化工进展 ›› 2022, Vol. 41 ›› Issue (12): 6285-6294.DOI: 10.16085/j.issn.1000-6613.2022-0321
水合物储氢的研究进展
李昊阳1,2( ), 张炜1,3, 李小森1(
), 张炜1,3, 李小森1( ), 徐纯刚1(
), 徐纯刚1( )
)
- 1.中国科学院广州能源研究所,广东 广州 510640
2.中国科学院大学,北京 100049
3.中国科学技术大学;能源科学与技术学院,安徽 合肥 230026
-
收稿日期:2022-03-03修回日期:2022-04-14出版日期:2022-12-20发布日期:2022-12-29 -
通讯作者:李小森,徐纯刚 -
作者简介:李昊阳(1997—),男,硕士研究生,研究方向为天然气水合物开采及综合技术研发。E-mail:lihy1@ms.giec.ac.cn。 -
基金资助:广东省自然科学基金面上项目(2019A1515011490);2022年省级促进经济高质量发展(海洋经济发展)海洋六大产业专项项目(GDME-2022D043)
Research process of hydrate-based hydrogen storage
LI Haoyang1,2( ), ZHANG Wei1,3, LI Xiaosen1(
), ZHANG Wei1,3, LI Xiaosen1( ), XU Chungang1(
), XU Chungang1( )
)
- 1.Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, Guangdong, China
2.University of Chinese Academy of Sciences, Beijing 100049, China
3.School of Engineering Science, University of Science and Technology of China, Hefei 230026, Anhui, China
-
Received:2022-03-03Revised:2022-04-14Online:2022-12-20Published:2022-12-29 -
Contact:LI Xiaosen, XU Chungang
摘要:
氢作为一种清洁能源,越来越受到人们的重视,氢能利用技术的需求日益迫切。氢能的利用关键挑战在于氢气的储运,促进剂作用下氢气水合物可使氢气在相对温和的温压条件下安全、长期地储存,为储氢提供了一种选择。水合物储氢因其安全环保的特性具有巨大的工业化应用潜力,其目前工业化应用的两个关键问题即为储氢密度与储氢速率。本文首先回顾了氢气水合物的研究历程,阐述了几种常见氢气水合物的相平衡数据,然后归纳了不同晶型氢气水合物的储氢密度,最后总结了物理方法强化与化学方法强化对水合物储氢速率的影响,通过对近年来水合物储氢评估与总结,提出了当前水合物储氢存在的问题与未来研究方向,以期为水合物储气的工业化应用和氢气水合物的研究提供参考。
中图分类号:
引用本文
李昊阳, 张炜, 李小森, 徐纯刚. 水合物储氢的研究进展[J]. 化工进展, 2022, 41(12): 6285-6294.
LI Haoyang, ZHANG Wei, LI Xiaosen, XU Chungang. Research process of hydrate-based hydrogen storage[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6285-6294.
| 晶型 | 孔穴 | 表述 | 孔穴数 | 孔穴直径/Å | 配位数 | 理想表达式 |
|---|---|---|---|---|---|---|
| sI | 小 | 512 | 2 | 3.95 | 20 | 6X·2Y·46H2O |
| 大 | 51262 | 6 | 4.33 | 24 | ||
| sII | 小 | 512 | 16 | 3.91 | 20 | 8X·16Y·136·H2O |
| 大 | 51264 | 8 | 4.73 | 28 | ||
| sH | 小 | 512 | 3 | 3.91 | 20 | 1X·3Y·2Z·34H2O |
| 中 | 435663 | 2 | 4.06 | 20 | ||
| 大 | 51268 | 1 | 5.71 | 36 |
表1 三种水合物结构的有关参数[2]
| 晶型 | 孔穴 | 表述 | 孔穴数 | 孔穴直径/Å | 配位数 | 理想表达式 |
|---|---|---|---|---|---|---|
| sI | 小 | 512 | 2 | 3.95 | 20 | 6X·2Y·46H2O |
| 大 | 51262 | 6 | 4.33 | 24 | ||
| sII | 小 | 512 | 16 | 3.91 | 20 | 8X·16Y·136·H2O |
| 大 | 51264 | 8 | 4.73 | 28 | ||
| sH | 小 | 512 | 3 | 3.91 | 20 | 1X·3Y·2Z·34H2O |
| 中 | 435663 | 2 | 4.06 | 20 | ||
| 大 | 51268 | 1 | 5.71 | 36 |
| 方法 | 存储条件 | 理论储氢密度 (质量分数) | 安全性 | 文献 |
|---|---|---|---|---|
| 高压气态储氢 | 298K,35~70MPa | 5.7%(70MPa) | 危险 | [ |
| 低温液化储氢 | 23K,0.3~0.5MPa | 7.6% | 危险 | [ |
| 固体材料储氢 | 413K,0.001MPa | 4.0%(MgH2) | 安全 | [ |
| 氢气水合物 | 280K,300MPa | 3.77%~4.97%(H2/H2O) | 安全 | [ |
表2 几种储氢方法比较
| 方法 | 存储条件 | 理论储氢密度 (质量分数) | 安全性 | 文献 |
|---|---|---|---|---|
| 高压气态储氢 | 298K,35~70MPa | 5.7%(70MPa) | 危险 | [ |
| 低温液化储氢 | 23K,0.3~0.5MPa | 7.6% | 危险 | [ |
| 固体材料储氢 | 413K,0.001MPa | 4.0%(MgH2) | 安全 | [ |
| 氢气水合物 | 280K,300MPa | 3.77%~4.97%(H2/H2O) | 安全 | [ |
