化工进展 ›› 2024, Vol. 43 ›› Issue (5): 2760-2775.DOI: 10.16085/j.issn.1000-6613.2024-0098
• 二氧化碳捕集与资源化利用 • 上一篇
二氧化碳电催化还原反应制合成气催化剂研究进展
黄澎1( ), 邹颖2, 王宝焕2, 王逍妍2, 赵勇1, 梁鑫2(
), 邹颖2, 王宝焕2, 王逍妍2, 赵勇1, 梁鑫2( ), 胡迪1
), 胡迪1
- 1.煤炭科学技术研究院有限公司,北京 100013
2.北京化工大学化学工程学院化工资源有效利用国家重点实验室,北京 100029
-
收稿日期:2024-01-14修回日期:2024-04-25出版日期:2024-05-15发布日期:2024-06-15 -
通讯作者:梁鑫 -
作者简介:黄澎(1982—),男,博士,研究员,研究方向为煤炭洁净转化、固废利用、CO2还原和精细化学品等。E-mail:squallok@qq.com。 -
基金资助:天地科技创业资金项目(2022-2-TD-MS001);国家自然科学基金(21571012)
Research progress of electrocatalysts towards electrocatalytic reduction reaction of carbon dioxide to syngas
HUANG Peng1( ), ZOU Ying2, WANG Baohuan2, WANG Xiaoyan2, ZHAO Yong1, LAING Xin2(
), ZOU Ying2, WANG Baohuan2, WANG Xiaoyan2, ZHAO Yong1, LAING Xin2( ), HU Di1
), HU Di1
- 1.China Coal Research Institute Corporation Company Limited, Beijing 100013, China
2.State Key Laboratory of Chemical Resource Engineering, College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
-
Received:2024-01-14Revised:2024-04-25Online:2024-05-15Published:2024-06-15 -
Contact:LAING Xin
摘要:
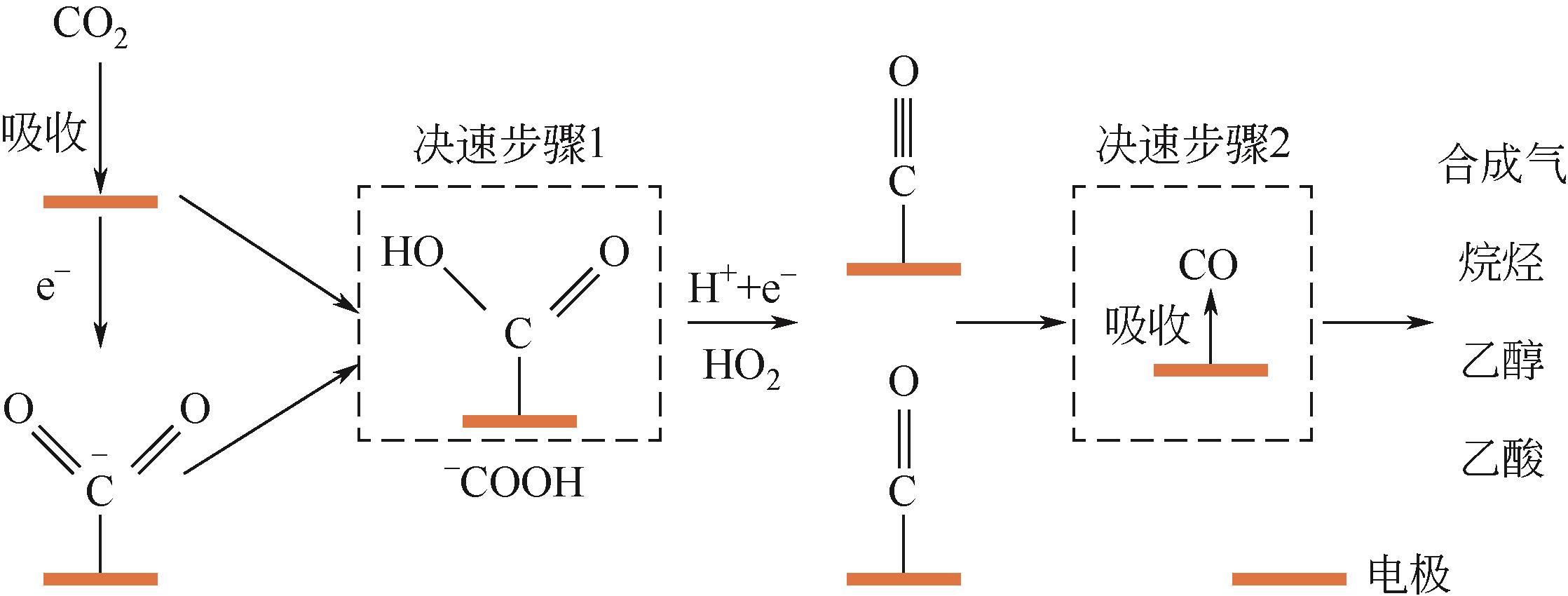
利用电催化二氧化碳还原反应(CRR)将CO2转变为有价值的合成气(CO/H2)受到广泛关注。CRR电催化剂的开发对于高效、精确制备合成气至关重要。本文综述了CRR制备合成气的反应过程、反应机理以及催化剂等方面的研究进展,介绍了现有CRR催化剂的种类、优点、存在的问题以及发展方向,具体分析了催化剂的掺杂元素种类和比例对反应中间体的影响,指出了掺杂非金属元素的金属原子边缘和活性位对CRR的作用,探讨了催化剂设计和反应条件调节对CO和H2比例的精确调控。本文也讨论了增加反应活性位点、降低中间体的反应能垒等促进CRR以及调节合成气碳氢比的方式。此外,提出了可通过催化剂多级形貌调控、多活性位点设计、CO2还原与阳极反应耦合等途径,提升CRR制合成气效率的策略。最后,探讨和展望了CRR制合成气在未来工业化生产中存在的挑战和问题。
中图分类号:
引用本文
黄澎, 邹颖, 王宝焕, 王逍妍, 赵勇, 梁鑫, 胡迪. 二氧化碳电催化还原反应制合成气催化剂研究进展[J]. 化工进展, 2024, 43(5): 2760-2775.
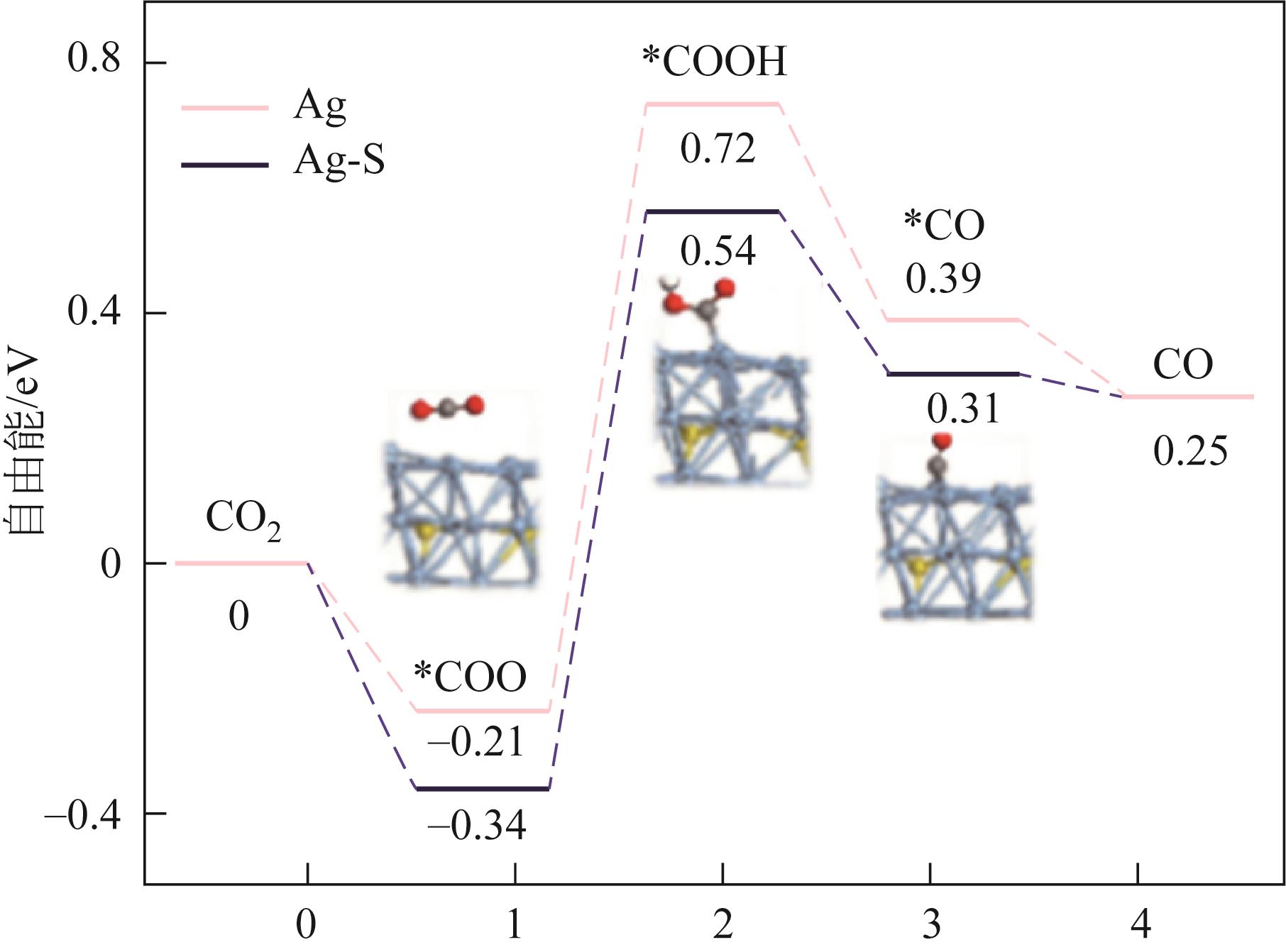
HUANG Peng, ZOU Ying, WANG Baohuan, WANG Xiaoyan, ZHAO Yong, LAING Xin, HU Di. Research progress of electrocatalysts towards electrocatalytic reduction reaction of carbon dioxide to syngas[J]. Chemical Industry and Engineering Progress, 2024, 43(5): 2760-2775.
