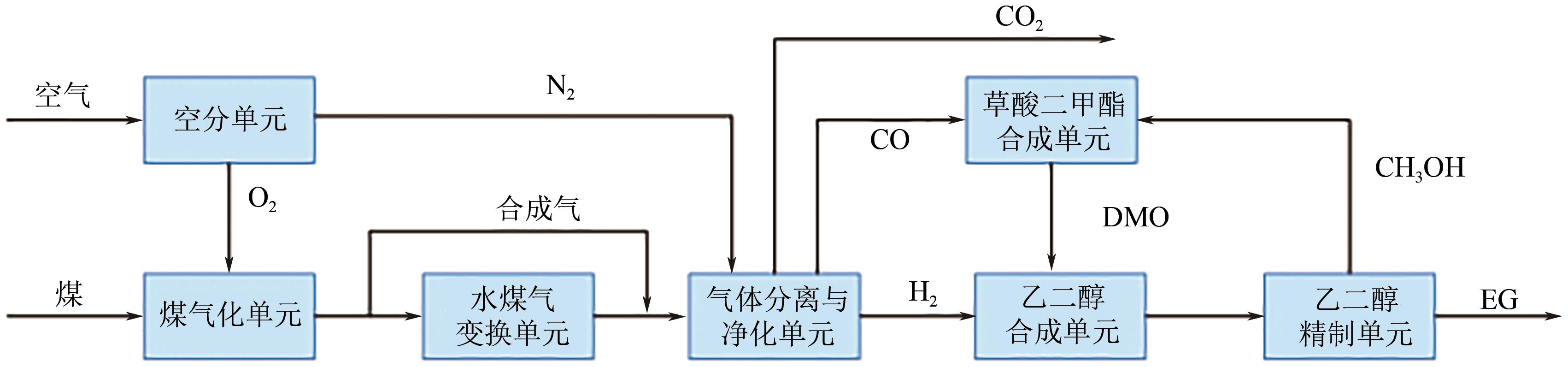
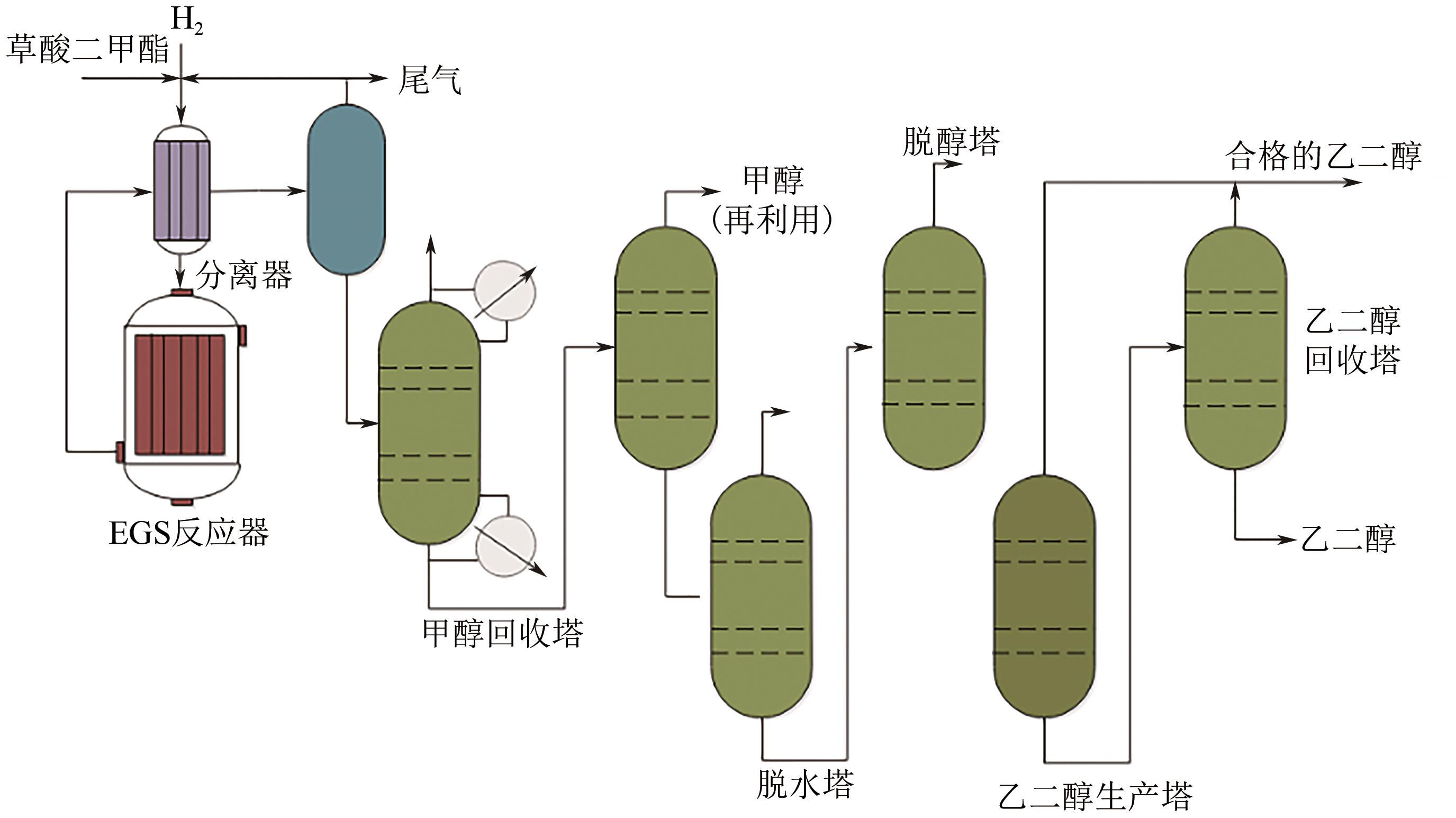
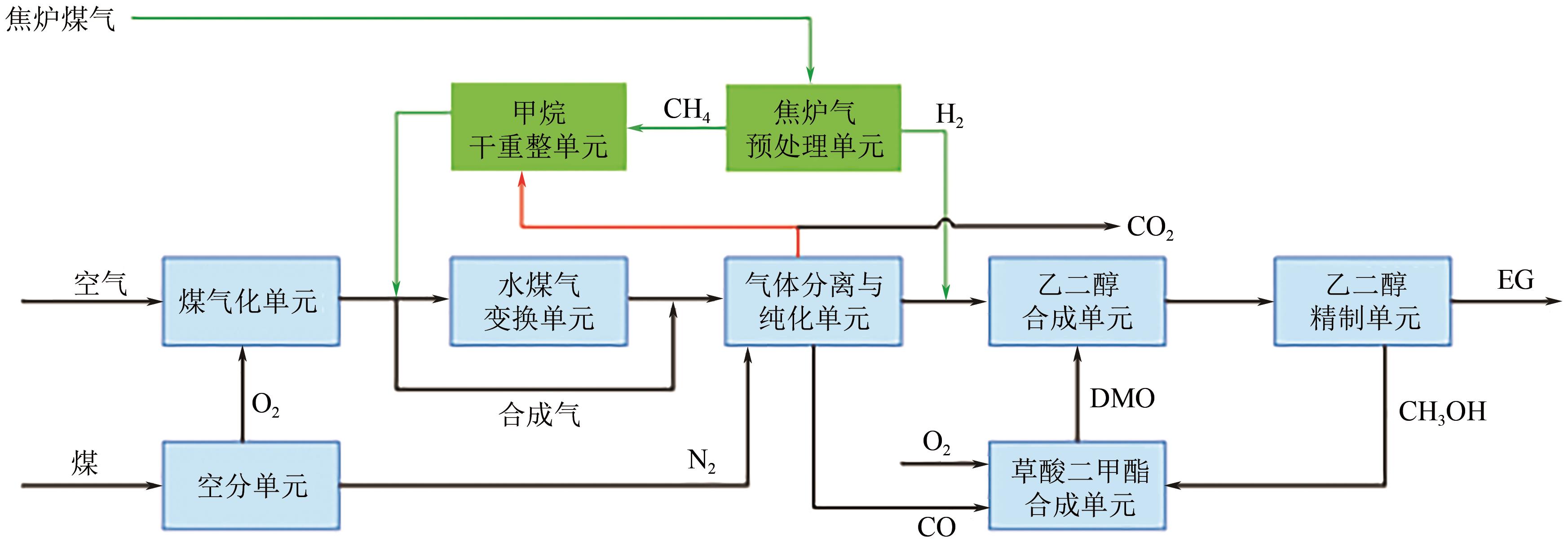
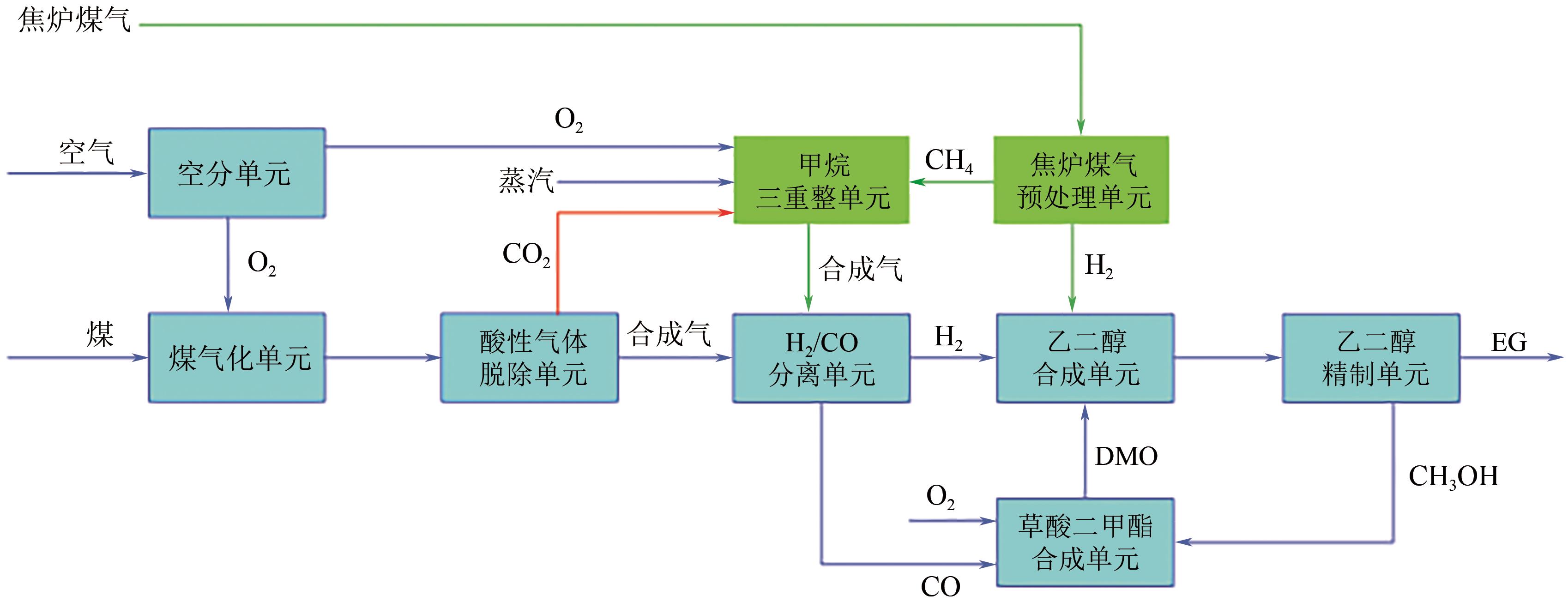
我国乙二醇对外依存度居高不下,而富煤少油的资源特性使得我国煤制乙二醇技术具有较好的成本与原料优势,发展迅速。本文综述了国内外煤制乙二醇技术的技术现状和发展趋势,重点介绍了煤气化、草酸二甲酯合成和乙二醇合成与精制等关键单元技术的技术特征、工艺流程和技术进展,并分析了相关单元对整个煤制乙二醇系统技术经济性能的影响。针对现有煤制乙二醇技术存在能耗高、质能效率低和CO2排放大的问题,着重讨论了集成CO2高效利用的煤与富氢资源联供制乙二醇集成工艺的进展,包括耦合焦炉气、页岩气和绿氢等资源的新工艺等。以焦炉气为例,集成不同重整技术的新工艺使得传统工艺的碳效率和?效率分别提升了23.35%~39.17%和4.25%~10.12%,生产成本降低了8.73%~19.88%,内部收益率提高了3.6%~9.6%。因此,集成富氢资源与CO2高效利用的煤制乙二醇创新工艺是该行业向高效-经济-清洁可持续发展的重要方向。
中图分类号:
引用本文
储根云, 范英杰, 张大伟, 高明林, 梅树美, 杨庆春. 煤制乙二醇关键单元技术与低碳集成工艺的研究进展[J]. 化工进展, 2022, 41(3): 1654-1666.
CHU Genyun, FAN Yingjie, ZHANG Dawei, GAO Minglin, MEI Shumei, YANG Qingchun. Progress in key unit technologies and low-carbon integrated processes of coal to ethylene glycol process[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1654-1666.
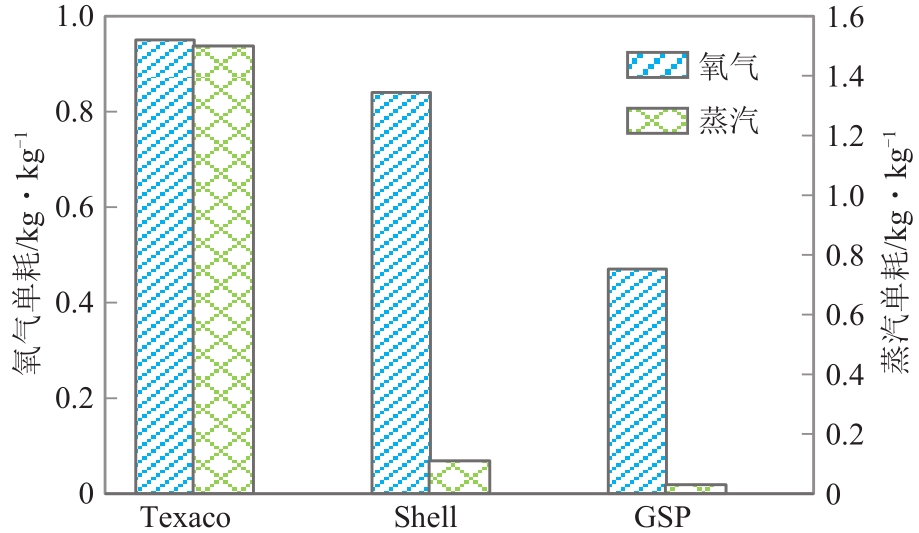
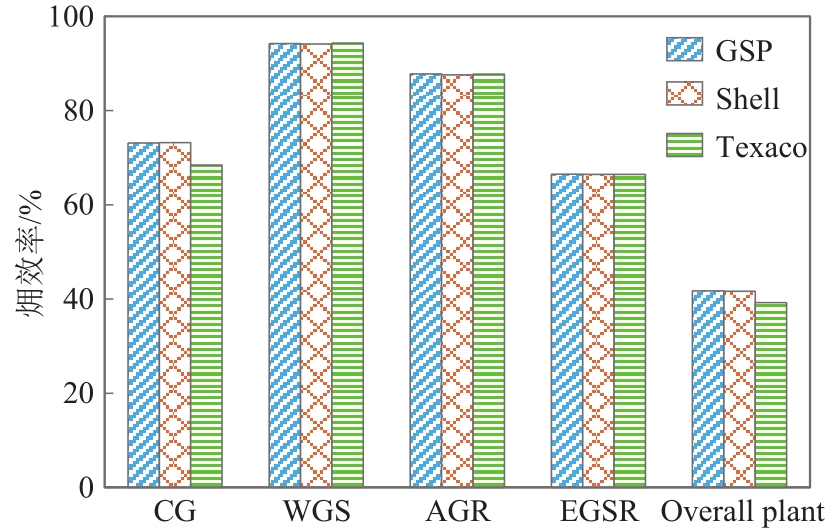
| 气化技术 | Texaco | Shell | GSP |
|---|---|---|---|
| 反应床类型 | 气流床 | 气流床 | 气流床 |
| 气化温度/℃ | 1350~1400 | 1400~1600 | 1400~1600 |
| 气化压力 /MPa | 2.6~8.5 | 2~4 | 2.5~4.0 |
| 出口温度/℃ | 约210 | 约170 | 约220 |
| 煤种 | 烟煤、次烟煤等 | 褐煤、烟煤等 | 褐煤、烟煤等 |
| 进料方式 | 60%~65%水煤浆 | 150~200目干煤粉 | 250~500目干煤粉 |
| 湿度/% | <8 | 烟煤<2;褐煤<8 | 烟煤<2;褐煤<8 |
| 灰熔点/℃ | <1300 | <1500 | <1500 |
| 灰分/% | <13 | 1~20 | 1~20 |
| 单炉投煤量 /t·d-1 | 2000 | 2800 | 720 |
| 气化剂 | 纯氧 | 纯氧+水蒸气 | 纯氧+水蒸气 |
| 有效气体积 分数/% | 77.5 | 96.6 | 88.8 |
| 国产化水平 | 基本国产化 | 关键设备需引进 | 关键设备需引进 |
| 投资 | 低 | 高 | 较高 |
表1 不同气w化技术的主要参数
| 气化技术 | Texaco | Shell | GSP |
|---|---|---|---|
| 反应床类型 | 气流床 | 气流床 | 气流床 |
| 气化温度/℃ | 1350~1400 | 1400~1600 | 1400~1600 |
| 气化压力 /MPa | 2.6~8.5 | 2~4 | 2.5~4.0 |
| 出口温度/℃ | 约210 | 约170 | 约220 |
| 煤种 | 烟煤、次烟煤等 | 褐煤、烟煤等 | 褐煤、烟煤等 |
| 进料方式 | 60%~65%水煤浆 | 150~200目干煤粉 | 250~500目干煤粉 |
| 湿度/% | <8 | 烟煤<2;褐煤<8 | 烟煤<2;褐煤<8 |
| 灰熔点/℃ | <1300 | <1500 | <1500 |
| 灰分/% | <13 | 1~20 | 1~20 |
| 单炉投煤量 /t·d-1 | 2000 | 2800 | 720 |
