化工进展 ›› 2024, Vol. 43 ›› Issue (8): 4421-4431.DOI: 10.16085/j.issn.1000-6613.2023-1200
• 工业催化 • 上一篇
甲酸为氢源硝基苯转移加氢合成对氨基苯酚
王雨菲1( ), 贾宇1, 张议升1, 薛伟1,2, 李芳1,2(
), 贾宇1, 张议升1, 薛伟1,2, 李芳1,2( ), 王延吉1,2,3
), 王延吉1,2,3
- 1.河北工业大学化工学院河北省绿色化工与高效节能重点实验室,天津 300400
2.天津市本质安全化工技术重点实验室,天津 300400
3.河北省绿色化学工业产业研究院,河北 沧州 061000
-
收稿日期:2023-07-14修回日期:2023-12-10出版日期:2024-08-15发布日期:2024-09-02 -
通讯作者:李芳 -
作者简介:王雨菲(1999—),女,硕士研究生,研究方向为绿色化工。E-mail:961283910@qq.com。 -
基金资助:国家自然科学基金(U21A20306);河北省自然科学基金(B2022202077)
Synthesis of p-aminophenol by transfer hydrogenation of nitrobenzene using formic acid as hydrogen source
WANG Yufei1( ), JIA Yu1, ZHANG Yisheng1, XUE Wei1,2, LI Fang1,2(
), JIA Yu1, ZHANG Yisheng1, XUE Wei1,2, LI Fang1,2( ), WANG Yanji1,2,3
), WANG Yanji1,2,3
- 1.Hebei Provincial Key Laboratory of Green Chemical Technology and High Efficient Energy Saving, School of Chemical Engineering and Technology, Hebei University of Technology, Tianjin 300400, China
2.Tianjin Key Laboratory of Chemical Process Safety, Tianjin 300400, China
