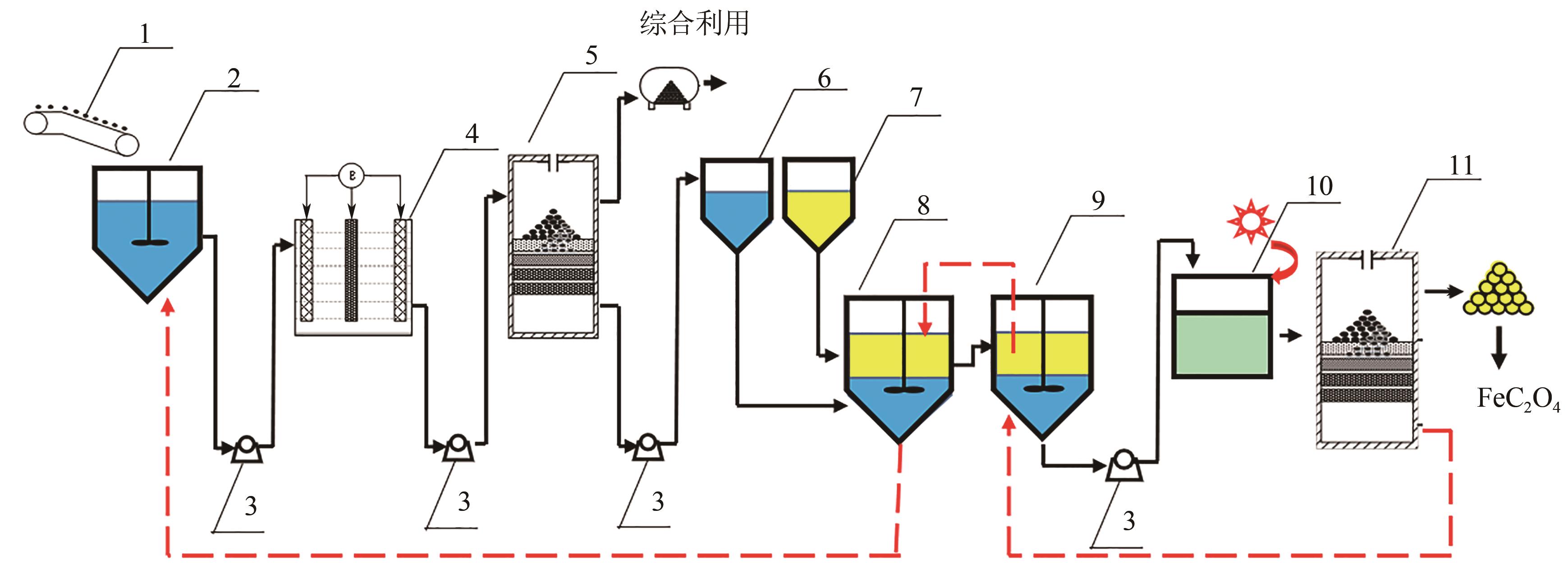
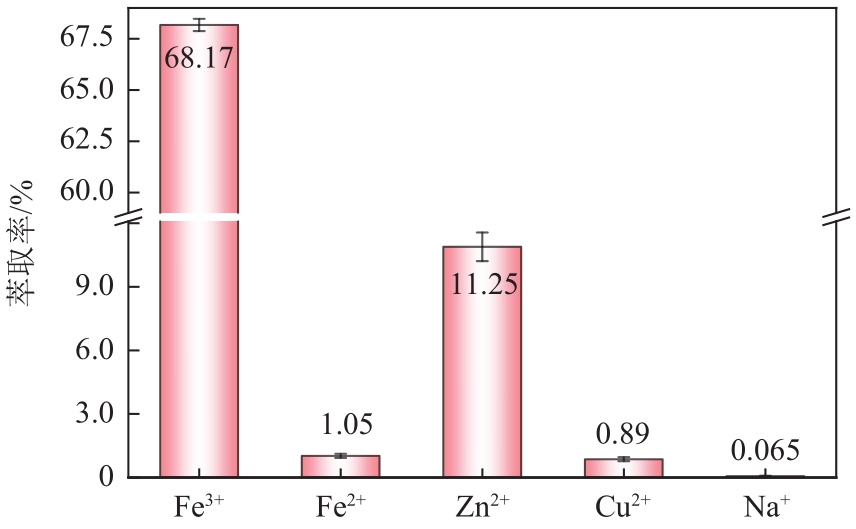
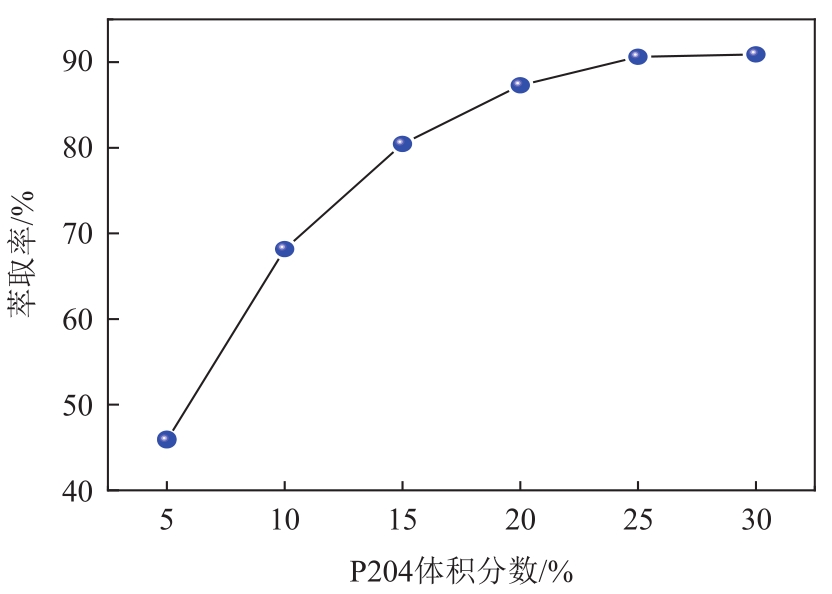
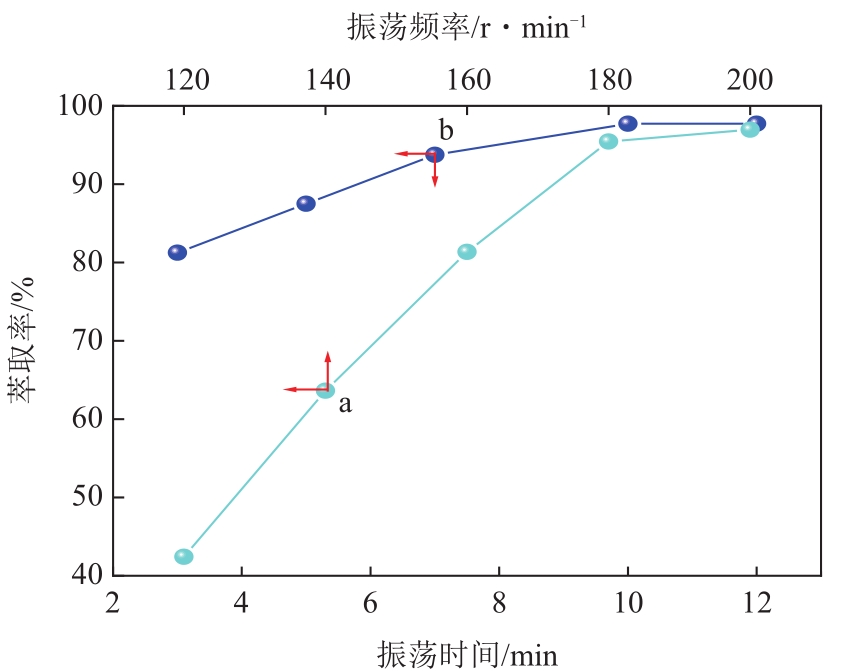
| 4 |
CHEN Yi, SONG Yonghui, CHEN Yao, et al. Comparative experimental study on the harmless treatment of cyanide tailings through slurry electrolysis[J]. Separation and Purification Technology, 2020, 251: 117314.
|
| 5 |
董萍, 宋永辉, 张红菊, 等. 利用矿浆电解技术处理氰化尾渣[J]. 环境工程学报, 2021, 15(5): 1662-1669.
|
|
DONG Ping, SONG Yonghui, ZHANG Hongju, et al. Treatment of cyanide tailings with slurry electrolysis technology[J]. Chinese Journal of Environmental Engineering, 2021, 15(5): 1662-1669.
|
| 6 |
LI Feifan, CHEN Mengjun, SHU Jianchen, et al. Copper and gold recovery from CPU sockets by one-step slurry electrolysis[J]. Journal of Cleaner Production, 2019, 213: 673-679.
|
| 7 |
VINCO J H, BOTELHO A B, DUARTE H A, et al. Purification of an iron contaminated vanadium solution through ion exchange resins[J]. Minerals Engineering, 2022, 176: 107337.
