化工进展 ›› 2022, Vol. 41 ›› Issue (8): 4288-4302.DOI: 10.16085/j.issn.1000-6613.2021-2037
基于MOFs材料的低碳烃(C1~C3)分离研究进展
张雨珂1,2( ), 刘倩1,2, 段媛媛1,2, 赵英杰1,2, 崔阳1,2, 史利娟2,3,4, 李向远4, 李剑川4, 范海明5, 易群2,3(
), 刘倩1,2, 段媛媛1,2, 赵英杰1,2, 崔阳1,2, 史利娟2,3,4, 李向远4, 李剑川4, 范海明5, 易群2,3( )
)
- 1.太原理工大学省部共建煤基能源清洁高效利用国家重点实验室,山西 太原 030024
2.太原理工大学环境科学与工程学院,山西 太原 030024
3.武汉工程大学化工与制药学院,湖北 武汉 430205
4.天脊煤化工集团股份有限公司,山西 长治 046000
5.中国石油大学(华东),山东省油田化学重点实验室,山东 青岛 266580
-
收稿日期:2021-09-28修回日期:2021-11-12出版日期:2022-08-25发布日期:2022-08-22 -
通讯作者:易群 -
作者简介:张雨珂(1995—),女,博士研究生。E-mail:zhangyuke0145@link.tyut.edu.cn 。 -
基金资助:国家自然科学基金(U1810125);山西省自然科学基金面上基金(201901D111082);山西省科技计划揭榜招标项目(20191102002);山东省油田化学重点实验室开放基金;中央高校基本科研业务费专项(19CX05006A)
Research progress of low-carbon hydrocarbon(C1~C3) separation based on MOFs
ZHANG Yuke1,2( ), LIU Qian1,2, DUAN Yuanyuan1,2, ZHAO Yingjie1,2, CUI Yang1,2, SHI Lijuan2,3,4, LI Xiangyuan4, LI Jianchuan4, FAN Haiming5, YI Qun2,3(
), LIU Qian1,2, DUAN Yuanyuan1,2, ZHAO Yingjie1,2, CUI Yang1,2, SHI Lijuan2,3,4, LI Xiangyuan4, LI Jianchuan4, FAN Haiming5, YI Qun2,3( )
)
- 1.State Key Laboratory of Clean and Efficient Utilization of Coal-based Energy, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
2.College of Environmental Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
3.School of Chemical Engineering and Pharmacy, Wuhan Institute of Technology, Wuhan 430205, Hubei, China
4.Tianji Coal Chemical Group Company Limited, Changzhi 046000, Shanxi, China
5.Shandong Key Laboratory of Oilfield Chemistry, China University of Petroleum-East China, Qingdao 266580, Shangdong, China
-
Received:2021-09-28Revised:2021-11-12Online:2022-08-25Published:2022-08-22 -
Contact:YI Qun
摘要:
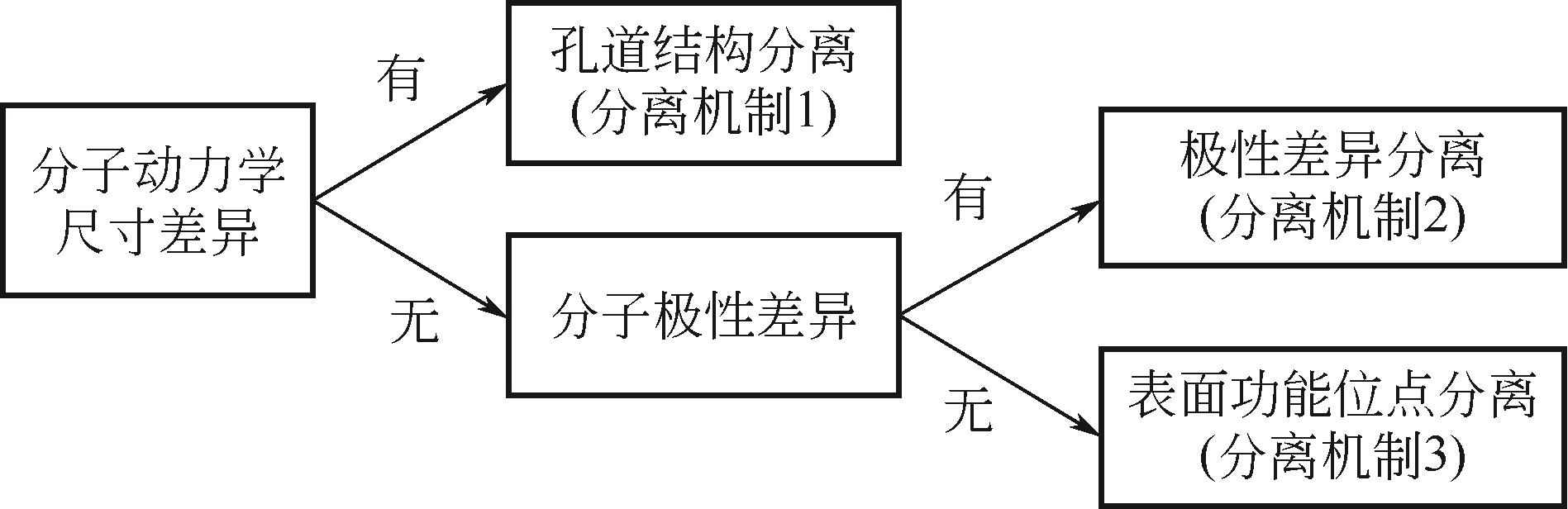
低碳烃(C1~C3)混合物的分离和纯化是化工过程中最重要且耗能最大的过程单元之一,开发温和条件下低能耗高选择性吸附分离C1~C3分子的固体吸附材料迫在眉睫。金属有机框架材料(MOFs)作为一类相对新颖的多孔有机-无机杂化材料,因其可控的拓扑结构和多样的化学微环境,在低碳烃分离和纯化领域受到广泛关注。本文概述了MOFs作为分离和纯化低碳烃气体吸附剂的特性,重点关注了MOFs材料在C1(CO2/CH4)、C2、C3烯/烷烃以及烯/炔烃分离领域的应用进展。首先归纳了MOFs材料在C1~C3烃类物质分离过程中的三种常见分离机制,并据此回顾了近年来MOFs材料对常见C1~C3烃类分子的吸附及分离性能;分析了MOFs材料在C1~C3烃类物质分离过程中的构效关系,总结了MOFs材料的孔道尺寸/形状、骨架柔性和表面功能的调控理念与方法,并提出MOFs材料成本高、水热稳定性差、主客体关系难以精准探测等制约其应用发展的现状。文章指出未来研究重点为开发低成本多样化专一性的新型配体,构造复合型吸附剂,并明确吸附分离过程中分离体系主客体性质,为MOFs材料用于低碳烃分离的定向设计提供了探索方向。
中图分类号:
引用本文
张雨珂, 刘倩, 段媛媛, 赵英杰, 崔阳, 史利娟, 李向远, 李剑川, 范海明, 易群. 基于MOFs材料的低碳烃(C1~C3)分离研究进展[J]. 化工进展, 2022, 41(8): 4288-4302.
