化工进展 ›› 2024, Vol. 43 ›› Issue (6): 3051-3060.DOI: 10.16085/j.issn.1000-6613.2023-0843
• 工业催化 • 上一篇
铜基催化剂电还原二氧化碳为甲酸研究进展
- 东南大学能源与环境学院,江苏 南京 210096
-
收稿日期:2023-05-22修回日期:2023-09-12出版日期:2024-06-15发布日期:2024-07-02 -
通讯作者:仲兆平 -
作者简介:陈富强(1999—),男,硕士研究生,研究方向为铜基电催化二氧化碳还原。E-mail:220210560@seu.edu.cn。 -
基金资助:国家重点研发计划(2018YFB1501405)
Research progress on copper-based catalysts for electrochemical reduction of carbon dioxide to formic acid
CHEN Fuqiang( ), ZHONG Zhaoping(
), ZHONG Zhaoping( ), QI Renzhi
), QI Renzhi
- School of Energy and Environment, Southeast University, Nanjing 210096, Jiangsu, China
-
Received:2023-05-22Revised:2023-09-12Online:2024-06-15Published:2024-07-02 -
Contact:ZHONG Zhaoping
摘要:
电化学二氧化碳还原(ECO2RR)通过可再生能源制备高能量化学品或燃料对碳中和具有巨大价值。尤其是以铜基催化剂进行的ECO2RR,其成本优势和卓越的催化活性使其成为最有前景的策略。在ECO2RR的各种产物中,甲酸作为优秀的储氢材料和内燃机燃料展现出工业化生产潜力。本文针对近年来过渡金属族的铜基催化剂在制备甲酸的研究进展进行了全面的总结,从ECO2RR制甲酸机理出发,综述了铜基催化剂在ECO2RR制甲酸领域取得的重要研究进展,其中以典型催化剂为例分析ECO2RR生成甲酸的策略,包括形貌结构、表面价态、合金化、晶面效应、空位和碳载体等,重点讨论了活性位点数量以及关键中间体*OCHO的形成对甲酸产物选择性的影响,最后总结了该领域面临的挑战以及从原位表征、科学计算和反应条件等角度的展望。
中图分类号:
引用本文
陈富强, 仲兆平, 戚仁志. 铜基催化剂电还原二氧化碳为甲酸研究进展[J]. 化工进展, 2024, 43(6): 3051-3060.
CHEN Fuqiang, ZHONG Zhaoping, QI Renzhi. Research progress on copper-based catalysts for electrochemical reduction of carbon dioxide to formic acid[J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3051-3060.
| 催化剂 | 策略 | 电流密度/mA·cm-2 | 参考文献 | |
|---|---|---|---|---|
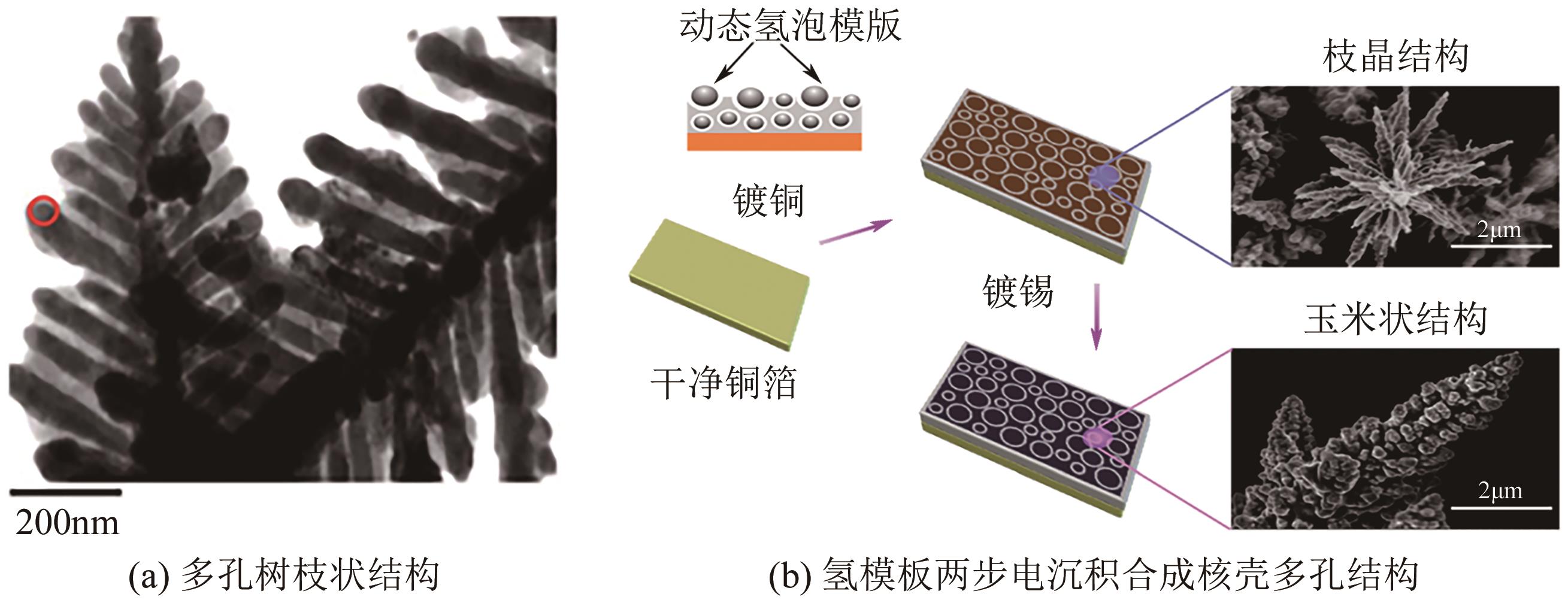
| 多孔树枝Cu | 形貌调整 | 87 | 6.5 | [ |
| 三维核壳Cu@Sn | 形貌调整 | 100 | 16.52 | [ |
| 中空纤维Cu | 形貌调整 | 77.1 | 34.7 | [ |
| Pd5Cu1 | 合金化 | 64 | — | [ |
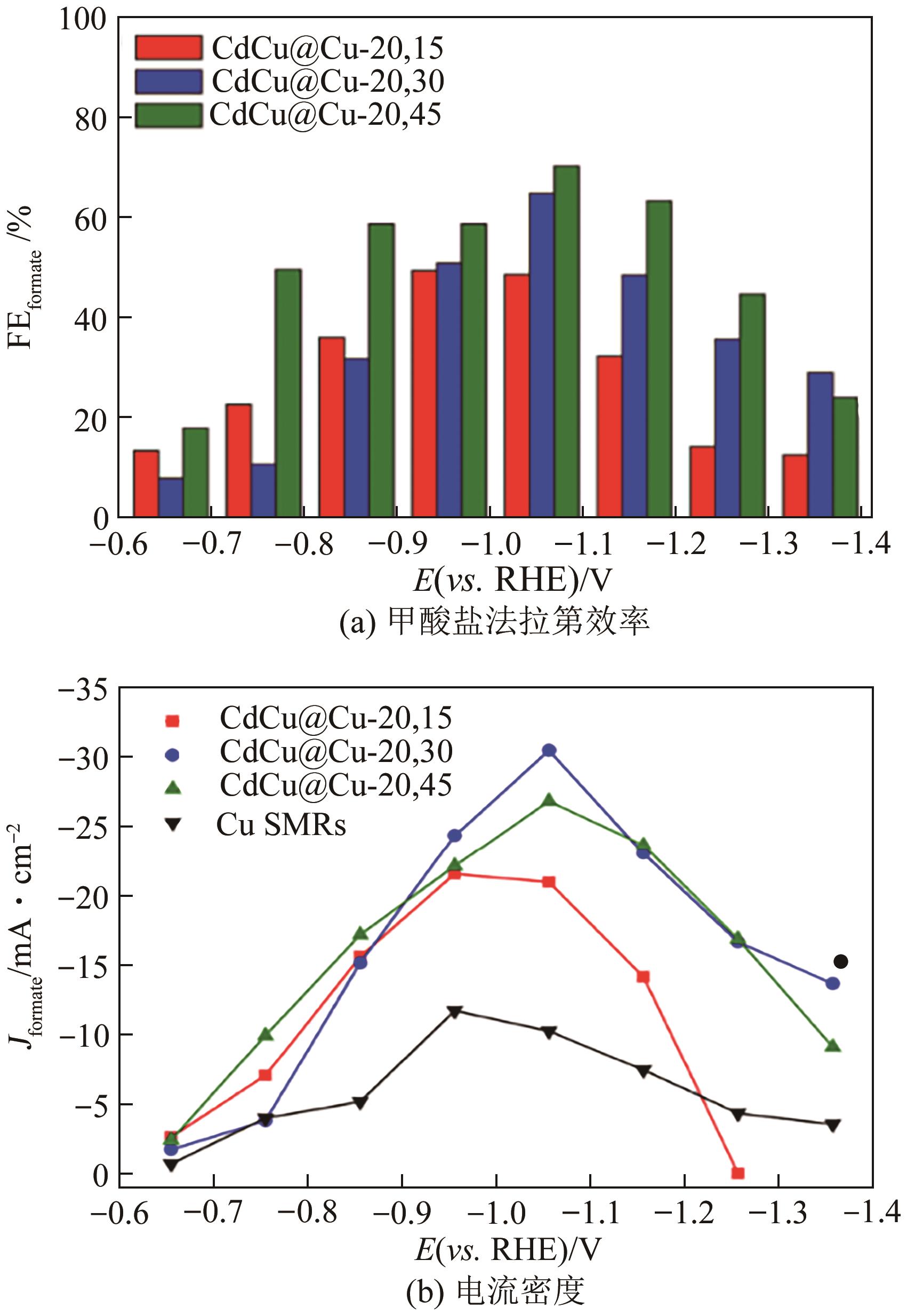
| CdCu@Cu | 合金化 | 70.5 | 30.5 | [ |
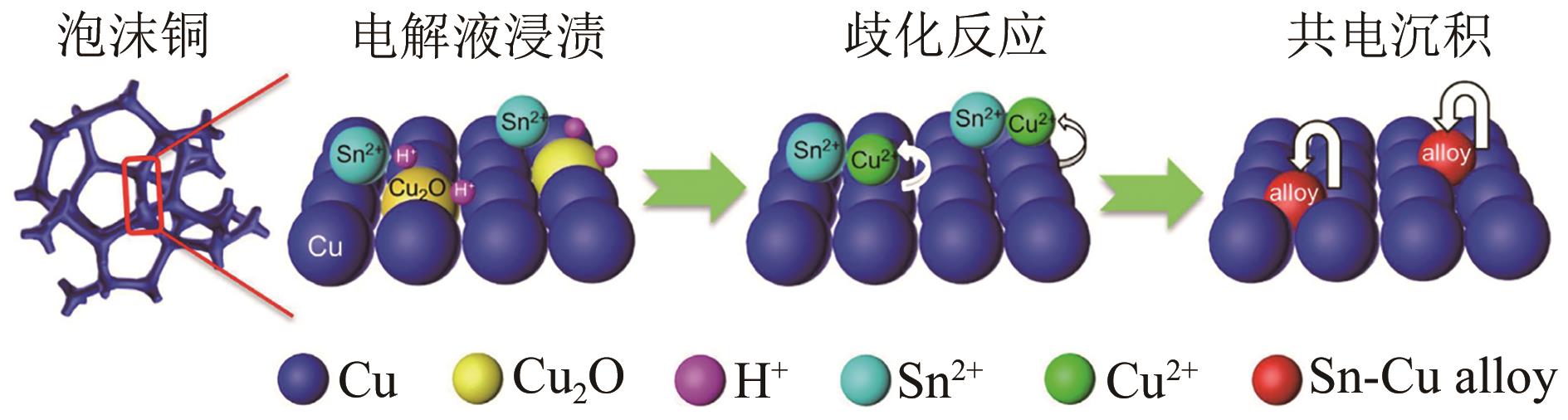
| Sn-Cu | 合金化 | 84.4 | 79 | [ |
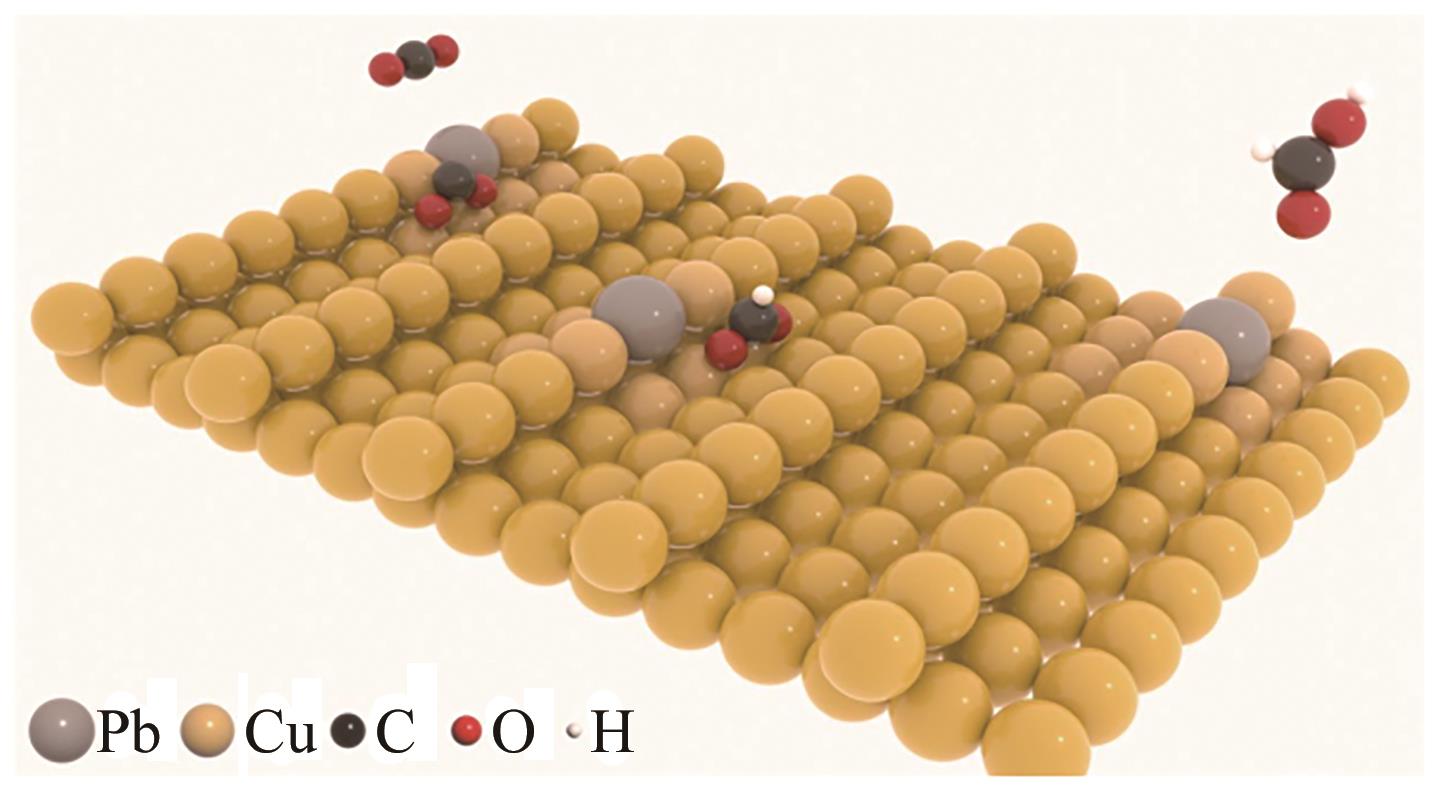
| Pb1Cu | 合金化 | 96 | 1000 | [ |
| Cu2S/Cu | 硫掺杂 | 85 | 5.3 | [ |
| 超薄多孔Cu-S NFs | 硫掺杂 | 89.8 | 404.1 | [ |
| 磷酸盐调控Cu | 磷掺杂 | 79 | — | [ |
| Cu2O(111) | 晶面暴露 | 90 | — | [ |
