化工进展 ›› 2023, Vol. 42 ›› Issue (1): 526-537.DOI: 10.16085/j.issn.1000-6613.2022-0596
模拟煤炭气化废水中苯酚的微生物降解
付佳1( ), 谌伦建1, 徐冰1,2(
), 谌伦建1, 徐冰1,2( ), 华绍烽1, 李从强1, 杨明坤1, 邢宝林1,2, 仪桂云1,2
), 华绍烽1, 李从强1, 杨明坤1, 邢宝林1,2, 仪桂云1,2
- 1.河南理工大学化学化工学院,河南省煤炭绿色转化重点实验室,河南 焦作 454003
2.煤炭安全生产与清洁高效利用省部共建协同创新中心,河南 焦作 454003
-
收稿日期:2022-04-08修回日期:2022-05-24出版日期:2023-01-25发布日期:2023-02-20 -
通讯作者:徐冰 -
作者简介:付佳(1997—),女,硕士研究生,研究方向为生物化工。E-mail:fujia3700@163.com。 -
基金资助:国家自然科学基金(U1803114);河南省科技攻关项目(212102311069);河南省高校重点科研计划(20A530003)
Microbial degradation of phenol in simulated coal gasification wastewater
FU Jia1( ), CHEN Lunjian1, XU Bing1,2(
), CHEN Lunjian1, XU Bing1,2( ), HUA Shaofeng1, LI Congqiang1, YANG Mingkun1, XING Baolin1,2, YI Guiyun1,2
), HUA Shaofeng1, LI Congqiang1, YANG Mingkun1, XING Baolin1,2, YI Guiyun1,2