3.Hebei Industrial Technology Research Institute of Green Chemical Industry, Cangzhou 061000, Hebei, China
-
Received:2023-07-14Revised:2023-12-10Online:2024-08-15Published:2024-09-02 -
Contact:LI Fang
摘要:
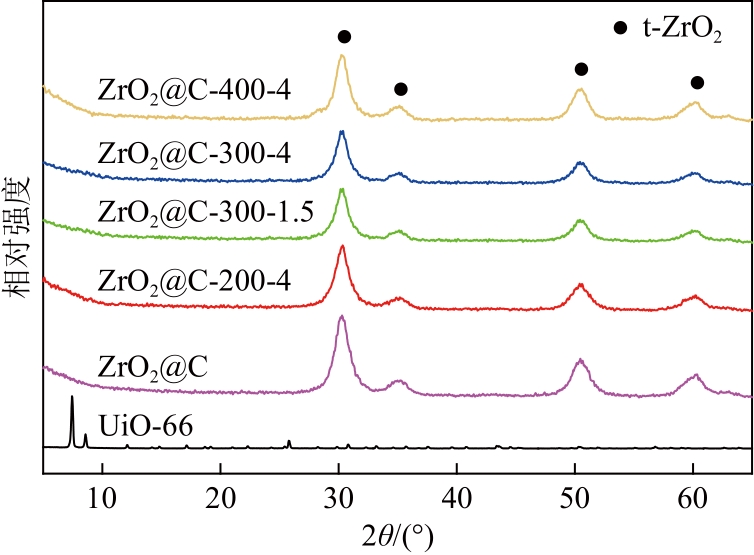
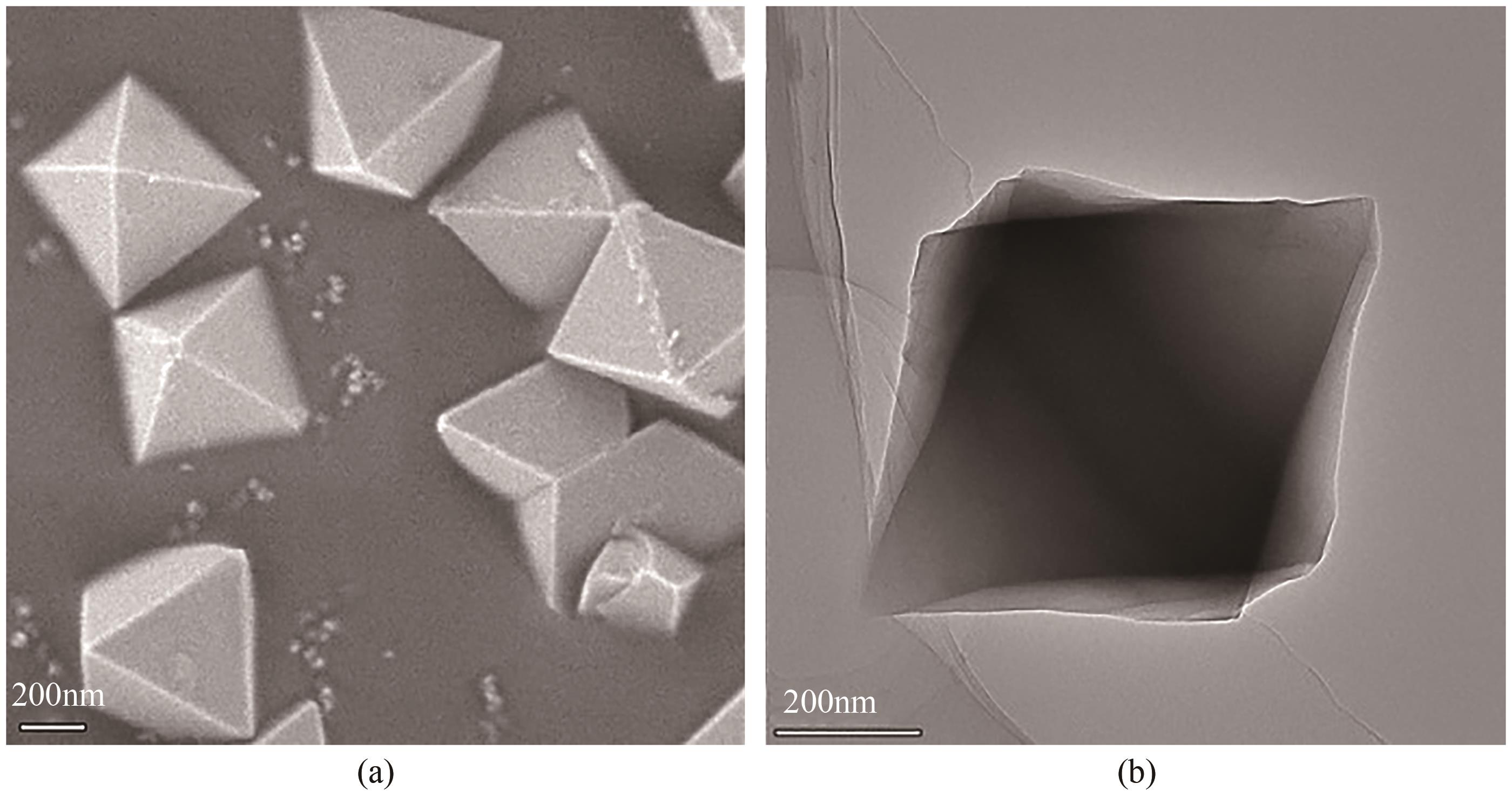
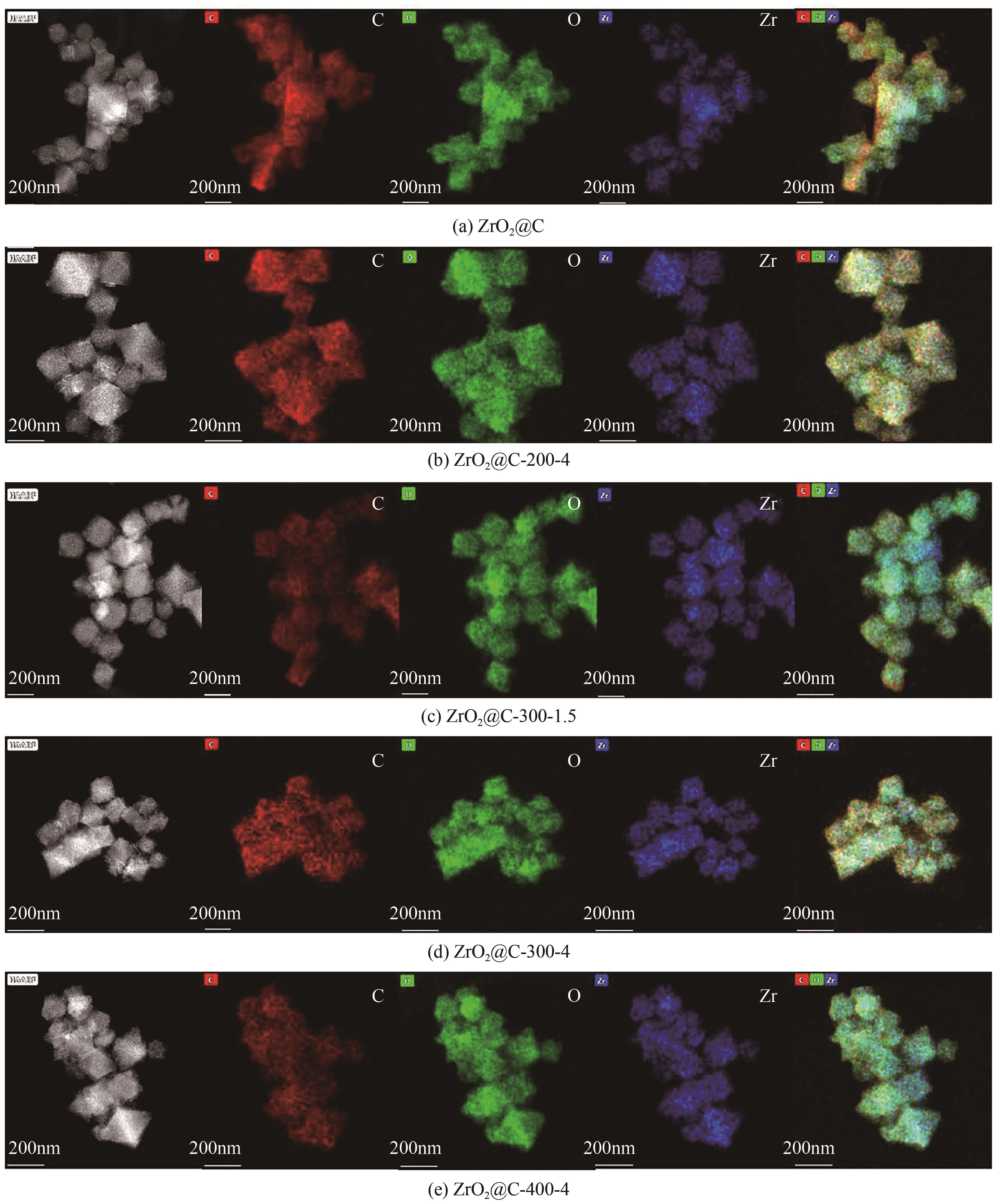
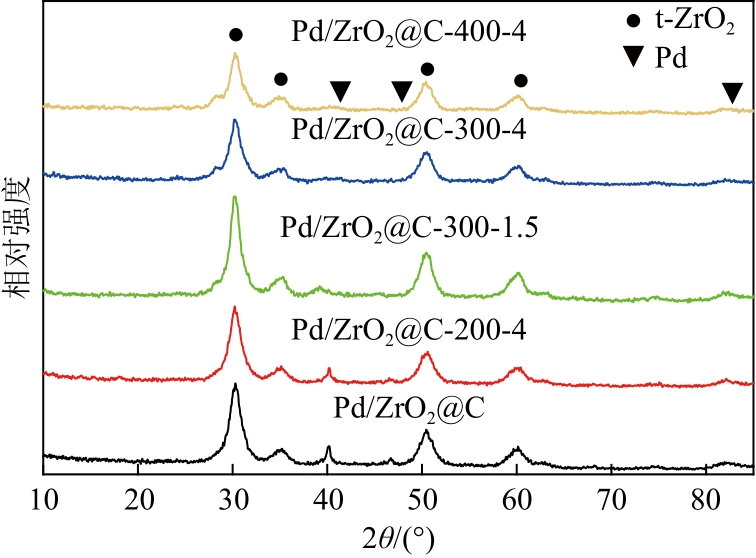
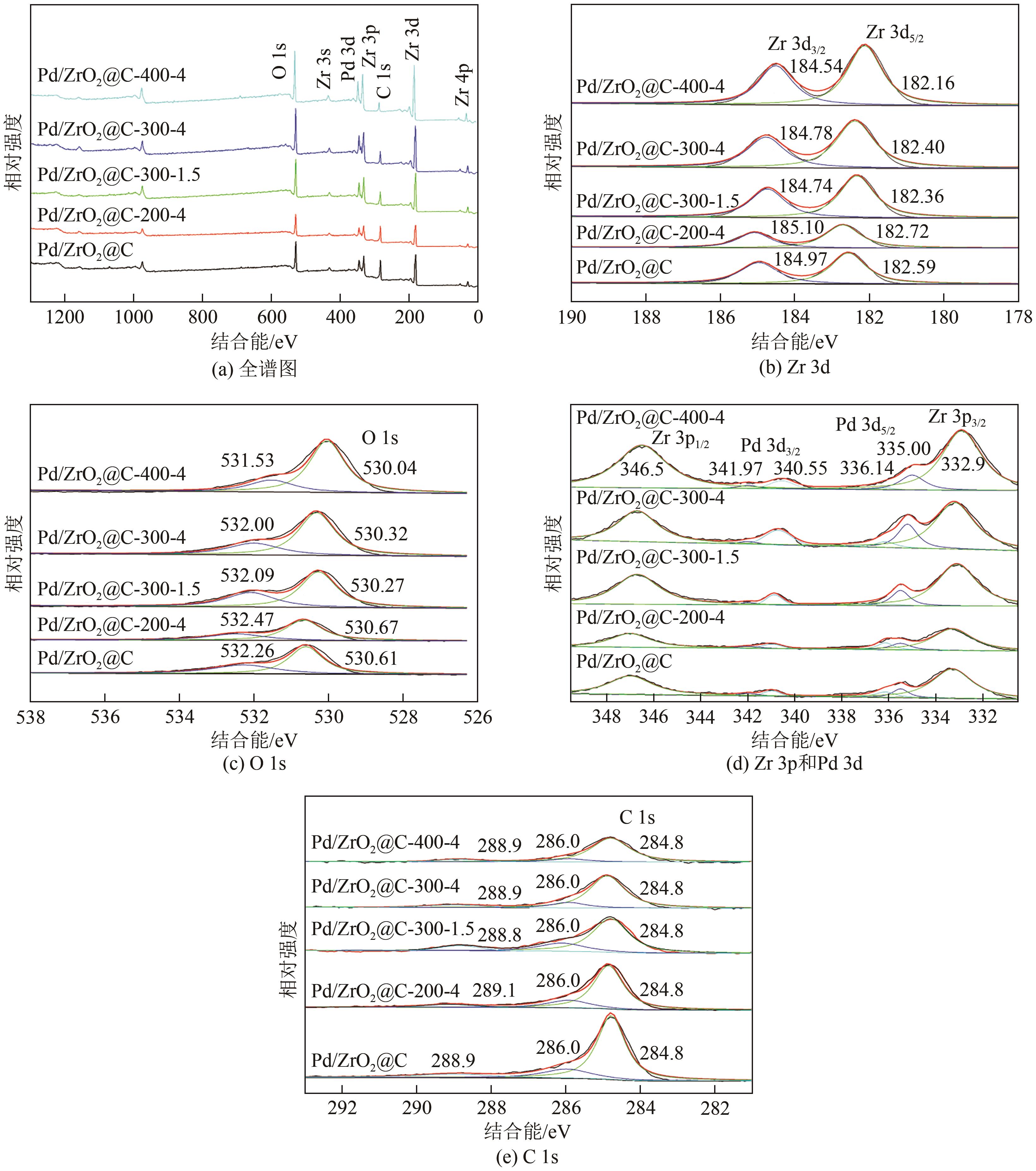
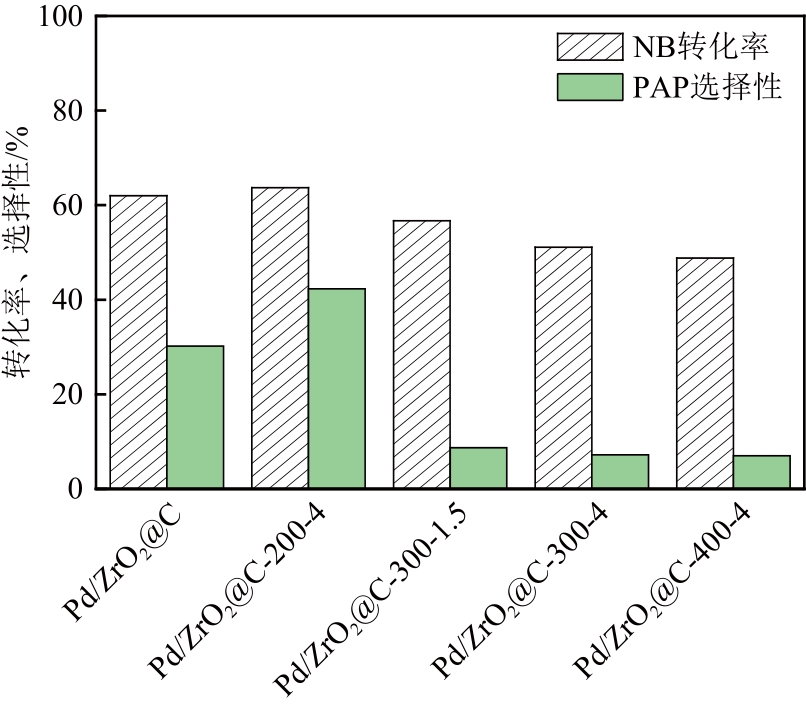
