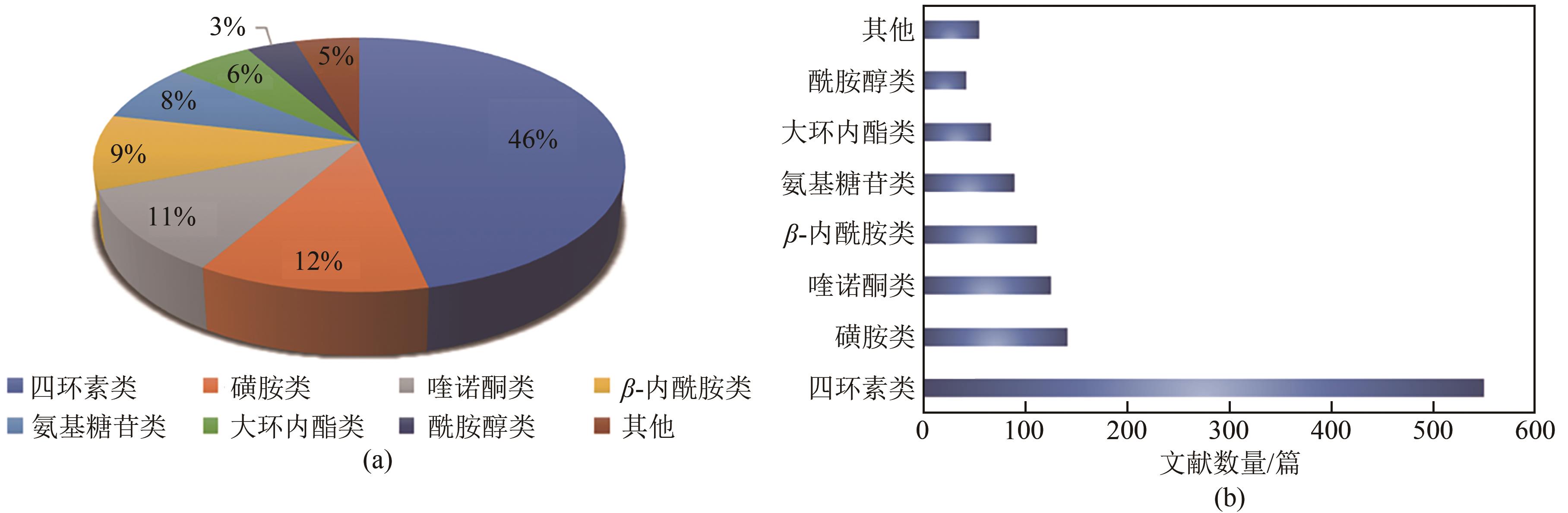
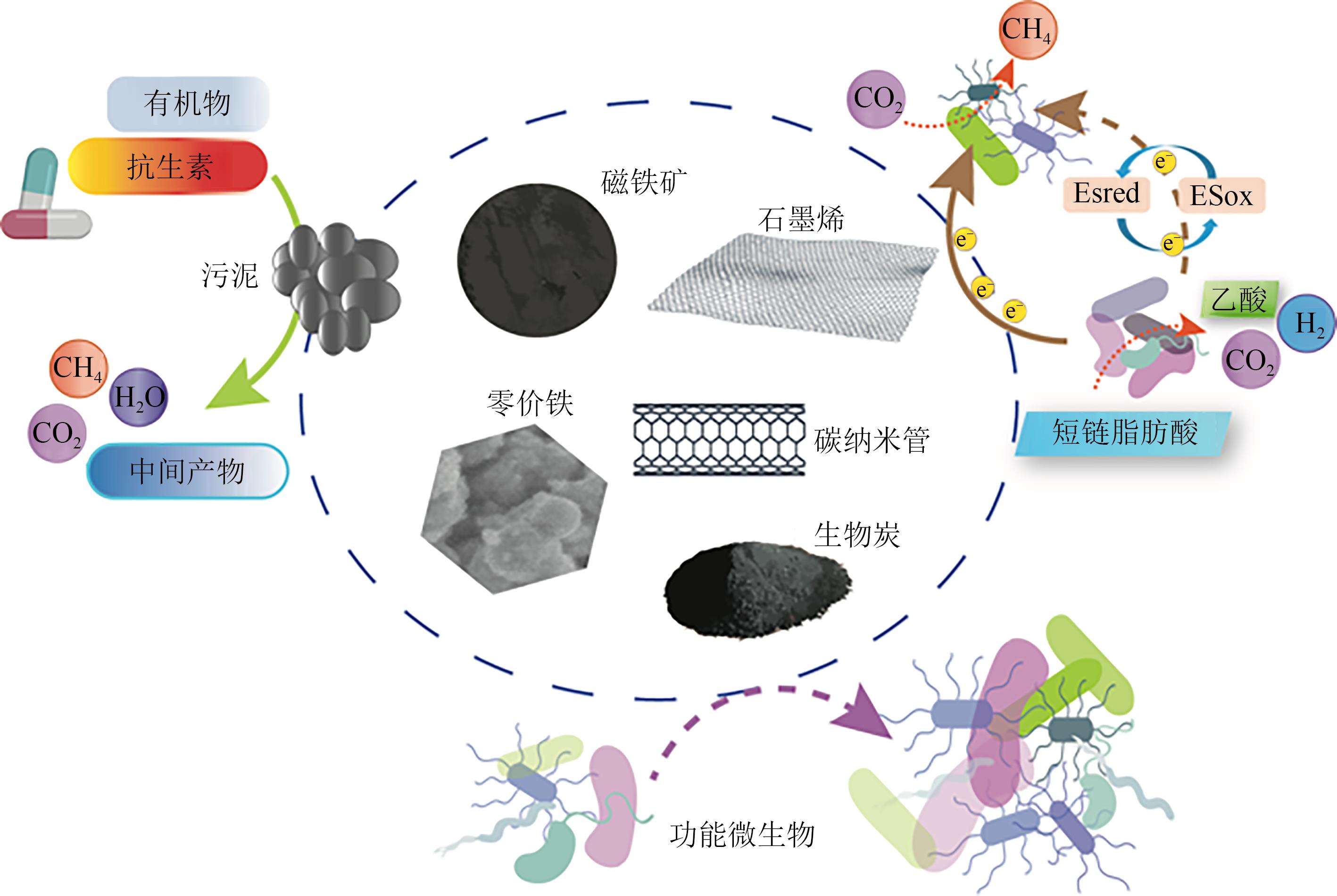
化工进展 ›› 2023, Vol. 42 ›› Issue (2): 1008-1019.DOI: 10.16085/j.issn.1000-6613.2022-0707
基于导电材料强化抗生素胁迫厌氧消化的研究进展
祝佳欣1( ), 朱雯喆1, 徐俊1, 谢靖1, 王文标2, 谢丽1(
), 朱雯喆1, 徐俊1, 谢靖1, 王文标2, 谢丽1( )
)
- 1.长江水环境教育部重点实验室,同济大学环境科学与工程学院,上海 200092
2.上海泓济环保科技股份有限公司,上海 200082
-
收稿日期:2022-04-20修回日期:2022-05-30出版日期:2023-02-25发布日期:2023-03-13 -
通讯作者:谢丽 -
作者简介:祝佳欣(1998—),女,硕士研究生,研究方向为厌氧生物处理技术。E-mail:2030580@tongji.edu.cn。 -
基金资助:国家自然科学基金(51978487)
Enhancement of anaerobic digestion under antibiotics stress via conductive materials application: A review
ZHU Jiaxin1( ), ZHU Wenzhe1, XU Jun1, XIE Jing1, WANG Wenbiao2, XIE Li1(
), ZHU Wenzhe1, XU Jun1, XIE Jing1, WANG Wenbiao2, XIE Li1( )
)
