化工进展 ›› 2023, Vol. 42 ›› Issue (1): 40-52.DOI: 10.16085/j.issn.1000-6613.2022-1545
代谢工程改造微生物固定二氧化碳研究进展
- 1.江南大学食品科学与技术国家重点实验室,江苏 无锡 214122
2.江南大学生命科学与健康工程学院,江苏 无锡 214122
-
收稿日期:2022-08-22修回日期:2022-10-29出版日期:2023-01-25发布日期:2023-02-20 -
通讯作者:陈修来 -
作者简介:陶雨萱(1997—),女,博士研究生,研究方向为微生物代谢工程。E-mail:15106192105@163.com。 -
基金资助:国家重点研发计划(2021YFC2103500);国家自然科学基金(22122806);江苏省自然科学基金(BK20211529)
Progress in metabolic engineering of microorganisms for CO2 fixation
TAO Yuxuan1( ), GUO Liang1, GAO Cong1, SONG Wei2, CHEN Xiulai1(
), GUO Liang1, GAO Cong1, SONG Wei2, CHEN Xiulai1( )
)
- 1.State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, Jiangsu, China
2.School of Life Sciences and Health Engineering, Jiangnan University, Wuxi 214122, Jiangsu, China
-
Received:2022-08-22Revised:2022-10-29Online:2023-01-25Published:2023-02-20 -
Contact:CHEN Xiulai
摘要:
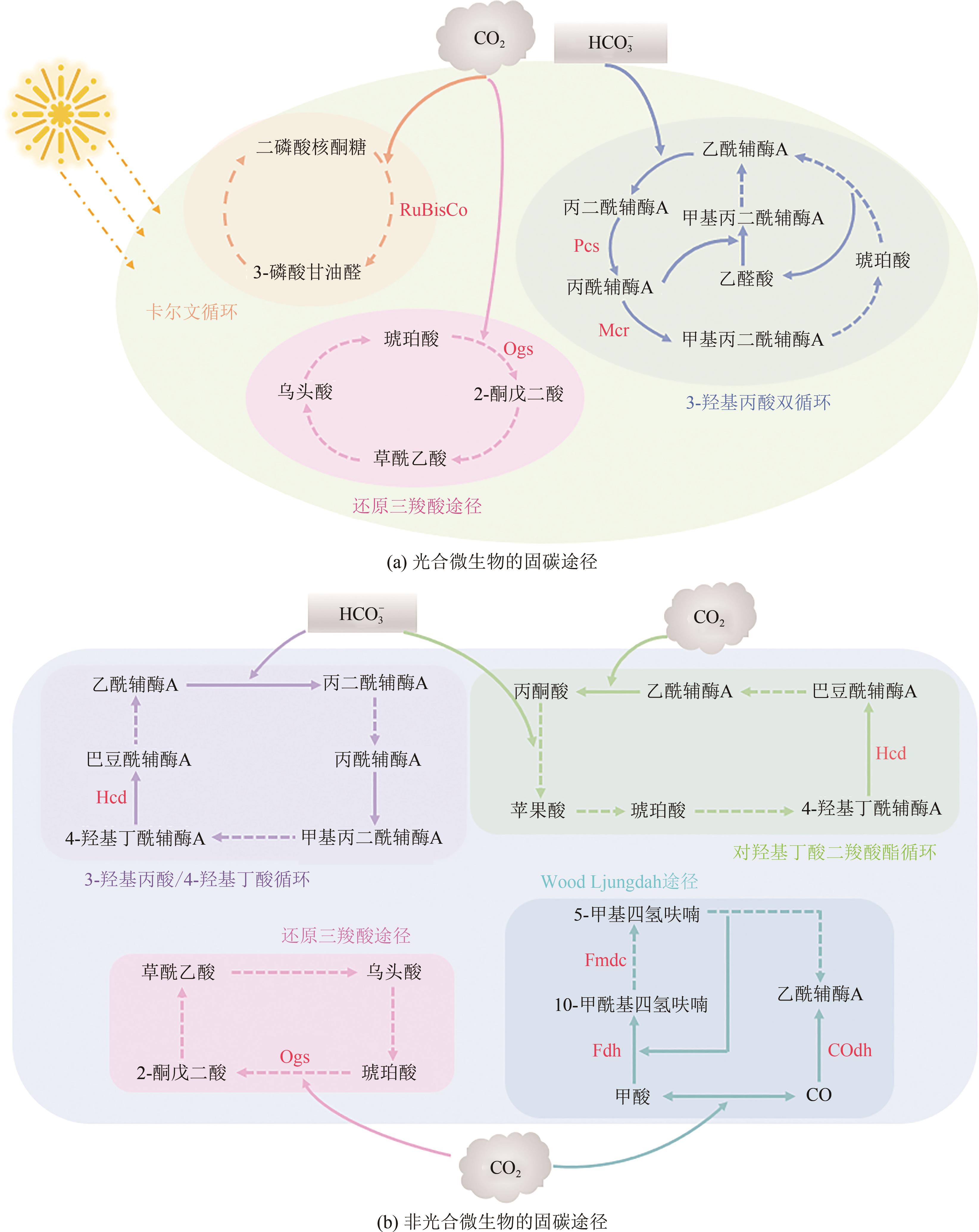
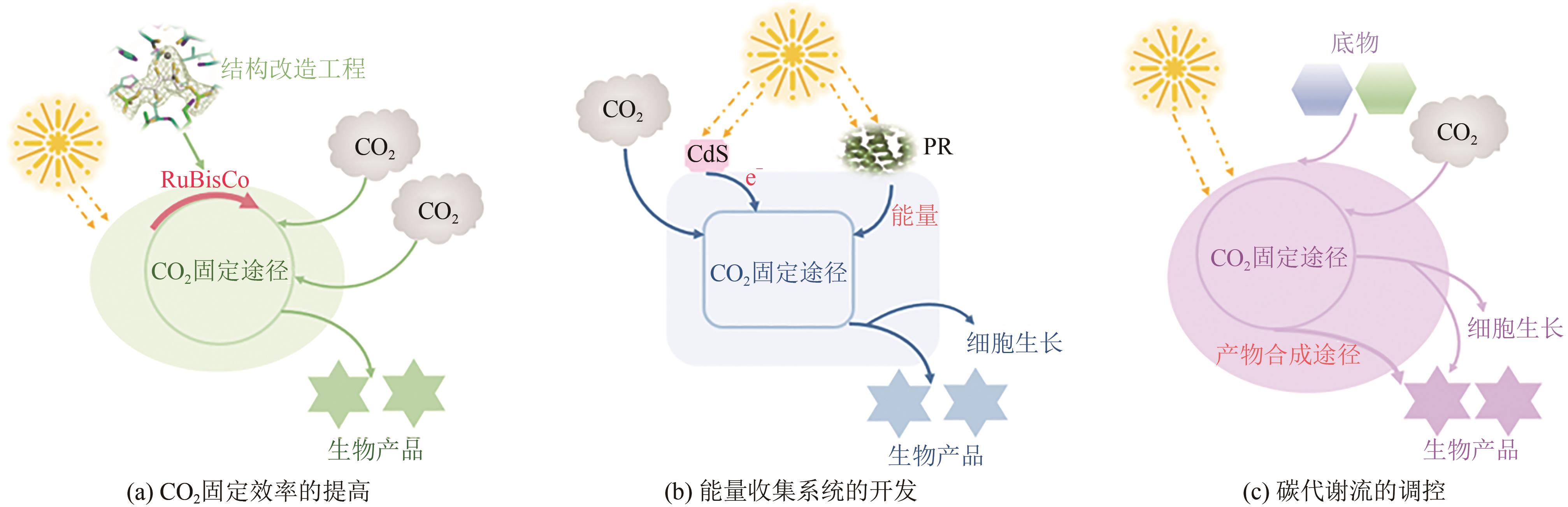
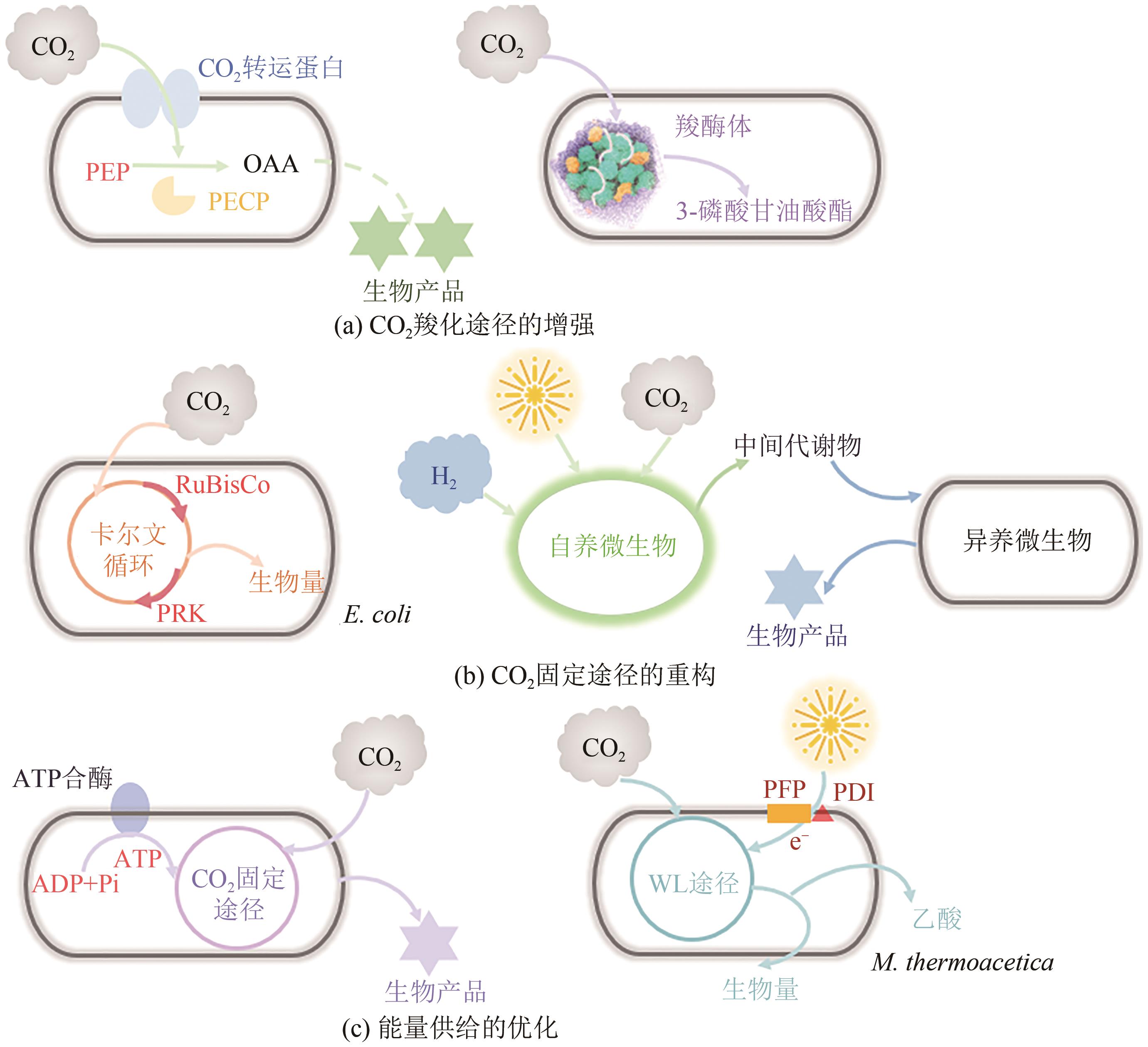
