化工进展 ›› 2022, Vol. 41 ›› Issue (8): 4277-4287.DOI: 10.16085/j.issn.1000-6613.2021-2138
高镍正极材料微裂纹诱导容量衰减的应对策略研究进展
- 1.河南科技大学化工与制药学院,河南 洛阳 471000
2.贵州理工学院化学工程学院,贵州 贵阳 550003
3.中国科学院成都有机化学有限公司,四川 成都 610041
-
收稿日期:2021-10-18修回日期:2021-11-16出版日期:2022-08-25发布日期:2022-08-22 -
通讯作者:葛武杰 -
作者简介:李想(1990—),男,博士,讲师,研究方向为锂离子电池正负极材和光催化。E-mail:lixiang@haust.edu.cn 。 -
基金资助:河南省高等学校重点科研项目计划(21A150018);国家自然科学基金(21805053);贵州理工学院高层次人才科研启动项目(XJGC20190901);贵州省科技计划(黔科合基础[2019]1132号);郑州市基础与应用基础研究专项基金(ZZSZX202001)
Research progress on countermeasures for microcrack-induced capacity degradation of Ni-rich cathode materials
LI Xiang1( ), GE Wujie2(
), GE Wujie2( ), MA Xianguo2, PENG Gongchang3
), MA Xianguo2, PENG Gongchang3
- 1.Chemical Engineering & Pharmaceutics School, Henan University of Science & Technology, Luoyang 471000, Henan, China
2.School of Chemical Engineering, Guizhou Institute of Technology, Guiyang 550003, Guizhou, China
3.Chengdu Organic Chemicals Company Limited, Chinese Academy of Sciences, Chengdu 610041, Sichuan, China
-
Received:2021-10-18Revised:2021-11-16Online:2022-08-25Published:2022-08-22 -
Contact:GE Wujie
摘要:
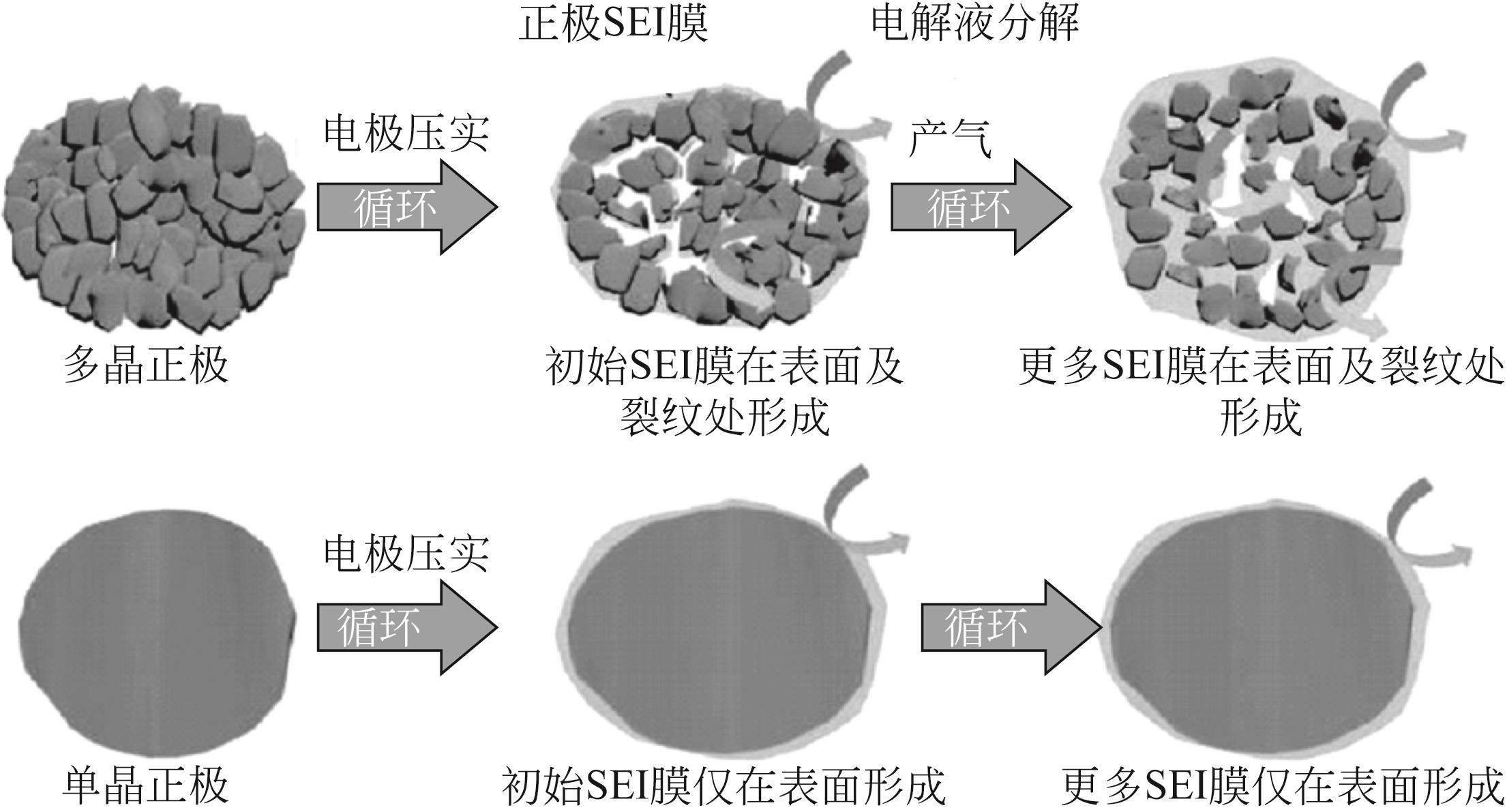
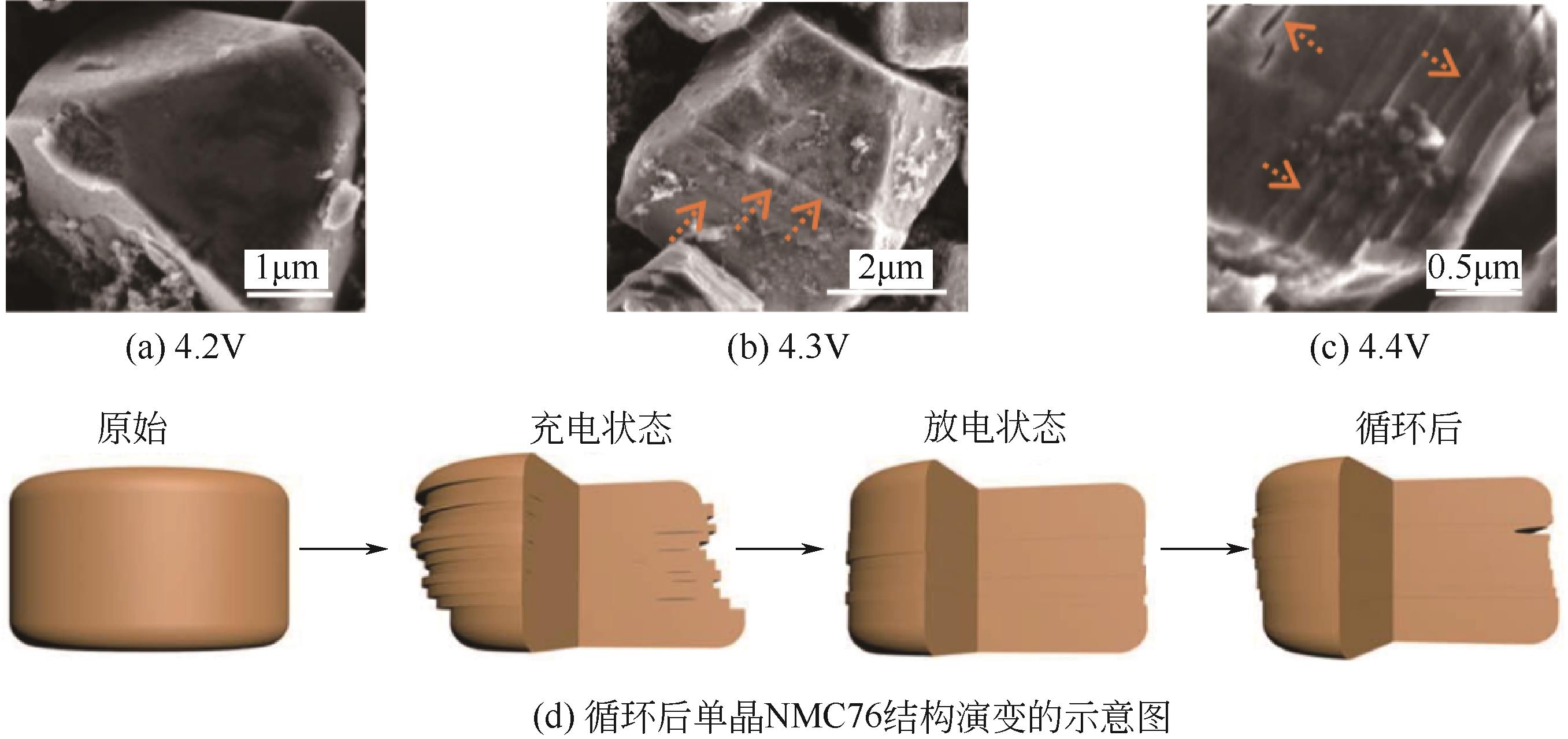
随着锂离子电池在电动汽车、储能等领域的广泛应用,其正极材料尤其是钴酸锂、镍钴锰酸锂及镍钴铝酸锂三元正极材料的需求量也随之剧增。然而由于钴资源稀缺,“高镍低钴化”成为近年来锂离子电池行业的重要关注点和发展方向。高镍正极材料(Ni的摩尔分数大于60%)凭借着容量高、成本低廉等优势获得了广泛的关注和研发,其产业化步伐逐渐加快。然而其仍然面临着诸多限制其大规模应用的问题,其中微裂纹的产生诱发的快速容量衰减问题被越来越多的研究证明是常规球状高镍正极材料容量衰减的首要因素。本文综述了近年来针对这一问题的几种典型应对策略的研究进展,包括填隙包覆处理、径向有序设计以及采用高镍单晶正极材料。本文对以上典型应对策略的技术手段、工艺参数和电化学性能进行了总结和归纳。最后对于进一步的研究方向进行了展望。
中图分类号:
引用本文
李想, 葛武杰, 马先果, 彭工厂. 高镍正极材料微裂纹诱导容量衰减的应对策略研究进展[J]. 化工进展, 2022, 41(8): 4277-4287.
LI Xiang, GE Wujie, MA Xianguo, PENG Gongchang. Research progress on countermeasures for microcrack-induced capacity degradation of Ni-rich cathode materials[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4277-4287.
| 方法 | 解决的关键问题 | 优点 | 缺陷 | 技术手段 | 典型改性原料及工艺参数 |
|---|---|---|---|---|---|
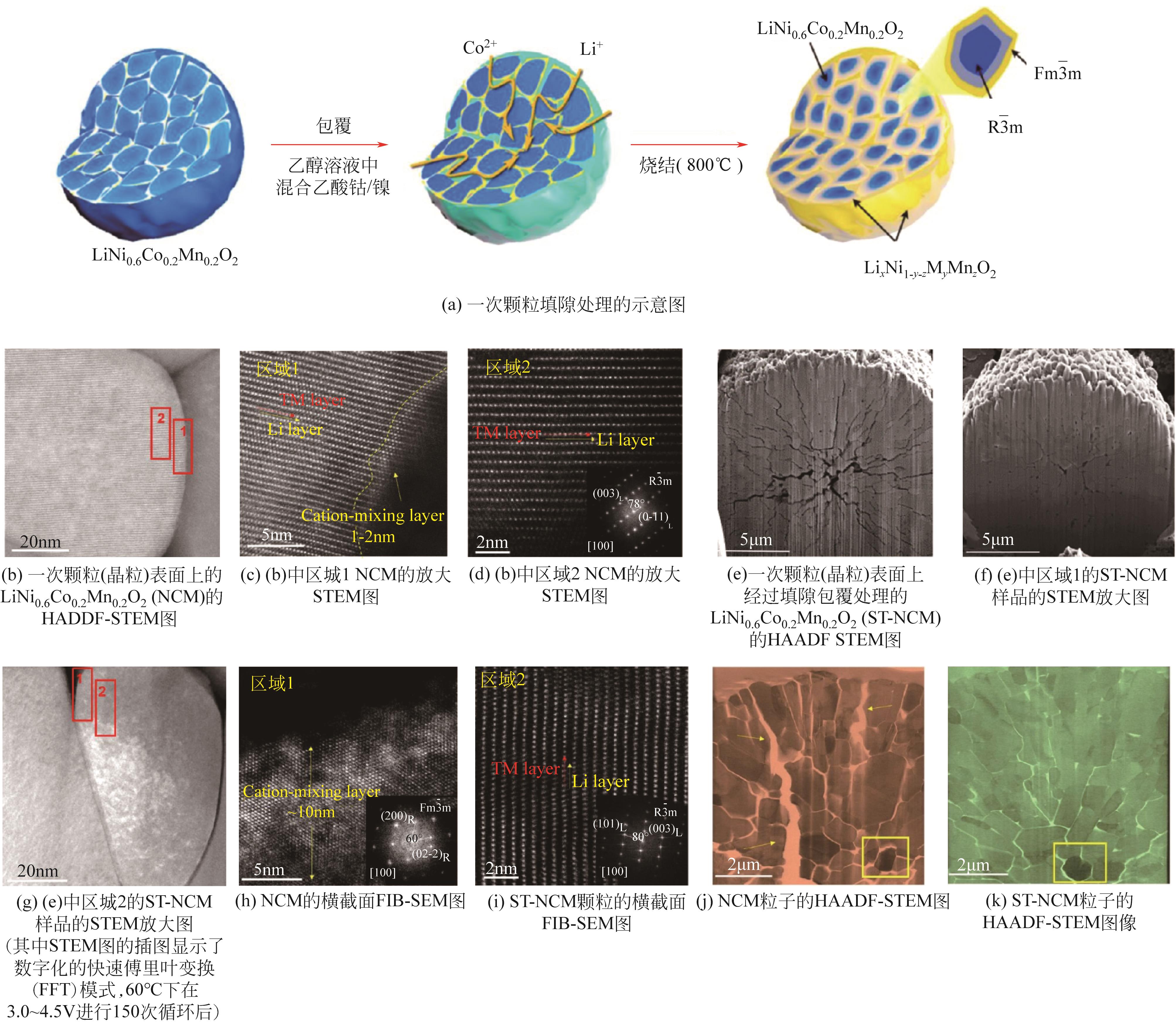