| 气化剂 | 纯氧 | 纯氧+水蒸气 | 纯氧+水蒸气 |
| 有效气体积 分数/% | 77.5 | 96.6 | 88.8 |
| 国产化水平 | 基本国产化 | 关键设备需引进 | 关键设备需引进 |
| 投资 | 低 | 高 | 较高 |
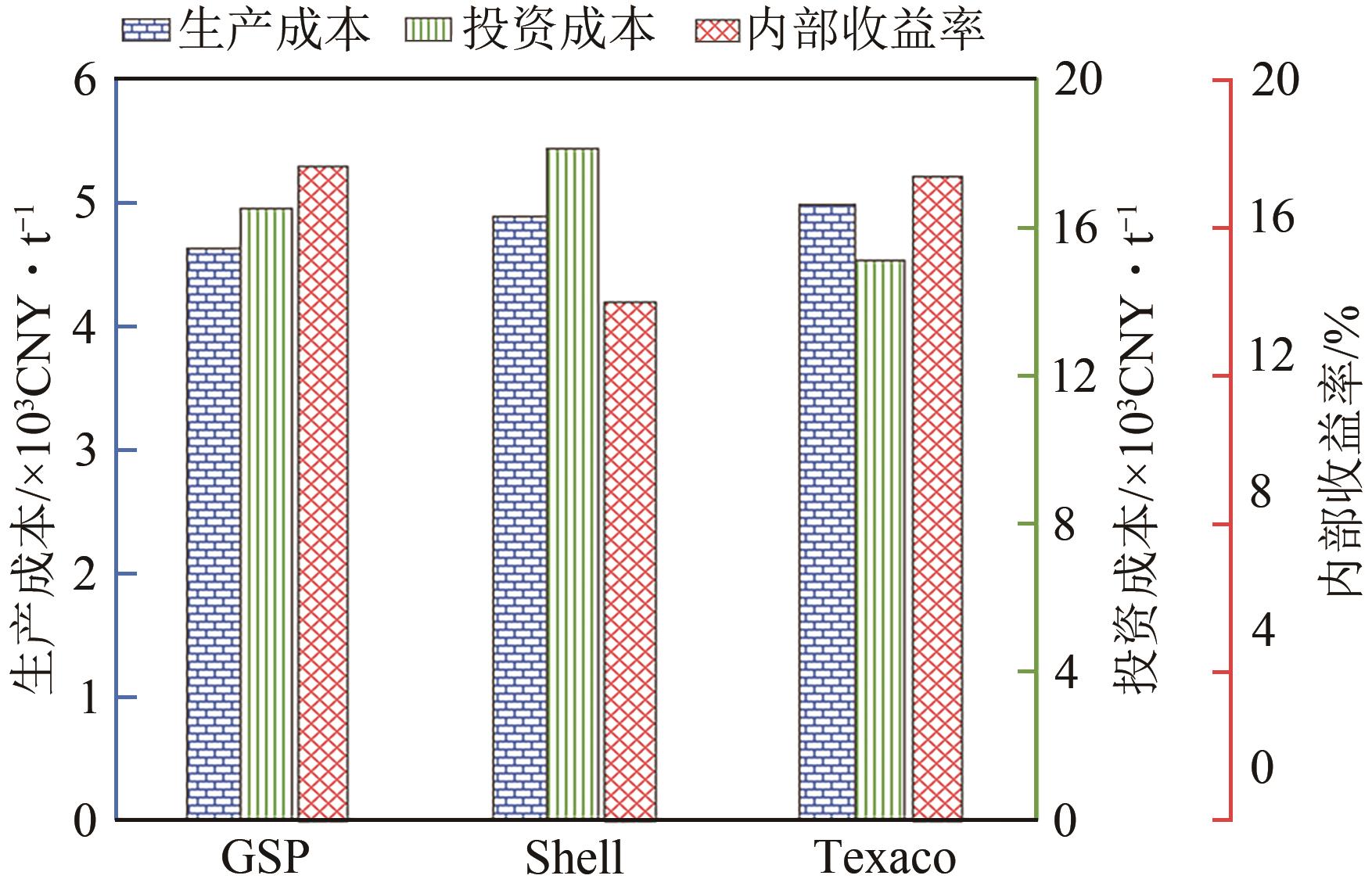
| 工艺 | 煤/t·t EG-1 | 焦炉煤气/kmol·t-1 | 电/kW·t-1 | 碳效率/% | ?效率/% | 总投资/CNY·t-1·a-1 | 总生产成本/CNY·t-1 | 内部收益率/% |
|---|---|---|---|---|---|---|---|---|
| CtEG | 3.17 | — | 999.07 | 21.23 | 30.68 | 16142 | 5142 | 9.25 |
| CtEG-SMR | 1.18 | 40.20 | 765.07 | 44.58 | 34.93 | 13591 | 4693 | 12.85 |
| CtEG-DMR | 0.90 | 41.51 | 736.40 | 53.56 | 38.75 | 14147 | 4600 | 13.01 |
| CtEG-S&DMR | 0.69 | 48.55 | 655.33 | 60.44 | 39.46 | 12604 | 4377 | 16.45 |
| CtEG-TR | 0.87 | 48.88 | 678.13 | 55.51 | 40.80 | 12114 | 4120 | 18.85 |
表2 典型的焦炉气辅助煤制乙二醇创新工艺的综合性能对比[45]
| 工艺 | 煤/t·t EG-1 | 焦炉煤气/kmol·t-1 | 电/kW·t-1 | 碳效率/% | ?效率/% | 总投资/CNY·t-1·a-1 | 总生产成本/CNY·t-1 | 内部收益率/% |
|---|---|---|---|---|---|---|---|---|
| CtEG | 3.17 | — | 999.07 | 21.23 | 30.68 | 16142 | 5142 | 9.25 |
| CtEG-SMR | 1.18 | 40.20 | 765.07 | 44.58 | 34.93 | 13591 | 4693 | 12.85 |
| CtEG-DMR | 0.90 | 41.51 | 736.40 | 53.56 | 38.75 | 14147 | 4600 | 13.01 |
| CtEG-S&DMR | 0.69 | 48.55 | 655.33 | 60.44 | 39.46 | 12604 | 4377 | 16.45 |
| CtEG-TR | 0.87 | 48.88 | 678.13 | 55.51 | 40.80 | 12114 | 4120 | 18.85 |
| 项目 | CtEG | D-SCtEG | S-SCtEG | D+S-SCtEG |
|---|---|---|---|---|
| 原煤/t·t EG-1 | 3.17 | 0.57 | 0.57 | 0.44 |
| 页岩气/kmol?h-1·t EG-1 | 0 | 1.58 | 1.64 | 2.14 |
| 总碳输入/kmol·h-1 | 5699.48 | 2034.64 | 2068.02 | 2336.36 |
| 总?输入/MW | 661.56 | 419.53 | 395.34 | 457.75 |
| 乙二醇/Mt·a-1 | 0.3 | 0.3 | 0.3 | 0.3 |
| 碳元素利用效率/% | 21.23 | 59.47 | 58.51 | 59.33 |
| ?效率/% | 30.68 | 48.31 | 55.98 | 50.94 |
| 总投资/CNY·t-1·a-1 | 16142 | 15360 | 13280 | 13530 |
| 总生产成本/CNY·t-1 | 5142 | 4565 | 4425 | 4636 |
| 内部收益率/% | 9.25 | 13.64 | 18.65 | 16.43 |
| 直接CO2排放/t·t EG-1 | 2.58 | 0 | 0.57 | 0.06 |
表3 典型页岩气辅助煤制乙二醇创新工艺性能对比结果[46]
| 项目 | CtEG | D-SCtEG | S-SCtEG | D+S-SCtEG |
|---|---|---|---|---|
| 原煤/t·t EG-1 | 3.17 | 0.57 | 0.57 | 0.44 |