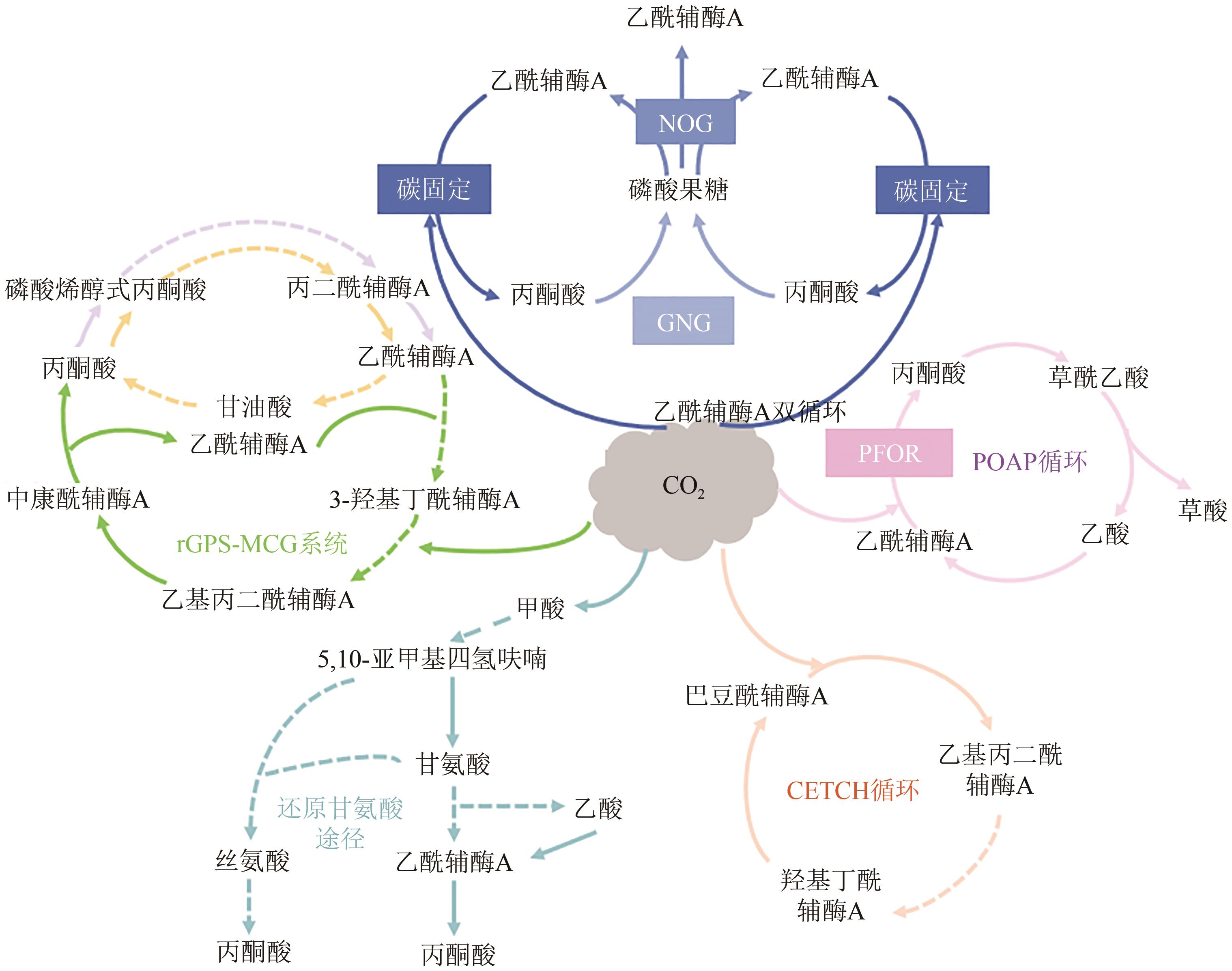
微生物固定CO2是实现CO2资源化利用的有效策略之一,为固碳减排、节能生产与绿色合成提供了借鉴。然而,微生物在固定CO2过程中存在底物利用效率低、能量需求量大、路径难优化等问题。为了解决这些问题,本文总结了6种天然CO2固定途径与5种人工CO2固定途径,并从自养微生物、异养微生物和人工微生物三个方面系统分析了代谢工程改造微生物固定CO2合成化学品的最新进展。在自养微生物固定CO2方面,采用的策略主要包括提高CO2固定途径效率、开发能量捕集系统与调节碳代谢流分布;在异养微生物固定CO2方面,常用的方法主要有强化羧化途径、重构CO2固定途径与优化能量供给;在人工微生物固定CO2方面,主要的研究思路是设计人工CO2固定途径与构建人工CO2固定微生物。最后,从CO2固定的关键酶、途径和微生物三方面展望了进一步提高CO2固定效率的发展方向。
中图分类号:
引用本文
陶雨萱, 郭亮, 高聪, 宋伟, 陈修来. 代谢工程改造微生物固定二氧化碳研究进展[J]. 化工进展, 2023, 42(1): 40-52.
