化工进展 ›› 2022, Vol. 41 ›› Issue (6): 3279-3292.DOI: 10.16085/j.issn.1000-6613.2021-1439
废弃PET化学回收及制备不饱和聚酯树脂的研究进展
- 1.昆明理工大学化学工程学院,云南 昆明 650504
2.老挝南塔师范学院自然科学部,南塔 南塔 03000
-
收稿日期:2021-07-08修回日期:2021-08-30出版日期:2022-06-10发布日期:2022-06-21 -
通讯作者:唐辉 -
作者简介:李志斌(1990—),男,硕士研究生,研究方向为高分子材料。E-mail:269056953@qq.com 。 -
基金资助:国家自然科学基金地区基金(51167008);校企合作科技基金(kkk0201705038)
Progress in chemical recycling of waste PET and preparation of unsaturated polyester resins
LI Zhibin1( ), TANG Hui1(
), TANG Hui1( ), LUO Dawei1,2, YING Qiao1
), LUO Dawei1,2, YING Qiao1
- 1.Faculty of Chemical Engineering, Kunming University of Science and Technology, Kunming 650504, Yunnan, China
2.Natural Science Office, Louangnamtha Teacher Training College, Louangnamtha 03000, Louangnamtha, Laos
-
Received:2021-07-08Revised:2021-08-30Online:2022-06-10Published:2022-06-21 -
Contact:TANG Hui
摘要:
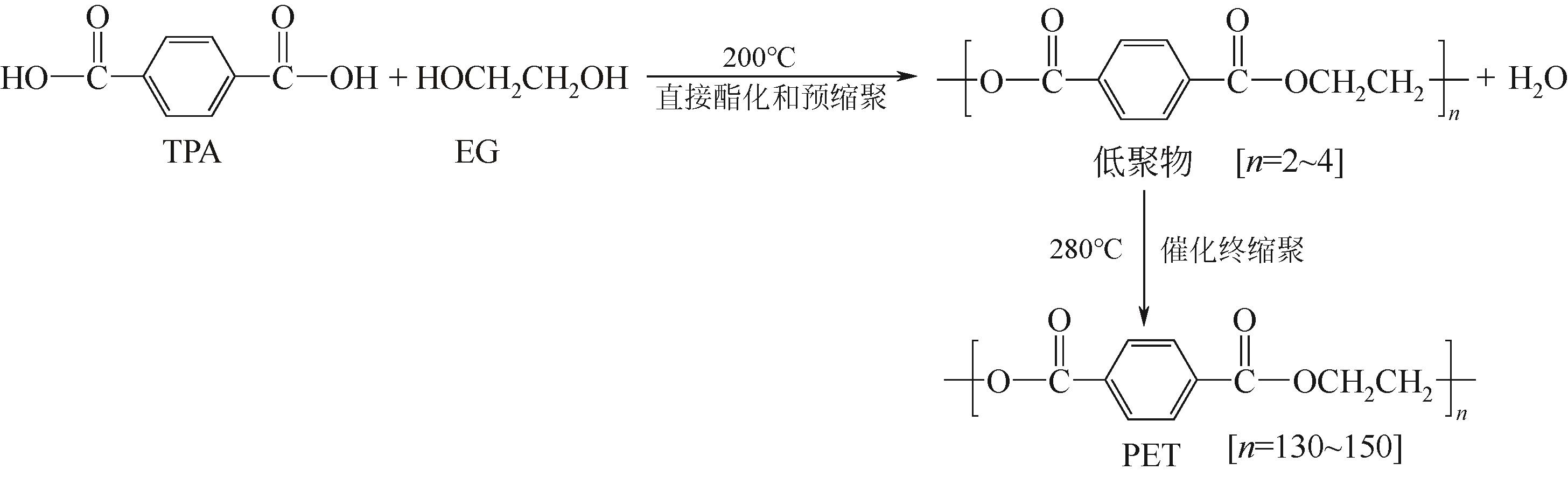
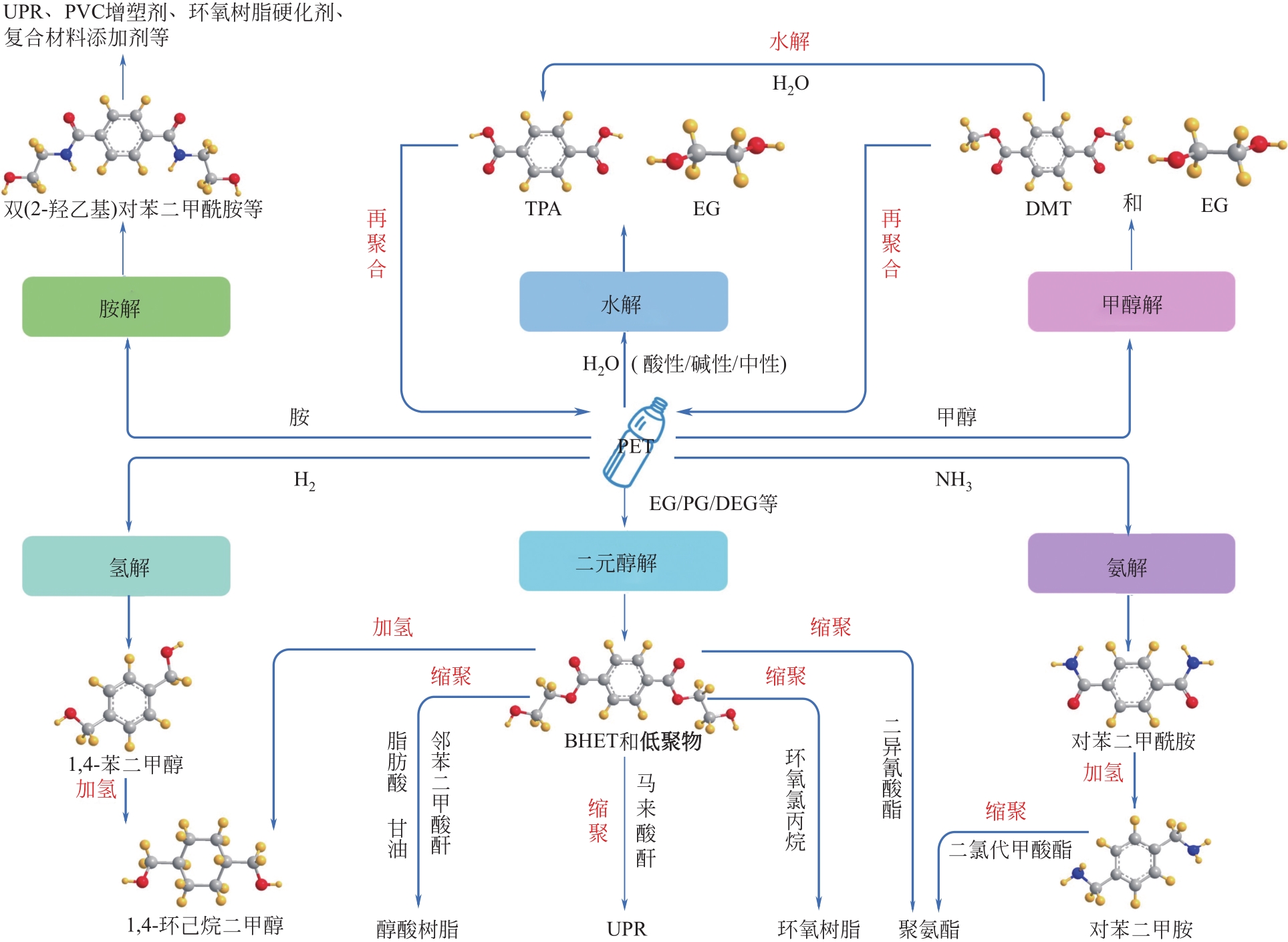
随着聚对苯二甲酸乙二醇酯(PET)材料用量的大幅增长,大量废弃PET制品堆积造成的环境污染问题日益突出,其回收利用技术也随之广受关注。在不同的PET回收方法中,将PET降解为单体或低聚物的化学回收是效率最高、产物利用价值最大的方法,但也存在反应条件苛刻、产物收率低等问题。本文详细梳理了水解法、甲醇醇解法、二元醇醇解法、胺解法和氨解法等化学回收方法的主要特点以及微波加热、离子液体、纳米技术等新兴技术在PET化学回收过程中的应用概况。通过对各种化学回收工艺的比较,文中得出二元醇醇解法是最具商业应用价值方法的结论。在此基础上,文中重点介绍了PET的二元醇解以及进一步制备不饱和聚酯树脂的化学过程、发展现状、制约因素和改进措施。分析表明,由PET二元醇解产物制备不饱和聚酯树脂是提高废弃PET资源化效率、丰富原料供给、推动产品升级的重要途径,开发高效、廉价、环保的新型催化剂或酶催化技术是废弃PET回收领域今后主要的发展方向。
中图分类号:
引用本文
李志斌, 唐辉, 罗大伟, 应俏. 废弃PET化学回收及制备不饱和聚酯树脂的研究进展[J]. 化工进展, 2022, 41(6): 3279-3292.