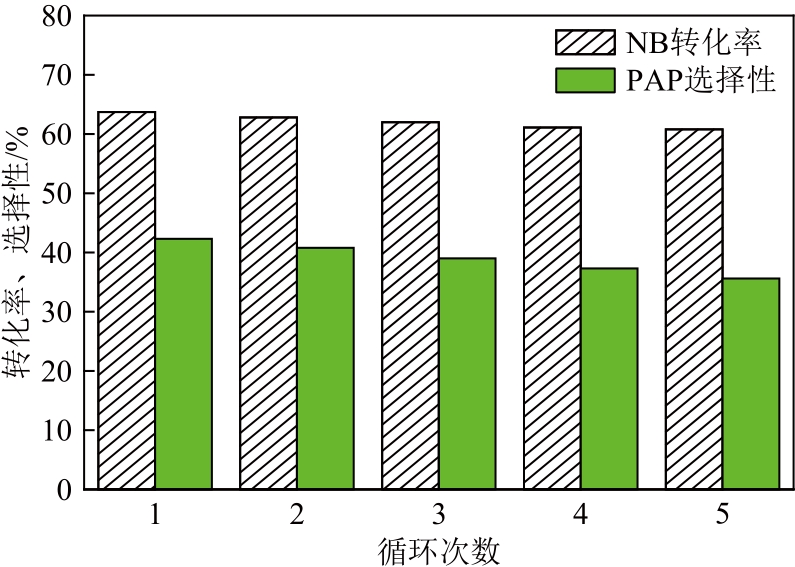
以UiO-66为前体,在N2气氛下焙烧制备了ZrO2@C,以其为载体,利用浸渍法制备了Pd/ZrO2@C催化剂。以Pd/ZrO2@C+SO42-/ZrO2为催化剂,对以甲酸(FA)为氢源、硝基苯(NB)转移加氢合成对氨基苯酚(PAP)的反应进行了研究。通过表征发现,Pd/ZrO2@C载体中的ZrO2为四方相ZrO2,包埋在无定形C中。随着ZrO2@C在空气中焙烧温度的升高和焙烧时间的增加,其C含量和比表面积随之下降,同时Pd颗粒尺寸变大。Pd/ZrO2@C催化剂中Pd以Pd0和Pd2+两种形式存在,且随着C含量的减少,Pd0含量增加,Pd2+含量减少;当Pd0含量明显高于Pd2+,其对PAP的选择性明显下降。在140℃、反应6h的条件下,Pd/ZrO2@C-200-4+SO42-/ZrO2具有较好的催化性能,NB转化率为63.7%,PAP选择性为42.3%。
中图分类号:
引用本文
王雨菲, 贾宇, 张议升, 薛伟, 李芳, 王延吉. 甲酸为氢源硝基苯转移加氢合成对氨基苯酚[J]. 化工进展, 2024, 43(8): 4421-4431.
