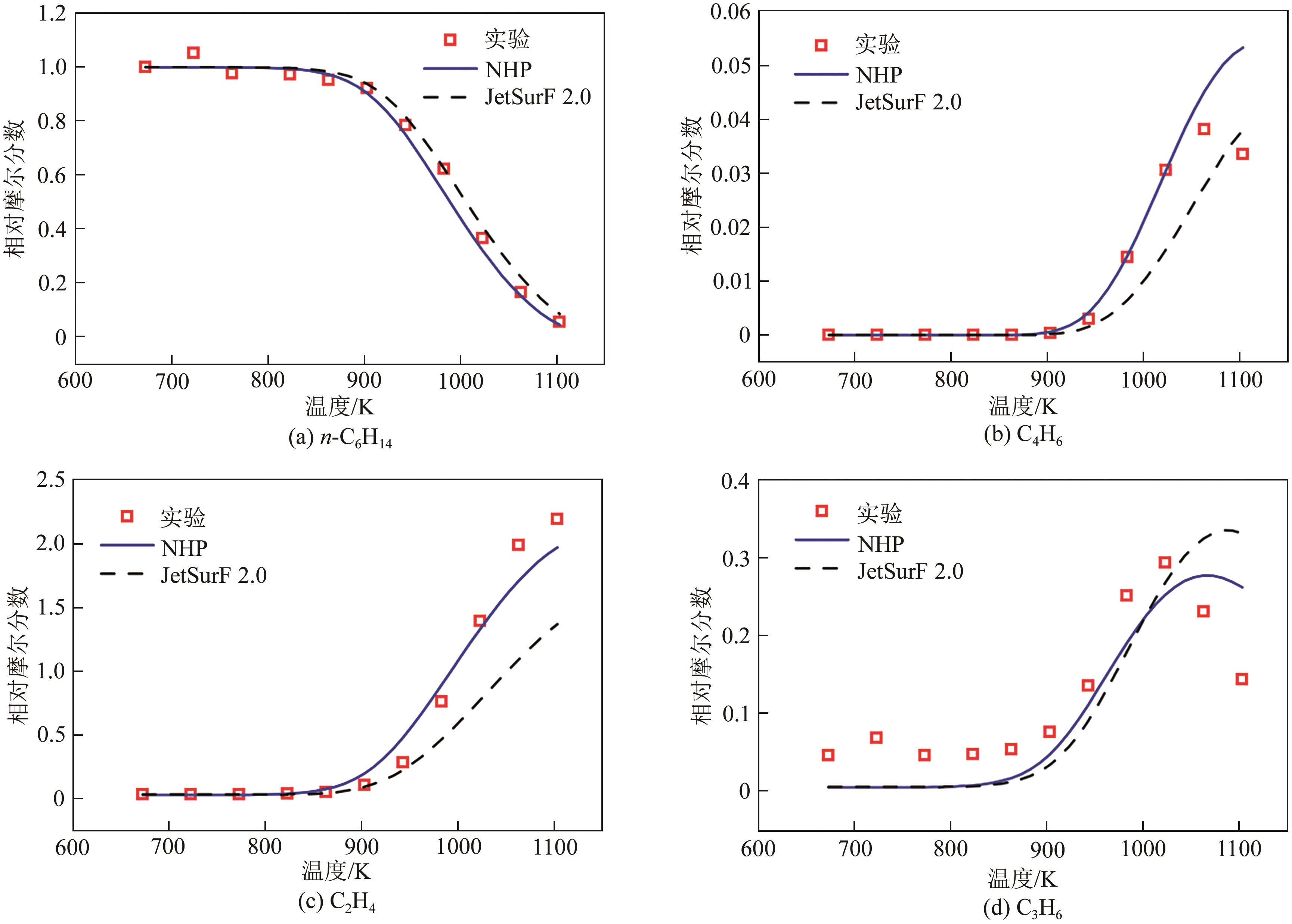
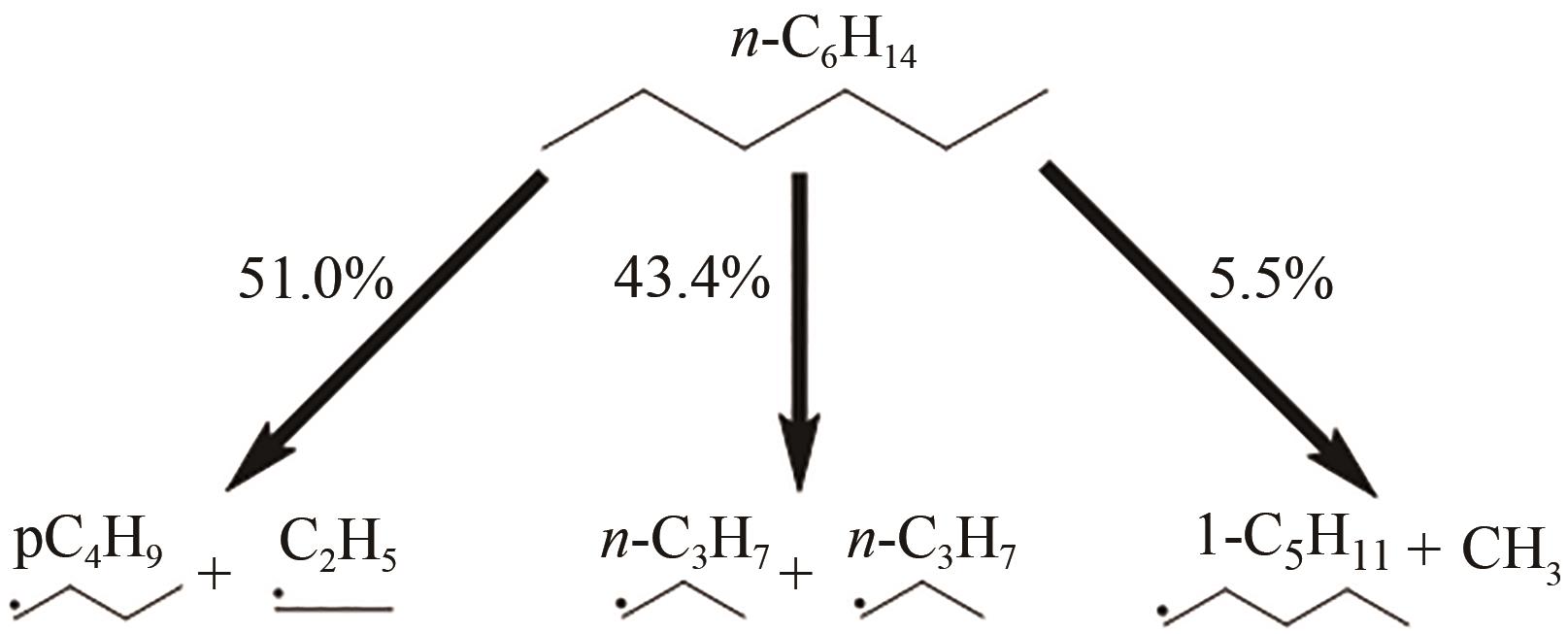
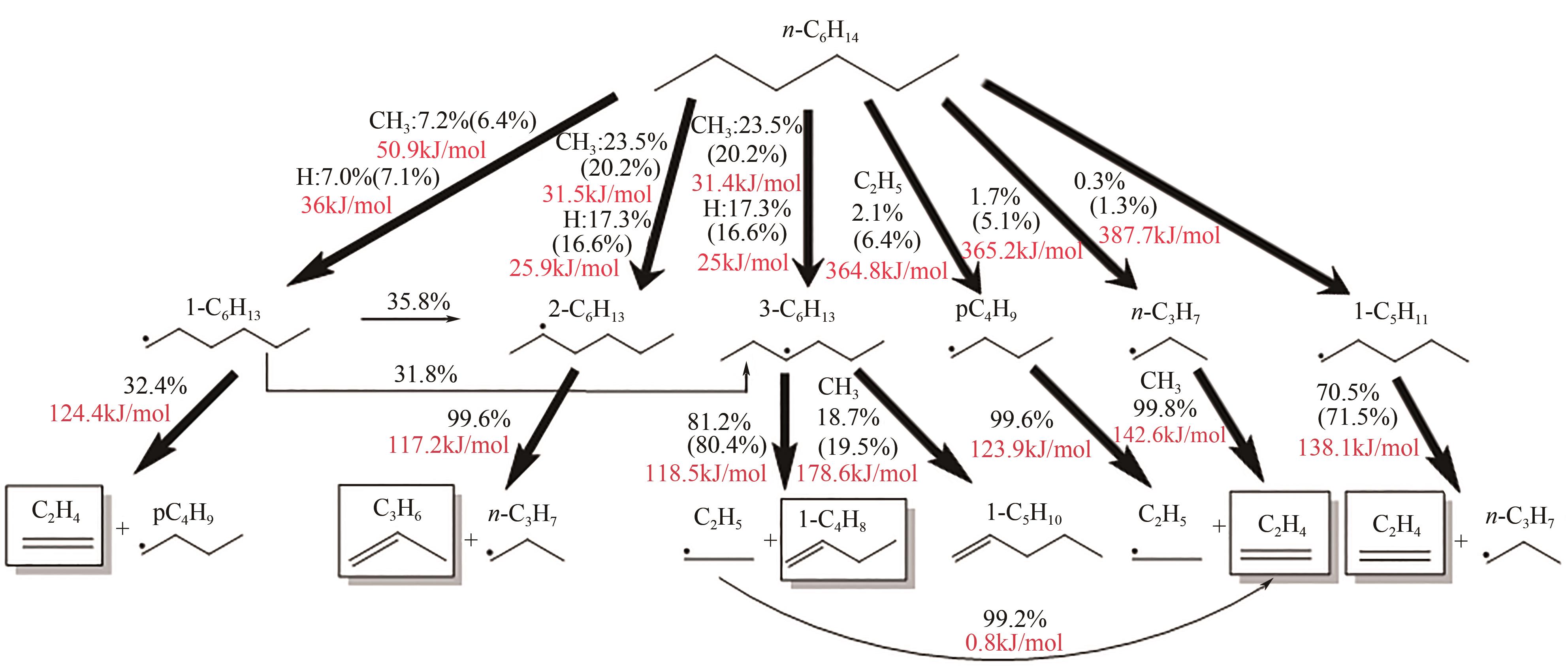
| 1 |
张乐乐, 钱运东, 朱华曈, 等. 加氢原料煤焦油脱水除盐预处理工艺优化限值[J]. 化工进展, 2023, 42(5): 2298-2305.
|
|
ZHANG Lele, QIAN Yundong, ZHU Huatong, et al. Study on optimization limits of dehydration and desalination pretreatment of hydrogenated coal tar[J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2298-2305.
|
| 2 |
王龙, 刘永峰, 毕贵军, 等. 基于量子化学计算柴油在CO2/O2氛围下的燃烧特性[J]. 化工进展, 2022, 41(6): 2948-2958.
|
|