| 电化学半反应 | 电极电位(vs. SHE)/ V |
|---|---|
| 2H+ + 2e- | -0.420 |
| CO2 (g) + 2H+ + 2e- | -0.250 |
| CO2 (g) + H2O (l) + e- | -1.078 |
| CO2 (g) + 2H+ + 2e- | -0.106 |
| CO2 (g) + H2O (l) + 2e- | -0.934 |
表1 在标准大气压和25℃下,部分CRR产物及其在中性水溶液中相对标准氢电极(SHE)的平衡电势[16]
| 电化学半反应 | 电极电位(vs. SHE)/ V |
|---|---|
| 2H+ + 2e- | -0.420 |
| CO2 (g) + 2H+ + 2e- | -0.250 |
| CO2 (g) + H2O (l) + e- | -1.078 |
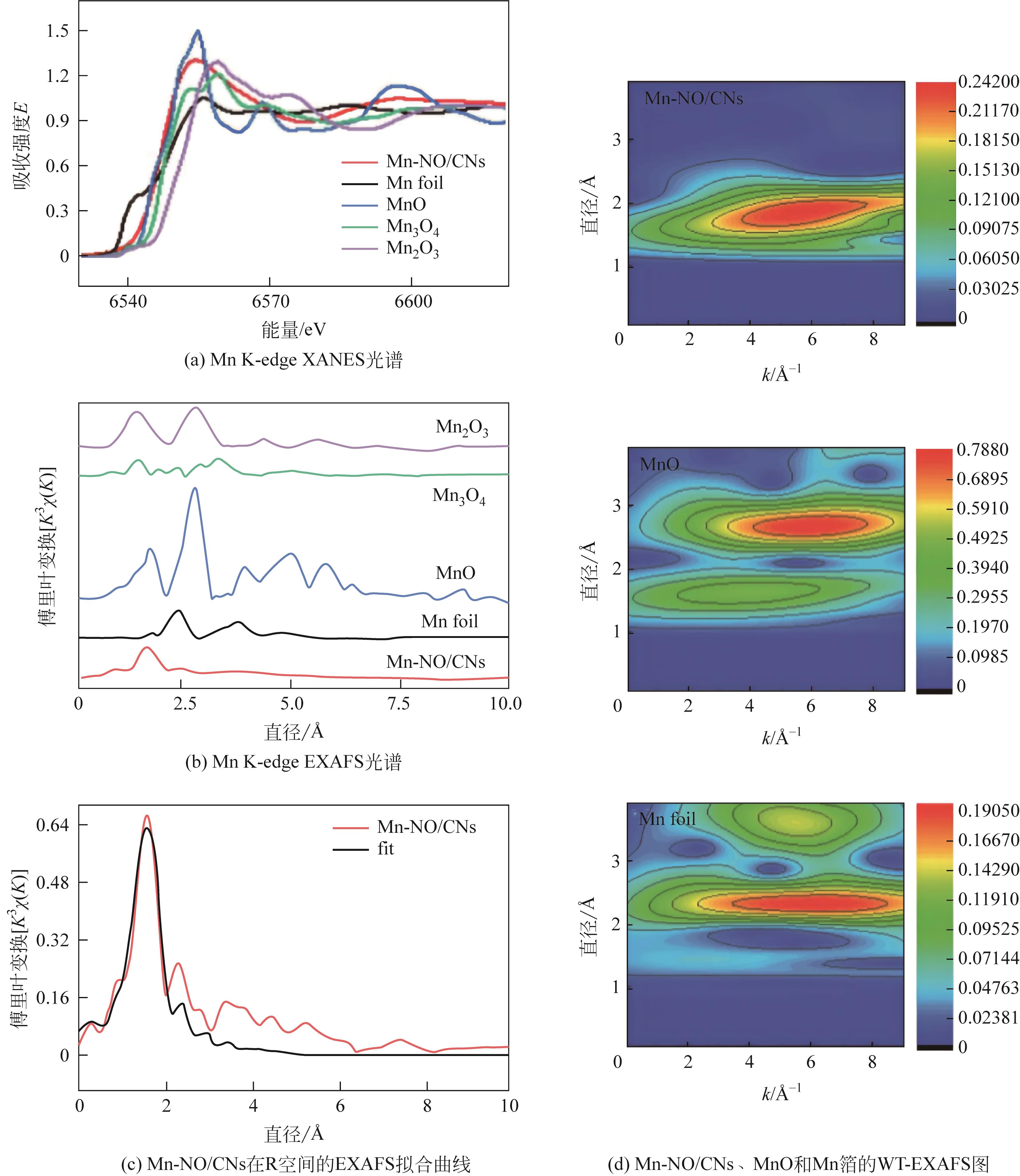
| CO2 (g) + 2H+ + 2e- | -0.106 |
| CO2 (g) + H2O (l) + 2e- | -0.934 |
| 催化剂名称 | 过电位(vs. RHE)/V | 电流密度/mA·cm-2 | 电解质 | 法拉第效率/% | 参考文献 |
|---|---|---|---|---|---|
| Fe3+-N-C | -0.45 | 15 | 0.5mol/L KHCO3 | 90 | [ |
| Mn-NO/CNs | -0.46 | 8 | 0.5mol/L KHCO3 | 96 | [ |
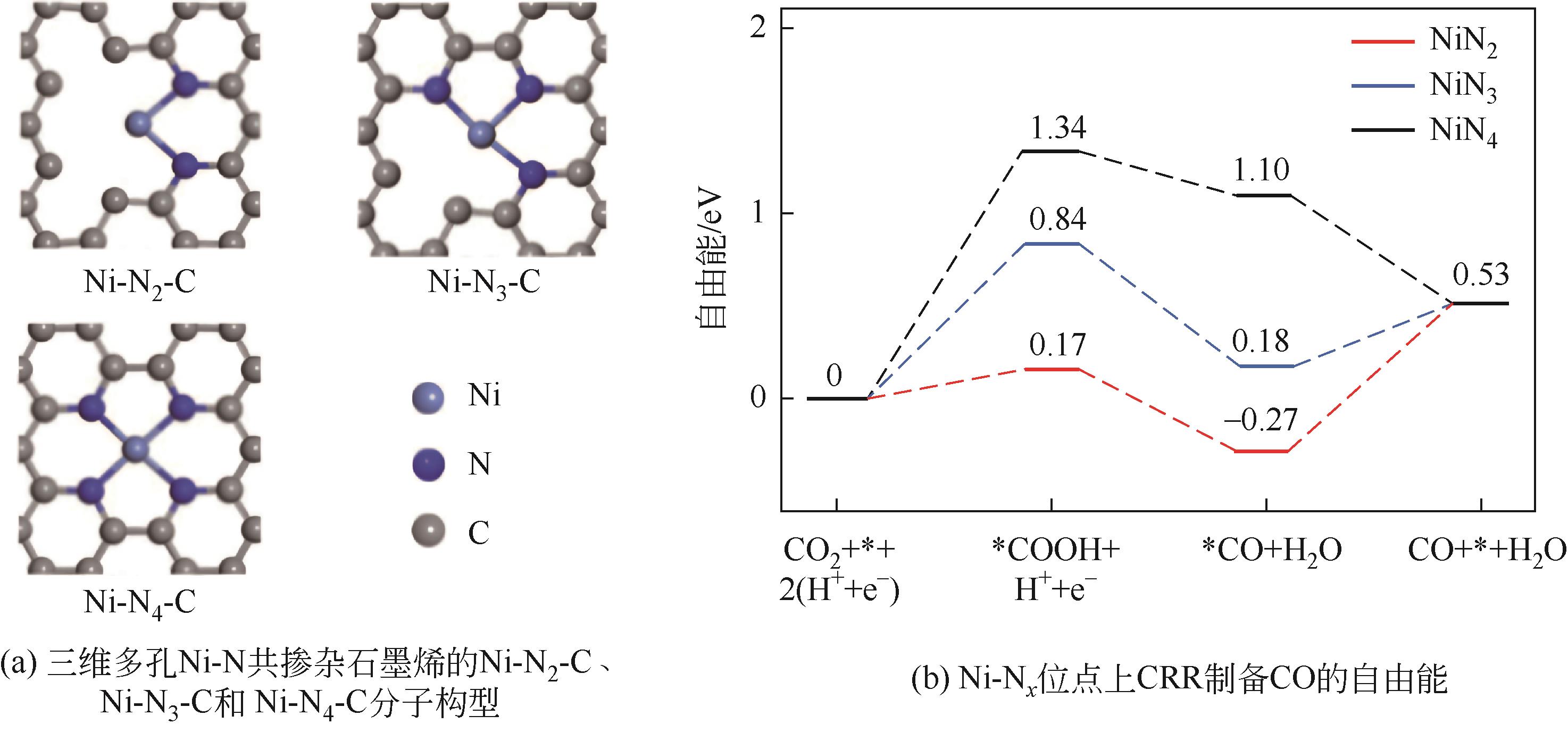
| Ni-N4-C | -0.4 | 8 | 0.5mol/L KHCO3 | 90.2 | [ |
| P/Ni-0@Ni-N-C | -0.8 | 20 | 0.5mol/L KHCO3 | 91 | [ |
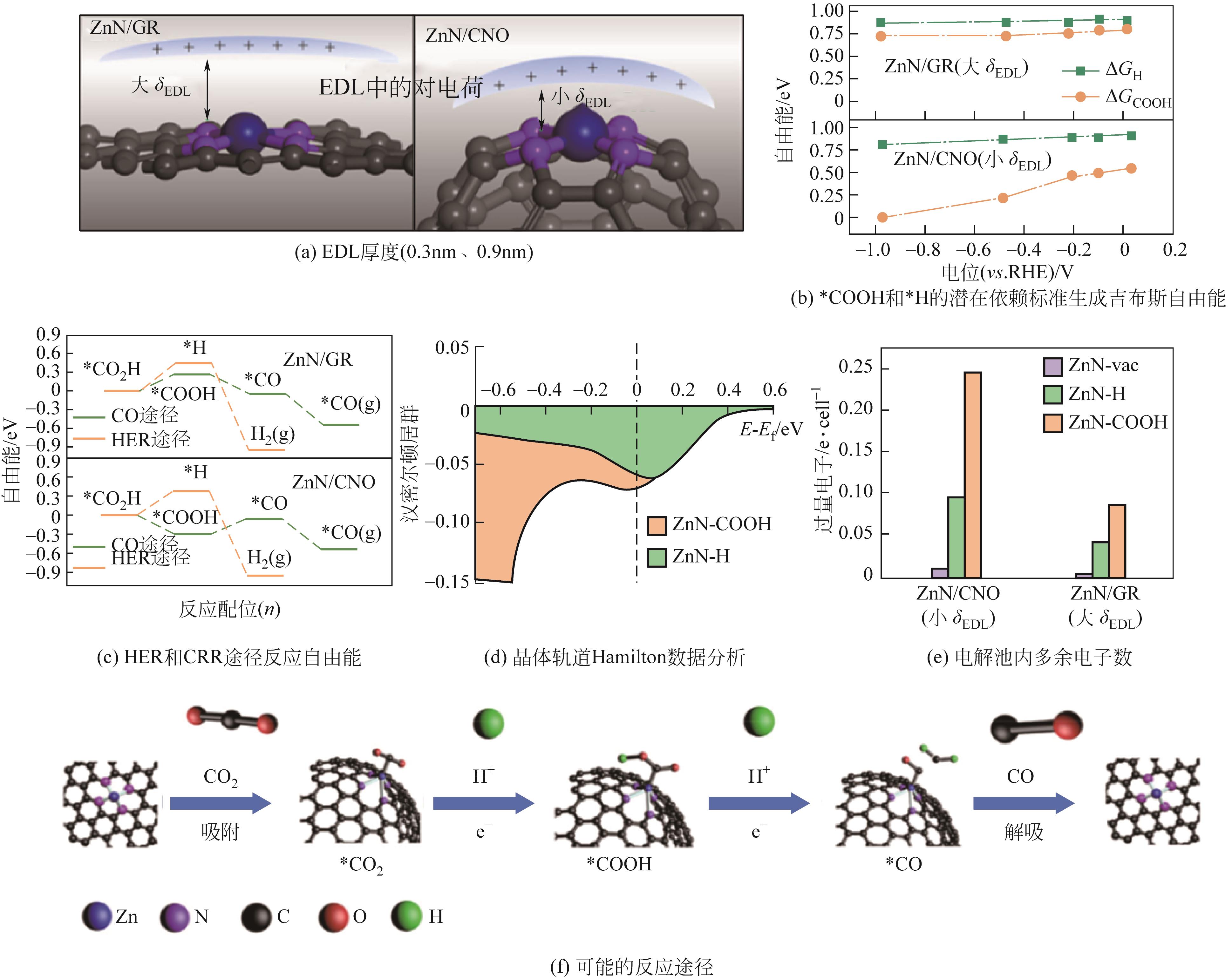
| ZnN/CNO | -0.47 | 5.5 | 0.5mol/L KHCO3 | 97 | [ |
| Fe1-Ni1-N-C | -0.5 | 7 | 0.5mol/L KHCO3 | 96.2 | [ |
| Au NWs | -0.35 | 8 | 0.5mol/L KHCO3 | 94 | [ |
| DDT-Au | -1.1 | 2.4 | 0.1mol/L KHCO3+3.4μmol/L EDTA | 40 | [ |
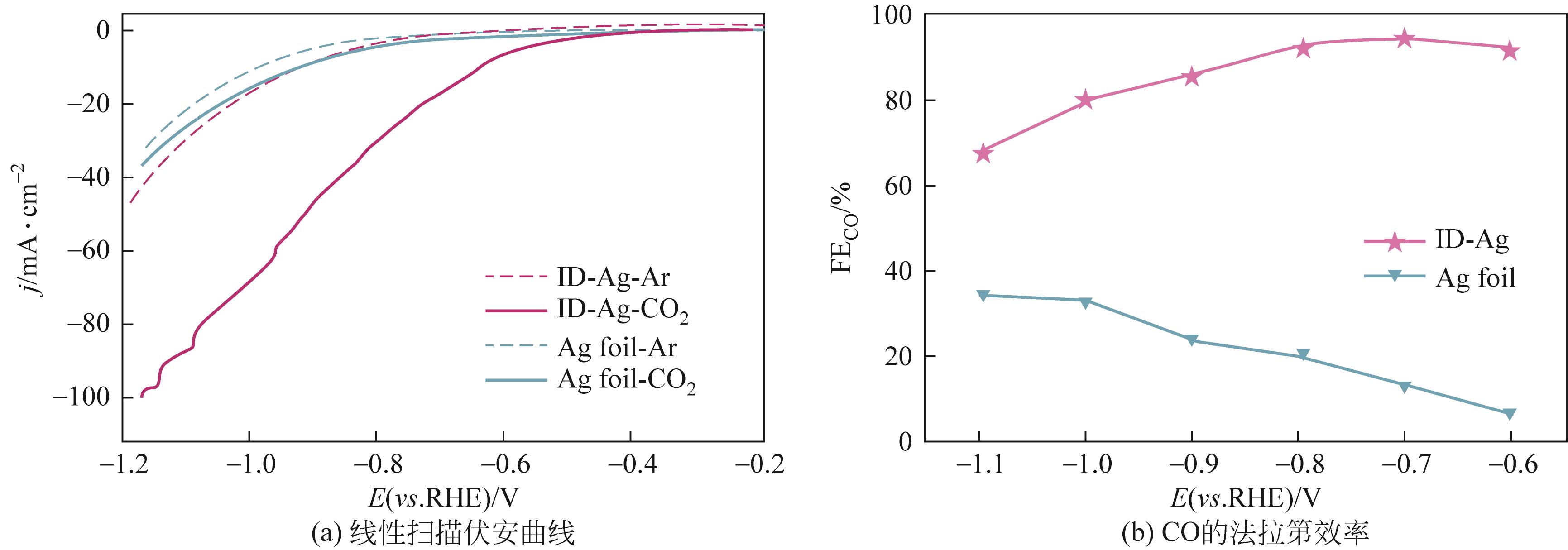
| ID-Ag | -0.7 | 18 | 0.5mol/L KHCO3 | 94.5 | [ |
| Ag | -0.49 | 3 | 0.1mol/L KHCO3 | 80 | [ |
| AuCu/CNT | -0.4 | 2 | 0.5mol/L KHCO3 | 95.2 | [ |
| Cu/Ni(OH)2 | -0.39 | 4.3 | 0.5mol/L NaHCO3 | 92 | [ |
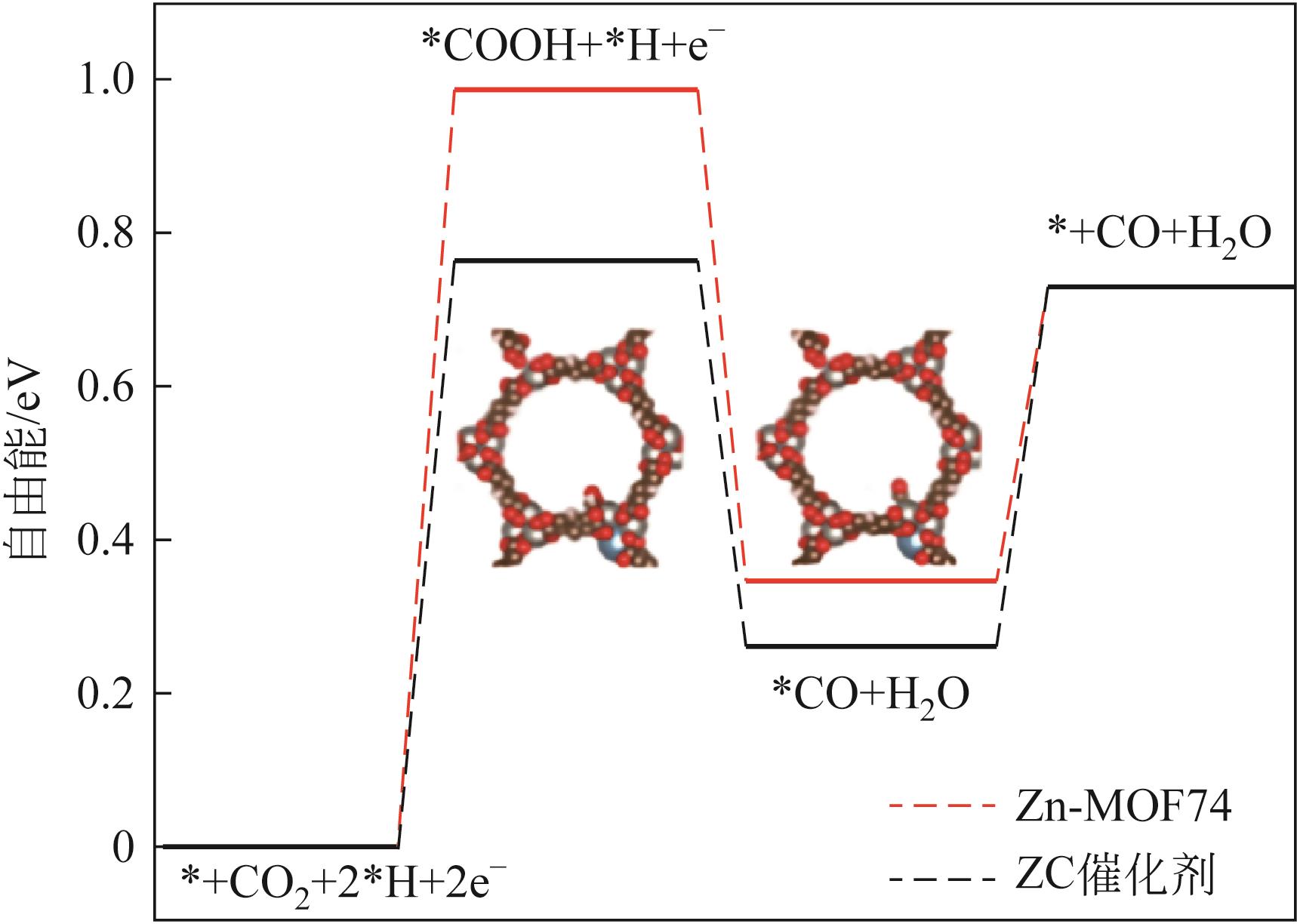
| ZnCa-MOF74 | -1.9V (vs.SCE) | 4 | 0.1mol/L KHCO3 | 93 | [ |
| Zn x Cd1-x @S-胺 | -1.16 | 25 | 0.5mol/L NaHCO3 | 60 | [ |
| p-Cu@Zn(101) | -0.39 | 11.36 | 0.1mol/L KHCO3 | 85 | [ |
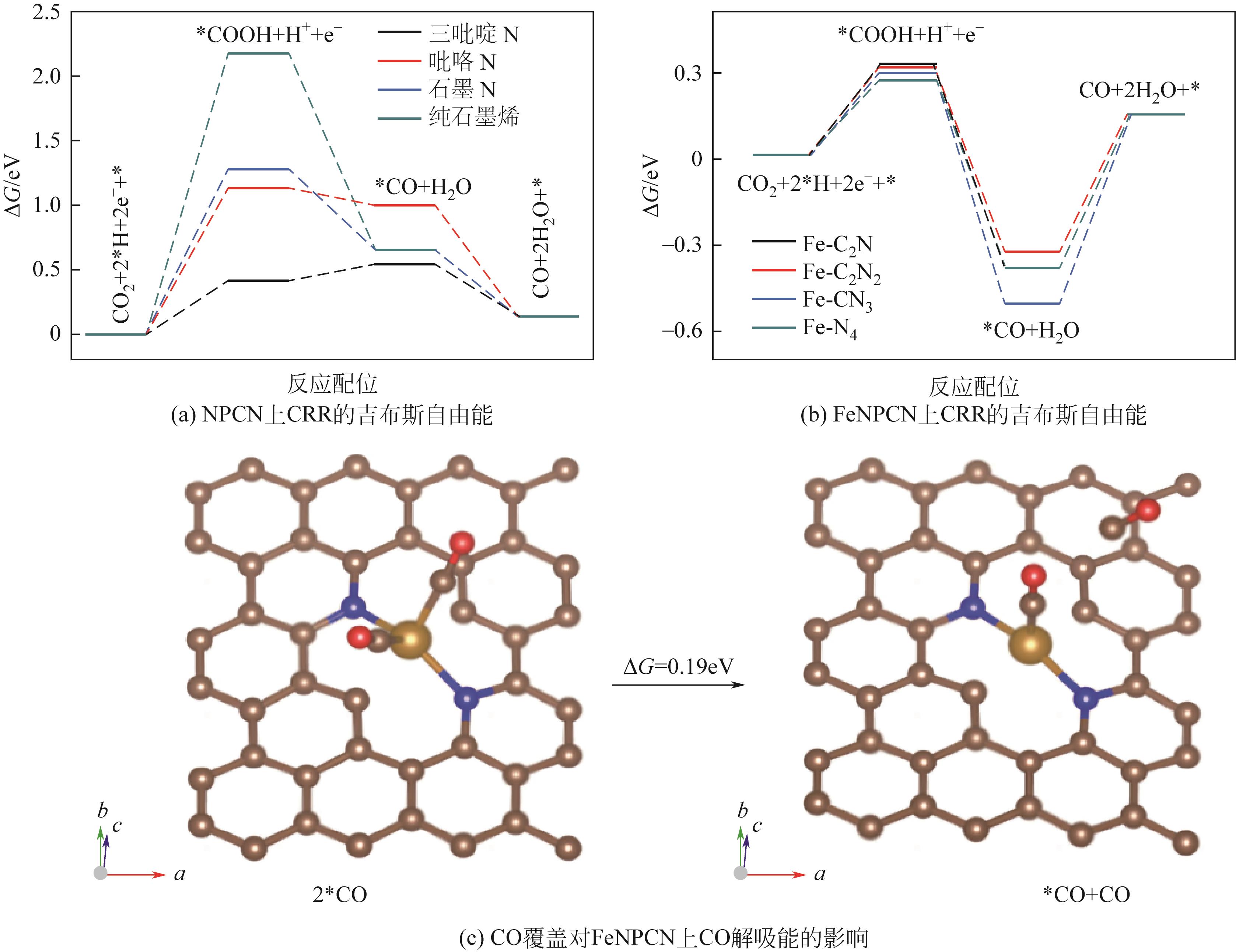
| FeNPCN | -0.5 | 3 | 0.1mol/L KHCO3 | 94 | [ |
| Ni SAs/NCNTs | -0.75 | 22 | 0.5mol/L KHCO3 | 95 | [ |
| CoPc/CNT | -0.52 | 15 | 0.1mol/L KHCO3 | 96 | [ |
| NC-900 | -0.35 | 5 | 0.5mol/L NaHCO3 | 90 | [ |
| 1D/2D NR/CS-900 | -0.45 | 16 | 0.5mol/L KHCO3 | 94.2 | [ |
| NSHPC | -0.6 | 5.58 | 0.1mol/L KHCO3 | 87.8 | [ |
| SD-AgPMRs | -0.38 | 2.3 | 0.1mol/L KHCO3 | 80 | [ |
| NP-C | -0.59 | 5.5 | 0.1mol/L KHCO3 | 81 | [ |
| BPNC | -0.5 | 3 | 0.1mol/L KHCO3 | 81.8 | [ |
表2 文中催化剂汇总
| 催化剂名称 | 过电位(vs. RHE)/V | 电流密度/mA·cm-2 | 电解质 | 法拉第效率/% | 参考文献 |
|---|---|---|---|---|---|
| Fe3+-N-C | -0.45 | 15 | 0.5mol/L KHCO3 | 90 | [ |
| Mn-NO/CNs | -0.46 | 8 | 0.5mol/L KHCO3 | 96 | [ |
| Ni-N4-C | -0.4 | 8 | 0.5mol/L KHCO3 | 90.2 | [ |
| P/Ni-0@Ni-N-C | -0.8 | 20 | 0.5mol/L KHCO3 | 91 | [ |
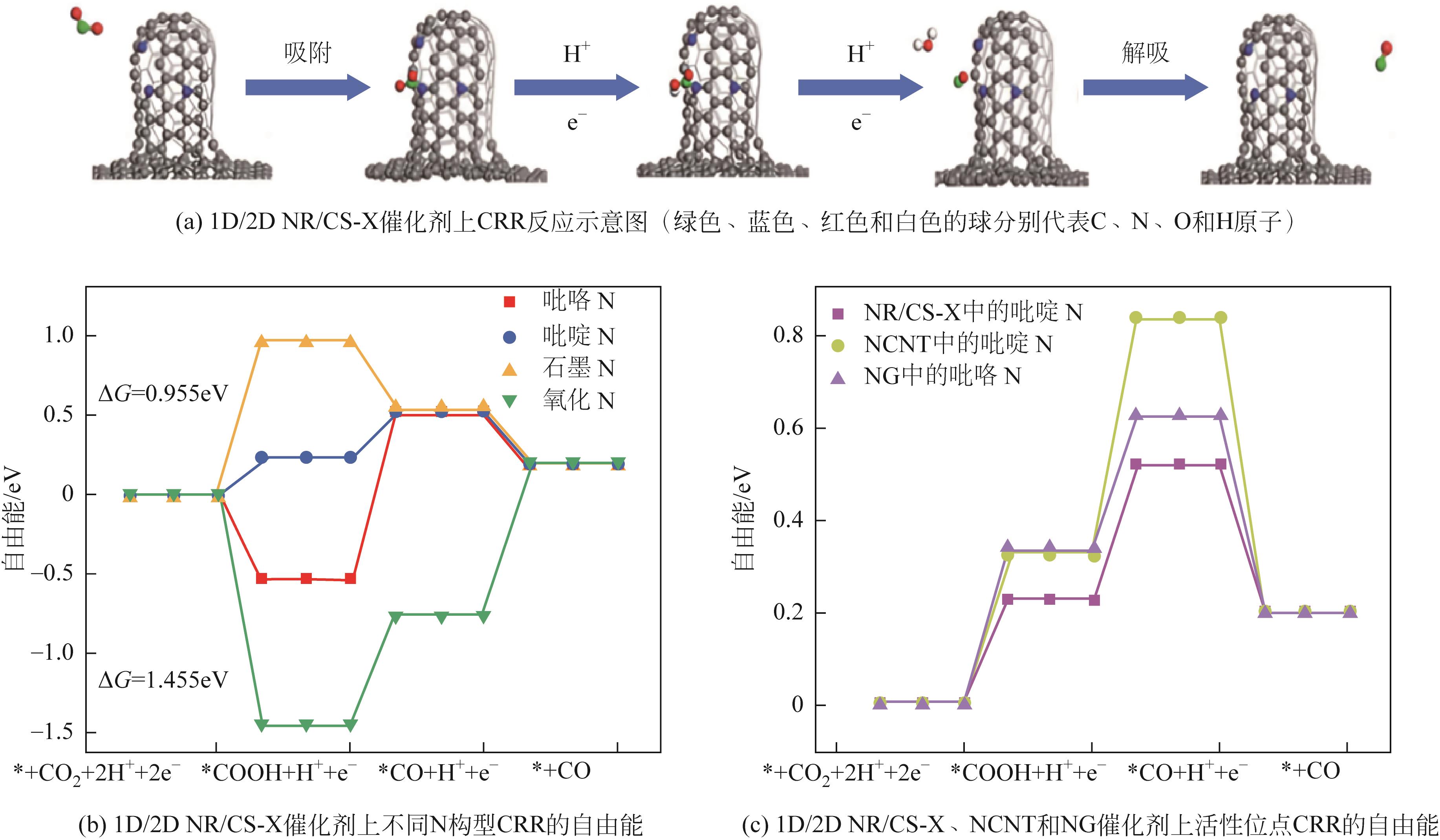
| ZnN/CNO | -0.47 | 5.5 | 0.5mol/L KHCO3 | 97 | [ |
| Fe1-Ni1-N-C | -0.5 | 7 | 0.5mol/L KHCO3 | 96.2 | [ |
| Au NWs | -0.35 | 8 | 0.5mol/L KHCO3 | 94 | [ |
| DDT-Au | -1.1 | 2.4 | 0.1mol/L KHCO3+3.4μmol/L EDTA | 40 | [ |
| ID-Ag | -0.7 | 18 | 0.5mol/L KHCO3 | 94.5 | [ |
| Ag | -0.49 | 3 | 0.1mol/L KHCO3 | 80 | [ |
| AuCu/CNT | -0.4 | 2 | 0.5mol/L KHCO3 | 95.2 | [ |
| Cu/Ni(OH)2 | -0.39 | 4.3 | 0.5mol/L NaHCO3 | 92 | [ |
| ZnCa-MOF74 | -1.9V (vs.SCE) | 4 | 0.1mol/L KHCO3 | 93 | [ |
| Zn x Cd1-x @S-胺 | -1.16 | 25 | 0.5mol/L NaHCO3 | 60 | [ |
| p-Cu@Zn(101) | -0.39 | 11.36 | 0.1mol/L KHCO3 | 85 | [ |
| FeNPCN | -0.5 | 3 | 0.1mol/L KHCO3 | 94 | [ |
| Ni SAs/NCNTs | -0.75 | 22 | 0.5mol/L KHCO3 | 95 | [ |
| CoPc/CNT | -0.52 | 15 | 0.1mol/L KHCO3 | 96 | [ |
| NC-900 | -0.35 | 5 | 0.5mol/L NaHCO3 | 90 | [ |
| 1D/2D NR/CS-900 | -0.45 | 16 | 0.5mol/L KHCO3 | 94.2 | [ |
| NSHPC | -0.6 | 5.58 | 0.1mol/L KHCO3 | 87.8 | [ |
| SD-AgPMRs | -0.38 | 2.3 | 0.1mol/L KHCO3 | 80 | [ |
| NP-C | -0.59 | 5.5 | 0.1mol/L KHCO3 | 81 | [ |
| BPNC | -0.5 | 3 | 0.1mol/L KHCO3 | 81.8 | [ |
| 1 | LI Xiang, DAMARTZIS Theodoros, STADLER Zoe, et al. Decarbonization in complex energy systems: A study on the feasibility of carbon neutrality for Switzerland in 2050[J]. Frontiers in Energy Research, 2020, 8: 549615. |