LI Zhibin, TANG Hui, LUO Dawei, YING Qiao. Progress in chemical recycling of waste PET and preparation of unsaturated polyester resins[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3279-3292.
| EG/PET配比 | 催化剂 | 催化剂/PET配比 | 温度/℃ | 醇解时间/min | BHET收率/% | 参考文献 |
|---|---|---|---|---|---|---|
| 7.6∶1(mol/mol) | Zn(OAc)2 | 0.0026(mol/mol) | 196 | 60 | 64 | [ |
| 7.6∶1(mol/mol) | Na2CO3 | 0.0041(mol/mol) | 196 | 60 | 65 | |
| 4∶1(w/w) | 尿素 | 0.1(w/w) | 180 | 180 | 78 | [ |
| 10∶1(mL/g) | ZrCl4 | 0.01(w/w) | 190 | 180 | 69 | [ |
| 10∶1(mL/g) | CoCl2·6H2O | 0.01(w/w) | 190 | 180 | 65 | |
| 10∶1(mL/g) | [Co(dcype)Cl2] | 0.03(mol/mol) | 190 | 180 | 82 | |
| 4∶1(w/w) | K6SiW11ZnO39(H2O) | 0.0013(mol/mol) | 185 | 30 | 84.1 | [ |
| 4∶1(w/w) | Na12[WZn3(H2O)2(ZnW9O34)2] | 0.00018(mol/mol) | 190 | 40 | 84.5 | [ |
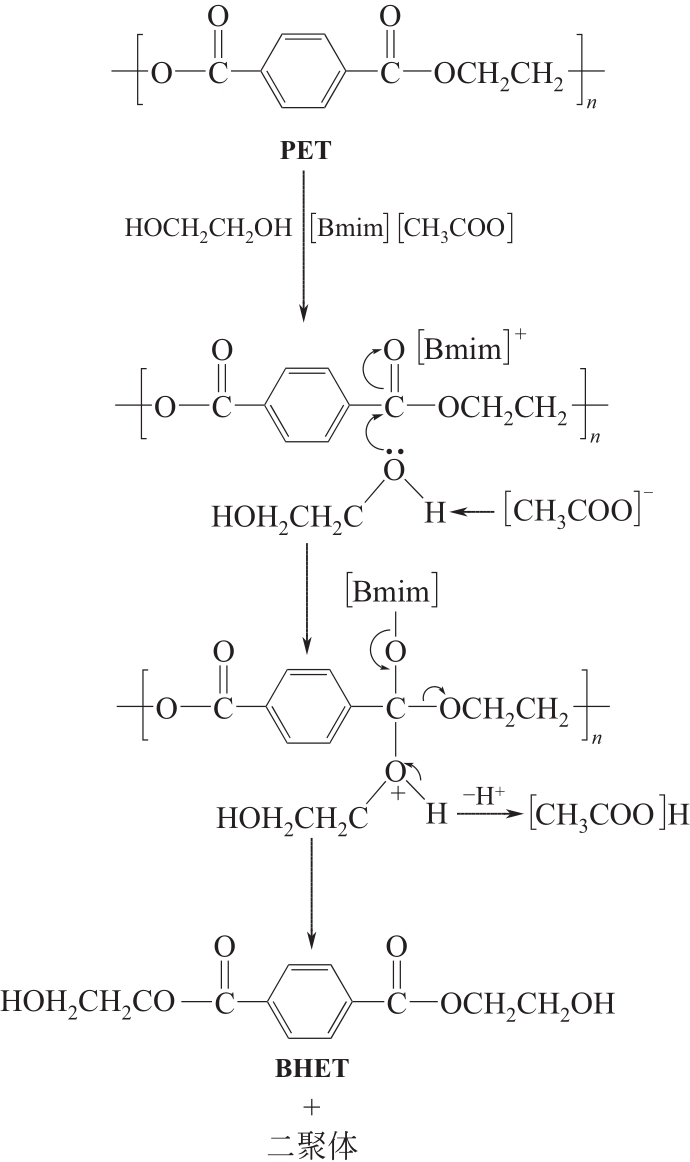
| 10∶1(w/w) | [Bmim]OH | 0.05(w/w) | 190 | 120 | 71.2 | [ |
| 11∶1(w/w) | [Bmim]ZnCl3 | 0.0125(w/w) | 190 | 120 | 84.9 | [ |
| 4∶1(w/w) | [Amim][ZnCl3] | 0.1(w/w) | 175 | 75 | 80.1 | [ |
| 11.7∶1(w/w) | [Bmim]2[CoCl4] | 0.17(w/w) | 175 | 90 | 81.1 | [ |
| 6.7∶1(w/w) | [Bmim][OAc] | 0.33(w/w) | 190 | 180 | 58.2 | [ |
| 6.7∶1(w/w) | [Bmim-Fe][(OAc)3]/膨润土 | 0.33(w/w) | 190 | 180 | 44 | [ |
| 4∶1(w/w) | [Ch]3[PO4] | 0.05(w/w) | 180 | 180 | 60.6 | [ |
| 10∶1(w/w) | 1,3-二甲基咪唑-2-羧酸盐 | 0.15(w/w) | 185 | 60 | 60 | [ |
| 10∶1(w/w) | Fe3O4增强多壁碳纳米管 | 0.05(w/w) | 190 | 120 | 100 | [ |
| 10∶1(mL/g) | Fe3O4@SiO2@(mim)[FeCl4] | 0.15(w/w) | 180 | 1440 | 100 | [ |
| 4∶1(w/w) | 尿素/ZnCl2 DES | 0.05(w/w) | 170 | 30 | 82.8 | [ |
| 4∶1(w/w) | 1,3-二甲基脲/Zn(OAc)2 DES | 0.05(w/w) | 190 | 20 | 82 | [ |
| 5∶1(w/w) | 乙酰胺/ZnCl2@ZIF-8 | 0.004(w/w) | 195 | 25 | 83.2 | [ |
表1 使用不同催化剂乙二醇解PET的最佳条件
| EG/PET配比 | 催化剂 | 催化剂/PET配比 | 温度/℃ | 醇解时间/min | BHET收率/% | 参考文献 |
|---|---|---|---|---|---|---|
| 7.6∶1(mol/mol) | Zn(OAc)2 | 0.0026(mol/mol) | 196 | 60 | 64 | [ |
| 7.6∶1(mol/mol) | Na2CO3 | 0.0041(mol/mol) | 196 | 60 | 65 | |
| 4∶1(w/w) | 尿素 | 0.1(w/w) | 180 | 180 | 78 | [ |
| 10∶1(mL/g) | ZrCl4 | 0.01(w/w) | 190 | 180 | 69 | [ |
| 10∶1(mL/g) | CoCl2·6H2O | 0.01(w/w) | 190 | 180 | 65 | |
| 10∶1(mL/g) | [Co(dcype)Cl2] | 0.03(mol/mol) | 190 | 180 | 82 | |
| 4∶1(w/w) | K6SiW11ZnO39(H2O) | 0.0013(mol/mol) | 185 | 30 | 84.1 | [ |
| 4∶1(w/w) | Na12[WZn3(H2O)2(ZnW9O34)2] | 0.00018(mol/mol) | 190 | 40 | 84.5 | [ |
| 10∶1(w/w) | [Bmim]OH | 0.05(w/w) | 190 | 120 | 71.2 | [ |
| 11∶1(w/w) | [Bmim]ZnCl3 | 0.0125(w/w) | 190 | 120 | 84.9 | [ |
| 4∶1(w/w) | [Amim][ZnCl3] | 0.1(w/w) | 175 | 75 | 80.1 | [ |
| 11.7∶1(w/w) | [Bmim]2[CoCl4] | 0.17(w/w) | 175 | 90 | 81.1 | [ |
| 6.7∶1(w/w) | [Bmim][OAc] | 0.33(w/w) | 190 | 180 | 58.2 | [ |
| 6.7∶1(w/w) | [Bmim-Fe][(OAc)3]/膨润土 | 0.33(w/w) | 190 | 180 | 44 | [ |
| 4∶1(w/w) | [Ch]3[PO4] | 0.05(w/w) | 180 | 180 | 60.6 | [ |
| 10∶1(w/w) | 1,3-二甲基咪唑-2-羧酸盐 | 0.15(w/w) | 185 | 60 | 60 | [ |
| 10∶1(w/w) | Fe3O4增强多壁碳纳米管 | 0.05(w/w) | 190 | 120 | 100 | [ |
| 10∶1(mL/g) | Fe3O4@SiO2@(mim)[FeCl4] | 0.15(w/w) | 180 | 1440 | 100 | [ |
| 4∶1(w/w) | 尿素/ZnCl2 DES | 0.05(w/w) | 170 | 30 | 82.8 | [ |
| 4∶1(w/w) | 1,3-二甲基脲/Zn(OAc)2 DES | 0.05(w/w) | 190 | 20 | 82 | [ |
| 5∶1(w/w) | 乙酰胺/ZnCl2@ZIF-8 | 0.004(w/w) | 195 | 25 | 83.2 | [ |
| 回收方法 | 解聚试剂 | 操作条件 | 回收产物 | 优点 | 不足与改进 |
|---|---|---|---|---|---|