| 文献 | 体系 | 促进剂浓度 (摩尔分数) | 温度 /K | 压力 /MPa |
|---|---|---|---|---|
| Smirnov等[ | H2/H2O | 260 | 200 | |
| 258 | 100 | |||
| 178 | 50 | |||
| Hashimoto等[ | H2/THF | 5.56% | 277.5 | 0.33 |
| 277.6 | 0.55 | |||
| 277.8 | 1.03 | |||
| 278.0 | 1.55 | |||
| 278.2 | 2.13 | |||
| 279.2 | 4.87 | |||
| 280.1 | 8.30 | |||
| 280.8 | 11.3 | |||
| 281.4 | 13.3 | |||
| H2/TBAB | 3.6% | 285.4 | 0.18 | |
| 285.5 | 0.70 | |||
| 285.8 | 1.19 | |||
| 286.1 | 3.27 | |||
| 286.3 | 6.05 | |||
| 286.6 | 7.93 | |||
| 287.2 | 13.4 | |||
| Du等[ | H2/叔丁胺 | 5.56% | 268.4 | 9.54 |
| 269.2 | 12.53 | |||
| 269.9 | 15.09 | |||
| 271.5 | 20.85 | |||
| 273.2 | 26.12 | |||
| 274.0 | 29.95 | |||
| 8.86% | 270.4 | 11.75 | ||
| 271.3 | 15.01 | |||
| 271.8 | 17.14 | |||
| 272.6 | 19.83 | |||
| 273.1 | 21.63 | |||
| 274.0 | 27.72 | |||
| Wang等[ | H2/C3H8 | 33%(体积分数) | 279.05 | 1.60 |
| 278.25 | 1.40 | |||
| 277.84 | 1.20 | |||
| 276.83 | 1.00 | |||
| 275.90 | 0.80 | |||
| 274.71 | 0.60 | |||
| 273.72 | 0.40 |
表3 不同体系下氢气水合物相平衡数据
| 文献 | 体系 | 促进剂浓度 (摩尔分数) | 温度 /K | 压力 /MPa |
|---|---|---|---|---|
| Smirnov等[ | H2/H2O | 260 | 200 | |
| 258 | 100 | |||
| 178 | 50 | |||
| Hashimoto等[ | H2/THF | 5.56% | 277.5 | 0.33 |
| 277.6 | 0.55 | |||
| 277.8 | 1.03 | |||
| 278.0 | 1.55 | |||
| 278.2 | 2.13 | |||
| 279.2 | 4.87 | |||
| 280.1 | 8.30 | |||
| 280.8 | 11.3 | |||
| 281.4 | 13.3 | |||
| H2/TBAB | 3.6% | 285.4 | 0.18 | |
| 285.5 | 0.70 | |||
| 285.8 | 1.19 | |||
| 286.1 | 3.27 | |||
| 286.3 | 6.05 | |||
| 286.6 | 7.93 | |||
| 287.2 | 13.4 | |||
| Du等[ | H2/叔丁胺 | 5.56% | 268.4 | 9.54 |
| 269.2 | 12.53 | |||
| 269.9 | 15.09 | |||
| 271.5 | 20.85 | |||
| 273.2 | 26.12 | |||
| 274.0 | 29.95 | |||
| 8.86% | 270.4 | 11.75 | ||
| 271.3 | 15.01 | |||
| 271.8 | 17.14 | |||
| 272.6 | 19.83 | |||
| 273.1 | 21.63 | |||
| 274.0 | 27.72 | |||
| Wang等[ | H2/C3H8 | 33%(体积分数) | 279.05 | 1.60 |
| 278.25 | 1.40 | |||
| 277.84 | 1.20 | |||
| 276.83 | 1.00 | |||
| 275.90 | 0.80 | |||
| 274.71 | 0.60 | |||
| 273.72 | 0.40 |
晶型 结构 | 体系 | 生成温压条件 | 储氢密度(质量分数) |
|---|---|---|---|
| sI | H2/CO2 | 200MPa,270K | 0.37% |
| sII | 纯水 | 200~300MPa,240~249K | 5.3% |
| sII | H2/THF | 6.5MPa,270K | 1% |
| sH | H2/MTBE | 100MPa,270K | 1.4% |
| sc | H2/TBAB | 16MPa,281.15K | 0.046% |
表4 几种结构的水合物的生成温压条件与储氢密度对比[5,19,28,51,53]
晶型 结构 | 体系 | 生成温压条件 | 储氢密度(质量分数) |
|---|---|---|---|
| sI | H2/CO2 | 200MPa,270K | 0.37% |
| sII | 纯水 | 200~300MPa,240~249K | 5.3% |
| sII | H2/THF | 6.5MPa,270K | 1% |
| sH | H2/MTBE | 100MPa,270K | 1.4% |
| sc | H2/TBAB | 16MPa,281.15K | 0.046% |
| 文献 | 强化方法 | 研究课题 |
|---|---|---|
| 谢应明等[ | 机械搅拌 | 搅拌式强化以TBAB为促进剂储氢 |
| 吕秋楠等[ | 鼓泡 | 研究鼓泡器中水合物的生成动力学 |
| 郝文峰[ | 喷雾 | 利用半连续喷淋式反应釜生成水合物 |
| Zhong等[ | 表面活性剂 | 利用十二烷基硫酸钠(SDS)促进生成水合物 |
| 吴宛青等[ | 表面活性剂 | 研究不同表面活性剂促进水合物生成的动力学 |
| Samad等[ | 纳米粒子 | 研究银纳米颗粒对水合物生成的促进效果 |
| 胡腾等[ | 多孔介质 | 研究石英砂对水合物生成的影响 |
表5 几种动力学强化方法