| 2 | Simelys HERNÁNDEZ, AMIN FARKHONDEHFAL M, SASTRE Francesc, et al. Syngas production from electrochemical reduction of CO2: Current status and prospective implementation[J]. Green Chemistry, 2017, 19(10): 2326-2346. |
| 3 | 王深, 吕连宏, 张保留, 等. 基于多目标模型的中国低成本碳达峰、碳中和路径[J]. 环境科学研究, 2021, 34(9): 2044-2055. |
| WANG Shen, Lianhong LÜ, ZHANG Baoliu, et al. Multi objective programming model of low-cost path for China’s peaking carbon dioxide emissions and carbon neutrality[J]. Research of Environmental Sciences, 2021, 34(9): 2044-2055. | |
| 4 | HE Yajun, CHEN Xin, HUANG Chi, et al. Encapsulation of Co single sites in covalent triazine frameworks for photocatalytic production of syngas[J]. Chinese Journal of Catalysis, 2021, 42(1): 123-130. |
| 5 | REZAEI Ebrahim, DZURYK Stephen. Techno-economic comparison of reverse water gas shift reaction to steam and dry methane reforming reactions for syngas production[J]. Chemical Engineering Research and Design, 2019, 144: 354-369. |
| 6 | MORENO-GONZÁLEZ M, BERGER Angelina, Tory BORSBOOM-HANSON, et al. Carbon-neutral fuels and chemicals: Economic analysis of renewable syngas pathways via CO2 electrolysis[J]. Energy Conversion and Management, 2021, 244: 114452. |
| 7 | JIAO Long, ZHU Juntong, ZHANG Yan, et al. Non-bonding interaction of neighboring Fe and Ni single-atom pairs on MOF-derived N-doped carbon for enhanced CO2 electroreduction[J]. Journal of the American Chemical Society, 2021, 143(46): 19417-19424. |
| 8 | WANG Yangang, LI Tian, YAO Yonggang, et al. Dramatic enhancement of CO2 photoreduction by biodegradable light-management paper[J]. Advanced Energy Materials, 2018, 8(16): 1703136. |
| 9 | APPEL Aaron M, BERCAW John E, BOCARSLY Andrew B, et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation[J]. Chemical Reviews, 2013, 113(8): 6621-6658. |
| 10 | XIA Wenjie, CHEN Rui, LI Yang, et al. Photo-driven heterogeneous microbial consortium reducing CO2 to hydrocarbons fuel[J]. Journal of Cleaner Production, 2021, 326: 129397. |
| 11 | PAN Fuping, YANG Yang. Designing CO2 reduction electrode materials by morphology and interface engineering[J]. Energy & Environmental Science, 2020, 13(8): 2275-2309. |
| 12 | CAI Bin, Alexander EYCHMÜLLER. Promoting electrocatalysis upon aerogels[J]. Advanced Materials, 2019, 31(31): e1804881. |
| 13 | XIE Huan, WANG Tanyuan, LIANG Jiashun, et al. Cu-based nanocatalysts for electrochemical reduction of CO2 [J]. Nano Today, 2018, 21: 41-54. |
| 14 | DUTTA Abhijit, MORSTEIN Carina Elisabeth, RAHAMAN Motiar, et al. Beyond copper in CO2 electrolysis: Effective hydrocarbon production on silver-nanofoam catalysts[J]. ACS Catalysis, 2018, 8(9): 8357-8368. |
| 15 | ZHANG Wenjun, HU Yi, MA Lianbo, et al. Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals[J]. Advanced Science, 2018, 5(1): 1700275. |
| 16 | AGARWAL Arun S, ZHAI Yumei, HILL Davion, et al. The electrochemical reduction of carbon dioxide to formate/formic acid: Engineering and economic feasibility[J]. ChemSusChem, 2011, 4(9): 1301-1310. |
| 17 | HORI Yoshio, WAKEBE Hidetoshi, TSUKAMOTO Toshio, et al. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media[J]. Electrochimica Acta, 1994, 39(11/12): 1833-1839. |
| 18 | ZHU Dong dong, LIU Jin long, QIAO Shi zhang. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide[J]. Advanced Materials, 2016, 28(18): 3423-3452. |
| 19 | SHEN Jing, KORTLEVER Ruud, Recep KAS, et al. Electrocatalytic reduction of carbon dioxide to carbon monoxide and methane at an immobilized cobalt protoporphyrin[J]. Nature Communications, 2015, 6: 8177. |
| 20 | BIRDJA Yuvraj Y, Elena PÉREZ-GALLENT, FIGUEIREDO Marta C, et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels[J]. Nature Energy, 2019, 4: 732-745. |