- 1.The Yangtze River Water Environment Key Laboratory of the Ministry of Education, College of Environmental Science and Engineering, Tongji University, Shanghai 200092, China
2.Shanghai Honess Environmental Technology Co. , Ltd. , Shanghai 200082, China
-
Received:2022-04-20Revised:2022-05-30Online:2023-02-25Published:2023-03-13 -
Contact:XIE Li
摘要:
厌氧消化是处理含抗生素有机废物的常用技术手段,但高浓度抗生素会抑制厌氧微生物菌群活性,从而干扰厌氧消化效能和抗生素自身降解效率。近年来,导电材料强化含抗生素有机废物厌氧消化取得了良好效果,有机废物资源回收效率得到进一步提升。本文从抗生素使用现状和对厌氧消化代谢过程的影响出发,讨论了抗生素在厌氧消化中的迁移转化机制,重点阐述了铁基和碳基导电材料在抗生素胁迫厌氧消化系统中的应用及生化作用机理。研究表明:通过富集功能性微生物、强化微生物种间电子传递以及削减厌氧消化系统中的抗生素和抗生素抗性基因,导电材料可以提升厌氧消化产甲烷效能、降低抗生素污染的环境风险。最后,从构建生物信息网络、开发优化新型材料和处理多元污染物方面对导电材料强化技术的发展方向进行了展望。
中图分类号:
引用本文
祝佳欣, 朱雯喆, 徐俊, 谢靖, 王文标, 谢丽. 基于导电材料强化抗生素胁迫厌氧消化的研究进展[J]. 化工进展, 2023, 42(2): 1008-1019.