ZHANG Yuke, LIU Qian, DUAN Yuanyuan, ZHAO Yingjie, CUI Yang, SHI Lijuan, LI Xiangyuan, LI Jianchuan, FAN Haiming, YI Qun. Research progress of low-carbon hydrocarbon(C1~C3) separation based on MOFs[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4288-4302.
| 类型 | 分子大小/? | 极性 | 极化率/10-25cm3 | 偶极矩/10-18esu·cm | 分子结构模型 |
|---|---|---|---|---|---|
| C1 | |||||
| CO2 | 3.3 | 无 | 29.1 | 0 |  |
| CH4 | 3.8 | 无 | 25.9 | 0 |  |
| C2 | |||||
| C2H6 | 4.4 | 无 | 44.3~44.7 | 0.0995 |  |
| C2H4 | 4.2(3.28×4.18×4.84) | 无 | 42.5 | 0.0866 |  |
| C2H2 | 3.3(3.32×3.34×5.70) | 无 | — | 0.178 |  |
| C3 | |||||
| C3H8 | 4.3~5.1 | 有 | 62.9~63.7 | 0.084 |  |
| C3H6 | 4.7 | 有 | 62.6 | 0.366 |  |
| C3H4 | 4.2(4.16×4.01×6.51) | 有 | 51 | 0.781 |  |
表1 C1~C3分子大小与极性对比
| 类型 | 分子大小/? | 极性 | 极化率/10-25cm3 | 偶极矩/10-18esu·cm | 分子结构模型 |
|---|---|---|---|---|---|
| C1 | |||||
| CO2 | 3.3 | 无 | 29.1 | 0 |  |
| CH4 | 3.8 | 无 | 25.9 | 0 |  |
| C2 | |||||
| C2H6 | 4.4 | 无 | 44.3~44.7 | 0.0995 |  |
| C2H4 | 4.2(3.28×4.18×4.84) | 无 | 42.5 | 0.0866 |  |
| C2H2 | 3.3(3.32×3.34×5.70) | 无 | — | 0.178 |  |
| C3 | |||||
| C3H8 | 4.3~5.1 | 有 | 62.9~63.7 | 0.084 |  |
| C3H6 | 4.7 | 有 | 62.6 | 0.366 |  |
| C3H4 | 4.2(4.16×4.01×6.51) | 有 | 51 | 0.781 |  |
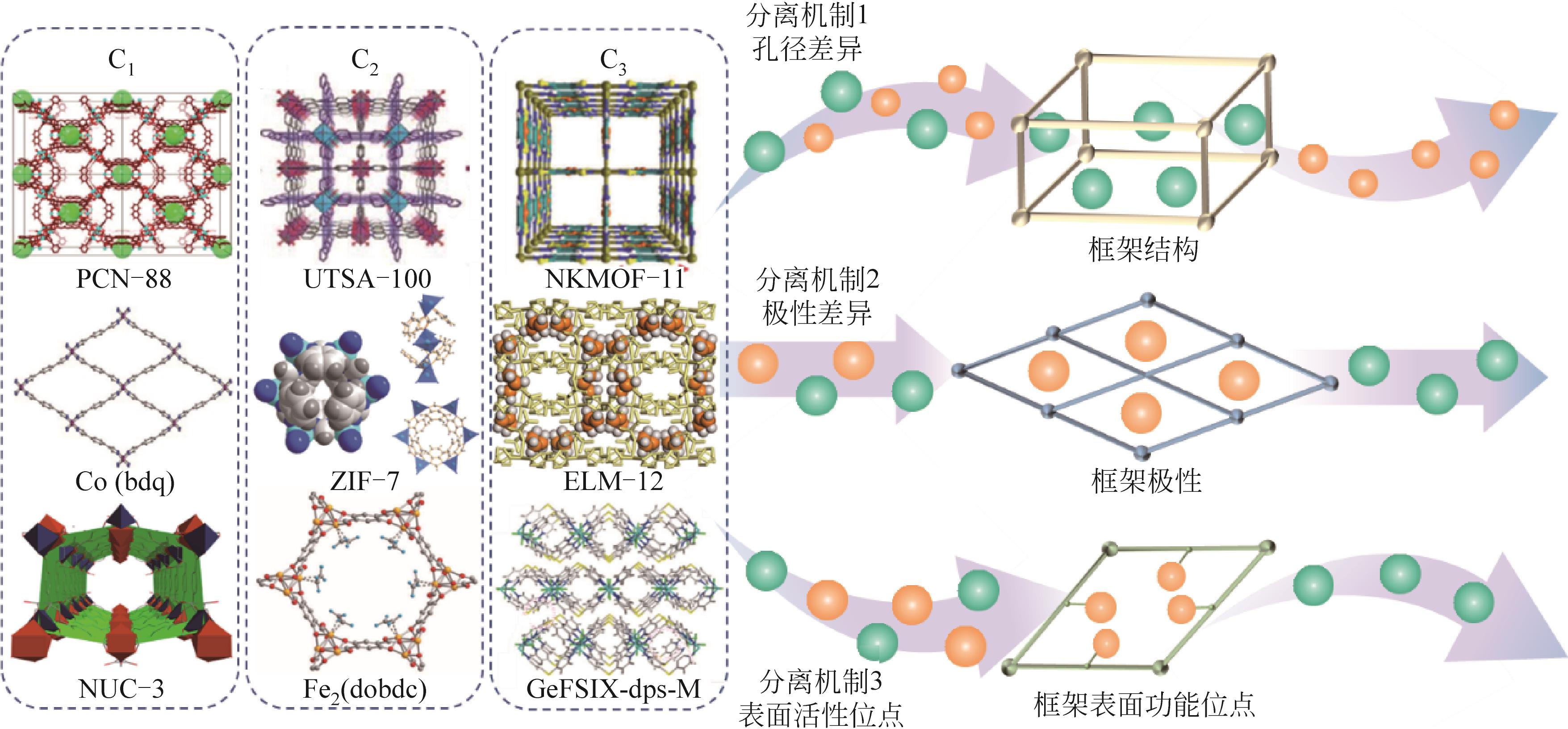
| MOFs | 合成方法 | 分离对象 | 分离效果 | 分离机制 | 稳定性 |
|---|---|---|---|---|---|
| MIL-53(Al)[ | 水热合成法 | CO2(CH4) | α①=7(5bar,303K) | CO2和CH4的不同吸附强度 | — |
| UTSA-16[ | 溶剂热合成法 | CO2(CH4) | IAST②≈50(296K,1bar) | 孔隙笼和末端的水分子与CO2分子之间的相互作用 | 水稳定性好,暴露在空气中3天,仍然保持相同的CO2吸附和解吸能力 |
| SIFSIX-3-Zn[ | 扩散法 | CO2(CH4) | CO2为112mg·g-1(298K,1bar);α=8.8 | 通过晶体工程和网状化学方法精确控制孔径的大小;SiF | 水稳定性:在相对高的湿度下SIFSIX-2-Cu-i结构不变,而SIFSIX-3-Zn经历可逆相变 |
| Co(bdp)[ | 溶剂热合成法 | CH4(CO2) | CO2/CH4(46∶54、42∶58、43∶57),S③=100%;CO2/CH4(6∶94),S③=100% | 通过柔性控制其1D孔径的收缩与扩张 | — |
| ZIF-7x-8[ | 快速电流驱动法 | CO2(CH4) | αi/j④=25 | 采用混合配体,“刚性和缩孔”同步进行的双策略 | 180h长期分离连续循环运行(398K) |
| NUC-3[ | 水(溶剂)热合成法 | CO2(CH4) | IAST=28.7(298K) | 功能性内表面 | 框架在663K之前保持稳定,表明NUC-3具有极高的热稳定性。在48h水中处理后没有结构坍塌 |
表2 C1吸附分离剂与分离效果
| MOFs | 合成方法 | 分离对象 | 分离效果 | 分离机制 | 稳定性 |
|---|---|---|---|---|---|
| MIL-53(Al)[ | 水热合成法 | CO2(CH4) | α①=7(5bar,303K) | CO2和CH4的不同吸附强度 | — |
| UTSA-16[ | 溶剂热合成法 | CO2(CH4) | IAST②≈50(296K,1bar) | 孔隙笼和末端的水分子与CO2分子之间的相互作用 | 水稳定性好,暴露在空气中3天,仍然保持相同的CO2吸附和解吸能力 |
| SIFSIX-3-Zn[ | 扩散法 | CO2(CH4) | CO2为112mg·g-1(298K,1bar);α=8.8 | 通过晶体工程和网状化学方法精确控制孔径的大小;SiF | 水稳定性:在相对高的湿度下SIFSIX-2-Cu-i结构不变,而SIFSIX-3-Zn经历可逆相变 |
| Co(bdp)[ | 溶剂热合成法 | CH4(CO2) | CO2/CH4(46∶54、42∶58、43∶57),S③=100%;CO2/CH4(6∶94),S③=100% | 通过柔性控制其1D孔径的收缩与扩张 | — |
| ZIF-7x-8[ | 快速电流驱动法 | CO2(CH4) | αi/j④=25 | 采用混合配体,“刚性和缩孔”同步进行的双策略 | 180h长期分离连续循环运行(398K) |
| NUC-3[ | 水(溶剂)热合成法 | CO2(CH4) | IAST=28.7(298K) | 功能性内表面 | 框架在663K之前保持稳定,表明NUC-3具有极高的热稳定性。在48h水中处理后没有结构坍塌 |
| MOFs | 合成方法 | 分离对象 | 分离效果 | 分离机制 | 稳定性 |
|---|---|---|---|---|---|
| ZIF-7[ | 水热合成法 | C2H6(C2H4) | — | 客体分子和ZIF-7中苯并咪唑配体之间的相互作用决定了烷烯烃的开门压力的不同 | — |
| RPM3-Zn[ | 溶剂热合成法 | C2H6(C2H4) | C2H6为15cm3·g-1(273K,0.6atm) | C2H6中—CH3与2D层中存在的骨架单齿羧酸酯基发生非特异性相互作用,形成了弱氢键 | — |
| Fe-MOF-74[ | 微波辅助法 | C2H4(C2H6) | αi,j①=11.1;IAST②=2.1 | 灵活的开放Fe2+金属特性 | — |
| NOTT-300[ | 水热合成法 | C2H4(C2H6) | IAST=48.7 | 具有软官能团和Al(Ⅲ)处的饱和配位点 | 对水和其他有机蒸气表现出优异的结构稳定性,并且在再生时保留孔隙率 |
| MAF-49[ | 水热合成法 | C2H6(C2H4) | C2H6为35cm3·g-1 C2H4为15cm3·g-1(316K,0.01bar) | 多个氢键受体和位于孔道表面偶极排斥基团的协同作用 | — |
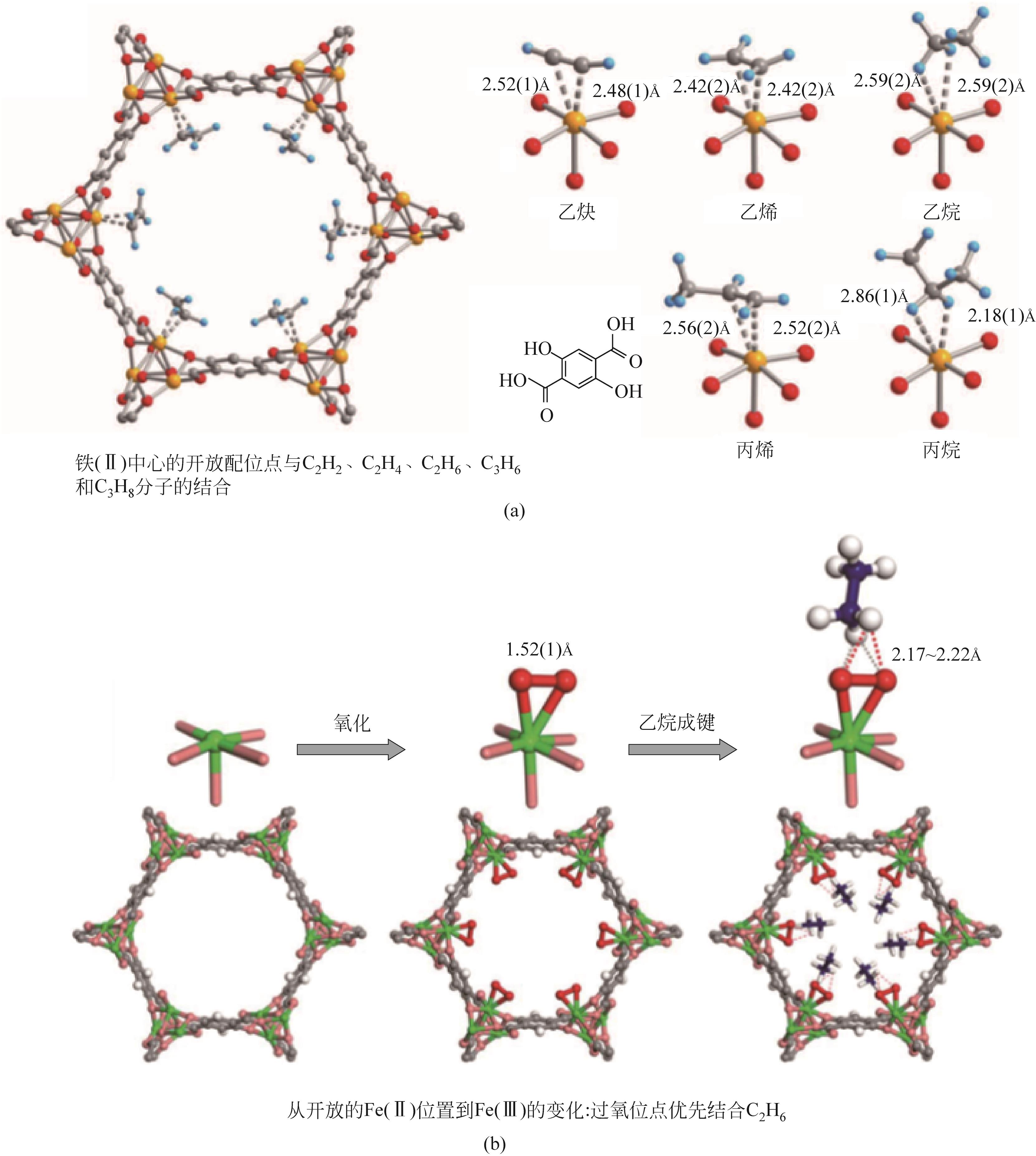
| Fe2(O2)(dobdc)[ | 普通溶液法 | C2H6(C2H4) | IAST②=4.1 | Fe过氧位点与C2H6更好地结合 | 需在惰性条件下进行特殊处理,但在突破循环测试后仍保持其稳定性 |
| MUF-15[ | 水热合成法 | C2H6(C2H4) | C2H6为4.69mmol·g-1(298K,1bar) | 孔隙尺寸诱导了客体和骨架表面之间的范德华相互作用 | 673K以上氮气条件下分解;环境温度下可稳定暴露于大气(约80%湿度)至少1周 |
| Mg2V-bdc-tpt[ | 溶剂热合成法 | C2H6(C2H4) | C2H6为7.45mmol·g-1 C2H4为1.73mmol·g-1 | C2H6分子的孔隙空间中密度分布非常分散 | 热稳定性:高达723K;水稳定性:在水中浸泡24h后仍能保持其高结晶度 |
| Fe2(m-dobdc)[ | 溶剂热合成法 | C2H6(C2H4) | IAST=44 C2H4为7mmol·g-1 | 烯烃与材料中高浓度存在的配位不饱和金属位点结合 | 温和条件再生 |
| M-gallate[ | 溶剂热合成法 | C2H6(C2H4) | IAST=52 C2H4为3.37mmol·g-1 | 合适的孔径尺寸在乙烯和乙烷的截面尺寸最小交叉范围内,提供了乙烯相对于乙烷的高选择性 | 对水蒸气高稳定性 |
| MMOF-3[ | 溶剂热合成法 | C2H2(C2H4) | α=5.23(295K,100kPa) | 调整微孔并固定功能位点,特异性识别,从而分离小分子 | — |