| 21 | JIN Song, HAO Zhimeng, ZHANG Kai, et al. Advances and challenges for the electrochemical reduction of CO2 to CO: From fundamentals to industrialization[J]. Angewandte Chemie International Edition, 2021, 60(38): 20627-20648. |
| 22 | ROSS Michael B, DE LUNA Phil, LI Yifan, et al. Designing materials for electrochemical carbon dioxide recycling[J]. Nature Catalysis, 2019, 2: 648-658. |
| 23 | ZHANG Lei, ZHAO Zhijian, GONG Jinlong. Nanostructured materials for heterogeneous electrocatalytic CO2 reduction and their related reaction mechanisms[J]. Angewandte Chemie International Edition, 2017, 56(38): 11326-11353. |
| 24 | PAN Fuping, LI Boyang, SARNELLO Erik, et al. Pore-edge tailoring of single-atom iron-nitrogen sites on graphene for enhanced CO2 reduction[J]. ACS Catalysis, 2020, 10(19): 10803-10811. |
| 25 | QIAO Jinli, LIU Yuyu, HONG Feng, et al. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels[J]. Chemical Society Reviews, 2014, 43(2): 631-675. |
| 26 | KORTLEVER Ruud, SHEN Jing, SCHOUTEN Klaas Jan P, et al. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide[J]. The Journal of Physical Chemistry Letters, 2015, 6(20): 4073-4082. |
| 27 | LIU Subiao, TAO Hongbiao, ZENG Li, et al. Shape-dependent electrocatalytic reduction of CO2 to CO on triangular silver nanoplates[J]. Journal of the American Chemical Society, 2017, 139(6): 2160-2163. |
| 28 | Michael Shincheon JEE, KIM Haeri, JEON Hyo Sang, et al. Stable surface oxygen on nanostructured silver for efficient CO2 electroreduction[J]. Catalysis Today, 2017, 288: 48-53. |
| 29 | DENG Yilin, HUANG Yun, REN Dan, et al. On the role of sulfur for the selective electrochemical reduction of CO2 to formate on CuS x catalysts[J]. ACS Applied Materials & Interfaces, 2018, 10(34): 28572-28581. |
| 30 | ROSEN Jonathan, HUTCHINGS Gregory S, LU Qi, et al. Mechanistic insights into the electrochemical reduction of CO2 to CO on nanostructured Ag surfaces[J]. ACS Catalysis, 2015, 5(7): 4293-4299. |
| 31 | MA Ming, TRZEŚNIEWSKI Bartek J, XIE Jie, et al. Selective and efficient reduction of carbon dioxide to carbon monoxide on oxide-derived nanostructured silver electrocatalysts[J]. Angewandte Chemie International Edition, 2016, 55(33): 9748-9752. |
| 32 | CHENG Cheng, FANG Weihai, LONG Run, et al. Water splitting with a single-atom Cu/TiO2 photocatalyst: Atomistic origin of high efficiency and proposed enhancement by spin selection[J]. JACS Au, 2021, 1(5): 550-559. |
| 33 | WANG Yuchao, XU Liang, ZHAN Longsheng, et al. Electron accumulation enables Bi efficient CO2 reduction for formate production to boost clean Zn-CO2 batteries[J]. Nano Energy, 2022, 92: 106780. |
| 34 | ZHU Chengzhou, SHI Qiurong, FENG Shuo, et al. Single-atom catalysts for electrochemical water splitting[J]. ACS Energy Letters, 2018, 3(7): 1713-1721. |
| 35 | CHEN Yuanjun, JI Shufang, CHEN Chen, et al. Single-atom catalysts: Synthetic strategies and electrochemical applications[J]. Joule, 2018, 2(7): 1242-1264. |
| 36 | JONES John, XIONG Haifeng, DELARIVA Andrew T, et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping[J]. Science, 2016, 353(6295): 150-154. |
| 37 | DENG Jiao, LI Haobo, XIAO Jianping, et al. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping[J]. Energy & Environmental Science, 2015, 8(5): 1594-1601. |
| 38 | WANG Chunhua, SHI Huimin, LIU Huaizhi, et al. Quasi-atomic-scale platinum anchored on porous titanium nitride nanorod arrays for highly efficient hydrogen evolution[J]. Electrochimica Acta, 2018, 292: 727-735. |
| 39 | YAN Chengcheng, LI Haobo, YE Yifan, et al. Coordinatively unsaturated nickel-nitrogen sites towards selective and high-rate CO2 electroreduction[J]. Energy & Environmental Science, 2018, 11(5): 1204-1210. |
