化工进展 ›› 2025, Vol. 44 ›› Issue (10): 5975-5990.DOI: 10.16085/j.issn.1000-6613.2024-1441
• 资源与环境化工 • 上一篇
过硫酸盐体系中非自由基路径的调控研究进展
胡芝璇1,2( ), 熊婷1,3(
), 熊婷1,3( ), 蒋龙波2,3(
), 蒋龙波2,3( ), 袁兴中2,3
), 袁兴中2,3
- 1.湖南工商大学前沿交叉学院,湖南 长沙 410205
2.湖南大学环境科学与工程学院,湖南 长沙 410082
3.湘江实验室,湖南 长沙 410205
-
收稿日期:2024-09-03修回日期:2024-10-22出版日期:2025-10-25发布日期:2025-11-10 -
通讯作者:熊婷,蒋龙波 -
作者简介:胡芝璇(2001—),女,硕士研究生,研究方向为环境修复、环境功能材料。E-mail:zhixuan0413@163.com。 -
基金资助:湘江实验室重大项目(24XJ01003);湘江实验室开放基金(23XJ03013);湖南工商大学“数智+”学科交叉研究项目(2023SZJ05);湖南省骨干教师项目;湖南省自然科学基金(2023JJ30136);湖南省生态环境厅环保科研项目(HBKYXM-2023011);湖南省教育厅科研项目(23B0039);湖南省教育厅科研项目(22B0670)
Advances in the regulation of non-free radical pathways in persulfate systems
HU Zhixuan1,2( ), XIONG Ting1,3(
), XIONG Ting1,3( ), JIANG Longbo2,3(
), JIANG Longbo2,3( ), YUAN Xingzhong2,3
), YUAN Xingzhong2,3
- 1.Frontier Crossing College, Hunan Technology and Business University, Changsha 410205, Hunan, China
2.College of Environmental Science and Engineering, Hunan University, Changsha 410082, Hunan, China
3.Xiangjiang Laboratory, Changsha 410205, Hunan, China
-
Received:2024-09-03Revised:2024-10-22Online:2025-10-25Published:2025-11-10 -
Contact:XIONG Ting, JIANG Longbo
摘要:
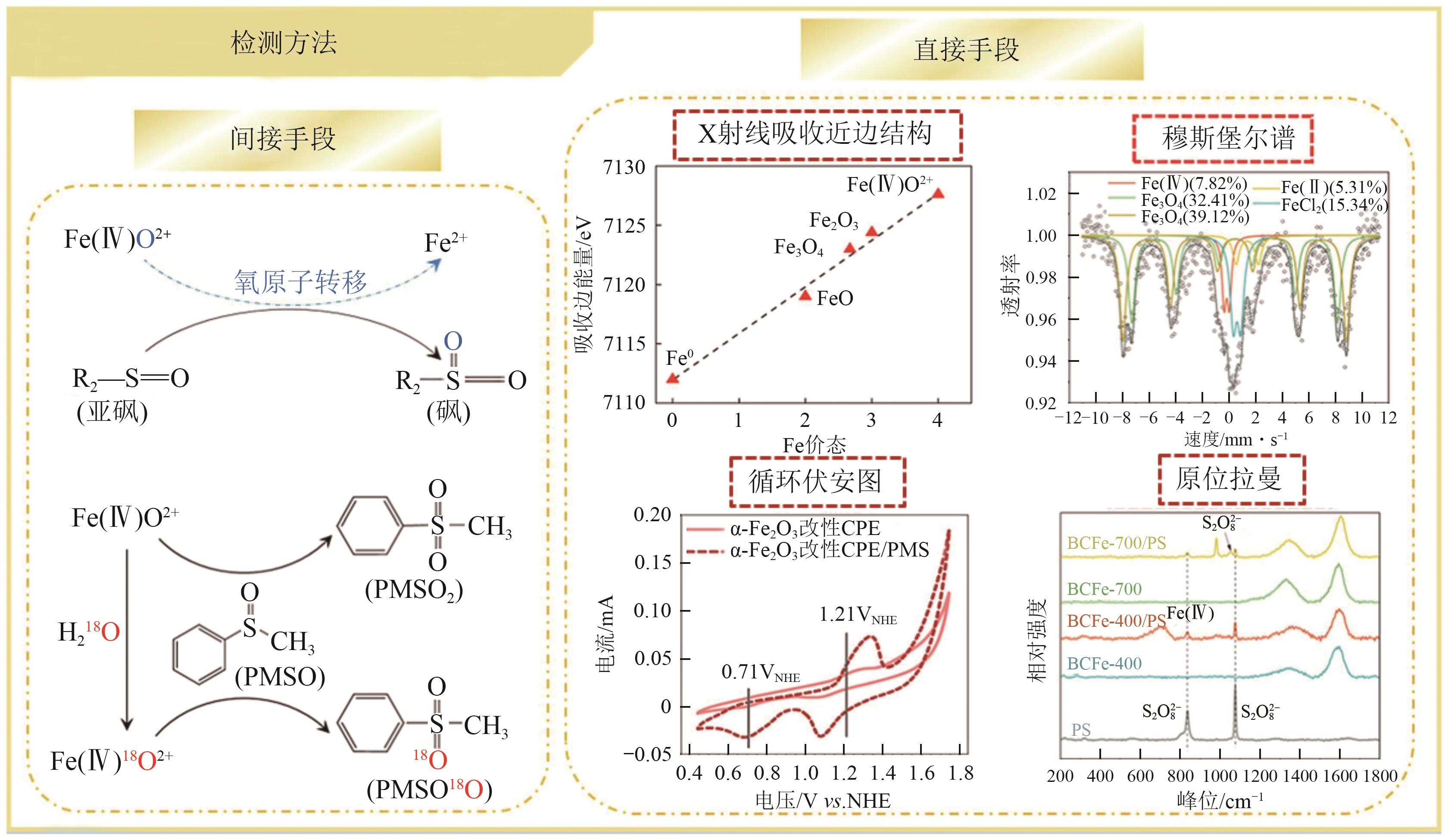
近年来,过硫酸盐基高级氧化技术(PS-AOPs)已被广泛用于废水中有机污染物的去除,其净水效果与催化剂诱导的活性氧物种类型密切相关。PS-AOPs体系可通过自由基路径(·OH和·SO4-等)或非自由基路径(1O2、电子转移和高价金属种等)降解污染物。自由基在非均相体系中存在无选择性、作用时间短、易于与卤素离子反应生成有毒副产物等问题,且废水中的多种干扰离子(如OH-、Cl-、CO32-等)会消耗自由基,减少其与有机污染物的作用量,从而影响处理效果。非自由基对富电子污染物的高选择性以及对卤素离子不敏感等优点使其成为当下研究热点。然而,国内外还没有针对催化剂结构/性质与PS-AOPs体系中非自由基路径调控的系统综述。基于此,本文介绍了1O2、电子转移和高价金属种三种典型非自由基路径的特点、生成机制及鉴定方法,并概括了缩小催化剂活性位点尺寸、在催化剂中引入杂原子和改变催化剂物理化学特性三种实现非自由基定向调控的方法及机理,最后展望了非自由基调控应往提升催化剂稳定性、完善自由基/非自由基贡献度区分方法、明确非自由基物种氧化有机污染物的机制以及深入研究活性物种生成路径或污染物降解路径的方向发展,为实现非自由基路径高效活化PS降解有机污染物提供理论依据。
中图分类号:
引用本文
胡芝璇, 熊婷, 蒋龙波, 袁兴中. 过硫酸盐体系中非自由基路径的调控研究进展[J]. 化工进展, 2025, 44(10): 5975-5990.