| 碱性水解 | NaOH/KOH水溶液 | 200~250℃,带压操作[ | TPA、EG | 水解产物TPA和EG可直接用于PET的生产;相转移催化剂的应用能使反应在100℃以下、常压的温和条件下进行 | 生成的TPA需额外的酸化步骤后沉淀得到,需做好废液处理;水的亲核性较弱、水解速率慢,可通过超声波辅助、微波加热技术提高反应速率 |
| 酸性水解 | 强酸(H2SO4、HNO3) | H2SO4:25~100℃[ HNO3:70~100℃[ | TPA、EG | 无需高温高压条件 | 普遍存在腐蚀问题,温度和酸浓度过低也会使水解速率很慢,可引入微波加热技术、开发新型催化体系解决速率和废液的问题 |
| 中性水解 | 水或蒸汽 | 200~300℃,1~4MPa | TPA、EG | 无废液问题,具有生态友好型特征 | 高温高压的操作条件会增加生产成本,亟需开发出高效催化剂以改善操作条件,如H+@ZSM-5-25型分子筛 |
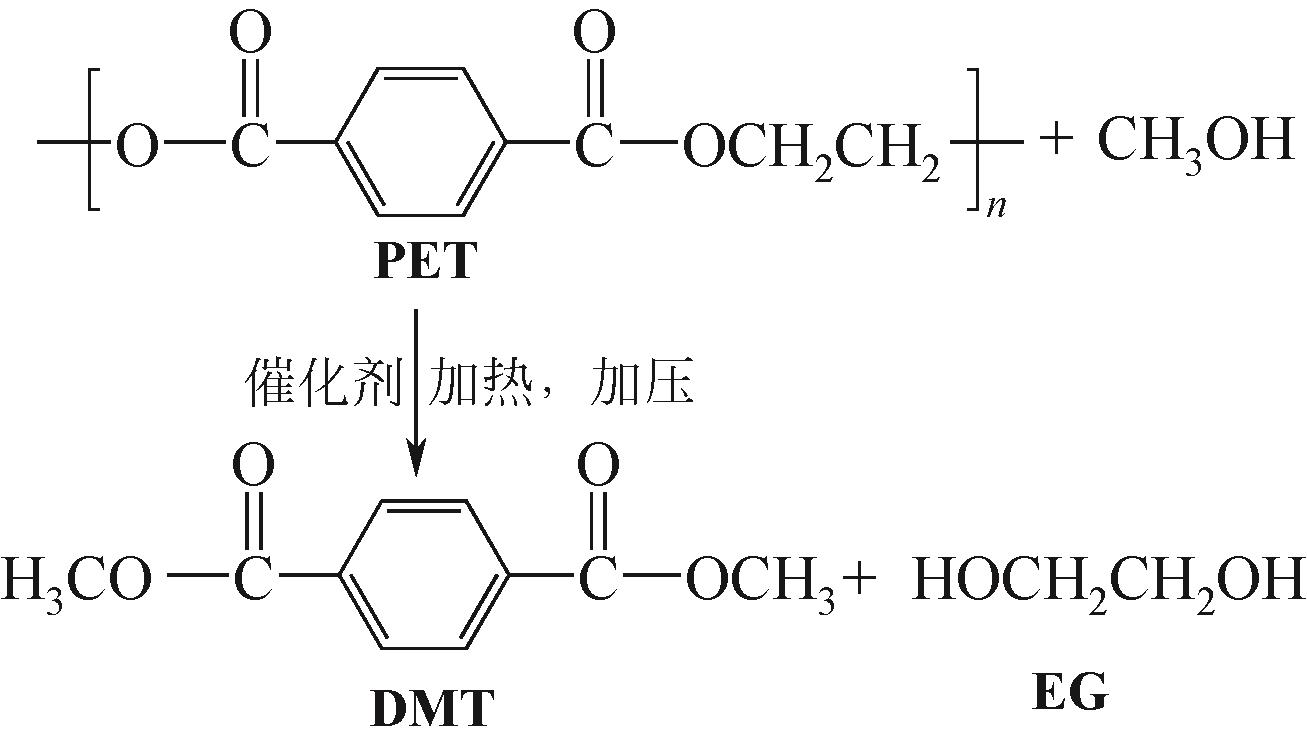
| 甲醇解 | 甲醇 | 180~280℃,2~4MPa | DMT、EG | 对污染物的耐受性更强,可以处理低质量的原料;高温下的气相甲醇还可以作为载体将气相中的单体组分与液相中的低聚物分离 | 高温高压的操作条件增加了生产成本,加剧了甲醇解的强腐蚀性,发展催化甲醇解更有利于降低PET解聚温度,减少能耗;产物分离困难,预蒸馏和无溶剂熔融结晶相结合的新型分离工艺将大大降低投资和运行成本,提高甲醇解的可持续性 |
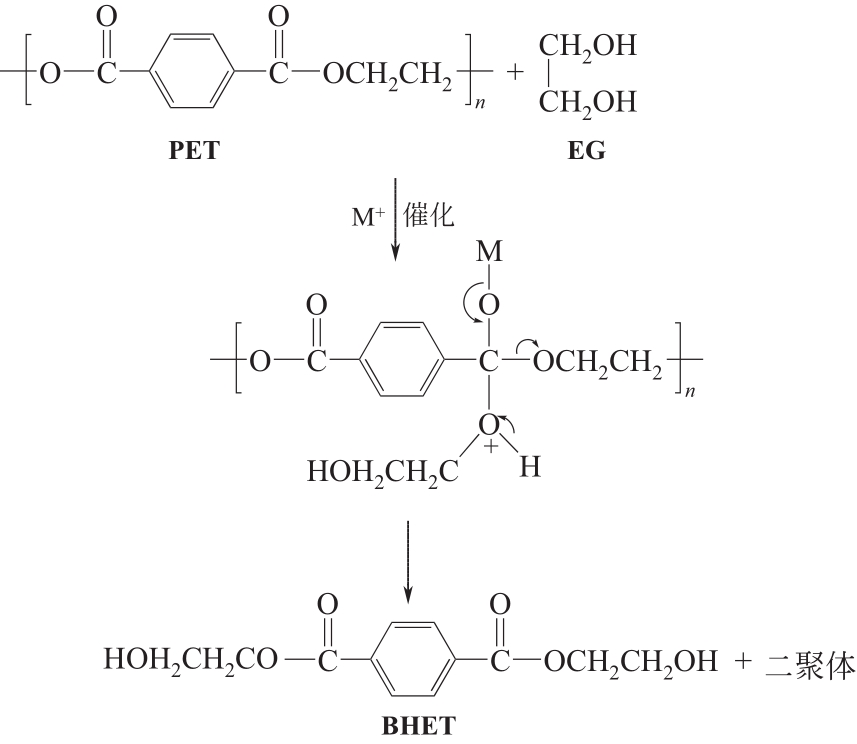
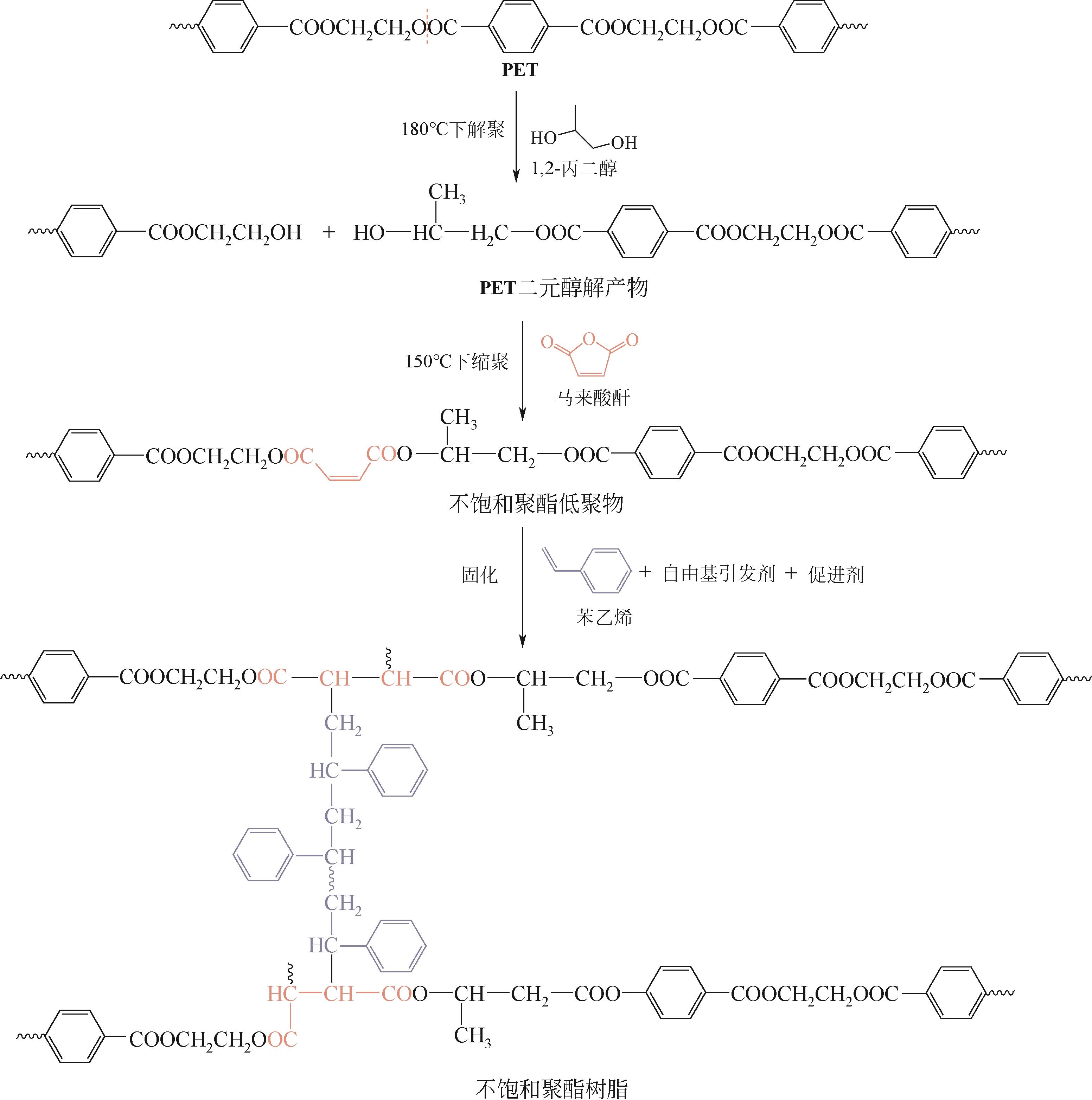
| 二元醇解 | 二元醇(EG、PG、DEG等) | 180~240℃,常压 | BHET及其低聚物、EG、PG、DEG等 | 反应温度适中且多在常压下进行,简单、灵活、易于工业化扩大;以少量的二元醇为解聚剂,能做到重复利用,解聚及催化剂回收过程对环境危害性小;可按需求选择不同种类的催化剂;产物用途广泛,可生产包括UPR、PU、醇酸树脂、环氧树脂在内的多种高分子材料,用于UPR、PU等的生产时可不用从混合产物中分离出二元醇 | 因为存在聚合可能,解聚单体不能通过真空蒸馏等常规方法提纯,可采用闪蒸柱和结晶器两级蒸发工艺进行单体提纯[ |
| 胺解 | 甲胺、乙胺、乙醇胺、 乙二胺等 | 20~100℃,常压 | TPA的二酰胺、EG | 反应条件很温和,已确定部分胺解在增强PET性能方面的应用,如纤维表面改性 | 在PET化学回收工业中很少应用,部分胺解产物用作增塑剂、添加剂,产品附加值较低 |
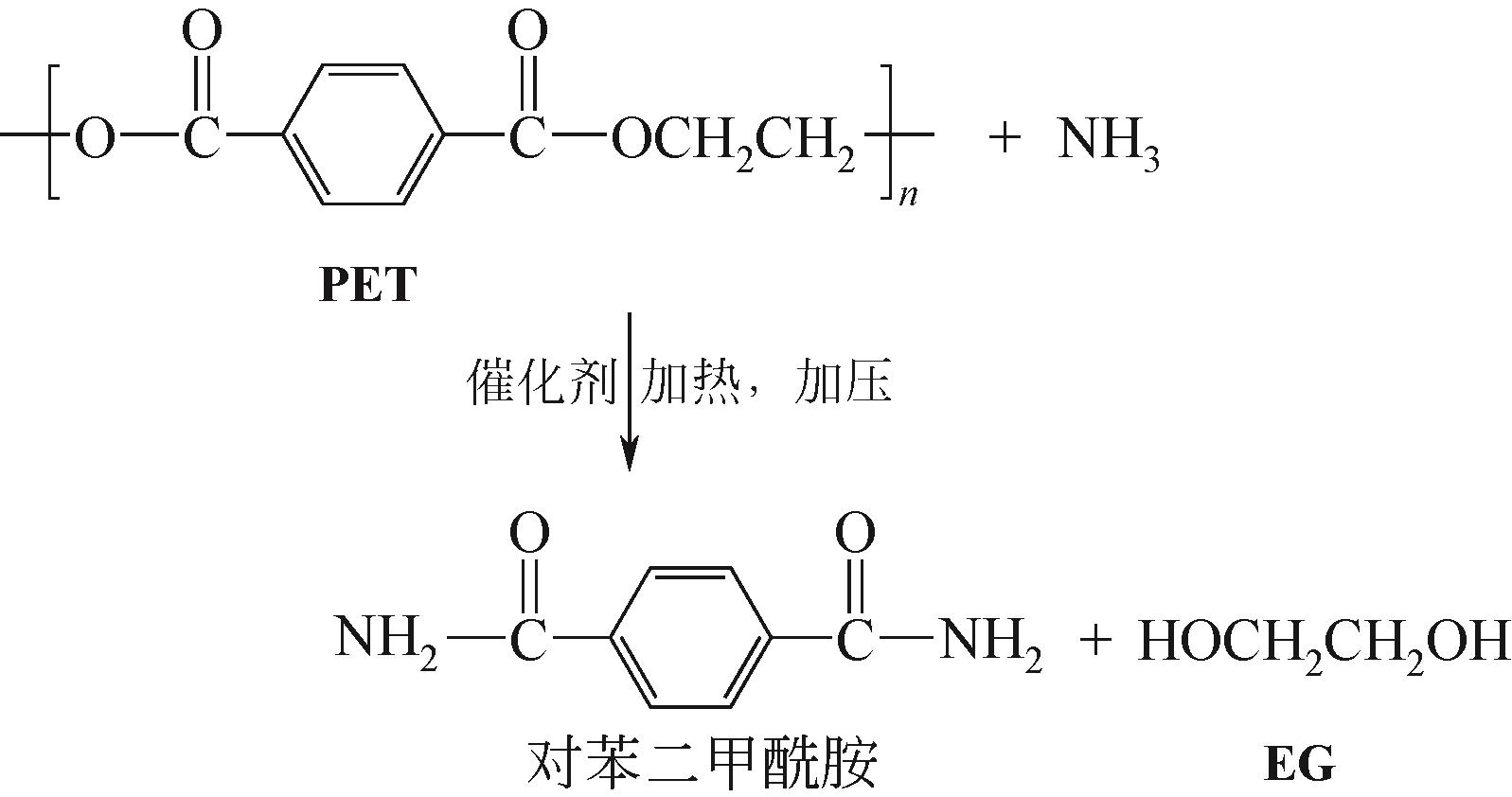
| 氨解 | NH3 | 70~180℃,中压、低压 | 对苯二甲酰胺、EG | 温度、压力要求适中,产品收率较高;产物对苯二甲酰胺经加氢可制备对苯二甲酰胺,用于生产环氧树脂、聚氨酯涂料等 | 解聚时间较长,产品提纯步骤繁复,需简化 |
表2 PET化学回收方法比较
| 回收方法 | 解聚试剂 | 操作条件 | 回收产物 | 优点 | 不足与改进 |
|---|---|---|---|---|---|
| 碱性水解 | NaOH/KOH水溶液 | 200~250℃,带压操作[ | TPA、EG | 水解产物TPA和EG可直接用于PET的生产;相转移催化剂的应用能使反应在100℃以下、常压的温和条件下进行 | 生成的TPA需额外的酸化步骤后沉淀得到,需做好废液处理;水的亲核性较弱、水解速率慢,可通过超声波辅助、微波加热技术提高反应速率 |
| 酸性水解 | 强酸(H2SO4、HNO3) | H2SO4:25~100℃[ HNO3:70~100℃[ | TPA、EG | 无需高温高压条件 | 普遍存在腐蚀问题,温度和酸浓度过低也会使水解速率很慢,可引入微波加热技术、开发新型催化体系解决速率和废液的问题 |
| 中性水解 | 水或蒸汽 | 200~300℃,1~4MPa | TPA、EG | 无废液问题,具有生态友好型特征 | 高温高压的操作条件会增加生产成本,亟需开发出高效催化剂以改善操作条件,如H+@ZSM-5-25型分子筛 |
| 甲醇解 | 甲醇 | 180~280℃,2~4MPa | DMT、EG | 对污染物的耐受性更强,可以处理低质量的原料;高温下的气相甲醇还可以作为载体将气相中的单体组分与液相中的低聚物分离 | 高温高压的操作条件增加了生产成本,加剧了甲醇解的强腐蚀性,发展催化甲醇解更有利于降低PET解聚温度,减少能耗;产物分离困难,预蒸馏和无溶剂熔融结晶相结合的新型分离工艺将大大降低投资和运行成本,提高甲醇解的可持续性 |
| 二元醇解 | 二元醇(EG、PG、DEG等) | 180~240℃,常压 | BHET及其低聚物、EG、PG、DEG等 | 反应温度适中且多在常压下进行,简单、灵活、易于工业化扩大;以少量的二元醇为解聚剂,能做到重复利用,解聚及催化剂回收过程对环境危害性小;可按需求选择不同种类的催化剂;产物用途广泛,可生产包括UPR、PU、醇酸树脂、环氧树脂在内的多种高分子材料,用于UPR、PU等的生产时可不用从混合产物中分离出二元醇 | 因为存在聚合可能,解聚单体不能通过真空蒸馏等常规方法提纯,可采用闪蒸柱和结晶器两级蒸发工艺进行单体提纯[ |
| 胺解 | 甲胺、乙胺、乙醇胺、 乙二胺等 | 20~100℃,常压 | TPA的二酰胺、EG | 反应条件很温和,已确定部分胺解在增强PET性能方面的应用,如纤维表面改性 | 在PET化学回收工业中很少应用,部分胺解产物用作增塑剂、添加剂,产品附加值较低 |
| 氨解 | NH3 | 70~180℃,中压、低压 | 对苯二甲酰胺、EG | 温度、压力要求适中,产品收率较高;产物对苯二甲酰胺经加氢可制备对苯二甲酰胺,用于生产环氧树脂、聚氨酯涂料等 | 解聚时间较长,产品提纯步骤繁复,需简化 |
| 63 | YUE Q F, WANG C X, ZHANG L N, et al. Glycolysis of poly(ethylene terephthalate) (PET) using basic ionic liquids as catalysts[J]. Polymer Degradation and Stability, 2011, 96(4): 399-403. |
| 64 | JU Z Y, XIAO W H, LU X M, et al. Theoretical studies on glycolysis of poly(ethylene terephthalate) in ionic liquids[J]. RSC Advances, 2018, 8(15): 8209-8219. |
| 65 | WANG R, WANG T L, YU G R, et al. A new class of catalysts for the glycolysis of PET: deep eutectic solvent@ZIF-8 composite[J]. Polymer Degradation and Stability, 2021, 183: 109463. |
| 66 | WANG Q, YAO X Q, TANG S F, et al. Urea as an efficient and reusable catalyst for the glycolysis of poly(ethylene terephthalate) wastes and the role of hydrogen bond in this process[J]. Green Chemistry, 2012, 14(9): 2559-2566. |