| 文献 | 强化方法 | 研究课题 |
|---|---|---|
| 谢应明等[ | 机械搅拌 | 搅拌式强化以TBAB为促进剂储氢 |
| 吕秋楠等[ | 鼓泡 | 研究鼓泡器中水合物的生成动力学 |
| 郝文峰[ | 喷雾 | 利用半连续喷淋式反应釜生成水合物 |
| Zhong等[ | 表面活性剂 | 利用十二烷基硫酸钠(SDS)促进生成水合物 |
| 吴宛青等[ | 表面活性剂 | 研究不同表面活性剂促进水合物生成的动力学 |
| Samad等[ | 纳米粒子 | 研究银纳米颗粒对水合物生成的促进效果 |
| 胡腾等[ | 多孔介质 | 研究石英砂对水合物生成的影响 |
| 1 | Carolyn A KOH, SLOAN E Dendy, Amadeu K SUM, et al. Fundamentals and applications of gas hydrates[J]. Annual Review of Chemical and Biomolecular Engineering, 2011, 2: 237-257. |
| 2 | RIPMEESTER John A, John S TSE, RATCLIFFE Christopher I, et al. A new clathrate hydrate structure[J]. Nature, 1987, 325(6100): 135-136. |
| 3 | PARK Youngjune, KIM Do-Youn, LEE Jong-Won, et al. Sequestering carbon dioxide into complex structures of naturally occurring gas hydrates[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(34): 12690-12694. |
| 4 | VELUSWAMY Hari Prakash, KUMAR Asheesh, SEO Yutaek, et al. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates[J]. Applied Energy, 2018, 216: 262-285. |
| 5 | STROBEL Timothy A, HESTER Keith C, Carolyn A KOH, et al. Properties of the clathrates of hydrogen and developments in their applicability for hydrogen storage[J]. Chemical Physics Letters, 2009, 478(4/5/6): 97-109. |
| 6 | YU Yisong, ZHANG Xianwei, LIU Jianwu, et al. Natural gas hydrate resources and hydrate technologies: a review and analysis of the associated energy and global warming challenges[J]. Energy & Environmental Science, 2021, 14(11): 5611-5668. |
| 7 | BABU Ponnivalavan, LINGA Praveen, KUMAR Rajnish, et al. A review of the hydrate based gas separation (HBGS) process for carbon dioxide pre-combustion capture[J]. Energy, 2015, 85: 261-279. |
| 8 | XU Chungang, LI Xiaosen, CAI Jing, et al. Hydrate-based carbon dioxide capture from simulated integrated gasification combined cycle gas[J]. Journal of Natural Gas Chemistry, 2012, 21(5): 501-507. |
| 9 | ZHONG Dongliang, ENGLEZOS Peter. Methane separation from coal mine methane gas by tetra-n-butyl ammonium bromide semiclathrate hydrate formation[J]. Energy & Fuels, 2012, 26(4): 2098-2106. |
| 10 | BABU Ponnivalavan, NAMBIAR Abhishek, CHONG Zheng rong, et al. Hydrate-based desalination (HyDesal) process employing a novel prototype design[J]. Chemical Engineering Science, 2020, 218: 115563. |
| 11 | BABU Ponnivalavan, NAMBIAR Abhishek, HE Tianbiao, et al. A review of clathrate hydrate based desalination to strengthen energy-water nexus[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 8093-8107. |
| 12 | HO-VAN S, BOUILLOT B, DOUZET J, et al. Cyclopentane hydrates—A candidate for desalination? [J]. Journal of Environmental Chemical Engineering, 2019, 7(5): 103359. |
| 13 | ENGLEZOS Peter. Extraction of methane hydrate energy by carbon dioxide injection-key challenges and a paradigm shift[J]. Chinese Journal of Chemical Engineering, 2019, 27(9): 2044-2048. |