HU Zhixuan, XIONG Ting, JIANG Longbo, YUAN Xingzhong. Advances in the regulation of non-free radical pathways in persulfate systems[J]. Chemical Industry and Engineering Progress, 2025, 44(10): 5975-5990.
| 催化剂类型 | 催化剂 | 非自由基路径 | 活性位点 | 实验条件 | 催化效率 | 参考文献 |
|---|---|---|---|---|---|---|
| 过渡金属基催化剂 | Co、N掺杂石墨碳(CZIF) | 1O2 | Co3+和Co0、石墨N、吡咯N、酮基(C | [催化剂]0=0.05g/L,[卡马西平] 0=5mg/L, [PMS] 0=0.4mmol/L | 96.8% | [ |
| CuO和钴/氮掺杂的碳质框架(Cu@Co-N-C) | 1O2、电子转移 | Cu和Co位点、羟基(—OH)、酮基(C | [催化剂]0=0.025g/L,[萘普生]0=11.5mg/L, [PMS] 0=0.5mmol/L | 100% | [ | |
| 生物炭铜单原子催化剂(SACu30@NC) | 电子转移 | Cu(Ⅰ)、Cu(Ⅱ)和Cu(Ⅲ) | [催化剂]0=0.1g/L,[双酚A]0=20mg/L, [PDS]0=0.2g/L | 100% | [ | |
| 二元金属-有机骨架衍生的催化剂(Mn-Co3O4) | (中性和酸性)电子转移、1O2、高价钴氧 | Co和Mn位点、氧空位 | [催化剂]0=0.05g/L,[磺胺甲𫫇唑]0=10mg/L, [PDS]0=0.5mmol/L | 100% | [ | |
| 含铁氮化碳(Fe-CN)单原子催化剂 | 1O2、高价铁氧 | Fe-N3O1位点 | [催化剂]0=0.1g/L,[磺胺甲𫫇唑]0=10mg/L, [PMS]0=1mmol/L | 99.97% | [ | |
| FeO和Fe2O3 | 1O2、高价铁氧 | FeO(220) | [催化剂]0=0.1g/L,[四环素]0=20mg/L, [PMS]0=0.1g/L | 99% | [ | |
| 碳基催化剂 | 氮掺杂的碳化聚苯胺(N-CPANI) | 1O2、电子转移 | 石墨N、酮基(C | [催化剂]0=0.75g/L,[强力霉素]0=20mg/L, [PDS]0=0.5g/L | 91.66% | [ |
| 石墨化氮掺杂生物炭纤维(PGBF-N) | 1O2、电子转移 | 酮基(C | [催化剂]0=0.1g/L,[四环素]0=20mg/L, [PMS]0=1mmol/L | 96.5% | [ | |
| 氮掺杂多孔碳材料(NPCs) | 1O2、电子转移 | 石墨N、sp2杂化碳 | [催化剂]0=0.1g/L,[罗丹明B]0=50mg/L, [PMS]0=0.3g/L | 99.25% | [ | |
| 富氮层系多孔石墨碳(NHC) | 1O2、电子转移 | 缺陷边、酮基(C | [催化剂]0=0.05g/L,[四环素]0=20mg/L, [PDS]0=5mmol/L | 94.4% | [ |
表1 非自由基路径主导氧化降解有机污染物研究进展
| 催化剂类型 | 催化剂 | 非自由基路径 | 活性位点 | 实验条件 | 催化效率 | 参考文献 |
|---|---|---|---|---|---|---|
| 过渡金属基催化剂 | Co、N掺杂石墨碳(CZIF) | 1O2 | Co3+和Co0、石墨N、吡咯N、酮基(C | [催化剂]0=0.05g/L,[卡马西平] 0=5mg/L, [PMS] 0=0.4mmol/L | 96.8% | [ |
| CuO和钴/氮掺杂的碳质框架(Cu@Co-N-C) | 1O2、电子转移 | Cu和Co位点、羟基(—OH)、酮基(C | [催化剂]0=0.025g/L,[萘普生]0=11.5mg/L, [PMS] 0=0.5mmol/L | 100% | [ | |
| 生物炭铜单原子催化剂(SACu30@NC) | 电子转移 | Cu(Ⅰ)、Cu(Ⅱ)和Cu(Ⅲ) | [催化剂]0=0.1g/L,[双酚A]0=20mg/L, [PDS]0=0.2g/L | 100% | [ | |
| 二元金属-有机骨架衍生的催化剂(Mn-Co3O4) | (中性和酸性)电子转移、1O2、高价钴氧 | Co和Mn位点、氧空位 | [催化剂]0=0.05g/L,[磺胺甲𫫇唑]0=10mg/L, [PDS]0=0.5mmol/L | 100% | [ | |
| 含铁氮化碳(Fe-CN)单原子催化剂 | 1O2、高价铁氧 | Fe-N3O1位点 | [催化剂]0=0.1g/L,[磺胺甲𫫇唑]0=10mg/L, [PMS]0=1mmol/L | 99.97% | [ | |
| FeO和Fe2O3 | 1O2、高价铁氧 | FeO(220) | [催化剂]0=0.1g/L,[四环素]0=20mg/L, [PMS]0=0.1g/L | 99% | [ | |
| 碳基催化剂 | 氮掺杂的碳化聚苯胺(N-CPANI) | 1O2、电子转移 | 石墨N、酮基(C | [催化剂]0=0.75g/L,[强力霉素]0=20mg/L, [PDS]0=0.5g/L | 91.66% | [ |
| 石墨化氮掺杂生物炭纤维(PGBF-N) | 1O2、电子转移 | 酮基(C | [催化剂]0=0.1g/L,[四环素]0=20mg/L, [PMS]0=1mmol/L | 96.5% | [ | |
| 氮掺杂多孔碳材料(NPCs) | 1O2、电子转移 | 石墨N、sp2杂化碳 | [催化剂]0=0.1g/L,[罗丹明B]0=50mg/L, [PMS]0=0.3g/L | 99.25% | [ | |
| 富氮层系多孔石墨碳(NHC) | 1O2、电子转移 | 缺陷边、酮基(C | [催化剂]0=0.05g/L,[四环素]0=20mg/L, [PDS]0=5mmol/L | 94.4% | [ |
| [1] | ZHENG Xiaoxian, NIU Xiaojun, ZHANG Dongqing, et al. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review[J]. Chemical Engineering Journal, 2022, 429: 132323. |
| [2] | 刘路明, 高志敏, 邓兆雄, 等. 过硫酸盐的活化及其在氧化降解水中抗生素的机理和应用[J]. 环境化学, 2022, 41(5): 1702-1717. |
| LIU Luming, GAO Zhimin, DENG Zhaoxiong, et al. Activation of persulfate and its mechanism and application in oxidative degradation of antibiotics in water[J]. Environmental Chemistry, 2022, 41(5): 1702-1717. | |
| [3] | 郭若男, 吕宁磬. 单原子催化剂在过硫酸盐氧化体系中的研究进展[J]. 环境化学, 2024, 43(3): 834-845. |
| GUO Ruonan, Ningqing LYU. Research progress of single-atom catalysts in persulfate activation[J]. Environmental Chemistry, 2024, 43(3): 834-845. | |
| [4] | 罗瑞. 过硫酸盐高级氧化过程中活性氧物种的研究进展[J]. 当代化工研究, 2024(4): 6-8. |
| LUO Rui. Research progress of reactive oxygen species in persulfate activation[J]. Modern Chemical Research, 2024(4): 6-8. | |
| [5] | RODRIGUES Carmen S D, AZIZ Sofia n A, PEREIRA M F R, et al. Degradation of p-nitrophenol by activated persulfate with carbon-based materials[J]. Journal of Environmental Management, 2023, 343: 118140. |
| [6] | VENÂNCIO João P F, RODRIGUES Carmen S D, NUNES Olga C, et al. Application of iron-activated persulfate for municipal wastewater disinfection[J]. Journal of Hazardous Materials, 2022, 426: 127989. |
| [7] | WANG Baowei, WANG Yu. A comprehensive review on persulfate activation treatment of wastewater[J]. Science of the Total Environment, 2022, 831: 154906. |
| [8] | DONG Luyu, XIA Yujin, HU Zhixin, et al. Research progress of persulfate activation technology[J]. Environmental Science and Pollution Research International, 2024, 31(22): 31771-31786. |
| [9] | JAWAD Ali, ZHAN Kun, WANG Haibin, et al. Tuning of persulfate activation from a free radical to a nonradical pathway through the incorporation of non-redox magnesium oxide[J]. Environmental Science & Technology, 2020, 54(4): 2476-2488. |
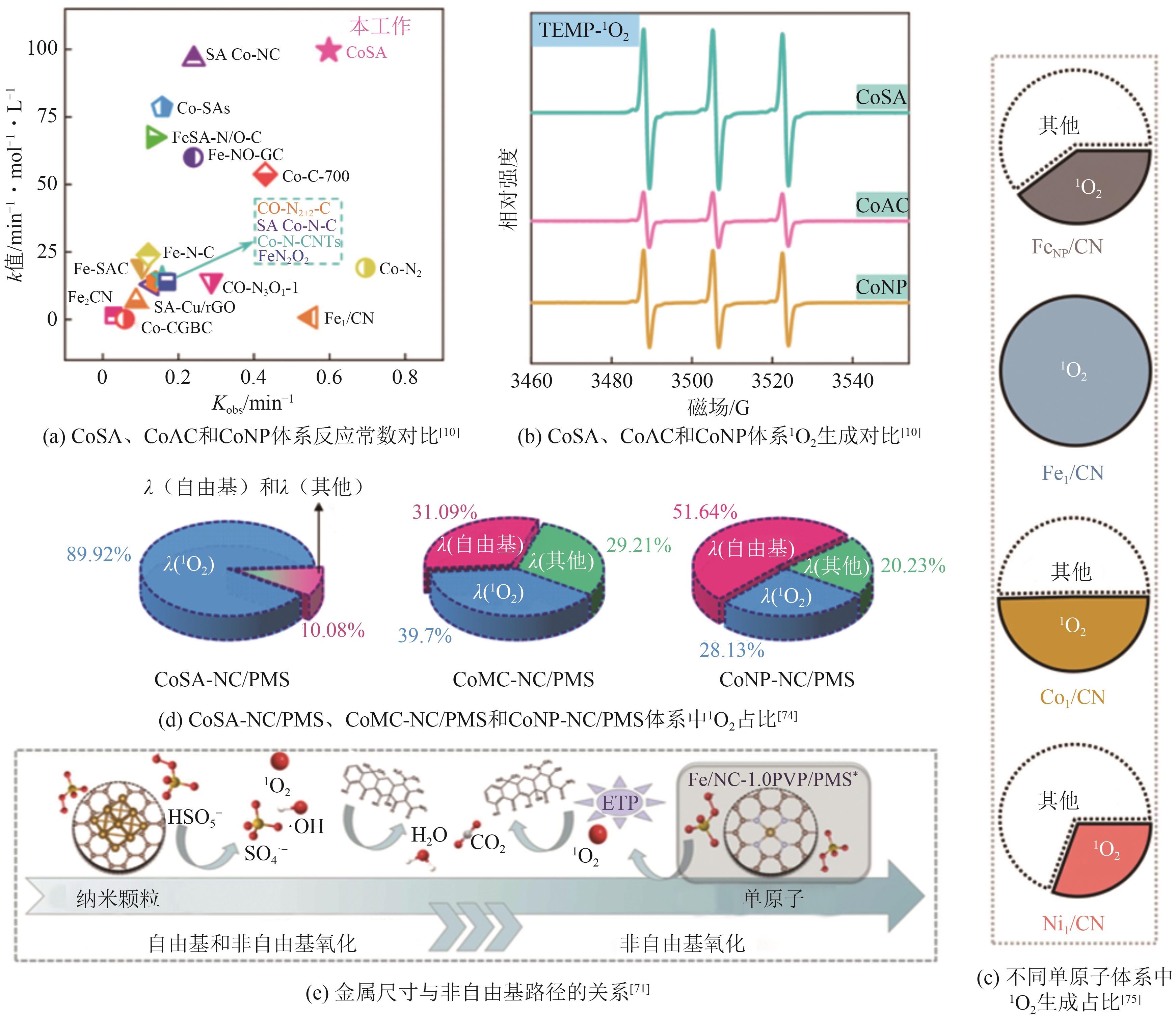
| [10] | WU Zelin, XIONG Zhaokun, LIU Wen, et al. Active center size-dependent Fenton-like chemistry for sustainable water decontamination[J]. Environmental Science & Technology, 2023, 57(50): 21416-21427. |
