化工进展 ›› 2022, Vol. 41 ›› Issue (6): 3263-3278.DOI: 10.16085/j.issn.1000-6613.2021-1614
介孔金属有机框架材料吸附水中重金属离子研究进展
唐朝春( ), 王顺藤, 黄从新, 冯文涛, 阮以宣, 史纯菁
), 王顺藤, 黄从新, 冯文涛, 阮以宣, 史纯菁
- 华东交通大学土木建筑学院,江西 南昌 330013
-
收稿日期:2021-07-30修回日期:2021-10-28出版日期:2022-06-10发布日期:2022-06-21 -
通讯作者:唐朝春 -
作者简介:唐朝春(1964—),男,硕士,教授,主要从事水处理理论与技术研究。E-mail:tangcc1964@163.com 。 -
基金资助:国家自然科学基金(51768018);江西省自然科学基金(2013BAB203033)
Research progress on adsorption of heavy metal ions in water by mesoporous metal organic framework materials
TANG Chaochun( ), WANG Shunteng, HUANG Congxin, FENG Wentao, RUAN Yixuan, SHI Chunjing
), WANG Shunteng, HUANG Congxin, FENG Wentao, RUAN Yixuan, SHI Chunjing
- School of Civil Engineering and Architecture, East China Jiaotong University, Nanchang 330013, Jiangxi, China
-
Received:2021-07-30Revised:2021-10-28Online:2022-06-10Published:2022-06-21 -
Contact:TANG Chaochun
摘要:
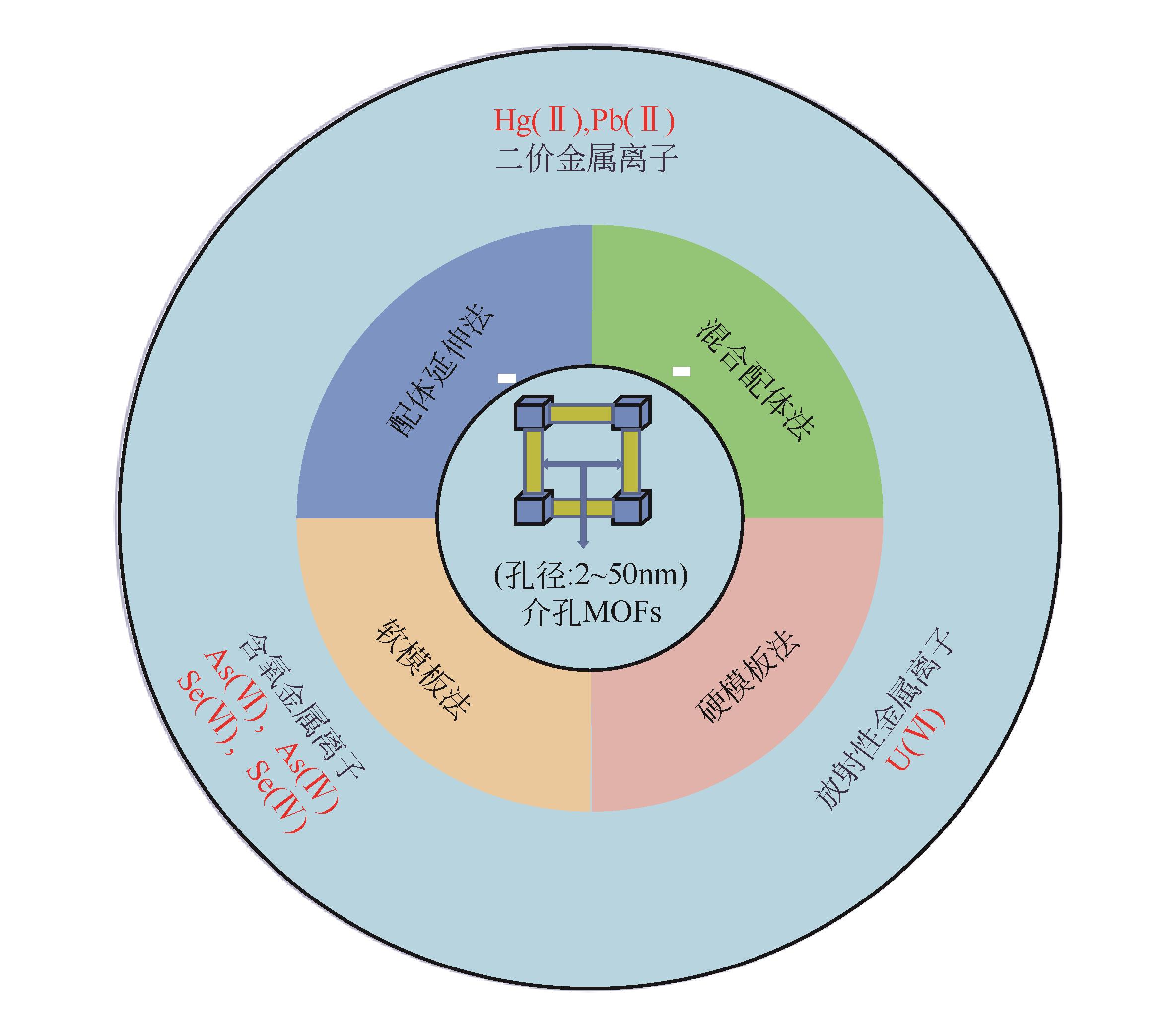
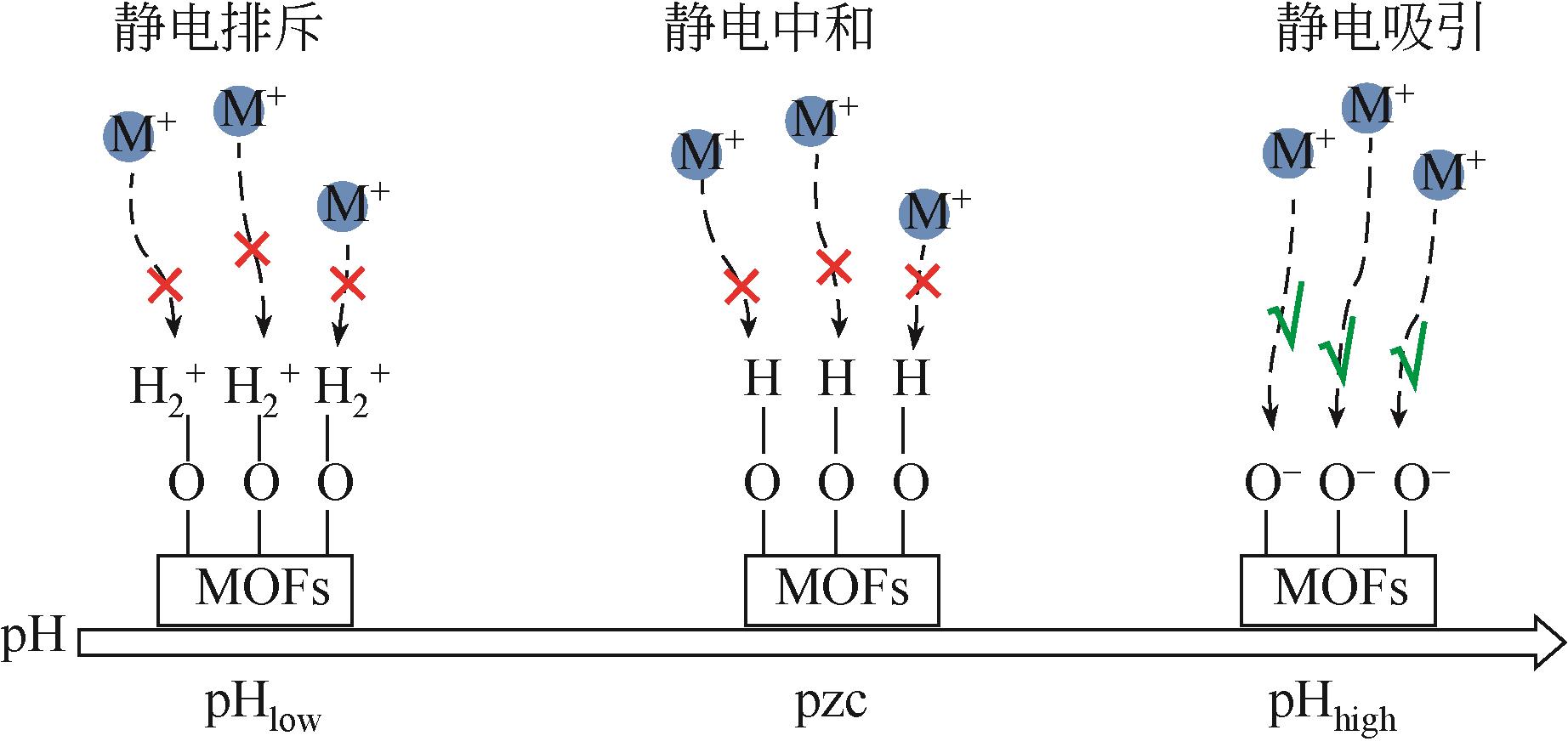
介孔金属有机框架材料(介孔MOFs)相较于传统吸附剂具有孔径大、孔隙率可调、比表面积大、官能团丰富,便于功能化改性修饰等优点,可高效地吸附水体中重金属污染物。本文介绍介孔MOFs的特性、合成策略及四种合成介孔MOFs的方法,重点分析四种方法的介孔形成机理及其所面临的问题,并将四种合成方法的优劣进行了比较。详述介孔MOFs吸附去除水中重金属离子、类重金属阴离子以及放射性金属离子的研究进展;介绍了介孔MOFs在吸附去除重金属离子方面的可重复利用性;阐述介孔MOFs吸附去除水中重金属污染物的作用机理。对介孔MOFs成本高昂、合成条件苛刻、回收利用难等问题提出了优化方向,指出提高介孔MOFs的水稳定性、易回收利用、简便绿色合成技术以及痕量去除将是未来的研究方向。
中图分类号:
引用本文
唐朝春, 王顺藤, 黄从新, 冯文涛, 阮以宣, 史纯菁. 介孔金属有机框架材料吸附水中重金属离子研究进展[J]. 化工进展, 2022, 41(6): 3263-3278.
TANG Chaochun, WANG Shunteng, HUANG Congxin, FENG Wentao, RUAN Yixuan, SHI Chunjing. Research progress on adsorption of heavy metal ions in water by mesoporous metal organic framework materials[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3263-3278.
| 制备方法 | 制备要素 | 介孔形成机理 | 缺点 |
|---|---|---|---|
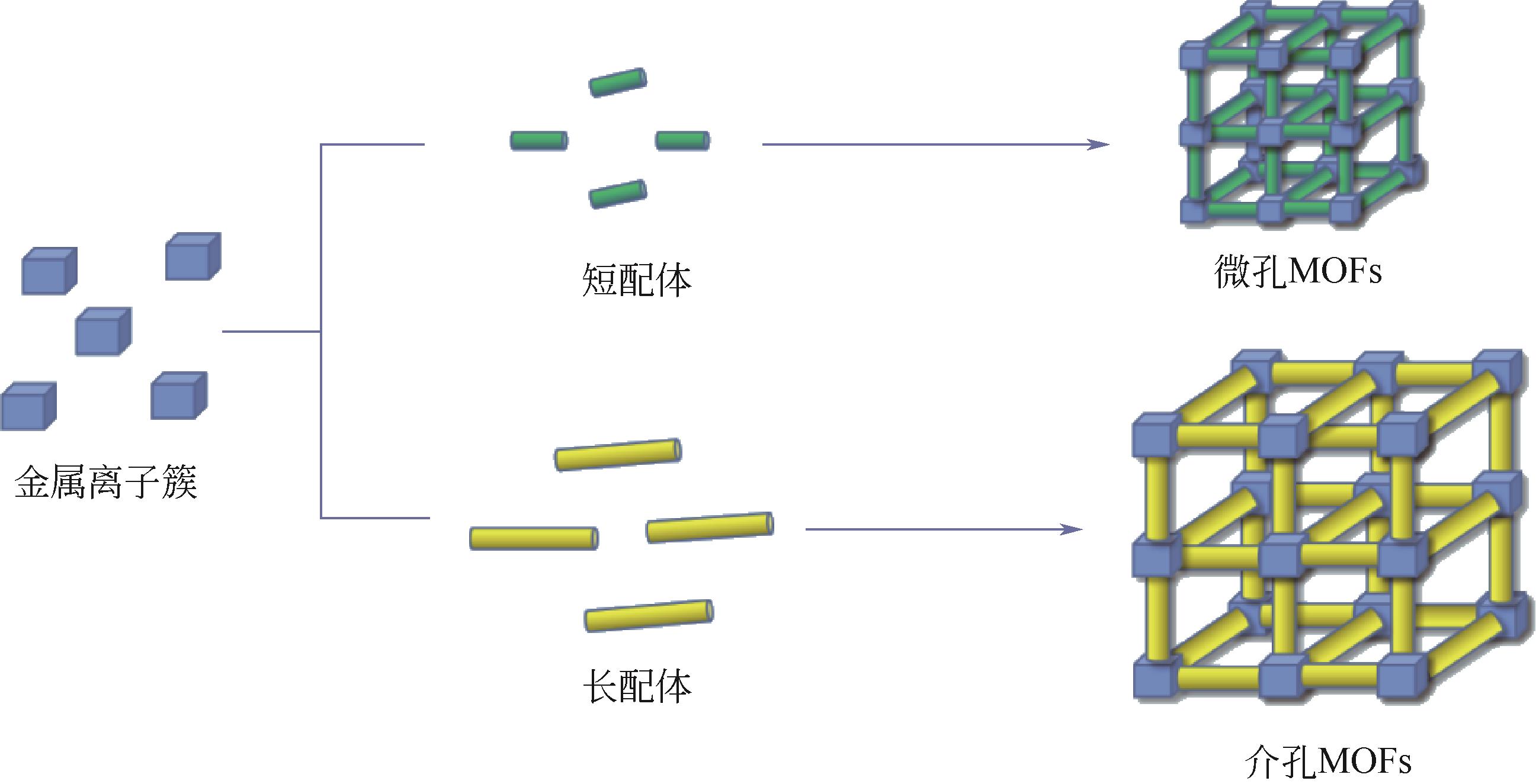
| 配体延伸法 | 需要有一定结构强度的长配体 | 延长配体长度以扩大孔径范围 | 配体相互渗透影响材料结构;受配体柔性结构的影响,结构易坍塌 |
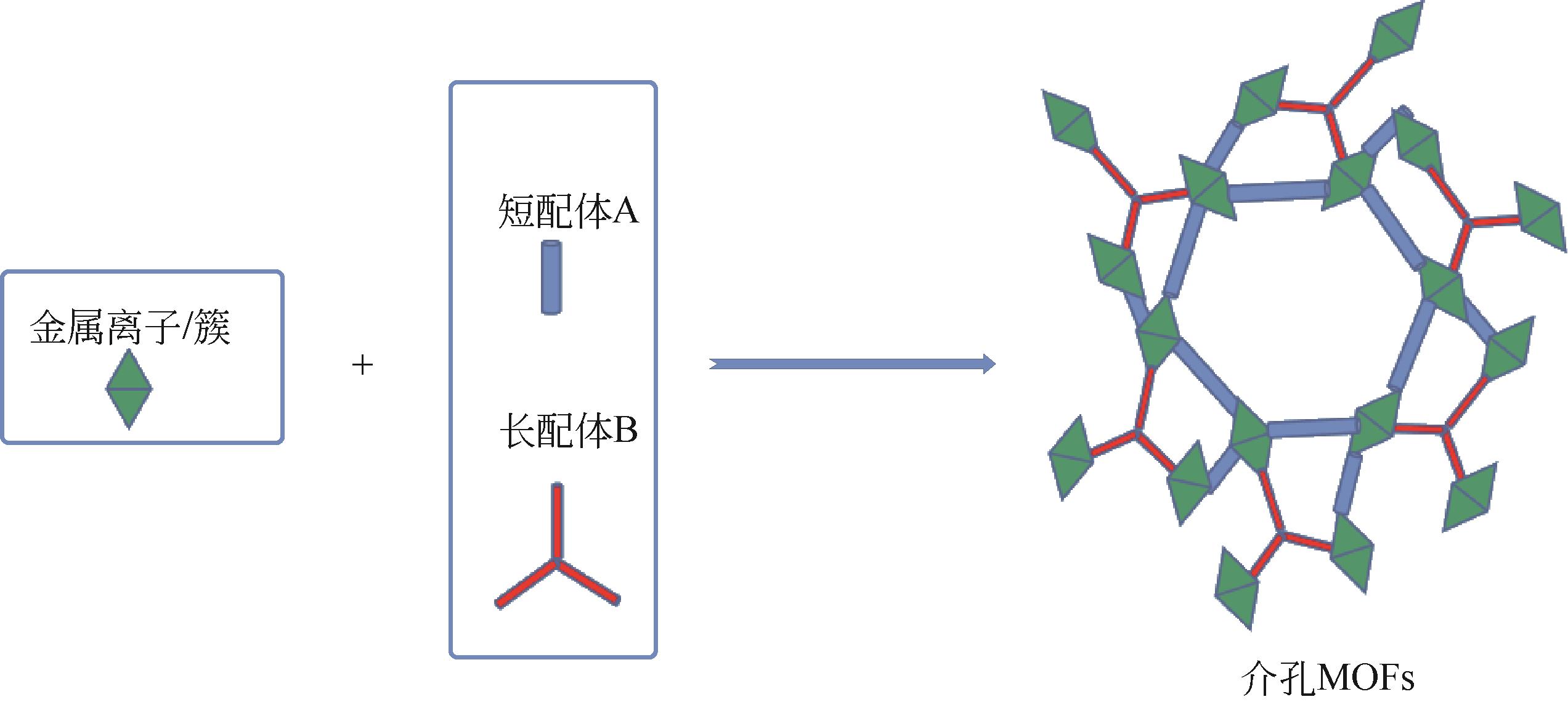
| 混合配体法 | 将两种或多种结构长度不同有机配体混合 | 长配体构建MOFs介孔道,短配体稳定框架结构 | 易形成单体共聚的混合物,不易检测其配位模式及骨架结构 |
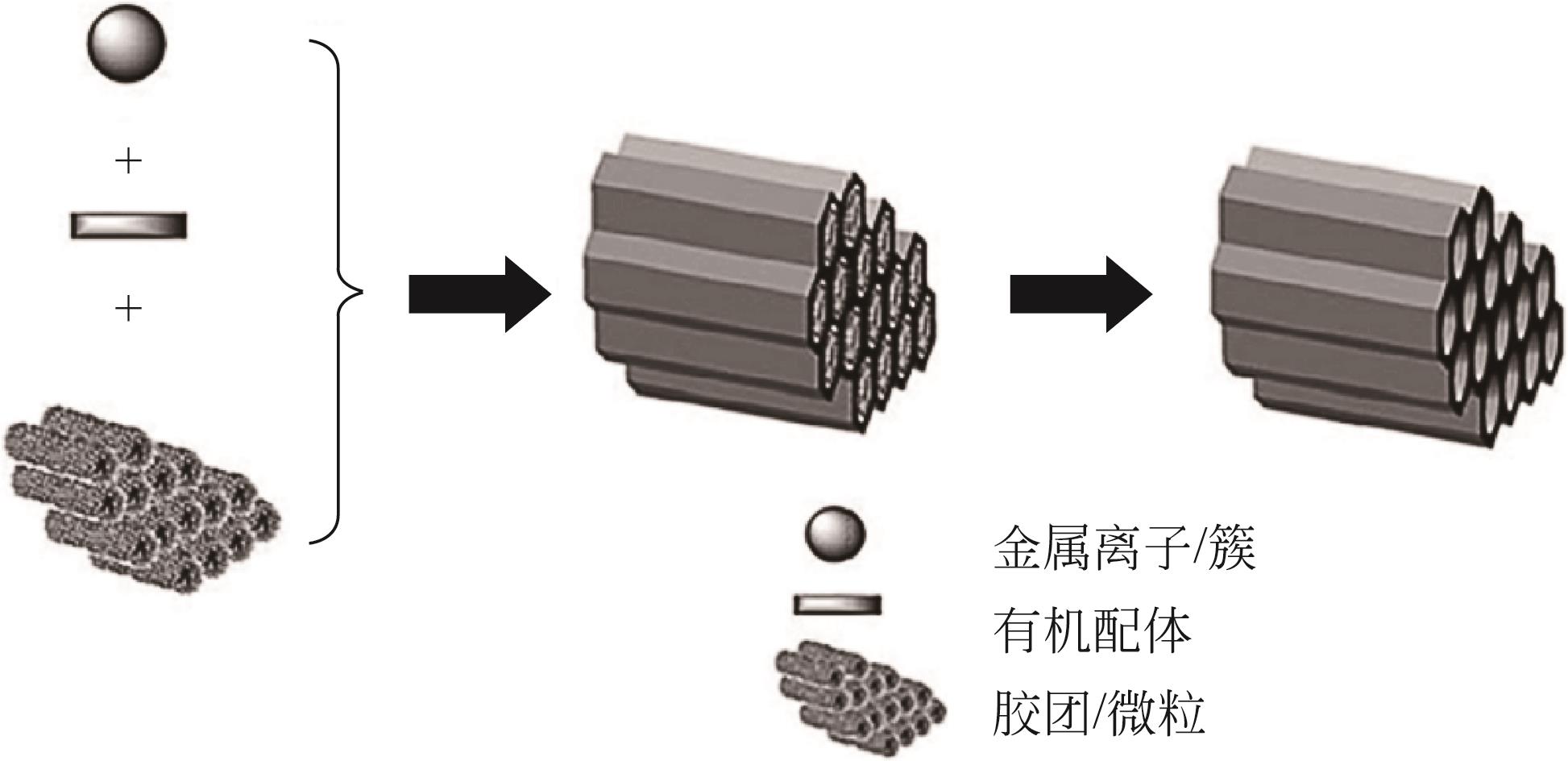
| 软模板法 | 以表面活性剂或者嵌段共聚物,超临界CO2为结构导向剂 | 利用SDAs在反应体系中构建介孔架构进而形成介孔孔道 | 有机溶剂的大量使用增加成本同时污染环境 |
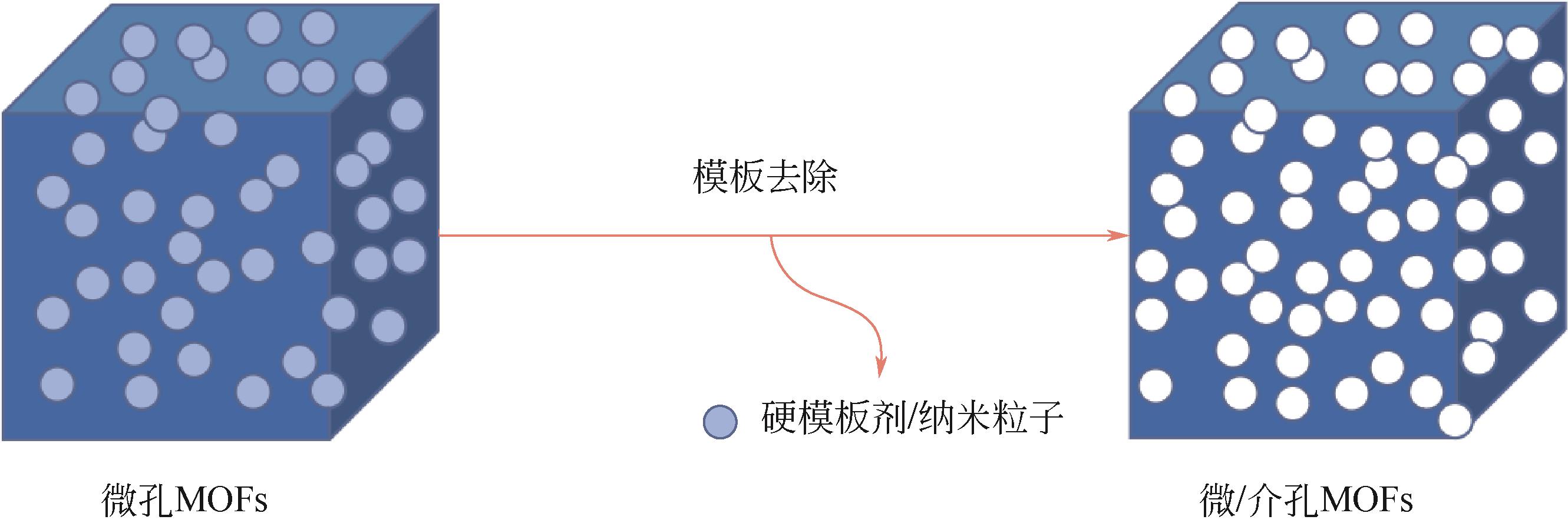
| 硬模板法 | 以纳米粒子或不稳定的组装体为牺牲模板 | 移除被引入的纳米粒子进而形成介孔孔道 | 硬模板剂不易去除 |
表1 几种介孔MOFs的制备方法比较
| 制备方法 | 制备要素 | 介孔形成机理 | 缺点 |
|---|---|---|---|
| 配体延伸法 | 需要有一定结构强度的长配体 | 延长配体长度以扩大孔径范围 | 配体相互渗透影响材料结构;受配体柔性结构的影响,结构易坍塌 |
| 混合配体法 | 将两种或多种结构长度不同有机配体混合 | 长配体构建MOFs介孔道,短配体稳定框架结构 | 易形成单体共聚的混合物,不易检测其配位模式及骨架结构 |
| 软模板法 | 以表面活性剂或者嵌段共聚物,超临界CO2为结构导向剂 | 利用SDAs在反应体系中构建介孔架构进而形成介孔孔道 | 有机溶剂的大量使用增加成本同时污染环境 |
| 硬模板法 | 以纳米粒子或不稳定的组装体为牺牲模板 | 移除被引入的纳米粒子进而形成介孔孔道 | 硬模板剂不易去除 |
| 金属离子 | MOFs | 介孔大小/nm | 吸附量/mg·g-1 | 主要吸附机理 | 文献来源 |
|---|---|---|---|---|---|
| Hg2+ | PCN-221 | — | 277 | 卟啉簇中吡咯功能位点同Hg(Ⅱ)之间的配位作用 | |
| Hg2+ | JUC-62 | 5.0833 | 836.7 | 静电作用以及配位作用 | |
| Hg2+ | MOF-808 | 13.6~17.1 | 343.6 | 锆金属簇同Cr2O | |
| Hg2+ | MOF-808(AO) | 3.5 | 383.8 | 配位作用及MOF-808(AO)中AO基团与Hg(Ⅱ)成键作用 | |
| Pb2+ | melamine-MOFs | 2.0 | 205 | —NH2与Pb(Ⅱ)之间的配位作用 | |
| Pb2+ | MOF-MA | 5.7 | 510 | 巯基配体和Pb(Ⅱ)之间的强亲和力 | |
| Cr2O | BUT-39 | 2.0 | 215 | BUT-39中的锆金属簇同Cr2O | |