| 页岩气/kmol?h-1·t EG-1 | 0 | 1.58 | 1.64 | 2.14 |
| 总碳输入/kmol·h-1 | 5699.48 | 2034.64 | 2068.02 | 2336.36 |
| 总?输入/MW | 661.56 | 419.53 | 395.34 | 457.75 |
| 乙二醇/Mt·a-1 | 0.3 | 0.3 | 0.3 | 0.3 |
| 碳元素利用效率/% | 21.23 | 59.47 | 58.51 | 59.33 |
| ?效率/% | 30.68 | 48.31 | 55.98 | 50.94 |
| 总投资/CNY·t-1·a-1 | 16142 | 15360 | 13280 | 13530 |
| 总生产成本/CNY·t-1 | 5142 | 4565 | 4425 | 4636 |
| 内部收益率/% | 9.25 | 13.64 | 18.65 | 16.43 |
| 直接CO2排放/t·t EG-1 | 2.58 | 0 | 0.57 | 0.06 |
| 1 | YUE H R, ZHAO Y J, MA X B, et al. Ethylene glycol: properties, synthesis, and applications[J]. Chemical Society Reviews, 2012, 41(11): 4218. |
| 2 | 陈冬燕. 中国煤制乙二醇行业发展现状及未来趋势[J]. 上海化工, 2018, 43(11): 43-45. |
| CHEN Dongyan. Development and trends of China’s coal-to-ethylene glycol industry[J]. Shanghai Chemical Industry, 2018, 43(11): 43-45. | |
| 3 | 安超. 全球乙二醇供需分析与预测[J]. 世界石油工业, 2021, 28(5): 38-46. |
| AN Chao. Analysis and forecast of global ethylene glycol supply and demand[J]. World Petroleum Industry, 2021, 28(5): 38-46. | |
| 4 | 黄格省, 李振宇, 王建明. 我国现代煤化工产业发展现状及对石油化工产业的影响[J]. 化工进展, 2015, 34(2): 295-302. |
| HUANG Gesheng, LI Zhenyu, WANG Jianming. Development status of coal chemical industry in China and its influence on petrochemical industry[J]. Chemical Industry and Engineering Progress, 2015, 34(2): 295-302. | |
| 5 | 陈嵩嵩, 张国帅, 霍锋, 等. 煤基大宗化学品市场及产业发展趋势[J]. 化工进展, 2020, 39(12): 5009-5020. |
| CHEN Songsong, ZHANG Guoshuai, HUO Feng, et al. Market and technology development trends of coal-based bulk chemicals[J]. Chemical Industry and Engineering Progress, 2020, 39(12): 5009-5020. | |
| 6 | YANG Q, YANG Q C, XU S M, et al. Technoeconomic and environmental analysis of ethylene glycol production from coal and natural gas compared with oil-based production[J]. Journal of Cleaner Production, 2020, 273: 123120. |
| 7 | 张丽君. 煤制乙二醇技术经济性分析研究[J]. 能源化工, 2016, 37(2): 1-6. |
| ZHANG Lijun. Techno-economic analysis and research for coal-based ethylene glycol route[J]. Energy Chemical Industry, 2016, 37(2): 1-6. | |
