化工进展 ›› 2023, Vol. 42 ›› Issue (1): 30-39.DOI: 10.16085/j.issn.1000-6613.2022-1490
一碳资源在酵母中的利用与转化
郭峰1( ), 张尚杰1, 蒋羽佳1, 姜万奎1, 信丰学1,2, 章文明1,2(
), 张尚杰1, 蒋羽佳1, 姜万奎1, 信丰学1,2, 章文明1,2( ), 姜岷1,2(
), 姜岷1,2( )
)
- 1.南京工业大学生物与制药工程学院,材料化学工程国家重点实验室,江苏 南京 211816
2.南京工业大学江苏先进生物与化学制造协同创新中心(SICAM),江苏 南京 211816
-
收稿日期:2022-08-12修回日期:2022-09-16出版日期:2023-01-25发布日期:2023-02-20 -
通讯作者:章文明,姜岷 -
作者简介:郭峰(1996—),博士研究生,研究方向为甲醇酵母细胞工厂的构建。E-mail:202062112028@njtech.edu.cn。 -
基金资助:国家重点研发计划(2018YFA0901500);国家自然科学基金(22078151);中国科协青年人才托举工程(YESS20200174)
Biotransformation of one-carbon resources by yeast
GUO Feng1( ), ZHANG Shangjie1, JIANG Yujia1, JIANG Wankui1, XIN Fengxue1,2, ZHANG Wenming1,2(
), ZHANG Shangjie1, JIANG Yujia1, JIANG Wankui1, XIN Fengxue1,2, ZHANG Wenming1,2( ), JIANG Min1,2(
), JIANG Min1,2( )
)
- 1.State Key Laboratory of Materials-Oriented Chemical Engineering, College of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing 211816, Jiangsu, China
2.Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing Tech University, Nanjing 211816, Jiangsu, China
-
Received:2022-08-12Revised:2022-09-16Online:2023-01-25Published:2023-02-20 -
Contact:ZHANG Wenming, JIANG Min
摘要:
在当前日趋严重的气候与能源危机下,传统的化工行业亟需减少对化石燃料的依赖,转而开发利用更为清洁可持续的原料,如包括甲醇、甲酸和二氧化碳等在内的一碳资源。作为真核生物的酵母由于细胞器的存在能够隔绝一碳化合物代谢过程中产生的有毒物质渗入细胞质对细胞产生毒害,在一碳化合物的生物转化中极具潜力。近年来,随着对甲醇代谢途径愈发深入地了解以及遗传操作工具的逐步完善,利用天然甲醇酵母以甲醇作为碳源生产化学品取得了重要进展。同时,开发常用的工业酵母如酿酒酵母、解脂耶氏酵母为人工甲基营养型酵母,实现将一碳资源进行高值化转化也取得了不俗的成就。本文着重讨论了酵母底盘在转化利用一碳原料方面的研究进展,在此基础上讨论并分析了目前一碳资源生物转化存在的瓶颈以及潜在的解决方案,指出随着更多精准高效的工具的开发以及对细胞代谢网络的阐释,依托酵母细胞进行一碳资源的生物转化将在未来的绿色生物制造中扮演越来越重要的角色。
中图分类号:
引用本文
郭峰, 张尚杰, 蒋羽佳, 姜万奎, 信丰学, 章文明, 姜岷. 一碳资源在酵母中的利用与转化[J]. 化工进展, 2023, 42(1): 30-39.