| Cr2O | FJI-C11 | 2.4~3.1 | 551 | Cr2O | |
| AsO | MIL-100(Fe) | 2.5~2.9 | 110 | 通过末端羟基的取代形成Fe—O—As配合物 | |
| AsO | CoFe2O4@MIL-100(Fe) | 20.6 | 115 | 通过末端羟基的取代形成Fe—O—As配合物 | |
| AsO | CoFe2O4@MIL-100(Fe) | 20.6 | 144 | 通过末端羟基的取代形成Fe—O—As配合物 | |
| U6+ | MIL-100(Al) | 2.5 | 110 | 配位作用 |
表2 各种介孔MOFs吸附重金属污染物效能对比
| 金属离子 | MOFs | 介孔大小/nm | 吸附量/mg·g-1 | 主要吸附机理 | 文献来源 |
|---|---|---|---|---|---|
| Hg2+ | PCN-221 | — | 277 | 卟啉簇中吡咯功能位点同Hg(Ⅱ)之间的配位作用 | |
| Hg2+ | JUC-62 | 5.0833 | 836.7 | 静电作用以及配位作用 | |
| Hg2+ | MOF-808 | 13.6~17.1 | 343.6 | 锆金属簇同Cr2O | |
| Hg2+ | MOF-808(AO) | 3.5 | 383.8 | 配位作用及MOF-808(AO)中AO基团与Hg(Ⅱ)成键作用 | |
| Pb2+ | melamine-MOFs | 2.0 | 205 | —NH2与Pb(Ⅱ)之间的配位作用 | |
| Pb2+ | MOF-MA | 5.7 | 510 | 巯基配体和Pb(Ⅱ)之间的强亲和力 | |
| Cr2O | BUT-39 | 2.0 | 215 | BUT-39中的锆金属簇同Cr2O | |
| Cr2O | FJI-C11 | 2.4~3.1 | 551 | Cr2O | |
| AsO | MIL-100(Fe) | 2.5~2.9 | 110 | 通过末端羟基的取代形成Fe—O—As配合物 | |
| AsO | CoFe2O4@MIL-100(Fe) | 20.6 | 115 | 通过末端羟基的取代形成Fe—O—As配合物 | |
| AsO | CoFe2O4@MIL-100(Fe) | 20.6 | 144 | 通过末端羟基的取代形成Fe—O—As配合物 | |
| U6+ | MIL-100(Al) | 2.5 | 110 | 配位作用 |
| 污染物 | 吸附剂 | 解吸剂 | 再生性能 | 文献来源 |
|---|---|---|---|---|
| Hg2+ | PCN-221 | 0.01mol/L硝酸溶液 | 75%(3次) | [ |
| Hg2+ | NENU-401 | 乙腈和硫代乙二醇 | 90%(4次) | [ |
| Pb2+ | AMCA-MIL-53(Al) | 0.1mol/L盐酸溶液、硫酸溶液、硝酸溶液 | 79.5%(1次) | [ |
| Cr2O | FJI-C11 | 0.1mol/L盐酸溶液 | 93%(3次) | [ |
| AsO | MIL-100(Fe) | 0.1mol/L盐酸溶液 | 86%(3次) | [ |
表3 各种介孔MOFs可重复利用性对比
| 污染物 | 吸附剂 | 解吸剂 | 再生性能 | 文献来源 |
|---|---|---|---|---|
| Hg2+ | PCN-221 | 0.01mol/L硝酸溶液 | 75%(3次) | [ |
| Hg2+ | NENU-401 | 乙腈和硫代乙二醇 | 90%(4次) | [ |
| Pb2+ | AMCA-MIL-53(Al) | 0.1mol/L盐酸溶液、硫酸溶液、硝酸溶液 | 79.5%(1次) | [ |
| Cr2O | FJI-C11 | 0.1mol/L盐酸溶液 | 93%(3次) | [ |
| AsO | MIL-100(Fe) | 0.1mol/L盐酸溶液 | 86%(3次) | [ |
| 1 | AZIMI A, AZARI A, REZAKAZEMI M, et al. Removal of heavy metals from industrial wastewaters: a review[J]. ChemBioEng Reviews, 2017, 4(1): 37-59. |
| 2 | XU S Z, LYU Y, ZENG X F, et al. ZIF-derived nitrogen-doped porous carbons as highly efficient adsorbents for removal of organic compounds from wastewater[J]. Chemical Engineering Journal, 2017, 323: 502-511. |
| 3 | 刘贤力, 侯昭胤. 重金属污染土壤回转窑协同处置和资源化利用[J]. 化工进展, 2020, 39(S1): 287-291. |
| LIU Xianli, HOU Zhaoyin. Ccooperative disposal and resource utilization of heavy metal contaminated soil by rotary kiln[J]. Chemical Industry and Engineering Progress, 2020, 39(S1): 287-291. | |
| 4 | 张申平, 王艺蒙, 葛宇, 等. 基于孔材料的多元复合光催化剂降解抗生素[J]. 化工进展, 2021, 40(6): 3287-3299. |
| ZHANG Shenping, WANG Yimeng, GE Yu, et al. Degradation of antibiotics by porous composite photocatalyst[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3287-3299. | |
| 5 | 唐朝春, 敖永波, 刘占孟, 等. 矿化垃圾静态吸附处理模拟印染废水试验研究[J]. 华东交通大学学报, 2009, 26(3): 11-15. |
| TANG Chaochun, AO Yongbo, LIU Zhanmeng, et al. Experimental study on the treatment of simulation dyeing wastewater by aged-refuse with static absorption[J]. Journal of East China Jiaotong University, 2009, 26(3): 11-15. | |
| 6 | 周立, 钟宏, 杨湘洪. 气液固三相流化床内氧化吸附组合联用处理含酚废水[J]. 化工进展, 2012, 31(2): 458-461, 472. |