| Cu2O (100) | 晶面暴露 | 90 | 260 | [ |
| Cu@NC | 氮掺杂碳材料 | 41 | — | [ |
| Cu2S/ NDg-C3N4 | 含氮缺陷的石墨相氮化碳 | 82.3 | 5.24 | [ |
| Cu-CDots | 碳点载体 | 79 | — | [ |
表1 铜基催化剂改良策略与对应的ECO2RR性能
| 催化剂 | 策略 | 电流密度/mA·cm-2 | 参考文献 | |
|---|---|---|---|---|
| 多孔树枝Cu | 形貌调整 | 87 | 6.5 | [ |
| 三维核壳Cu@Sn | 形貌调整 | 100 | 16.52 | [ |
| 中空纤维Cu | 形貌调整 | 77.1 | 34.7 | [ |
| Pd5Cu1 | 合金化 | 64 | — | [ |
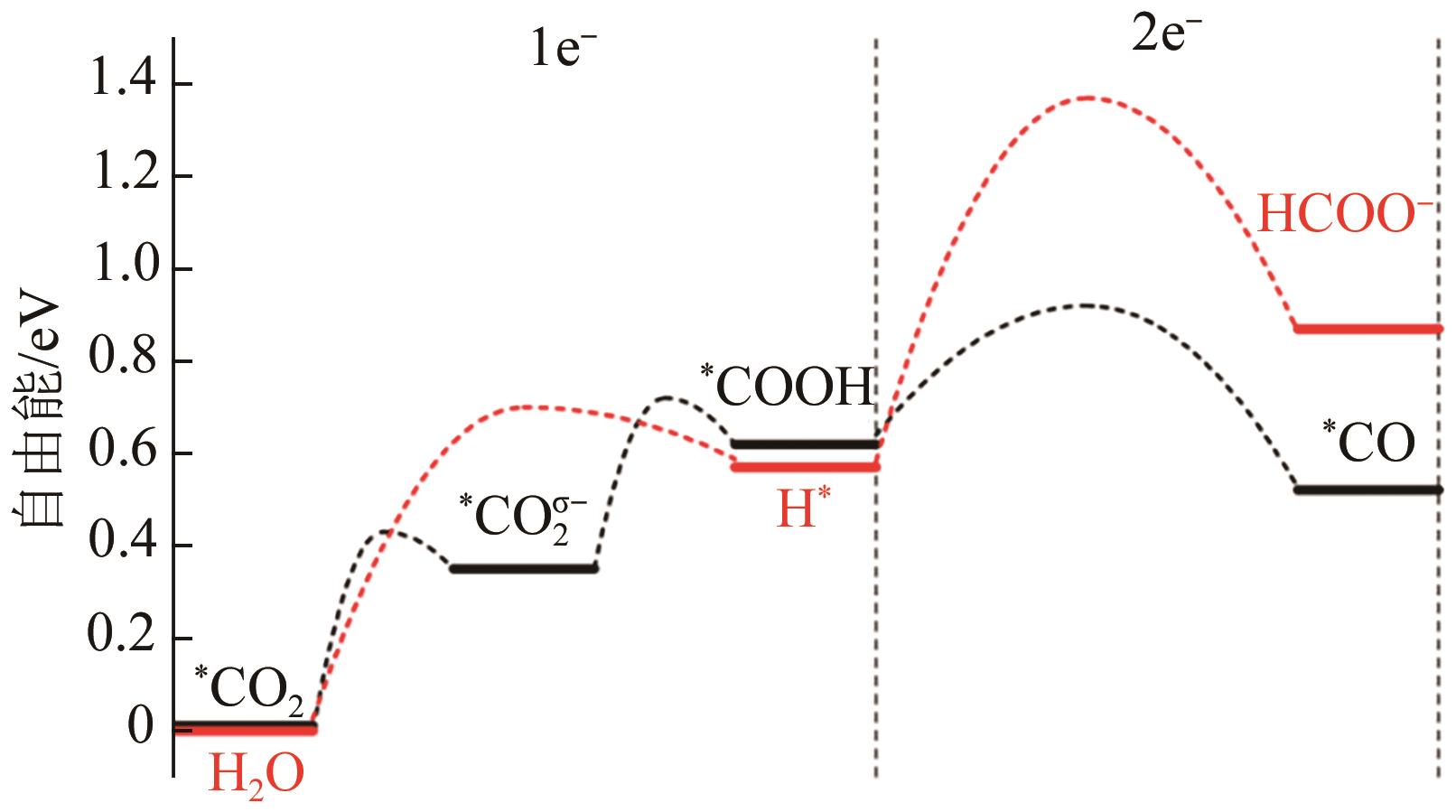
| CdCu@Cu | 合金化 | 70.5 | 30.5 | [ |
| Sn-Cu | 合金化 | 84.4 | 79 | [ |
| Pb1Cu | 合金化 | 96 | 1000 | [ |
| Cu2S/Cu | 硫掺杂 | 85 | 5.3 | [ |
| 超薄多孔Cu-S NFs | 硫掺杂 | 89.8 | 404.1 | [ |
| 磷酸盐调控Cu | 磷掺杂 | 79 | — | [ |
| Cu2O(111) | 晶面暴露 | 90 | — | [ |
| Cu2O (100) | 晶面暴露 | 90 | 260 | [ |
| Cu@NC | 氮掺杂碳材料 | 41 | — | [ |
| Cu2S/ NDg-C3N4 | 含氮缺陷的石墨相氮化碳 | 82.3 | 5.24 | [ |
| Cu-CDots | 碳点载体 | 79 | — | [ |
| 1 | WEI Jian, YAO Ruwei, HAN Yu, et al. Towards the development of the emerging process of CO2 heterogenous hydrogenation into high-value unsaturated heavy hydrocarbons[J]. Chemical Society Reviews, 2021, 50(19): 10764-10805. |
| 2 | YAASHIKAA P R, SENTHIL KUMAR P, VARJANI Sunita J, et al. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products[J]. Journal of CO2 Utilization, 2019, 33: 131-147. |
| 3 | DUARAH Prangan, HALDAR Dibyajyoti, YADAV VSK, et al. Progress in the electrochemical reduction of CO2 to formic acid: A review on current trends and future prospects[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106394. |
| 4 | 张轩, 黄耀桢, 邵秀丽, 等. 结构化铜基催化剂电化学还原CO2为多碳产物研究进展[J]. 化工进展, 2021, 40(7): 3736-3746. |
| ZHANG Xuan, HUANG Yaozhen, SHAO Xiuli, et al. Recent progress in structured Cu-based catalysts for electrochemical CO2 reduction to C2+ products[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3736-3746. | |
| 5 | 李喆, 李泽洋, 杨宇森, 等. 电化学二氧化碳还原制甲酸催化剂的研究进展[J]. 化工进展, 2023, 42(1): 53-66. |
| LI Zhe, LI Zeyang, YANG Yusen, et al. Research progress on catalysts for electrocatalytic reduction of carbon dioxide to formic acid[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 53-66. | |