| 40 | GU Jun, HSU Chia-Shuo, BAI Lichen, et al. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO[J]. Science, 2019, 364(6445): 1091-1094. |
| 41 | DONG Wenfei, ZHANG Nan, LI Sanxiu, et al. A Mn single atom catalyst with Mn-N2O2 sites integrated into carbon nanosheets for efficient electrocatalytic CO2 reduction[J]. Journal of Materials Chemistry A, 2022, 10(20): 10892-10901. |
| 42 | MOU Kaiwen, CHEN Zhipeng, ZHANG Xinxin, et al. Highly efficient electroreduction of CO2 on nickel single-atom catalysts: Atom trapping and nitrogen anchoring[J]. Small, 2019, 15(49): e1903668. |
| 43 | YE Chengyu, YU Xiaofei, LI Wencui, et al. Engineering of bifunctional nickel Phosphide@Ni-N-C catalysts for selective electroreduction of CO2-H2O to syngas[J]. Acta Physico Chimica Sinica, 2020, 38(4): 107-117. |
| 44 | HAO Zhongjing, CHEN Junxiang, ZHANG Dafeng, et al. Coupling effects of Zn single atom and high curvature supports for improved performance of CO2 reduction[J]. Science Bulletin, 2021, 66(16): 1649-1658. |
| 45 | WANG Zhongli, LI Cuiling, YAMAUCHI Yusuke. Nanostructured nonprecious metal catalysts for electrochemical reduction of carbon dioxide[J]. Nano Today, 2016, 11(3): 373-391. |
| 46 | ZHU Wenlei, MICHALSKY Ronald, Önder METIN, et al. Monodisperse Au nanoparticles for selective electrocatalytic reduction of CO2 to CO[J]. Journal of the American Chemical Society, 2013, 135(45): 16833-16836. |
| 47 | ZHU Wenlei, ZHANG Yinjia, ZHANG Hongyi, et al. Active and selective conversion of CO2 to CO on ultrathin Au nanowires[J]. Journal of the American Chemical Society, 2014, 136(46): 16132-16135. |
| 48 | SHANG Hongyu, WALLENTINE Spencer K, HOFMANN Daniel M, et al. Effect of surface ligands on gold nanocatalysts for CO2 reduction[J]. Chemical Science, 2020, 11(45): 12298-12306. |
| 49 | ZHANG Ya, JI Lei, QIU Weibin, et al. Iodide-derived nanostructured silver promotes selective and efficient carbon dioxide conversion into carbon monoxide[J]. Chemical Communications, 2018, 54(21): 2666-2669. |
| 50 | MAURICE V, KLEIN L H, H-H STREHBLOW, et al. In situ STM study of the surface structure, dissolution, and early stages of electrochemical oxidation of the Ag(111) electrode[J]. The Journal of Physical Chemistry C, 2007, 111(44): 16351-16361. |
| 51 | CHEN Hao, LI Zihao, ZHANG Zihao, et al. Synthesis of composition-tunable syngas from efficiently electrochemical conversion of CO2 over AuCu/CNT bimetallic catalyst[J]. Industrial & Engineering Chemistry Research, 2019, 58(34): 15425-15431. |
| 52 | LIU Anmin, GAO Mengfan, REN Xuefeng, et al. Current progress in electrocatalytic carbon dioxide reduction to fuels on heterogeneous catalysts[J]. Journal of Materials Chemistry A, 2020, 8(7): 3541-3562. |
| 53 | DAI Lei, QIN Qing, WANG Pei, et al. Ultrastable atomic copper nanosheets for selective electrochemical reduction of carbon dioxide[J]. Science Advances, 2017, 3(9): e1701069. |
| 54 | VAN PHUC Tran, KANG Sung Gu, CHUNG Jin Suk, et al. Highly CO selective Ca and Zn hybrid metal-organic framework electrocatalyst for the electrochemical reduction of CO2 [J]. Current Applied Physics, 2021, 27: 31-37. |
| 55 | BHUGUN Iqbal, LEXA Doris, Jean-Michel SAVÉANT. Catalysis of the electrochemical reduction of carbon dioxide by iron(0) porphyrins. Synergistic effect of Lewis acid cations[J]. The Journal of Physical Chemistry, 1996, 100(51): 19981-19985. |
| 56 | KARLSEN Elly J, NYGREN Martin A, PETTERSSON Lars G M. Comparative study on structures and energetics of NO x, SO x, and CO x adsorption on alkaline-earth-metal oxides[J]. The Journal of Physical Chemistry B, 2003, 107(31): 7795-7802. |
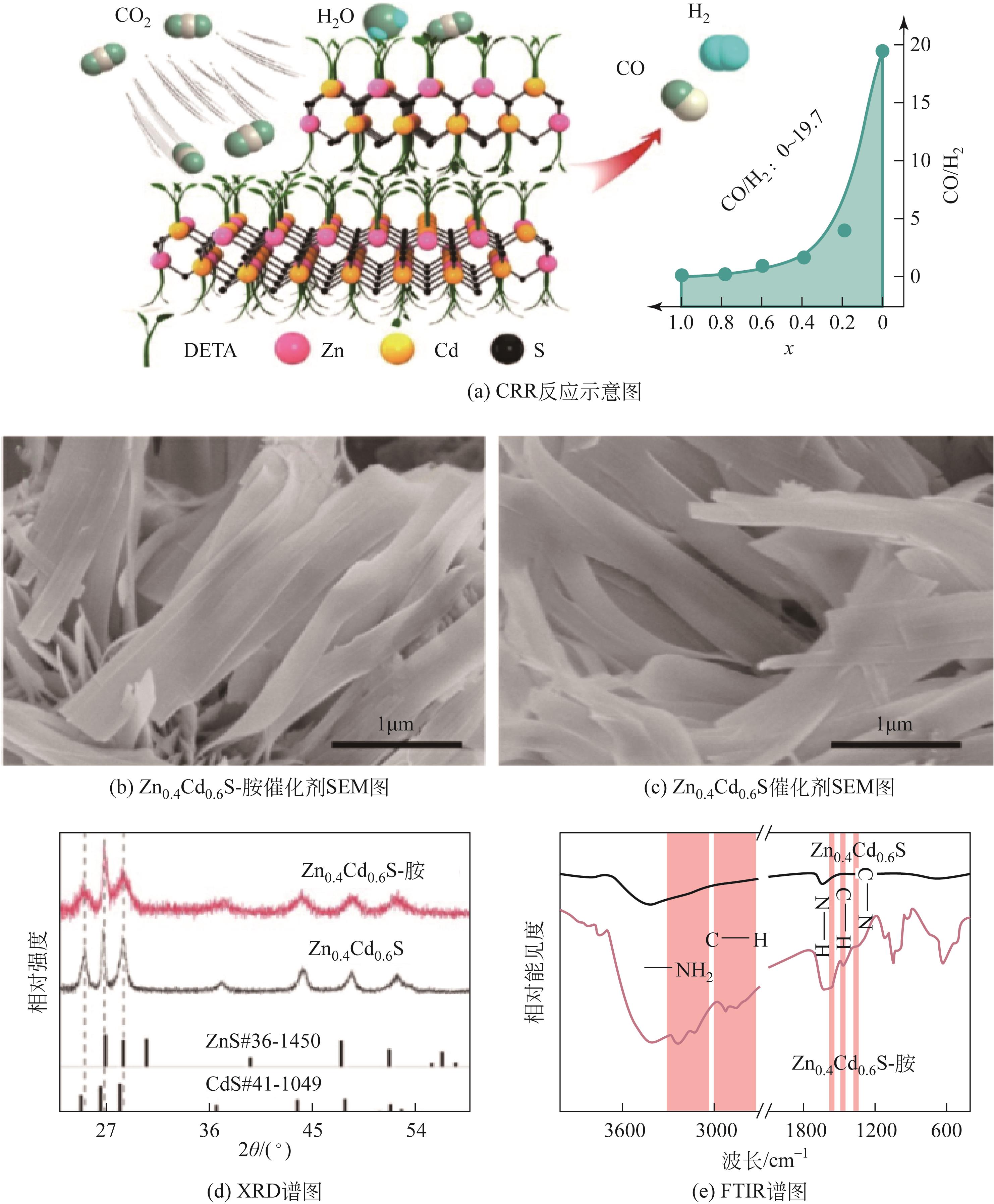