| ZHOU Li, ZHONG Hong, YANG Xianghong. Phenolic wastewater treatment by combination of oxidation combined with adsorption and in a gas-liquid-solid three-phase fluidized bed reactor[J]. Chemical Industry and Engineering Progress, 2012, 31(2): 458-461, 472. | |
| 7 | 徐艳艳, 刘伟, 舒婷, 等. 泡沫浮选耦合膜过滤回收有机废水中纳米TiO2光催化剂的工艺[J]. 化工进展, 2019, 38(10): 4773-4779. |
| XU Yanyan, LIU Wei, SHU Ting, et al. Recovery of nano-sized TiO2 photocatalyst from its organic wastewater by using a technology of froth flotation coupled with ultrafiltration[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4773-4779. | |
| 8 | 钟静, 陆旗玮, 林森, 等. 锂铝层状吸附剂超低品位卤水提锂冲洗和解吸过程[J]. 化工进展, 2021, 40(8): 4638-4646. |
| ZHONG Jing, LU Qiwei, LIN Sen, et al. Washing and desorption procedures research on granulated lithium aluminum layered double hydroxides for lithium recovery from low-grade brine[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4638-4646. | |
| 9 | 李红艳, 严铁尉, 崔建国, 等. 废菌渣活性炭的制备及对废水中硝基苯的吸附[J]. 化工进展, 2020, 39(11): 4702-4707. |
| LI Hongyan, YAN Tiewei, CUI Jianguo, et al. Preparation of mushroom residue activated carbon and its adsorption mechanism of nitrobenzene in waste water[J]. Chemical Industry and Engineering Progress, 2020, 39(11): 4702-4707. | |
| 10 | PAN Z Z, ZHU X M, SATPATHY A, et al. Cr( Ⅵ ) adsorption on engineered iron oxide nanoparticles: exploring complexation processes and water chemistry[J]. Environmental Science & Technology, 2019, 53(20): 11913-11921. |
| 11 | ZHOU J M, CHEN S, LIU J, et al. Adsorption kinetic and species variation of arsenic for As(Ⅴ) removal by biologically mackinawite (FeS)[J]. Chemical Engineering Journal, 2018, 354: 237-244. |
| 12 | WU Y H, PANG H W, LIU Y, et al. Environmental remediation of heavy metal ions by novel-nanomaterials: a review[J]. Environmental Pollution, 2019, 246: 608-620. |
| 13 | SING K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984)[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619. |
| 14 | XUAN W M, ZHU C F, LIU Y, et al. Mesoporous metal-organic framework materials[J]. Chemical Society Reviews, 2012, 41(5): 1677-1695. |
| 15 | ZOU K Y, L I Z X. Controllable syntheses for MOF-derived materials[J]. Journal of Substance Abuse Treatment. 2018, 13(3): 287-288. |
| 16 | SHARMA A, KUMAR A, LI C N, et al. A cannabidiol-loaded Mg-gallate metal-organic framework-based potential therapeutic for glioblastomas[J]. Journal of Materials Chemistry B, 2021, 9(10): 2505-2514. |
| 17 | LU D F, WANG Z W, WANG F, et al. Phosphorescent calcium-based metal-organic framework with second-scale long afterglow[J]. Inorganic Chemistry, 2021, 60(14): 10075-10078. |
| 18 | ZHANG Z, LIU J, WANG Z, et al. Efficient capture of gaseous elemental mercury based on novel copper-based metal-organic frameworks[J]. Fuel, 2021, 289: 119791. |
| 19 | 杨建成, 王诗宁, 杨硕, 等. 金属有机框架材料吸附VOCs影响因素研究进展[J]. 化工进展, 2021, 40(1): 463-476. |
| YANG Jiancheng, WANG Shining, YANG Shuo, et al. Influence factors of VOCs adsorption on metal-organic frameworks: the reviews[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 463-476. | |