| 8 | GONG M H, YI Q, HUANG Y, et al. Coke oven gas to methanol process integrated with CO2 recycle for high energy efficiency, economic benefits and low emissions[J]. Energy Conversion and Management, 2017, 133: 318-331. |
| 9 | HAO Y H, HUANG Y, GONG M H, et al. A polygeneration from a dual-gas partial catalytic oxidation coupling with an oxygen-permeable membrane reactor[J]. Energy Conversion and Management, 2015, 106: 466-478. |
| 10 | YANG Q C, ZHANG C W, ZHANG D W, et al. Development of a coke oven gas assisted coal to ethylene glycol process for high techno-economic performance and low emission[J]. Industrial & Engineering Chemistry Research, 2018, 57(22): 7600-7612. |
| 11 | YANG Q C, LIU X, ZHU S, et al. Efficient utilization of CO2 in a coal to ethylene glycol process integrated with dry/steam-mixed reforming: conceptual design and technoeconomic analysis[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(3): 3496-3510. |
| 12 | 唐宏青. 现代煤化工新技术[M]. 2版. 北京: 化学工业出版社, 2016. |
| TANG Hongqing. Modern coal chemical industry new technology[M]. 2nd ed. Beijing: Chemical Industry Press, 2016. | |
| 13 | YANG Q C, ZHANG D W, ZHOU H R, et al. Process simulation, analysis and optimization of a coal to ethylene glycol process[J]. Energy, 2018, 155: 521-534. |
| 14 | 谢书胜, 邹佩良, 史瑾燕. 德士古水煤浆气化、Shell气化和GSP气化工艺对比[J]. 当代化工, 2008, 37(6): 666-668. |
| XIE Shusheng, ZOU Peiliang, SHI Jinyan. Comparison the coal gasification technologies—Texaco, Shell and GSP[J]. Contemporary Chemical Industry, 2008, 37(6): 666-668. | |
| 15 | 王辅臣, 于广锁, 龚欣, 等. 大型煤气化技术的研究与发展[J]. 化工进展, 2009, 28(2): 173-180. |
| WANG Fuchen, YU Guangsuo, GONG Xin, et al. Research and development of large-scale coal gasification technology[J]. Chemical Industry and Engineering Progress, 2009, 28(2): 173-180. | |
| 16 | 张海峰, 张敏. 不同煤气化技术合成气发酵法制乙醇的可行性探讨[J]. 现代化工, 2020, 40(S1): 279-283. |