| [11] | WANG Liangjie, XIAO Ke, ZHAO Huazhang. The debatable role of singlet oxygen in persulfate-based advanced oxidation processes[J]. Water Research, 2023, 235: 119925. |
| [12] | ZHANG Tao, CHEN Yin, WANG Yuru, et al. Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation[J]. Environmental Science & Technology, 2014, 48(10): 5868-5875. |
| [13] | LIU Xinyue, YAN Xinyi, LIU Wenyuan, et al. Switching of radical and nonradical pathways through the surface defects of Fe3O4/MoO x S y in a Fenton-like reaction[J]. Science Bulletin, 2023, 68(6): 603-612. |
| [14] | ZHANG Jiali, WEI Jian, XIONG Zhaokun, et al. Coupled adsorption and non-radical dominated mechanisms in Co,N-doped graphite via peroxymonosulfate activation for efficiently degradation of carbamazepine[J]. Separation and Purification Technology, 2023, 309: 122981. |
| [15] | ZHANG Jin, ZENG Hanxuan, BU Lingjun, et al. Cu0 incorporated cobalt/nitrogen doped carbonaceous frameworks derived from ZIF-67 (Cu@Co-N-C) as PMS activator for efficient degradation of naproxen: Direct electron transfer and 1O2 dominated nonradical mechanisms[J]. Chemical Engineering Journal, 2023, 454: 139989. |
| [16] | PAN Jingwen, WANG Xinyuan, YANG Xinyu, et al. Insights into the enhanced oxidation of organic micropollutants by single-atom Cu catalyst activated peroxydisulfate: Valence-dominated nonradical pathway[J]. Applied Catalysis B: Environment and Energy, 2024, 351: 123997. |
| [17] | CHEN Yanling, CHEN Dandan, BAI Xue. Binary MOFs-derived Mn-Co3O4 for efficient peroxymonosulfate activation to remove sulfamethoxazole: Oxygen vacancy-assisted high-valent cobalt-oxo species generation[J]. Chemical Engineering Journal, 2024, 479: 147886. |
| [18] | ZENG Yuxi, DENG Jie, ZHOU Nan, et al. Mediated peroxymonosulfate activation at the single atom Fe-N3O1 sites: Synergistic degradation of antibiotics by two non-radical pathways[J]. Small, 2024, 20(32): 2311552. |
| [19] | WANG Guangfu, HUANG Danlian, CHENG Min, et al. The surface confinement of FeO assists in the generation of singlet oxygen and high-valent metal-oxo species for enhanced Fenton-like catalysis[J]. Small, 2024, 20(37): 2401970. |
| [20] | CHENG Minxian, MA Rui, CHAI Guodong, et al. Nitrogen-doped carbonized polyaniline (N-CPANI) for peroxydisulfate (PDS) activation towards efficient degradation of doxycycline (DOX) via the non-radical pathway dominated by electron transfer[J]. Chemical Engineering Journal, 2023, 453: 139810. |
| [21] | YE Shujing, ZENG Guangming, TAN Xiaofei, et al. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer[J]. Applied Catalysis B: Environmental, 2020, 269: 118850. |
| [22] | YANG Juan, SUN Yuxing, CHANG Xiaojuan, et al. MOF-derived N-doped porous carbons activate peroxomonosulfate to efficiently degrade organic pollutants via non-radical pathways[J]. Journal of Water Process Engineering, 2023, 56: 104295. |
| [23] | PI Zhoujie, HOU Kunjie, YAO Fubing, et al. Nonradical-dominated peroxydisulfate activation by nitrogen-rich hierarchical porous graphite carbon for efficient degradation of tetracycline[J]. Carbon, 2022, 196: 736-748. |
| [24] | WANG Yue, LIN Yan, YANG Chunping, et al. Calcination temperature regulates non-radical pathways of peroxymonosulfate activation via carbon catalysts doped by iron and nitrogen[J]. Chemical Engineering Journal, 2023, 451: 138468. |
| [25] | PENG Xin, FAN Zhengyi, WANG Qingyuan, et al. Recent advances on the role of high-valent metals formed during persulfate activation[J]. Separation and Purification Technology, 2024, 345: 127365. |
| [26] | ZHOU Xiaoyue, YIN Renli, KANG Jieqiong, et al. Atomic cation-vacancy modulated peroxymonosulfate nonradical oxidation of sulfamethoxazole via high-valent iron-oxo species[J]. Applied Catalysis B: Environmental, 2023, 330: 122640. |
| [27] | ZHANG Chi, DING Ning, PAN Yuwei, et al. The degradation pathways of contaminants by reactive oxygen species generated in the Fenton/Fenton-like systems[J]. Chinese Chemical Letters, 2024, 35(10): 109579. |
| [28] | LIU Zonghao, TAN Chaoqun, ZHAO Yan, et al. Singlet oxygen in biochar-based catalysts-activated persulfate process: From generation to detection and selectivity removing emerging contaminants[J]. Chemical Engineering Journal, 2024, 485: 149724. |
| [29] | YAN Yiqi, WEI Zongsu, DUAN Xiaoguang, et al. Merits and limitations of radical vs. nonradical pathways in persulfate-based advanced oxidation processes[J]. Environmental Science & Technology, 2023, 57(33): 12153-12179. |
| [30] | WANG Yingjun, BAO Shuangyou, LIU Xinyang, et al. Regulating the peroxymonosulfate activation on N doped δ-MnO2 nanosheets for tetracycline degradation: N species as the degradation pathways switcher to convert radical to nonradical[J]. Chemical Engineering Journal, 2023, 477: 147050. |