ZHU Jiaxin, ZHU Wenzhe, XU Jun, XIE Jing, WANG Wenbiao, XIE Li. Enhancement of anaerobic digestion under antibiotics stress via conductive materials application: A review[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1008-1019.
| 抗生种类 | 结构特点 | 抗菌机理 | 主要用途 | |||
|---|---|---|---|---|---|---|
| 临床 | 养殖 | 农业 | ||||
| β-内酰胺类 | ||||||
| 青霉素 | 含有β-内酯胺环 | 抑制细胞壁中黏肽合成酶,对动物毒性低 | √ | √ | ||
| 头孢菌素 | √ | √ | ||||
| 四环素类 | ||||||
| 金霉素 | 具有并四苯基 | 抑制蛋白质合成,阻断肽链的延长 | √ | √ | ||
| 土霉素 | √ | √ | √ | |||
| 强力霉素 | √ | √ | ||||
| 四环素 | √ | √ | ||||
| 喹诺酮类 | ||||||
| 环丙沙星 | 具有相似4-喹诺酮母核 | 抑制细菌DNA旋转酶或拓扑异构酶活性 | √ | |||
| 诺氟沙星 | √ | √ | ||||
| 恩诺沙星 | √ | √ | ||||
| 氧氟沙星 | √ | |||||
| 大环内酯类 | ||||||
| 阿奇霉素 | 具有不少于12个碳原子的内酯环 | 核糖体水平上抑制细菌蛋白质合成 | √ | √ | ||
| 红霉素 | √ | √ | ||||
| 克拉霉素 | √ | |||||
| 罗红霉素 | √ | √ | ||||
| 磺胺类 | ||||||
| 磺胺嘧啶 | 具有对氨基苯磺酰胺结构 | 阻止细菌的二氢叶酸合成 | √ | √ | ||
| 磺胺甲𫫇唑 | √ | √ | ||||
| 磺胺甲嘧啶 | √ | √ | ||||
| 磺胺二甲嘧啶 | √ | √ | ||||
| 林可酰胺类 | ||||||
| 林可霉素 | 含有氨基酸和糖苷部分,并通过肽键相连 | 与50S核糖体亚基结合,阻止原核翻译 | √ | √ | ||
| 克林霉素 | √ | |||||
| 多肽类 | ||||||
| 黏杆菌素 | 由肽键将各氨基酸结合成锁链状 | 抑制细菌的生长和繁殖 | √ | √ | ||
| 杆菌肽 | √ | √ | ||||
| 酰胺醇类 | ||||||
| 氯霉素 | 由对硝基苯基、丙二醇与二氯乙酰胺组成 | 抑制细菌70S核糖体与50S亚基结合及肽酰基转移酶和肽链延伸,干扰蛋白质合成 | √ | √ | ||
| 甲砜霉素 | √ | |||||
| 氨基糖苷类 | ||||||
| 链霉素 | 由氨基糖与氨基环醇组成 | 抑制蛋白质合成,对厌氧菌不具有抗菌作用 | √ | √ | ||
| 卡那霉素 | √ | |||||
| 庆大霉素 | √ | √ | ||||
| 新霉素 | √ | √ | ||||
表1 常见抗生素的分类、结构特点、抗菌机理及用途[2, 8-10, 16-19]
| 抗生种类 | 结构特点 | 抗菌机理 | 主要用途 | |||
|---|---|---|---|---|---|---|
| 临床 | 养殖 | 农业 | ||||
| β-内酰胺类 | ||||||
| 青霉素 | 含有β-内酯胺环 | 抑制细胞壁中黏肽合成酶,对动物毒性低 | √ | √ | ||
| 头孢菌素 | √ | √ | ||||
| 四环素类 | ||||||
| 金霉素 | 具有并四苯基 | 抑制蛋白质合成,阻断肽链的延长 | √ | √ | ||
| 土霉素 | √ | √ | √ | |||
| 强力霉素 | √ | √ | ||||
| 四环素 | √ | √ | ||||
| 喹诺酮类 | ||||||
| 环丙沙星 | 具有相似4-喹诺酮母核 | 抑制细菌DNA旋转酶或拓扑异构酶活性 | √ | |||
| 诺氟沙星 | √ | √ | ||||
| 恩诺沙星 | √ | √ | ||||
| 氧氟沙星 | √ | |||||
| 大环内酯类 | ||||||
| 阿奇霉素 | 具有不少于12个碳原子的内酯环 | 核糖体水平上抑制细菌蛋白质合成 | √ | √ | ||
| 红霉素 | √ | √ | ||||
| 克拉霉素 | √ | |||||
| 罗红霉素 | √ | √ | ||||
| 磺胺类 | ||||||
| 磺胺嘧啶 | 具有对氨基苯磺酰胺结构 | 阻止细菌的二氢叶酸合成 | √ | √ | ||
| 磺胺甲𫫇唑 | √ | √ | ||||
| 磺胺甲嘧啶 | √ | √ | ||||
| 磺胺二甲嘧啶 | √ | √ | ||||
| 林可酰胺类 | ||||||
| 林可霉素 | 含有氨基酸和糖苷部分,并通过肽键相连 | 与50S核糖体亚基结合,阻止原核翻译 | √ | √ | ||
| 克林霉素 | √ | |||||
| 多肽类 | ||||||
| 黏杆菌素 | 由肽键将各氨基酸结合成锁链状 | 抑制细菌的生长和繁殖 | √ | √ | ||
| 杆菌肽 | √ | √ | ||||
| 酰胺醇类 | ||||||
| 氯霉素 | 由对硝基苯基、丙二醇与二氯乙酰胺组成 | 抑制细菌70S核糖体与50S亚基结合及肽酰基转移酶和肽链延伸,干扰蛋白质合成 | √ | √ | ||