WANG Long, LIU Yongfeng, BI Guijun, et al. Characteristics of diesel combustion under CO2/O2 atmosphere by quantum chemistry calculations[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 2948-2958.
|
| 3 |
ZHENG Ming, HAN Xiaoye, ASAD Usman, et al. Investigation of butanol-fuelled HCCI combustion on a high efficiency diesel engine[J]. Energy Conversion and Management, 2015, 98: 215-224.
|
| 4 |
HE Yongdi, SONG Jinou, YANG Jiuzhong, et al. Experimental study on n-hexane pyrolysis initiated by 1-nitropropane in a low-pressure flow reactor[J]. Journal of the Energy Institute, 2023, 109: 101290.
|
| 5 |
Bilal AYDOĞAN. An experimental examination of the effects of n-hexane and n-heptane fuel blends on combustion, performance and emissions characteristics in a HCCI engine[J]. Energy, 2020, 192: 116600.
|
| 6 |
HIRAI Hirotoshi. Molecular dynamics simulations for initial formation process of polycyclic aromatic hydrocarbons in n-hexane and cyclohexane combustion[J]. Chemical Physics, 2021, 548: 111225.
|
| 7 |
BAYINDIRLI Cihan, CELIK Mehmet. Investigation of combustion and emission characteristics of n-hexane and n-hexadecane additives in diesel fuel[J]. Journal of Mechanical Science and Technology, 2019, 33(4): 1937-1946.