| 6 | WU Yahui, CHEN Chunjun, YAN Xupeng, et al. Enhancing CO2 electroreduction to CH4 over Cu nanoparticles supported on N-doped carbon[J]. Chemical Science, 2022, 13(28): 8388-8394. |
| 7 | RASUL Shahid, ANJUM Dalaver H, JEDIDI Abdesslem, et al. A highly selective copper-indium bimetallic electrocatalyst for the electrochemical reduction of aqueous CO2 to CO[J]. Angewandte Chemie International Edition, 2015, 54(7): 2146-2150. |
| 8 | Yeongdong MUN, LEE Seunghyun, CHO Ara, et al. Cu-Pd alloy nanoparticles as highly selective catalysts for efficient electrochemical reduction of CO2 to CO[J]. Applied Catalysis B: Environmental, 2019, 246: 82-88. |
| 9 | ZHANG Qiang, YUE Ruirui, JIANG Fengxing, et al. Au as an efficient promoter for electrocatalytic oxidation of formic acid and carbon monoxide: A comparison between Pt-on-Au and PtAu alloy catalysts[J]. Gold Bulletin, 2013, 46(3): 175-184. |
| 10 | Sally Fae HO, Adriana MENDOZA-GARCIA, GUO Shaojun, et al. A facile route to monodisperse MPd (M = Co or Cu) alloy nanoparticles and their catalysis for electrooxidation of formic acid[J]. Nanoscale, 2014, 6(12): 6970-6973. |
| 11 | LI Sirui, ZUO Yu, TANG Yuming, et al. The electroplated Pd-Co alloy film on 316L stainless steel and the corrosion resistance in boiling acetic acid and formic acid mixture with stirring[J]. Applied Surface Science, 2014, 321: 179-187. |
| 12 | Elías MARDONES-HERRERA, Carmen CASTRO-CASTILLO, NANDA Kamala Kanta, et al. Reduced graphene oxide overlayer on copper nanocube electrodes steers the selectivity towards ethanol in electrochemical reduction of carbon dioxide[J]. ChemElectroChem, 2022, 9(10): e202200259. |
| 13 | BAEK Yeji, SONG Hakhyeon, HONG Deokgi, et al. Electrochemical carbon dioxide reduction on copper-zinc alloys: Ethanol and ethylene selectivity analysis[J]. Journal of Materials Chemistry A, 2022, 10(17): 9393-9401. |