| UTSA-100[ | 溶剂热合成法 | C2H2(C2H4) | IAST=10.1 (296K,100kPa) | Z字形纳米通道以及氨基和四唑功能化的内壁,与酸性更强的C2H2分子之间的酸碱作用 | — |
| SIFSIX-2-Cu-i[ | 普通溶液法 | C2H2(C2H4) | IAST=39.7 (298K,100kPa) | 有机配体的尺寸调整孔道大小的调控,形成双重互穿结构;一个C2H2分子可以通过协作的C—H···F键同时被来自不同网络的两个F原子同时键合 | 298K连续吸附分离160min |
| UTSA-300[ | 普通溶液法 | C2H2(C2H4) | C2H2为76.5cm3·g-1 (S③>99%,273K,1bar) | 利用具有强结合位点和动态孔结构的尺寸匹配的孔来结合目标分子进行高选择性气体分离;框架内的各向异性吸附位点仅允许C2H2通过,形成强的主客体相互作用来打开孔结构 | 空气中稳定存在 |
| UTSA-220[ | 普通溶液法 | C2H2(C2H4) | IAST②=10 C2H2为3.4mmol·g-1 | Cu原子横向与四个氮原子配位,在轴向与四个双齿配体配位,双向互穿的三维网络,得到3.0?×3.2?和4.0?×6.5?两种孔径,同时利用H原子和F原子之间的相互作用 | — |
| ZJU-74a[ | 普通溶液法 | C2H2(C2H4) | IAST=24.2 | 利用两个金属Ni2+与C C的相互作用、H原子与[Ni(CN)4]-之间的协同作用 C的相互作用、H原子与[Ni(CN)4]-之间的协同作用 | 暴露在不同的化学环境中(水、沸水、pH介于1和12之间的水溶液、6mol/L HCl和18mol/L H2SO4)3天稳定存在;573K仍然表现出优异的热稳定性,且不发生相变 |
| ZU-62-Ni[ | 柱制备法 | C2H2(C2H4) | C2H2为3mmol·g-1 IAST=37.2 | 吡啶环的旋转,使其具有合适孔径,从而限制C2H4的进入 | 对湿度和水均表现出很高的耐受性 |
| ATC-Cu[ | 溶剂热合成法 | C2H2(CO2) | C2H2为2.54mmol·g-1 IAST=53.6 | 超强C2H2纳米陷阱可有效捕获C2H2分子 | — |
表3 MOFs基C2吸附分离剂与分离效果
| MOFs | 合成方法 | 分离对象 | 分离效果 | 分离机制 | 稳定性 |
|---|---|---|---|---|---|
| ZIF-7[ | 水热合成法 | C2H6(C2H4) | — | 客体分子和ZIF-7中苯并咪唑配体之间的相互作用决定了烷烯烃的开门压力的不同 | — |
| RPM3-Zn[ | 溶剂热合成法 | C2H6(C2H4) | C2H6为15cm3·g-1(273K,0.6atm) | C2H6中—CH3与2D层中存在的骨架单齿羧酸酯基发生非特异性相互作用,形成了弱氢键 | — |
| Fe-MOF-74[ | 微波辅助法 | C2H4(C2H6) | αi,j①=11.1;IAST②=2.1 | 灵活的开放Fe2+金属特性 | — |
| NOTT-300[ | 水热合成法 | C2H4(C2H6) | IAST=48.7 | 具有软官能团和Al(Ⅲ)处的饱和配位点 | 对水和其他有机蒸气表现出优异的结构稳定性,并且在再生时保留孔隙率 |
| MAF-49[ | 水热合成法 | C2H6(C2H4) | C2H6为35cm3·g-1 C2H4为15cm3·g-1(316K,0.01bar) | 多个氢键受体和位于孔道表面偶极排斥基团的协同作用 | — |
| Fe2(O2)(dobdc)[ | 普通溶液法 | C2H6(C2H4) | IAST②=4.1 | Fe过氧位点与C2H6更好地结合 | 需在惰性条件下进行特殊处理,但在突破循环测试后仍保持其稳定性 |
| MUF-15[ | 水热合成法 | C2H6(C2H4) | C2H6为4.69mmol·g-1(298K,1bar) | 孔隙尺寸诱导了客体和骨架表面之间的范德华相互作用 | 673K以上氮气条件下分解;环境温度下可稳定暴露于大气(约80%湿度)至少1周 |
| Mg2V-bdc-tpt[ | 溶剂热合成法 | C2H6(C2H4) | C2H6为7.45mmol·g-1 C2H4为1.73mmol·g-1 | C2H6分子的孔隙空间中密度分布非常分散 | 热稳定性:高达723K;水稳定性:在水中浸泡24h后仍能保持其高结晶度 |
| Fe2(m-dobdc)[ | 溶剂热合成法 | C2H6(C2H4) | IAST=44 C2H4为7mmol·g-1 | 烯烃与材料中高浓度存在的配位不饱和金属位点结合 | 温和条件再生 |
| M-gallate[ | 溶剂热合成法 | C2H6(C2H4) | IAST=52 C2H4为3.37mmol·g-1 | 合适的孔径尺寸在乙烯和乙烷的截面尺寸最小交叉范围内,提供了乙烯相对于乙烷的高选择性 | 对水蒸气高稳定性 |
| MMOF-3[ | 溶剂热合成法 | C2H2(C2H4) | α=5.23(295K,100kPa) | 调整微孔并固定功能位点,特异性识别,从而分离小分子 | — |
| UTSA-100[ | 溶剂热合成法 | C2H2(C2H4) | IAST=10.1 (296K,100kPa) | Z字形纳米通道以及氨基和四唑功能化的内壁,与酸性更强的C2H2分子之间的酸碱作用 | — |
| SIFSIX-2-Cu-i[ | 普通溶液法 | C2H2(C2H4) | IAST=39.7 (298K,100kPa) | 有机配体的尺寸调整孔道大小的调控,形成双重互穿结构;一个C2H2分子可以通过协作的C—H···F键同时被来自不同网络的两个F原子同时键合 | 298K连续吸附分离160min |
| UTSA-300[ | 普通溶液法 | C2H2(C2H4) | C2H2为76.5cm3·g-1 (S③>99%,273K,1bar) | 利用具有强结合位点和动态孔结构的尺寸匹配的孔来结合目标分子进行高选择性气体分离;框架内的各向异性吸附位点仅允许C2H2通过,形成强的主客体相互作用来打开孔结构 | 空气中稳定存在 |
| UTSA-220[ | 普通溶液法 | C2H2(C2H4) | IAST②=10 C2H2为3.4mmol·g-1 | Cu原子横向与四个氮原子配位,在轴向与四个双齿配体配位,双向互穿的三维网络,得到3.0?×3.2?和4.0?×6.5?两种孔径,同时利用H原子和F原子之间的相互作用 | — |
| ZJU-74a[ | 普通溶液法 | C2H2(C2H4) | IAST=24.2 | 利用两个金属Ni2+与C C的相互作用、H原子与[Ni(CN)4]-之间的协同作用 C的相互作用、H原子与[Ni(CN)4]-之间的协同作用 | 暴露在不同的化学环境中(水、沸水、pH介于1和12之间的水溶液、6mol/L HCl和18mol/L H2SO4)3天稳定存在;573K仍然表现出优异的热稳定性,且不发生相变 |
| ZU-62-Ni[ | 柱制备法 | C2H2(C2H4) | C2H2为3mmol·g-1 IAST=37.2 | 吡啶环的旋转,使其具有合适孔径,从而限制C2H4的进入 | 对湿度和水均表现出很高的耐受性 |
| ATC-Cu[ | 溶剂热合成法 | C2H2(CO2) | C2H2为2.54mmol·g-1 IAST=53.6 | 超强C2H2纳米陷阱可有效捕获C2H2分子 | — |
| MOFs | 合成方法 | 分离对象 | 分离效果 | 分离机制 | 稳定性 |
|---|---|---|---|---|---|
| ZIFs[ | 溶剂热合成法 | C3H6(C3H8) | — | 通过扩散速率的差异实现分离 | — |
| MIL-100(Fe)[ | 水热合成法 | C3H6(C3H8) | α①=28.9 | 配位不饱和金属位点(CUS) | 在523K之前完全稳定 |
| Co-MOF-74[ | 溶剂热合成法 | C3H6(C3H8) | IAST②≈46 | Co2+对C3H6的强大吸附作用 | — |
| KAUST-7[ | 水热合成法 | C3H8(C3H6) | C3H8为0.6mol·kg-1(298K,1bar) | 材料孔尺寸的精确控制 | 在材料暴露于H2O或H2S中,未观察到结晶度损失和相变。在水溶液中浸泡6个月以上材料的性能没有改变。热稳定性:298K,1bar循环10次 |
| ELM-12[ | 普通溶液法 | C3H6(C3H8) | C3H6为62mg·g-1 C3H8为60mg·g-1 IAST=83(298K,1bar) | 曲折的2D通道和合适的 孔径 | 环境条件下储存3年以上可以保持其结构完整性和气体吸收能力 |
| Cu-MOF-74[ | 水热合成法 | C3H6(C3H8) | IAST②=12.7(7.66mmol·g-1) | 开放金属位点,C3H6表现出比C3H8高的亲和力 | — |