| 填隙包覆 | 保护晶界,避免开裂后与电解液直接接触,减少阻碍锂离子传导的惰性相的形成 | 承袭表面包覆技术方法,较为简便,利于规模化应用 | 仍停留在表面,无法从根本上减弱应力应变的程度;改性物质选择范围有限,对其含量要求苛刻,使用过多会导致容量明显下降 | ①湿法共混-球磨-烧结; ②干法共混-球磨-烧结 | ①乙酸钴+乙酸锂(NCM622)[ |
| 径向有序设计 | 调整各向异性应力应变方向,减弱开裂的程度 | 可以大幅度减弱应力应变的累积,提高电压稳定性 | 合成过程较为复杂,工艺难度较大 | ①水/溶剂热法;②控制结晶-共沉淀法;③烧结助剂法 | ①硝酸镍+硝酸钴+聚乙烯吡咯烷酮[ |
| 单晶制备 | 进一步减弱一次颗粒之间的应力应变作用,极大减少开裂现象 | 可以从根本上避免球形二次颗粒内部出现应力应变累积现象,极大提高整体稳定性;同时能避免加工过程的挤压破碎 | 单晶颗粒内部锂离子传导距离较长,对倍率性能有较大影响;需要较长煅烧时间和较多的烧结助剂 | ①成品球磨-烧结;②共沉淀-助剂-烧结法;③熔盐法;④喷雾热解法 | ①碳酸锂,Li/TM=1.05,940℃-5h(NCM622)[ |
表1 不同应对策略拟解决的关键问题、优缺点、技术手段和工艺参数汇总
| 方法 | 解决的关键问题 | 优点 | 缺陷 | 技术手段 | 典型改性原料及工艺参数 |
|---|---|---|---|---|---|
| 填隙包覆 | 保护晶界,避免开裂后与电解液直接接触,减少阻碍锂离子传导的惰性相的形成 | 承袭表面包覆技术方法,较为简便,利于规模化应用 | 仍停留在表面,无法从根本上减弱应力应变的程度;改性物质选择范围有限,对其含量要求苛刻,使用过多会导致容量明显下降 | ①湿法共混-球磨-烧结; ②干法共混-球磨-烧结 | ①乙酸钴+乙酸锂(NCM622)[ |
| 径向有序设计 | 调整各向异性应力应变方向,减弱开裂的程度 | 可以大幅度减弱应力应变的累积,提高电压稳定性 | 合成过程较为复杂,工艺难度较大 | ①水/溶剂热法;②控制结晶-共沉淀法;③烧结助剂法 | ①硝酸镍+硝酸钴+聚乙烯吡咯烷酮[ |
| 单晶制备 | 进一步减弱一次颗粒之间的应力应变作用,极大减少开裂现象 | 可以从根本上避免球形二次颗粒内部出现应力应变累积现象,极大提高整体稳定性;同时能避免加工过程的挤压破碎 | 单晶颗粒内部锂离子传导距离较长,对倍率性能有较大影响;需要较长煅烧时间和较多的烧结助剂 | ①成品球磨-烧结;②共沉淀-助剂-烧结法;③熔盐法;④喷雾热解法 | ①碳酸锂,Li/TM=1.05,940℃-5h(NCM622)[ |
| 1 | SCHIPPER F, ERICKSON E M, ERK C, et al. Review—Recent advances and remaining challenges for lithium ion battery cathodes[J]. Journal of the Electrochemical Society, 2016, 164(1): A6220-A6228. |
| 2 | WANG T, REN K L, HE M, et al. Synthesis and manipulation of single-crystalline lithium nickel manganese cobalt oxide cathodes: a review of growth mechanism[J]. Frontiers in Chemistry, 2020, 8: 747-755. |
| 3 | 李想, 葛武杰, 王昊, 等. 高镍系三元层状氧化物正极材料容量衰减机理的研究进展[J]. 无机材料学报, 2017, 32(2): 113-121. |
| LI X, GE W J, WANG H, et al. Research progress on the capacity fading mechanisms of high-nickel ternary layered oxide cathode materials[J]. Journal of Inorganic Materials, 2017, 32(2): 113-121. | |
| 4 | WATANABE S, KINOSHITA M, HOSOKAWA T, et al. Capacity fade of LiAl y Ni1- x- y Co x O2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (surface analysis of LiAl y Ni1- x- y Co x O2 cathode after cycle tests in restricted depth of discharge ranges) [J]. Journal of Power Sources, 2014, 258: 210-217. |
