化工进展 ›› 2024, Vol. 43 ›› Issue (7): 4118-4127.DOI: 10.16085/j.issn.1000-6613.2023-0935
人工仿真菱铁矿及其衍生材料催化修复PAHs污染土壤
杨昕1,2( ), 钟承韡1, 杨志山1,3, 朱韦韦1, 王文浩1,2, 余江1,2,3(
), 钟承韡1, 杨志山1,3, 朱韦韦1, 王文浩1,2, 余江1,2,3( )
)
- 1.四川大学建筑与环境学院,四川 成都 610065
2.四川大学新能源与低碳技术研究院,四川 成都 610065
3.四川大学宜宾产业技术研究院,四川 宜宾 644000
-
收稿日期:2023-06-06修回日期:2023-07-13出版日期:2024-07-25发布日期:2024-08-14 -
通讯作者:余江 -
作者简介:杨昕(2000—),女,硕士研究生,研究方向为土壤污染治理与修复技术。E-mail:yyyxin@stu.scu.edu.cn。 -
基金资助:国家重点研发计划(2018YFC1802605);四川省区域创新项目(2022YFQ0081);成都市科技局重点研发支撑计划(2022-YF05-00357-SN)
Catalytic remediation of polycyclic aromatic hydrocarbons contaminated soil by synthetic siderite and its derivatives
YANG Xin1,2( ), ZHONG Chengwei1, YANG Zhishan1,3, ZHU Weiwei1, WANG Wenhao1,2, YU Jiang1,2,3(
), ZHONG Chengwei1, YANG Zhishan1,3, ZHU Weiwei1, WANG Wenhao1,2, YU Jiang1,2,3( )
)
- 1.College of Architecture and Environment, Sichuan University, Chengdu 610065, Sichuan, China
2.Institute of New Energy and Low Carbon Technology, Sichuan University, Chengdu 610065, Sichuan, China