GUO Feng, ZHANG Shangjie, JIANG Yujia, JIANG Wankui, XIN Fengxue, ZHANG Wenming, JIANG Min. Biotransformation of one-carbon resources by yeast[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 30-39.
| 产品 | 宿主 | 共底物 | 产量 | 参考文献 |
|---|---|---|---|---|
| 达马烯二醇-Ⅱ | 毕赤酵母 | 无 | 1mg/g | [ |
| 洛伐他汀 | 毕赤酵母 | 甘油 | 419mg/L | [ |
| 莫纳可林 J | 毕赤酵母 | 甘油 | 594mg/L | [ |
| 苹果酸 | 毕赤酵母 | 无 | 0.75g/L | [ |
| 苹果酸 | 毕赤酵母 | 酵母粉 | 2.79g/L | [ |
| 脂肪酸 | 毕赤酵母 | 无 | 23.4g/L | [ |
| 脂肪醇 | 毕赤酵母 | 无 | 2.0g/L | [ |
| 谷胱甘肽 | 多形汉逊酵母 | 无 | 0.25g/L | [ |
| 脂肪酸 | 多形汉逊酵母 | 无 | 15.9g/L | [ |
表1 改造甲醇酵母利用甲醇合成部分化学品
| 产品 | 宿主 | 共底物 | 产量 | 参考文献 |
|---|---|---|---|---|
| 达马烯二醇-Ⅱ | 毕赤酵母 | 无 | 1mg/g | [ |
| 洛伐他汀 | 毕赤酵母 | 甘油 | 419mg/L | [ |
| 莫纳可林 J | 毕赤酵母 | 甘油 | 594mg/L | [ |
| 苹果酸 | 毕赤酵母 | 无 | 0.75g/L | [ |
| 苹果酸 | 毕赤酵母 | 酵母粉 | 2.79g/L | [ |
| 脂肪酸 | 毕赤酵母 | 无 | 23.4g/L | [ |
| 脂肪醇 | 毕赤酵母 | 无 | 2.0g/L | [ |
| 谷胱甘肽 | 多形汉逊酵母 | 无 | 0.25g/L | [ |
| 脂肪酸 | 多形汉逊酵母 | 无 | 15.9g/L | [ |

图2 在酵母中表达一碳资源利用途径红色箭头表示甲醇利用途径;蓝色箭头表示甲酸利用途径;绿色箭头表示二氧化碳利用途径Ru5P—核酮糖-5-磷酸;F6P—果糖-6-磷酸;FBP—果糖-1,6-二磷酸;DHA—二羟丙酮;DHAP—磷酸二羟丙酮;GAP—甘油醛-3-磷酸;R5P—核糖-5-磷酸;Xu5P—木酮糖-5-磷酸;E4P—赤藻糖-4-磷酸;S1,7BP—景天庚酮糖-1,7-磷酸;S7P—景天庚酮糖-7-磷酸;RuBP—核酮糖-1,5二磷酸;3PG—甘油酸-3-磷酸;1,3BPG—甘油酸-1,3-二磷酸;GLY—甘氨酸;SER—丝氨酸;PYR—丙酮酸;GCS—甘氨酸裂解体系(glycine cleavage system);RuBisCO—1,5-二磷酸核酮糖羧化/加氧酶;Prk—磷酸核酮糖激酶;Aox1/2—醇氧化酶;Das1/2—二羟基丙酮合酶;Dak—二羟丙酮激酶;Fba1/2—果糖-1,6-二磷酸醛缩酶;Fbp1—果糖-1,6-二磷酸酶;Shb17—景天庚酮糖二磷酸酶;Rpi1/2—核糖-5-磷酸异构酶;Rpe1/2—核酮糖-3-磷酸差向异构酶;Pgk1—磷酸甘油酸激酶;Tdh3—甘油醛-3-磷酸脱氢酶;Tpi1—磷酸丙糖异构酶;Msi—三功能甲酰基-THF合成酶、亚甲基THF环化水解酶和亚甲基-THF脱氢酶;GcvH/T/P—甘氨酸裂解酶;5,10-methylene-THF—5,10-亚甲基四氢叶酸
| 底物 | 共底物 | 宿主 | 研究策略 | 成果 | 参考文献 |
|---|---|---|---|---|---|
| 甲醇 | 酵母粉 | 酿酒酵母 | 异源引入来源于毕赤酵母的甲醇同化途径 | 消耗2.35g/L甲醇,细胞生长增长11.70%,积累0.26g/L丙酮酸 | [ |
| 甲醇 | 酵母粉 | 酿酒酵母 | 实验室适应性驯化 | 揭示了酿酒酵母存在天然的甲醇同化机制 | [ |
| 甲醇 | 无 | 解脂耶氏酵母 | 异源表达杂合的RuMP和XuMP途径,敲除甲醛脱氢酶,强化Ru5P前体再生,适应性驯化 | 同化1.1g/L甲醇,在甲醇培养基下维持细胞不凋亡 | [ |
| 甲酸,二氧化碳 | 葡萄糖 | 酿酒酵母 | 在甘氨酸缺陷型菌株中强化表达内源性还原性甘氨酸途径 | 实现甲酸依赖型的生长 | [ |
| 二氧化碳 | 葡萄糖 | 酿酒酵母 | 异源引入一部分CBB循环基因 | 产物乙醇的产量提高10%,副产物甘油积累下降90% | [ |
| 二氧化碳 | 木糖 | 酿酒酵母 | 异源引入一部分CBB循环基因 | 产物乙醇的产量提高10%,副产物木糖醇积累下降90% | [ |
| 二氧化碳 | 甲醇 | 毕赤酵母 | 引入完整的CBB循环,敲除甲醇同化途径,适应性驯化 | 完全利用二氧化碳合成生物质,实现半自养 | [ |