- 1.Henan Key Laboratory of Coal Green Conversion, College of Chemistry and Chemical Engineering, Henan Polytechnic University, Jiaozuo 454003, Henan, China
2.Collaborative Innovation Center of Coal Work Safety and Clean High Efficiency Utilization, Jiaozuo 454003, Henan, China
-
Received:2022-04-08Revised:2022-05-24Online:2023-01-25Published:2023-02-20 -
Contact:XU Bing
摘要:
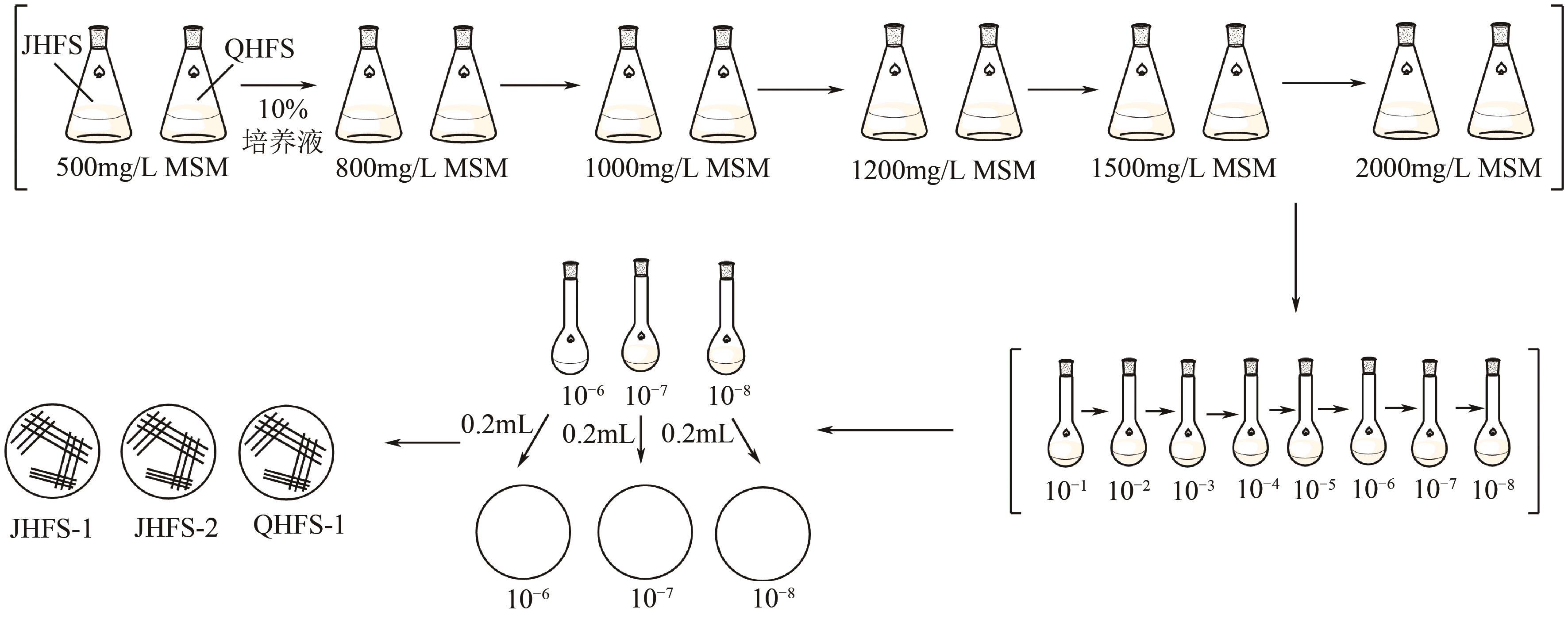
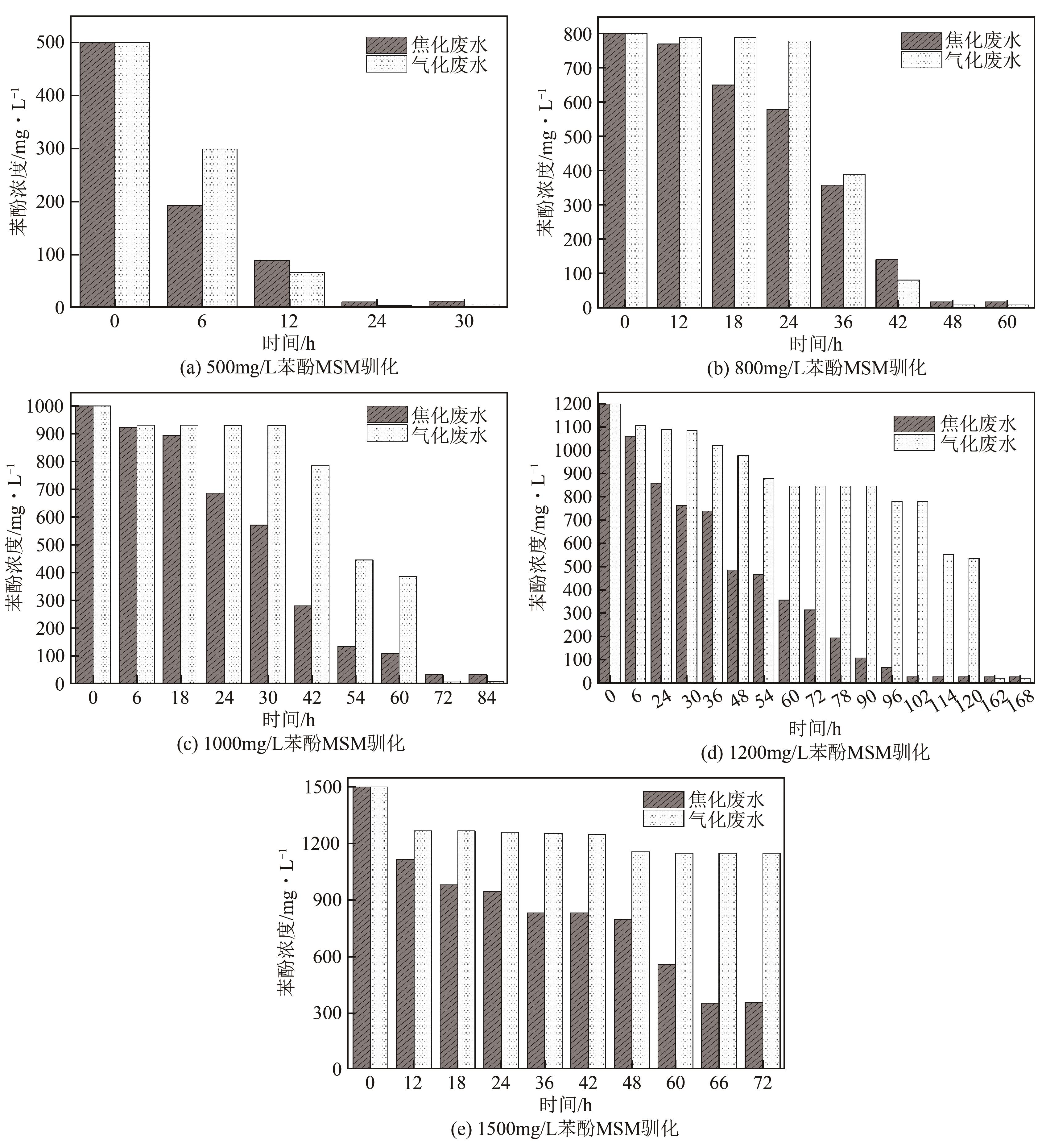