3.Yibin Institute of Industrial Technology, Sichuan University, Yibin 644000, Sichuan, China
-
Received:2023-06-06Revised:2023-07-13Online:2024-07-25Published:2024-08-14 -
Contact:YU Jiang
摘要:
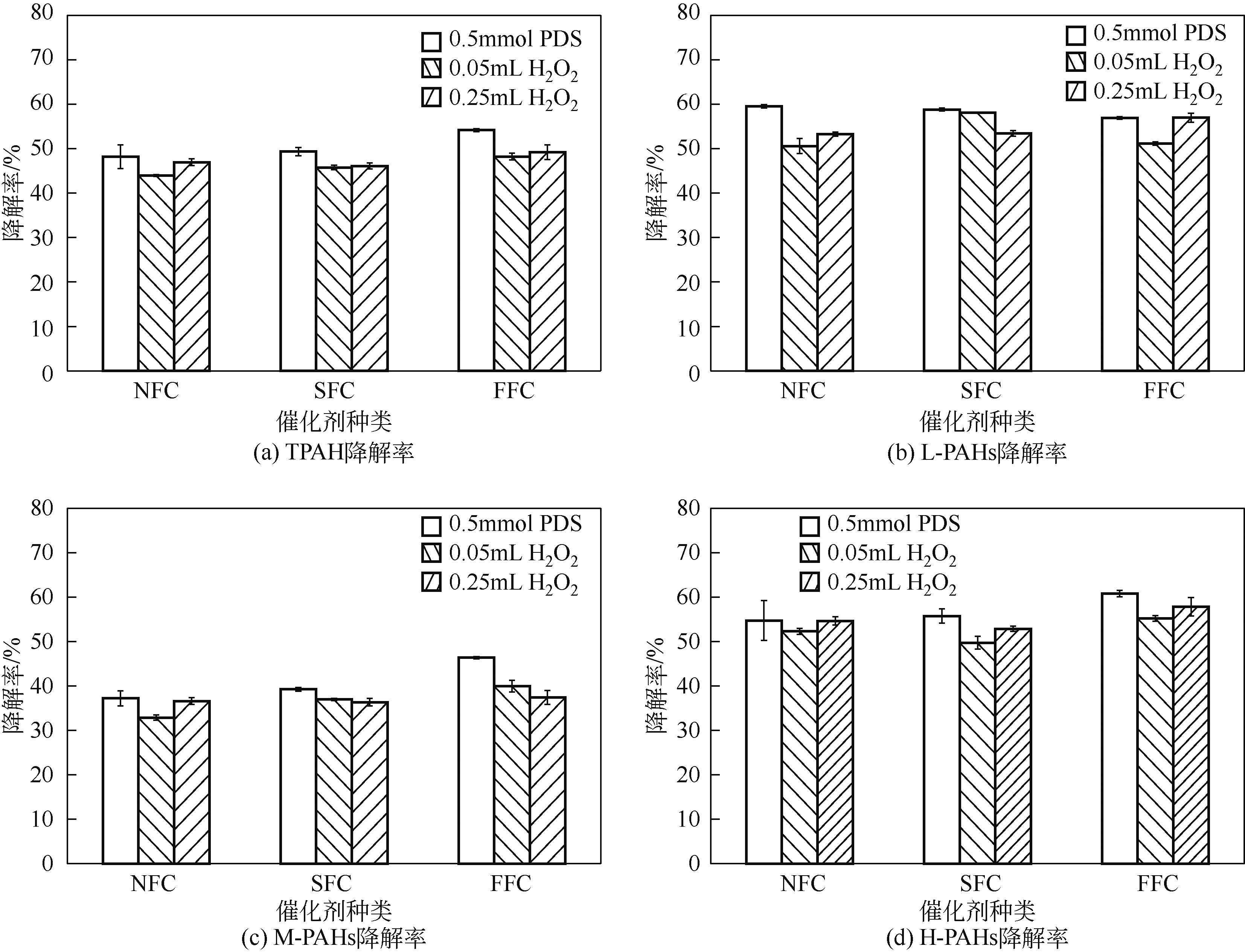
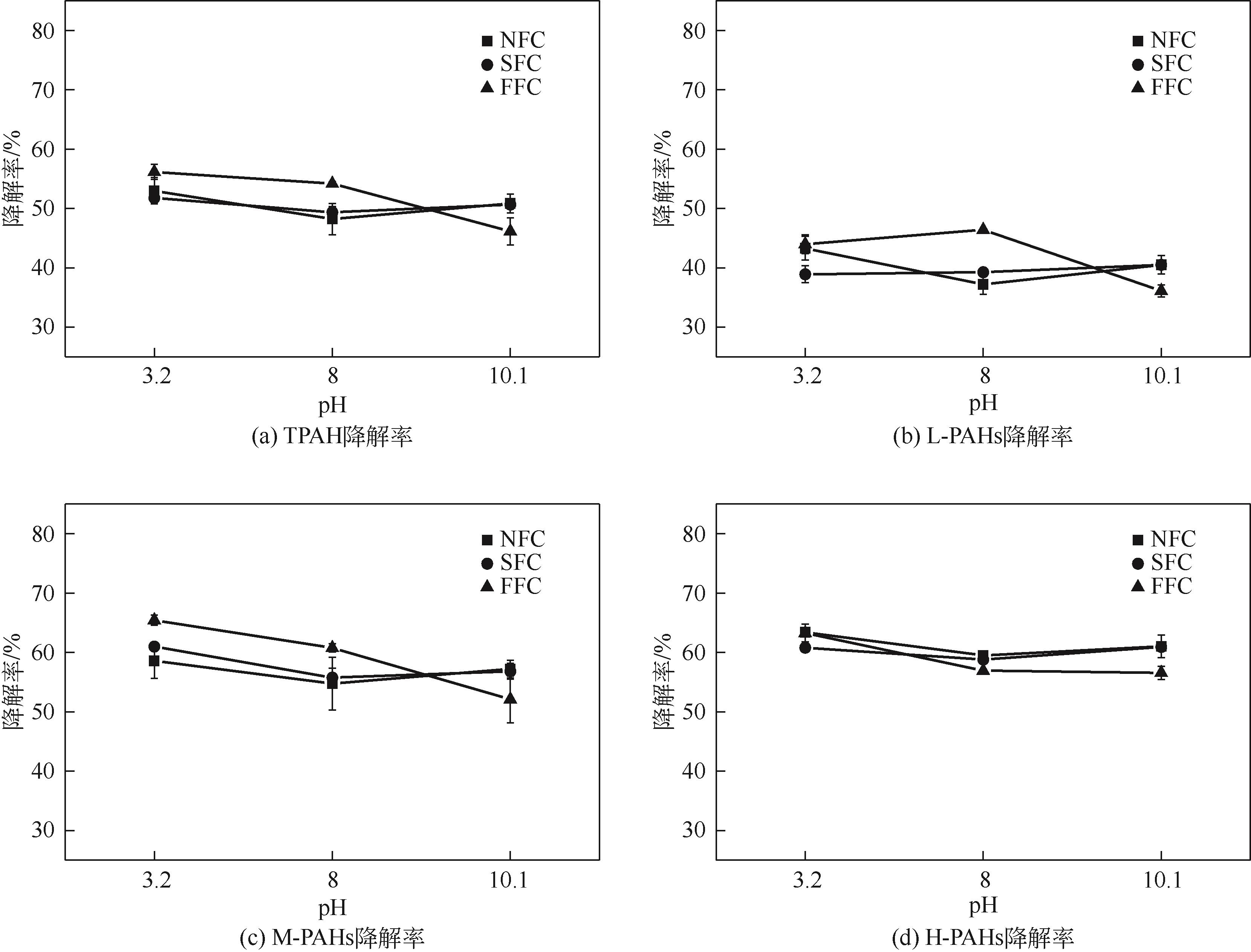
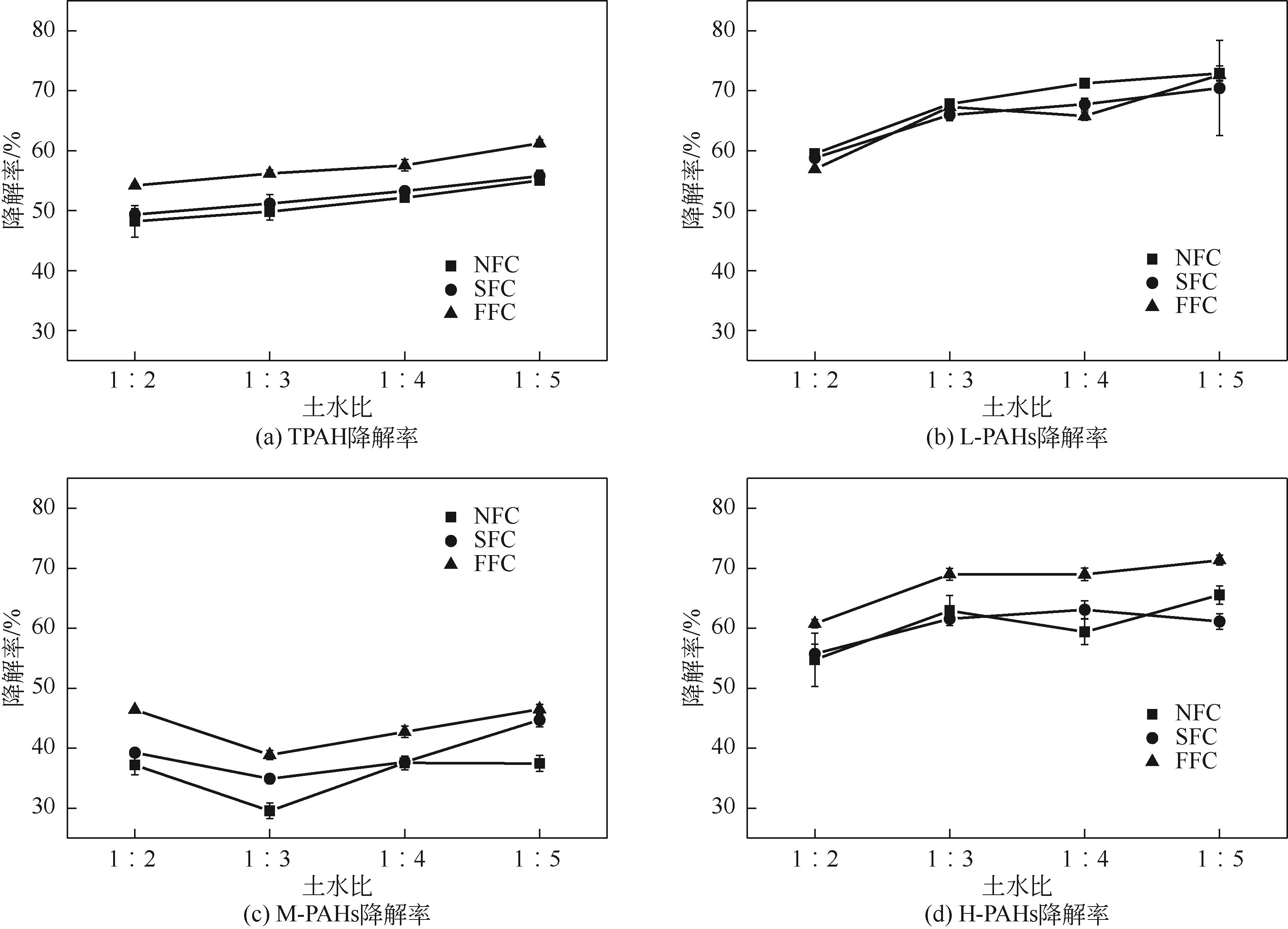
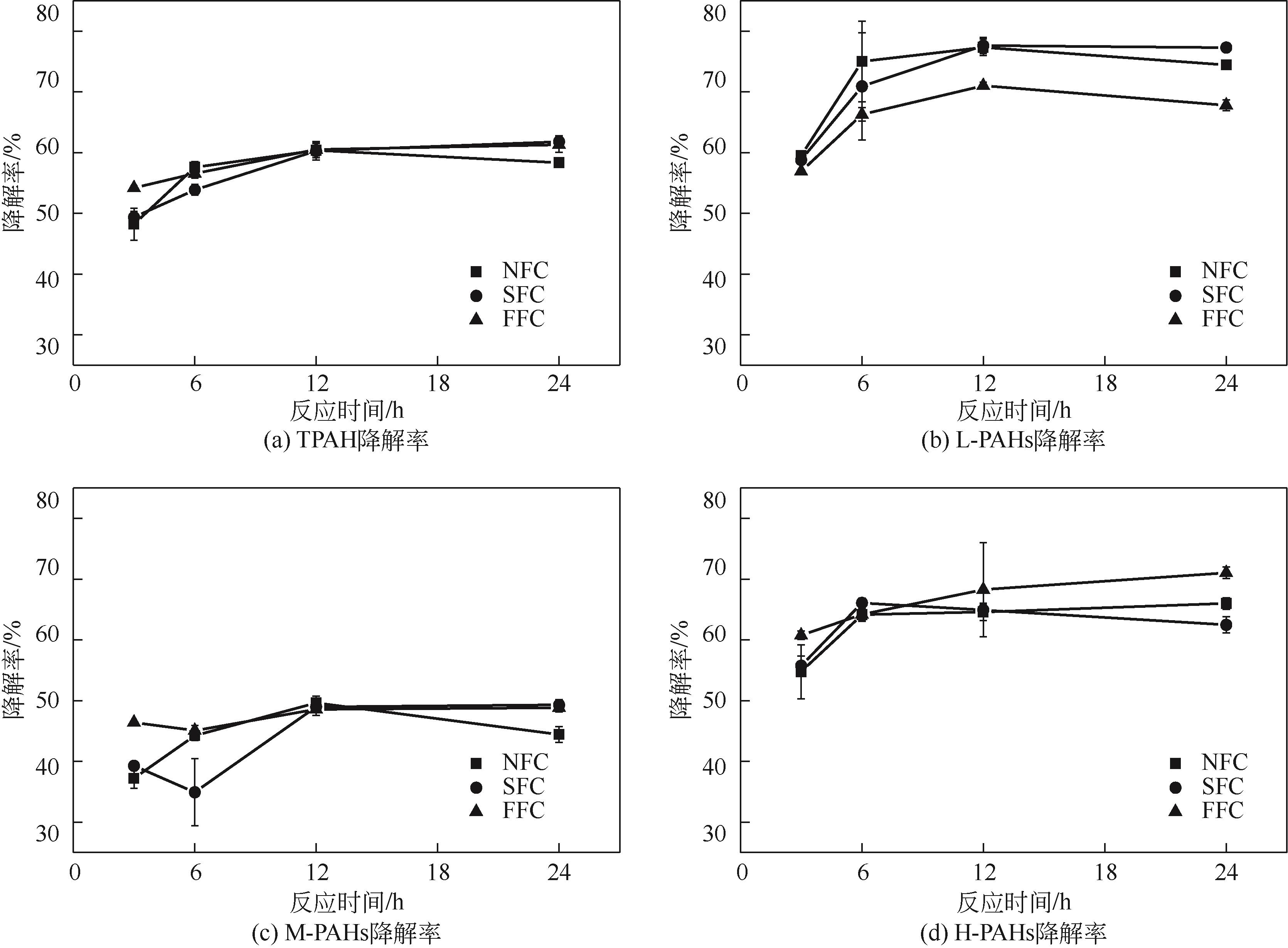
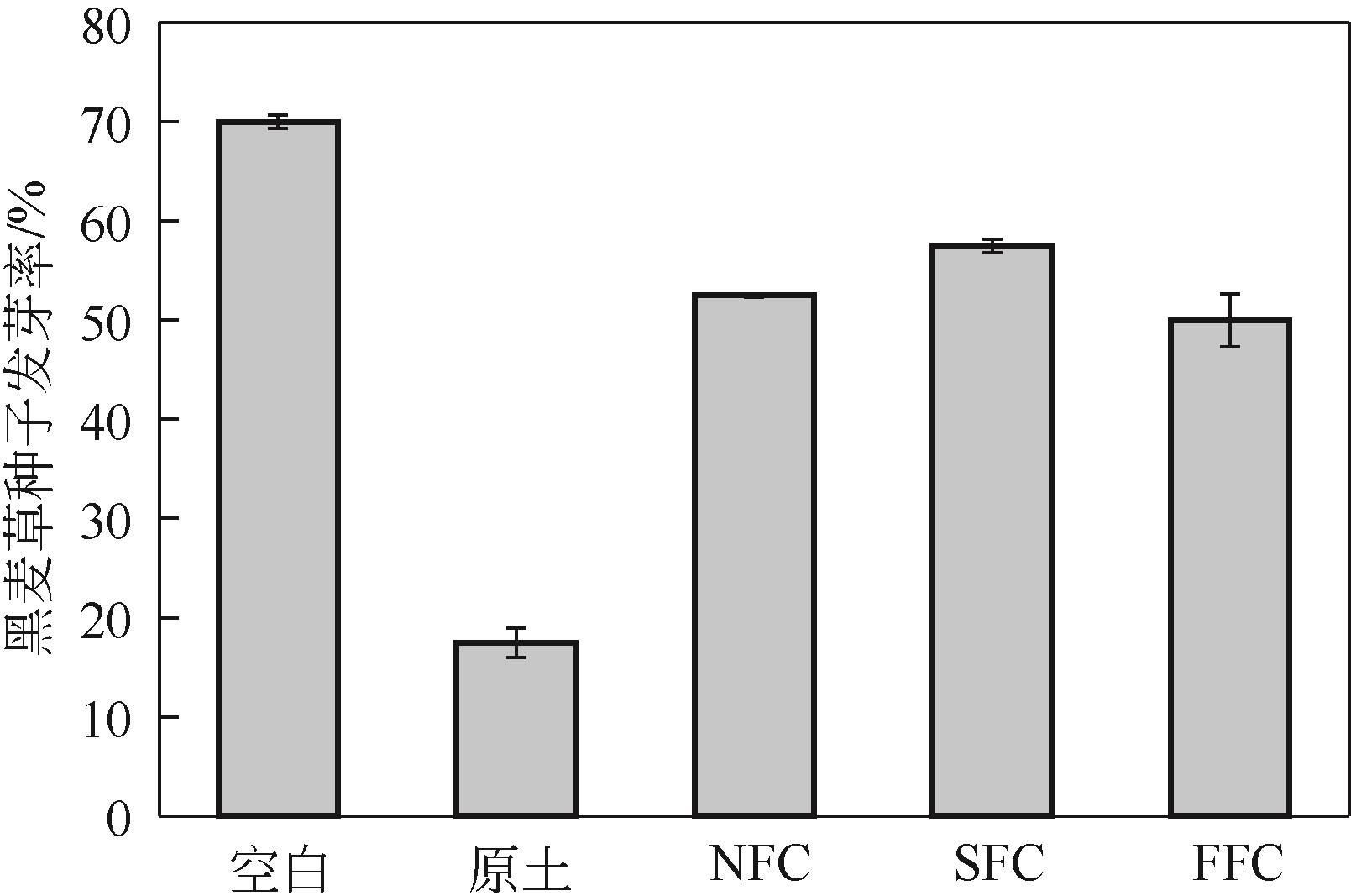
为考察不同氧化体系及运行参数对土壤多环芳烃降解性能的影响,本文通过人工仿真合成菱铁矿(SFC)并进一步引入纳米零价铁(nZVI)制备改性菱铁矿(FFC),催化氧化修复实际多环芳烃(PAHs)污染场地土壤。实验结果表明,SFC和FFC催化过硫酸钠(PDS)体系均能有效降解PAHs。通过优化运行参数包括降低初始pH、提高土水比和延长反应时间等可进一步提高土壤PAHs降解率。而等价处理实验结果显示,采用H2O2等价代替PDS虽可以降低治理成本,但PAHs降解率却有所下降。同时,土壤毒性评估结果发现经SFC/PDS和FFC/PDS处理后的黑麦草种子发芽率得到显著提高。本研究表明SFC和FFC催化体系可有效修复PAHs污染土壤,并缓减土壤生态毒性。
中图分类号:
引用本文
杨昕, 钟承韡, 杨志山, 朱韦韦, 王文浩, 余江. 人工仿真菱铁矿及其衍生材料催化修复PAHs污染土壤[J]. 化工进展, 2024, 43(7): 4118-4127.
YANG Xin, ZHONG Chengwei, YANG Zhishan, ZHU Weiwei, WANG Wenhao, YU Jiang. Catalytic remediation of polycyclic aromatic hydrocarbons contaminated soil by synthetic siderite and its derivatives[J]. Chemical Industry and Engineering Progress, 2024, 43(7): 4118-4127.