| 67 | GENG Y R, DONG T, FANG P T, et al. Fast and effective glycolysis of poly(ethylene terephthalate) catalyzed by polyoxometalate[J]. Polymer Degradation and Stability, 2015, 117: 30-36. |
| 68 | WANG Q, GENG Y R, LU X M, et al. First-row transition metal-containing ionic liquids as highly active catalysts for the glycolysis of poly(ethylene terephthalate) (PET)[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(2): 340-348. |
| 69 | AL-SABAGH A M, YEHIA F Z, ESHAQ G, et al. Ionic liquid-coordinated ferrous acetate complex immobilized on bentonite as a novel separable catalyst for PET glycolysis[J]. Industrial & Engineering Chemistry Research, 2015, 54(50): 12474-12481. |
| 70 | AL-SABAGH A M, YEHIA F Z, HARDING D R K, et al. Fe3O4-boosted MWCNT as an efficient sustainable catalyst for PET glycolysis[J]. Green Chemistry, 2016, 18(14): 3997-4003. |
| 71 | IMRAN M, KIM B K, HAN M, et al. Sub- and supercritical glycolysis of polyethylene terephthalate (PET) into the monomer bis(2-hydroxyethyl) terephthalate (BHET)[J]. Polymer Degradation and Stability, 2010, 95(9): 1686-1693. |
| 72 | TAWFIK M E, ESKANDER S B. Chemical recycling of poly(ethylene terephthalate) waste using ethanolamine. sorting of the end products[J]. Polymer Degradation and Stability, 2010, 95(2): 187-194. |
| 73 | 王明, 赵涛. 离子液体催化PET胺解反应的研究[J]. 印染助剂, 2015, 32(5): 12-15. |
| WANG Ming, ZHAO Tao. Study on the PET aminolysis catalyzed by ionic liquid[J]. Textile Auxiliaries, 2015, 32(5): 12-15. | |
| 1 | PARK S H, KIM S H. Poly(ethylene terephthalate) recycling for high value added textiles[J]. Fashion and Textiles, 2014, 1: 1. |
| 2 | PUDACK C, STEPANSKI M, FASSLER P. PET recycling – contributions of crystallization to sustainability[J]. Chemie Ingenieur Technik, 2020, 92(4): 452-458. |
| 74 | ZHOU J F, LI M, ZHONG L, et al. Aminolysis of polyethylene terephthalate fabric by a method involving the gradual concentration of dilute ethylenediamine[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017, 513: 146-152. |
| 75 | RAHEEM A B, HASSAN, A B, NOOR Z Z, et al. Process simulation of bis(2-hydroxyethyl) terephthalate and its recovery using two-stage evaporation systems[J]. Chemical Engineering Transactions, 2018, 63: 655-660. |
| 76 | GAO Y, ROMERO P, ZHANG H L, et al. Unsaturated polyester resin concrete: a review[J]. Construction and Building Materials, 2019, 228: 116709. |
| 77 | BARBOZA E S, LOPEZ D R, AMICO S C, et al. Determination of a recyclability index for the PET glycolysis[J]. Resources, Conservation and Recycling, 2009, 53(3): 122-128. |
| 78 | DUQUE-INGUNZA I, LÓPEZ-FONSECA R, RIVAS B D, et al. Synthesis of unsaturated polyester resin from glycolysed postconsumer PET wastes[J]. Journal of Material Cycles and Waste Management, 2013, 15(3): 256-263. |
| 79 | ABDULLAH N M, AHMAD I. Fire-retardant polyester composites from recycled polyethylene terephthalate (PET) wastes reinforced with coconut fibre[J]. Sains Malaysiana, 2013, 42(6): 811-818. |
| 80 | SUH D J, PARK O O, YOON K H. The properties of unsaturated polyester based on the glycolyzed poly(ethylene terephthalate) with various glycol compositions[J]. Polymer, 2000, 41(2): 461-466. |
| 81 | KARAYANNIDIS G P, NIKOLAIDIS A K, SIDERIDOU I D, et al. Chemical recycling of PET by glycolysis: polymerization and characterization of the dimethacrylated glycolysate[J]. Macromolecular Materials and Engineering, 2006, 291(11): 1338-1347. |