| [31] | WANG Anna, ZHU Benzhan, HUANG Chunhua, et al. Generation mechanism of singlet oxygen from the interaction of peroxymonosulfate and chloride in aqueous systems[J]. Water Research, 2023, 235: 119904. |
| [32] | SHAO Penghui, TIAN Jiayu, YANG Feng, et al. Identification and regulation of active sites on nanodiamonds: Establishing a highly efficient catalytic system for oxidation of organic contaminants[J]. Advanced Functional Materials, 2018, 28(13): 1705295. |
| [33] | ZHOU Yang, JIANG Jin, GAO Yuan, et al. Activation of peroxymonosulfate by benzoquinone: A novel nonradical oxidation process[J]. Environmental Science & Technology, 2015, 49(21): 12941-12950. |
| [34] | ZHU Lijun, WANG Huan, SUN Jian, et al. Sulfur vacancies in pyrite trigger the path to nonradical singlet oxygen and spontaneous sulfamethoxazole degradation: Unveiling the hidden potential in sediments[J]. Environmental Science & Technology, 2024, 58(15): 6753-6762. |
| [35] | WANG Yue, LIN Yan, HE Shanying, et al. Singlet oxygen: Properties, generation, detection, and environmental applications[J]. Journal of Hazardous Materials, 2024, 461: 132538. |
| [36] | ZHOU Xinquan, ZHAO Qindi, WANG Jia, et al. Nonradical oxidation processes in PMS-based heterogeneous catalytic system: Generation, identification, oxidation characteristics, challenges response and application prospects[J]. Chemical Engineering Journal, 2021, 410: 128312. |
| [37] | CHENG Minxian, ZHANG Yichu, LAI Bo, et al. Nitrogen and phosphorus co-doped porous carbons (NPCs) for peroxydisulfate (PDS) activation towards tetracycline degradation: Defects enhanced adsorption and non-radical mechanism dominated by electron transfer[J]. Chemical Engineering Journal, 2023, 455: 140615. |
| [38] | SUN Fuwei, CHEN Tianhu, LIU Haibo, et al. The pH-dependent degradation of sulfadiazine using natural siderite activating PDS: The role of singlet oxygen[J]. Science of the Total Environment, 2021, 784: 147117. |
| [39] | CHENG Xin, GUO Hongguang, ZHANG Yongli, et al. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes[J]. Water Research, 2017, 113: 80-88. |
| [40] | CHENG Xin, GUO Hongguang, ZHANG Yongli, et al. Insights into the mechanism of nonradical reactions of persulfate activated by carbon nanotubes: Activation performance and structure-function relationship[J]. Water Research, 2019, 157: 406-414. |
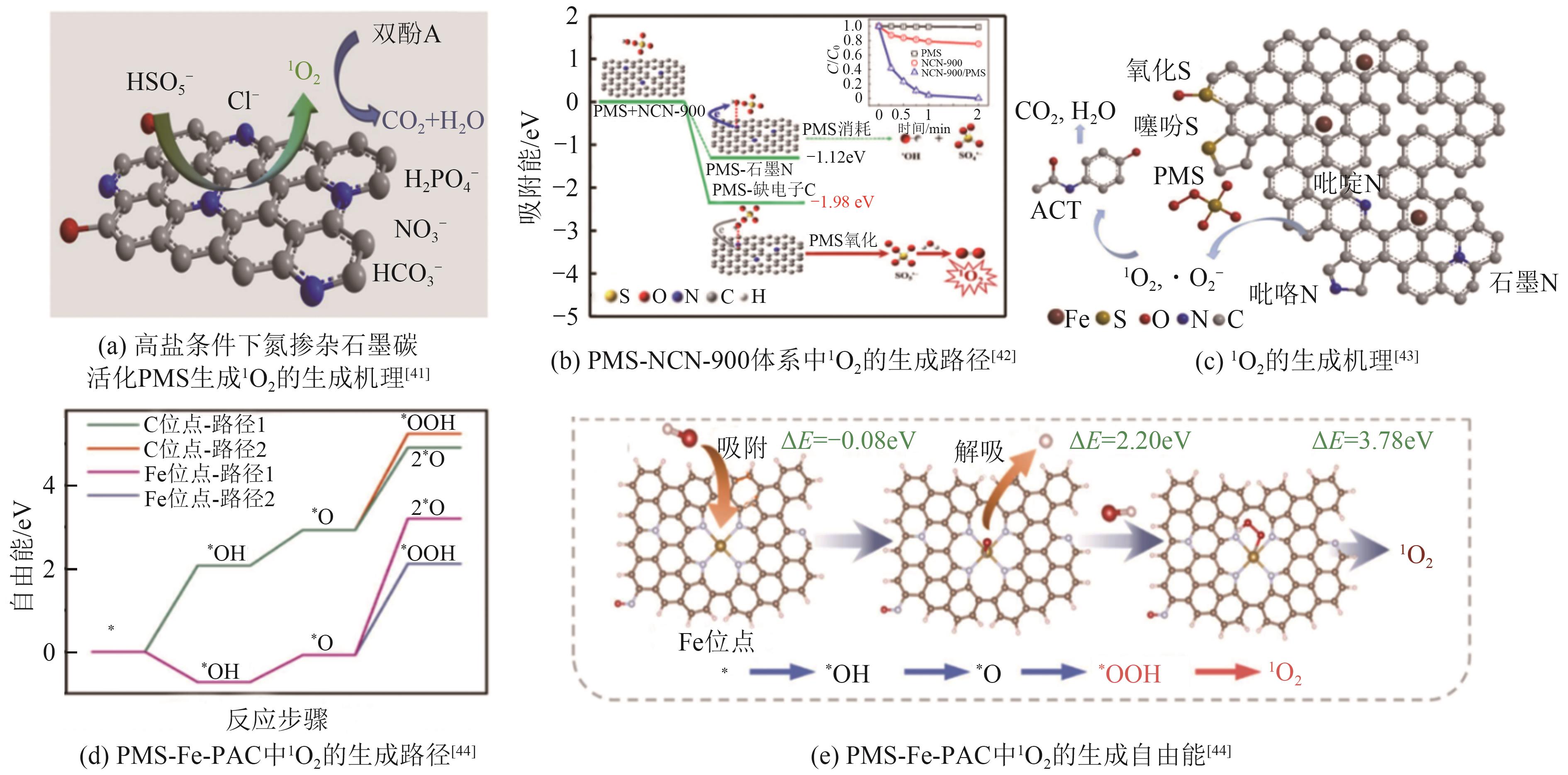
| [41] | LUO Rui, LI Miaoqing, WANG Chaohai, et al. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition[J]. Water Research, 2019, 148: 416-424. |
| [42] | GAO Yaowen, CHEN Zhenhuan, ZHU Yue, et al. New insights into the generation of singlet oxygen in the metal-free peroxymonosulfate activation process: Important role of electron-deficient carbon atoms[J]. Environmental Science & Technology, 2020, 54(2): 1232-1241. |
| [43] | CHEN Yuyu, LIANG Qianwei, DENG Yuhui, et al. Peroxymonosulfate activation by concave porous S/N co-doped carbon: Singlet oxygen-dominated non-radical efficient oxidation of organics[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107933. |
| [44] | LIU Yunlong, ZHOU Hongyan, JIN Can, et al. Bio-porphyrin supported single-atom iron catalyst boosting peroxymonosulfate activation for pollutants degradation: A singlet oxygen-dominated nonradical pathway[J]. Applied Catalysis B: Environmental, 2023, 338: 123061. |
| [45] | YANG Zhaoyi, YANG Xiaofang, ZHANG Weijun, et al. Asymmetrically coordinated Mn-S1N3 configuration induces localized electric field-driven peroxymonosulfate activation for remarkably efficient generation of 1O2 [J]. Small, 2024, 20(32): 2311642. |
| [46] | ZHOU Yang, JIANG Jin, GAO Yuan, et al. Activation of peroxymonosulfate by phenols: Important role of quinone intermediates and involvement of singlet oxygen[J]. Water Research, 2017, 125: 209-218. |
| [47] | YU Jiangfang, TANG Lin, PANG Ya, et al. Non-radical oxidation in environmental catalysis: Recognition, identification, and perspectives[J]. Chemical Engineering Journal, 2022, 433: 134385. |
| [48] | WANG Zhiwei, WANG Wenlong, WANG Jin, et al. High-valent iron-oxo species mediated cyclic oxidation through single-atom Fe-N6 sites with high peroxymonosulfate utilization rate[J]. Applied Catalysis B: Environmental, 2022, 305: 121049. |
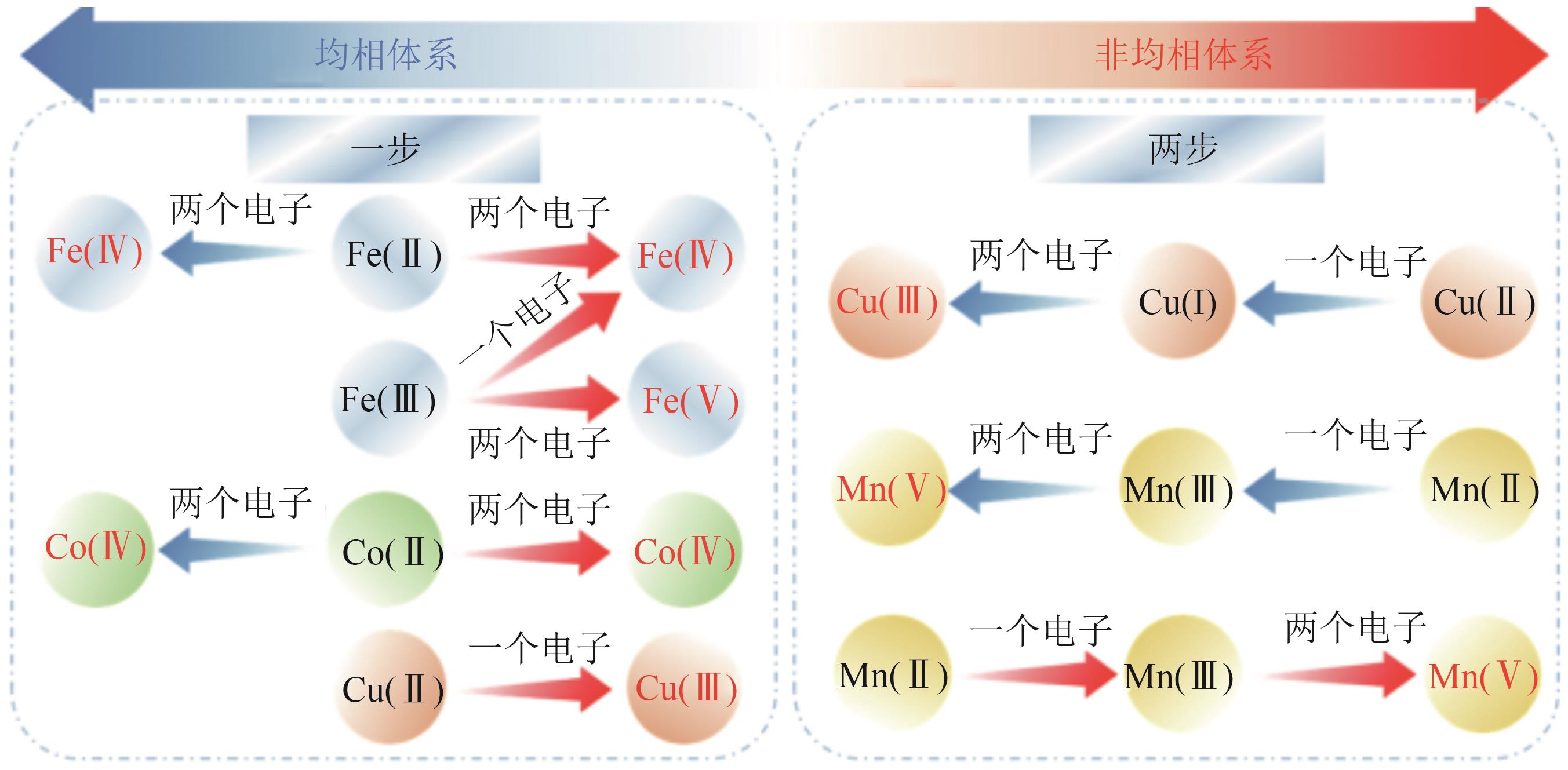
| [49] | PESTOVSKY Oleg, BAKAC Andreja. Aqueous ferryl(Ⅳ) ion: Kinetics of oxygen atom transfer to substrates and oxo exchange with solvent water[J]. Inorganic Chemistry, 2006, 45(2): 814-820. |
| [50] | PESTOVSKY Oleg, STOIAN Sebastian, BOMINAAR Emile L, et al. Aqueous FeⅣ ̿ O: Spectroscopic identification and oxo-group exchange[J]. Angewandte Chemie International Edition, 2005, 44(42): 6871-6874. |