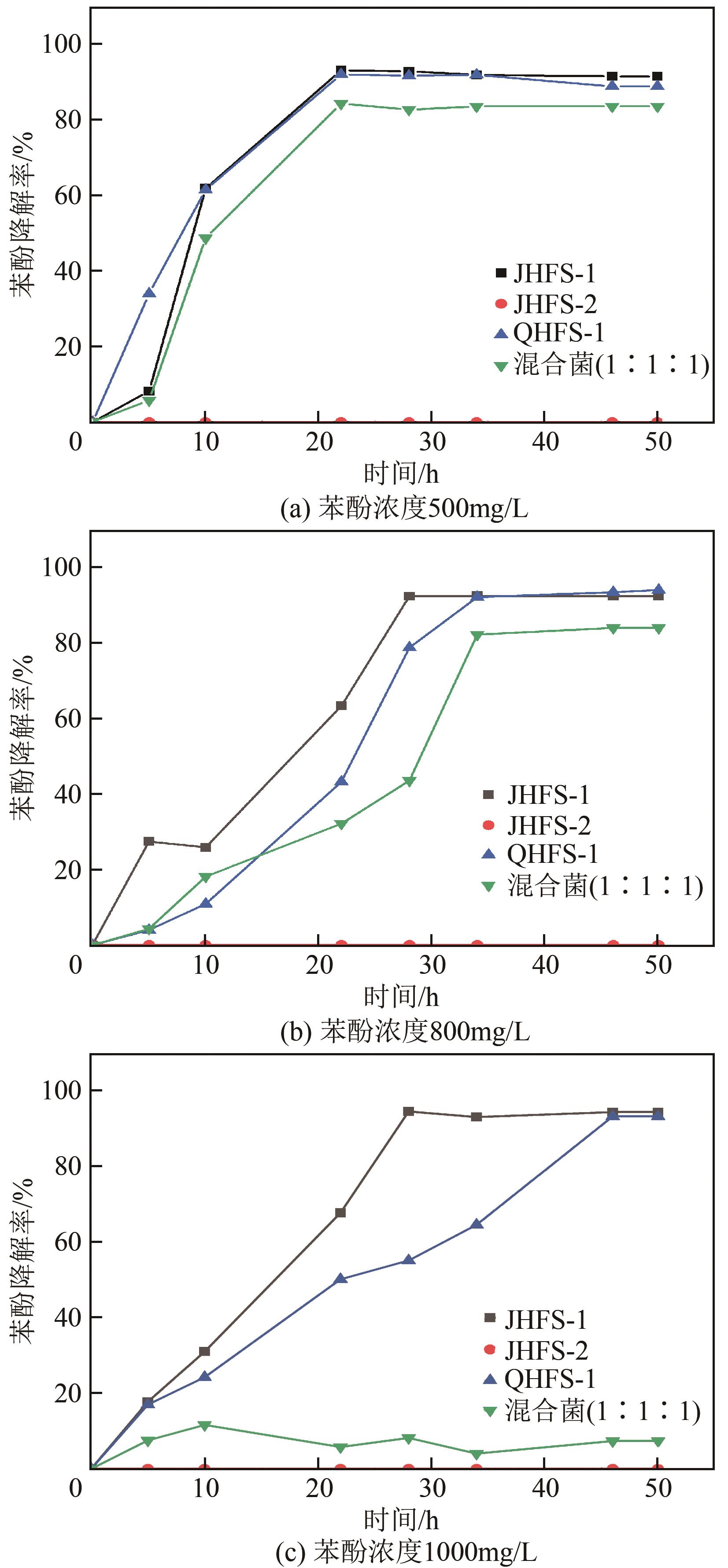
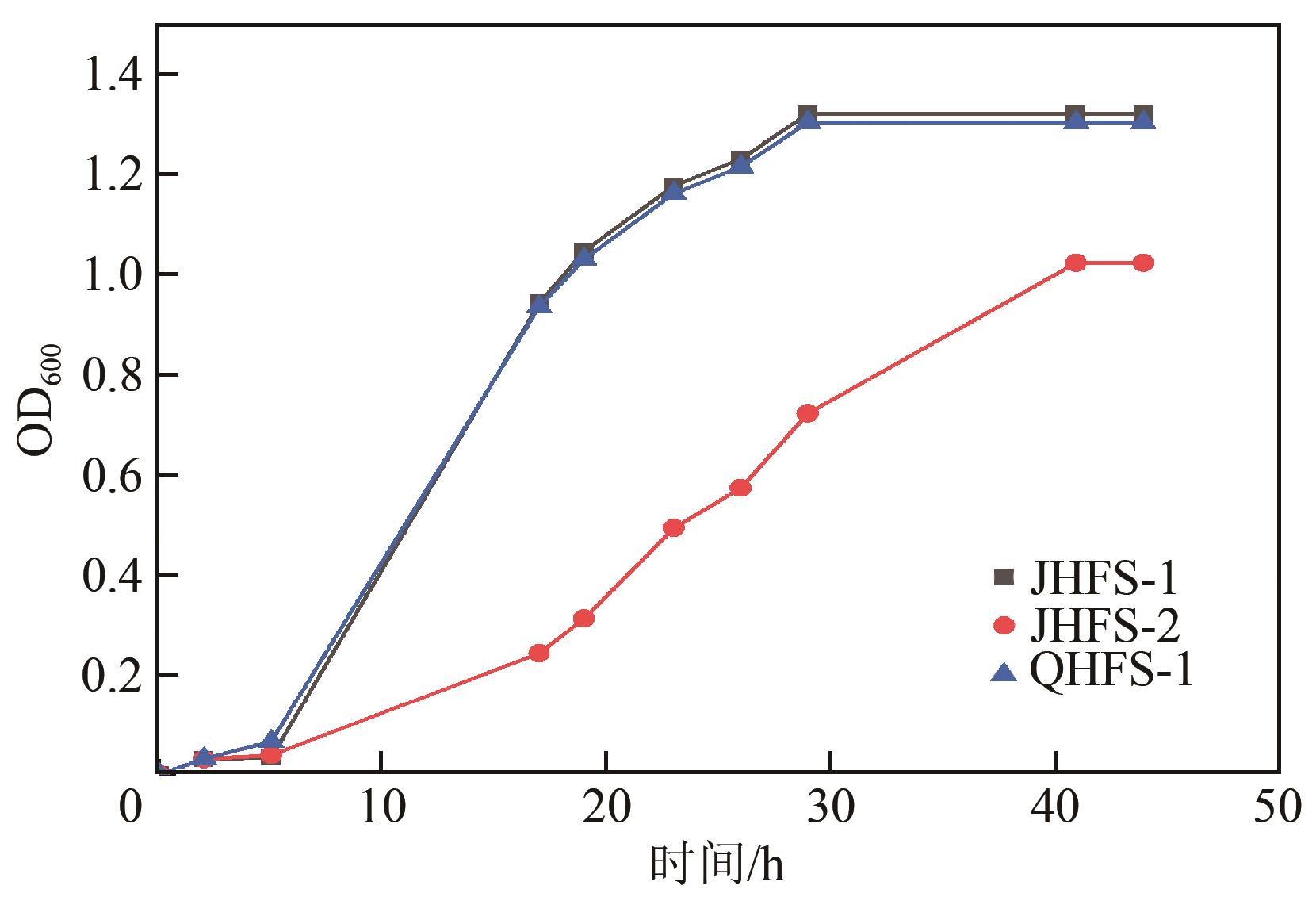
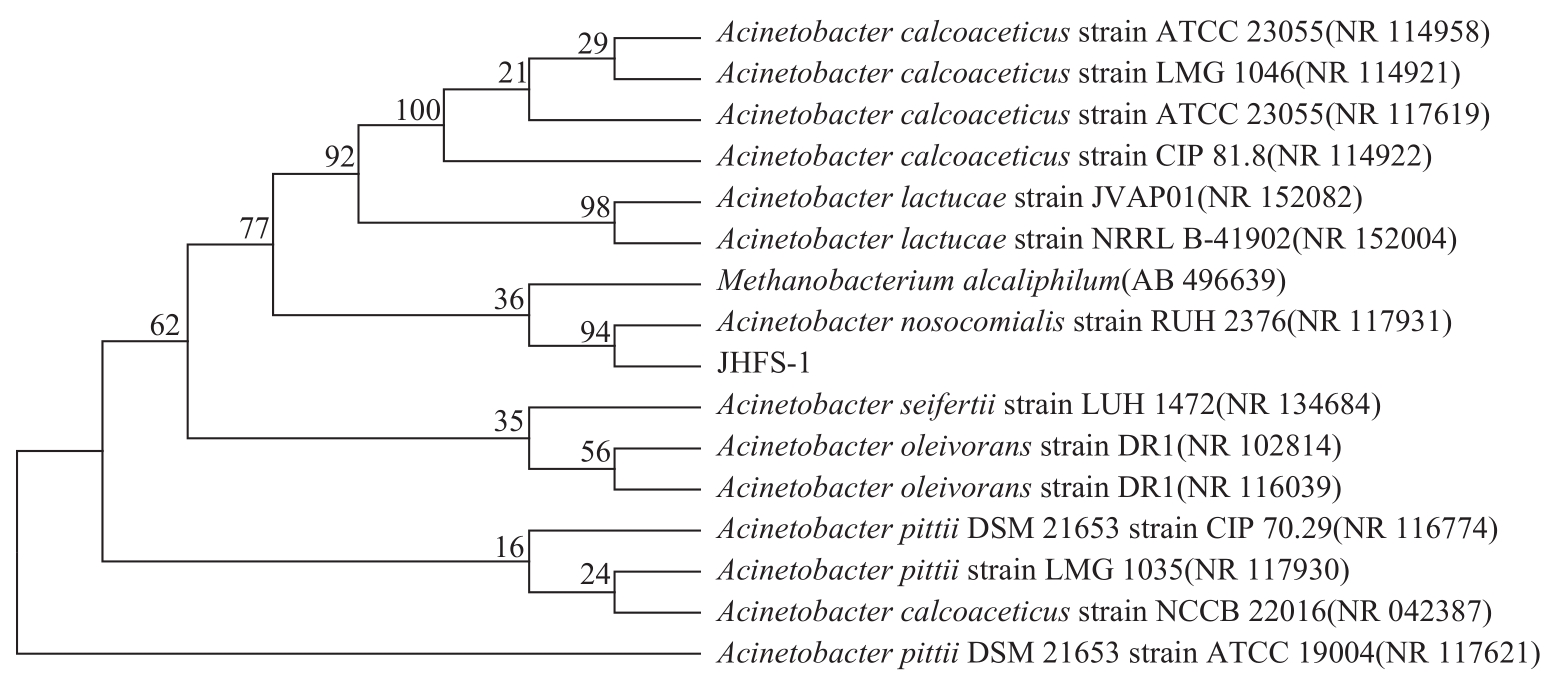
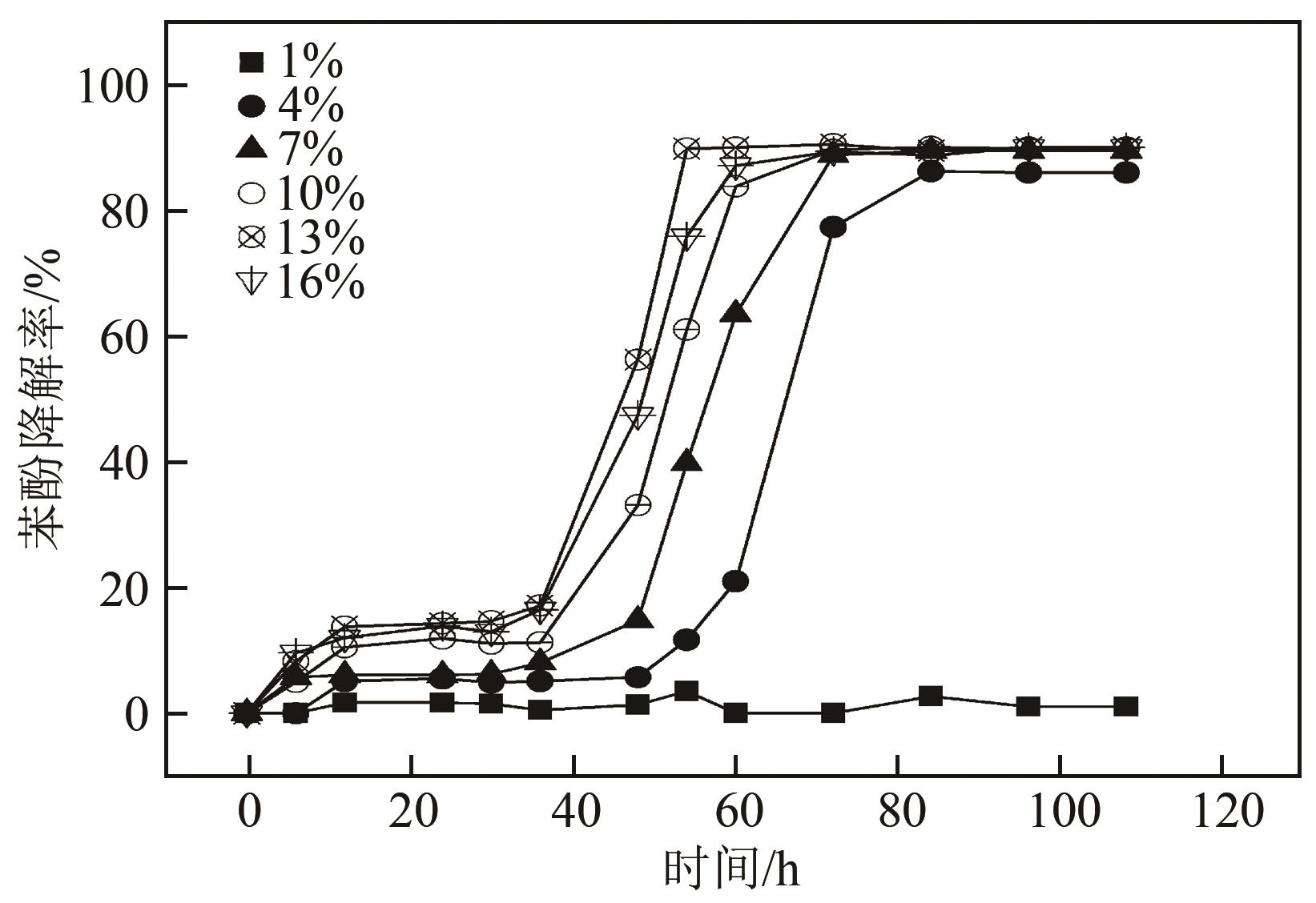
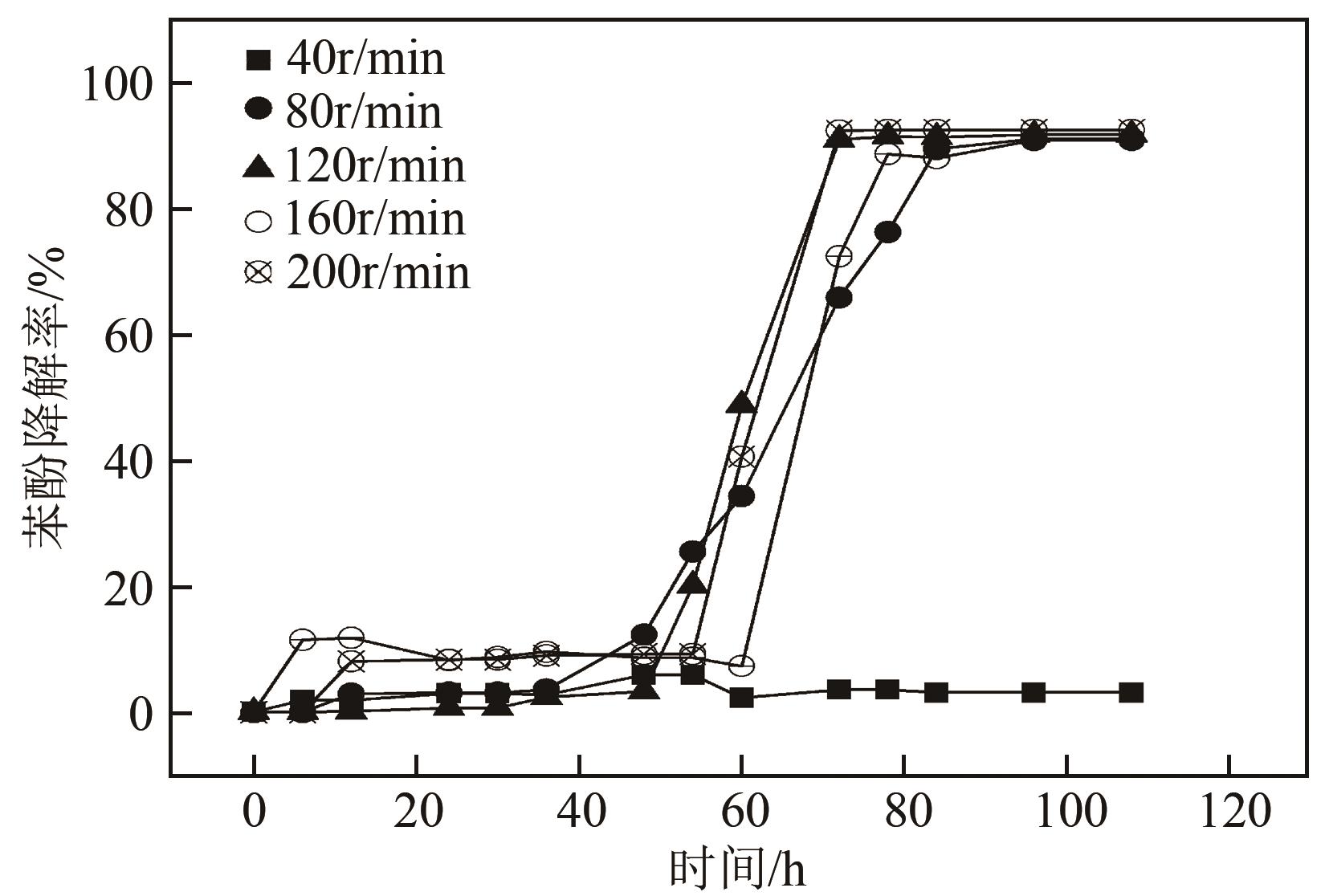
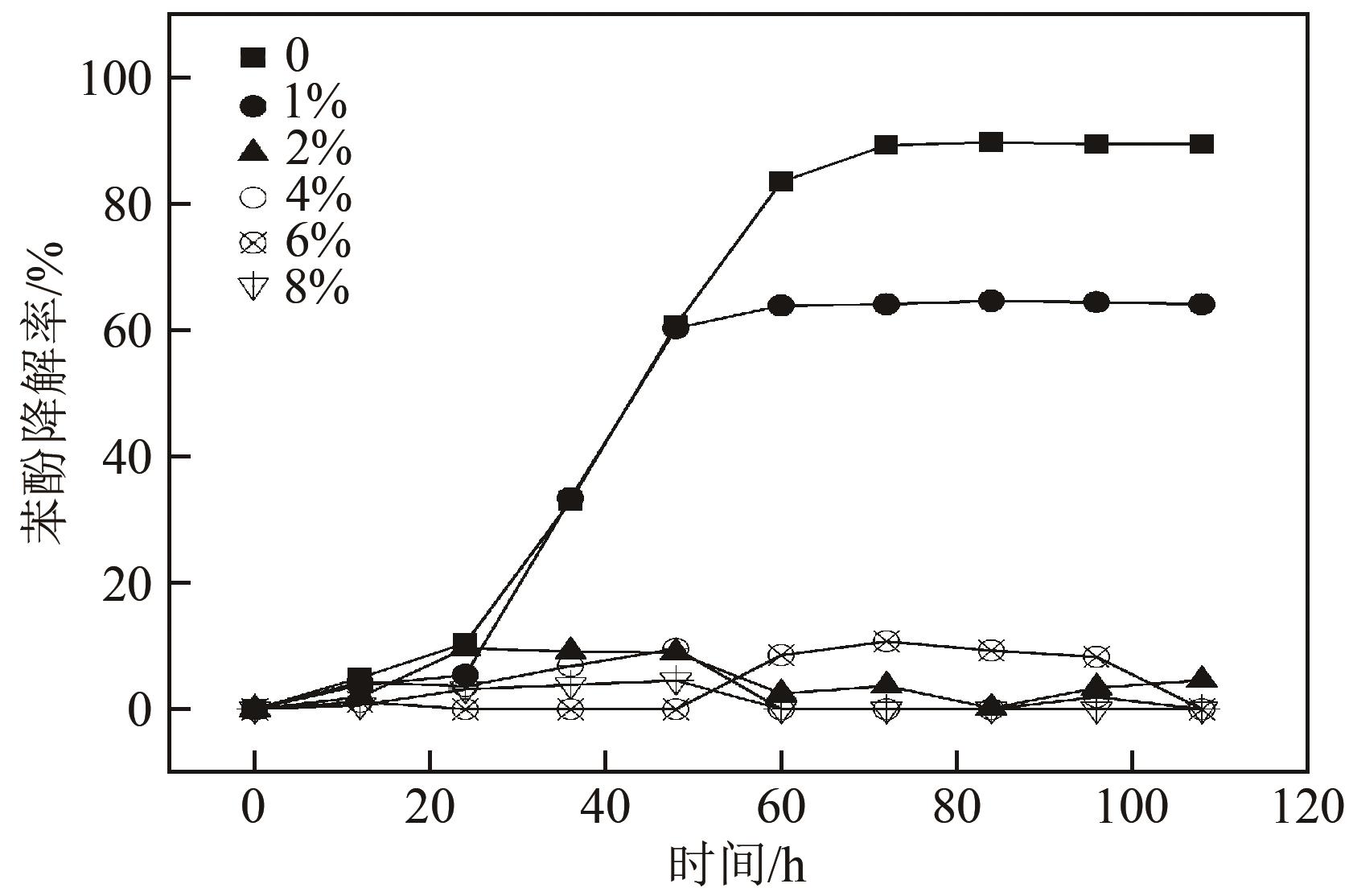
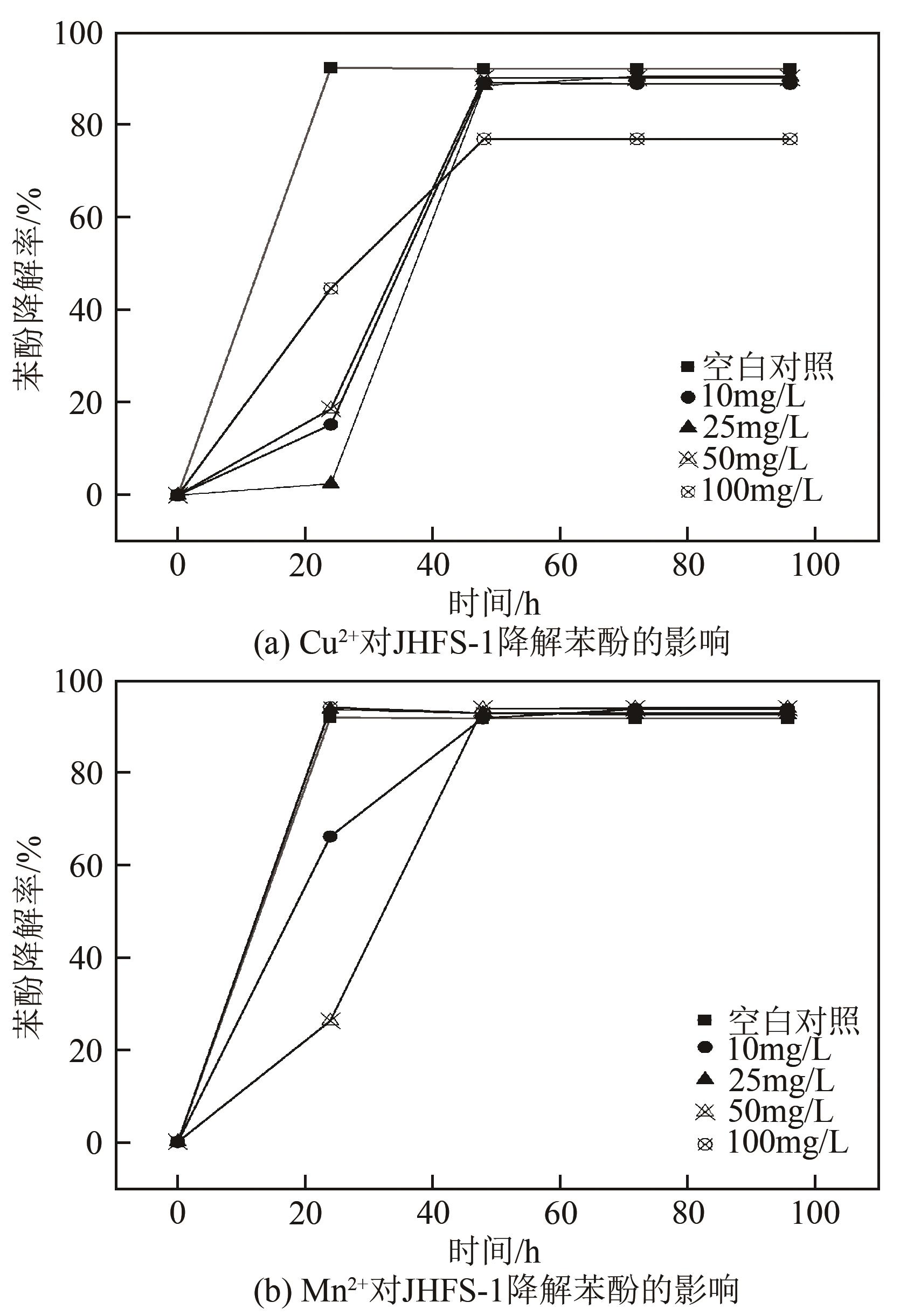
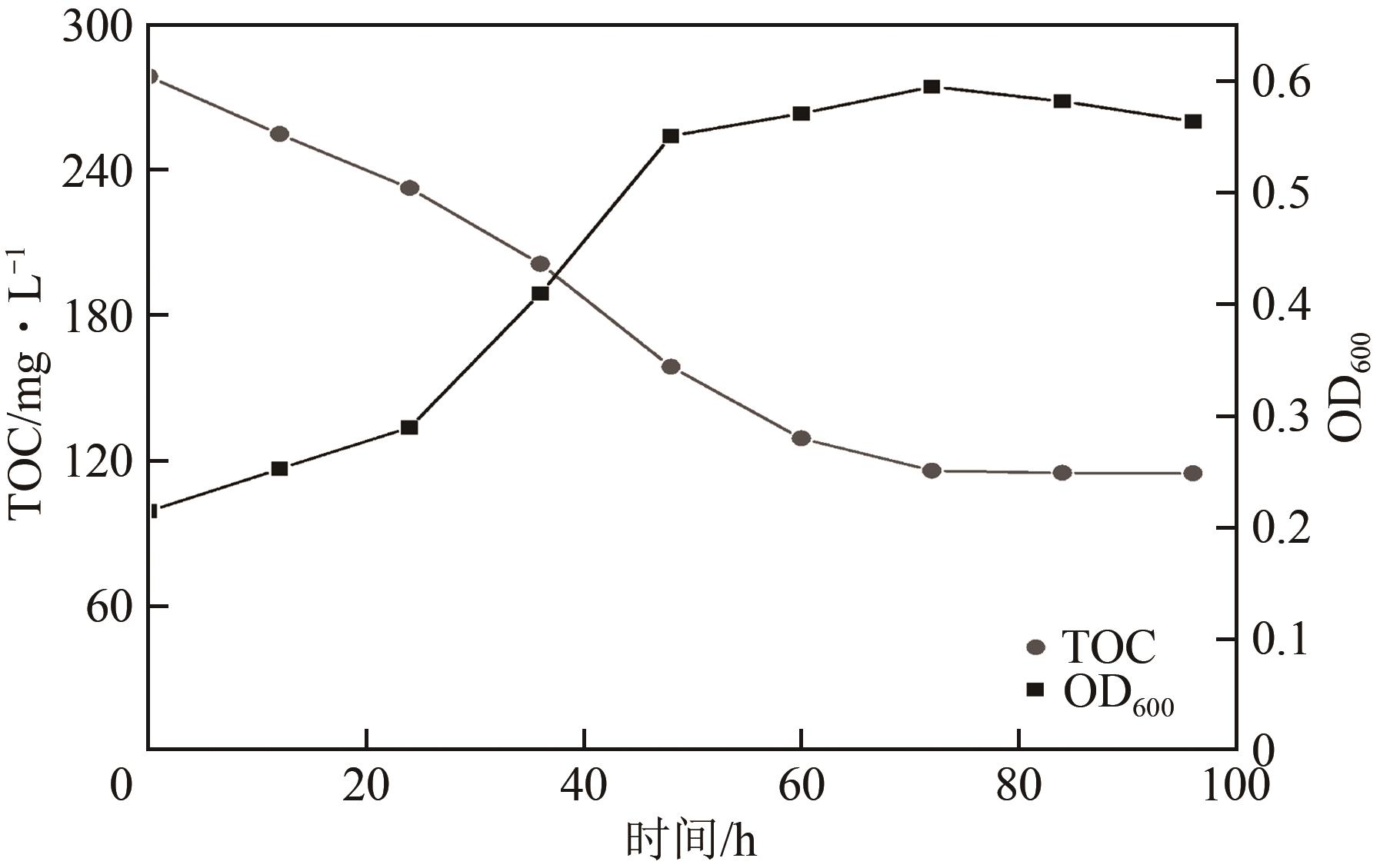
苯酚是煤炭气化废水中一种典型的有机污染物,其处理受到广泛关注和研究。本文采用连续驯化和平板划线法从焦化废水和气化废水中筛选出两种苯酚高效降解菌株,分别命名为JHFS-1和QHFS-1;通过苯酚溶液的微生物降解实验研究了温度、pH、摇床转速、细菌接种量、Cu2+和Mn2+等对苯酚降解效果的影响,还考察了模拟煤炭气化产生的煤气洗涤水的微生物净化修复效果。结果发现:经16S rDNA基因测序和微生物学鉴定,两种菌株均为醋酸钙不动杆菌(Acinetobacter calcoaceticus);30℃、pH=6.0、摇床转速120r/min、接种量13%是苯酚的最优降解条件,经24h处理,苯酚降解率可达94.31%;Cu2+对JHFS-1降解苯酚有一定的抑制作用,Mn2+一定程度上促进JHFS-1对苯酚的降解;微生物对苯酚的降解遵从羟基化途径和羧基化途径;JHFS-1菌可有效降解煤气洗涤水中的有机污染物,其总有机碳(TOC)降解率达58.43%。
中图分类号:
引用本文
付佳, 谌伦建, 徐冰, 华绍烽, 李从强, 杨明坤, 邢宝林, 仪桂云. 模拟煤炭气化废水中苯酚的微生物降解[J]. 化工进展, 2023, 42(1): 526-537.