| 82 | KÁRPÁTI L, FOGARASSY F, KOVÁCSIK D, et al. One-pot depolymerization and polycondensation of PET based random oligo- and polyesters[J]. Journal of Polymers and the Environment, 2019, 27(10): 2167-2181. |
| 3 | SINHA V, PATEL M R, PATEL J V. Pet waste management by chemical recycling: a review[J]. Journal of Polymers and the Environment, 2010, 18(1): 8-25. |
| 4 | HO N T L, NGO D M, CHO J, et al. Enhanced catalytic glycolysis conditions for chemical recycling of glycol-modified poly(ethylene terephthalate)[J]. Polymer Degradation and Stability, 2018, 155: 15-21. |
| 5 | REIS V F, THIAGO P D S C, PEREIRA M M, et al. Ultrasmall cobalt nanoparticles as a catalyst for PET glycolysis: a green protocol for pure hydroxyethyl terephthalate precipitation without water[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12017-12024. |
| 6 | 牛梅, 杨雅茹, 王欣, 等. CMSs/PET微胶囊阻燃PET纤维制备与表征[J]. 材料工程, 2016, 44(6): 63-69. |
| NIU Mei, YANG Yaru, WANG Xin, et al. Preparation and characterization of flame retardant PET fiber with microencapsulated CMSs/PET[J]. Journal of Materials Engineering, 2016, 44(6): 63-69. | |
| 7 | NISTICÒ R. Polyethylene terephthalate (PET) in the packaging industry[J]. Polymer Testing, 2020, 90: 106707. |
| 8 | BORNSCHEUER U T. Feeding on plastic[J]. Science, 2016, 351(6278): 1154-1155. |
| 9 | CANO I, MARTIN C, FERNANDES J A, et al. Paramagnetic ionic liquid-coated SiO2@Fe3O4 nanoparticles — The next generation of magnetically recoverable nanocatalysts applied in the glycolysis of PET[J]. Applied Catalysis B: Environmental, 2020, 260: 118110. |
| 10 | TOURNIER V, TOPHAM C M, GILLES A, et al. An engineered PET depolymerase to break down and recycle plastic bottles[J]. Nature, 2020, 580(7802): 216-219. |
| 11 | GIBB B C. Plastics are forever[J]. Nature Chemistry, 2019, 11(5): 394-395. |
| 12 | TANIGUCHI I, YOSHIDA S, HIRAGA K, et al. Biodegradation of PET: current status and application aspects[J]. ACS Catalysis, 2019, 9(5): 4089-4105. |
| 13 | 王翠芳, 黎焕敏, 随献伟, 等. 废弃塑料的回收及高值化再利用[J]. 高分子材料科学与工程, 2021, 37(1): 335-342. |
| WANG Cuifang, LI Huanmin, SUI Xianwei, et al. Recycling and value-added utilization of waste plastics[J]. Polymer Materials Sciene and Engineering, 2021, 37(1): 335-342. | |
| 14 | RAHIMI A, GARCÍA J M. Chemical recycling of waste plastics for new materials production[J]. Nature Reviews Chemistry, 2017, 1: 0046. |
| 15 | THIOUNN T, SMITH R C. Advances and approaches for chemical recycling of plastic waste[J]. Journal of Polymer Science, 2020, 58(10): 1347-1364. |
| 16 | RAHEEM A B, NOOR Z Z, HASSAN A, et al. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: a review[J]. Journal of Cleaner Production, 2019, 225: 1052-1064. |
| 17 | WESTHUES S, IDEL J, KLANKERMAYER J. Molecular catalyst systems as key enablers for tailored polyesters and polycarbonate recycling concepts[J]. Science Advances, 2018, 4: 9669. |
| 18 | GARCÍA K E, NAVARRO R, RAMÍREZ-HERNÁNDEZ A, et al. New routes to difunctional macroglycols using ethylene carbonate: reaction with bis-(2-hydroxyethyl) terephthalate and degradation of poly(ethylene terephthalate)[J]. Polymer Degradation and Stability, 2017, 144: 195-206. |
| 19 | ZHOU X, WANG C X, FANG C Q, et al. Structure and thermal properties of various alcoholysis products from waste poly(ethylene terephthalate)[J]. Waste Management, 2019, 85: 164-174. |