| 20 | JONGKIND M K, RIVERA-TORRENTE M, NIKOLOPOULOS N, et al. Influence of pore structure and metal-node geometry on the polymerization of ethylene over Cr-based metal-organic frameworks[J]. Chemistry—A European Journal, 2021, 27(18): 5769-5781. |
| 21 | OPANASENKO M, DHAKSHINAMOORTHY A, HWANG Y K, et al. Superior performance of metal-organic frameworks over zeolites as solid acid catalysts in the prins reaction: green synthesis of nopol[J]. ChemSusChem, 2013, 6(5): 865-871. |
| 22 | HAMON L, SERRE C, DEVIC T, et al. Comparative study of hydrogen sulfide adsorption in the MIL-53(Al, Cr, Fe), MIL-47(Ⅴ), MIL-100(Cr), and MIL-101(Cr) metal-organic frameworks at room temperature[J]. Journal of the American Chemical Society, 2009, 131(25): 8775-8777. |
| 23 | JEYASEELAN A, VISWANATHAN N. Design of amino-functionalized benzene-1,4-dicarboxylic acid-fabricated lanthanum-based metal-organic frameworks for defluoridation of water[J]. Journal of Chemical & Engineering Data, 2020, 65(11): 5328-5340. |
| 24 | LI Z, ZENG H C. Armored MOFs: enforcing soft microporous MOF nanocrystals with hard mesoporous silica[J]. Journal of the American Chemical Society, 2014, 136(15): 5631-5639. |
| 25 | LIU D X, ZOU D T, ZHU H L, et al. Mesoporous metal-organic frameworks: synthetic strategies and emerging applications[J]. Small, 2018, 14(37): 1801454. |
| 26 | GUAN H Y, LEBLANC R J, XIE S Y, et al. Recent progress in the syntheses of mesoporous metal-organic framework materials[J]. Coordination Chemistry Reviews, 2018, 369: 76-90. |
| 27 | GULCAY E, IACOMI P, KO Y, et al. Breaking the upper bound of siloxane uptake: metal-organic frameworks as an adsorbent platform[J]. Journal of Materials Chemistry A, 2021. DOI: 10.1039/d1ta02275j . |
| 28 | XIANG Z H, HU Z, CAO D P, et al. Metal-organic frameworks with incorporated carbon nanotubes: improving carbon dioxide and methane storage capacities by lithium doping[J]. Angewandte Chemie International Edition, 2011, 50(2): 491-494. |
| 29 | MURRAY L J, DINCĂ M, LONG J R. Hydrogen storage in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1294. |
| 30 | XU H, GAO J K, QIAN X F, et al. Metal-organic framework nanosheets for fast-response and highly sensitive luminescent sensing of Fe3+ [J]. Journal of Materials Chemistry A, 2016, 4(28): 10900-10905. |
| 31 | LIU J Y, YU H J, WANG L, et al. Two-dimensional metal-organic frameworks nanosheets: synthesis strategies and applications[J]. Inorganica Chimica Acta, 2018, 483: 550-564. |
| 32 | WANG J, LI N, XU Y X, et al. Two-dimensional MOF and COF nanosheets: synthesis and applications in electrochemistry[J]. Chemistry - A European Journal, 2020, 26(29): 6402-6422. |
| 33 | WEN J, LI Y W, GAO J K. Two-dimensional metal-organic frameworks and derivatives for electrocatalysis[J]. Chemical Research in Chinese Universities, 2020, 36(4): 662-679. |
| 34 | 刘幸, 韩勇, 张亚如, 等. 介孔金属-有机框架(MesoMOFs)的合成研究进展[J]. 合成化学, 2019, 27(7): 568-575. |