| 14 | XIANG Kaisong, LIU Yucheng, LI Chaofang, et al. Microenvironmental feeding and stabilization of C2H4 intermediates by iodide-doped copper nanowire arrays to boost C2H6 formation[J]. Energy & Fuels, 2021, 35(19): 15987-15994. |
| 15 | SHAN Jingjing, SHI Yaoxuan, LI Huiyi, et al. Effective CO2 electroreduction toward C2H4 boosted by Ce-doped Cu nanoparticles[J]. Chemical Engineering Journal, 2022, 433: 133769. |
| 16 | LAN Yangchun, NIU Gaoqiang, WANG Fei, et al. SnO2-modified two-dimensional CuO for enhanced electrochemical reduction of CO2 to C2H4 [J]. ACS Applied Materials & Interfaces, 2020, 12(32): 36128-36136. |
| 17 | BULUSHEV Dmitri A, ROSS Julian R H. Towards sustainable production of formic acid[J]. ChemSusChem, 2018, 11(5): 821-836. |
| 18 | ZHENG Tingting, LIU Chunxiao, GUO Chenxi, et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying[J]. Nature Nanotechnology, 2021, 16(12): 1386-1393. |
| 19 | VENKATA Sai Sriram Mosali, ZHANG Xiaolong, ZHANG Ying, et al. Electrocatalytic CO2 reduction to formate on Cu based surface alloys with enhanced selectivity[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(24): 19453-19462. |
| 20 | AI Ling, Sue-Faye NG, Wee-Jun ONG. Carbon dioxide electroreduction into formic acid and ethylene: A review[J]. Environmental Chemistry Letters, 2022, 20(6): 3555-3612. |
| 21 | HUAN Tran Ngoc, SIMON Philippe, ROUSSE Gwenaëlle, et al. Porous dendritic copper: An electrocatalyst for highly selective CO2 reduction to formate in water/ionic liquid electrolyte[J]. Chemical Science, 2017, 8(1): 742-747. |
| 22 | HOU Xiaofan, CAI Yixiao, ZHANG Dan, et al. 3D core-shell porous-structured Cu@Sn hybrid electrodes with unprecedented selective CO2-into-formate electroreduction achieving 100%[J]. Journal of Materials Chemistry A, 2019, 7(7): 3197-3205. |
| 23 | LIU Defei, HU Yan, SHOKO Elvis, et al. High selectivity of CO2 conversion to formate by porous copper hollow fiber: Microstructure and pressure effects[J]. Electrochimica Acta, 2021, 365: 137343. |