| 甲砜霉素 | √ | |||||
| 氨基糖苷类 | ||||||
| 链霉素 | 由氨基糖与氨基环醇组成 | 抑制蛋白质合成,对厌氧菌不具有抗菌作用 | √ | √ | ||
| 卡那霉素 | √ | |||||
| 庆大霉素 | √ | √ | ||||
| 新霉素 | √ | √ | ||||
| 种类 | 浓度 | 底物 | 甲烷产量/% | 挥发性脂肪酸变化情况 | 微生物相对丰度变化 | 参考文献 | |
|---|---|---|---|---|---|---|---|
| 四环素类 | |||||||
| 四环素 | 20mg/L | 合成废水 | -31.3 | 丙酸累积浓度260mg/L | Porphyromonas pogonae③(+5.6%)、 Proteiniphilum acetatigenes③(+0.3%)、 Syntrophobacter wolinii④(-0.09%)、 Methanomassiliicoccus⑥(+0.1%) | [ | |
| 金霉素 | 28mg/L | 猪粪 | -27.8 | TVFA①产量+88.8% | Methanosaetaceae⑤、 Methanosarcinaceae spp.⑦减少 | [ | |
| 氯四环素 | 40mg/L | 丁酸钠 | -40.4 | 丁酸降解速率-44.2% | Syntrophomonas④表现出耐受性、Tepidanaerobacter④被抑制 | [ | |
| 大环内酯类 | |||||||
| 红霉素 | 25mg/L | 污泥 | -40.6 | 乙酸、丙酸和丁酸分别累积0.9倍、0.3倍和2.5倍 | —② | [ | |
| 红霉素 | 500mg/L | 红霉素 | +13.0 | — | Sedimentibacter③(+33%)、 Methanosarcina⑦(+2800%) | [ | |
| 克拉霉素 | 1000mg/ kg TSS | 污泥 | — | 乙酸、丙酸和丁酸降解速率分别减少-28.0%、9.3%和-15.5% | 水解发酵菌+0.64%、 Gammaproteobacteria④(-2.1%) | [ | |
| 林可酰胺类 | |||||||
| 林可霉素(LCM) | 25mg/L | 污泥 | -29.4 | 丁酸累积6.2倍 | — | [ | |
| 1000mg/L | 葡萄糖 | +20.8 | LCM浓度≥200mg/L,乙酸和丙酸显着积累 | — | [ | ||
| 磺胺类 | |||||||
| 磺胺甲𫫇唑(SMX) | 25mg/L | 短链 脂肪酸 | -35.8 | 当SMX由250mg/L增加到1000mg/L,丙酸和丁酸利用率 分别减少41.0%和30.3% | — | [ | |
| 磺胺二甲嘧啶 | 500mg/kg | 污泥 | +5.0~9.1 | — | Methanosaetaceae⑤(-9.6%)、Methanosarcinaceae⑦(-19.0%) | [ | |
| 喹诺酮类 | |||||||
| 环丙沙星(CIP) | 0.5~50mg/L | 葡萄糖、肉提取物、蛋白胨 | -4.8~32.0 | 50mg/L CIP出现丙酸积累 | Syntrophobacter④、Methanosaeta⑤减少 | [ | |
| 诺氟沙星 | 0.5mmol/L | — | -77.4 | 理论总挥发性脂肪酸(TVFA) -61.2% | Methanosaeta⑤(-22.7%)、 Methanobacterium⑥(-1.3%)、 Methanoculleus⑥(+10.4%) | [ | |
| 诺氟沙星 | 500mg/kg | 污泥 | -11.3 | — | Methanosaetaceae⑤(-9.4%)、 Methanosarcinaceae⑦(-39.9%) | [ | |
| 恩诺沙星 | 8mg/kg TS | 鸡粪 | +15.3 | TVFA+5.9% | Methanosaeta⑤(-12.6%)、 Methanobrevibacter⑥(+48.6%) | [ | |
表2 抗生素对厌氧消化效能的影响
| 种类 | 浓度 | 底物 | 甲烷产量/% | 挥发性脂肪酸变化情况 | 微生物相对丰度变化 | 参考文献 | |
|---|---|---|---|---|---|---|---|
| 四环素类 | |||||||
| 四环素 | 20mg/L | 合成废水 | -31.3 | 丙酸累积浓度260mg/L | Porphyromonas pogonae③(+5.6%)、 Proteiniphilum acetatigenes③(+0.3%)、 Syntrophobacter wolinii④(-0.09%)、 Methanomassiliicoccus⑥(+0.1%) | [ | |
| 金霉素 | 28mg/L | 猪粪 | -27.8 | TVFA①产量+88.8% | Methanosaetaceae⑤、 Methanosarcinaceae spp.⑦减少 | [ | |
| 氯四环素 | 40mg/L | 丁酸钠 | -40.4 | 丁酸降解速率-44.2% | Syntrophomonas④表现出耐受性、Tepidanaerobacter④被抑制 | [ | |
| 大环内酯类 | |||||||
| 红霉素 | 25mg/L | 污泥 | -40.6 | 乙酸、丙酸和丁酸分别累积0.9倍、0.3倍和2.5倍 | —② | [ | |