| LIU Xing, HAN Yong, ZHANG Yaru, et al. Research progress on synthesis of mesoporous metal-organic frameworks(MesoMOFs)[J]. Chinese Journal of Synthetic Chemistry, 2019, 27(7): 568-575. | |
| 35 | 段崇雄. 多级孔金属-有机骨架材料的制备、形成机制及吸附性能研究[D]. 广州: 华南理工大学, 2019. |
| DUAN Chongxiong. Synthesis, formation mechanism and adsorption performance of hierarchically porous metal-organic frameworks[D]. Guangzhou: South China University of Technology, 2019. | |
| 36 | ZHAO D, TIMMONS D J, YUAN D Q, et al. Tuning the topology and functionality of metal–organic frameworks by ligand design[J]. Accounts of Chemical Research, 2011, 44(2): 123-133. |
| 37 | EDDAOUDI M, KIM J, ROSI N, et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage[J]. Science, 2002, 295(5554): 469-472. |
| 38 | SONG L F, ZHANG J, SUN L X, et al. Mesoporous metal-organic frameworks: design and applications[J]. Energy & Environmental Science, 2012, 5(6): 7508. |
| 39 | GOMEZ-GUALDRON D A, GUTOV O V, KRUNGLEVICIUTE V, et al. Computational design of metal-organic frameworks based on stable zirconium building units for storage and delivery of methane[J]. Chemistry of Materials, 2014, 26(19): 5632-5639. |
| 40 | LI P, MOON S Y, GUELTA M A, et al. Nanosizing a metal-organic framework enzyme carrier for accelerating nerve agent hydrolysis[J]. ACS Nano, 2016, 10(10): 9174-9182. |
| 41 | MONDLOCH J E, BURY W, FAIREN-JIMENEZ D, et al. Vapor-phase metalation by atomic layer deposition in a metal-organic framework[J]. Journal of the American Chemical Society, 2013, 135(28): 10294-10297. |
| 42 | XU W L, THAPA K B, JU Q, et al. Heterogeneous catalysts based on mesoporous metal-organic frameworks[J]. Coordination Chemistry Reviews, 2018, 373: 199-232. |
| 43 | CHEN X, ZHANG Q Y. Recent advances in mesoporous metal-organic frameworks[J]. Particuology, 2019, 45: 20-34. |
| 44 | KOH K, WONG-FOY A, MATZGER A. A crystalline mesoporous coordination copolymer with high microporosity[J]. Angewandte Chemie, 2008, 120(4): 689-692. |
| 45 | KLEIN N, SENKOVSKA I, GEDRICH K, et al. A mesoporous metal-organic framework[J]. Angewandte Chemie International Edition, 2009, 48(52): 9954-9957. |
| 46 | KLEIN N, SENKOVSKA I, BABURIN I A, et al. Route to a family of robust, non-interpenetrated metal-organic frameworks with pto-like topology[J]. Chemistry - A European Journal, 2011, 17(46): 13007-13016. |
| 47 | LIU B Y, LI Y Y, OH S C, et al. Fabrication of a hierarchically structured HKUST-1 by a mixed-ligand approach[J]. RSC Advances, 2016, 6(66): 61006-61012. |
| 48 | KIM S Y, KIM A R, YOON J W, et al. Creation of mesoporous defects in a microporous metal-organic framework by an acetic acid-fragmented linker co-assembly and its remarkable effects on methane uptake[J]. Chemical Engineering Journal, 2018, 335: 94-100. |
| 49 | QIU L G, XU T, LI Z Q, et al. Hierarchically micro- and mesoporous metal-organic frameworks with tunable porosity[J]. Angewandte Chemie International Edition, 2008, 47(49): 9487-9491. |