| 24 | TAKASHIMA Toshihiro, SUZUKI Tomohiro, IRIE Hiroshi. Electrochemical reduction of carbon dioxide to formate on palladium-copper alloy nanoparticulate electrode[J]. Electrochemistry, 2019, 87(2): 134-138. |
| 25 | YE Ke, CAO Ang, SHAO Jiaqi, et al. Synergy effects on Sn-Cu alloy catalyst for efficient CO2 electroreduction to formate with high mass activity[J]. Science Bulletin, 2020, 65(9): 711-719. |
| 26 | ZHU Qinggong, SUN Xiaofu, KANG Xinchen, et al. Cu2S on Cu foam as highly efficient electrocatalyst for reduction of CO2 to formic acid[J]. Acta Physico-Chimica Sinica, 2016, 32(1): 261-266. |
| 27 | LIU Lixia, LI Xiang, CAI Yanming, et al. Hierarchical S-modified Cu porous nanoflakes for efficient CO2 electroreduction to formate[J]. Nanoscale, 2022, 14(37): 13679-13688. |
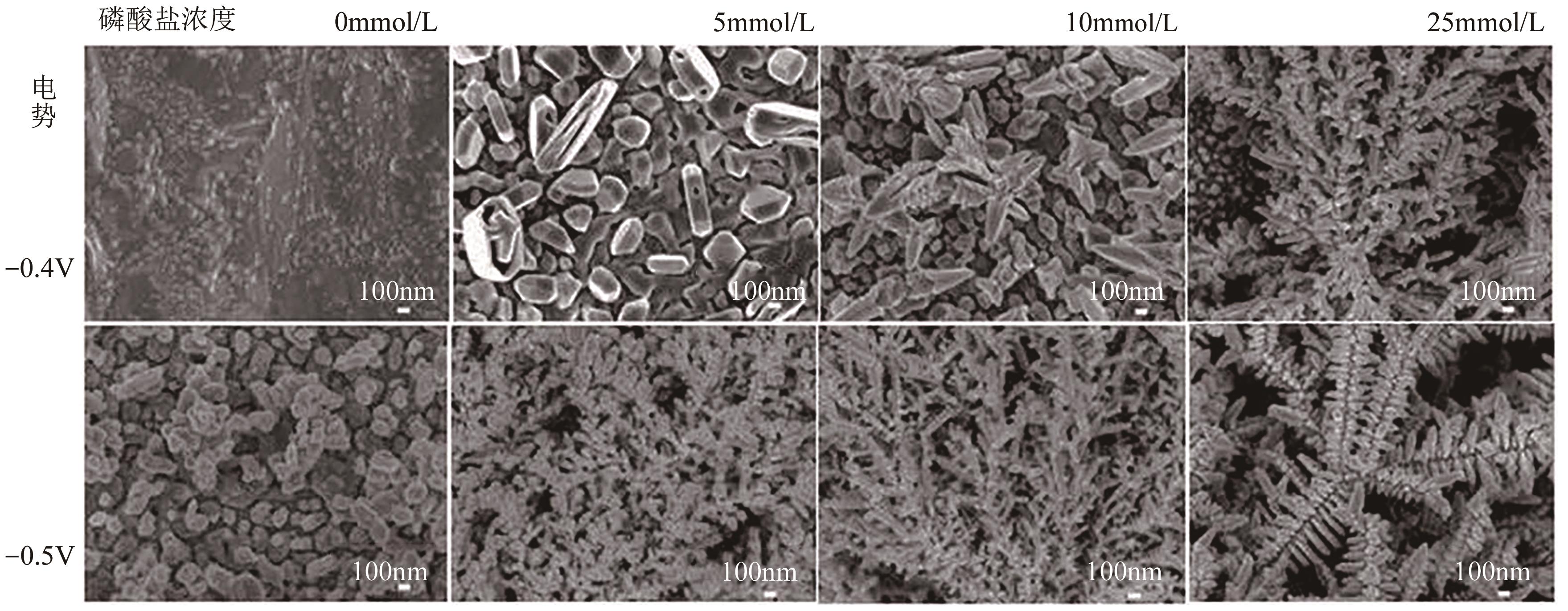
| 28 | ZHAO Jian, SUN Libo, CANEPA Silvia, et al. Phosphate tuned copper electrodeposition and promoted formic acid selectivity for carbon dioxide reduction[J]. Journal of Materials Chemistry A, 2017, 5(23): 11905-11916. |
| 29 | QIU Yanling, XU Wenbin, YAO Pengfei, et al. Electrochemical production of formic acid from CO2 with cetyltrimethylammonium bromide-assisted copper-based catalysts[J]. ChemSusChem, 2021, 14(8): 1962-1969. |
| 30 | MA Xintao, ZHANG Yinggan, FAN Tingting, et al. Facet dopant regulation of Cu2O boosts electrocatalytic CO2 reduction to formate[J]. Advanced Functional Materials, 2023, 33(16): 2213145. |
| 31 | JIANG Chengjie, HOU Yue, LIU Hua, et al. CO2 electrocatalytic reduction on Cu nanoparticles loaded on nitrogen-doped carbon[J]. Journal of Electroanalytical Chemistry, 2022, 915: 116353. |
| 32 | HU Shengnan, TIAN Na, LI Mengying, et al. Sulfur-modified copper synergy with nitrogen-defect sites for the electroreduction of CO2 to formate at low overpotentials[J]. Electrochimica Acta, 2022, 422: 140557. |