表2 改造非甲基酵母为人工甲基酵母利用一碳原料
| 底物 | 共底物 | 宿主 | 研究策略 | 成果 | 参考文献 |
|---|---|---|---|---|---|
| 甲醇 | 酵母粉 | 酿酒酵母 | 异源引入来源于毕赤酵母的甲醇同化途径 | 消耗2.35g/L甲醇,细胞生长增长11.70%,积累0.26g/L丙酮酸 | [ |
| 甲醇 | 酵母粉 | 酿酒酵母 | 实验室适应性驯化 | 揭示了酿酒酵母存在天然的甲醇同化机制 | [ |
| 甲醇 | 无 | 解脂耶氏酵母 | 异源表达杂合的RuMP和XuMP途径,敲除甲醛脱氢酶,强化Ru5P前体再生,适应性驯化 | 同化1.1g/L甲醇,在甲醇培养基下维持细胞不凋亡 | [ |
| 甲酸,二氧化碳 | 葡萄糖 | 酿酒酵母 | 在甘氨酸缺陷型菌株中强化表达内源性还原性甘氨酸途径 | 实现甲酸依赖型的生长 | [ |
| 二氧化碳 | 葡萄糖 | 酿酒酵母 | 异源引入一部分CBB循环基因 | 产物乙醇的产量提高10%,副产物甘油积累下降90% | [ |
| 二氧化碳 | 木糖 | 酿酒酵母 | 异源引入一部分CBB循环基因 | 产物乙醇的产量提高10%,副产物木糖醇积累下降90% | [ |
| 二氧化碳 | 甲醇 | 毕赤酵母 | 引入完整的CBB循环,敲除甲醇同化途径,适应性驯化 | 完全利用二氧化碳合成生物质,实现半自养 | [ |
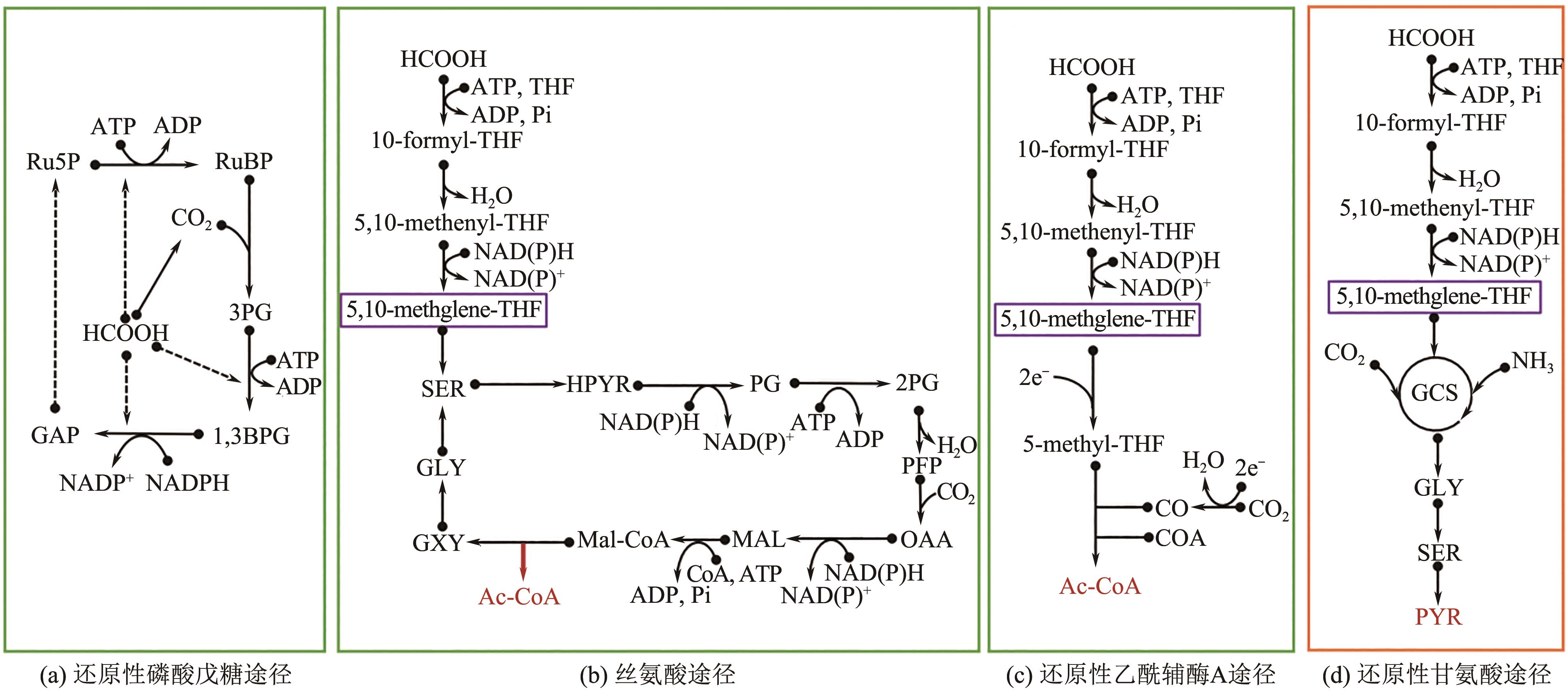
图3 天然与人工甲酸代谢途径10-formyl-THF—10-甲酰基四氢叶酸;10-formyl-THF—10-甲酰基四氢叶酸;5,10-methenyl-THF—5,10-亚甲酰基四氢叶酸;GXY—乙醛酸;Mal-CoA—苹果酰基辅酶A;HPYR—羟基丙酮酸;PG—甘油酸;2PG—2-磷酸甘油酸;PEP—磷酸烯醇式丙酮酸;OAA—草酰乙酸;Mal—苹果酸;AC-CoA—乙酰辅酶A
| 1 | ZHANG Wenming, SONG Meng, YANG Qiao, et al. Current advance in bioconversion of methanol to chemicals[J]. Biotechnology for Biofuels, 2018, 11: 260. |
| 2 | 陶雨萱, 张尚杰, 景艺文, 等. 甲基营养型大肠杆菌构建策略的研究进展[J]. 化工进展, 2021, 40(7): 3932-3941. |
| TAO Yuxuan, ZHANG Shangjie, JING Yiwen, et al. Recent advances in the construction strategy of methylotrophic Escherichia coli [J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3932-3941. | |
| 3 | 高教琪, 周雍进. 甲醇生物转化的机遇与挑战[J]. 合成生物学, 2020, 1(2): 158-173. |
| GAO Jiaoqi, ZHOU Yongjin. Advances in methanol bio-transformation[J]. Synthetic Biology Journal, 2020, 1(2): 158-173. | |
| 4 | ZHANG Wenming, ZHANG Ting, SONG Meng, et al. Metabolic engineering of Escherichia coli for high yield production of succinic acid driven by methanol[J]. ACS Synthetic Biology, 2018, 7(12): 2803-2811. |
| 5 | TUYISHIME Philibert, WANG Yu, FAN Liwen, et al. Engineering Corynebacterium glutamicum for methanol-dependent growth and glutamate production[J]. Metabolic Engineering, 2018, 49: 220-231. |
| 6 | CHEN F Y H, JUNG H W, TSUEI C Y, et al. Converting Escherichia coli to a synthetic methylotroph growing solely on methanol[J]. Cell, 2020, 182(4): 933-946.e14. |
| 7 | KIM S, LINDNER S N, ASLAN S, et al. Growth of E. coli on formate and methanol via the reductive Glycine pathway[J]. Nature Chemical Biology, 2020, 16(5): 538-545. |