| 50 | ALIZADEH S, NEMATOLLAHI D. Electrochemically assisted self-assembly technique for the fabrication of mesoporous metal-organic framework thin films: composition of 3D hexagonally packed crystals with 2D honeycomb-like mesopores[J]. Journal of the American Chemical Society, 2017, 139(13): 4753-4761. |
| 51 | YU H N, XU D D, XU Q. Dual template effect of supercritical CO2 in ionic liquid to fabricate a highly mesoporous cobalt metal-organic framework[J]. Chemical Communications, 2015, 51(67): 13197-13200. |
| 52 | DO X D, HOANG V T, KALIAGUINE S. MIL-53(Al) mesostructured metal-organic frameworks[J]. Microporous and Mesoporous Materials, 2011, 141(1/2/3): 135-139. |
| 53 | 李彦彦. 中微双孔金属—有机骨架材料HKUST-1的合成及表征[D]. 广州: 华南理工大学, 2016. |
| LI Yanyan. Synthesis and characterization of micro-mesoporous metal-organic framework HKUST-1[D]. Guangzhou: South China University of Technology, 2016. | |
| 54 | SENKOVSKA I, KASKEL S. Ultrahigh porosity in mesoporous MOFs: promises and limitations[J]. Chemical Communications, 2014, 50(54): 7089. |
| 55 | CAO S, GODY G, ZHAO W, et al. Hierarchical bicontinuous porosity in metal–organic frameworks templated from functional block co-oligomer micelles[J]. Chemical Science, 2013, 4(9): 3573. |
| 56 | ZHANG W N, LIU Y Y, LU G, et al. Mesoporous metal-organic frameworks with size-, shape-, and space-distribution-controlled pore structure[J]. Advanced Materials, 2015, 27(18): 2923-2929. |
| 57 | KIM Y, YANG T, YUN G, et al. Hydrolytic transformation of microporous metal-organic frameworks to hierarchical micro- and mesoporous MOFs[J]. Angewandte Chemie International Edition, 2015, 54(45): 13273-13278. |
| 58 | HUANG H L, LI J R, WANG K K, et al. An in situ self-assembly template strategy for the preparation of hierarchical-pore metal-organic frameworks[J]. Nature Communications, 2015, 6: 8847. |
| 59 | SEYFI HASANKOLA Z, RAHIMI R, SHAYEGAN H, et al. Removal of Hg2+ heavy metal ion using a highly stable mesoporous porphyrinic zirconium metal-organic framework[J]. Inorganica Chimica Acta, 2020, 501: 119264. |
| 60 | WU Y Z, XU G H, WEI F D, et al. Determination of Hg (Ⅱ) in tea and mushroom samples based on metal-organic frameworks as solid phase extraction sorbents[J]. Microporous and Mesoporous Materials, 2016, 235: 204-210. |
| 61 | LIU Q F, ZHANG Q, LIU B R, et al. A new synthesis and adsorption mechanism of ZrO2 based metal-organic frames for efficient removal of mercury ions from aqueous solution[J]. Ceramics International, 2019, 45(12): 15720-15724. |
| 62 | BHATTACHARJEE S, LEE Y R, AHN W S. Post-synthesis functionalization of a zeolitic imidazolate structure ZIF-90: a study on removal of Hg( Ⅱ ) from water and epoxidation of alkenes[J]. CrystEngComm, 2015, 17(12): 2575-2582. |
| 63 | REBELO F M, CALDAS E D. Arsenic, lead, mercury and cadmium: toxicity, levels in breast milk and the risks for breastfed infants[J]. Environmental Research, 2016, 151: 671-688. |
| 64 | DING L, LUO X B, SHAO P H, et al. Thiol-functionalized Zr-based metal-organic framework for capture of Hg( Ⅱ ) through a proton exchange reaction[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(7): 8494-8502. |