FU Jia, CHEN Lunjian, XU Bing, HUA Shaofeng, LI Congqiang, YANG Mingkun, XING Baolin, YI Guiyun. Microbial degradation of phenol in simulated coal gasification wastewater[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 526-537.
| 试验项目 | 试验结果 | 试验项目 | 试验结果 |
|---|---|---|---|
| 革兰氏染色 | G- | 淀粉水解试验 | + |
| 明胶液化试验 | - | 氧化酶试验 | + |
| 甲基红试验 | - | 过氧化氢酶试验 | + |
| 吲哚试验 | - | V-P试验 | + |
| 硝酸盐还原试验 | - |
表1 菌株JHFS-1生理生化试验结果
| 试验项目 | 试验结果 | 试验项目 | 试验结果 |
|---|---|---|---|
| 革兰氏染色 | G- | 淀粉水解试验 | + |
| 明胶液化试验 | - | 氧化酶试验 | + |
| 甲基红试验 | - | 过氧化氢酶试验 | + |
| 吲哚试验 | - | V-P试验 | + |
| 硝酸盐还原试验 | - |
| 保留时间/min | 匹配项名称 | 质量分数/% | 相对浓度/mg·L-1 |
|---|---|---|---|
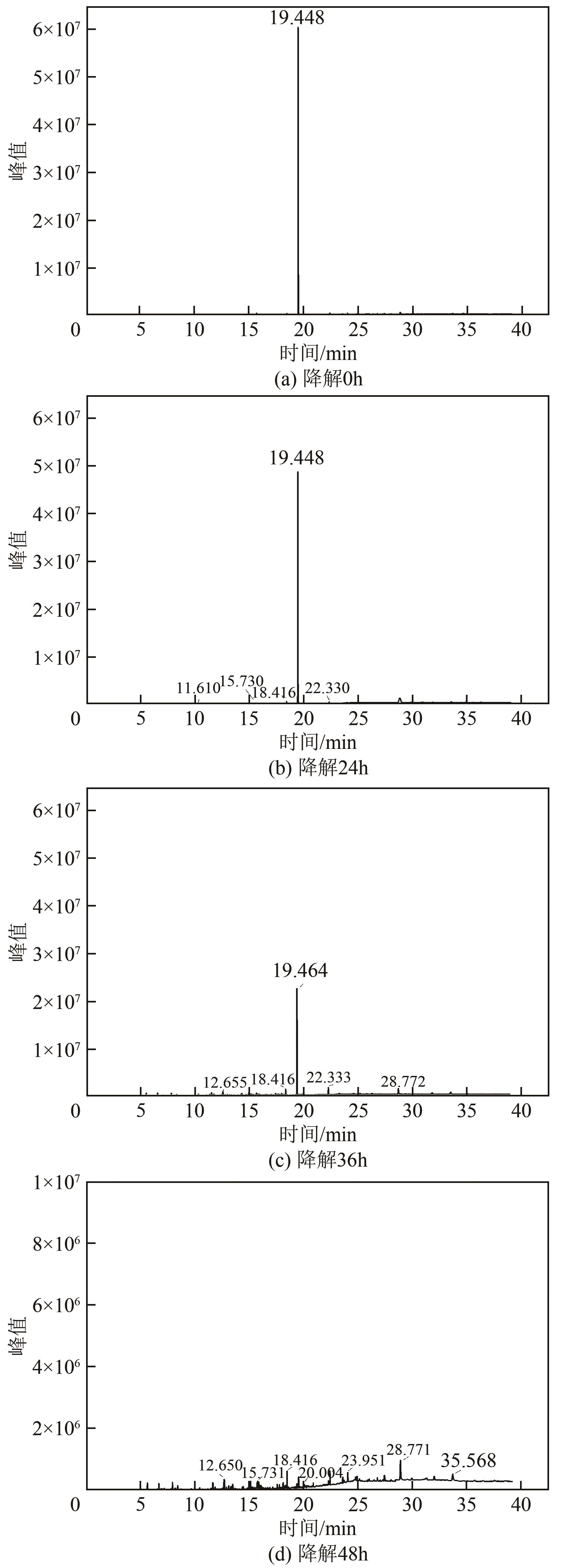
| 19.448 | 苯酚 | 95.82 | 437.13 |
表2 苯酚降解0h过程产物
| 保留时间/min | 匹配项名称 | 质量分数/% | 相对浓度/mg·L-1 |
|---|---|---|---|
| 19.448 | 苯酚 | 95.82 | 437.13 |
| 保留时间/min | 匹配项名称 | 质量分数/% | 相对浓度/mg·L-1 |
|---|---|---|---|
| 11.532 | 乙二酸酯 | 0.17 | 0.77 |
| 15.730 | 邻苯二酚 | 0.14 | 0.63 |
| 18.416 | 琥珀酸盐 | 0.52 | 2.32 |
| 19.448 | 苯酚 | 89.36 | 397.81 |
| 22.330 | 5-羟基戊酸盐 | 0.33 | 1.47 |
表3 苯酚降解24h过程产物
| 保留时间/min | 匹配项名称 | 质量分数/% | 相对浓度/mg·L-1 |
|---|---|---|---|
| 11.532 | 乙二酸酯 | 0.17 | 0.77 |
| 15.730 | 邻苯二酚 | 0.14 | 0.63 |
| 18.416 | 琥珀酸盐 | 0.52 | 2.32 |
| 19.448 | 苯酚 | 89.36 | 397.81 |
| 22.330 | 5-羟基戊酸盐 | 0.33 | 1.47 |
| 保留时间/min | 匹配项名称 | 质量分数/% | 相对浓度/mg·L-1 |
|---|---|---|---|
| 11.532 | 乙二酸酯 | 0.39 | 0.93 |
| 18.416 | 琥珀酸盐 | 2.83 | 6.80 |
| 19.448 | 苯酚 | 54.03 | 129.77 |
| 22.330 | 5-羟基戊酸盐 | 3.44 | 8.27 |
表4 苯酚降解36h过程产物
| 保留时间/min | 匹配项名称 | 质量分数/% | 相对浓度/mg·L-1 |
|---|---|---|---|
| 11.532 | 乙二酸酯 | 0.39 | 0.93 |
| 18.416 | 琥珀酸盐 | 2.83 | 6.80 |
| 19.448 | 苯酚 | 54.03 | 129.77 |
| 22.330 | 5-羟基戊酸盐 | 3.44 | 8.27 |
| 保留时间/min | 匹配项名称 | 质量分数/% | 相对浓度/mg·L-1 |
|---|---|---|---|
| 7.780 | 2-氧代戊酸 | 0.91 | 0.28 |
| 11.532 | 乙二酸酯 | 2.69 | 0.82 |
| 15.330 | 丁酸 | 0.39 | 0.12 |
| 15.840 | 苯甲酸 | 3.30 | 1.01 |
| 18.416 | 琥珀酸盐 | 4.76 | 1.45 |
| 19.448 | 苯酚 | 3.60 | 1.10 |
| 20.230 | 富马酸 | 0.38 | 0.13 |
| 20.800 | 龙胆酸 | 1.35 | 0.41 |
| 22.330 | 5-羟基戊酸盐 | 3.78 | 1.16 |
| 27.390 | 甲酸 | 0.17 | 0.05 |
| 28.771 | 乙二酸 | 15.20 | 4.64 |
表5 苯酚降解48h过程产物
| 保留时间/min | 匹配项名称 | 质量分数/% | 相对浓度/mg·L-1 |
|---|---|---|---|
| 7.780 | 2-氧代戊酸 | 0.91 | 0.28 |
| 11.532 | 乙二酸酯 | 2.69 | 0.82 |
| 15.330 | 丁酸 | 0.39 | 0.12 |
| 15.840 | 苯甲酸 | 3.30 | 1.01 |
| 18.416 | 琥珀酸盐 | 4.76 | 1.45 |
| 19.448 | 苯酚 | 3.60 | 1.10 |
| 20.230 | 富马酸 | 0.38 | 0.13 |
| 20.800 | 龙胆酸 | 1.35 | 0.41 |
| 22.330 | 5-羟基戊酸盐 | 3.78 | 1.16 |
| 27.390 | 甲酸 | 0.17 | 0.05 |
| 28.771 | 乙二酸 | 15.20 | 4.64 |
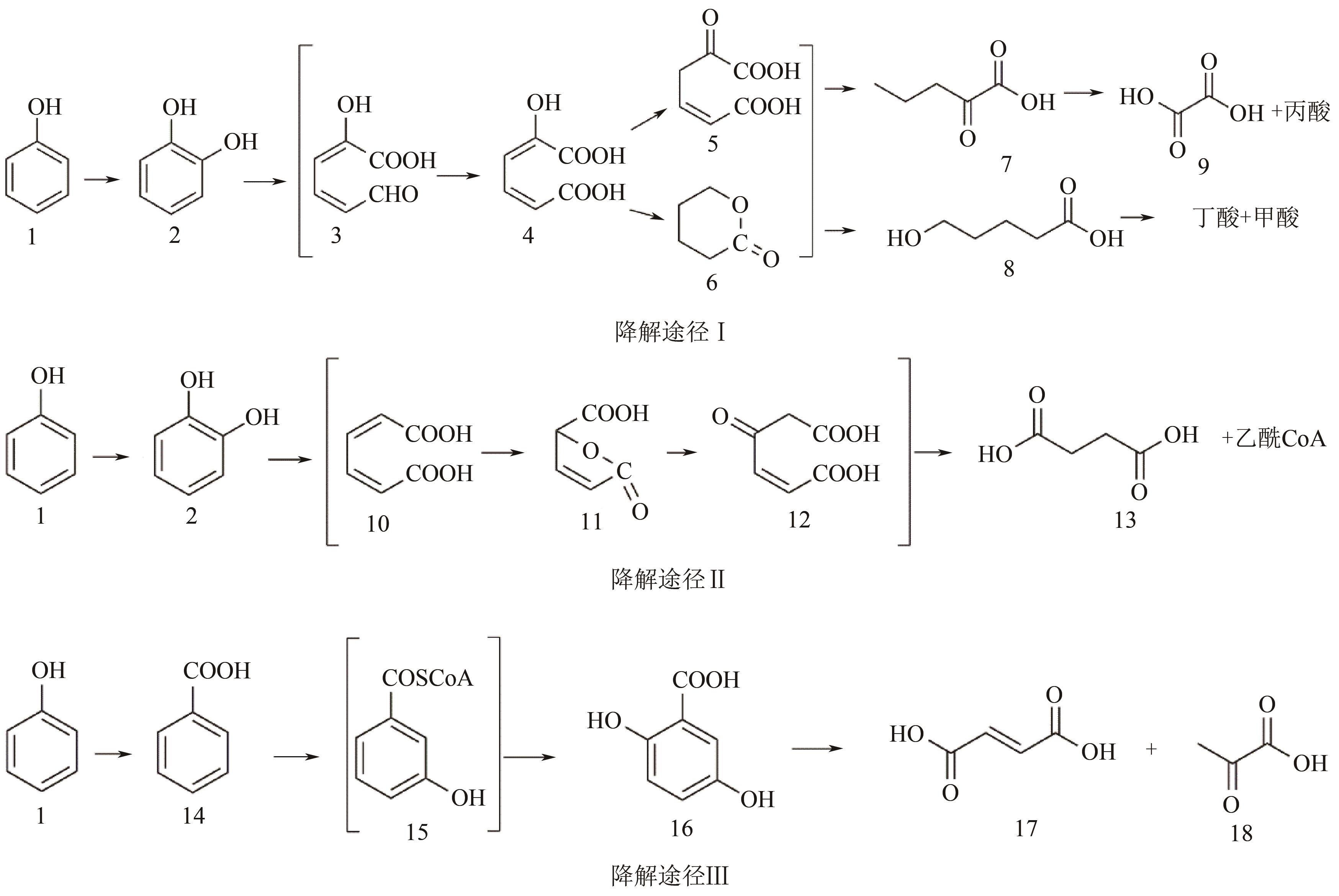
图18 JHFS-1降解苯酚的途径1—苯酚;2—邻苯二酚;3—2-羟基黏糠酸半醛;4—2-羟基黏酸;5—4-氧代己二酸;6—内酯;7—2-氧代戊酸;8—5-羟基戊酸;9—乙二酸;10—黏酸;11—黏酸内酯;12—β-酮基己二酸;13—琥珀酸;14—苯甲酸;15—3-羟基苯甲酰辅酶A;16—龙胆酸;17—富马酸;18—丙酮酸
| 1 | 杨国辉, 褚夫奎, 李磊. 煤炭气化技术的比较与分析[J]. 山东化工, 2021, 50(23): 61-64. |
| YANG Guohui, CHU Fukui, LI Lei. Comparison and analysis of coal gasification technology[J]. Shandong Chemical Industry, 2021, 50(23): 61-64. | |
| 2 | 李玉龙, 梁栋宇, 盛训超, 等. 煤炭地下气化残留物中微量元素分布及富集特性[J]. 化工进展, 2018, 37(4): 1590-1598. |
| LI Yulong, LIANG Dongyu, SHENG Xunchao, et al. Distribution and enrichment characteristics of trace elements during underground coal gasification[J]. Chemical Industry and Engineering Progress, 2018, 37(4): 1590-1598. | |
| 3 | 张乐, 谌伦建, 苏毓, 等. 褐煤气化半焦对地下水有机污染的模拟脱除[J]. 化工进展, 2016, 35(10): 3337-3343. |
| ZHANG Le, CHEN Lunjian, SU Yu, et al. Experimental studies on removal of organic contaminants in groundwater by UCG using semi-coke[J]. Chemical Industry and Engineering Progress, 2016, 35(10): 3337-3343. | |
| 4 | HEMIDOUCHE S, FAVIER L, AMRANE A, et al. Successful biodegradation of a refractory pharmaceutical compound by an indigenous phenol-tolerant pseudomonas aeruginosa strain[J]. Water, Air, & Soil Pollution, 2018, 229(3): 1-16. |