| ZHANG Haifeng, ZHANG Min. Discussion on feasibility of fermentation to produce ethanol by syngas with different coal gasification technologies[J]. Modern Chemical Industry, 2020, 40(S1): 279-283. | |
| 17 | 许世森, 王保民. 两段式干煤粉加压气化技术及工程应用[J]. 化工进展, 2010, 29(S1): 290-294. |
| XU Shisen, WANG Baomin. Pressure gasification technology and engineering application of two-stage dry pulverized coal [J]. Chemical Industry and Engineering Progress, 2010, 29(S1): 290-294. | |
| 18 | 高丽. 德士古水煤浆加压气化技术的应用[J]. 煤炭技术, 2010, 29(7): 161-162. |
| GAO Li. Application of texaco coal-water slurry presurized gasification[J]. Coal Technology, 2010, 29(7): 161-162. | |
| 19 | WANG H P, CHEN Z C, ZHANG B, et al. Thermal-calculation method for entrained-flow coal gasifiers[J]. Energy, 2019, 166: 373-379. |
| 20 | 汪家铭. Shell煤气化技术及其在我国的应用[J]. 煤炭加工与综合利用, 2007(2):37-39. |
| WANG Jiaming. Shell coal gasification technology and its use in China[J]. Coal Processing and Comprehensive Utilization, 2007(2): 37-39. | |
| 21 | 范为鹏. GSP气化技术的发展与优化[J]. 石油化工应用, 2012, 31(7): 77-79, 98. |
| FAN Weipeng. Development and optimization of GSP gasification technology [J]. Petrochemical Industry Application, 2012, 31(7): 77-79, 98. | |
| 22 | YANG Q C, ZHU S, YU P J, et al. Thermodynamic and techno-economic analysis of coal to ethylene glycol process (CtEG) with different coal gasifiers[J]. Energy Conversion and Management, 2019, 191: 80-92. |
| 23 | JU Y, LEE C H. Evaluation of the energy efficiency of the shell coal gasification process by coal type[J]. Energy Conversion and Management, 2017, 143: 123-136. |
| 24 | KUNZE C, Modelling SPLIETHOFF H., comparison and operation experiences of entrained flow gasifier[J]. Energy Conversion and Management, 2011, 52(5): 2135-2141. |
| 25 | SARKIS R BET, ZARE V. Proposal and analysis of two novel integrated configurations for hybrid solar-biomass power generation systems: thermodynamic and economic evaluation[J]. Energy Conversion and Management, 2018, 160: 411-425. |