| 57 | MENG Nannan, LIU Cuibo, LIU Yang, et al. Efficient electrosynthesis of syngas with tunable CO/H2 ratios over Zn x Cd1– x S-amine inorganic-organic hybrids[J]. Angewandte Chemie International Edition, 2019, 58(52): 18908-18912. |
| 58 | QIN Binhao, LI Yuhang, FU Hongquan, et al. Electrochemical reduction of CO2 into tunable syngas production by regulating the crystal facets of earth-abundant Zn catalyst[J]. ACS Applied Materials & Interfaces, 2018, 10(24): 20530-20539. |
| 59 | XIONG Bo, YANG Yingju, LIU Jing, et al. Crystal orientation effects on the electrochemical conversion of CO2 to syngas over Cu-M (M=Ag, Ni, Zn, Cd, and Pd) bimetal catalysts[J]. Applied Surface Science, 2021, 567: 150839. |
| 60 | WU Zhiyi, IQBAL Zafar, WANG Xianqin. Metal-free, carbon-based catalysts for oxygen reduction reactions[J]. Frontiers of Chemical Science and Engineering, 2015, 9(3): 280-294. |
| 61 | WU Jingjie, LIU Mingjie, SHARMA Pranav P, et al. Incorporation of nitrogen defects for efficient reduction of CO2 via two-electron pathway on three-dimensional graphene foam[J]. Nano Letters, 2016, 16(1): 466-470. |
| 62 | SONG Yanfang, CHEN Wei, ZHAO Chengcheng, et al. Metal-free nitrogen-doped mesoporous carbon for electroreduction of CO2 to ethanol[J]. Angewandte Chemie International Edition, 2017, 56(36): 10840-10844. |
| 63 | LIU Yanming, CHEN Shuo, QUAN Xie, et al. Efficient electrochemical reduction of carbon dioxide to acetate on nitrogen-doped nanodiamond[J]. Journal of the American Chemical Society, 2015, 137(36): 11631-11636. |
| 64 | KUMAR Bijandra, ASADI Mohammad, PISASALE Davide, et al. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction[J]. Nature Communications, 2013, 4: 2819. |
| 65 | SHARMA Pranav P, WU Jingjie, YADAV Ram Manohar, et al. Nitrogen-doped carbon nanotube arrays for high-efficiency electrochemical reduction of CO2: On the understanding of defects, defect density, and selectivity[J]. Angewandte Chemie International Edition, 2015, 54(46): 13701-13705. |
| 66 | WANG Wei, BORSE Rahul Anil, XIE Jiafang, et al. Spontaneously producing syngas from MFC-MEC coupling system based on biocompatible bifunctional metal-free electrocatalyst[J]. Science China Materials, 2021, 64(3): 592-600. |
| 67 | GONG Kuanping, DU Feng, XIA Zhenhai, et al. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction[J]. Science, 2009, 323(5915): 760-764. |
| 68 | WANG Xingpu, LI Xueyan, DING Shaosong, et al. Constructing ample active sites in nitrogen-doped carbon materials for efficient electrocatalytic carbon dioxide reduction[J]. Nano Energy, 2021, 90: 106541. |
| 69 | WANG Yue, ZHANG Chen, LI Xinjian, et al. Metal-free carbon-based nanomaterials for electrochemical nitrogen and carbon dioxide reductions[J]. Materials Research Bulletin, 2021, 140: 111294. |
| 70 | ZHANG Zheng, YU Liang, TU Yunchuan, et al. Unveiling the active site of metal-free nitrogen-doped carbon for electrocatalytic carbon dioxide reduction[J]. Cell Reports Physical Science, 2020, 1(8): 100145. |
| 71 | ZHOU Wenyang, SHEN Haoming, WANG Qian, et al. N-doped peanut-shaped carbon nanotubes for efficient CO2 electrocatalytic reduction[J]. Carbon, 2019, 152: 241-246. |
| 72 | LI Fengwang, XUE Mianqi, KNOWLES Gregory P, et al. Porous nitrogen-doped carbon derived from biomass for electrocatalytic reduction of CO2 to CO[J]. Electrochimica Acta, 2017, 245: 561-568. |
| 73 | HAO Xiaoqiong, AN Xiaowei, PATIL Amar M, et al. Biomass-derived N-doped carbon for efficient electrocatalytic CO2 reduction to CO and Zn-CO2 batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(3): 3738-3747. |