| ZU-62[ | 柱制备法 | C3H6(C3H4) | C3H4为1.87mmol·g-1 IAST=48(298K,1bar) | 特殊的非对称配位几何结构(O/F)以及互穿结构使得在0.2~0.5?尺度上可进行孔径微调,从而成功构建窄分布的多个结合位点 | 可稳定暴露于水中一周;高达230℃表现出良好的热稳定性 |
| UTSA-200[ | 溶剂热合成法 | C3H4(C3H6) | C3H6为95cm3·g-1 IAST>20000(298K,0.01bar) | 孔内吡啶环的旋转有效地阻止较大的C3H6分子进入,而孔洞中暴露的SiF | — |
| NKMOF-11[ | 普通溶液法 | C3H4(C3H6) | C3H4为1.78mmol·g-1 IAST=1074(298K,0.01bar) | 硫醇基团的氢键(HC C—CH3···S)的作用和吡嗪基团与C3H4中C C—CH3···S)的作用和吡嗪基团与C3H4中C C的π-π相互作用 C的π-π相互作用 | 具有水稳定性,在循环5次中穿透曲线几乎重叠并且保留了NKMOF-11的结晶度 |
表4 C3吸附分离剂与分离效果
| MOFs | 合成方法 | 分离对象 | 分离效果 | 分离机制 | 稳定性 |
|---|---|---|---|---|---|
| ZIFs[ | 溶剂热合成法 | C3H6(C3H8) | — | 通过扩散速率的差异实现分离 | — |
| MIL-100(Fe)[ | 水热合成法 | C3H6(C3H8) | α①=28.9 | 配位不饱和金属位点(CUS) | 在523K之前完全稳定 |
| Co-MOF-74[ | 溶剂热合成法 | C3H6(C3H8) | IAST②≈46 | Co2+对C3H6的强大吸附作用 | — |
| KAUST-7[ | 水热合成法 | C3H8(C3H6) | C3H8为0.6mol·kg-1(298K,1bar) | 材料孔尺寸的精确控制 | 在材料暴露于H2O或H2S中,未观察到结晶度损失和相变。在水溶液中浸泡6个月以上材料的性能没有改变。热稳定性:298K,1bar循环10次 |
| ELM-12[ | 普通溶液法 | C3H6(C3H8) | C3H6为62mg·g-1 C3H8为60mg·g-1 IAST=83(298K,1bar) | 曲折的2D通道和合适的 孔径 | 环境条件下储存3年以上可以保持其结构完整性和气体吸收能力 |
| Cu-MOF-74[ | 水热合成法 | C3H6(C3H8) | IAST②=12.7(7.66mmol·g-1) | 开放金属位点,C3H6表现出比C3H8高的亲和力 | — |
| ZU-62[ | 柱制备法 | C3H6(C3H4) | C3H4为1.87mmol·g-1 IAST=48(298K,1bar) | 特殊的非对称配位几何结构(O/F)以及互穿结构使得在0.2~0.5?尺度上可进行孔径微调,从而成功构建窄分布的多个结合位点 | 可稳定暴露于水中一周;高达230℃表现出良好的热稳定性 |
| UTSA-200[ | 溶剂热合成法 | C3H4(C3H6) | C3H6为95cm3·g-1 IAST>20000(298K,0.01bar) | 孔内吡啶环的旋转有效地阻止较大的C3H6分子进入,而孔洞中暴露的SiF | — |
| NKMOF-11[ | 普通溶液法 | C3H4(C3H6) | C3H4为1.78mmol·g-1 IAST=1074(298K,0.01bar) | 硫醇基团的氢键(HC C—CH3···S)的作用和吡嗪基团与C3H4中C C—CH3···S)的作用和吡嗪基团与C3H4中C C的π-π相互作用 C的π-π相互作用 | 具有水稳定性,在循环5次中穿透曲线几乎重叠并且保留了NKMOF-11的结晶度 |
| 1 | FU M Y, WANG Y T, WANG X K, et al. Metal-organic framework materials for light hydrocarbon separation[J]. ChemPlusChem, 2021, 86(3): 387-395. |
| 2 | 王皓, 王建国. MTO烯烃分离回收技术与烯烃转化技术[J]. 煤化工, 2011, 39(2): 5-8. |
| WANG Hao, WANG Jianguo. MTO recovery technology and OCT integration help maximize MTO plant profit[J]. Coal Chemical Industry, 2011, 39(2): 5-8. | |
| 3 | SPEIGHT J G. Sources of hydrocarbons[M]//Handbook of Industrial Hydrocarbon Processes. Amsterdam: Elsevier, 2011: 43-83. |
| 4 | MIDDLETON R S, GUPTA R, HYMAN J D, et al. The shale gas revolution: barriers, sustainability, and emerging opportunities[J]. Applied Energy, 2017, 199: 88-95. |
| 5 | LI X Y, PEI C L, GONG J L. Shale gas revolution: catalytic conversion of C1—C3 light alkanes to value-added chemicals[J]. Chem, 2021, 7(7): 1755-1801. |
| 6 | YUAN Y N, WANG T J, LI Q X. Production of low-carbon light olefins from catalytic cracking of crude bio-oil[J]. Chinese Journal of Chemical Physics, 2013, 26(2): 237-244. |
| 7 | LI J R, KUPPLER R J, ZHOU H C. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477. |
| 8 | ZHANG L H, LIU J B, LI X G, et al. Separations of different binary hydrocarbon mixtures using pressure swing thermally coupled distillation process[J]. Separation Science and Technology, 2015, 50(1): 148-157. |
| 9 | BENALI M, AYDIN B. Ethane/ethylene and propane/propylene separation in hybrid membrane distillation systems: optimization and economic analysis[J]. Separation and Purification Technology, 2010, 73(3): 377-390. |
| 10 | TIMOSHENKO A, ANOKHINA E, AKHAPKINA O. Energy-saving hydrocarbon distillation with coupled heat and material flows[J]. Chemical Engineering & Technology, 2016, 39(12): 2251-2264. |
| 11 | WANG J, KRISHNA R, YANG T, et al. Nitrogen-rich microporous carbons for highly selective separation of light hydrocarbons[J]. Journal of Materials Chemistry A, 2016, 4(36): 13957-13966. |
| 12 | YANG Y X, BURKE N, ALI S, et al. Experimental studies of hydrocarbon separation on zeolites, activated carbons and MOFs for applications in natural gas processing[J]. RSC Advances, 2017, 7(21): 12629-12638. |
| 13 | LIN R B, XIANG S C, XING H B, et al. Exploration of porous metal-organic frameworks for gas separation and purification[J]. Coordination Chemistry Reviews, 2019, 378: 87-103. |
| 14 | BERECIARTUA P J, CANTÍN Á, CORMA A, et al. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene[J]. Science, 2017, 358(6366): 1068-1071. |