| 26 | QIN S Y, CHANG S Y, YAO Q. Modeling, thermodynamic and techno-economic analysis of coal-to-liquids process with different entrained flow coal gasifiers[J]. Applied Energy, 2018, 229: 413-432. |
| 27 | WANG Z Q, SUN J, XU Z N, et al. CO direct esterification to dimethyl oxalate and dimethyl carbonate: the key functional motifs for catalytic selectivity[J]. Nanoscale, 2020, 12(39): 20131-20140. |
| 28 | WANG C Z, XU W S, QIN Z X, et al. Low-temperature synthesis of α-alumina nanosheets on microfibrous-structured Al-fibers for Pd-catalyzed CO oxidative coupling to dimethyl oxalate[J]. Catalysis Today, 2020, 354: 158-166. |
| 29 | XING W C, AN J M, LYU J, et al. Highly active Pd-Fe/α-Al2O3 catalyst with the bayberry tannin as chelating promoter for CO oxidative coupling to diethyl oxalate[J]. Chinese Chemical Letters, 2021, 32(2): 796-800. |
| 30 | YANG L, PAN Z D, WANG D, et al. Highly effective Pd/MgO/γ-Al2O3 catalysts for CO oxidative coupling to dimethyl oxalate: the effect of MgO coating on γ-Al2O3 [J]. ACS Applied Materials & Interfaces, 2021, 13(24): 28064-28071. |
| 31 | WANG Q, QIU L, DING D, et al. Performance enhancement of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol through zinc incorporation[J]. Catalysis Communications, 2018, 108: 68-72. |
| 32 | CUI G Q, MENG X Y, ZHANG X, et al. Low-temperature hydrogenation of dimethyl oxalate to ethylene glycol via ternary synergistic catalysis of Cu and acid-base sites[J]. Applied Catalysis B: Environmental, 2019, 248: 394-404. |
| 33 | WANG M L, YAO D W, LI A T, et al. Enhanced selectivity and stability of Cu/SiO2 catalysts for dimethyl oxalate hydrogenation to ethylene glycol by using silane coupling agents for surface modification[J]. Industrial & Engineering Chemistry Research, 2020, 59(20): 9414-9422. |
| 34 | YE R P, LIN L, WANG L C, et al. Perspectives on the active sites and catalyst design for the hydrogenation of dimethyl oxalate[J]. ACS Catalysis, 2020, 10(8): 4465-4490. |