| [51] | WANG Zhen, JIANG Jin, PANG Suyan, et al. Is sulfate radical really generated from peroxydisulfate activated by iron(Ⅱ) for environmental decontamination?[J]. Environmental Science & Technology, 2018, 52(19): 11276-11284. |
| [52] | BAO Yan, LIAN Cheng, HUANG Kai, et al. Generating high-valent iron-oxo ≡≡FeⅣ ̿ O complexes in neutral microenvironments through peroxymonosulfate activation by Zn-Fe layered double hydroxides[J]. Angewandte Chemie International Edition, 2022, 61(42): e202209542. |
| [53] | CHEN Yiqun, LONG Liying, LUO Yingxi, et al. Insights into persulfate activation by FeO for phenol removal: The production and effect of Fe(Ⅳ)[J]. Chemical Engineering Journal, 2023, 468: 143842. |
| [54] | WANG Zhen, QIU Wei, PANG Suyan, et al. Aqueous iron(Ⅳ)-oxo complex: An emerging powerful reactive oxidant formed by iron(Ⅱ)-based advanced oxidation processes for oxidative water treatment[J]. Environmental Science & Technology, 2022, 56(3): 1492-1509. |
| [55] | PIERPONT Aaron W, CUNDARI Thomas R. Computational study of methane C-H activation by first-row late transition metal L n M ̿ E (M: Fe, Co, Ni) complexes[J]. Inorganic Chemistry, 2010, 49(5): 2038-2046. |
| [56] | LARSON Virginia A, BATTISTELLA Beatrice, Kallol RAY, et al. Iron and manganese oxo complexes, oxo wall and beyond[J]. Nature Reviews Chemistry, 2020, 4(8): 404-419. |
| [57] | JIANG Jingjing, ZHAO Ziqing, GAO Jiaying, et al. Nitrogen vacancy-modulated peroxymonosulfate nonradical activation for organic contaminant removal via high-valent cobalt-oxo species[J]. Environmental Science & Technology, 2022, 56(9): 5611-5619. |
| [58] | ZOU Yixiao, LI Jie, TAN Jie, et al. High-valent cobalt-oxo species triggers singlet oxygen for rapid contaminants degradation along with mild peroxymonosulfate decomposition in single Co atom-doped g-C3N4 [J]. Chemical Engineering Journal, 2023, 471: 144531. |
| [59] | GAO Yuan, ZHOU Yang, PANG Suyan, et al. Enhanced peroxymonosulfate activation via complexed Mn(Ⅱ): A novel non-radical oxidation mechanism involving manganese intermediates[J]. Water Research, 2021, 193: 116856. |
| [60] | WEI Yan, MIAO Jie, GE Jianxin, et al. Ultrahigh peroxymonosulfate utilization efficiency over CuO nanosheets via heterogeneous Cu(Ⅲ) formation and preferential electron transfer during degradation of phenols[J]. Environmental Science & Technology, 2022, 56(12): 8984-8992. |
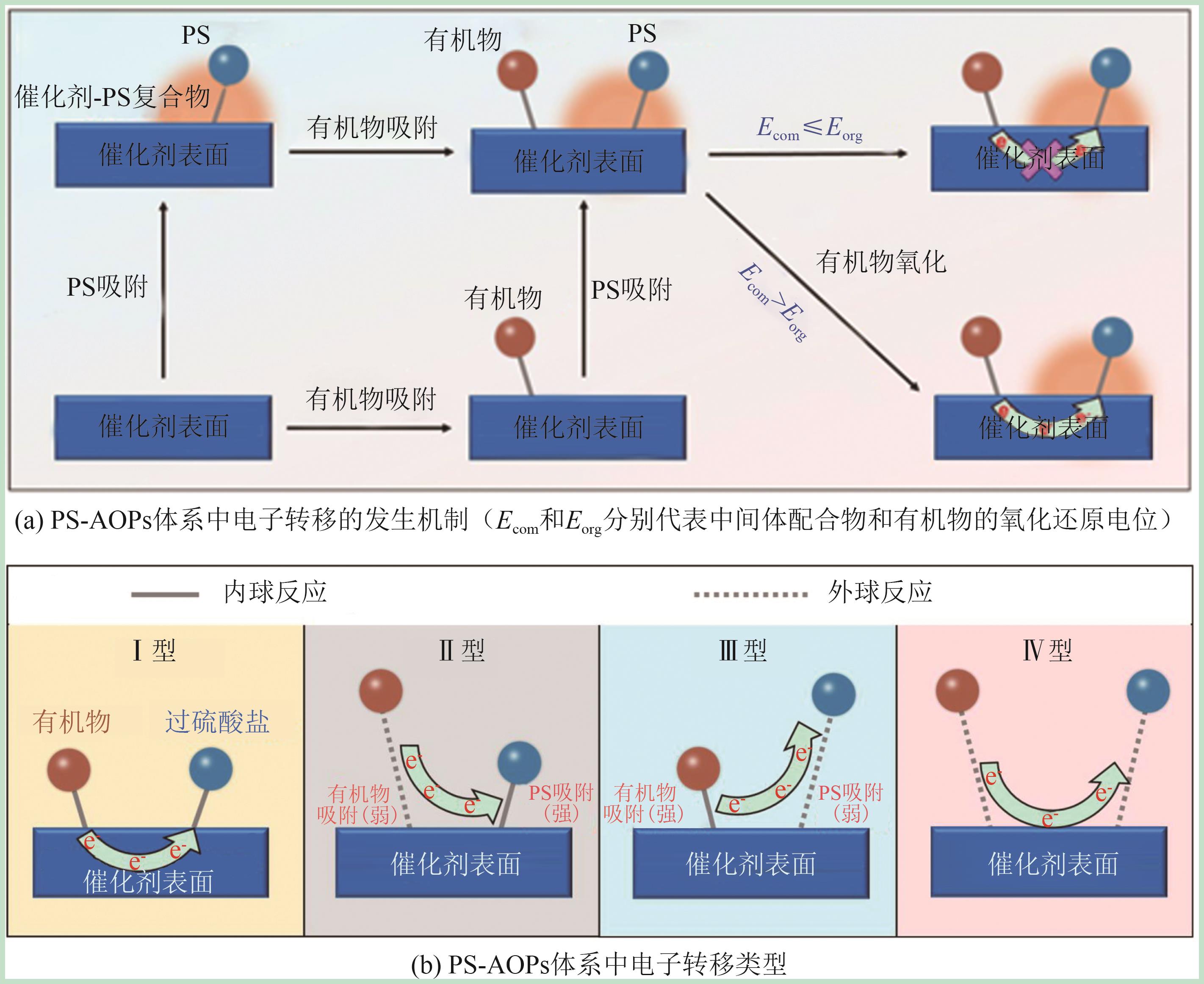
| [61] | REN Wei, CHENG Cheng, SHAO Penghui, et al. Origins of electron-transfer regime in persulfate-based nonradical oxidation processes[J]. Environmental Science & Technology, 2022, 56(1): 78-97. |
| [62] | ZUO Shiyu, LI Dongya, GUAN Zeyu, et al. Rapid electron transfer boosts CuO-mediated nonradical oxidation pathways for efficient removal of Bisphenol A[J]. Journal of Cleaner Production, 2022, 336: 130480. |
| [63] | ZUO Shiyu, GUAN Zeyu, XIA Dongsheng, et al. Polarized heterogeneous CuO-CN for peroxymonosulfate nonradical activation: An enhancement mechanism of mediated electron transfer[J]. Chemical Engineering Journal, 2021, 420: 127619. |
| [64] | ZHU Hongqing, MA Hui, YU Jingyang, et al. Encapsulating MnFe LDH in biochar tunes persulfate activation from radical to nonradical pathway: Significant role of electron transfer[J]. ACS ES&T Water, 2023, 3(10): 3343-3356. |
| [65] | GUO Yaoping, ZENG Zequan, ZHU Youcai, et al. Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulfur-doped hierarchically porous carbon derived from thiophene[J]. Applied Catalysis B: Environmental, 2018, 220: 635-644. |
| [66] | PENG Haihao, XIONG Weiping, YANG Zhaohui, et al. Insights into the mechanism of persulfate activation by hollow MOF-derived carbon: Electron transfer-triggered non-radical oxidization for antibiotic removal[J]. Environmental Science: Nano, 2024, 11(1): 216-228. |
| [67] | JIANG Jingjing, YUE Chenli, ZHAO Ziqing, et al. Breaking hydrogen bond in metal free carbon nitride to induce peroxymonosulfate nonradical activation: Surface-mediated electron transfer[J]. Applied Catalysis B: Environment and Energy, 2024, 355: 124153. |
| [68] | HUANG Bingkun, WU Zelin, ZHOU Hongyu, et al. The structure-performance relationships in active center size-dependent Fenton-like catalysis: From nanoparticles to single atoms[J]. Applied Catalysis B: Environment and Energy, 2024, 355: 124157. |
| [69] | GUO Wenxin, WANG Zhiyuan, WANG Xiaoqian, et al. General design concept for single-atom catalysts toward heterogeneous catalysis[J]. Advanced Materials, 2021, 33(34): 2004287. |
| [70] | ZHANG Wei, LI Mu, LUO Jingwen, et al. Modulating the coordination environment of Co single-atom catalysts through sulphur doping to efficiently enhance peroxymonosulfate activation for degradation of carbamazepine[J]. Chemical Engineering Journal, 2023, 474: 145377. |