| 项目 | 组别 | 氧化剂 | 浓度 /mmol·g-1 | 用量 | 单价 /CNY·g-1 |
|---|---|---|---|---|---|
| CK | 对照组 | 过硫酸钠 | 0.5~1.0 | 0.119~0.238g·g-1 | 0.025~0.05 |
| 1 | 等浓度组 | 30%过氧化氢 | 0.5~1.0 | 0.05~0.1mL·g-1 | 0.005~0.01 |
| 2 | 等单价组 | 30%过氧化氢 | 2.5~5.0 | 0.25~0.5mL·g-1 | 0.025~0.05 |
表1 氧化剂用量
| 项目 | 组别 | 氧化剂 | 浓度 /mmol·g-1 | 用量 | 单价 /CNY·g-1 |
|---|---|---|---|---|---|
| CK | 对照组 | 过硫酸钠 | 0.5~1.0 | 0.119~0.238g·g-1 | 0.025~0.05 |
| 1 | 等浓度组 | 30%过氧化氢 | 0.5~1.0 | 0.05~0.1mL·g-1 | 0.005~0.01 |
| 2 | 等单价组 | 30%过氧化氢 | 2.5~5.0 | 0.25~0.5mL·g-1 | 0.025~0.05 |
| 项目 | 土样 /g | 催化剂 /mg·g-1 | 氧化剂 /mmol·g-1 | 温度 /℃ | 初始pH | 土水比 | 时间 /min |
|---|---|---|---|---|---|---|---|
| NFC | 10 | 50 | 1.0 | 45 | 本底值 | 1∶2 | 180 |
| SFC | 10 | 50 | 1.0 | 45 | 本底值 | 1∶2 | 180 |
| FFC | 10 | 50 | 0.5 | 45 | 本底值 | 1∶2 | 180 |
表2 PAHs污染场地降解实验
| 项目 | 土样 /g | 催化剂 /mg·g-1 | 氧化剂 /mmol·g-1 | 温度 /℃ | 初始pH | 土水比 | 时间 /min |
|---|---|---|---|---|---|---|---|
| NFC | 10 | 50 | 1.0 | 45 | 本底值 | 1∶2 | 180 |
| SFC | 10 | 50 | 1.0 | 45 | 本底值 | 1∶2 | 180 |
| FFC | 10 | 50 | 0.5 | 45 | 本底值 | 1∶2 | 180 |
| pH | 含水率 /% | 湿密度 /g·cm-3 | 干密度 /g·cm-3 | 孔隙比 | 饱和度 /% | 土粒占比 /% |
|---|---|---|---|---|---|---|
| 8.9 | 13.3 | 2.11 | 1.86 | 0.44 | 81.1 | 2.6 |
表3 土壤理化性质
| pH | 含水率 /% | 湿密度 /g·cm-3 | 干密度 /g·cm-3 | 孔隙比 | 饱和度 /% | 土粒占比 /% |
|---|---|---|---|---|---|---|
| 8.9 | 13.3 | 2.11 | 1.86 | 0.44 | 81.1 | 2.6 |
| PAHs | 污染物 | 环数 | 浓度/mg·kg-1 | 占比/% | 一类用地筛选值/mg·kg-1 | 超标倍数 |
|---|---|---|---|---|---|---|
| L-PAHs | 萘(ANP) | 2 | 1.12 | 0.55 | 25 | — |
| 苊烯(ANY) | 3 | 1.37 | 0.67 | — | — | |
| 苊(ANA) | 3 | 未检出 | 0 | — | — | |
| 芴(FLU) | 3 | 1.15 | 0.57 | — | — | |
| 菲(PHE) | 3 | 20.16 | 9.91 | — | — | |
| 蒽(ANT) | 3 | 8.40 | 4.13 | — | — | |
| M-PAHs | 荧蒽(FLT) | 4 | 25.24 | 12.40 | — | — |
| 芘(PYR) | 4 | 25.11 | 12.34 | — | — | |
| 苯并(a)蒽(BaA) | 4 | 19.75 | 9.71 | 5.5 | 3.59 | |
| 䓛(CHR) | 4 | 14.56 | 7.16 | 490 | — | |
| H-PAHs | 苯并(b)荧蒽(BaE) | 5 | 22.71 | 11.16 | 5.5 | 4.13 |
| 苯并(k)荧蒽(BKF) | 5 | 15.61 | 7.67 | 55 | — | |
| 苯并(a)芘(BaP) | 5 | 22.84 | 11.23 | 0.55 | 41.53 | |
| 茚并(1.2.3-c,d)芘(IPY) | 6 | 12.34 | 6.06 | 5.5 | 2.24 | |
| 二苯并(a,h)蒽(DBA) | 6 | 1.99 | 0.98 | 0.55 | 3.62 | |
| 苯并(g,h,i)苝(BPE) | 6 | 11.12 | 5.47 | 5.5 | — | |
| TPAH | 203.47 | 100 |
表4 化工场地PAHs污染土壤组分一览表
| PAHs | 污染物 | 环数 | 浓度/mg·kg-1 | 占比/% | 一类用地筛选值/mg·kg-1 | 超标倍数 |
|---|---|---|---|---|---|---|
| L-PAHs | 萘(ANP) | 2 | 1.12 | 0.55 | 25 | — |
| 苊烯(ANY) | 3 | 1.37 | 0.67 | — | — | |
| 苊(ANA) | 3 | 未检出 | 0 | — | — | |
| 芴(FLU) | 3 | 1.15 | 0.57 | — | — | |
| 菲(PHE) | 3 | 20.16 | 9.91 | — | — | |
| 蒽(ANT) | 3 | 8.40 | 4.13 | — | — | |
| M-PAHs | 荧蒽(FLT) | 4 | 25.24 | 12.40 | — | — |
| 芘(PYR) | 4 | 25.11 | 12.34 | — | — | |
| 苯并(a)蒽(BaA) | 4 | 19.75 | 9.71 | 5.5 | 3.59 | |
| 䓛(CHR) | 4 | 14.56 | 7.16 | 490 | — | |
| H-PAHs | 苯并(b)荧蒽(BaE) | 5 | 22.71 | 11.16 | 5.5 | 4.13 |
| 苯并(k)荧蒽(BKF) | 5 | 15.61 | 7.67 | 55 | — | |
| 苯并(a)芘(BaP) | 5 | 22.84 | 11.23 | 0.55 | 41.53 | |
| 茚并(1.2.3-c,d)芘(IPY) | 6 | 12.34 | 6.06 | 5.5 | 2.24 | |
| 二苯并(a,h)蒽(DBA) | 6 | 1.99 | 0.98 | 0.55 | 3.62 | |
| 苯并(g,h,i)苝(BPE) | 6 | 11.12 | 5.47 | 5.5 | — | |
| TPAH | 203.47 | 100 |
| PAHs | 初始pH | 土水比 | 反应时间 | |||
|---|---|---|---|---|---|---|
| R值 | P值 | R值 | P值 | R值 | P值 | |
| TPAH | -0.214 | 0.231 | 0.172 | 0.338 | 0.700② | 0.000 |
| L-PAHs | -0.096 | 0.596 | 0.344 | 0.050 | 0.641② | 0.000 |
| M-PAHs | -0.130 | 0.472 | 0.155 | 0.390 | 0.588② | 0.000 |
| H-PAHs | -0.266 | 0.134 | 0.353 | 0.044① | 0.463② | 0.007 |
表5 试验条件与PAHs的相关性分析
| PAHs | 初始pH | 土水比 | 反应时间 | |||
|---|---|---|---|---|---|---|
| R值 | P值 | R值 | P值 | R值 | P值 | |