| 65 | YIN N, WANG K, XIA Y A, et al. Novel melamine modified metal-organic frameworks for remarkably high removal of heavy metal Pb (Ⅱ)[J]. Desalination, 2018, 430: 120-127. |
| 66 | WANG C, LIN G, XI Y H, et al. Development of mercaptosuccinic anchored MOF through one-step preparation to enhance adsorption capacity and selectivity for Hg(Ⅱ) and Pb(Ⅱ)[J]. Journal of Molecular Liquids, 2020, 317: 113896. |
| 67 | ALQADAMI A A, KHAN M A, SIDDIQUI M R, et al. Development of citric anhydride anchored mesoporous MOF through post synthesis modification to sequester potentially toxic lead ( Ⅱ ) from water[J]. Microporous and Mesoporous Materials, 2018, 261: 198-206. |
| 68 | RAO A, BANKAR A, KUMAR A R, et al. Removal of hexavalent chromium ions by Yarrowia lipolytica cells modified with phyto-inspired Fe0/Fe3O4 nanoparticles[J]. Journal of Contaminant Hydrology, 2013, 146: 63-73. |
| 69 | SIDIRAS D, POLITI D, BATZIAS F, et al. Efficient removal of hexavalent chromium from aqueous solutions using autohydrolyzed Scots Pine (Pinus Sylvestris) sawdust as adsorbent[J]. International Journal of Environmental Science and Technology, 2013, 10(6): 1337-1348. |
| 70 | SANDHU A, MASUDA H, ORAL A, et al. Room temperature magnetic imaging of magnetic storage media and garnet epilayers in the presence of external magnetic fields using a sub-micron GaAs SHPM[J]. Journal of Crystal Growth, 2001, 227/228: 899-905. |
| 71 | KHEDR S, SHOUMAN M N, FATHY N, et al. Effect of physical and chemical activation on the removal of hexavalent chromium ions using palm tree branches[J]. ISRN Environmental Chemistry, 2014, 2014: 1-10. |
| 72 | HE T, ZHANG Y Z, KONG X J, et al. Zr(Ⅳ)-based metal-organic framework with T-shaped ligand: unique structure, high stability, selective detection, and rapid adsorption of Cr2O 7 2 - in water[J]. ACS Applied Materials & Interfaces, 2018, 10(19): 16650-16659. |
| 73 | 李茹霞, 钟文彬, 谢林华, 等. 金属有机框架材料对Cr(Ⅵ)离子的吸附去除研究进展[J]. 无机化学学报, 2021, 37(3): 385-400. |
| LI Ruxia, ZHONG Wenbin, XIE Linhua, et al. Recent advances in adsorptive removal of Cr( Ⅵ ) ions by metal-organic frameworks[J]. Chinese Journal of Inorganic Chemistry, 2021, 37(3): 385-400. | |
| 74 | ZOU Y H, LIANG J, HE C, et al. A mesoporous cationic metal-organic framework with a high density of positive charge for enhanced removal of dichromate from water[J]. Dalton Transactions, 2019, 48(20): 6680-6684. |
| 75 | LIU B, JIAN M P, WANG H, et al. Comparing adsorption of arsenic and antimony from single-solute and bi-solute aqueous systems onto ZIF-8[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 538: 164-172. |
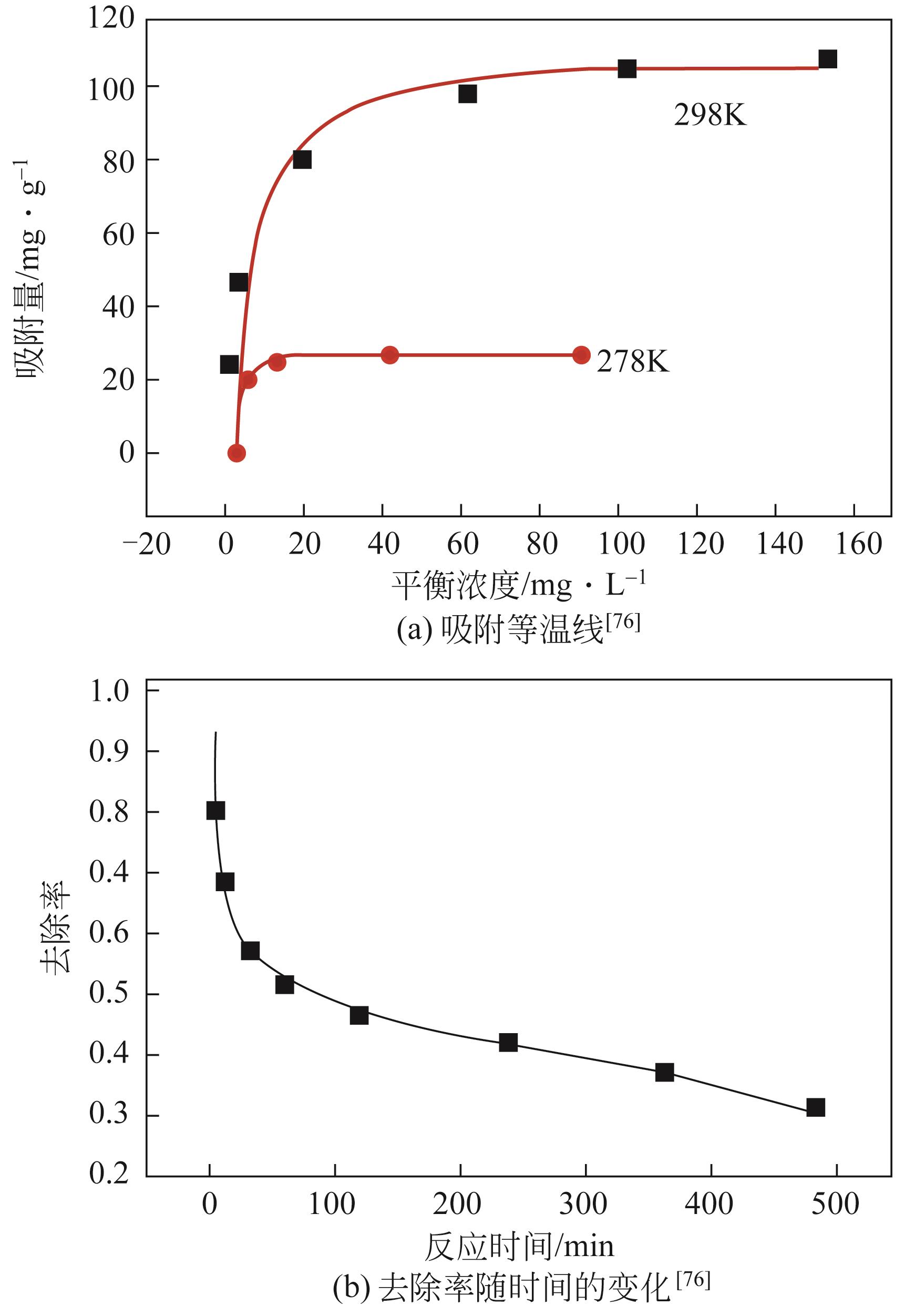
| 76 | CAI J H, WANG X Y, ZHOU Y, et al. Selective adsorption of arsenate and the reversible structure transformation of the mesoporous metal-organic framework MIL-100(Fe)[J]. Physical Chemistry Chemical Physics, 2016, 18(16): 10864-10867. |
| 77 | YANG J C, YIN X B. CoFe2O4@MIL-100(Fe) hybrid magnetic nanoparticles exhibit fast and selective adsorption of arsenic with high adsorption capacity[J]. Scientific Reports, 2017, 7: 40955. |