WANG Yufei, JIA Yu, ZHANG Yisheng, XUE Wei, LI Fang, WANG Yanji. Synthesis of p-aminophenol by transfer hydrogenation of nitrobenzene using formic acid as hydrogen source[J]. Chemical Industry and Engineering Progress, 2024, 43(8): 4421-4431.
| 样品 | C质量 分数/% | 比表面积 /m2·g-1 | 孔容积 /cm3·g-1 | 孔径/nm |
|---|---|---|---|---|
| ZrO2@C | 24.38 | 240.95 | 0.23 | 3.82 |
| ZrO2@C-200-4 | 22.74 | 246.94 | 0.25 | 4.01 |
| ZrO2@C-300-1.5 | 7.56 | 206.56 | 0.20 | 3.92 |
| ZrO2@C-300-4 | 2.77 | 173.74 | 0.26 | 6.02 |
| ZrO2@C-400-4 | 0.50 | 118.44 | 0.26 | 8.84 |
表1 不同ZrO2@C载体的C含量及织构参数
| 样品 | C质量 分数/% | 比表面积 /m2·g-1 | 孔容积 /cm3·g-1 | 孔径/nm |
|---|---|---|---|---|
| ZrO2@C | 24.38 | 240.95 | 0.23 | 3.82 |
| ZrO2@C-200-4 | 22.74 | 246.94 | 0.25 | 4.01 |
| ZrO2@C-300-1.5 | 7.56 | 206.56 | 0.20 | 3.92 |
| ZrO2@C-300-4 | 2.77 | 173.74 | 0.26 | 6.02 |
| ZrO2@C-400-4 | 0.50 | 118.44 | 0.26 | 8.84 |
| 样品 | O 1s/% | Zr 3d/% | O/Zr |
|---|---|---|---|
| ZrO2@C | 30.65 | 10.88 | 2.82 |
| ZrO2@C-200-4 | 33.67 | 11.64 | 2.89 |
| ZrO2@C-300-1.5 | 43.74 | 15.34 | 2.85 |
| ZrO2@C-300-4 | 51.55 | 18.34 | 2.81 |
| ZrO2@C-400-4 | 59.73 | 21.97 | 2.72 |
表2 不同ZrO2@C载体的原子比例
| 样品 | O 1s/% | Zr 3d/% | O/Zr |
|---|---|---|---|
| ZrO2@C | 30.65 | 10.88 | 2.82 |
| ZrO2@C-200-4 | 33.67 | 11.64 | 2.89 |
| ZrO2@C-300-1.5 | 43.74 | 15.34 | 2.85 |
| ZrO2@C-300-4 | 51.55 | 18.34 | 2.81 |
| ZrO2@C-400-4 | 59.73 | 21.97 | 2.72 |
| 样品 | 比表面积/m2·g-1 | 孔容积/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| Pd/ZrO2@C | 234.37 | 0.26 | 4.51 |
| Pd/ZrO2@C-200-4 | 223.02 | 0.29 | 5.15 |
| Pd/ZrO2@C-300-1.5 | 168.93 | 0.24 | 5.62 |
| Pd/ZrO2@C-300-4 | 160.99 | 0.21 | 5.10 |
| Pd/ZrO2@C-400-4 | 115.89 | 0.25 | 8.69 |
表3 不同Pd/ZrO2@C催化剂的织构参数
| 样品 | 比表面积/m2·g-1 | 孔容积/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| Pd/ZrO2@C | 234.37 | 0.26 | 4.51 |
| Pd/ZrO2@C-200-4 | 223.02 | 0.29 | 5.15 |
| Pd/ZrO2@C-300-1.5 | 168.93 | 0.24 | 5.62 |
| Pd/ZrO2@C-300-4 | 160.99 | 0.21 | 5.10 |
| Pd/ZrO2@C-400-4 | 115.89 | 0.25 | 8.69 |
| 样品 | Pd物种分布/% | Pd2+/Pd0 | |
|---|---|---|---|
| Pd0 | Pd2+ | ||
| Pd/ZrO2@C | 57.0 | 43.0 | 0.75 |
| Pd/ZrO2@C-200-4 | 55.7 | 44.3 | 0.80 |
| Pd/ZrO2@C-300-1.5 | 80.2 | 19.8 | 0.25 |
| Pd/ZrO2@C-300-4 | 81.7 | 18.3 | 0.22 |
| Pd/ZrO2@C-400-4 | 83.3 | 16.7 | 0.20 |
表4 不同Pd/ZrO2@C催化剂的Pd物种分布
| 样品 | Pd物种分布/% | Pd2+/Pd0 | |
|---|---|---|---|
| Pd0 | Pd2+ | ||
| Pd/ZrO2@C | 57.0 | 43.0 | 0.75 |
| Pd/ZrO2@C-200-4 | 55.7 | 44.3 | 0.80 |
| Pd/ZrO2@C-300-1.5 | 80.2 | 19.8 | 0.25 |
| Pd/ZrO2@C-300-4 | 81.7 | 18.3 | 0.22 |
| Pd/ZrO2@C-400-4 | 83.3 | 16.7 | 0.20 |
| 催化剂 | TOF/h-1 |
|---|---|
| Pd/ZrO2@C | 306.4 |
| Pd/ZrO2@C-200-4 | 331.1 |
| Pd/ZrO2@C-300-1.5 | 252.7 |
| Pd/ZrO2@C-300-4 | 182.9 |
| Pd/ZrO2@C-400-4 | 160.0 |
表5 不同Pd/ZrO2@C催化剂催化FA分解活性比较
| 催化剂 | TOF/h-1 |
|---|---|
| Pd/ZrO2@C | 306.4 |
| Pd/ZrO2@C-200-4 | 331.1 |
| Pd/ZrO2@C-300-1.5 | 252.7 |
| Pd/ZrO2@C-300-4 | 182.9 |
| Pd/ZrO2@C-400-4 | 160.0 |
| 催化剂 | S/% | Pd/% | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 孔径 /nm |
|---|---|---|---|---|---|