| 红霉素 | 500mg/L | 红霉素 | +13.0 | — | Sedimentibacter③(+33%)、 Methanosarcina⑦(+2800%) | [ | |
| 克拉霉素 | 1000mg/ kg TSS | 污泥 | — | 乙酸、丙酸和丁酸降解速率分别减少-28.0%、9.3%和-15.5% | 水解发酵菌+0.64%、 Gammaproteobacteria④(-2.1%) | [ | |
| 林可酰胺类 | |||||||
| 林可霉素(LCM) | 25mg/L | 污泥 | -29.4 | 丁酸累积6.2倍 | — | [ | |
| 1000mg/L | 葡萄糖 | +20.8 | LCM浓度≥200mg/L,乙酸和丙酸显着积累 | — | [ | ||
| 磺胺类 | |||||||
| 磺胺甲𫫇唑(SMX) | 25mg/L | 短链 脂肪酸 | -35.8 | 当SMX由250mg/L增加到1000mg/L,丙酸和丁酸利用率 分别减少41.0%和30.3% | — | [ | |
| 磺胺二甲嘧啶 | 500mg/kg | 污泥 | +5.0~9.1 | — | Methanosaetaceae⑤(-9.6%)、Methanosarcinaceae⑦(-19.0%) | [ | |
| 喹诺酮类 | |||||||
| 环丙沙星(CIP) | 0.5~50mg/L | 葡萄糖、肉提取物、蛋白胨 | -4.8~32.0 | 50mg/L CIP出现丙酸积累 | Syntrophobacter④、Methanosaeta⑤减少 | [ | |
| 诺氟沙星 | 0.5mmol/L | — | -77.4 | 理论总挥发性脂肪酸(TVFA) -61.2% | Methanosaeta⑤(-22.7%)、 Methanobacterium⑥(-1.3%)、 Methanoculleus⑥(+10.4%) | [ | |
| 诺氟沙星 | 500mg/kg | 污泥 | -11.3 | — | Methanosaetaceae⑤(-9.4%)、 Methanosarcinaceae⑦(-39.9%) | [ | |
| 恩诺沙星 | 8mg/kg TS | 鸡粪 | +15.3 | TVFA+5.9% | Methanosaeta⑤(-12.6%)、 Methanobrevibacter⑥(+48.6%) | [ | |
| 材料 | 基质 | 投加量 | 甲烷产量 | 初始抗生素浓度 | 抗生素去除率 | 参考文献 |
|---|---|---|---|---|---|---|
| 零价铁 | 污泥 | 0.8g/L 1g/L 1.2g/L | +20.2% +80.9% +47.3% | 20μg/L① | 磺胺甲𫫇唑:+21.1% 磺胺嘧啶:+56.0% | [ |
| 零价铁 | 猪粪 | 15g/L | +4.7%(37℃) +3.9%(55℃) | —② | 抗生素抗性基因(ARGs) 还原+33.3%(37℃) | [ |
| 纳米零价铁 | 葡萄糖 | Fe∶VS=0.5 | +441.0% | 80mg/L | 四环素:+3.7% | [ |
| 纳米零价铁 | 模拟废水 | 1g/L | +200%以上 | 50mg/L | 氯霉素:+52.7% | [ |
| 纳米零价铁(NZVI)+ 活性炭(AC) | 模拟废水 | 1.06g NZVI/L、1.24g AC/L | — | 100mg/L | 环丙沙星:+5.0% | [ |
| 磁铁矿 | 葡萄糖 | 0.1~5g/L | +15.7%~28.9% | 100mg/L | 四环素:+6.3%~40.4% | [ |
| 纳米磁铁矿 | 污泥 | 20mmol/L | — | 50mg/L | 环丙沙星+35%~77% | [ |
| 碳纳米管 | 乙醇 | 0.1g/L | 产率-3.8% | 1mmol/L | 环丙沙星:+2% | [ |
| 生物炭 | 养殖废水 | 0.5g/L | — | 100μg/L② | 磺胺甲𫫇唑:+28.8%~33.1%; 磺胺嘧啶:+39.4%~61.2%; 磺胺二甲嘧啶:+44.4%~61.2% | [ |
| 石墨烯气凝胶 | 氯霉素 | 0.5g/L | — | 50mg/L | 氯霉素:+51.6%③ | [ |
表3 导电材料强化抗生素厌氧消化效能
| 材料 | 基质 | 投加量 | 甲烷产量 | 初始抗生素浓度 | 抗生素去除率 | 参考文献 |
|---|---|---|---|---|---|---|
| 零价铁 | 污泥 | 0.8g/L 1g/L 1.2g/L | +20.2% +80.9% +47.3% | 20μg/L① | 磺胺甲𫫇唑:+21.1% 磺胺嘧啶:+56.0% | [ |
| 零价铁 | 猪粪 | 15g/L | +4.7%(37℃) +3.9%(55℃) | —② | 抗生素抗性基因(ARGs) 还原+33.3%(37℃) | [ |
| 纳米零价铁 | 葡萄糖 | Fe∶VS=0.5 | +441.0% | 80mg/L | 四环素:+3.7% | [ |
| 纳米零价铁 | 模拟废水 | 1g/L | +200%以上 | 50mg/L | 氯霉素:+52.7% | [ |
| 纳米零价铁(NZVI)+ 活性炭(AC) | 模拟废水 | 1.06g NZVI/L、1.24g AC/L | — | 100mg/L | 环丙沙星:+5.0% | [ |
| 磁铁矿 | 葡萄糖 | 0.1~5g/L | +15.7%~28.9% | 100mg/L | 四环素:+6.3%~40.4% | [ |