| 20 | ABDULLAH N M, AHMAD I. Potential of using polyester reinforced coconut fiber composites derived from recycling polyethylene terephthalate (PET) waste[J]. Fibers and Polymers, 2013, 14(4): 584-590. |
| 21 | KÁRPÁTI L, SZARKA G, HARTMAN M, et al. Oligoester and polyester production via acido-alcoholysis of PET waste[J]. Periodica Polytechnica Chemical Engineering, 2018, 62(3): 336-344. |
| 22 | ATTA A M, EL-GHAZAWY R A, EL-SAEED A M. Corrosion protective coating based on alkyd resins derived from recycled poly(ethylene terephthalate) waste for carbon steel[J]. International Journal of Electrochemical Science, 2013, 8(4): 5136-5152. |
| 23 | ATTA A M, EL-KAFRAWY A F, ALY M H, et al. New epoxy resins based on recycled poly(ethylene terephthalate) as organic coatings[J]. Progress in Organic Coatings, 2007, 58(1): 13-22. |
| 24 | PUROHIT J, CHAWADA G, CHOUBISA B, et al. Polyester polyol derived from waste poly(ethylene terephthalate) for coating application on mild steel [J]. Chemical Sciences Journal, 2012, 76: 1-7. |
| 25 | SHUKLA S R, KAPADI P U, MHASKE S T, et al. Synthesis of a secondary plasticizer for poly(vinyl chloride) by recycling of poly(ethylene terephthalate) bottle waste through aminolytic depolymerization[J]. Journal of Vinyl and Additive Technology, 2017, 23(2): 152-160. |
| 26 | SOGANCIOGLU M, YUCEL A, YEL E, et al. Production of epoxy composite from the pyrolysis char of washed PET wastes[J]. Energy Procedia, 2017, 118: 216-220. |
| 27 | LENG Z, PADHAN R K, SREERAM A. Production of a sustainable paving material through chemical recycling of waste PET into crumb rubber modified asphalt[J]. Journal of Cleaner Production, 2018, 180: 682-688. |
| 28 | LENG Z, SREERAM A, PADHAN R K, et al. Value-added application of waste PET based additives in bituminous mixtures containing high percentage of reclaimed asphalt pavement (RAP)[J]. Journal of Cleaner Production, 2018, 196: 615-625. |
| 29 | 胡浩斌, 武芸, 朱治明. 响应面法优化废PET塑料降解制备TPA的工艺[J]. 化工进展, 2016, 35(7): 2243-2250. |
| HU Haobin, WU Yun, ZHU Zhiming. Optimization of TPA preparation technology from waste PET by response surface methodology[J]. Chemical Industry and Engineering Progress, 2016, 35(7): 2243-2250. | |
| 30 | DIAZ-SILVARREY L S, MCMAHON A, PHAN A N. Benzoic acid recovery via waste poly(ethylene terephthalate) (PET) catalytic pyrolysis using sulphated zirconia catalyst[J]. Journal of Analytical and Applied Pyrolysis, 2018, 134: 621-631. |
| 31 | LIU B, LU X M, JU Z Y, et al. Ultrafast homogeneous glycolysis of waste polyethylene terephthalate via a dissolution-degradation strategy[J]. Industrial & Engineering Chemistry Research, 2018, 57(48): 16239-16245. |
| 32 | RAGAERT K, DELVA L, GEEM K VAN. Mechanical and chemical recycling of solid plastic waste[J]. Waste Management, 2017, 69: 24-58. |
| 33 | 胡肖霞, 王亮, 冯杰. 利用KH560和纳米SiO2采用“一步法”反应挤出增黏PET[J]. 化工进展, 2020, 39(7): 2768-2774. |
| HU Xiaoxia, WANG Liang, FENG Jie. Increase the viscosity of PET by one-step reaction extrusion with KH560 and nano-SiO2 as chain extender[J]. Chemical Industry and Engineering Progress, 2020, 39(7): 2768-2774. | |
| 34 | YOSHIDA S, HIRAGA K, TAKEHANA T, et al. A bacterium that degrades and assimilates poly(ethylene terephthalate)[J]. Science, 2016, 351(6278): 1196-1199. |