| 5 | MILLER D J, PROFF C, WEN J G, et al. Observation of microstructural evolution in Li battery cathode oxide particles by in situ electron microscopy[J]. Advanced Energy Materials, 2013, 3(8): 1098-1103. |
| 6 | LIU H, WOLF M, KARKI K, et al. Intergranular cracking as a major cause of long-term capacity fading of layered cathodes[J]. Nano Letters, 2017, 17(6): 3452-3457. |
| 7 | KIM H, KIM M G, JEONG H Y, et al. A new coating method for alleviating surface degradation of LiNi0.6Co0.2Mn0.2O2 cathode material: nanoscale surface treatment of primary particles[J]. Nano Letters, 2015, 15: 2111-2119. |
| 8 | DU F H, SUN P P, ZHOU Q, et al. Interlinking primary grains with lithium boron oxide to enhance the stability of LiNi0.8Co0.15Al0.05O2 [J]. ACS Applied Materials & Interfaces, 2020, 12(51): 56963-56973. |
| 9 | YOON M, DONG Y H, HWANG J, et al. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries[J]. Nature Energy, 2021, 6(4): 362-371. |
| 10 | YANG C K, SHAO R W, MI Y Y, et al. Stable interstitial layer to alleviate fatigue fracture of high nickel cathode for lithium-ion batteries[J]. Journal of Power Sources, 2018, 376: 200-206. |
| 11 | YAN P F, ZHENG J M, LIU J, et al. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries[J]. Nature Energy, 2018, 3(7): 600-605. |
| 12 | CHENG X P, ZHENG J M, LU J X, et al. Realizing superior cycling stability of Ni-rich layered cathode by combination of grain boundary engineering and surface coating[J]. Nano Energy, 2019, 62: 30-37. |
| 13 | DAS H, URBAN A, HUANG W X, et al. First-principles simulation of the (Li-Ni-vacancy)O phase diagram and its relevance for the surface phases in Ni-rich Li-ion cathode materials[J]. Chemistry of Materials, 2017, 29(18): 7840-7851. |
| 14 | XIANG W, LIU Y, ZHANG J, et al. Controlled synthesis of nickel-rich layered oxide cathodes with preferentially exposed {010} active facets for high rate and long cycling stable lithium-ion batteries[J]. Journal of Alloys and Compounds, 2019, 775:72-80. |