| [71] | LI Haoyue, WANG Na, LI Han, et al. Polyvinylpyrrolidone-induced size-dependent catalytic behavior of Fe sites on N-doped carbon substrate and mechanism conversion in Fenton-like oxidation reaction[J]. Applied Catalysis B: Environmental, 2024, 341: 123323. |
| [72] | MA Chenyang, GUO Yajie, ZHANG Daofang, et al. Metal-nitrogen-carbon catalysts for peroxymonosulfate activation to degrade aquatic organic contaminants: Rational design, size-effect description, applications and mechanisms[J]. Chemical Engineering Journal, 2023, 454: 140216. |
| [73] | ZENG Qingming, WEN Yanjun, DUAN Xiaoguang, et al. Single-atom Ni-N4 sites coordinate dual nonradical oxidation pathways via peroxymonosulfate activation: Computational insights and in situ spectroscopic analyses[J]. Applied Catalysis B: Environment and Energy, 2024, 346: 123752. |
| [74] | WANG Xinhao, XIONG Zhaokun, SHI Hongle, et al. Switching the reaction mechanisms and pollutant degradation routes through active center size-dependent Fenton-like catalysis[J]. Applied Catalysis B: Environmental, 2023, 329: 122569. |
| [75] | ZHANG Longshuai, JIANG Xunheng, ZHONG Ziai, et al. Carbon nitride supported high-loading Fe single-atom catalyst for activation of peroxymonosulfate to generate 1O2 with 100% selectivity[J]. Angewandte Chemie International Edition, 2021, 60(40): 21751-21755. |
| [76] | YANG Mengxue, HOU Zexi, ZHANG Xin, et al. Unveiling the origins of selective oxidation in single-atom catalysis via Co-N4-C intensified radical and nonradical pathways[J]. Environmental Science & Technology, 2022, 56(16): 11635-11645. |
| [77] | FAN Mengmeng, CUI Jiewu, WU Jingjie, et al. Improving the catalytic activity of carbon-supported single atom catalysts by polynary metal or heteroatom doping[J]. Small, 2020, 16(22): 1906782. |
| [78] | FAN Xindan, LIN Qintie, XU Kehuan, et al. Activation of peroxydisulfate degradation of ciprofloxacin by nitrogen-doped modified graphitized iron-based carbon materials: The transition from free to nonfree radicals[J]. Separation and Purification Technology, 2023, 316: 123783. |
| [79] | REN Wei, NIE Gang, ZHOU Peng, et al. The intrinsic nature of persulfate activation and N-doping in carbocatalysis[J]. Environmental Science & Technology, 2020, 54(10): 6438-6447. |
| [80] | TUAN Nguyen Thanh, KIM Do Gun, Seok Oh KO. Catalytic degradation of acetaminophen by C and O co-doped graphitic carbon nitride: Peroxymonosulfate vs. peroxydisulfate[J]. Chemical Engineering Journal, 2024, 480: 148348. |
| [81] | DUAN Xiaoguang, AO Zhimin, ZHOU Li, et al. Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation[J]. Applied Catalysis B: Environmental, 2016, 188: 98-105. |
| [82] | ZHU Shishu, HUANG Xiaochen, MA Fang, et al. Catalytic removal of aqueous contaminants on N-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanisms[J]. Environmental Science & Technology, 2018, 52(15): 8649-8658. |
| [83] | ZHANG Jingyang, YANG Shuyi, ZHOU Kexin, et al. Preparation of co-doped biochar to improve electron transfer and modulate 1O2 generation: Unraveling the radical-unradical mechanism[J]. Chemical Engineering Journal, 2024, 491: 151985. |
| [84] | WANG Rupeng, HE Zixiang, CHEN Honglin, et al. Deciphering N-doped biochar design for non-radical pathways through hierarchical machine learning[J]. ACS ES&T Engineering, 2024, 4(7): 1738-1747. |
| [85] | WAN Zhonghao, SUN Yuqing, TSANG Daniel C W, et al. Customised fabrication of nitrogen-doped biochar for environmental and energy applications[J]. Chemical Engineering Journal, 2020, 401: 126136. |
| [86] | DOU Jibo, CHENG Jie, LU Zhijiang, et al. Biochar co-doped with nitrogen and boron switching the free radical based peroxydisulfate activation into the electron-transfer dominated nonradical process[J]. Applied Catalysis B: Environmental, 2022, 301: 120832. |
| [87] | XU Shizhe, WANG Pengfei, MI Xueyue, et al. N, S, and Cl tri-doped carbon boost the switching of radical to non-radical pathway in Fenton-like reactions: Synergism of N species and defects[J]. Journal of Hazardous Materials, 2024, 466: 133321. |
| [88] | LI Yin, HU Jiahui, ZOU Yubin, et al. Catalytic activity enhancement by P and S co-doping of a single-atom Fe catalyst for peroxymonosulfate-based oxidation[J]. Chemical Engineering Journal, 2023, 453: 139890. |
| [89] | LI Xintong, WANG Jun, DUAN Xiaoguang, et al. Fine-tuning radical/nonradical pathways on graphene by porous engineering and doping strategies[J]. ACS Catalysis, 2021, 11(8): 4848-4861. |
| [90] | ZHOU Xinquan, LUO Mengyi, XIE Chuyi, et al. Tunable S doping from Co3O4 to Co9S8 for peroxymonosulfate activation: Distinguished radical/nonradical species and generation pathways[J]. Applied Catalysis B: Environmental, 2021, 282: 119605. |