| 8 | GLEIZER S, BEN-NISSAN R, BAR-ON Y M, et al. Conversion of Escherichia coli to generate all biomass carbon from CO2 [J]. Cell, 2019, 179(6): 1255-1263.e12. |
| 9 | ANTONOVSKY N, GLEIZER S, NOOR E, et al. Sugar synthesis from CO2 in Escherichia coli [J]. Cell, 2016, 166(1): 115-125. |
| 10 | GUO Feng, DAI Zhongxue, PENG Wenfang, et al. Metabolic engineering of Pichia pastoris for malic acid production from methanol[J]. Biotechnology and Bioengineering, 2021, 118(1): 357-371. |
| 11 | DAI Zhongxue, GU Honglian, ZHANG Shangjie, et al. Metabolic construction strategies for direct methanol utilization in Saccharomyces cerevisiae [J]. Bioresource Technology, 2017, 245: 1407-1412. |
| 12 | ESPINOSA M I, GONZALEZ-GARCIA R A, VALGEPEA K, et al. Adaptive laboratory evolution of native methanol assimilation in Saccharomyces cerevisiae [J]. Nature Communications, 2020, 11(1): 5564. |
| 13 | WANG Guokun, OLOFSSON-DOLK M, HANSSON F G, et al. Engineering yeast Yarrowia lipolytica for methanol assimilation[J]. ACS Synthetic Biology, 2021, 10(12): 3537-3550. |
| 14 | FABARIUS J T, WEGAT V, ROTH A, et al. Synthetic methylotrophy in yeasts: towards a circular bioeconomy[J]. Trends in Biotechnology, 2021, 39(4): 348-358. |
| 15 | GUO Feng, WU Min, ZHANG Shangjie, et al. Improved succinic acid production through the reconstruction of methanol dissimilation in Escherichia coli [J]. Bioresources and Bioprocessing, 2022, 9: 62. |
| 16 | GREGORY G J, BENNETT R K, PAPOUTSAKIS E T. Recent advances toward the bioconversion of methane and methanol in synthetic methylotrophs[J]. Metabolic Engineering, 2022, 71: 99-116. |
| 17 | 高琳惠, 蔡鹏, 周雍进. 甲醇酵母代谢工程研究进展[J]. 生物工程学报, 2021, 37(3): 966-979. |
| GAO Linhui, CAI Peng, ZHOU Yongjin. Advances in metabolic engineering of methylotrophic yeasts[J]. Chinese Journal of Biotechnology, 2021, 37(3): 966-979. | |
| 18 | MATTANOVICH D, SAUER M, GASSER B. Industrial microorganisms: Pichia pastoris [M]//Industrial Biotechnology. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, 2016: 687-714. |