TAO Yuxuan, GUO Liang, GAO Cong, SONG Wei, CHEN Xiulai. Progress in metabolic engineering of microorganisms for CO2 fixation[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 40-52.
| CO2固定途径 | 固定CO2/HCO | 消耗能量 | 重要产物 | 氧气需求 | 参考文献 |
|---|---|---|---|---|---|
| CBB循环 | 3mol CO2 | 9mol ATP + 6mol NAD(P)H | 3-磷酸甘油醛 | 好氧 | [ |
| RTCA途径 | 2mol CO2 | 2mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧/微氧 | [ |
| 3-HP双循环 | 3mol HCO | 5mol ATP + 5mol NAD(P)H | 丙酮酸 | 好氧 | [ |
| WL途径 | 2mol CO2 | 1mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧 | [ |
| DC-4HB循环 | 1mol CO2 + 1mol HCO | 3mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧/微氧 | [ |
| 3HP-4HB循环 | 2mol HCO | 4mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 好氧 | [ |
表1 天然CO2固定途径
| CO2固定途径 | 固定CO2/HCO | 消耗能量 | 重要产物 | 氧气需求 | 参考文献 |
|---|---|---|---|---|---|
| CBB循环 | 3mol CO2 | 9mol ATP + 6mol NAD(P)H | 3-磷酸甘油醛 | 好氧 | [ |
| RTCA途径 | 2mol CO2 | 2mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧/微氧 | [ |
| 3-HP双循环 | 3mol HCO | 5mol ATP + 5mol NAD(P)H | 丙酮酸 | 好氧 | [ |
| WL途径 | 2mol CO2 | 1mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧 | [ |
| DC-4HB循环 | 1mol CO2 + 1mol HCO | 3mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 厌氧/微氧 | [ |
| 3HP-4HB循环 | 2mol HCO | 4mol ATP + 4mol NAD(P)H | 乙酰辅酶A | 好氧 | [ |
| CO2固定途径 | 固定CO2/HCO3- | 消耗能量 | 固碳效率 | 氧气需求 | 参考文献 |
|---|---|---|---|---|---|
| CETCH循环 | 1mol CO2 | 1mol ATP + 4mol NAD(P)H | 5nmol CO2/(mg 蛋白·min) | 好氧 | [ |
| rGly途径 | 1mol CO2 | 2mol ATP + 4mol NAD(P)H | — | 好氧 | [ |
| POAP循环 | 2mol CO2 | 2mol ATP和1mol NAD(P)H | 8.0nmol CO2/(mg CO2固定酶·min) | 厌氧 | [ |
| acetyl-CoA双循环 | 2mol CO2 | 消耗2mol乙酰辅酶A并生产3mol | — | 厌氧 | [ |
| rGPS-MCG系统 | 2mol HCO3– | 5mol ATP + 5mol NAD(P)H | 28.5nmol CO2/(mg核心蛋白·min) | 好氧/厌氧 | [ |
表2 人工CO2固定途径
| CO2固定途径 | 固定CO2/HCO3- | 消耗能量 | 固碳效率 | 氧气需求 | 参考文献 |
|---|---|---|---|---|---|
| CETCH循环 | 1mol CO2 | 1mol ATP + 4mol NAD(P)H | 5nmol CO2/(mg 蛋白·min) | 好氧 | [ |
| rGly途径 | 1mol CO2 | 2mol ATP + 4mol NAD(P)H | — | 好氧 | [ |
| POAP循环 | 2mol CO2 | 2mol ATP和1mol NAD(P)H | 8.0nmol CO2/(mg CO2固定酶·min) | 厌氧 | [ |
| acetyl-CoA双循环 | 2mol CO2 | 消耗2mol乙酰辅酶A并生产3mol | — | 厌氧 | [ |
| rGPS-MCG系统 | 2mol HCO3– | 5mol ATP + 5mol NAD(P)H | 28.5nmol CO2/(mg核心蛋白·min) | 好氧/厌氧 | [ |
| 1 | LIU Z, WANG K, CHEN Y, Third-generation biorefineries as the means to produce fuels and chemicals from CO2 [J]. Nature Catalysis, 2020, 3: 274-288. |
| 2 | HUGHES T P, BAIRD A H, BELLWOOD D R, et al. Climate change, human impacts, and the resilience of coral reefs[J]. Science, 2003, 301(5635): 929-933. |
| 3 | XU X Y, KENTISH S E and MARTIN G J O. Direct air capture of CO2 by microalgae with buoyant beads encapsulating carbonic anhydrase[J]. ACS Sustainable Chemistry and Engineering, 2021, 9(29): 9698-9706. |
| 4 | DE VISSER E, HENDRIKS C, BARRIO M, et al. Dynamis CO2 quality recommendations[J]. International Journal of Greenhouse Gas Control, 2008, 2(4): 478-484. |
| 5 | WALL T F. Combustion processes for carbon capture[J]. Proceedings of the Combustion Institute, 2007, 31(1): 31-47. |
| 6 | BRUNETTI A, SCURA F, BARBIERI G, et al. Membrane technologies for CO2 separation[J]. Journal of Membrane Science, 2010, 359: 115-125. |
| 7 | LEUNG D Y C, CARAMANNA G and MAROTO-VALER M M. An overview of current status of carbon dioxide capture and storage technologies[J]. Renewable and Sustainable Energy Reviews, 2014, 39: 426-443. |
| 8 | LIANG F, ENGLUND E, LINDBERG P, et al. Engineered cyanobacteria with enhanced growth show increased ethanol production and higher biofuel to biomass ratio[J]. Metabolic Engineering, 2018, 46: 51-59. |