| TPAH | -0.214 | 0.231 | 0.172 | 0.338 | 0.700② | 0.000 |
| L-PAHs | -0.096 | 0.596 | 0.344 | 0.050 | 0.641② | 0.000 |
| M-PAHs | -0.130 | 0.472 | 0.155 | 0.390 | 0.588② | 0.000 |
| H-PAHs | -0.266 | 0.134 | 0.353 | 0.044① | 0.463② | 0.007 |
| PAHs | TPAH | L-PAHs | M-PAHs | H-PAHs | ||||
|---|---|---|---|---|---|---|---|---|
| R值 | P值 | R值 | P值 | R值 | P值 | R值 | P值 | |
| TPAH | 1 | — | 0.748② | 0.000 | 0.790② | 0.000 | 0.854② | 0.000 |
| L-PAHs | 0.748② | 0.000 | 1 | — | 0.333 | 0.059 | 0.660② | 0.000 |
| M-PAHs | 0.790② | 0.000 | 0.333 | 0.059 | 1 | — | 0.433① | 0.012 |
| H-PAHs | 0.854② | 0.000 | 0.660② | 0.000 | 0.433① | 0.012 | 1 | — |
表6 不同环数PAHs的相关性分析
| PAHs | TPAH | L-PAHs | M-PAHs | H-PAHs | ||||
|---|---|---|---|---|---|---|---|---|
| R值 | P值 | R值 | P值 | R值 | P值 | R值 | P值 | |
| TPAH | 1 | — | 0.748② | 0.000 | 0.790② | 0.000 | 0.854② | 0.000 |
| L-PAHs | 0.748② | 0.000 | 1 | — | 0.333 | 0.059 | 0.660② | 0.000 |
| M-PAHs | 0.790② | 0.000 | 0.333 | 0.059 | 1 | — | 0.433① | 0.012 |
| H-PAHs | 0.854② | 0.000 | 0.660② | 0.000 | 0.433① | 0.012 | 1 | — |
| 1 | 余湛, 郑阳, 胡佳晨, 等. 典型氯代烃污染场地地下水化学氧化修复实验分析[J]. 环境保护科学, 2019, 45(5): 122-126. |
| YU Zhan, ZHENG Yang, HU Jiachen, et al. Chemical oxidation remediation analysis of groundwater in a typical chlorinated hydrocarbons contaminated site with a lab-scale[J]. Environmental Protection Science, 2019, 45(5): 122-126. | |
| 2 | 廖长君, 杨建建, 赵志勇, 等. 化工场地氯代烃污染地下水氧化修复研究[J]. 广东化工, 2020, 47(4): 57-60, 44. |
| LIAO Changjun, YANG Jianjian, ZHAO Zhiyong, et al. Study on remediation of groundwater polluted by chlorinated hydrocarbons by alkali activated sodium persulfate and modified Fenton oxidation system[J]. Guangdong Chemical Industry, 2020, 47(4): 57-60, 44. | |
| 3 | 王甫洋, 田珺, 夏晶, 等. 南京某化工企业搬迁场地土壤有机污染调查及健康风险评价研究[J]. 四川环境, 2020, 39(1): 105-111. |
| WANG Fuyang, TIAN Jun, XIA Jing, et al. Pollution investigation and health risk assessment of organic pollutants in the soil of a chemical factory in Nanjing[J]. Sichuan Environment, 2020, 39(1): 105-111. | |
| 4 | 周芷嫣, 张秀秀, 王飞, 等. 不同土地利用类型土壤多环芳烃的纵向污染特征及来源解析[J]. 环境科学, 2023, 44(3): 1583-1592. |
| ZHOU Zhiyan, ZHANG Xiuxiu, WANG Fei, et al. Vertical pollution characteristics and source analysis of soil PAHs in different land use types[J]. Environmental Science, 2023, 44(3): 1583-1592. | |
| 5 | 郭建, 罗孝俊, 管克兰, 等. 石化工业园员工PAHs的皮肤暴露及健康风险[J]. 中国环境科学, 2022, 42(11): 5427-5435. |
| GUO Jian, LUO Xiaojun, GUAN Kelan, et al. A study on employees' skin exposure to polycyclic aromatic hydrocarbons and health risk in a petrochemical industrial park[J]. China Environmental Science, 2022, 42(11): 5427-5435. | |
| 6 | 李伟, 王华伟, 孟祥宇, 等. 3种氧化剂对焦化场地多环芳烃的修复效果与土著微生物的响应关系[J]. 环境科学, 2023, 44(12): 6992-7003. |
| LI Wei, WANG Huawei, MENG Xiangyu, et al. Remediation of three oxidants on polycyclic aromatic hydrocarbons in coking contaminated soil and its response to indigenous microorganisms[J]. Environmental Science, 2023, 44(12): 6992-7003. | |
| 7 | 孙勇, 张祥, 吕树光. 基于过硫酸盐的多环芳烃污染场地原位修复技术研究进展[J]. 环境污染与防治, 2023, 45(1): 105-112, 121. |
| SUN Yong, ZHANG Xiang, Shuguang LYU. Research progress of in situ remediation technology based on persulfate for polycyclic aromatic hydrocarbons contaminated site[J]. Environmental Pollution & Control, 2023, 45(1): 105-112, 121. | |
| 8 | 韩跃鸣, 代朝猛, 段艳平, 等. 含过氧键化合物在土壤及地下水PAHs污染修复中的应用进展[J]. 材料导报, 2024, 38(6): 254-260. |
| Yueming HAH, DAI Chaomeng, DUAN Yanping, et al. Application progress of peroxybond compounds in remediation of PAHs pollution in soil and groundwater[J]. Materials Reports, 2024, 38(6): 254-260. | |