| 19 | DONG Ce, QIAO Jie, WANG Xinping, et al. Engineering Pichia pastoris with surface-display minicellulosomes for carboxymethyl cellulose hydrolysis and ethanol production[J]. Biotechnology for Biofuels, 2020, 13: 108. |
| 20 | KATA I, SEMKIV M V, RUCHALA J, et al. Overexpression of the genes PDC1 and ADH1 activates glycerol conversion to ethanol in the thermotolerant yeast Ogataea (Hansenula) polymorpha[J]. Yeast, 2016, 33(8): 471-478. |
| 21 | GONÇALVES D B, BATISTA A F, RODRIGUES M Q R B, et al. Ethanol production from macaúba (Acrocomia aculeata) presscake hemicellulosic hydrolysate by Candida boidinii UFMG14[J]. Bioresource Technology, 2013, 146: 261-266. |
| 22 | CAI Peng, WU Xiaoyan, DENG Jun, et al. Methanol biotransformation toward high-level production of fatty acid derivatives by engineering the industrial yeast Pichia pastoris [J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(29): e2201711119. |
| 23 | GAO Jiaoqi, LI Yunxia, YU Wei, et al. Rescuing yeast from cell death enables overproduction of fatty acids from sole methanol[J]. Nature Metabolism, 2022, 4(7): 932-943. |
| 24 | MEESAPYODSUK D, CHEN Yan, NG S H, et al. Metabolic engineering of Pichia pastoris to produce ricinoleic acid, a hydroxy fatty acid of industrial importance[J]. Journal of Lipid Research, 2015, 56(11): 2102-2109. |
| 25 | CAI Peng, LI Yunxia, ZHAI Xiaoxin, et al. Microbial synthesis of long-chain α-alkenes from methanol by engineering Pichia pastoris [J]. Bioresources and Bioprocessing, 2022, 9: 58. |
| 26 | LI Cheng, LIN Ying, ZHENG Xueyun, et al. Recycling of a selectable marker with a self-excisable plasmid in Pichia pastoris [J]. Scientific Reports, 2017, 7: 11113. |
| 27 | WRIESSNEGGER T, AUGUSTIN P, ENGLEDER M, et al. Production of the sesquiterpenoid (+)-nootkatone by metabolic engineering of Pichia pastoris [J]. Metabolic Engineering, 2014, 24: 18-29. |
| 28 | LIU Xinbin, LIU Min, TAO Xinyi, et al. Metabolic engineering of Pichia pastoris for the production of dammarenediol-Ⅱ[J]. Journal of Biotechnology, 2015,216: 47-55. |
| 29 | CAI Peng, DUAN Xingpeng, WU Xiaoyan, et al. Recombination machinery engineering facilitates metabolic engineering of the industrial yeast Pichia pastoris [J]. Nucleic Acids Research, 2021, 49(13): 7791-7805. |