|
| 8 |
LENG Xinke, ZHONG Yanjun, XU Dehua, et al. Mechanism and kinetics study on removal of Iron from phosphoric acid by cation exchange resin[J]. Chinese Journal of Chemical Engineering, 2019, 27(5): 1050-1057.
|
| 9 |
LIANG Guangquan, ZHAO Qiang, LIU Bin, et al. Treatment and reuse of process water with high suspended solids in low-grade iron ore dressing[J]. Journal of Cleaner Production, 2021, 278: 123493.
|
| 10 |
WOJCIECHOWSKA Irmina, WIESZCZYCKA Karolina, WOJCIECHOWSKA Aleksandra, et al. Ether derivatives—Efficient Fe(Ⅲ) extractants from HCl solution[J]. Separation and Purification Technology, 2019, 209: 756-763.
|
| 11 |
刘铭, 周雍茂. 用N235-TBP混合体系从硫酸盐溶液中协同萃取除铁[J]. 中国有色金属学报, 2005, 15(10): 1648-1654.
|
|
LIU Ming, ZHOU Yongmao. Removal of Fe(Ⅲ) from sulphate solutions by synergistic extraction using N235-TBP mixed solvent systems[J]. The Chinese Journal of Nonferrous Metals, 2005, 15(10): 1648-1654.
|
| 12 |
熊泽威, 刘家辉, 李义兵, 等. 铟在黄钠铁矾除铁过程中的沉淀行为[J]. 湿法冶金, 2018, 37(5): 416-419.
|
|
XIONG Zewei, LIU Jiahui, LI Yibing, et al. Precipitation behavior of indium during removal of iron by jarosite[J]. Hydrometallurgy of China, 2018, 37(5): 416-419.
|
| 13 |
TORKAMAN R, MOOSAVIAN M A, SAFDARI J, et al. Synergistic extraction of gadolinium from nitrate media by mixtures of bis (2, 4, 4-trimethylpentyl) dithiophosphinic acid and di-(2-ethylhexyl) phosphoric acid[J]. Annals of Nuclear Energy, 2013, 62: 284-290.
|
| 14 |
QIN Zhifeng, ZHANG Guoquan, LUO Dongmei, et al. Separation of titanium from vanadium and iron in leach solutions of vanadium slag by solvent extraction with trioctyl tertiary amine (N235)[J]. Hydrometallurgy, 2019, 188: 216-221.
|
| 15 |
ZENG Luqi, YANG Tianzu, YI Xintao, et al. Separation of molybdenum and tungsten from iron in hydrochloric-phosphoric acid solutions using solvent extraction with TBP and P507[J]. Hydrometallurgy, 2020, 198: 105500.
|
| 16 |
YANG Bin, WU Suozhi, LIU Xinyu, et al. Solid-phase extraction and separation of heavy rare earths from chloride media using P227-impregnated resins[J]. Rare Metals, 2021, 40(9): 2633-2644.
|
| 17 |
WANG Xingyao, CONG Ma, LIU Juan. Studies of selective removal of iron (Ⅲ) from the simulated bauxite hydrochloric acid leaching liquor by solvent extraction[J]. Advanced Materials Research, 2013, 746: 31-34.
|
| 18 |
张晓峰, 李林艳, 张覃. 盐酸体系中二(2-乙基己基)次膦酸(P227)萃取铁的性能研究[J]. 湿法冶金, 2017, 36(3): 203-207.
|
|
ZHANG Xiaofeng, LI Linyan, ZHANG Qin. Extraction of iron(Ⅲ) in medium of hydrochloric acid using P227[J]. Hydrometallurgy of China, 2017, 36(3): 203-207.
|
| 19 |
WANG Lijuan, WANG Ying, CUI Li, et al. A sustainable approach for advanced removal of iron from CFA sulfuric acid leach liquor by solvent extraction with P507[J]. Separation and Purification Technology, 2020, 251: 117371.
|
| 20 |
HU Guoping, WU Yue, CHEN Desheng, et al. Selective removal of iron(Ⅲ) from highly salted chloride acidic solutions by solvent extraction using di(2-ethylhexyl) phosphate[J]. Frontiers of Chemical Science and Engineering, 2021, 15(3): 528-537.
|
| 21 |
AZIZITORGHABEH Atefeh, RASHCHI Fereshteh, BABAKHANI Ataollah. Stoichiometry and structural studies of Fe(Ⅲ) and Zn(Ⅱ) solvent extraction using D2EHPA/TBP[J]. Separation and Purification Technology, 2016, 171: 197-205.