| 15 | WANG Z, LIU H, WU J, et al. Hierarchical LiNi0.8Co0.15Al0.05O2 plates with exposed {010} active Planes as a high performance cathode material for Li-ion batteries[J]. RSC Advances, 2016, 6(38): 32365-32369. |
| 16 | SU Y F, CHEN G, CHEN L, et al. Exposing the {010} Planes by oriented self-assembly with nanosheets to improve the electrochemical performances of Ni-rich Li[Ni0.8Co0.1Mn0.1]O2 microspheres[J]. ACS Applied Materials & Interfaces, 2018, 10: 6407-6414. |
| 17 | XU X, HUO H, JIAN J Y, et al. Radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries[J]. Advanced Energy Materials, 2019, 9(15): 1803963. |
| 18 | YANG C K, QI L Y, ZUO Z C, et al. Insights into the inner structure of high-nickel agglomerate as high-performance lithium-ion cathodes[J]. Journal of Power Sources, 2016, 331: 487-494. |
| 19 | PARK S, KU H, LEE K J, et al. The effect of NH3 concentration during co-precipitation of precursors from leachate of lithium-ion battery positive electrode active materials[J]. Journal of the Korean Institute of Resources Recycling, 2015, 24(6): 9-16. |
| 20 | DU B D, MO Y, JIN H F, et al. Radially microstructural design of LiNi0.8Co0.1Mn0.1O2 cathode material toward long-term cyclability and high rate capability at high voltage[J]. ACS Applied Energy Materials, 2020, 3(7): 6657-6669. |
| 21 | NOH H J, CHEN Z H, YOON C S, et al. Cathode material with nanorod structure—An application for advanced high-energy and safe lithium batteries[J]. Chemistry of Materials, 2013, 25(10): 2109-2115. |
| 22 | LEE E J, CHEN Z H, NOH H J, et al. Development of microstrain in aged lithium transition metal oxides[J]. Nano Letters, 2014, 14(8): 4873-4880. |
| 23 | PARK K J, JUNG H G, KUO L Y, et al. Improved cycling stability of Li[Ni0.90Co0.05Mn0.05]O2 through microstructure modification by boron doping for Li-ion batteries[J]. Advanced Energy Materials, 2018, 8(25): 1801202. |