| 74 | MUKHERJEE Shreya, CULLEN David A, KARAKALOS Stavros, et al. Metal-organic framework-derived nitrogen-doped highly disordered carbon for electrochemical ammonia synthesis using N2 and H2O in alkaline electrolytes[J]. Nano Energy, 2018, 48: 217-226. |
| 75 | WANG Riming, SUN Xiaohui, Samy OULD-CHIKH, et al. Metal-organic-framework-mediated nitrogen-doped carbon for CO2 electrochemical reduction[J]. ACS Applied Materials & Interfaces, 2018, 10(17): 14751-14758. |
| 76 | ZHU Ying, LV Kuilin, WANG Xingpu, et al. 1D/2D nitrogen-doped carbon nanorod arrays/ultrathin carbon nanosheets: Outstanding catalysts for the highly efficient electroreduction of CO2 to CO[J]. Journal of Materials Chemistry A, 2019, 7(24): 14895-14903. |
| 77 | YE Lin, YING Yiran, SUN Dengrong, et al. Highly efficient porous carbon electrocatalyst with controllable N-species content for selective CO2 reduction[J]. Angewandte Chemie International Edition, 2020, 59(8): 3244-3251. |
| 78 | NING Hui, GUO Dianliang, WANG Xiaoshan, et al. Efficient CO2 electroreduction over N-doped hieratically porous carbon derived from petroleum pitch[J]. Journal of Energy Chemistry, 2021, 56: 113-120. |
| 79 | LI Yao, LIU Nan, ZHANG Tao, et al. Highly microporous nitrogen-doped carbons from anthracite for effective CO2 capture and CO2/CH4 separation[J]. Energy, 2020, 211: 118561. |
| 80 | LIU Song, YANG Hongbin, HUANG Xiang, et al. Identifying active sites of nitrogen-doped carbon materials for the CO2 reduction reaction[J]. Advanced Functional Materials, 2018, 28(21): 1800499. |
| 81 | ZHONG Haixia, MENG Fanlu, ZHANG Qi, et al. Highly efficient and selective CO2 electro-reduction with atomic Fe-C-N hybrid coordination on porous carbon nematosphere[J]. Nano Research, 2019, 12(9): 2318-2323. |
| 82 | LU Peilong, YANG Yijun, YAO Jiannian, et al. Facile synthesis of single-nickel-atomic dispersed N-doped carbon framework for efficient electrochemical CO2 reduction[J]. Applied Catalysis B: Environmental, 2019, 241: 113-119. |
| 83 | ZHANG Xing, WU Zishan, ZHANG Xiao, et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures[J]. Nature Communications, 2017, 8: 14675. |
| 84 | YAO Pengfei, QIU Yanling, ZHANG Taotao, et al. N-doped nanoporous carbon from biomass as a highly efficient electrocatalyst for the CO2 reduction reaction[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(5): 5249-5255. |
| 85 | LI Ruru, LIU Feng, ZHANG Yihao, et al. Nitrogen, sulfur co-doped hierarchically porous carbon as a metal-free electrocatalyst for oxygen reduction and carbon dioxide reduction reaction[J]. ACS Applied Materials & Interfaces, 2020, 12(40): 44578-44587. |
| 86 | YANG Jinman, DU Huishuang, YU Qing, et al. Porous silver microrods by plasma vulcanization activation for enhanced electrocatalytic carbon dioxide reduction[J]. Journal of Colloid and Interface Science, 2022, 606(Pt 1): 793-799. |
| 87 | LIU Tianfu, Sajjad ALI, LIAN Zan, et al. Phosphorus-doped onion-like carbon for CO2 electrochemical reduction: The decisive role of the bonding configuration of phosphorus[J]. Journal of Materials Chemistry A, 2018, 6(41): 19998-20004. |
| 88 | HAN Juan, DENG Ximing, CHEN Keyu, et al. Electrochemical conversion of CO2 into tunable syngas on a B, P, N tri-doped carbon[J]. Renewable Energy, 2021, 177: 636-642. |
| 89 | FENG Caixia, ZHANG Xiaodong, JIN Huige, et al. Integrating carbon vacancy modified carbon quantum dots with carbon nitride for efficient photocatalytic CO2 reduction to syngas with tunable hydrogen to carbon monoxide ratio[J]. Carbon, 2023, 203: 671-685. |
| 90 | WEI Bing, HAO Jinhui, GE Baoxin, et al. Highly efficient electrochemical carbon dioxide reduction to syngas with tunable ratios over pyridinic-nitrogen rich ultrathin carbon nanosheets[J]. Journal of Colloid and Interface Science, 2022, 608(Pt 3): 2650-2659. |