| 纳米磁铁矿 | 污泥 | 20mmol/L | — | 50mg/L | 环丙沙星+35%~77% | [ |
| 碳纳米管 | 乙醇 | 0.1g/L | 产率-3.8% | 1mmol/L | 环丙沙星:+2% | [ |
| 生物炭 | 养殖废水 | 0.5g/L | — | 100μg/L② | 磺胺甲𫫇唑:+28.8%~33.1%; 磺胺嘧啶:+39.4%~61.2%; 磺胺二甲嘧啶:+44.4%~61.2% | [ |
| 石墨烯气凝胶 | 氯霉素 | 0.5g/L | — | 50mg/L | 氯霉素:+51.6%③ | [ |
| 反应式 | ΔG/kJ·mol-1 | 参考文献 |
|---|---|---|
| -136 | [ | |
| -5.02 | [ | |
| +3.5 | [ | |
| -139 | [ | |
| +774 | [ |
表4 厌氧消化中与铁有关的反应
| 反应式 | ΔG/kJ·mol-1 | 参考文献 |
|---|---|---|
| -136 | [ | |
| -5.02 | [ | |
| +3.5 | [ | |
| -139 | [ | |
| +774 | [ |
| 55 | 汪彩琴. 磁铁矿对废水厌氧生物处理过程直接种间电子传递(DIET)的调控机理研究[D]. 杭州: 浙江大学, 2020. |
| WANG Caiqin. Regulation mechanism of magnetite on direct interspecies electron transfer in wastewater anaerobic biological treatment process[D]. Hangzhou: Zhejiang University, 2020. | |
| 56 | ZHOU Haidong, CAO Zhengcao, ZHANG Minquan, et al. Zero-valent iron enhanced in situ advanced anaerobic digestion for the removal of antibiotics and antibiotic resistance genes in sewage sludge[J]. Science of the Total Environment, 2021, 754: 142077. |
| 57 | PAN Xiaofang, Nan LYU, LI Chunxing, et al. Impact of nano zero valent iron on tetracycline degradation and microbial community succession during anaerobic digestion[J]. Chemical Engineering Journal, 2019, 359: 662-671. |
| 58 | LI Jiahuan, GUO Ning, ZHAO Shan, et al. Mechanisms of metabolic performance enhancement and ARGs attenuation during nZVI-assisted anaerobic chloramphenicol wastewater treatment[J]. Journal of Hazardous Materials, 2021, 419: 126508. |
| 59 | ZHOU Mingdian, LI Chunxing, ZHAO Lixin, et al. Synergetic effect of nano zero-valent iron and activated carbon on high-level ciprofloxacin removal in hydrolysis-acidogenesis of anaerobic digestion[J]. Science of the Total Environment, 2021, 752: 142261. |
| 60 | ZHAO Zisheng, ZHANG Guangyi, ZHANG Yaobin, et al. Fe3O4 accelerates tetracycline degradation during anaerobic digestion: Synergistic role of adsorption and microbial metabolism[J]. Water Research, 2020, 185: 116225. |
| 61 | YANG Zhiman, XU Xiaohui, DAI Meng, et al. Accelerated ciprofloxacin biodegradation in the presence of magnetite nanoparticles[J]. Chemosphere, 2017, 188: 168-173. |
| 62 | CHENG Dongle, Huu Hao NGO, GUO Wenshan, et al. Applying a new pomelo peel derived biochar in microbial fell cell for enhancing sulfonamide antibiotics removal in swine wastewater[J]. Bioresource Technology, 2020, 318: 123886. |