| 9 | MOON S Y, HONG S H, KIM T Y, et al. Metabolic engineering of Escherichia coli for the production of malic acid[J]. Biochemical Engineering Journal, 2008, 40: 312-320. |
| 10 | GASSLER T, SAUER M, GASSER B, et al. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2 [J]. Nature Biotechnology, 2020, 38: 210-216. |
| 11 | GONG F, ZHU H, ZHANG Y, et al. Biological carbon fixation: From natural to synthetic[J]. Journal of CO2 Utilization, 2018, 28: 221-227. |
| 12 | GONG F, CAI Z, LI Y. Synthetic biology for CO2 fixation[J]. Science China Life Sciences, 2016, 59(11): 1106-1114. |
| 13 | CALVIN M, BENSO A A. The path of carbon in photosynthesis[J]. Science, 1948, 107: 476-480. |
| 14 | ALTAŞ N, ASLAN A S, KARATAŞ E, et al. Heterologous production of extreme alkaline thermostable NAD+- dependent formate dehydrogenase with wide-range pH activity from Myceliophthora thermophila [J]. Process Biochemistry, 2017, 61: 110-118. |
| 15 | HUGLER M, MENENDEZ C, SCHAGGER H, et al. Malonyl-coenzyme A reductase from Chloroflexus aurantiacus, a key enzyme of the 3-hydroxypropionate cycle for autotrophic CO2 fixation[J]. Journal of Bacteriology, 2002, 184(9): 2404-2410. |
| 16 | KUMAR M, SUNDARAM S, GNANSOUNOU E, et al. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: A review[J]. Bioresource Technology, 2018, 247: 1059-1068. |
| 17 | HUBER H, GALLENBERGER M, LAHN U, et al. A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis [J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(22): 7851-7856. |
| 18 | BERG I A, KOCKELKORN D, BUCKEL W, et al. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea [J]. Science, 2007, 318: 1782-1786. |
| 19 | MOHAN S V, MODESTRA J A, AMULYA K, et al. A circular bioeconomy with biobased products from CO2 sequestration[J]. Trends in Biotechnology, 2016, 34(6): 506-519. |
| 20 | SHI L X, THEG S M. The chloroplast protein import system: from algae to trees[J]. BBA Molecular Cell Research, 2013, 1833(2): 314-331. |
| 21 | FUCHS G. Alternative pathways of carbon dioxide fixation: Insights into the early evolution of life?[J]. Annual Review of Microbiology, 2011, 65: 631-658. |
| 22 | JAJESNIAK P, ALI H E M O and WONG T S. Carbon dioxide capture and utilization using biological systems: opportunities and challenges[J]. Journal of Bioprocessing and Biotechniques, 2014, 4(3): 1000155. |
| 23 | ZHAO T, LI Y, ZHANG Y. Biological carbon fixation: A thermodynamic perspective[J]. Green Chemistry, 2021, 23(20): 7852-7864. |
| 24 | BARENHOLZ U, DAVIDI D, REZNIK E, et al. Design principles of autocatalytic cycles constrain enzyme kinetics and force low substrate saturation at flux branch points[J]. Elife, 2017, 6: e20667. |
| 25 | HU G, LI Y, YE C, et al. Engineering microorganisms for enhanced CO2 sequestration[J]. Trends in Biotechnology, 2019, 37(5): 532-547. |
| 26 | LIANG F, LINDBLAD P. Synechocystis PCC 6803 overexpressing RuBisCo grow faster with increased photosynthesis[J]. Metabolic Engineering Communications, 2017, 4: 29-36. |
| 27 | ANDREWS T J, LORIMER G H. Rubisco structure, mechanisms, and prospects for improvement[J]. The Biochemistry of Plants, 1987, 10: 131-218. |
| 28 | NISHITANI Y, YOSHIDA S, FUJIHASHI M, et al. Structure-based catalytic optimization of a type Ⅲ RuBisCo from a hyperthermophile[J]. The Journal of Biological Chemistry, 2010, 285(50): 39339-39347. |
| 29 | DURAO P, AIGNER H, NAGY P, et al. Opposing effects of folding and assembly chaperones on evolvability of Rubisco[J]. Nature Chemical Biology, 2015, 11(2): 148-155. |
| 30 | YANG F, ZHANG J, CAI Z, et al. Exploring the oxygenase function of Form II Rubisco for production of glycolate from CO2 [J]. AMB Express, 2021, 11(65). |