| 30 | GAO Jiaoqi, GAO Ning, ZHAI Xiaoxin, et al. Recombination machinery engineering for precise genome editing in methylotrophic yeast Ogataea polymorpha [J]. iScience, 2021, 24(3): 102168. |
| 31 | LIU Yiqi, TU Xiaohu, XU Qin, et al. Engineered monoculture and co-culture of methylotrophic yeast for de novo production of monacolin J and lovastatin from methanol[J]. Metabolic Engineering, 2018, 45: 189-199. |
| 32 | LIU Yiqi, BAI Chenxiao, XU Qin, et al. Improved methanol-derived lovastatin production through enhancement of the biosynthetic pathway and intracellular lovastatin efflux in methylotrophic yeast[J]. Bioresources and Bioprocessing, 2018, 5: 22. |
| 33 | UBIYVOVK V M, ANANIN V M, MALYSHEV A Y, et al. Optimization of glutathione production in batch and fed-batch cultures by the wild-type and recombinant strains of the methylotrophic yeast Hansenula polymorpha DL-1[J]. BMC Biotechnology, 2011, 11: 8. |
| 34 | ESPINOSA M I, WILLIAMS T C, PRETORIUS I S, et al. Benchmarking two Saccharomyces cerevisiae laboratory strains for growth and transcriptional response to methanol[J]. Synthetic and Systems Biotechnology, 2019, 4(4): 180-188. |
| 35 | GONZÁLEZ DE LA CRUZ J, MACHENS F, MESSERSCHMIDT K, et al. Core catalysis of the reductive Glycine pathway demonstrated in yeast[J]. ACS Synthetic Biology, 2019, 8(5): 911-917. |
| 36 | GUADALUPE-MEDINA V, WISSELINK H W, LUTTIK M A, et al. Carbon dioxide fixation by Calvin-Cycle enzymes improves ethanol yield in yeast[J]. Biotechnology for Biofuels, 2013, 6(1): 125. |
| 37 | PAPAPETRIDIS I, GOUDRIAAN M, VÁZQUEZ VITALI M, et al. Optimizing anaerobic growth rate and fermentation kinetics in Saccharomyces cerevisiae strains expressing Calvin-cycle enzymes for improved ethanol yield[J]. Biotechnology for Biofuels, 2018, 11: 17. |
| 38 | GASSLER T, SAUER M, GASSER B, et al. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2 [J]. Nature Biotechnology, 2020, 38(2): 210-216. |
| 39 | COTTON C A, CLAASSENS N J, BENITO-VAQUERIZO S, et al. Renewable methanol and formate as microbial feedstocks[J]. Current Opinion in Biotechnology, 2020, 62: 168-180. |