| [91] | ZHANG Ting, WU Shuang, LI Ning, et al. Applications of vacancy defect engineering in persulfate activation: Performance and internal mechanism[J]. Journal of Hazardous Materials, 2023, 449: 130971. |
| [92] | XIE Lan, HAO Jiajia, WU Yinsu, et al. Non-radical activation of peroxymonosulfate with oxygen vacancy-rich amorphous MnO x for removing sulfamethoxazole in water[J]. Chemical Engineering Journal, 2022, 436: 135260. |
| [93] | WU Liying, SUN Zhiqiang, ZHEN Yufei, et al. Oxygen vacancy-induced nonradical degradation of organics: Critical trigger of oxygen (O2) in the Fe-Co LDH/peroxymonosulfate system[J]. Environmental Science & Technology, 2021, 55(22): 15400-15411. |
| [94] | WANG Jun, DUAN Xiaoguang, GAO Jian, et al. Roles of structure defect, oxygen groups and heteroatom doping on carbon in nonradical oxidation of water contaminants[J]. Water Research, 2020, 185: 116244. |
| [95] | ZHU Jingyi, ZHU Yixin, ZHOU Wenjun. Cu-doped Ni-LDH with abundant oxygen vacancies for enhanced methyl 4-hydroxybenzoate degradation via peroxymonosulfate activation: Key role of superoxide radicals[J]. Journal of Colloid and Interface Science, 2022, 610: 504-517. |
| [96] | LIU Shiqi, YIN Siyuan, ZHANG Zichen, et al. Regulation of defects and nitrogen species on carbon nanotube by plasma-etching for peroxymonosulfate activation: Inducing non-radical/radical oxidation of organic contaminants[J]. Journal of Hazardous Materials, 2023, 441: 129905. |
| [97] | WANG Jinpeng, YAO Jia, LI Yubiao, et al. S vacancies-introduced chalcopyrite switch radical to non-radical pathways via peroxymonosulfate activation: Vital roles of S vacancies[J]. Journal of Hazardous Materials, 2024, 467: 133751. |
| [98] | PHAM Van Luan, KIM Do-Gun, Seok-Oh KO. Advanced oxidative degradation of acetaminophen by carbon catalysts: Radical vs. non-radical pathways[J]. Environmental Research, 2020, 188: 109767. |
| [99] | SONG Hui, GUAN Zeyu, CHEN Luyi, et al. Role of curvature in a carbon electronic structure under spatial confinement: Conversion of nonradicals to radicals[J]. Carbon, 2021, 180: 22-30. |
| [100] | ZENG Hanxuan, ZHU Hao, DENG Jing, et al. Tunable peroxymonosulfate activation by (-111) crystal plane exposed δ-MnO2: Oxidant concentration induced intrinsic mechanisms transformation[J]. Chemical Engineering Journal, 2023, 473: 145222. |
| [101] | ZHAO Huanxin, LIU Xinyue, LIU Yuqi, et al. Directionally inducing non-radical pathways for peroxymonosulfate activation by regulating the exposed crystal plane of MnO2 [J]. Process Safety and Environmental Protection, 2023, 177: 947-958. |
| [102] | HAN Chen, DUAN Xiaoguang, ZHANG Mingjie, et al. Role of electronic properties in partition of radical and nonradical processes of carbocatalysis toward peroxymonosulfate activation[J]. Carbon, 2019, 153: 73-80. |
| [103] | ALMEIDA Eduardo A, MIYAMOTO Sayuri, MARTINEZ Glaucia R, et al. Direct evidence of singlet molecular oxygen[O2 (1Δg)] production in the reaction of acetonitrile with hydrogen peroxide in alkaline solutions[J]. Analytica Chimica Acta, 2003, 482(1): 99-104. |
| [104] | SHAO Penghui, JING Yunpeng, DUAN Xiaoguang, et al. Revisiting the graphitized nanodiamond-mediated activation of peroxymonosulfate: Singlet oxygenation versus electron transfer[J]. Environmental Science & Technology, 2021, 55(23): 16078-16087. |
| [105] | YOU Youngmin. Chemical tools for the generation and detection of singlet oxygen[J]. Organic & Biomolecular Chemistry, 2018, 16(22): 4044-4060. |
| [106] | SCHLACHTER Adrien, ASSELIN Paul, CHATELAIN Axel, et al. Best practices to directly assess heterogeneous singlet oxygen photosensitization by phosphorescence[J]. Advanced Functional Materials, 2024: ,34(41) 2404111. |
| [107] | ZONG Yang, CHEN Long, ZENG Yunqiao, et al. Do we appropriately detect and understand singlet oxygen possibly generated in advanced oxidation processes by electron paramagnetic resonance spectroscopy?[J]. Environmental Science & Technology, 2023, 57(25): 9394-9404. |
| [108] | XIE Zhihui, HE Chuanshu, PEI Danni, et al. Review of characteristics, generation pathways and detection methods of singlet oxygen generated in advanced oxidation processes (AOPs)[J]. Chemical Engineering Journal, 2023, 468: 143778. |
| [109] | VICTÓRIA Henrique F V, FERREIRA Daniele C, FILHO José B G, et al. Detection of singlet oxygen by EPR: The instability of the nitroxyl radicals[J]. Free Radical Biology and Medicine, 2022, 180: 143-152. |
| [110] | WENG Zonglin, LIN Yuanfang, GUO Siyuan, et al. Site engineering of covalent organic frameworks for regulating peroxymonosulfate activation to generate singlet oxygen with 100% selectivity[J]. Angewandte Chemie International Edition, 2023, 62(43): e202310934. |