|
| 22 |
ZHAO Zesen, CUI Li, GUO Yanxia, et al. Recovery of gallium from sulfuric acid leach liquor of coal fly ash by stepwise separation using P507 and Cyanex 272[J]. Chemical Engineering Journal, 2020, 381: 122699.
|
| 23 |
SONG Yonghui, LI Yifan, HE Xihong, et al. Recycling of residual valuable metals in cyanide-leached gold wastewater using the N263-TBP system[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106774.
|
| 1 |
吕翠翠, 丁剑, 付国燕, 等. 氰化尾渣中有价元素回收现状与展望[J]. 化工学报, 2016, 67(4): 1079-1089.
|
|
Cuicui LYU, DING Jian, FU Guoyan, et al. Present situation and prospect of recovering valuable elements from cyanidation tailing[J]. CIESC Journal, 2016, 67(4): 1079-1089.
|
| 2 |
李育彪, 陈坤, 郑仁军. 氰化尾渣脱氰技术及有价金属回收研究进展[J]. 矿产保护与利用, 2021, 41(1): 91-101.
|
|
LI Yubiao, CHEN Kun, ZHENG Renjun. Research progress on decyanation of cyanide tailings and recovery of valuable metal resources[J]. Conservation and Utilization of Mineral Resources, 2021, 41(1): 91-101.
|
| 3 |
袁嘉声, 畅永锋, 郑春龙, 等. 氰化尾渣脱氰技术综述[J]. 中国有色金属学报, 2021, 31(6): 1568-1581.
|
|
YUAN Jiasheng, CHANG Yongfeng, ZHENG Chunlong, et al. Review on treatment technologies of cyanide tailing[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(6): 1568-1581.
|
| 24 |
ZHANG Yan, JIANG Jianguo, CHEN Maozhe. MINTEQ modeling for evaluating the leaching behavior of heavy metals in MSWI fly ash[J]. Journal of Environmental Sciences, 2008, 20(11): 1398-1402.
|
| 25 |
JIN Yang, MA Yujing, WENG Yanling, et al. Solvent extraction of Fe3+ from the hydrochloric acid route phosphoric acid by D2EHPA in kerosene[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 3446-3452.
|
| 26 |
SUN Qi, YANG Limei, HUANG Songtao, et al. Synergistic solvent extraction of nickel by 2-hydroxy-5-nonylacetophenone oxime mixed with neodecanoic acid and bis(2-ethylhexyl) phosphoric acid: stoichiometry and structure investigation[J]. Minerals Engineering, 2019, 132: 284-292.
|
| 27 |
党晓娥, 刘安全, 边道超. 草酸配位浸出二段焙砂中包裹金的赤铁矿[J]. 贵金属, 2020, 41(2): 29-35.
|
|
DANG Xiao’e, LIU Anquan, BIAN Daochao. Oxalic acid coordination leaching of hematite wrapped with gold in two-stage calcine[J]. Precious Metals, 2020, 41(2): 29-35.
|
| 28 |
邓强, 杨林, 王辛龙, 等. 草酸反萃萃取剂中铁离子的工艺研究[J]. 应用化工, 2021, 50(2): 433-437.
|
|
DENG Qiang, YANG Lin, WANG Xinlong, et al. Technical study on stripping ferric iron from extractant by oxalic acid[J]. Applied Chemical Industry, 2021, 50(2): 433-437.
|
| 29 |
袁飞刚. 草酸强化三价铁离子的反萃性能[J]. 化工进展, 2019, 38(10): 4437-4443.
|
|
YUAN Feigang. Intensification of the stripping characteristics of iron(Ⅲ) using oxalic acid[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4437-4443.
|
| 30 |
Harald MÜLLER, BOURCET Léa, HANFLAND Michael. Iron(Ⅱ)oxalate dihydrate—Humboldtine: synthesis, spectroscopic and structural properties of a versatile precursor for high pressure research[J]. Minerals, 2021, 11(2): 113.
|
 ), 宋永辉1,2(
), 宋永辉1,2( ), 董萍1,2, 李一凡1,2, 朱荣燕1,2, 廖龙1,2
), 董萍1,2, 李一凡1,2, 朱荣燕1,2, 廖龙1,2
 ), SONG Yonghui1,2(
), SONG Yonghui1,2( ), DONG Ping1,2, LI Yifan1,2, ZHU Rongyan1,2, LIAO Long1,2
), DONG Ping1,2, LI Yifan1,2, ZHU Rongyan1,2, LIAO Long1,2