| 9 | FU Caixia, YAN Miao, WANG Zhuoyue, et al. New insights into the degradation and detoxification of methylene blue using heterogeneous-Fenton catalyzed by sustainable siderite[J]. Environmental Research, 2023, 216: 114819. |
| 10 | SONG Wei, LI Ji, ZHANG Xiaolei, et al. A feasible approach for azo-dye methyl orange degradation in siderite/H2O2 assisted by persulfate: Optimization using response surface methodology and pathway[J]. Journal of Environmental Management, 2022, 308: 114397. |
| 11 | FENG Yong, WU Deli, LI Hailong, et al. Activation of persulfates using siderite as a source of ferrous ions: Sulfate radical production, stoichiometric efficiency, and implications[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(3): 3624-3631. |
| 12 | SUN Fuwei, CHEN Tianhu, LIU Haibo, et al. The pH-dependent degradation of sulfadiazine using natural siderite activating PDS: The role of singlet oxygen[J]. Science of the Total Environment, 2021, 784: 147117. |
| 13 | SUN Fuwei, LIU Haibo, WANG Hanlin, et al. A novel discovery of a heterogeneous Fenton-like system based on natural siderite: A wide range of pH values from 3 to 9[J]. Science of the Total Environment, 2020, 698: 134293. |
| 14 | YAN Ni, LIU Fei, HUANG Weiying. Interaction of oxidants in siderite catalyzed hydrogen peroxide and persulfate system using trichloroethylene as a target contaminant[J]. Chemical Engineering Journal, 2013, 219: 149-154. |
| 15 | 李梦姣, 刘菲, 陈鸿汉, 等. 菱铁矿催化过氧化氢-过硫酸钠修复地下水中1,2-二氯乙烷污染[J]. 环境工程学报, 2014, 8(4): 1434-1438. |
| LI Mengjiao, LIU Fei, CHEN Honghan, et al. Removal of 1,2 - dichloroethane in groundwater with siderite-catalyzed hydrogen peroxide and persulfate system[J]. Chinese Journal of Environmental Engineering, 2014, 8(4): 1434-1438. | |
| 16 | ZHONG Chengwei, JIANG Yinying, LIU Quanfeng, et al. Natural siderite derivatives activated peroxydisulfate toward oxidation of organic contaminant: A green soil remediation strategy[J]. Journal of Environmental Sciences, 2023, 127: 615-627. |
| 17 | GAO Yue, XUE Yanan, JI Jing, et al. Remediation of industrial site soil contaminated with PAHs using stage persulfate oxidation activated by Fe2+ chelated with sodium citrate[J]. Chemosphere, 2023, 313: 137450. |
| 18 | KIM Cheolyong, Jun-Young AHN, KIM Tae Yoo, et al. Activation of persulfate by nanosized zero-valent iron (NZVI): Mechanisms and transformation products of NZVI[J]. Environmental Science & Technology, 2018, 52(6): 3625-3633. |
| 19 | 冉宗信, 陈靖宇, 王亚婷, 等. 典型工业区土壤多环芳烃污染特征及影响因素[J]. 环境科学, 2019, 40(10): 4594-4603. |
| RAN Zongxin, CHEN Jingyu, WANG Yating, et al. Characteristics and influencing factors of polycyclic aromatic hydrocarbons in surface soils from typical industrial areas of Chengdu[J]. Environmental Science, 2019, 40(10): 4594-4603. | |
| 20 | 周佳靖, 柳修楚, 郭瑾, 等. 纳米氧化铁与氧化剂对多环芳烃污染农田土壤修复和蔬菜健康风险的影响[J]. 环境污染与防治, 2021, 43(2): 223-228. |
| ZHOU Jiajing, LIU Xiuchu, GUO Jin, et al. Effects of nano-Fe2O3 and oxidants on soil remediation and health risk of polycyclic aromatic hydrocarbon in vegetable from contaminated farmland[J]. Environmental Pollution & Control, 2021, 43(2): 223-228. | |
| 21 | 翟雄. 烯烃的过氧化氢氧化研究[D]. 大连: 大连理工大学, 2005. |
| ZHAI Xiong. Olefin oxidation with hydrogen peroxide[D]. Dalian: Dalian University of Technology, 2005. | |
| 22 | 张维国. 稠环芳烃分子结构对其碳化产物结构的影响[D]. 北京: 北京化工大学, 2015. |
| ZHANG Weiguo. Influence of molecular structure polycyclic aromatic hydrocarbons on the carbonized product[D]. Beijing: Beijing University of Chemical Technology, 2015. | |
| 23 | 王春艳. PAHs污染土壤的化学氧化修复技术研究[D]. 北京: 北京化工大学, 2012. |