| 35 | KAWAI F, KAWABATA T, ODA M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields[J]. Applied Microbiology and Biotechnology, 2019, 103(11): 4253-4268. |
| 36 | GEYER B, LORENZ G, KANDELBAUER A. Recycling of poly(ethylene terephthalate)—A review focusing on chemical methods[J]. Express Polymer Letters, 2016, 10(7): 559-586. |
| 37 | MOHSIN M A, ALNAQBI M A, BUSHEER R M, et al. Sodium methoxide catalyzed depolymerization of waste polyethylene terephthalate under microwave irradiation[J]. Catalysis in Industry, 2018, 10(1): 41-48. |
| 38 | LIU Q L, LI R S, FANG T. Investigating and modeling PET methanolysis under supercritical conditions by response surface methodology approach[J]. Chemical Engineering Journal, 2015, 270: 535-541. |
| 39 | PASSOS J S D, GLASIUS M, BILLER P. Screening of common synthetic polymers for depolymerization by subcritical hydrothermal liquefaction[J]. Process Safety and Environmental Protection, 2020, 139: 371-379. |
| 40 | YUE Q F, XIAO L F, ZHANG M L, et al. The glycolysis of poly(ethylene terephthalate) waste: lewis acidic ionic liquids as high efficient catalysts[J]. Polymers, 2013, 5(4): 1258-1271. |
| 41 | SUN J, LIU D J, YOUNG R P, et al. High loading solubilization and upgrading of poly(ethylene terephthalate) in low cost bifunctional ionic liquid[J]. ChemSusChem, 2018, 11(4): 781-792. |
| 42 | WANG L, NELSON G A, TOLAND J, et al. Glycolysis of PET using 1,3-dimethylimidazolium-2-carboxylate as an organocatalyst[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(35): 13362-13368. |
| 43 | WANG Q, YAO X Q, GENG Y R, et al. Deep eutectic solvents as highly active catalysts for the fast and mild glycolysis of poly(ethylene terephthalate)(PET)[J]. Green Chemistry, 2015, 17(4): 2473-2479. |
| 44 | LIU B, FU W Z, LU X M, et al. Lewis acid-base synergistic catalysis for polyethylene terephthalate degradation by 1,3-dimethylurea/ Zn(OAc)2 deep eutectic solvent[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(3): 3292-3300. |
| 45 | KARAYANNIDIS G P, ACHILIAS D S. Chemical recycling of poly(ethylene terephthalate)[J]. Macromolecular Materials and Engineering, 2007, 292(2): 128-146. |
| 46 | PALIWAL N R, MUNGRAY A K. Ultrasound assisted alkaline hydrolysis of poly(ethylene terephthalate) in presence of phase transfer catalyst[J]. Polymer Degradation and Stability, 2013, 98(10): 2094-2101. |
| 47 | IKENAGA K, INOUE T, KUSAKABE K. Hydrolysis of PET by combining direct microwave heating with high pressure[J]. Procedia Engineering, 2016, 148: 314-318. |
| 48 | WANG Y Q, ZHANG Y, SONG H Y, et al. Zinc-catalyzed ester bond cleavage: chemical degradation of polyethylene terephthalate[J]. Journal of Cleaner Production, 2019, 208: 1469-1475. |
| 49 | AL-SABAGH A M, YEHIA F Z, ESHAQ G, et al. Greener routes for recycling of polyethylene terephthalate[J]. Egyptian Journal of Petroleum, 2016, 25(1): 53-64. |
| 50 | LIU Y P, WANG M X, PAN Z Y. Catalytic depolymerization of polyethylene terephthalate in hot compressed water[J]. The Journal of Supercritical Fluids, 2012, 62: 226-231. |
| 51 | KANG M J, YU H J, JEGAL J, et al. Depolymerization of PET into terephthalic acid in neutral media catalyzed by the ZSM-5 acidic catalyst[J]. Chemical Engineering Journal, 2020, 398: 125655. |
| 52 | DU J T, SUN Q, ZENG X F, et al. ZnO nanodispersion as pseudohomogeneous catalyst for alcoholysis of polyethylene terephthalate[J]. Chemical Engineering Science, 2020, 220: 115642. |