| 24 | RYU H H, PARK N Y, SEO J H, et al. A highly stabilized Ni-rich NCA cathode for high-energy lithium-ion batteries[J]. Materials Today, 2020, 36: 73-82. |
| 25 | KIM U H, PARK G T, SON B K, et al. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge[J]. Nature Energy, 2020, 5(11): 860-869. |
| 26 | ZHU J, SHARIFI-ASL S, GARCIA J C, et al. Atomic-level understanding of surface reconstruction based on Li[Ni x Mn y Co1- x- y ]O2 single-crystal studies[J]. ACS Applied Energy Materials, 2020, 3(5): 4799-4811. |
| 27 | LIANG C P, LONGO R C, KONG F T, et al. Ab initio study on surface segregation and anisotropy of Ni-rich LiNi1-2 y Co y Mn y O2 (NCM) (y≤0.1) cathodes[J]. ACS Applied Materials & Interfaces, 2018, 10(7): 6673-6680. |
| 28 | 肖建伟,刘良彬,符泽卫,等. 单晶LiNi x Co y Mn1- x- y O2三元正极材料研究进展[J]. 电池工业, 2017, 21(2): 51-54. |
| XIAO J W, LIU L B, FU Z W, et al. Research progress in the single crystal LiNi x Co y Mn1- x- y O2 ternary cathode materials[J]. Chinese Battery Industry, 2017, 21(2): 51-54. | |
| 29 | 刘帅,宋顺林,刘亚飞,等. 锂离子电池用高镍单晶正极材料的研究进展[J]. 山东化工, 2018, 47(16): 46-49. |
| LIU S, SONG S L, LIU Y F, et al. Research progress in the single crystal of high nickel cathode materials for lithium-ion batteries[J], Shandong Chemical Industry, 2018, 47(16): 46-49. | |
| 30 | BI Y J, TAO J H, WU Y Q, et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode[J]. Science, 2020, 370(6522):1313-1317. |
| 31 | YOON C S, RYU H H, PARK G T, et al. Extracting maximum capacity from Ni-rich Li[Ni0.95Co0.025Mn0.025]O2 cathodes for high-energy-density lithium-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(9): 4126-4132. |
| 32 | KIM U H, MYUNG S T, YOON C S, et al. Extending the battery life using an Al-doped Li[Ni0.76Co0.09Mn0.15]O2 cathode with concentration gradients for lithium ion batteries[J]. ACS Energy Letters, 2017, 2(8): 1848-1854. |
| 33 | LI H Y, LIU A, ZHANG N, et al. An unavoidable challenge for Ni-rich positive electrode materials for lithium-ion batteries[J]. Chemistry of Materials, 2019, 31(18): 7574-7583. |