| 15 | HERM Z R, WIERS B M, MASON J A, et al. Separation of hexane isomers in a metal-organic framework with triangular channels[J]. Science, 2013, 340(6135): 960-964. |
| 16 | LIAO P Q, HUANG N Y, ZHANG W X, et al. Controlling guest conformation for efficient purification of butadiene[J]. Science, 2017, 356(6343): 1193-1196. |
| 17 | CUI X L, CHEN K J, XING H B, et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene[J]. Science, 2016, 353(6295): 141-144. |
| 18 | BALASUBRAMANIAN G, NEUMANN P, TWITCHEN D, et al. Ultralong spin coherence time in isotopically engineered diamond[J]. Nature Materials, 2009, 8(5): 383-387. |
| 19 | KUZNICKI S M, BELL V A, NAIR S, et al. A titanosilicate molecular sieve with adjustable pores for size-selective adsorption of molecules[J]. Nature, 2001, 412(6848): 720-724. |
| 20 | PARRAVANO G, FRIEDRICK H G, BOUDART M. The slow step in chemisorption the possible role of the solid adsorbent[J]. The Journal of Physical Chemistry, 1959, 63(7): 1144-1147. |
| 21 | YANG R T. Gas separation by adsorption processes[M]. Oxford: Butterworth-Heinemann, 1987. |
| 22 | GRANDE C A, BASALDELLA E, RODRIGUES A E. Crystal size effect in vacuum pressure-swing adsorption for propane/propylene separation[J]. Industrial & Engineering Chemistry Research, 2004, 43(23): 7557-7565. |
| 23 | RUTHVEN D M, REYES S C. Adsorptive separation of light olefins from paraffins[J]. Microporous and Mesoporous Materials, 2007, 104(1/2/3): 59-66. |
| 24 | FURUKAWA H, CORDOVA K E, O'KEEFFE M, et al. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341(6149): 1230444. |
| 25 | KARAKURT I, AYDIN G, AYDINER K. Sources and mitigation of methane emissions by sectors: a critical review[J]. Renewable Energy, 2012, 39(1): 40-48. |
| 26 | DE QUEIROZ FERNANDES ARAÚJO, ODE CARVALHO REIS A, DE MEDEIROS J L, et al. Comparative analysis of separation technologies for processing carbon dioxide rich natural gas in ultra-deepwater oil fields[J]. Journal of Cleaner Production, 2017, 155: 12-22. |
| 27 | ANSON A, LIN C C H, KUZNICKI S M, et al. Adsorption of carbon dioxide, ethane, and methane on titanosilicate type molecular sieves[J]. Chemical Engineering Science, 2009, 64(16): 3683-3687. |
| 28 | NUGENT P, BELMABKHOUT Y, BURD S D, et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation[J]. Nature, 2013, 495(7439): 80-84. |
| 29 | MASON J A, OKTAWIEC J, TAYLOR M K, et al. Methane storage in flexible metal-organic frameworks with intrinsic thermal management[J]. Nature, 2015, 527(7578): 357-361. |
| 30 | TAYLOR M K, RUNČEVSKI T, OKTAWIEC J, et al. Near-perfect CO2/CH4 selectivity achieved through reversible guest templating in the flexible metal-organic framework Co(bdp)[J]. Journal of the American Chemical Society, 2018, 140(32): 10324-10331. |
| 31 | HOU Q Q, WU Y, ZHOU S, et al. Ultra-tuning of the aperture size in stiffened ZIF-8_Cm frameworks with mixed-linker strategy for enhanced CO2/CH4 separation[J]. Angewandte Chemie International Edition, 2019, 58(1): 327-331. |
| 32 | XIANG S C, HE Y B, ZHANG Z J, et al. Microporous metal-organic framework with potential for carbon dioxide capture at ambient conditions[J]. Nature Communications, 2012, 3: 954. |
| 33 | CHEN H T, FAN L M, ZHANG X T, et al. Robust heterometallic TbⅢ/MnⅡ-organic framework for CO2/CH4 separation and I2 adsorption[J]. ACS Applied Nano Materials, 2020, 3(3): 2680-2686. |
| 34 | FINSY V, MA L, ALAERTS L, et al. Separation of CO2/CH4 mixtures with the MIL-53(Al) metal-organic framework[J]. Microporous and Mesoporous Materials, 2009, 120(3): 221-227. |
| 35 | AGUADO S, BERGERET G, DANIEL C, et al. Absolute molecular sieve separation of ethylene/ethane mixtures with silver zeolite A[J]. Journal of the American Chemical Society, 2012, 134(36): 14635-14637. |
| 36 | GEIER S J, MASON J A, BLOCH E D, et al. Selective adsorption of ethylene over ethane and propylene over propane in the metal-organic frameworks M2(dobdc) (M = Mg, Mn, Fe, Co, Ni, Zn)[J]. Chemical Science, 2013, 4(5): 2054. |