| 5 | ZHAO Tiantao, GAO Yanhui, YU Tiantian, et al. Biodegradation of phenol by a highly tolerant strain Rhodococcus ruber C1: biochemical characterization and comparative genome analysis[J]. Ecotoxicology and Environmental Safety, 2021, 208: 111709. |
| 6 | DAYANA PRIYADHARSHINI S, BAKTHAVATSALAM A K. A comparative study on growth and degradation behavior of C. pyrenoidosa on synthetic phenol and phenolic wastewater of a coal gasification plant[J]. Journal of Environmental Chemical Engineering, 2019, 7(3): 103079. |
| 7 | BASHA K M, RAJENDRAN A, THANGAVELU V. Recent advances in the biodegradation of phenol: a review[J]. Asian Journal of Experimental Biological Science, 2010, 1(2): 219-234. |
| 8 | HE Qiangli, LIU Wenbin, YANG Haijun, et al. Isolation, identification of a phenol-degradaing strain and optimization for phenol degradation using response surface methodology[J]. Acta Scientiae Circumstantiae, 2016, 36(1): 112-123. |
| 9 | ZHOU Le’an, YAN Xuejun, YAN Yuqing, et al. Electrode potential regulates phenol degradation pathways in oxygen-diffused microbial electrochemical system[J]. Chemical Engineering Journal, 2020, 381: 122663. |
| 10 | 杨丙衡, 安路阳, 张立涛, 等. 中间层为聚吡咯的复合电极深度处理焦化废水[J]. 化工进展, 2020, 39(10): 4256-4267. |
| YANG Bingheng, AN Luyang, ZHANG Litao, et al. Treatment of coking wastewater with composite electrode with PPy as middle layer[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4256-4267. | |
| 11 | 王韬, 李鑫钢, 杜启云. 含酚废水治理技术研究进展[J]. 化工进展, 2008, 27(2): 231-235. |
| WANG Tao, LI Xingang, DU Qiyun. Research progress of phenol-containing waste water disposal technique[J]. Chemical Industry and Engineering Progress, 2008, 27(2): 231-235. | |
| 12 | SAHOO S K, BHATTACHARYA S, SAHOO N K. Photocatalytic degradation of biological recalcitrant pollutants: a green chemistry approach[J]. Biointerface Research in Applied Chemistry, 2020, 10(2): 5048-5060. |
| 13 | DU Juanshan, SUN Bo, ZHANG Jing, et al. Parabola-like shaped pH-rate profile for phenols oxidation by aqueous permanganate[J]. Environmental Science & Technology, 2012, 46(16): 8860-8867. |
| 14 | LI Haiping, CHENG Rongqing, LIU Zhiliang, et al. Waste control by waste: Fenton-like oxidation of phenol over Cu modified ZSM-5 from coal gangue[J]. Science of the Total Environment, 2019, 683: 638-647. |
| 15 | SUN Chen, CHEN Tong, HUANG Qunxing, et al. Activation of persulfate by CO2-activated biochar for improved phenolic pollutant degradation: performance and mechanism[J]. Chemical Engineering Journal, 2020, 380: 122519. |
| 16 | WANG Xinyue, SUN Yingnan, YANG Lu, et al. Novel photocatalytic system Fe-complex/TiO2 for efficient degradation of phenol and norfloxacin in water[J]. Science of the Total Environment, 2019, 656: 1010-1020. |
| 17 | RAJASULOCHANA P, PREETHY V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review[J]. Resource-Efficient Technologies, 2016, 2(4): 175-184. |
| 18 | PANIGRAHY N, BARIK M, SAHOO N K. Kinetics of phenol biodegradation by an indigenous Pseudomonas citronellolis NS1 isolated from coke oven wastewater[J]. Journal of Hazardous, Toxic, and Radioactive Waste, 2020, 24(3): 04020019. |
| 19 | SINGH U, ARORA N K, SACHAN P. Simultaneous biodegradation of phenol and cyanide present in coke-oven effluent using immobilized Pseudomonas putida and Pseudomonas stutzeri [J]. Brazilian Journal of Microbiology, 2018, 49(1): 38-44. |
| 20 | KE Qian, ZHANG Yunge, WU Xilin, et al. Sustainable biodegradation of phenol by immobilized Bacillus sp. SAS19 with porous carbonaceous gels as carriers[J]. Journal of Environmental Management, 2018, 222: 185-189. |
| 21 | VAN DEXTER S, BOOPATHY R. Biodegradation of phenol by Acinetobacter tandoii isolated from the gut of the termite[J]. Environmental Science and Pollution Research International, 2019, 26(33): 34067-34072. |