| 新鲜催化剂 | 5.90 | 0.33 | 81.24 | 0.31 | 11.72 |
| 循环5次后 的催化剂 | 4.03 | 0.30 | 80.15 | 0.23 | 8.05 |
表6 新鲜催化剂与循环5次后催化剂的对比
| 催化剂 | S/% | Pd/% | 比表面积 /m2·g-1 | 孔容 /cm3·g-1 | 孔径 /nm |
|---|---|---|---|---|---|
| 新鲜催化剂 | 5.90 | 0.33 | 81.24 | 0.31 | 11.72 |
| 循环5次后 的催化剂 | 4.03 | 0.30 | 80.15 | 0.23 | 8.05 |
| 1 | WU Shutao, HUANG Xun, ZHANG Hongliang, et al. Efficient electrochemical hydrogenation of nitroaromatics into arylamines on a CuCo2O4 spinel cathode in an alkaline electrolyte[J]. ACS Catalysis, 2022, 12(1): 58-65. |
| 2 | ABDELHAMID Hani Nasser. High performance and ultrafast reduction of 4-nitrophenol using metal-organic frameworks[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104404. |
| 3 | ATTIA Yasser A, MOHAMED Yasser M A. Silicon-grafted Ag/AgX/rGO nanomaterials (X = Cl or Br) as dip-photocatalysts for highly efficient p-nitrophenol reduction and paracetamol production[J]. Applied Organometallic Chemistry, 2019, 33(3): 1-11. |
| 4 | YU Yeonhwa, JUNG Euiyoung, KIM Hyun Jin, et al. Protein particles decorated with Pd nanoparticles for the catalytic reduction of p-nitrophenol to p-aminophenol[J]. ACS Applied Nano Materials, 2020, 3(10): 10487-10496. |
| 5 | ZHANG Tingting, JIANG Jingyang, WANG Yanhua. Green route for the preparation of p-aminophenol from nitrobenzene by catalytic hydrogenation in pressurized CO2/H2O system[J]. Organic Process Research & Development, 2015, 19(12): 2050-2054. |
| 6 | ZOU Luyao, CUI Yuanyuan, DAI Weilin. Highly efficient Au/TiO2 catalyst for one-pot conversion of nitrobenzene to p-aminophenol in water media[J]. Chinese Journal of Chemistry, 2014, 32(3): 257-262. |
| 7 | DONG Zhen, WANG Tao, ZHAO Jie, et al. Ni-Silicides nanoparticles as substitute for noble metals for hydrogenation of nitrobenzene to p-aminophenol in sulfuric acid[J]. Applied Catalysis A: General, 2016, 520: 151-156. |
| 8 | RODE Chandrashekhar V, VAIDYA Manisha J, CHAUDHARI Raghunath V. Synthesis of p-aminophenol by catalytic hydrogenation of nitrobenzene[J]. Organic Process Research & Development, 1999, 3(6): 465-470. |
| 9 | 王淑芳, 高杨, 王延吉, 等. 负载型纳米Pt催化剂的制备及其催化合成对氨基苯酚[J]. 石油化工, 2009, 38(4): 361-366. |
| WANG Shufang, GAO Yang, WANG Yanji, et al. Preparation of supported nano-Pt catalyst for synthesis of p-aminophenol[J]. Petrochemical Technology, 2009, 38(4): 361-366. | |
| 10 | 王淑芳, 王延吉, 高杨, 等. SAPO-5分子筛的制备及其催化合成对氨基苯酚[J]. 催化学报, 2010, 31(6): 637-644. |
| WANG Shufang, WANG Yanji, GAO Yang, et al. Preparation of SAPO-5 and its catalytic synthesis of p-aminophenol[J]. Chinese Journal of Catalysis, 2010, 31(6): 637-644. | |
| 11 | WANG Shufang, JIN Yadan, HE Beibei, et al. Synthesis of bifunctional Pt/MgAPO-5 catalysts and their catalytic performance in the hydrogenation of nitrobenzene to p-aminophenol[J]. Science China Chemistry, 2010, 53(7): 1514-1519. |
| 12 | 宋丽娟, 王利, 张晓彤, 等. 一种非酸介质中硝基苯选择加氢制对氨基苯酚的催化剂:CN102600891A[P]. 2012-07-25. |
| SONG Lijuan, WANG Li, ZHANG Xiaotong, et al. A catalyst for selective hydrogenation of nitrobenzene to p-aminophenol in a non-acid medium:CN102600891A[P]. 2012-07-25. | |