| 34 | POUILLERIE C, CROGUENNEC L, BIENSAN P, et al. Synthesis and characterization of new LiNi1- y Mg y O2 positive electrode materials for lithium-ion batteries[J]. Journal of the Electrochemical Society, 2000, 147(6): 2061-2067. |
| 35 | KONDO H, TAKEUCHI Y, SASAKI T, et al. Effects of Mg-substitution in Li(Ni,Co,Al)O2 positive electrode materials on the crystal structure and battery performance[J]. Journal of Power Sources, 2007, 174(2): 1131-1136. |
| 36 | MUTO S, TATSUMI K, KOJIMA Y, et al. Effect of Mg-doping on the degradation of LiNiO2-based cathode materials by combined spectroscopic methods[J]. Journal of Power Sources, 2012, 205: 449-455. |
| 37 | REN Z M, SHEN C, LIU M, et al. Improving LiNi0.9Co0.08Mn0.02O2’s cyclic stability via abating mechanical damages[J]. Energy Storage Materials, 2020, 28: 1-9. |
| 38 | HE T, LU Y, SU Y F, et al. Sufficient utilization of zirconium ions to improve the structure and surface properties of the Ni-rich cathode materials for lithium ion batteries[J]. ChemSusChem, 2018, 11(10): 1639-1648. |
| 39 | ZOU L F, ZHAO W G, JIA H P, et al. The role of secondary particle structures in surface phase transitions of Ni-rich cathodes[J]. Chemistry of Materials, 2020, 32(7): 2884-2892. |
| 40 | LI S, YAO Z P, ZHENG J M, et al. Direct observation of defect-aided structural evolution in a nickel-rich layered cathode[J]. Angewandte Chemie International Edition, 2020, 59:22092-22099. |
| 41 | HUANG B, WANG M, ZHANG X W, et al. Optimized preparation of LiNi0.6Mn0.2Co0.2O2 with single crystal morphology cathode material for lithium-ion batteries[J]. Ionics, 2020, 26(6): 2689-2698. |
| 42 | HUANG B, WANG M, ZUO Y X, et al. The effects of reheating process on the electrochemical properties of single crystal LiNi0.6Mn0.2Co0.2O2 [J]. Solid State Ionics, 2020, 345: 115200. |
| 43 | LI H Y, LI J, MA X W, et al. Synthesis of single crystal LiNi0.6Mn0.2Co0.2O2 with enhanced electrochemical performance for lithium ion batteries[J]. Journal of The Electrochemical Society, 2018, 165(5): A1038-A1045. |