| 78 | ZHAO X L, YU X Z, WANG X Y, et al. Recent advances in metal-organic frameworks for the removal of heavy metal oxoanions from water[J]. Chemical Engineering Journal, 2021, 407: 127221. |
| 79 | HOWARTH A J, KATZ M J, WANG T C, et al. High efficiency adsorption and removal of selenate and selenite from water using metal-organic frameworks[J]. Journal of the American Chemical Society, 2015, 137(23): 7488-7494. |
| 80 | FALAISE C, VOLKRINGER C, GIOVINE R, et al. Capture of actinides (Th4+, [UO2]2+) and surrogating lanthanide (Nd3+) in porous metal-organic framework MIL-100(Al) from water: selectivity and imaging of embedded nanoparticles[J]. Dalton Transactions, 2017, 46(36): 12010-12014. |
| 81 | YAN X Y, LI P X, SONG X M, et al. Recent progress in the removal of mercury ions from water based MOFs materials[J]. Coordination Chemistry Reviews, 2021, 443: 214034. |
| 82 | JIANG S Y, HE W W, LI S L, et al. Introduction of molecular building blocks to improve the stability of metal-organic frameworks for efficient mercury removal[J]. Inorganic Chemistry, 2018, 57(10): 6118-6123. |
| 83 | KHAN N A, HASAN Z, JHUNG S H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): a review[J]. Journal of Hazardous Materials, 2013, 244/245: 444-456. |
| 84 | WEN J, FANG Y, ZENG G M. Progress and prospect of adsorptive removal of heavy metal ions from aqueous solution using metal-organic frameworks: a review of studies from the last decade[J]. Chemosphere, 2018, 201: 627-643. |
| 85 | AHMED I, JHUNG S H. Adsorptive desulfurization and denitrogenation using metal-organic frameworks[J]. Journal of Hazardous Materials, 2016, 301: 259-276. |
| 86 | LIU Z M, WU S H, JIA S Y, et al. Novel hematite nanorods and magnetite nanoparticles prepared from MIL-100(Fe) template for the removal of As(Ⅴ)[J]. Materials Letters, 2014, 132: 8-10. |
| 87 | WANG C H, LIU X L, CHEN J P, et al. Superior removal of arsenic from water with zirconium metal-organic framework UiO-66[J]. Scientific Reports, 2015, 5: 16613. |
| 88 | ZHANG J M, XIONG Z H, LI C, et al. Exploring a thiol-functionalized MOF for elimination of lead and cadmium from aqueous solution[J]. Journal of Molecular Liquids, 2016, 221: 43-50. |
| [1] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [2] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [3] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [4] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [5] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [6] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [9] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [10] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [11] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [12] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [13] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [14] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [15] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||