|
| 8 |
HAN Dong, ZHAI Jiaqi, HUANG Zhen. Autoignition of n-hexane, cyclohexane, and methylcyclohexane in a constant volume combustion chamber[J]. Energy & Fuels, 2019, 33(4): 3576-3583.
|
| 9 |
VLASOV P A, SMIRNOV V N, TEREZA A M, et al. Effect of pressure on soot formation in the pyrolysis of n-hexane and the oxidation of fuel-rich mixtures of n-heptane behind reflected shock waves[J]. Russian Journal of Physical Chemistry B, 2016, 10(6): 912-921.
|
| 10 |
BILLAUD F, ELYAHYAOUI K, BARONNET F. Mechanistic modeling of the pyrolysis of n-hexane[J]. Journal of Analytical and Applied Pyrolysis, 1991, 19: 29-40.
|
| 11 |
YASUNAGA Kenji, YAMADA Hiroshi, OSHITA Hidekazu, et al. Pyrolysis of n-pentane, n-hexane and n-heptane in a single pulse shock tube[J]. Combustion and Flame, 2017, 185: 335-345.
|
| 12 |
田原宇, 乔英云. 石油热解过程中自由基调控反应机理的构建与应用[J]. 化工进展, 2021, 40(5): 2928-2932.
|
|
TIAN Yuanyu, QIAO Yingyun. Construction and application of the radical regulation reaction mechanism in petroleum pyrolysis[J]. Chemical Industry and Engineering Progress, 2021, 40(5): 2928-2932.
|
| 13 |
XU Minggao, ZHU Baozhong, ZHAO Long, et al. Atmospheric-pressure pyrolysis study of chlorobenzene using synchrotron radiation photoionization mass spectrometry[J]. The Journal of Physical Chemistry A, 2021, 125(9): 1949-1957.
|
| 14 |
JIA Zhenjian, WANG Zhandong, CHENG Zhanjun, et al. Experimental and modeling study on pyrolysis of n-decane initiated by nitromethane[J]. Combustion and Flame, 2016, 165: 246-258.
|
| 15 |
QI Fei. Combustion chemistry probed by synchrotron VUV photoionization mass spectrometry[J]. Proceedings of the Combustion Institute, 2013, 34(1): 33-63.
|
| 16 |
YUAN Tao, ZHANG Lidong, ZHOU Zhongyue, et al. Pyrolysis of n-heptane: Experimental and theoretical study[J]. The Journal of Physical Chemistry A, 2011, 115(9): 1593-1601.
|
| 17 |
LI Wei, ZHANG Yan, MEI Bowen, et al. Experimental and kinetic modeling study of n-propanol and i-propanol combustion: Flow reactor pyrolysis and laminar flame propagation[J]. Combustion and Flame, 2019, 207: 171-185.
|
| 18 |
HE Yongdi, SONG Jinou, YANG Jiuzhong, et al. Experimental investigation on the low-pressure pyrolysis of cyclohexane initiated by 1-nitropropane in a flow tube reactor[J]. Journal of Analytical and Applied Pyrolysis, 2023, 170: 105902.
|
| 19 |
LI Wang, YANG Jiuzhong, ZHAO Long, et al. Pyrolysis investigation of n-propylamine with synchrotron photoionization and molecular-beam mass spectrometry[J]. Combustion and Flame, 2021, 232: 111511.
|
| 20 |
ZHENG Zhihao, LI Wang, WU Lingnan, et al. Pyrolysis study of iso-propylamine with SVUV-photoionization molecular-beam mass spectrometry[J]. Combustion and Flame, 2022, 244: 112232.
|
| 21 |
JING Yixuan, CUI Jintao, LIU Bingzhi, et al. Pyrolysis and kinetic study of dimethyl methylphosphonate (DMMP) by synchrotron photoionization mass spectrometry[J]. Combustion and Flame, 2023, 255: 112919.
|
| 22 |
LI Wei, YE Lili, FANG Qilong, et al. Exploration on thermal decomposition of cyclopentanone: A flow reactor pyrolysis and kinetic modeling study[J]. Energy & Fuels, 2021, 35(17): 14023-14034.