| 44 | DUAN J G, WU C, CAO Y B, et al. Enhanced compacting density and cycling performance of Ni-riched electrode via building mono dispersed micron scaled morphology[J]. Journal of Alloys and Compounds, 2017, 695: 91-99. |
| 45 | QIAN G N, ZHANG Y T, LI L S, et al. Single-crystal nickel-rich layered-oxide battery cathode materials: synthesis, electrochemistry, and intra-granular fracture[J]. Energy Storage Materials, 2020, 27: 140-149. |
| 46 | ZHU J, ZHENG J C, CAO G L, et al. Flux-free synthesis of single-crystal LiNi0.8Co0.1Mn0.1O2 boosts its electrochemical performance in lithium batteries[J]. Journal of Power Sources, 2020, 464: 228207. |
| 47 | YAN W W, JIA X B, YANG S Y, et al. Synthesis of single crystal LiNi0.92Co0.06Mn0.01Al0.01O2 cathode materials with superior electrochemical performance for lithium ion batteries[J]. Journal of the Electrochemical Society, 2020, 167: 120514. |
| 48 | LI H Y, LI J, ZAKER N, et al. Synthesis of single crystal LiNi0.88Co0.09Al0.03O2 with a two-step lithiation method[J]. Journal of the Electrochemical Society, 2019, 166(10): A1956-A1963. |
| 49 | RYU H H, PARK N Y, YOON D R, et al. New class of Ni-rich cathode materials Li[Ni x Co y B1- x- y ]O2 for next lithium batteries[J]. Advanced Energy Materials, 2020, 10(25): 2000495. |
| 50 | LEE S H, JIN B S, KIM H S. Superior performances of B-doped LiNi0.84Co0.10Mn0.06O2 cathode for advanced LIBs[J]. Scientific Reports, 2019, 9: 17541. |
| 51 | JAMIL S, YU R Z, WANG Q, et al. Enhanced cycling stability of nickel-rich layered oxide by tantalum doping[J]. Journal of Power Sources, 2020, 473: 228597. |
| 52 | LI W J, ZHANG J, ZHOU Y, et al. Regulating the grain orientation and surface structure of primary particles through tungsten modification to comprehensively enhance the performance of nickel-rich cathode materials[J]. ACS Applied Materials & Interfaces, 2020, 12(42): 47513-47525. |