| 35 | WEI R X, YAN C L, YANG A, et al. Improved process design and optimization of 200kt/a ethylene glycol production using coal-based syngas[J]. Chemical Engineering Research and Design, 2018, 132: 551-563. |
| 36 | YI Q, LI W, FENG J, et al. Carbon cycle in advanced coal chemical engineering[J]. Chemical Society Reviews, 2015, 44(15): 5409-5445. |
| 37 | YI Q, GONG M H, HUANG Y, et al. Process development of coke oven gas to methanol integrated with CO2 recycle for satisfactory techno-economic performance[J]. Energy, 2016, 112: 618-628. |
| 38 | YANG S Y, YANG Q C, MAN Y, et al. Conceptual design and analysis of a natural gas assisted coal-to-olefins process for CO2 reuse[J]. Industrial & Engineering Chemistry Research, 2013, 52(40): 14406-14414. |
| 39 | YU B Y, CHIEN I L. Design and optimization of dimethyl oxalate (DMO) hydrogenation process to produce ethylene glycol (EG)[J]. Chemical Engineering Research and Design, 2017, 121: 173-190. |
| 40 | MAN Y, YANG S Y, QIAN Y. Integrated process for synthetic natural gas production from coal and coke-oven gas with high energy efficiency and low emission[J]. Energy Conversion and Management, 2016, 117: 162-170. |
| 41 | XIANG D, JIN T, LEI X R, et al. The high efficient synthesis of natural gas from a joint-feedstock of coke-oven gas and pulverized coke via a chemical looping combustion scheme[J]. Applied Energy, 2018, 212: 944-954. |
| 42 | RAZZAQ R, LI C S, ZHANG S J. Coke oven gas: availability, properties, purification, and utilization in China[J]. Fuel, 2013, 113: 287-299. |
| 43 | MAN Y, YANG S Y, XIANG D, et al. Environmental impact and techno-economic analysis of the coal gasification process with/without CO2 capture[J]. Journal of Cleaner Production, 2014, 71: 59-66. |
| 44 | YANG Q C, XU S M, ZHANG J L, et al. Thermodynamic and techno-economic analyses of a novel integrated process of coal gasification and methane tri-reforming to ethylene glycol with low carbon emission and high efficiency[J]. Energy, 2021, 229: 120713. |