| 63 | 宋艳芳, 张照韩, 孙沐晨, 等. 石墨烯气凝胶强化氯霉素废水厌氧生物处理效能及机制研究[J]. 环境科学学报, 2020, 40(10): 3749-3756. |
| SONG Yanfang, ZHANG Zhaohan, SUN Muchen, et al. Enhancing the anaerobic biological treatment efficiency and mechanism of chloramphenicol wastewater with graphene aerogel as exogenous mediator[J]. Acta Scientiae Circumstantiae, 2020, 40(10): 3749-3756. | |
| 64 | PAN Fei, ZHONG Xiaohan, XIA Dongsheng, et al. Nanoscale zero-valent iron/persulfate enhanced upflow anaerobic sludge blanket reactor for dye removal: Insight into microbial metabolism and microbial community[J]. Scientific Reports, 2017, 7: 44626. |
| 65 | WEI Jing, HAO Xiaodi, VAN LOOSDRECHT Mark C M, et al. Feasibility analysis of anaerobic digestion of excess sludge enhanced by iron: A review[J]. Renewable and Sustainable Energy Reviews, 2018, 89: 16-26. |
| 66 | 程佳琦. 外源介体强化四环素废水厌氧消化过程与效能研究[D]. 哈尔滨: 哈尔滨工业大学, 2016. |
| CHENG Jiaqi. Research on the enhanced process and performance during anaerobic digestion of tetracycline wastewater by redox mediator[D]. Harbin: Harbin Institute of Technology, 2016. | |
| 67 | ZHANG Junya, LU Tiedong, ZHONG Hui, et al. Zero valent iron improved methane production and specifically reduced aminoglycoside and tetracycline resistance genes in anaerobic digestion[J]. Waste Management, 2021, 136: 122-131. |
| 68 | 郝晓地, 魏静, 曹达啟. 废铁屑强化污泥厌氧消化产甲烷可行性分析[J]. 环境科学学报, 2016, 36(8): 2730-2740. |
| HAO Xiaodi, WEI Jing, CAO Daqi. Feasibility analysis of enhancing anaerobic digestion for methane production by waste iron scrap[J]. Acta Scientiae Circumstantiae, 2016, 36(8): 2730-2740. | |
| 69 | KONG Xin, NIU Jianan, ZHANG Wenjing, et al. Mini art review for zero valent iron application in anaerobic digestion and technical bottlenecks[J]. Science of the Total Environment, 2021, 791: 148415. |
| 70 | DONG Dandan, ALETA Prince, ZHAO Xin, et al. Effects of nanoscale zero valent iron (nZVI) concentration on the biochemical conversion of gaseous carbon dioxide (CO2) into methane (CH4)[J]. Bioresource Technology, 2019, 275: 314-320. |
| 71 | DANIELS L, BELAY N, RAJAGOPAL B S, et al. Bacterial methanogenesis and growth from CO2 with elemental iron as the sole source of electrons[J]. Science, 1987, 237(4814): 509-511. |
| 72 | 张永祥, 王晋昊, 井琦, 等. 地下水修复中纳米零价铁材料制备及应用综述[J]. 化工进展, 2021, 40(8): 4486-4496. |