| 14 | XU Chungang, YU Yisong, XIE Wenjun, et al. Study on developing a novel continuous separation device and carbon dioxide separation by process of hydrate combined with chemical absorption[J]. Applied Energy, 2019, 255: 113791. |
| 15 | LEE Yohan, KIM Yunju, LEE Jaehyoung, et al. CH4 recovery and CO2 sequestration using flue gas in natural gas hydrates as revealed by a micro-differential scanning calorimeter[J]. Applied Energy, 2015, 150: 120-127. |
| 16 | 曹军文, 覃祥富, 耿嘎, 等. 氢气储运技术的发展现状与展望[J]. 石油学报(石油加工), 2021, 37(6): 1461-1478. |
| CAO Junwen, QIN Xiangfu, GENG Ga, et al. Current status and prospects of hydrogen storage and transportation technology[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2021, 37(6): 1461-1478. | |
| 17 | WOLF Joachim. Liquid-hydrogen technology for vehicles[J]. MRS Bulletin, 2002, 27(9): 684-687. |
| 18 | ZHU Wen, REN Li, LU Chong, et al. Nanoconfined and in situ catalyzed MgH2 self-assembled on 3D Ti3C2 MXene folded nanosheets with enhanced hydrogen sorption performances[J]. ACS Nano, 2021, 15(11): 18494-18504. |
| 19 | MAO Wendy L, MAO Ho Kwang. Hydrogen storage in molecular compounds[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(3): 708-710. |
| 20 | 岳来群. 中国确定“十四五”规划及2035年远景目标 油气企业加快改革创新适应转型发展[J]. 国际石油经济, 2021, 29(1): 51-52. |
| YUE Laiqun. China’s 14th Five-Year Plan and long-term goal for 2035 promote oil and gas enterprises to adapt innovation and transformation[J]. International Petroleum Economics, 2021, 29(1): 51-52. | |
| 21 | ZHANG Ye, BHATTACHARJEE Gaurav, KUMAR Rajnish, et al. Solidified hydrogen storage (solid-HyStore) via clathrate hydrates[J]. Chemical Engineering Journal, 2022, 431: 133702. |
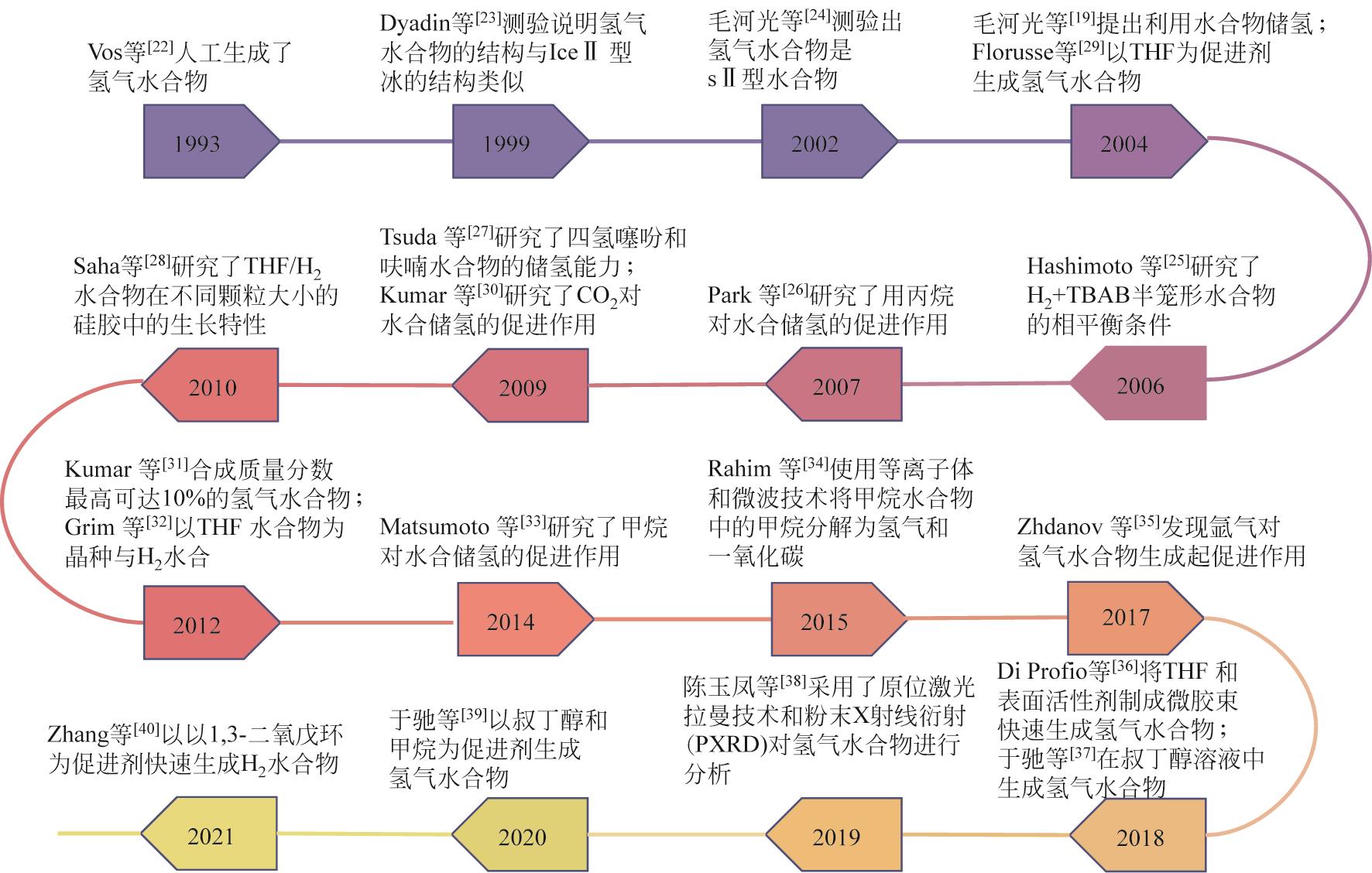
| 22 | VOS W L, FINGER L W, HEMLEY R J, et al. Novel H2-H2O clathrates at high pressures[J]. Physical Review Letters, 1993, 71(19): 3150-3153. |
| 23 | DYADIN Yuri A, LARIONOV Eduard G, MANAKOV Andrei Yu, et al. Clathrate hydrates of hydrogen and neon[J]. Mendeleev Communications, 1999, 9(5): 209-210. |
| 24 | MAO Wendy L, MAO Ho-Kwang, GONCHAROV Alexander F, et al. Hydrogen clusters in clathrate hydrate[J]. Science, 2002, 297(5590): 2247-2249. |
| 25 | HASHIMOTO S, MURAYAMA S, SUGAHARA T, et al. Thermodynamic and Raman spectroscopic studies on H2+tetrahydrofuran+water and H2+tetra-n-butyl ammonium bromide+water mixtures containing gas hydrates[J]. Chemical Engineering Science,2006, 61 (24): 7884-7888. |
| 26 | PARK Jeasung, LEE Huen. Spectroscopic evidences of the double hydrogen hydrates stabilized with ethane and propane[J]. Korean Journal of Chemical Engineering, 2007, 24(4): 624-627. |
| 27 | TSUDA Takaaki, OGATA Kyohei, HASHIMOTO Shunsuke, et al. Storage capacity of hydrogen in tetrahydrothiophene and furan clathrate hydrates[J]. Chemical Engineering Science, 2009, 64(19): 4150-4154. |