| 31 | ZHOU J, ZHANG F, MENG H, et al. Introducing extra NADPH consumption ability significantly increases the photosynthetic efficiency and biomass production of cyanobacteria[J]. Metabolic Engineering, 2016, 38: 217-227. |
| 32 | BAR-EVEN A. Daring metabolic designs for enhanced plant carbon fixation[J]. Plant Science, 2018, 273: 71-83. |
| 33 | BAR-EVEN A, NOOR E, FLAMHOLZ A, et al. Design and analysis of metabolic pathways supporting formatotrophic growth for electricity-dependent cultivation of microbes[J]. Biochimica et Biophysica Acta, 2013, 1827(8-9): 1039-1047. |
| 34 | BAR-EVEN A, NOOR E, LEWIS N E, et al. Design and analysis of synthetic carbon fixation pathways[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(19): 8889-8894. |
| 35 | DEVI M P, MOHAN S V. CO2 supplementation to domestic wastewater enhances microalgae lipid accumulation under mixotrophic microenvironment: Effect of sparging period and interval[J]. Bioresource Technology, 2012, 112: 116-123. |
| 36 | PALOVAARA J, AKRAM N, BALTAR F, et al. Stimulation of growth by proteorhodopsin phototrophy involves regulation of central metabolic pathways in marine planktonic bacteria[J]. Proceedings of the Combustion Institute, 2014, 111(35): E3650-3658. |
| 37 | CHEN Q, VAN DER STEEN J B, DEKKER H L, et al. Expression of holo-proteorhodopsin in Synechocystis sp. PCC 6803[J]. Metabolic Engineering, 2016, 35: 83-94. |
| 38 | KIRST H, FORMIGHIERI C, MELIS A. Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size[J]. Biochimica et Biophysica Acta, 2014, 1837(10): 1653-1664. |
| 39 | GARTZIA-RIVERO L, BANUELOS J, LOPEZ-ARBELOA I. Photoactive nanomaterials inspired by nature: ltl zeolite doped with laser dyes as artificial light harvesting systems[J]. Materials, 2017, 10(5): ma10050495. |
| 40 | CHOWDHURY F A, TRUDEAU M L, GUO H, et al. A photochemical diode artificial photosynthesis system for unassisted high efficiency overall pure water splitting[J]. Nature Communications, 2018, 9: 1707. |
| 41 | ZHANG X, WU Z, ZHANG X, et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures[J]. Nature Communications, 2017, 8: 14675. |
| 42 | SAKIMOTO K K, WONG A B, YANG P D. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production[J]. Science, 2016, 351(6268): 74-77. |
| 43 | DEMPO Y, OHTA E, NAKAYAMA Y, et al. Molar-based targeted metabolic profiling of cyanobacterial strains with potential for biological production[J]. Metabolites, 2014, 4(2): 499-516. |
| 44 | ANGERMAYR S A, GORCHS ROVIRA A, HELLINGWERF K J. Metabolic engineering of cyanobacteria for the synthesis of commodity products[J]. Trends in Biotechnology, 2015, 33(6): 352-361. |
| 45 | KUDOH K, KAWANO Y, HOTTA S, et al. Prerequisite for highly efficient isoprenoid production by cyanobacteria discovered through the over-expression of 1-deoxy-d-xylulose 5-phosphate synthase and carbon allocation analysis[J]. Journal of Bioscience and Bioengineering, 2014, 118(1): 20-28. |
| 46 | NGAN C Y, WONG C H, CHOI C, et al. Lineage-specific chromatin signatures reveal a regulator of lipid metabolism in microalgae[J]. Nature Plants, 2015, 1: 15107. |
| 47 | MATSON M M, ATSUMI S. Photomixotrophic chemical production in cyanobacteria[J]. Current Opinion in Biotechnology, 2018, 50: 65-71. |
| 48 | CLAASSENS N J, SOUSA D Z, DOS SANTOS V A, et al. Harnessing the power of microbial autotrophy[J]. Nature Reviews Microbiology, 2016, 14(11): 692-706. |
| 49 | HENARD C A, SMITH H, DOWE N, et al. Bioconversion of methane to lactate by an obligate methanotrophic bacterium[J]. Scientific Reports, 2016, 6: 21585. |