| [111] | NARDI Giacomo, MANET Ilse, MONTI Sandra, et al. Scope and limitations of the TEMPO/EPR method for singlet oxygen detection: The misleading role of electron transfer[J]. Free Radical Biology and Medicine, 2014, 77: 64-70. |
| [112] | LU Xiaohui, QIU Wei, MA Jun, et al. The overestimated role of singlet oxygen for pollutants degradation in some non-photochemical systems[J]. Chemical Engineering Journal, 2020, 401: 126128. |
| [113] | TANAKA Kumi, MIURA Tetsuo, UMEZAWA Naoki, et al. Rational design of fluorescein-based fluorescence probes. mechanism-based design of a maximum fluorescence probe for singlet oxygen[J]. Journal of the American Chemical Society, 2001, 123(11): 2530-2536. |
| [114] | 林大港. 焦磷酸钠/单过硫酸盐和碱/双氧水体系单线态氧鉴定方法初探[D]. 上海: 华东师范大学, 2023. |
| LIN Dagang. A preliminary study on the detection of singlet oxygen in the sodium pyrophosphate/monopersulfate and base-hydrogen peroxide systems[D]. Shanghai: East China Normal University, 2023. | |
| [115] | WU Haiyan, SONG Qijun, RAN Guoxia, et al. Recent developments in the detection of singlet oxygen with molecular spectroscopic methods[J]. TrAC Trends in Analytical Chemistry, 2011, 30(1): 133-141. |
| [116] | ZHANG Guanxin, LI Xiaohua, MA Huimin, et al. A selective and sensitive chemiluminescence reaction of 4,4’(5’)-bis[2-(9-anthryloxy)ethylthio]tetrathiafulvalene with singlet oxygen[J]. Chem Commun, 2004(18): 2072-2073. |
| [117] | LI Si, DAI Chaomeng, DUAN Yanping, et al. Non-radical pathways in peracetic acid-based micropollutant degradation: A comprehensive review of mechanisms, detection methods, and promising applications[J]. Separation and Purification Technology, 2024, 330: 125240. |
| [118] | MING Hongbo, BIAN Xiaoqiong, CHENG Jiajia, et al. Carbon nitride with a tailored electronic structure toward peroxymonosulfate activation: A direct electron transfer mechanism for organic pollutant degradation[J]. Applied Catalysis B: Environmental, 2024, 341: 123314. |
| [119] | YIN Kexin, WU Ruixian, SHANG Yanan, et al. Microenvironment modulation of cobalt single-atom catalysts for boosting both radical oxidation and electron-transfer process in Fenton-like system[J]. Applied Catalysis B: Environmental, 2023, 329: 122558. |
| [120] | KANG Hyeonseok, LEE Donghyun, LEE Ki-Myeong, et al. Nonradical activation of peroxymonosulfate by hematite for oxidation of organic compounds: A novel mechanism involving high-valent iron species[J]. Chemical Engineering Journal, 2021, 426: 130743. |
| [121] | LI Hong, QIU Rongliang, TANG Yetao, et al. Formation of Fe(Ⅳ) over a wide pH range via iron-carbon composite-catalyzed persulfate activation[J]. Chemical Engineering Journal, 2023, 461: 141951. |
| [122] | ZONG Yang, ZHANG Hua, ZHANG Xiaomeng, et al. High-valent cobalt-oxo species triggers hydroxyl radical for collaborative environmental decontamination[J]. Applied Catalysis B: Environmental, 2022, 300: 120722. |
| [123] | HE Yangzhuo, QIN Hong, WANG Ziwei, et al. Fe-Mn oxycarbide anchored on N-doped carbon for enhanced Fenton-like catalysis: Importance of high-valent metal-oxo species and singlet oxygen[J]. Applied Catalysis B: Environmental, 2024, 340: 123204. |
| [124] | WANG Zhen, QIU Wei, PANG Suyan, et al. Further understanding the involvement of Fe(Ⅳ) in peroxydisulfate and peroxymonosulfate activation by Fe(Ⅱ) for oxidative water treatment[J]. Chemical Engineering Journal, 2019, 371: 842-847. |
| [1] | 罗司玲, 艾建平, 李文魁, 王翼, 程丽红, 万芸, 黄隆, 李喜宝. 高级氧化技术降解典型抗生素的研究进展[J]. 化工进展, 2025, 44(7): 4169-4189. |
| [2] | 马丙瑞, 段学斌, 陈澄, 王松雪, 陈琳, 王守成, 李金成, 武桂芝, 闫博引. UV-氯联用降解卡马西平动力学及活性氯物种的功能[J]. 化工进展, 2025, 44(7): 4212-4222. |
| [3] | 王御豪, 蒋沁利, 徐西蒙. 表面修饰FeOCl活化过硫酸盐引发有机污染物非自由基降解[J]. 化工进展, 2025, 44(6): 3101-3111. |
| [4] | 王嘉, 孙丹卉, 乔一凡, 范秀方, 赵立东, 贺雷, 陆安慧. 乙醇催化转化制高值化学品研究进展[J]. 化工进展, 2025, 44(5): 2587-2597. |
| [5] | 杨群, 李红艳, 张峰, 毛立波, 崔佳丽, 董颖虹, 郭紫瑞. 钴氮共掺杂废菌棒生物炭活化PMS去除水中加替沙星[J]. 化工进展, 2025, 44(2): 1088-1099. |
| [6] | 廖旭, 王玮, 黄文婷, 熊文涛, 王泽宇, 覃佐东, 林金清. 生物质基催化剂在二氧化碳转化为环状碳酸酯中的研究进展[J]. 化工进展, 2025, 44(2): 834-846. |
| [7] | 高永平, 康家宁, 刘柏, 袁诗淇, 于泽广, 张志会, 高文秀. PCuMo11/MG-800多相催化硝基苯还原制苯胺[J]. 化工进展, 2025, 44(10): 5764-5770. |
| [8] | 武恩喜, 代怡, 张耀远, 吴芹, 史大昕, 陈康成, 黎汉生. 低碳烷烃无氧脱氢Co基催化剂的研究进展[J]. 化工进展, 2025, 44(10): 5730-5750. |
| [9] | 赵锡望, 高丽, 梁振明, 刘希涛. 微波协同高级氧化技术修复有机污染土壤的研究进展[J]. 化工进展, 2025, 44(10): 5911-5925. |
| [10] | 乔磊, 张亚新, 魏博, 冉文燊, 马金荣, 王峰. 氧热法气流床电石反应器烧嘴布置参数及操作参数优化[J]. 化工进展, 2025, 44(1): 145-157. |
| [11] | 张浩, 刘世钰, 沈卫华, 方云进. Ca-ZSM-5催化尿素脱水制备单氰胺[J]. 化工进展, 2024, 43(S1): 365-373. |
| [12] | 廖旭, 周骏, 罗杰, 曾瑞琳, 王泽宇, 李尊华, 林金清. 多孔离子聚合物催化二氧化碳环加成反应的研究进展[J]. 化工进展, 2024, 43(9): 4925-4940. |
| [13] | 张茜, 李皓芯, 张天阳, 李子富, 孙文俊, 敖秀玮. 基于紫外线的高级氧化或高级还原技术降解水中全氟或多氟烷基化合物[J]. 化工进展, 2024, 43(8): 4587-4600. |
| [14] | 何弈雪, 秦先超, 马伟芳. 过硫酸盐高级氧化原位修复地下水中卤代烃污染研究进展[J]. 化工进展, 2024, 43(7): 4072-4088. |
| [15] | 张子杭, 王树荣. 生物质热解转化与产物低碳利用研究进展[J]. 化工进展, 2024, 43(7): 3692-3708. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||