|
| 23 |
WANG H, DAMES E, SIRJEAN B, et al. A high-temperature chemical kinetic model of n-alkane (up to n-dodecane), cyclohexane, and methyl-, ethyl-, n-propyl and n-butyl-cyclohexane oxidation at high temperatures, JetSurF version 2.0[Z]. September 19, 2010.
|
| 24 |
WEIGEND Florian, AHLRICHS Reinhart. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy[J]. Physical Chemistry Chemical Physics, 2005, 7(18): 3297-3305.
|
| 25 |
CANNEAUX Sébastien, BOHR Frédéric, HENON Eric. KiSThelP: A program to predict thermodynamic properties and rate constants from quantum chemistry results[J]. Journal of Computational Chemistry, 2014, 35(1): 82-93.
|
| 26 |
PEPIOT-DESJARDINS P, PITSCH H. An efficient error-propagation-based reduction method for large chemical kinetic mechanisms[J]. Combustion and Flame, 2008, 154(1/2): 67-81.
|
| 27 |
LU Tianfeng, LAW Chung K. A directed relation graph method for mechanism reduction[J]. Proceedings of the Combustion Institute, 2005, 30(1): 1333-1341.
|
| 28 |
LU Tianfeng, LAW Chung K. Linear time reduction of large kinetic mechanisms with directed relation graph: n-Heptane and iso-octane[J]. Combustion and Flame, 2006, 144(1/2): 24-36.
|
| 29 |
ZHOU Zhongyue, DU Xuewei, YANG Jiuzhong, et al. The vacuum ultraviolet beamline/endstations at NSRL dedicated to combustion research[J]. Journal of Synchrotron Radiation, 2016, 23(4): 1035-1045.
|
| 30 |
XU Qiang, LIU Bingzhi, CHEN Weiye, et al. Comprehensive study of the low-temperature oxidation chemistry by synchrotron photoionization mass spectrometry and gas chromatography[J]. Combustion and Flame, 2022, 236: 111797.
|
| 31 |
ZHANG Taichang, ZHANG Lidong, HONG Xin, et al. An experimental and theoretical study of toluene pyrolysis with tunable synchrotron VUV photoionization and molecular-beam mass spectrometry[J]. Combustion and Flame, 2009, 156(11): 2071-2083.
|
| 32 |
MILLER James A, KLIPPENSTEIN Stephen J. Dissociation of propyl radicals and other reactions on a C3H7 potential[J]. The Journal of Physical Chemistry A, 2013, 117(13): 2718-2727.
|
| 33 |
马志豪, 吕恩雨, 董永超, 等. 碳氢燃料在激波管内的裂解试验与动力学研究[J]. 内燃机学报, 2022, 40(5): 420-429.
|
|
MA Zhihao, Enyu LYU, DONG Yongchao, et al. Experiment and kinetic study on pyrolysis of hydrocarbon fuel in shock tube[J]. Transactions of CSICE, 2022, 40(5): 420-429.
|
| 34 |
WU Junjun, NING Hongbo, MA Liuhao, et al. Accurate prediction of bond dissociation energies of large n-alkanes using ONIOM-CCSD(T)/CBS methods[J]. Chemical Physics Letters, 2018, 699: 139-145.
|
| 35 |
李青月. 正己烷热裂解一次自由基反应分子模拟及反应机理研究[D]. 大庆: 东北石油大学, 2015.
|
|
LI Qingyue. Molecular simulation and reaction mechanism of free radical reactions mechanism of n-hexane thermal cracking[D]. Daqing: Northeast Petroleum University, 2015.
|
 ), 刘永峰1(
), 刘永峰1( ), 陈睿哲1, 张璐1, 宋金瓯2, 刘海峰2
), 陈睿哲1, 张璐1, 宋金瓯2, 刘海峰2
 ), LIU Yongfeng1(
), LIU Yongfeng1( ), CHEN Ruizhe1, ZHANG Lu1, SONG Jin’ou2, LIU Haifeng2
), CHEN Ruizhe1, ZHANG Lu1, SONG Jin’ou2, LIU Haifeng2