| 50 | SCHWANDER T, BORZYSKOWSKI L S, BURGENER S, et al. A synthetic pathway for the fixation of carbon dioxide in vitro[J]. Science, 2016, 354(6314): 900-904. |
| 51 | BROWN S H, BASHKIROVA L, BERKA R, et al. Metabolic engineering of Aspergillus oryzae NRRL 3488 for increased production of L-malic acid[J]. Applied Microbiology and Biotechnology, 2013, 97(20): 8903-8912. |
| 52 | KUPRIYANOVA E, VILLAREJO A, MARKELOVA A, et al. Extracellular carbonic anhydrases of the stromatolite-forming cyanobacterium Microcoleus chthonoplastes [J]. Microbiology, 2007, 153(4): 1149-1156. |
| 53 | BONACCI W, TENG P K, AFONSO B, et al. Modularity of a carbon-fixing protein organelle[J]. Proceedings of the Combustion Institute, 2012, 109(2): 478-483. |
| 54 | ZHANG Y, ZHOU J, ZHANG Y, et al. Auxiliary module promotes the synthesis of carboxysomes in E. coli to achieve high-efficiency CO2 assimilation[J]. ACS Synthetic Biology, 2021, 10(4): 707-715. |
| 55 | MATTOZZI M, ZIESACK M, VOGES M J, et al. Expression of the sub-pathways of the Chloroflexus aurantiacus 3-hydroxypropionate carbon fixation bicycle in E. coli: Toward horizontal transfer of autotrophic growth[J]. Metabolic Engineering, 2013, 16: 130-139. |
| 56 | GONG F, LIU G, ZHAI X, et al. Quantitative analysis of an engineered CO2-fixing Escherichia coli reveals great potential of heterotrophic CO2 fixation[J]. Biotechnology for Biofuels, 2015, 8: 86. |
| 57 | ANTONOVSKY N, GLEIZER S, NOOR E, et al. Sugar synthesis from CO2 in Escherichia coli [J]. Cell, 2016, 166(1): 115-125. |
| 58 | FRADINHO J C, DOMINGOS J M, CARVALHO G, et al. Polyhydroxyalkanoates production by a mixed photosynthetic consortium of bacteria and algae[J]. Bioresource Technology, 2013, 132: 146-153. |
| 59 | SAID S BEN, TECON R, BORER B, et al. The engineering of spatially linked microbial consortia-potential and perspectives[J]. Current Opinion in Biotechnology, 2020, 62: 137-145. |
| 60 | HU P, CHAKRABORTY S, KUMAR A, et al. Integrated bioprocess for conversion of gaseous substrates to liquids[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(14): 3773-3778. |
| 61 | LOWE H, HOBMEIER K, MOOS M, et al. Photoautotrophic production of polyhydroxyalkanoates in a synthetic mixed culture of Synechococcus elongatus cscB and Pseudomonas putida cscAB [J]. Biotechnology for Biofuels, 2017, 10(190): 1-11. |
| 62 | TONG T, CHEN X, HU G, et al. Engineering microbial metabolic energy homeostasis for improved bioproduction[J]. Biotechnology Advances, 2021, 53: 107841. |
| 63 | BRAAKMAN R, SMITH E. Metabolic evolution of a deep-branching hyperthermophilic chemoautotrophic bacterium[J]. PLoS One, 2014, 9(2): e87950. |
| 64 | GUADALUPE-MEDINA V, WISSELINK H W, LUTTIK M A, et al. Carbon dioxide fixation by Calvin-Cycle enzymes improves ethanol yield in yeast[J]. Biotechnology for Biofuels, 2013, 6: 125. |
| 65 | HU G, ZHOU J, CHEN X, et al. Engineering synergetic CO2-fixing pathways for malate production[J]. Metabolic Engineering, 2018, 47: 496-504. |
| 66 | LIEW F, HENSTRA A M, KPKE M, et al. Metabolic engineering of Clostridium autoethanogenum for selective alcohol production[J]. Metabolic Engineering, 2017, 40: 104-114. |
| 67 | WU Z, WANG J, LIU J, et al. Engineering an electroactive Escherichia coli for the microbial electrosynthesis of succinate from glucose and CO2 [J]. Microbial Cell Factories, 2019, 18: 15. |
| 68 | HU G, LI Z, MA D, et al. Light-driven CO2 sequestration in Escherichia coli to achieve theoretical yield of chemicals[J]. Nature Catalysis, 2021, 4(5): 395-406. |
| 69 | GAI P, YU W, ZHAO H, et al. Solar-powered organic semiconductor-bacteria biohybrids for CO2 reduction into acetic acid[J]. Angewandte Chemie International Edition, 2020, 59(18): 7224-7229. |