| 22 | NAWAWI N M, AHMAD S A, SHUKOR M Y, et al. Statistical optimisation for improvement of phenol degradation by Rhodococcus sp. NAM 81[J]. Journal of Environmental Biology, 2016, 37(3): 443-451. |
| 23 | AZADI D, SHOJAEI H. Biodegradation of polycyclic aromatic hydrocarbons, phenol and sodium sulfate by Nocardia species isolated and characterized from Iranian ecosystems[J]. Scientific Reports, 2020, 10: 21860. |
| 24 | GOMES E SILVA N C, DE MACEDO A C, TELES PINHEIRO Á D, et al. Phenol biodegradation by Candida tropicalis ATCC 750 immobilized on cashew apple bagasse[J]. Journal of Environmental Chemical Engineering, 2019, 7(3): 103076. |
| 25 | DAN A, FUJII D, SODA S, et al. Removal of phenol, bisphenol A, and 4-tert-butylphenol from synthetic landfill leachate by vertical flow constructed wetlands[J]. Science of the Total Environment, 2017, 578: 566-576. |
| 26 | 王哲, 骆逸飞, 郑春丽, 等. 淋溶条件下生物炭对矿区土壤中重金属迁移的影响[J]. 化工进展, 2020, 39(2): 738-746. |
| WANG Zhe, LUO Yifei, ZHENG Chunli, et al. Effect of biochar on migration of heavy metals in mining soil under leaching conditions[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 738-746. | |
| 27 | MOORALITHARAN S, HANAFIAH Z M, MANAN T, et al. Optimization of mycoremediation treatment for the chemical oxygen demand (COD) and ammonia nitrogen (AN) removal from domestic effluent using wild-Serbian Ganoderma lucidum (WSGL)[J]. Environmental Science and Pollution Research International, 2021, 28(1): 32528-32544. |
| 28 | PANIGRAHY N, BARIK M, SAHOO R K, et al. Metabolic profile analysis and kinetics of p-cresol biodegradation by an indigenous Pseudomonas citronellolis NS1 isolated from coke oven wastewater[J]. International Biodeterioration & Biodegradation, 2020, 147: 104837. |
| 29 | 叶子兰, 吴生亮, 姜立春, 等. 苯酚降解菌Y_1的分离与鉴定[J]. 四川环境, 2022, 41(1): 24-29. |
| YE Zilan, WU Shengliang, JIANG Lichun, et al. Isolation and identification of phenol degrading bacterium named Y_1[J]. Sichuan Environment, 2022, 41(1): 24-29. | |
| 30 | HASSANSHAHIAN M, ABARIAN M, BAHRAMZADEH K, et al. Isolation and identification of phenol-degrading bacteria in the industrial wastewater from the coal tar mine of Zarand in Iran[J]. Desalination and Water Treatment, 2019, 147: 125-134. |
| 31 | 李从强, 杨明坤, 付佳, 等. 煤炭地下气化废水的微生物修复研究[J]. 河南理工大学学报(自然科学版), 2022, 41(6): 1-9. |
| LI Congqiang, YANG Mingkun, FU Jia, et al. Microbial remediation of underground coal gasification wastewater[J]. Journal of Polytechnic University (Natural Science), 2022, 41(6): 1-9. |
| [1] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [2] | 张宁, 吴海滨, 李钰, 李剑锋, 程芳琴. 漂浮型光催化材料的制备及其在水处理领域的应用研究进展[J]. 化工进展, 2023, 42(5): 2475-2485. |
| [3] | 刘雅娟. 浸没式PAC-AMBRs系统中PAC缓解膜污染的研究进展[J]. 化工进展, 2023, 42(1): 457-468. |
| [4] | 杨柳, 王名威, 张耀斌. 磁铁矿负载生物炭强化厌氧微生物处理2,4-二氯苯酚废水[J]. 化工进展, 2022, 41(9): 5065-5073. |
| [5] | 伊学农, 李京梅, 高玉琼. 紫外-高铁酸盐体系氧化降解水中的萘普生[J]. 化工进展, 2022, 41(8): 4562-4570. |
| [6] | 吕莹, 胡学武, 陈素素, 刘兴宇, 陈勃伟, 张明江. 多环芳烃污染土壤的微生物修复技术研究进展[J]. 化工进展, 2022, 41(6): 3249-3262. |
| [7] | 周永泉, 张艾, 刘亚男, 王铮. 等离子体射流耦合活性碳纤维去除水中糖皮质激素[J]. 化工进展, 2022, 41(4): 2209-2215. |
| [8] | 翟重渊, 赵丹荻, 何亚鹏, 黄惠, 陈步明, 郭忠诚. 掺硼金刚石阳极电催化降解新兴抗生素类污染物研究进展[J]. 化工进展, 2022, 41(12): 6615-6626. |
| [9] | 马云波, 王彦斐, 李鹏, 刘欢, 武海洋, 苏衡, 马宣宣, 夏传海. 有机胺对Pd/C催化氯酚加氢脱氯反应的影响[J]. 化工进展, 2022, 41(11): 6185-6194. |
| [10] | 王吉坤, 李阳, 陈贵锋, 刘敏, 寇丽红, 王琦, 何毅聪. 臭氧催化氧化降解煤化工高盐废水有机物的机理[J]. 化工进展, 2022, 41(1): 493-502. |
| [11] | 张永祥, 王晋昊, 井琦, 李雅君. 地下水修复中纳米零价铁材料制备及应用综述[J]. 化工进展, 2021, 40(8): 4486-4496. |
| [12] | 罗艳红, 岳秀萍, 姜悦如, 赵博玮, 高艳娟, 段燕青. 高级氧化技术降解吲哚的研究进展[J]. 化工进展, 2021, 40(2): 1025-1034. |
| [13] | 杨硕, 余薇薇, 杨伦, 杜邦昊, 谢明原, 赵晨菊, 万巧玲, 潘伟亮. 纳米零价铁降解水中17β-雌二醇的作用机制[J]. 化工进展, 2020, 39(9): 3826-3834. |
| [14] | 张兰河, 郭琳, 李佳宁, 陈子成, 贾艳萍, 李正, 关晓辉. Fe2O3/改性沸石催化剂的制备及其催化臭氧氧化对氯苯酚[J]. 化工进展, 2020, 39(8): 3086-3094. |
| [15] | 王宏宇, 李国强, 朱沛宇, 张舒婷, 赵永乐, 段丽媛, 张永发. 炭化活化条件对Cu/AC表面Cu价态及其催化降解苯酚的影响[J]. 化工进展, 2020, 39(11): 4462-4473. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||