| 40 | BAI Xiaofang, CHEN Wei, ZHAO Chengcheng, et al. Exclusive formation of formic acid from CO2 electroreduction by a tunable Pd-Sn alloy[J]. Angewandte Chemie, 2017, 129(40): 12387-12391. |
| 41 | BAR-EVEN A, NOOR E, FLAMHOLZ A, et al. Design and analysis of metabolic pathways supporting formatotrophic growth for electricity-dependent cultivation of microbes[J]. Biochimica et Biophysica Acta (BBA): Bioenergetics, 2013, 1827(8/9): 1039-1047. |
| 42 | BAR-EVEN A, NOOR E, MILO R. A survey of carbon fixation pathways through a quantitative lens[J]. Journal of Experimental Botany, 2011, 63(6): 2325-2342. |
| 43 | LI Han, OPGENORTH P H, WERNICK D G, et al. Integrated electromicrobial conversion of CO2 to higher alcohols[J]. Science, 2012, 335(6076): 1596. |
| 44 | YISHAI O, LINDNER S N, GONZALEZ DE LA CRUZ J, et al. The formate bio-economy[J]. Current Opinion in Chemical Biology, 2016, 35: 1-9. |
| 45 | SÁNCHEZ-ANDREA I, GUEDES I A, HORNUNG B, et al. The reductive Glycine pathway allows autotrophic growth of Desulfovibrio desulfuricans [J]. Nature Communications, 2020, 11(1): 5090. |
| 46 | CLAASSENS N J. Reductive Glycine pathway: a versatile route for one-carbon biotech[J]. Trends in Biotechnology, 2021, 39(4): 327-329. |
| 47 | PETER S C. Reduction of CO2 to chemicals and fuels: a solution to global warming and energy crisis[J]. ACS Energy Letters, 2018, 3(7): 1557-1561. |
| 48 | XU Suyun, QIAO Zihao, LUO Liwen, et al. On-site CO2 bio-sequestration in anaerobic digestion: current status and prospects[J]. Bioresource Technology, 2021, 332: 125037. |
| 49 | HA-TRAN D M, LAI Rouyin, NGUYEN T T M, et al. Construction of engineered RuBisCO Kluyveromyces marxianus for a dual microbial bioethanol production system[J]. PLoS One, 2021, 16(3): e0247135. |
| 50 | BAR-EVEN A, FLAMHOLZ A, NOOR E, et al. Thermodynamic constraints shape the structure of carbon fixation pathways[J]. Biochimica et Biophysica Acta (BBA): Bioenergetics, 2012, 1817(9): 1646-1659. |
| 51 | GUO Junling, SUÁSTEGUI M, SAKIMOTO K K, et al. Light-driven fine chemical production in yeast biohybrids[J]. Science, 2018, 362(6416): 813-816. |