| 37 | CHEN H T, ZHUANG G L, FAN L M, et al. A highly robust heterometallic TbⅢ/NiⅡ-organic framework for C2 hydrocarbon separation and capture[J]. Chemical Communications, 2020, 56(13): 2047-2050. |
| 38 | KISHIDA K, WATANABE Y, HORIKE S, et al. DRIFT and theoretical studies of ethylene/ethane separation on flexible and microporous [Cu2(2,3-pyrazinedicarboxylate)2(pyrazine)] n [J]. European Journal of Inorganic Chemistry, 2014, 2014(17): 2747-2752. |
| 39 | GÜCÜYENER C, VAN DEN BERGH J, GASCON J, et al. Ethane/ethene separation turned on its head: selective ethane adsorption on the metal-organic framework ZIF-7 through a gate-opening mechanism[J]. Journal of the American Chemical Society, 2010, 132(50): 17704-17706. |
| 40 | YANG L, WANG Y, CHEN Y, et al. Microporous metal-organic framework with specific functional sites for efficient removal of ethane from ethane/ethylene mixtures[J]. Chemical Engineering Journal, 2020, 387: 124137. |
| 41 | NIJEM N, WU H H, CANEPA P, et al. Tuning the gate opening pressure of metal-organic frameworks (MOFs) for the selective separation of hydrocarbons[J]. Journal of the American Chemical Society, 2012, 134(37): 15201-15204. |
| 42 | YANG S H, RAMIREZ-CUESTA A J, NEWBY R, et al. Supramolecular binding and separation of hydrocarbons within a functionalized porous metal-organic framework[J]. Nature Chemistry, 2015, 7(2): 121-129. |
| 43 | LIN R B, WU H, LI L, et al. Boosting ethane/ethylene separation within isoreticular ultramicroporous metal-organic frameworks[J]. Journal of the American Chemical Society, 2018, 140(40): 12940-12946. |
| 44 | QAZVINI O T, BABARAO R, SHI Z L, et al. A robust ethane-trapping metal-organic framework with a high capacity for ethylene purification[J]. Journal of the American Chemical Society, 2019, 141(12): 5014-5020. |
| 45 | YANG H J, WANG Y X, KRISHNA R, et al. Pore-space-partition-enabled exceptional ethane uptake and ethane-selective ethane-ethylene separation[J]. Journal of the American Chemical Society, 2020, 142(5): 2222-2227. |
| 46 | WU X F, BAO Z B, YUAN B, et al. Microwave synthesis and characterization of MOF-74 (M=Ni, Mg) for gas separation[J]. Microporous and Mesoporous Materials, 2013, 180: 114-122. |
| 47 | CHANG G G, HUANG M H, SU Y, et al. Immobilization of Ag(Ⅰ) into a metal-organic framework with —SO3H sites for highly selective olefin-paraffin separation at room temperature[J]. Chemical Communications, 2015, 51(14): 2859-2862. |
| 48 | LIAO P Q, ZHANG W X, ZHANG J P, et al. Efficient purification of ethene by an ethane-trapping metal-organic framework[J]. Nature Communications, 2015, 6: 8697. |
| 49 | BLOCH E D, QUEEN W L, KRISHNA R, et al. Hydrocarbon separations in a metal-organic framework with open iron(Ⅱ) coordination sites[J]. Science, 2012, 335(6076): 1606-1610. |
| 50 | LI L B, LIN R B, KRISHNA R, et al. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites[J]. Science, 2018, 362(6413): 443-446. |
| 51 | DIETZEL P D C, MORITA Y, BLOM R, et al. An in situ high-temperature single-crystal investigation of a dehydrated metal-organic framework compound and field-induced magnetization of one-dimensional metal-oxygen chains[J]. Angewandte Chemie International Edition, 2005, 44(39): 6354-6358. |
| 52 | LEE K, HOWE J D, LIN L C, et al. Small-molecule adsorption in open-site metal-organic frameworks: a systematic density functional theory study for rational design[J]. Chemistry of Materials, 2015, 27(3): 668-678. |
| 53 | ZHOU W, WU H, YILDIRIM T. Enhanced H2 adsorption in isostructural metal-organic frameworks with open metal sites: strong dependence of the binding strength on metal ions[J]. Journal of the American Chemical Society, 2008, 130(46): 15268-15269. |
| 54 | LUNA-TRIGUERO A, VICENT-LUNA J M, BECKER T M, et al. Effective model for olefin/paraffin separation using (Co, Fe, Mn, Ni)-MOF-74[J]. ChemistrySelect, 2017, 2(2): 665-672. |