| 33 | GUO Sijie, ZHAO Siqi, GAO Jin, et al. Cu-CDots nanocorals as electrocatalyst for highly efficient CO2 reduction to formate[J]. Nanoscale, 2017, 9(1): 298-304. |
| 34 | Weixin LYU, ZHOU Jing, BEI Jingjing, et al. Electrodeposition of tin based film on copper plate for electrocatalytic reduction of carbon dioxide to formate[J]. International Journal of Electrochemical Science, 2016, 11(7): 6183-6191. |
| 35 | Weixin LYU, ZHOU Jing, KONG Fenying, et al. Porous tin-based film deposited on copper foil for electrochemical reduction of carbon dioxide to formate[J]. International Journal of Hydrogen Energy, 2016, 41(3): 1585-1591. |
| 36 | CHEN Zhipeng, WANG Nailiang, YAO Shuyu, et al. The flaky Cd film on Cu plate substrate: An active and efficient electrode for electrochemical reduction of CO2 to formate[J]. Journal of CO2 Utilization, 2017, 22: 191-196. |
| 37 | LI Yinshi, HE Yaling, YANG Weiwei. A high-performance direct formate-peroxide fuel cell with palladium-gold alloy coated foam electrodes[J]. Journal of Power Sources, 2015, 278: 569-573. |
| 38 | Önder METIN, SUN Xiaolian, SUN Shouheng. Monodisperse gold-palladium alloy nanoparticles and their composition-controlled catalysis in formic acid dehydrogenation under mild conditions[J]. Nanoscale, 2013, 5(3): 910-912. |
| 39 | JIANG Junhua, KUCERNAK Anthony. Probing anodic reaction kinetics and interfacial mass transport of a direct formic acid fuel cell using a nanostructured palladium-gold alloy microelectrode[J]. Electrochimica Acta, 2009, 54(19): 4545-4551. |
| 40 | CHEN Guoqin, LI Yunhua, WANG Dong, et al. Carbon-supported PtAu alloy nanoparticle catalysts for enhanced electrocatalytic oxidation of formic acid[J]. Journal of Power Sources, 2011, 196(20): 8323-8330. |
| 41 | MARCINKOWSKI Matthew D, LIU Jilei, MURPHY Colin J, et al. Selective formic acid dehydrogenation on Pt-Cu single-atom alloys[J]. ACS Catalysis, 2017, 7(1): 413-420. |