| 53 | PARK N Y, RYU H H, KUO L Y, et al. High-energy cathodes via precision microstructure tailoring for next-generation electric vehicles[J]. ACS Energy Letters, 2021, 6(12): 4195-4202. |
| 54 | YANG C, ZHU Z H, WEI W F, et al. Superior cycle stability of single crystal nickel-rich layered oxides with micron-scale grain size as cathode material for lithium ion batteries[J]. International Journal of Electrochemical Science, 2020, 15: 5031-5041. |
| 55 | ZHU J, CHEN G Y. Single-crystal based studies for correlating the properties and high-voltage performance of Li[Ni x Mn y Co1- x- y ]O2 cathodes[J]. Journal of Materials Chemistry A, 2019, 7(10): 5463-5474. |
| 56 | 何康宇, 曹博凯, 莫岩, 等. 熔盐法制备LiNi0.8Co0.1Mn0.1O2单晶及其电化学性能[J]. 材料导报, 2021, 35(12): 12027-12031. |
| HE K Y, CAO B K, MO Y, et al. Synthesis of LiNi0.8Co0.1Mn0.1O2 single crystal by molten salt method and its electrochemical performance[J]. Materials Reports, 2021, 35(12): 12027-12031. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 张杰, 白忠波, 冯宝鑫, 彭肖林, 任伟伟, 张菁丽, 刘二勇. PEG及其复合添加剂对电解铜箔后处理的影响[J]. 化工进展, 2023, 42(S1): 374-381. |
| [4] | 雷伟, 姜维佳, 王玉高, 和明豪, 申峻. N、S共掺杂煤基碳量子点的电化学氧化法制备及用于Fe3+检测[J]. 化工进展, 2023, 42(9): 4799-4807. |
| [5] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [6] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [7] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [8] | 王帅晴, 杨思文, 李娜, 孙占英, 安浩然. 元素掺杂生物质炭材料在电化学储能中的研究进展[J]. 化工进展, 2023, 42(8): 4296-4306. |
| [9] | 王鑫, 王兵兵, 杨威, 徐志明. 金属表面PDA/PTFE超疏水涂层抑垢与耐腐蚀性能[J]. 化工进展, 2023, 42(8): 4315-4321. |
| [10] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [11] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| [12] | 吴展华, 盛敏. 绝热加速量热仪在反应安全风险评估应用中的常见问题[J]. 化工进展, 2023, 42(7): 3374-3382. |
| [13] | 薛凯, 王帅, 马金鹏, 胡晓阳, 种道彤, 王进仕, 严俊杰. 工业园区分布式综合能源系统的规划与调度[J]. 化工进展, 2023, 42(7): 3510-3519. |
| [14] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| [15] | 谢志伟, 吴张永, 朱启晨, 蒋佳骏, 梁天祥, 刘振阳. 植物油基Ni0.5Zn0.5Fe2O4磁流体的黏度特性及磁黏特性[J]. 化工进展, 2023, 42(7): 3623-3633. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||