| 28 | SAHA Dipendu, DENG Shuguang. Accelerated formation of THF-H2 clathrate hydrate in porous media[J]. Langmuir, 2010, 26(11): 8414-8418. |
| 29 | FLORUSSE Louw J, PETERS Cor J, SCHOONMAN Joop, et al. Stable low-pressure hydrogen clusters stored in a binary clathrate hydrate[J]. Science, 2004, 306(5695): 469-471. |
| 30 | KUMAR Rajnish, ENGLEZOS Peter, MOUDRAKOVSKI Igor. Structure and composition of CO2/H2 and CO2/H2/C3H8 hydrate in relation to simultaneous CO2 capture and H2 production[J]. AIChE Journal, 2009, 55(6): 1584-1594. |
| 31 | KUMAR Rajnish, KLUG Dennis D, RATCLIFFE Christopher I, et al. Low-pressure synthesis and characterization of hydrogen-filled ice Ic[J]. Angewandte Chemie International Edition, 2013, 52(5): 1531-1534. |
| 32 | Gary GRIM R, KERKAR Prasad B, SLOAN E Dendy, et al. Rapid hydrogen hydrate growth from non-stoichiometric tuning mixtures during liquid nitrogen quenching[J]. The Journal of Chemical Physics, 2012, 136(23): 234504. |
| 33 | MATSUMOTO Yuuki, Gary GRIM R, KHAN Naveed M, et al. Investigating the thermodynamic stabilities of hydrogen and methane binary gas hydrates[J]. The Journal of Physical Chemistry C, 2014, 118(7): 3783-3788. |
| 34 | RAHIM Ismail, NOMURA Shinfuku, MUKASA Shinobu, et al. Decomposition of methane hydrate for hydrogen production using microwave and radio frequency in-liquid plasma methods[J]. Applied Thermal Engineering, 2015, 90: 120-126. |
| 35 | ZHDANOV Ravil K, GETS Kirill V, BELOSLUDOV Rodion V, et al. Theoretical modeling of the thermodynamic properties and the phase diagram of binary gas hydrates of argon and hydrogen[J]. Fluid Phase Equilibria, 2017, 434: 87-92. |
| 36 | DI PROFIO Pietro, CANALE Valentino, GERMANI Raimondo, et al. Reverse micelles enhance the formation of clathrate hydrates of hydrogen[J]. Journal of Colloid and Interface Science, 2018, 516: 224-231. |
| 37 | 于驰. 新型水合物复合储氢技术研究[D]. 广州: 华南理工大学, 2018. |
| YU Chi. Study of A novel hydrate-based hybrid hydrogen storage technology[D]. Guangzhou: South China University of Technology, 2018. | |
| 38 | 陈玉凤, 周雪冰, 梁德青, 等. TBAB-CO2水合物形成过程的微观实验[J]. 光谱学与光谱分析, 2019, 39(9): 2889-2893. |
| CHEN Yufeng, ZHOU Xuebing, LIANG Deqing, et al. Microscopic experimental study on the crystallization of TBAB-CO2 hydrate[J]. Spectroscopy and Spectral Analysis, 2019, 39(9): 2889-2893. | |
| 39 | YU Chi, FAN Shuanshi, LANG Xuemei, et al. Hydrogen and chemical energy storage in gas hydrate at mild conditions[J]. International Journal of Hydrogen Energy, 2020, 45(29): 14915-14921. |
| 40 | ZHANG Ye, BHATTACHARJEE Gaurav, ZHENG Junjie, et al. Hydrogen storage as clathrate hydrates in the presence of 1, 3-dioxolane as a dual-function promoter[J]. Chemical Engineering Journal, 2022, 427: 131771. |