| WANG Chunyan. The research of remediation technology of PAHs contaminated soil by chemical oxidation[D]. Beijing: Beijing University of Chemical Technology, 2012. | |
| 24 | 赵丹, 廖晓勇, 阎秀兰, 等. 不同化学氧化剂对焦化污染场地多环芳烃的修复效果[J]. 环境科学, 2011, 32(3): 857-863. |
| ZHAO Dan, LIAO Xiaoyong, YAN Xiulan, et al. Chemical oxidants for remediation of soils contaminated with polycyclic aromatic hydrocarbons at a coking site[J]. Environmental Science, 2011, 32(3): 857-863. | |
| 25 | 占升, 郑义, 李森, 等. 不同氧化剂活化过硫酸钠对土壤中多环芳烃降解的影响[J]. 浙江农业学报, 2017, 29(1): 129-136. |
| ZHAN Sheng, ZHENG Yi, LI Sen, et al. Degradation of PAHs in soil by different oxidants activated sodium persulfate[J]. Acta Agriculturae Zhejiangensis, 2017, 29(1): 129-136. | |
| 26 | 徐源洲, 张力浩, 魏志敏, 等. 硫酸根自由基高级氧化技术对污染场地多环芳烃的修复效果研究[J]. 土壤, 2020, 52(3): 532-538. |
| XU Yuanzhou, ZHANG Lihao, WEI Zhimin, et al. Effects of sulfate radical advanced oxidation technology on PAHs remediation in contaminated sites[J]. Soils, 2020, 52(3): 532-538. | |
| 27 | HUSSAIN Imtyaz, LI Mingyu, ZHANG Yongqing, et al. Insights into the mechanism of persulfate activation with nZVI/BC nanocomposite for the degradation of nonylphenol[J]. Chemical Engineering Journal, 2017, 311: 163-172. |
| 28 | 张羽. 零价铁活化过硫酸钠降解土壤中多环芳烃[D]. 北京: 中国地质大学(北京), 2019. |
| ZHANG Yu. Degradation of PAHs in soil by zero-valent iron activated sodium persulfate[D]. Beijing: China University of Geosciences (Beijing), 2019. | |
| 29 | 李海红. 硫化纳米零价铁对水中诺氟沙星去除的机理研究[D]. 太原: 太原理工大学, 2020. |
| LI Haihong. Study on removal mechanism of norfloxacin in water by sulfidated nanoscale zero-valent iron[D]. Taiyuan: Taiyuan University of Technology, 2020. | |
| 30 | PELUFFO M, PARDO F, SANTOS A, et al. Use of different kinds of persulfate activation with iron for the remediation of a PAH-contaminated soil[J]. Science of the Total Environment, 2016, 563: 649-656. |
| 31 | ZHOU Zhou, LIU Xitao, MA Jun, et al. Activation of persulfate by vanadium oxide modified carbon nanotube for 17 β - e s t r a d i o l degradation in soil: Mechanism, application and ecotoxicity assessment[J]. Science of the Total Environment, 2023, 858: 159760. |
| [1] | 刘哲, 周顺利, 李永祥, 张成喜, 刘宜鹏. 烷基萘合成催化剂研究进展[J]. 化工进展, 2025, 44(S1): 144-158. |
| [2] | 林已杰, 乔鹏, 李心睿, 张宏斌, 王雪芹. TiO2纳米光催化剂的异质结构建策略与应用研究进展[J]. 化工进展, 2025, 44(S1): 159-177. |
| [3] | 王涛, 张雪冰, 张琪, 陈强, 张魁, 门卓武. 还原碳化温度和CO浓度对工业级费托合成沉淀铁催化剂性能的影响[J]. 化工进展, 2025, 44(S1): 178-184. |
| [4] | 包新德, 刘必烨, 黄仁伟, 洪宇豪, 关鑫, 林金国. 生物质基@CuNiOS复合催化剂的制备及其在有机染料还原中的应用[J]. 化工进展, 2025, 44(S1): 185-196. |
| [5] | 马晓彪, 刘晗, 王伟欢, 苗培培, 季莹辉, 陈博阳, 彭晓伟, 许强, 靳凤英, 马明超, 王银斌, 郭春垒. 酸和磷复合改性对ZSM-5分子筛催化裂解性能的影响[J]. 化工进展, 2025, 44(S1): 197-204. |
| [6] | 赵思阳, 李陈冉, 刘洋. 副产C4预积炭调控MTO再生催化剂双烯选择性的工艺优化[J]. 化工进展, 2025, 44(S1): 205-212. |
| [7] | 赵雨龙, 蔡凯, 于善青. 氧化铝孔结构对催化裂化烃类分子吸附扩散及反应性能的影响[J]. 化工进展, 2025, 44(S1): 213-221. |
| [8] | 李军良, 李悦, 孙道来. Cu/SiO2-Al2O3催化1,2-丁二醇加氢脱氧制备1-丁醇[J]. 化工进展, 2025, 44(S1): 222-231. |
| [9] | 刘超, 丁承奥, 吴宝顺, 雷欣宇, 王光应, 余正伟. TiO2载体粒度对RuO x -V2O5-WO3/TiO2催化剂脱硝及抗水硫中毒性能的影响[J]. 化工进展, 2025, 44(S1): 232-242. |
| [10] | 张涵林, 岳学海, 刘俊希, 殷逢俊. 钌锶铱电沉积构筑高稳定性析氧反应电催化剂[J]. 化工进展, 2025, 44(S1): 243-251. |
| [11] | 谭芳芳, 程安, 刘佳, 王渊博, 王军. 可见光驱动对甲氧基苯甲醛一步合成对甲氧基苯甲酸甲酯新方法[J]. 化工进展, 2025, 44(S1): 434-440. |
| [12] | 王伟豪, 吴贤豪, 周瑛, 冯向东, 胡达清, 卢晗锋. 水相耦合高级氧化法治理VOCs技术:机理、应用与挑战[J]. 化工进展, 2025, 44(S1): 478-491. |
| [13] | 陈子朝, 何方书, 胡强, 杨扬, 陈汉平, 杨海平. 甲烷干重整抗积炭Ni基催化剂研究进展[J]. 化工进展, 2025, 44(9): 4968-4978. |
| [14] | 王振, 张耀远, 吴芹, 史大昕, 陈康成, 黎汉生. 甲烷干重整用Ni/Al2O3基催化剂研究进展[J]. 化工进展, 2025, 44(9): 4979-4998. |
| [15] | 张海鹏, 秦珊珊, 王俣萱, 于海彪. 3.0F-Ag x Co催化剂的制备及其催化分解N2O[J]. 化工进展, 2025, 44(9): 4999-5005. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||