| 91 | STANLEY Philip M, SU Alice Y, RAMM Vanessa, et al. Photocatalytic CO2-to-syngas evolution with molecular catalyst metal-organic framework nanozymes[J]. Advanced Materials, 2023, 35(6): e2207380. |
| 92 | JIANG Yuhang, WANG Yating, CHEN Rongzhen, et al. Minireview and perspectives of bimetallic metal-organic framework electrocatalysts for carbon dioxide reduction[J]. Energy & Fuels, 2023, 37(23): 17951-17965. |
| 93 | MIN Zhaojun, CHANG Bing, SHAO Chunfeng, et al. Enhancing CO2 electroreduction to syngas by active protons of imidazolium ionic liquids: From performance to mechanism[J]. Applied Catalysis B: Environmental, 2023, 326: 122185. |
| 94 | ALIPOUR Zahra, BABU BORUGADDA Venu, WANG Hui, et al. Syngas production through dry reforming: A review on catalysts and their materials, preparation methods and reactor type[J]. Chemical Engineering Journal, 2023, 452: 139416. |
| 95 | POUREBRAHIMI Sina, PIROOZ Majid, AHMADI Shabnam, et al. Nanoengineering of metal-based electrocatalysts for carbon dioxide (CO2) reduction: A critical review[J]. Materials Today Physics, 2023, 38: 101250. |
| 96 | LIU Guangbo, YANG Guohui, PENG Xiaobo, et al. Recent advances in the routes and catalysts for ethanol synthesis from syngas[J]. Chemical Society Reviews, 2022, 51(13): 5606-5659. |
| 97 | TIMOFEEVA S S, KARAEVA J V, KOVALEV A A, et al. Steam gasification of digestate after anaerobic digestion and dark fermentation of lignocellulosic biomass to produce syngas with high hydrogen content[J]. International Journal of Hydrogen Energy, 2023, 48(21): 7559-7568. |
| 98 | SU Bo, ZHENG Mei, LIN Wei, et al. S-scheme Co9S8@Cd0.8Zn0.2S-DETA hierarchical nanocages bearing organic CO2 activators for photocatalytic syngas production[J]. Advanced Energy Materials, 2023, 13(15): 2203290. |
| 99 | CHEN Huimin, WANG Min, FAN Kaicai, et al. One stone three birds: An aqueous Mg-CO2 battery for generation of electricity, syngas, and high-value phosphate[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(7): 3123-3132. |
| 100 | WANG Linjie, LUO Zichang, FENG Sitong, et al. Synthesis of MOF-derived hybrids for efficient electrocatalytic reduction of CO2 to syngas[J]. Catalysis Letters, 2023, 153(5): 1527-1535. |
| [1] | 王嘉锐, 刘大伟, 邓耀, 徐瑾, 马晓迅, 徐龙. 载氧体在甲烷化学链重整反应中的研究进展[J]. 化工进展, 2024, 43(5): 2235-2253. |
| [2] | 周安宁, 江雨寒, 刘墨宣, 赵伟, 李振. 电解煤浆制氢过程中煤阶及矿物的影响与煤结构演化研究进展[J]. 化工进展, 2024, 43(5): 2294-2310. |
| [3] | 高凡翔, 刘阳, 张贵泉, 秦锋, 姚建涛, 金辉, 师进文. 燃煤烟气湿法协同脱硫脱碳技术研究进展[J]. 化工进展, 2024, 43(5): 2324-2342. |
| [4] | 吴达, 蒋淑娇, 魏强, 袁胜华, 杨刚, 张成. 能源转型中渣油高效利用技术的研究进展[J]. 化工进展, 2024, 43(5): 2343-2353. |
| [5] | 江安迪, 丁雪兴, 王世鹏, 丁俊华, 力宁. 超临界CO2干气密封热动力学性能研究进展[J]. 化工进展, 2024, 43(5): 2354-2369. |
| [6] | 桂鑫, 陈汇勇, 白柏杨, 贾永梁, 马晓迅. Mo掺杂改性NiC/Al-MCM-41的芘催化加氢性能[J]. 化工进展, 2024, 43(5): 2386-2395. |
| [7] | 丁思佳, 蒋淑娇, 杨占林, 彭绍忠, 蒋乾民. 基于氮化物结构与加氢行为关系设计重油加氢脱氮催化剂[J]. 化工进展, 2024, 43(5): 2436-2448. |
| [8] | 李思, 陶艺月, 肖振翀, 张亮, 李俊, 朱恂, 廖强. 热再生电池堆-二氧化碳电化学还原池系统耦合特性[J]. 化工进展, 2024, 43(5): 2568-2575. |
| [9] | 王欣宇, 王超, 张梦娟, 刘方正, 李晗旸, 王正林, 贾鑫, 宋兴飞, 许光文, 韩振南. 松木颗粒流态化两段气化制备清洁燃气的工艺稳定性验证[J]. 化工进展, 2024, 43(5): 2576-2586. |
| [10] | 段翔, 田野, 董文威, 宋松, 李新刚. 苯酐合成的反应网络及催化反应机制研究现状与展望[J]. 化工进展, 2024, 43(5): 2587-2599. |
| [11] | 方峣, 刘雷, 高志华, 黄伟, 左志军. 光辅助直接甲醇燃料电池阳极催化剂的研究进展[J]. 化工进展, 2024, 43(5): 2611-2628. |
| [12] | 张金鹏, 屈婷, 荆洁颖, 李文英. 吸附强化水气变换制氢复合催化剂研究进展[J]. 化工进展, 2024, 43(5): 2629-2644. |
| [13] | 刘思宇, 杨卷, 陈培, 陈祖田, 闫斌, 刘育红, 邱介山. 富氮多孔碳纳米片的氮掺杂构型调控及其储锌性能[J]. 化工进展, 2024, 43(5): 2673-2683. |
| [14] | 李娜, 赵婉彤, 凌丽霞, 王宝俊, 章日光. RhCu催化剂中限域环境调控合成气转化生成CH x 反应性能[J]. 化工进展, 2024, 43(5): 2684-2695. |
| [15] | 冯勇强, 王洁茹, 王超娴, 李芳, 苏婉婷, 孙宇, 赵彬然. γ-Al2O3 负载的Ni、Fe、Cu对介质阻挡放电等离子体转化CO2/CH4的影响[J]. 化工进展, 2024, 43(5): 2705-2713. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||