| 13 | Jeeva RATNAM K, Sudarshan REDDY R, SEKHAR N S, et al. Bamberger rearrangement on solid acids[J]. Applied Catalysis A: General, 2008, 348(1): 26-29. |
| 14 | DESHPANDE Abhay, FIGUERAS F, LAKSHMI KANTAM M, et al. Environmentally friendly hydrogenation of nitrobenzene to p-aminophenol using heterogeneous catalysts[J]. Journal of Catalysis, 2010, 275(2): 250-256. |
| 15 | 黄伟, 储政, 任磊, 等. 碳基固体酸在硝基苯加氢制备对氨基苯酚中的应用[J]. 化工进展, 2023, 42(1): 272-281. |
| HUANG Wei, CHU Zheng, REN Lei, et al. Application of carbon-based solid acid in hydrogenation of nitrobenzene to p-aminophenol[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 272-281. | |
| 16 | 刘迎新, 刘晓爽, 曾茂, 等. 硝基苯催化加氢合成对氨基苯酚的研究进展[J]. 石油化工, 2018, 47(1): 79-85. |
| LIU Yingxin, LIU Xiaoshuang, ZENG Mao, et al. Progress in synthesis of p-aminophenol by catalytic hydrogenation of nitrobenzene[J]. Petrochemical Technology, 2018, 47(1): 79-85. | |
| 17 | GU Jing, ZHANG Zhiyang, DING Liping, et al. Platinum nanoparticles encapsulated in HZSM-5 crystals as an efficient catalyst for green production of p-aminophenol[J]. Catalysis Communications, 2017, 97: 98-101. |
| 18 | 蒋文艳, 魏光涛, 刘子涵, 等. 催化转移加氢及其强化研究进展[J]. 现代化工, 2019, 39(3): 26-30. |
| JIANG Wenyan, WEI Guangtao, LIU Zihan, et al. Research progress in catalytic transfer hydrogenation and its intensification[J]. Modern Chemical Industry, 2019, 39(3): 26-30. | |
| 19 | 郑纯智, 张继炎, 王日杰. 催化转移加氢及其在有机合成中的应用[J]. 工业催化, 2004, 12(3): 29-35. |
| ZHENG Chunzhi, ZHANG Jiyan, WANG Rijie. Catalytic transfer hydrogenation and its application in organic synthesis[J]. Industrial Catalysis, 2004, 12(3): 29-35. | |
| 20 | MENG Qinglei, YANG Xiaolong, WANG Xian, et al. Preparation strategy using pre-nucleation coupled with in situ reduction for a high-performance catalyst towards selective hydrogen production from formic acid[J]. Catalysts, 2022, 12(3): 325. |
| 21 | 王显. 甲酸氢能转换过程中抗中毒催化剂的理性设计与精准制备[D]. 合肥: 中国科学技术大学, 2021. |
| WANG Xian. Rational design and precise preparation of anti-poisoning catalyst in the conversion of formic acid to hydrogen energy[D]. Hefei: University of Science and Technology of China, 2021. | |
| 22 | JIA Yu, LI Fang, ZHANG Yisheng, et al. A novel process for the synthesis of p-aminophenol by transfer hydrogenation of nitrobenzene using formic acid as hydrogen source[J]. Asia-Pacific Journal of Chemical Engineering, 2022, 17(5): 1-10. |
| 23 | DHAKSHINAMOORTHY Amarajothi, GARCIA Hermenegildo. Metal-organic frameworks as solid catalysts for the synthesis of nitrogen-containing heterocycles[J]. Chemical Society Reviews, 2014, 43(16): 5750-5765. |
| 24 | Raja DAS, PACHFULE Pradip, BANERJEE Rahul, et al. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): Finding the border of metal and metal oxides[J]. Nanoscale, 2012, 4(2): 591-599. |
| 25 | SHEN Kui, CHEN Xiaodong, CHEN Junying, et al. Development of MOF-derived carbon-based nanomaterials for efficient catalysis[J]. ACS Catalysis, 2016, 6(9): 5887-5903. |