| 70 | JONES S W, FAST A G, CARLSON E D, et al. CO2 fixation by anaerobic non-photosynthetic mixotrophy for improved carbon conversion[J]. Nature Communications, 2016, 7: 12800. |
| 71 | HU L, GUO S, WANG B, et al. Bio-valorization of C1 gaseous substrates into bioalcohols: Potentials and challenges in reducing carbon emissions[J]. Biotechnology Advances, 2022, 59: 107954. |
| 72 | XIAO L, LIU G, GONG F, et al. A Minimized synthetic carbon fixation cycle[J]. ACS Catalysis, 2021, 12(1): 799-808. |
| 73 | WU C, LO J, URBAN C, et al. Acetyl-CoA synthesis through a bicyclic carbon-fixing pathway in gas-fermenting bacteria[J]. Nature Synthesis, 2022, 1(8): 615-625. |
| 74 | LUO S, LIN P P, NIEH L Y, et al. A cell-free self-replenishing CO2-fixing system[J]. Nature Catalysis, 2022, 5(2): 154-162. |
| 75 | YISHAI O, BOUZON M, DORING V, et al. In vivo assimilation of one-carbon via a synthetic reductive glycine pathway in Escherichia coli [J]. ACS Synthetic Biology, 2018, 7(9): 2023-2028. |
| 76 | PAVAN M, REINMETS K, GARG S, et al. Advances in systems metabolic engineering of autotrophic carbon oxide-fixing biocatalysts towards a circular economy[J]. Metabolic Engineering, 2022, 71: 117-141. |
| 77 | RODRIGUES R M, GUAN X, IÑIGUEZ J A, et al. Perfluorocarbon nanoemulsion promotes the delivery of reducing equivalents for electricity-driven microbial CO2 reduction[J]. Nature Catalysis, 2019, 2(5): 407-414. |
| 78 | TREMBLAY P L, XU M, CHEN Y, et al. Nonmetallic abiotic-biological hybrid photocatalyst for visible water splitting and carbon dioxide reduction[J]. iScience, 2020, 23(1): 100784. |
| 79 | LI H, OPGENORTH P H, WERNICK D G, et al. Integrated electromicrobial conversion of CO2 to higher slcohols[J]. Science, 2012, 335(6076): 1596. |
| 80 | MILLER T E, BENEYTON T, SCHWANDER T, et al. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts[J]. Science, 2020, 368(6491): 649-654. |
| 81 | GLEIZER S, BEN-NISSAN R, BAR-ON Y M, et al. Conversion of Escherichia coli to generate all biomass carbon from CO2 [J]. Cell, 2019, 179(6): 1255-1263. |
| 82 | PENA D A, GASSER B, ZANGHELLINI J, et al. Metabolic engineering of Pichia pastoris [J]. Metabolic Engineering, 2018, 50: 2-15. |
| [1] | 陈翔宇, 卞春林, 肖本益. 温度分级厌氧消化工艺的研究进展[J]. 化工进展, 2023, 42(9): 4872-4881. |
| [2] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [3] | 高聪, 陈城虎, 陈修来, 刘立明. 代谢工程改造微生物合成生物基单体的进展与挑战[J]. 化工进展, 2023, 42(8): 4123-4135. |
| [4] | 蒋博龙, 崔艳艳, 史顺杰, 常嘉城, 姜楠, 谭伟强. 过渡金属Co3O4/ZnO-ZIF氧还原催化剂Co/Zn-ZIF模板法制备及其产电性能[J]. 化工进展, 2023, 42(6): 3066-3076. |
| [5] | 李白雪, 信欣, 朱羽蒙, 刘琴, 刘鑫. SASD-A体系构建及进水不同S/N对脱氮工艺的影响机制[J]. 化工进展, 2023, 42(6): 3261-3271. |
| [6] | 范思涵, 于国熙, 来超超, 何欢, 黄斌, 潘学军. 非生物改性对厌氧微生物产物光化学活性影响[J]. 化工进展, 2023, 42(4): 2180-2189. |
| [7] | 程荣, 邓子祺, 夏锦程, 李江, 石磊, 郑祥. 光催化系统灭活微生物气溶胶的研究进展[J]. 化工进展, 2023, 42(2): 957-968. |
| [8] | 胡璇, 陈滢. 聚酯纤维微塑料胁迫下活性污泥系统性能及微生物群落的变化情况[J]. 化工进展, 2023, 42(2): 1051-1060. |
| [9] | 郭峰, 张尚杰, 蒋羽佳, 姜万奎, 信丰学, 章文明, 姜岷. 一碳资源在酵母中的利用与转化[J]. 化工进展, 2023, 42(1): 30-39. |
| [10] | 刘雅娟. 浸没式PAC-AMBRs系统中PAC缓解膜污染的研究进展[J]. 化工进展, 2023, 42(1): 457-468. |
| [11] | 付佳, 谌伦建, 徐冰, 华绍烽, 李从强, 杨明坤, 邢宝林, 仪桂云. 模拟煤炭气化废水中苯酚的微生物降解[J]. 化工进展, 2023, 42(1): 526-537. |
| [12] | 刘亚利, 张宏伟, 康晓荣. 微塑料对污泥厌氧消化的影响和机理[J]. 化工进展, 2022, 41(9): 5037-5046. |
| [13] | 徐沛, 贾璇, 王勇, 亓雪娇, 赵玉娇, 李鸣晓. 流场对MEC生物阴极CO2还原性能与产物的影响[J]. 化工进展, 2022, 41(7): 3816-3823. |
| [14] | 陈加波, 周鑫, 李旭. 以活性污泥为接种污泥厌氧氨氧化工艺的快速启动及脱氮效能[J]. 化工进展, 2022, 41(7): 3900-3907. |
| [15] | 潘文政, 纪志永, 汪婧, 李淑明, 黄智辉, 郭小甫, 刘杰, 赵颖颖, 袁俊生. 微生物燃料电池处理偶氮含盐废水的产电性能和降解过程[J]. 化工进展, 2022, 41(6): 3306-3313. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||