| 52 | ZHANG Hao, LIU Hao, TIAN Zhiquan, et al. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production[J]. Nature Nanotechnology, 2018, 13(10): 900-905. |
| 53 | HONG Y, ARBTER P, WANG Wei, et al. Introduction of Glycine synthase enables uptake of exogenous formate and strongly impacts the metabolism in Clostridium pasteurianum [J]. Biotechnology and Bioengineering, 2021, 118(3): 1366-1380. |
| 54 | SCHWANDER T, VON BORZYSKOWSKI L S, BURGENER S, et al. A synthetic pathway for the fixation of carbon dioxide in vitro [J]. Science, 2016, 354(6314): 900-904. |
| 55 | LIEBAL U W, BLANK L M, EBERT B E. CO2 to succinic acid—Estimating the potential of biocatalytic routes[J]. Metabolic Engineering Communications, 2018, 7: e00075. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 许家珩, 李永胜, 罗春欢, 苏庆泉. 甲醇水蒸气重整工艺的优化[J]. 化工进展, 2023, 42(S1): 41-46. |
| [3] | 舒斌, 陈建宏, 熊健, 吴其荣, 喻江涛, 杨平. 碳中和目标下推动绿色甲醇发展的必要性分析[J]. 化工进展, 2023, 42(9): 4471-4478. |
| [4] | 刘炫麟, 王驿凯, 戴苏洲, 殷勇高. 热泵中氨基甲酸铵分解反应特性及反应器结构优化[J]. 化工进展, 2023, 42(9): 4522-4530. |
| [5] | 王子健, 柯明, 宋昭峥, 李佳涵, 童燕兵, 孙巾茹. 分子筛催化汽油烷基化降苯技术研究进展[J]. 化工进展, 2023, 42(5): 2371-2389. |
| [6] | 萧垚鑫, 张军, 胡升, 单锐, 袁浩然, 陈勇. 甲醇供氢体系铜锌双金属催化糠醛加氢转化[J]. 化工进展, 2023, 42(3): 1341-1352. |
| [7] | 黄起中, 刘冰, 马红鹏, 吕文杰. 基于新型微通道分离技术的甲醇制烯烃废水处理[J]. 化工进展, 2023, 42(2): 669-676. |
| [8] | 刘丹, 范云洁, 王慧敏, 严政, 李鹏飞, 李家成, 曹雪波. 基于废弃PET的高值化功能性多孔碳材料及其应用进展[J]. 化工进展, 2023, 42(2): 969-984. |
| [9] | 郭晓宇, 李冬晨, 赵炜, 杜朕屹, 李晓良. Au-Pd/MnO2催化剂的制备及其苯甲醇氧化性能[J]. 化工进展, 2023, 42(10): 5223-5231. |
| [10] | 陶雨萱, 郭亮, 高聪, 宋伟, 陈修来. 代谢工程改造微生物固定二氧化碳研究进展[J]. 化工进展, 2023, 42(1): 40-52. |
| [11] | 李喆, 李泽洋, 杨宇森, 卫敏. 电化学二氧化碳还原制甲酸催化剂的研究进展[J]. 化工进展, 2023, 42(1): 53-66. |
| [12] | 赵同心, 赵磊, 张延平, 李寅. 甲酸微生物转化研究进展[J]. 化工进展, 2023, 42(1): 67-72. |
| [13] | 尹爽, 梁伟杰, 陈沛嘉, 张志聪, 葛建芳. 聚己二酸丁二醇酯-共对苯二甲酸酯基可降解塑料改性研究进展[J]. 化工进展, 2022, 41(S1): 307-317. |
| [14] | 孟令玎, 毛梦雷, 廖奇勇, 孟子晖, 刘文芳. 碳酸酐酶和甲酸脱氢酶的稳定性研究进展[J]. 化工进展, 2022, 41(S1): 436-447. |
| [15] | 刘鹏龙, 许雄飞, 张玮, 许鑫, 张侃, 王俊文. 甲醇制芳烃K-means-PSO-SVR局部建模及优化[J]. 化工进展, 2022, 41(9): 4691-4700. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