| 42 | ZHAO Xi, DAI Pingwang, XU Dongyan, et al. Ultra\ufb01ne PdAg alloy nanoparticles anchored on NH2-functionalized 2D/2D TiO2 nanosheet/rGO composite as efficient and reusable catalyst for hydrogen release from additive-free formic acid at room temperature Journal of Energy Chemistry[J]. Journal of Energy Chemistry, 2021, 59: 455-464. |
| 43 | WANG Miao, LIU Shuai, CHEN Bo, et al. Synergistic geometric and electronic effects in Bi-Cu bimetallic catalysts for CO2 electroreduction to formate over a wide potential window[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(17): 5693-5701. |
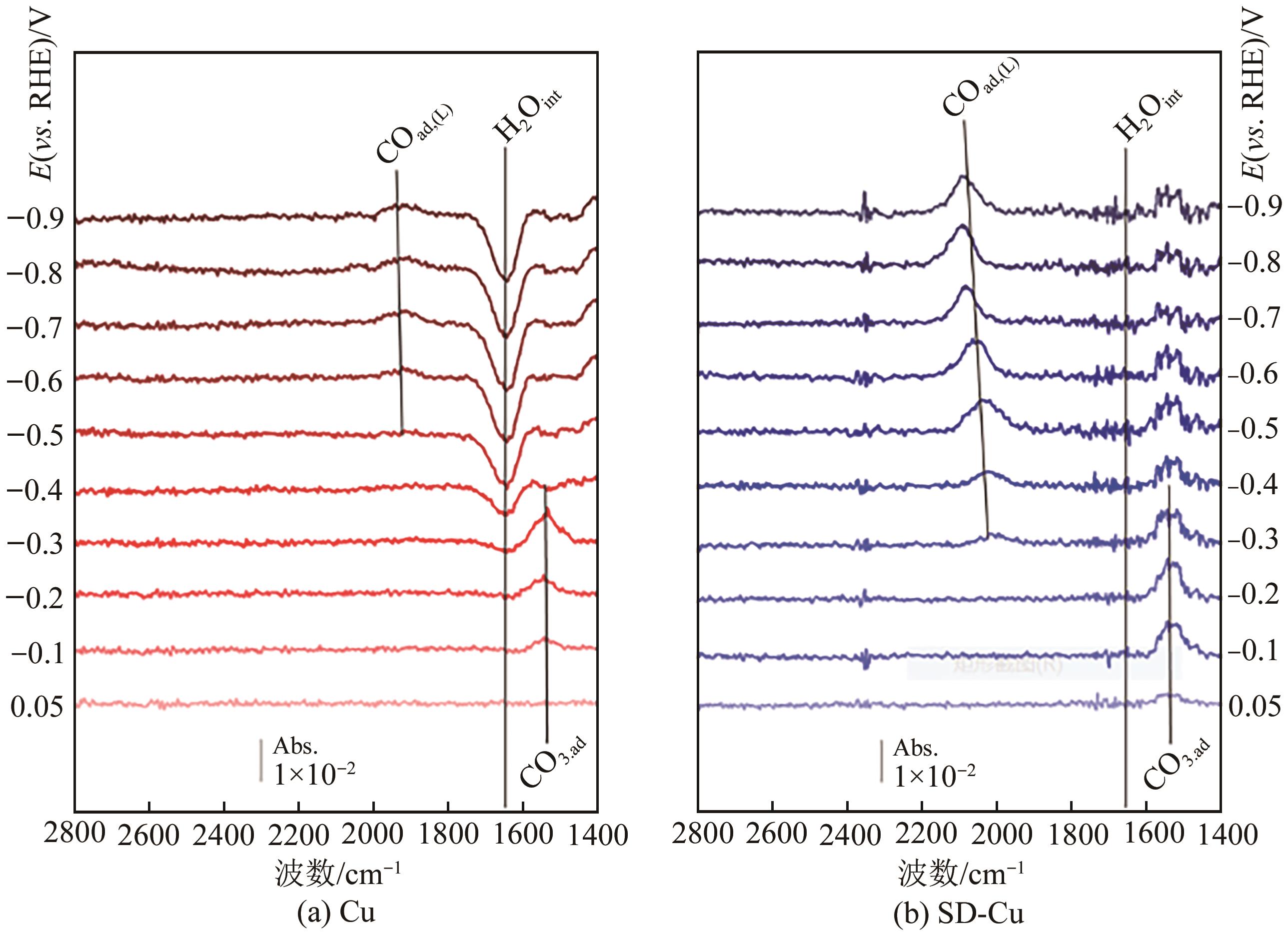
| 44 | PHILLIPS Katherine R, KATAYAMA Yu, HWANG Jonathan, et al. Sulfide-derived copper for electrochemical conversion of CO2 to formic acid[J]. The Journal of Physical Chemistry Letters, 2018, 9(15): 4407-4412. |
| 45 | XIAO Hai, GODDARD William A, CHENG Tao, et al. Cu metal embedded in oxidized matrix catalyst to promote CO2 activation and CO dimerization for electrochemical reduction of CO2 [J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(26): 6685-6688. |
| 46 | LAURSEN Anders B, CALVINHO Karin U D, GOETJEN Timothy A, et al. CO2 electro-reduction on Cu3P: Role of Cu(Ⅰ) oxidation state and surface facet structure in C1-formate production and H2 selectivity[J]. Electrochimica Acta, 2021, 391: 138889. |
| 47 | CHENG Tao, XIAO Hai, GODDARD William A. Reaction mechanisms for the electrochemical reduction of CO2 to CO and formate on the Cu(100) surface at 298 K from quantum mechanics free energy calculations with explicit water[J]. Journal of the American Chemical Society, 2016, 138(42): 13802-13805. |
| 48 | Lihui OU, HE Zixi. Potential-dependent competitive electroreduction of CO2 into CO and formate on Cu(111) from an improved H coverage-dependent electrochemical model with explicit solvent effect[J]. ACS Omega, 2020, 5(22): 12735-12744. |