| 53 | MALIK N, KUMAR P, SHRIVASTAVA S, et al. An overview on PET waste recycling for application in packaging[J]. International Journal of Plastics Technology, 2017, 21(1): 1-24. |
| 54 | GEORGE N, KURIAN T. Recent developments in the chemical recycling of postconsumer poly(ethylene terephthalate) waste[J]. Industrial & Engineering Chemistry Research, 2014, 53(37): 14185-14198. |
| 55 | 李梦娟, 李艳艳, 鲁静, 等. 聚酯醇解废液的脱色动力学[J]. 化工进展, 2018, 37(9): 3666-3674. |
| LI Mengjuan, LI Yanyan, LU Jing, et al. Dynamics study on decolorization of PET alcoholysis waste liquid [J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3666-3674. | |
| 56 | LÓPEZ-FONSECA R, DUQUE-INGUNZA I, RIVAS B D, et al. Chemical recycling of post-consumer PET wastes by glycolysis in the presence of metal salts[J]. Polymer Degradation and Stability, 2010, 95(6): 1022-1028. |
| 57 | WANG Q, LU X M, ZHOU X Y, et al. 1-Allyl-3-methylimidazolium halometallate ionic liquids as efficient catalysts for the glycolysis of poly(ethylene terephthalate)[J]. Journal of Applied Polymer Science, 2013, 129(6): 3574-3581. |
| 58 | AL-SABAGH A M, YEHIA F Z, EISSA A M M F, et al. Glycolysis of poly(ethylene terephthalate) catalyzed by the lewis base ionic liquid [Bmim][OAc][J]. Industrial & Engineering Chemistry Research, 2014, 53(48): 18443-18451. |
| 59 | PINGALE N D, PALEKAR V S, SHUKLA S R. Glycolysis of postconsumer polyethylene terephthalate waste[J]. Journal of Applied Polymer Science, 2010, 115(1): 249-254. |
| 60 | GHAEMY M, MOSSADDEGH K. Depolymerisation of poly(ethylene terephthalate) fibre wastes using ethylene glycol[J]. Polymer Degradation and Stability, 2005, 90(3): 570-576. |
| 61 | ESQUER R, GARCÍA J J. Metal-catalysed poly(ethylene) terephthalate and polyurethane degradations by glycolysis[J]. Journal of Organometallic Chemistry, 2019, 902: 120972. |
| 62 | FANG P T, LIU B, XU J L, et al. High-efficiency glycolysis of poly(ethylene terephthalate) by sandwich-structure polyoxometalate catalyst with two active sites[J]. Polymer Degradation and Stability, 2018, 156: 22-31. |
| [1] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [2] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [3] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [4] | 高彦静. 单原子催化技术国际研究态势分析[J]. 化工进展, 2023, 42(9): 4667-4676. |
| [5] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [6] | 钱思甜, 彭文俊, 张先明. PET熔融缩聚与溶液解聚形成环状低聚物的对比分析[J]. 化工进展, 2023, 42(9): 4808-4816. |
| [7] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [8] | 王报英, 王皝莹, 闫军营, 汪耀明, 徐铜文. 聚合物包覆膜在金属分离回收中的研究进展[J]. 化工进展, 2023, 42(8): 3990-4004. |
| [9] | 吕杰, 黄冲, 冯自平, 胡亚飞, 宋文吉. 基于余热回收的燃气热泵性能及控制系统[J]. 化工进展, 2023, 42(8): 4182-4192. |
| [10] | 胡亚飞, 冯自平, 田佳垚, 宋文吉. 空气源燃气热泵系统多制热运行模式下余热回收特性[J]. 化工进展, 2023, 42(8): 4204-4211. |
| [11] | 李润蕾, 王子彦, 王志苗, 李芳, 薛伟, 赵新强, 王延吉. CuO-CeO2/TiO 2 高效催化CO低温氧化反应性能[J]. 化工进展, 2023, 42(8): 4264-4274. |
| [12] | 杨静, 李博, 李文军, 刘晓娜, 汤刘元, 刘月, 钱天伟. 焦化污染场地中萘降解菌的分离及降解特性[J]. 化工进展, 2023, 42(8): 4351-4361. |
| [13] | 常印龙, 周启民, 王青月, 王文俊, 李伯耿, 刘平伟. 废弃聚烯烃的高值化学回收研究进展[J]. 化工进展, 2023, 42(8): 3965-3978. |
| [14] | 俞俊楠, 俞建峰, 程洋, 齐一搏, 化春键, 蒋毅. 基于深度学习的变宽度浓度梯度芯片性能预测[J]. 化工进展, 2023, 42(7): 3383-3393. |
| [15] | 徐伟, 李凯军, 宋林烨, 张兴惠, 姚舜华. 光催化及其协同电化学降解VOCs的研究进展[J]. 化工进展, 2023, 42(7): 3520-3531. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||