| 41 | DAVOODABADI Ali, MAHMOUDI Ashkan, GHASEMI Hadi. The potential of hydrogen hydrate as a future hydrogen storage medium[J]. iScience, 2021, 24(1): 101907. |
| 42 | SMIRNOV Grigory S, STEGAILOV Vladimir V. Toward determination of the new hydrogen hydrate clathrate structures[J]. The Journal of Physical Chemistry Letters, 2013, 4(21): 3560-3564. |
| 43 | DU Jianwei, LIANG Deqing, DAI Xingxue, et al. Hydrate phase equilibrium for the (hydrogen + tert-butylamine + water) system[J]. The Journal of Chemical Thermodynamics, 2011, 43(4): 617-621. |
| 44 | WANG Pengfei, LI Kehan, YANG Jianyu, et al. Experimental and theoretical study on dissociation thermodynamics and kinetics of hydrogen-propane hydrate[J]. Chemical Engineering Journal, 2021, 426: 131279. |
| 45 | Naveed KHAN M, WARRIER Pramod, PETERS Cor J, et al. Advancements in hydrate phase equilibria and modeling of gas hydrates systems[J]. Fluid Phase Equilibria, 2018, 463: 48-61. |
| 46 | KIM Do-Youn, LEE Huen. Spectroscopic identification of the mixed hydrogen and carbon dioxide clathrate hydrate[J]. Journal of the American Chemical Society, 2005, 127(28): 9996-9997. |
| 47 | Gary GRIM R, KERKAR Prasad B, SHEBOWICH Michele, et al. Synthesis and characterization of sI clathrate hydrates containing hydrogen[J]. The Journal of Physical Chemistry C, 2012, 116(34): 18557-18563. |
| 48 | BELOSLUDOV R V, ZHDANOV R K, SUBBOTIN O S, et al. Theoretical modelling of the phase diagrams of clathrate hydrates for hydrogen storage applications[J]. Molecular Simulation, 2012, 38(10): 773-780. |
| 49 | PAPADIMITRIOU N I, TSIMPANOGIANNIS I N, STUBOS A K. Monte Carlo study of sI hydrogen hydrates[J]. Molecular Simulation, 2010, 36(10): 736-744. |
| 50 | DUARTE A R C, SHARIATI A, ROVETTO L J, et al. Water cavities of sH clathrate hydrate stabilized by molecular hydrogen: phase equilibrium measurements[J]. The Journal of Physical Chemistry B, 2008, 112 (7): 1888-1889.. |
| 51 | STROBEL Timothy A, Carolyn A KOH, SLOAN E Dendy. Water cavities of sH clathrate hydrate stabilized by molecular hydrogen[J]. The Journal of Physical Chemistry B, 2008, 112(7): 1885-1887. |
| 52 | RODIONOVA Tatyana V, KOMAROV Vladislav Yu, VILLEVALD Galina V, et al. Calorimetric and structural studies of tetrabutylammonium bromide ionic clathrate hydrates[J]. The Journal of Physical Chemistry B, 2013, 117(36): 10677-10685. |
| 53 | TRUEBA Alondra Torres, RADOVIĆ Ivona R, ZEVENBERGEN John F, et al. Kinetics measurements and in situ Raman spectroscopy of formation of hydrogen-tetrabutylammonium bromide semi-hydrates[J]. International Journal of Hydrogen Energy, 2012, 37(7): 5790-5797. |
| 54 | ANIL Jugal N, BHAWANGIRKAR Dnyaneshwar R, SANGWAI Jitendra S. Effect of guest-dependent reference hydrate vapor pressure in thermodynamic modeling of gas hydrate phase equilibria, with various combinations of equations of state and activity coefficient models[J]. Fluid Phase Equilibria, 2022, 556: 113356. |