| 49 | ZHAO Xiuhui, CHEN Qingsong, ZHUO Dehuang, et al. Oxygen vacancies enriched Bi based catalysts for enhancing electrocatalytic CO2 reduction to formate[J]. Electrochimica Acta, 2021, 367: 137478. |
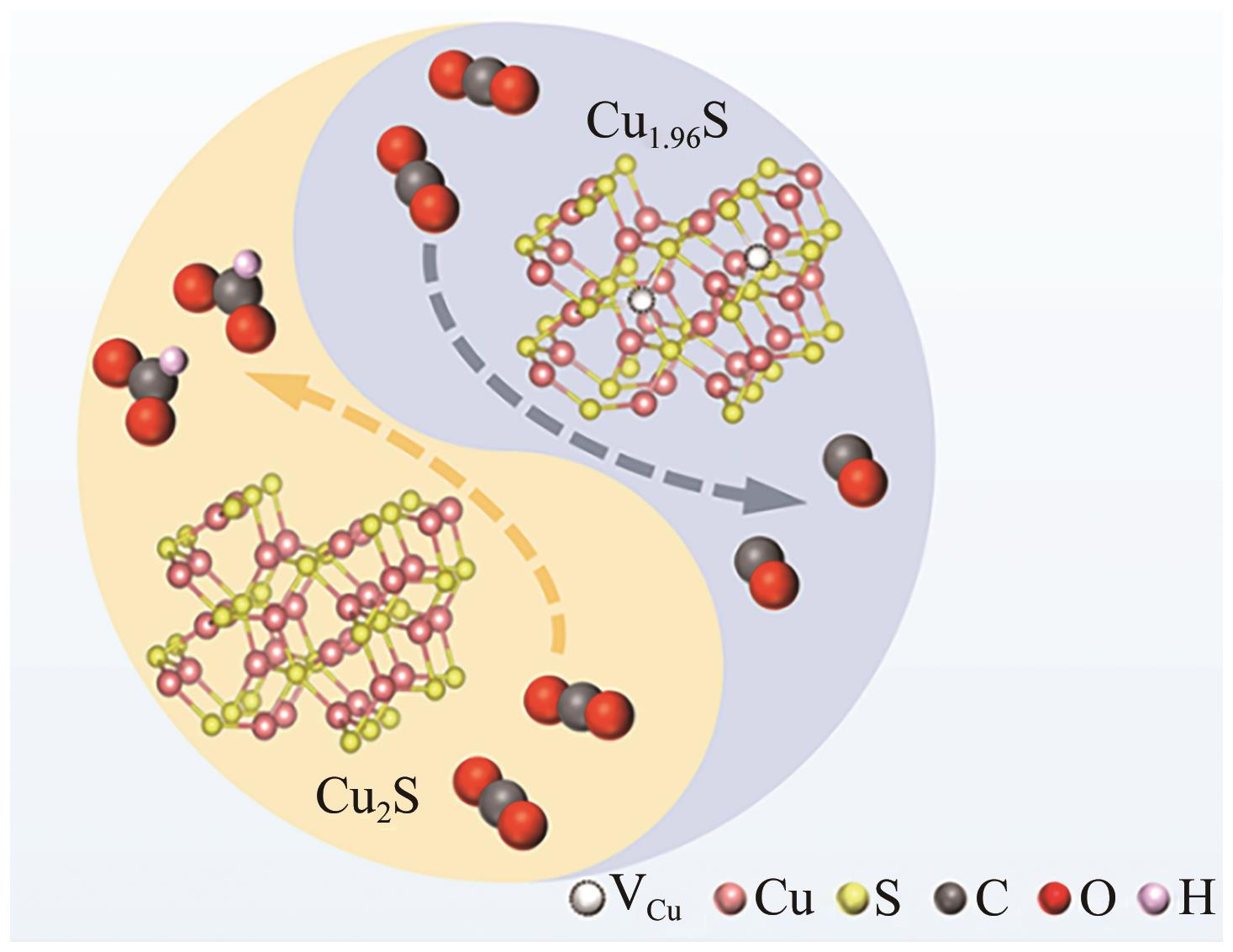
| 50 | LI Simeng, DUAN Huan, YU Jun, et al. Cu vacancy induced product switching from formate to CO for CO2 reduction on copper sulfide[J]. ACS Catalysis, 2022, 12(15): 9074-9082. |
| 51 | ZHOU Wenjing, YANG Huimin, GAO Nan, et al. Vacancies and electronic effects enhanced photoelectrochemical activity of Cu-doped Bi2Se3 for efficient CO2 reduction to formate[J]. Journal of Alloys and Compounds, 2022, 903: 163707. |
| [1] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [2] | 刘炫麟, 王驿凯, 戴苏洲, 殷勇高. 热泵中氨基甲酸铵分解反应特性及反应器结构优化[J]. 化工进展, 2023, 42(9): 4522-4530. |
| [3] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [4] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [5] | 符淑瑢, 王丽娜, 王东伟, 刘蕊, 张晓慧, 马占伟. 析氧助催化剂增强光阳极光电催化分解水性能研究进展[J]. 化工进展, 2023, 42(5): 2353-2370. |
| [6] | 刘丹, 范云洁, 王慧敏, 严政, 李鹏飞, 李家成, 曹雪波. 基于废弃PET的高值化功能性多孔碳材料及其应用进展[J]. 化工进展, 2023, 42(2): 969-984. |
| [7] | 黄世慧, 潘相玉, 张榴萍, 周丽绘, 王斯峥, 罗世龙, 张颖霞, 胡军. 分子印迹电化学传感器对食用油中塑化剂邻苯二甲酸二异壬酯的快速检测[J]. 化工进展, 2023, 42(12): 6469-6477. |
| [8] | 张会霞, 周立山, 张程蕾, 钱光磊, 谢陈鑫, 朱令之. Bi2S3/TiO2纳米锥光阳极的制备及其光电催化降解土霉素[J]. 化工进展, 2023, 42(10): 5548-5557. |
| [9] | 郭峰, 张尚杰, 蒋羽佳, 姜万奎, 信丰学, 章文明, 姜岷. 一碳资源在酵母中的利用与转化[J]. 化工进展, 2023, 42(1): 30-39. |
| [10] | 李喆, 李泽洋, 杨宇森, 卫敏. 电化学二氧化碳还原制甲酸催化剂的研究进展[J]. 化工进展, 2023, 42(1): 53-66. |
| [11] | 赵同心, 赵磊, 张延平, 李寅. 甲酸微生物转化研究进展[J]. 化工进展, 2023, 42(1): 67-72. |
| [12] | 孟令玎, 毛梦雷, 廖奇勇, 孟子晖, 刘文芳. 碳酸酐酶和甲酸脱氢酶的稳定性研究进展[J]. 化工进展, 2022, 41(S1): 436-447. |
| [13] | 寇佳伟, 程淑艳, 程芳琴. 类水滑石基催化剂光催化二氧化碳还原研究进展[J]. 化工进展, 2022, 41(S1): 190-198. |
| [14] | 尹爽, 梁伟杰, 陈沛嘉, 张志聪, 葛建芳. 聚己二酸丁二醇酯-共对苯二甲酸酯基可降解塑料改性研究进展[J]. 化工进展, 2022, 41(S1): 307-317. |
| [15] | 李志斌, 唐辉, 罗大伟, 应俏. 废弃PET化学回收及制备不饱和聚酯树脂的研究进展[J]. 化工进展, 2022, 41(6): 3279-3292. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||