| 26 | CAO Wenxiu, LUO Wenhao, GE Hongguang, et al. UiO-66 derived Ru/ZrO2@C as a highly stable catalyst for hydrogenation of levulinic acid to γ-valerolactone[J]. Green Chemistry, 2017, 19(9): 2201-2211. |
| 27 | ZHANG Weina, LU Guang, CUI Chenlong, et al. A family of metal-organic frameworks exhibiting size-selective catalysis with encapsulated noble-metal nanoparticles[J]. Advanced Materials, 2014, 26(24): 4056-4060. |
| 28 | ZHANG Xue, QIAO Jing, LIU Chang, et al. A MOF-derived ZrO2/C nanocomposite for efficient electromagnetic wave absorption[J]. Inorganic Chemistry Frontiers, 2020, 7(2): 385-393. |
| 29 | RAHMAN Md Anisur, ROUT S, THOMAS Joseph P, et al. Defect-rich dopant-free ZrO2 nanostructures with superior dilute ferromagnetic semiconductor properties[J]. Journal of the American Chemical Society, 2016, 138(36): 11896-11906. |
| 30 | QIAO Jing, ZHANG Xue, XU Dongmei, et al. Design and synthesis of TiO2/Co/carbon nanofibers with tunable and efficient electromagnetic absorption[J]. Chemical Engineering Journal, 2020, 380: 122591. |
| 31 | FENG Wei, WANG Yaming, CHEN Junchen, et al. Reduced graphene oxide decorated with in-situ growing ZnO nanocrystals: Facile synthesis and enhanced microwave absorption properties[J]. Carbon, 2016, 108: 52-60. |
| 32 | Qing LYU, MENG Qinglei, LIU Weiwei, et al. Pd-PdO interface as active site for HCOOH selective dehydrogenation at ambient condition[J]. The Journal of Physical Chemistry C, 2018, 122(4): 2081-2088. |
| 33 | WEI Qinhong, MA Qingxiang, ZUO Pingping, et al. Hollow structure and electron promotion effect of mesoporous Pd/CeO2 catalyst for enhanced catalytic hydrogenation[J]. ChemCatChem, 2018, 10(5): 1019-1026. |
| [1] | 胡婷霞, 赵立欣, 姚宗路, 霍丽丽, 贾吉秀, 谢腾. 双金属催化剂在生物质焦油催化蒸汽重整领域的研究进展[J]. 化工进展, 2024, 43(8): 4354-4365. |
| [2] | 吴泽亮, 管琦卉, 陈世霞, 王珺. 炔烃选择性加氢制烯烃反应的研究进展[J]. 化工进展, 2024, 43(8): 4366-4381. |
| [3] | 张叶素, 权燕红, 丁欣欣, 任军. 链状MFI型分子筛的合成与应用[J]. 化工进展, 2024, 43(8): 4382-4392. |
| [4] | 王嘉, 李文翠, 吴凡, 高新芊, 陆安慧. NiMo/Al2O3催化剂活性组分分布调控及其加氢脱硫应用[J]. 化工进展, 2024, 43(8): 4393-4402. |
| [5] | 付涛, 李立, 高莉宁, 朱富维, 曹炜烨, 陈华鑫. 水泥基硼掺杂石墨相氮化碳降解NO[J]. 化工进展, 2024, 43(8): 4403-4410. |
| [6] | 龙涛, 周锋, 张伟, 吴泓, 王建, 陈霖. CO-CO2体系制备氘代甲醇催化剂的合成与改性[J]. 化工进展, 2024, 43(8): 4411-4420. |
| [7] | 刘文津, 张玉明, 李家州, 张炜, 陈哲文. 典型石油热加工技术发展现状及展望[J]. 化工进展, 2024, 43(7): 3534-3550. |
| [8] | 张子杭, 王树荣. 生物质热解转化与产物低碳利用研究进展[J]. 化工进展, 2024, 43(7): 3692-3708. |
| [9] | 龚德成, 沈倩, 朱贤青, 黄云, 夏奡, 张敬苗, 朱恂, 廖强. 微藻超临界水气化制取富氢合成气的研究进展[J]. 化工进展, 2024, 43(7): 3709-3728. |
| [10] | 郭鹏, 李红伟, 李贵贤, 季东, 王东亮, 赵新红. 直接甲醇燃料电池阳极催化剂的失活机制及应对策略[J]. 化工进展, 2024, 43(7): 3812-3823. |
| [11] | 王颖杰, 祝新利. 溶胶-凝胶法制备高分散Ni-Cu/SiO2 促进间甲酚直接脱氧制甲苯[J]. 化工进展, 2024, 43(7): 3824-3833. |
| [12] | 罗丛佳, 豆义波, 卫敏. 水滑石光催化剂结构调控用于二氧化碳还原的研究进展[J]. 化工进展, 2024, 43(7): 3891-3909. |
| [13] | 龚勇, 潘忠文, 谢纯, 崔瑾, 王鲜. 碳酸钠催化制备纳米碳纤维[J]. 化工进展, 2024, 43(7): 3980-3986. |
| [14] | 张世蕊, 范朕连, 宋慧平, 张丽娜, 高宏宇, 程淑艳, 程芳琴. 粉煤灰负载光催化材料的研究进展[J]. 化工进展, 2024, 43(7): 4043-4058. |
| [15] | 何弈雪, 秦先超, 马伟芳. 过硫酸盐高级氧化原位修复地下水中卤代烃污染研究进展[J]. 化工进展, 2024, 43(7): 4072-4088. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||