| 45 | YANG Q C, ZHANG J L, CHU G Y, et al. Optimal design, thermodynamic and economic analysis of coal to ethylene glycol processes integrated with various methane reforming technologies for CO2 reduction[J]. Energy Conversion and Management, 2021, 244: 114538. |
| 46 | YANG Q C, YANG Q, XU S M, et al. Optimal design, exergy and economic analyses of coal-to-ethylene glycol process coupling different shale gas reforming technologies[J]. Energy, 2021, 228: 120535. |
| 47 | YANG Q, CHU G Y, YANG Q C, et al. Process development and technoeconomic analysis of different integration methods of coal-to-ethylene glycol process and solid oxide electrolysis cells[J]. Industrial & Engineering Chemistry Research, 2021, 60(40): 14519-14533. |
| [1] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [2] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [3] | 赵晨, 苗天泽, 张朝阳, 洪芳军, 汪大海. 负压状态窄缝通道乙二醇水溶液传热特性[J]. 化工进展, 2023, 42(S1): 148-157. |
| [4] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [5] | 刘炫麟, 王驿凯, 戴苏洲, 殷勇高. 热泵中氨基甲酸铵分解反应特性及反应器结构优化[J]. 化工进展, 2023, 42(9): 4522-4530. |
| [6] | 朱杰, 金晶, 丁正浩, 杨会盼, 侯封校. 化学链气化中准东煤灰对CaSO4载氧体改性及其作用机理[J]. 化工进展, 2023, 42(9): 4628-4635. |
| [7] | 王晨, 白浩良, 康雪. 大功率UV-LED散热与纳米TiO2光催化酸性红26耦合系统性能[J]. 化工进展, 2023, 42(9): 4905-4916. |
| [8] | 张丽宏, 金要茹, 程芳琴. 煤气化渣资源化利用[J]. 化工进展, 2023, 42(8): 4447-4457. |
| [9] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [10] | 李蓝宇, 黄新烨, 王笑楠, 邱彤. 化工科研范式智能化转型的思考与展望[J]. 化工进展, 2023, 42(7): 3325-3330. |
| [11] | 周龙大, 赵立新, 徐保蕊, 张爽, 刘琳. 电场-旋流耦合强化多相介质分离研究进展[J]. 化工进展, 2023, 42(7): 3443-3456. |
| [12] | 薛凯, 王帅, 马金鹏, 胡晓阳, 种道彤, 王进仕, 严俊杰. 工业园区分布式综合能源系统的规划与调度[J]. 化工进展, 2023, 42(7): 3510-3519. |
| [13] | 顾诗亚, 董亚超, 刘琳琳, 张磊, 庄钰, 都健. 考虑中间节点的碳捕集管路系统设计与优化[J]. 化工进展, 2023, 42(6): 2799-2808. |
| [14] | 李雪, 王艳君, 王玉超, 陶胜洋. 仿生表面用于雾水收集的最新研究进展[J]. 化工进展, 2023, 42(5): 2486-2503. |
| [15] | 黄能坤, 王梓雯, 王文耕, 王学峰, 谈继淮, 朱新宝. 新型氢化二聚酸乙二醇醚酯的合成及其增塑PVC性能[J]. 化工进展, 2023, 42(5): 2638-2646. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||