| 55 | DIETZEL P D C, BLOM R, FJELLVÅG H. Base-induced formation of two magnesium metal-organic framework compounds with a bifunctional tetratopic ligand[J]. European Journal of Inorganic Chemistry, 2008, 2008(23): 3624-3632. |
| 56 | ROSI N L, KIM J, EDDAOUDI M, et al. Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units[J]. Journal of the American Chemical Society, 2005, 127(5): 1504-1518. |
| 57 | YANG L F, QIAN S H, WANG X B, et al. Energy-efficient separation alternatives: metal–organic frameworks and membranes for hydrocarbon separation[J]. Chemical Society Reviews, 2020, 49(15): 5359-5406. |
| 58 | BACHMAN J E, KAPELEWSKI M T, REED D A, et al. M2(m-dobdc) (M = Mn, Fe, Co, Ni) metal-organic frameworks as highly selective, high-capacity adsorbents for olefin/paraffin separations[J]. Journal of the American Chemical Society, 2017, 139(43): 15363-15370. |
| 59 | BAO Z B, WANG J W, ZHANG Z G, et al. Molecular sieving of ethane from ethylene through the molecular cross-section size differentiation in gallate-based metal-organic frameworks[J]. Angewandte Chemie International Edition, 2018, 57(49): 16020-16025. |
| 60 | CHAI Y C, HAN X, LI W Y, et al. Control of zeolite pore interior for chemoselective alkyne/olefin separations[J]. Science, 2020, 368(6494): 1002-1006. |
| 61 | XIANG S C, ZHANG Z J, ZHAO C G, et al. Rationally tuned micropores within enantiopure metal-organic frameworks for highly selective separation of acetylene and ethylene[J]. Nature Communications, 2011, 2: 204. |
| 62 | HAZRA A, JANA S, BONAKALA S, et al. Separation/purification of ethylene from an acetylene/ethylene mixture in a pillared-layer porous metal-organic framework[J]. Chemical Communications, 2017, 53(36): 4907-4910. |
| 63 | LIN R B, LI L B, WU H, et al. Optimized separation of acetylene from carbon dioxide and ethylene in a microporous material[J]. Journal of the American Chemical Society, 2017, 139(23): 8022-8028. |
| 64 | HE Y B, KRISHNA R, CHEN B L. Metal-organic frameworks with potential for energy-efficient adsorptive separation of light hydrocarbons[J]. Energy & Environmental Science, 2012, 5(10): 9107. |
| 65 | HU T L, WANG H L, LI B, et al. Microporous metal-organic framework with dual functionalities for highly efficient removal of acetylene from ethylene/acetylene mixtures[J]. Nature Communications, 2015, 6: 7328. |
| 66 | LI H, LI L B, LIN R B, et al. Microporous metal-organic framework with dual functionalities for efficient separation of acetylene from light hydrocarbon mixtures[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 4897-4902. |
| 67 | PEI J Y, SHAO K, WANG J X, et al. A chemically stable Hofmann-type metal-organic framework with sandwich-like binding sites for benchmark acetylene capture[J]. Advanced Materials, 2020, 32(24): e1908275. |
| 68 | YANG L F, JIN A Y, GE L S, et al. A novel interpenetrated anion-pillared porous material with high water tolerance afforded efficient C2H2/C2H4 separation[J]. Chemical Communications, 2019, 55(34): 5001-5004. |
| 69 | NIU Z, CUI X L, PHAM T, et al. A MOF-based ultra-strong acetylene nano-trap for highly efficient C2H2/CO2 separation[J]. Angewandte Chemie International Edition, 2021, 60(10): 5283-5288. |
| 70 | LI K H, OLSON D H, SEIDEL J, et al. Zeolitic imidazolate frameworks for kinetic separation of propane and propene[J]. Journal of the American Chemical Society, 2009, 131(30): 10368-10369. |
| 71 | KWON H T, JEONG H K. In situ synthesis of thin zeolitic-imidazolate framework ZIF-8 membranes exhibiting exceptionally high propylene/propane separation[J]. Journal of the American Chemical Society, 2013, 135(29): 10763-10768. |
| 72 | KWON H T, JEONG H K, LEE A S, et al. Heteroepitaxially grown zeolitic imidazolate framework membranes with unprecedented propylene/propane separation performances[J]. Journal of the American Chemical Society, 2015, 137(38): 12304-12311. |