| 55 | PATCHKOVSKII Serguei, John S TSE. Thermodynamic stability of hydrogen clathrates[J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(25): 14645-14650. |
| 56 | INERBAEV Talgat M, BELOSLUDOV Vladimir R, BELOSLUDOV Rodion V, et al. Dynamics and equation of state of hydrogen clathrate hydrate as a function of cage occupation[J]. Computational Materials Science, 2006, 36(1/2): 229-233. |
| 57 | PARK Seongmin, Dong Yeun KOH, KANG Hyery, et al. Effect of molecular nitrogen on multiple hydrogen occupancy in clathrate hydrates[J]. The Journal of Physical Chemistry C, 2014, 118(35): 20203-20208. |
| 58 | CAI Jing, XU Chungang, CHEN Zhaoyang, et al. Recovery of methane from coal-bed methane gas mixture via hydrate-based methane separation method by adding anionic surfactants[J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2018, 40(9): 1019-1026. |
| 59 | VELUSWAMY Hari Prakash, LINGA Praveen. Macroscopic kinetics of hydrate formation of mixed hydrates of hydrogen/tetrahydrofuran for hydrogen storage[J]. International Journal of Hydrogen Energy, 2013, 38(11): 4587-4596. |
| 60 | YU Yisong, XU Chungang, LI Xiaosen. Crystal morphology-based kinetic study of carbon dioxide-hydrogen-tetra-n-butyl ammonium bromide hydrates formation in a static system[J]. Energy, 2018, 143: 546-553. |
| 61 | 代梦玲, 孙志高, 李娟, 等. 水合物储气促进技术研究进展[J]. 化工进展, 2020, 39(10): 3975-3986. |
| DAI Mengling, SUN Zhigao, LI Juan, et al. Progress on promotion technology for gas storage in hydrates[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 3975-3986. | |
| 62 | 谢应明, 龚金明, 汤涛, 等. 四丁基溴化铵水合物储存氢气的研究[J]. 石油化工,2012, 41(1): 22-26. |
| XIE Y M, GONG J M, TANG T, et al. Hydrogen storage with tetra-butyl ammonium bromide hydrate[J]. Petrochemical Technology, 2012, 41(1): 22-26. | |
| 63 | 吕秋楠, 宋永臣, 李小森. 鼓泡器中环戊烷-甲烷-盐水体系水合物的生成动力学[J]. 化工进展, 2016, 35(12): 3777-3782. |
| Qiunan LYU, SONG Yongchen, LI Xiaosen. Formation kinetics of cyclopentane-methane hydrate in NaCl solution with a bubbling equipment[J]. Chemical Industry and Engineering Progress, 2016, 35(12): 3777-3782. | |
| 64 | 郝文峰. 天然气水合物生成过程及其反应器特性研究[D]. 大连: 大连理工大学, 2006. |
| HAO Wenfeng. Study on natural gas hydrate formation with different reactors[D]. Dalian: Dalian University of Technology, 2006. | |
| 65 | ZHONG Y, ROGERS R E. Surfactant effects on gas hydrate formation[J]. Chemical Engineering Science, 2000, 55(19): 4175-4187. |
| 66 | 吴宛青, 宋学明, 魏方, 等. 不同表面活性剂对天然气水合物的生成影响实验[J]. 油气储运, 2021, 40(4): 411-416. |
| WU Wanqing, SONG Xueming, WEI Fang, et al. Experiment on effect of different surfactants on formation of gas hydrate[J]. Oil & Gas Storage and Transportation, 2021, 40(4): 411-416. | |
| 67 | ARJANG Samad, MANTEGHIAN Mehrdad, MOHAMMADI Abolfazl. Effect of synthesized silver nanoparticles in promoting methane hydrate formation at 4.7 MPa and 5.7 MPa[J]. Chemical Engineering Research and Design, 2013, 91(6): 1050-1054. |
| 68 | 胡腾. 石英砂、铜丝网存在下四氢呋喃水合物生长过程研究[D]. 广州: 华南理工大学, 2015. |
| HU Teng. Studies of tetrahydrofuran hydrate growth in the presence of quartz sand and cooper wire[D]. Guangzhou: South China University of Technology, 2015. | |
| 69 | 王林军, 邵磊, 张学民, 等. 促进二氧化碳水合物快速生成的方法与机理的研究进展[J]. 中国沼气, 2012, 30(3): 25-29, 33. |
| WNAG Linjun, SHAO Lei, ZHANG Xuemin, et al. Advances on methods promoting the rapid formation of carbon dioxide hydrate and mechanisms[J]. China Biogas, 2012, 30(3): 25-29, 33. | |
| 70 | HE Yan, SUN Mengting, CHEN Chen, et al. Surfactant-based promotion to gas hydrate formation for energy storage[J]. Journal of Materials Chemistry A, 2019, 7(38): 21634-21661. |
| 71 | FEDELE Laura, COLLA Laura, BOBBO Sergio. Viscosity and thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles[J]. International Journal of Refrigeration, 2012, 35(5): 1359-1366. |
| 72 | LIRIO Cláudia Ferreira da Silva, PESSOA Fernando Luiz Pellegrini, ULLER Angela Maria Cohen. Storage capacity of carbon dioxide hydrates in the presence of sodium dodecyl sulfate (SDS) and tetrahydrofuran (THF)[J]. Chemical Engineering Science, 2013, 96: 118-123. |
| 73 | 孙志高, 郭开华. H型天然气水合物形成实验[J]. 天然气工业, 2007, 27(9): 15-16, 126. |
| SUN Zhigao, GUO Kaihua. Experimental study on the formation of structure h hydrate[J]. Natural Gas Industry, 2007, 27(9): 15-16, 126. |
| [1] | 孙旭东, 赵玉莹, 李诗睿, 王琦, 李晓健, 张博. 我国地方性氢能发展政策的文本量化分析[J]. 化工进展, 2023, 42(7): 3478-3488. |
| [2] | 张巍, 王锐, 缪平, 田戈. 全球可再生能源电转甲烷的应用[J]. 化工进展, 2023, 42(3): 1257-1269. |
| [3] | 孙晖, 孟祥海, 魏景海, 周红军, 徐春明. 绿电制氢生产氨的新场景与实践[J]. 化工进展, 2023, 42(2): 1098-1102. |
| [4] | 王红霞, 徐婉怡, 张早校. 可再生电力电解制绿色氢能的发展现状与建议[J]. 化工进展, 2022, 41(S1): 118-131. |
| [5] | 周红军, 周颖, 徐春明. 中国碳中和目标下CO2转化的思考与实践[J]. 化工进展, 2022, 41(6): 3381-3385. |
| [6] | 周红军, 周颖, 徐春明. 中国碳达峰碳中和目标下炼化一体化新路径与实践[J]. 化工进展, 2022, 41(4): 2226-2230. |
| [7] | 王集杰, 韩哲, 陈思宇, 汤驰洲, 沙峰, 唐珊, 姚婷婷, 李灿. 太阳燃料甲醇合成[J]. 化工进展, 2022, 41(3): 1309-1317. |
| [8] | 陈伟锋, 尚娟, 邢百汇, 魏皓天, 顾超华, 花争立. 关于天然气管网安全掺氢比10%的商榷[J]. 化工进展, 2022, 41(3): 1487-1493. |
| [9] | 施文博, 蔡淳名, 李德威, 小野圭, 张剑波. ISO/IEC、美日中氢能技术标准化体系比较与建议[J]. 化工进展, 2022, 41(12): 6275-6284. |
| [10] | 张振扬, 妙丛, 王峰, 兰玉岐, 安刚, 杨申音. 规模化氢液化装置现状及未来技术路线分析[J]. 化工进展, 2022, 41(12): 6261-6274. |
| [11] | 张学民, 张梦军, 杨惠结, 李银辉, 李金平, 王英梅. 冰点以下多孔介质中气体水合物的生成动力学研究进展[J]. 化工进展, 2021, 40(S2): 101-108. |
| [12] | 岳国君, 林海龙, 彭元亭, 闵剑, 王梦, 熊强. 以生物质为原料的未来绿色氢能[J]. 化工进展, 2021, 40(8): 4678-4684. |
| [13] | 高佳佳, 米媛媛, 周洋, 周红军, 徐泉. 新型储氢材料研究进展[J]. 化工进展, 2021, 40(6): 2962-2971. |
| [14] | 郭博文, 罗聃, 周红军. 可再生能源电解制氢技术及催化剂的研究进展[J]. 化工进展, 2021, 40(6): 2933-2951. |
| [15] | 陈宏, 吴军, 陈晨, 涂智, 禹丽娥, 杨恩喆, 杨敏, 肖本益. 有机废弃物厌氧共发酵制氢研究进展[J]. 化工进展, 2021, 40(1): 440-450. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||