| 73 | LEE C Y, BAE Y S, JEONG N C, et al. Kinetic separation of propene and propane in metal-organic frameworks: controlling diffusion rates in plate-shaped crystals via tuning of pore apertures and crystallite aspect ratios[J]. Journal of the American Chemical Society, 2011, 133(14): 5228-5231. |
| 74 | LI L B, LIN R B, WANG X Q, et al. Kinetic separation of propylene over propane in a microporous metal-organic framework[J]. Chemical Engineering Journal, 2018, 354: 977-982. |
| 75 | CADIAU A, ADIL K, BHATT P M, et al. A metal-organic framework-based splitter for separating propylene from propane[J]. Science, 2016, 353(6295): 137-140. |
| 76 | LI L B, LIN R B, KRISHNA R, et al. Flexible-robust metal-organic framework for efficient removal of propyne from propylene[J]. Journal of the American Chemical Society, 2017, 139(23): 7733-7736. |
| 77 | YOON J W, SEO Y K, HWANG Y K, et al. Controlled reducibility of a metal-organic framework with coordinatively unsaturated sites for preferential gas sorption[J]. Angewandte Chemie International Edition, 2010, 49(34): 5949-5952. |
| 78 | WUTTKE S, BAZIN P, VIMONT A, et al. Discovering the active sites for C3 separation in MIL-100(Fe) by using operando IR spectroscopy[J]. Chemistry—A European Journal, 2012, 18(38): 11959-11967. |
| 79 | BAE Y S, LEE C Y, KIM K C, et al. High propene/propane selectivity in isostructural metal-organic frameworks with high densities of open metal sites[J]. Angewandte Chemie, 2012, 51(8): 1857-1860. |
| 80 | ABEDINI H, SHARIATI A, KHOSRAVI-NIKOU M R. Adsorption of propane and propylene on M-MOF-74 (M=Cu,Co): equilibrium and kinetic study[J]. Chemical Engineering Research and Design, 2020, 153: 96-106. |
| 81 | KE T, WANG Q J, SHEN J, et al. Molecular sieving of C2—C3 alkene from alkyne with tuned threshold pressure in robust layered metal-organic frameworks[J]. Angewandte Chemie International Edition, 2020, 59(31): 12725-12730. |
| 82 | YANG L F, CUI X L, ZHANG Z Q, et al. An asymmetric anion-pillared metal-organic framework as a multisite adsorbent enables simultaneous removal of propyne and propadiene from propylene[J]. Angewandte Chemie International Edition, 2018, 57(40): 13145-13149. |
| 83 | LI L B, WEN H M, HE C H, et al. A metal-organic framework with suitable pore size and specific functional sites for the removal of trace propyne from propylene[J]. Angewandte Chemie International Edition, 2018, 57(46): 15183-15188. |
| 84 | PENG Y L, WANG T, JIN C N, et al. A robust heterometallic ultramicroporous MOF with ultrahigh selectivity for propyne/propylene separation[J]. Journal of Materials Chemistry A, 2021, 9(5): 2850-2856. |
| 85 | 赵亚梅, 曹婷婷, 丁思奇, 等. 基于离子液体修饰的金属有机骨架材料在CO2分离与转化方面的研究进展[J]. 化学研究与应用, 2021, 33(9): 1633-1641. |
| ZHAO Yamei, CAO Tingting, DING Siqi, et al. Research progress of metal organic framework materials modified by ionic liquids in the field of CO2 separation and conversion[J]. Chemical Research and Application, 2021, 33(9): 1633-1641. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 贺美晋. 分子管理在炼油领域分离技术中的应用和发展趋势[J]. 化工进展, 2023, 42(S1): 260-266. |
| [3] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [4] | 李世霖, 胡景泽, 王毅霖, 王庆吉, 邵磊. 电渗析分离提取高值组分的研究进展[J]. 化工进展, 2023, 42(S1): 420-429. |
| [5] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [6] | 廖志新, 罗涛, 王红, 孔佳骏, 申海平, 管翠诗, 王翠红, 佘玉成. 溶剂脱沥青技术应用与进展[J]. 化工进展, 2023, 42(9): 4573-4586. |
| [7] | 潘宜昌, 周荣飞, 邢卫红. 高效分离同碳数烃的先进微孔膜:现状与挑战[J]. 化工进展, 2023, 42(8): 3926-3942. |
| [8] | 周龙大, 赵立新, 徐保蕊, 张爽, 刘琳. 电场-旋流耦合强化多相介质分离研究进展[J]. 化工进展, 2023, 42(7): 3443-3456. |
| [9] | 陈香李, 李倩倩, 张甜, 李彪, 李康康. 自愈合油水分离膜的研究进展[J]. 化工进展, 2023, 42(7): 3600-3610. |
| [10] | 娄宝辉, 吴贤豪, 张驰, 陈臻, 冯向东. 纳米流体用于二氧化碳吸收分离研究进展[J]. 化工进展, 2023, 42(7): 3802-3815. |
| [11] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [12] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [13] | 朱雅静, 徐岩, 简美鹏, 李海燕, 王崇臣. 金属有机框架材料用于海水提铀的研究进展[J]. 化工进展, 2023, 42(6): 3029-3048. |
| [14] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| [15] | 周磊, 孙晓岩, 陶少辉, 陈玉石, 项曙光. 基于分离因数法的简捷炼油塔模型开发及应用[J]. 化工进展, 2023, 42(6): 2819-2827. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||