| ZHANG Yongxiang, WANG Jinhao, JING Qi, et al. Preparation and application of modified nanoscale zero-valent iron (nZVI) in groundwater: A review[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4486-4496. | |
| 73 | 徐妍, 苑春刚. 纳米零价铁复合材料制备、稳定方法及其水处理应用[J]. 化学进展, 2022, 34(3): 717-742. |
| XU Yan, YUAN Chungang. Preparation, stabilization and applications of nano-zero-valent iron composites in water treatment[J]. Progress in Chemistry, 2022, 34(3): 717-742. | |
| 74 | ZHOU Shungui, XU Jielong, YANG Guiqin, et al. Methanogenesis affected by the co-occurrence of iron(III) oxides and humic substances[J]. FEMS Microbiology Ecology, 2014, 88(1): 107-120. |
| 75 | ANDRIAMANOHIARISOAMANANA Fetra J, IHARA Ikko, YOSHIDA Gen, et al. Tackling antibiotic inhibition in anaerobic digestion: The roles of Fe3+ and Fe3O4 on process performance and volatile fatty acids utilization pattern[J]. Bioresource Technology Reports, 2020, 11: 100460. |
| 76 | BAEK Gahyun, JUNG Heejung, KIM Jaai, et al. A long-term study on the effect of magnetite supplementation in continuous anaerobic digestion of dairy effluent-magnetic separation and recycling of magnetite[J]. Bioresource Technology, 2017, 241: 830-840. |
| 77 | ANDRIAMANOHIARISOAMANANA Fetra J, IKKO Ihara, Yoshida GEN, et al. Comparative effects of ferric hydroxide and (semi) conductive iron oxides on the anaerobic digestion of oxytetracycline-contaminated dairy manure[J]. Journal of Environmental Management, 2022, 310: 114731. |
| 78 | ZHANG Le, Kai-Chee LOH, ZHANG Jingxin. Jointly reducing antibiotic resistance genes and improving methane yield in anaerobic digestion of chicken manure by feedstock microwave pretreatment and activated carbon supplementation[J]. Chemical Engineering Journal, 2019, 372: 815-824. |
| 79 | XIAO Yeyuan, YAOHARI Hazarki, DE ARAUJO Cecilia, et al. Removal of selected pharmaceuticals in an anaerobic membrane bioreactor (AnMBR) with/without powdered activated carbon (PAC)[J]. Chemical Engineering Journal, 2017, 321: 335-345. |
| 80 | 唐梦园, 赵佳奇, 邱春生, 等. 生物炭理化特性及其对厌氧消化效率提升的研究进展[J]. 环境工程, 2021, 39(9): 138-145. |
| TANG Mengyuan, ZHAO Jiaqi, QIU Chunsheng, et al. Research progress on physicochemical characteristics of biochar and its improvement effect on anaerobic digestion efficiency[J]. Environmental Engineering, 2021, 39(9): 138-145. | |
| 81 | LUO Jiwei, LI Xue, GE Chengjun, et al. Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems[J]. Bioresource Technology, 2018, 263: 385-392. |
| 82 | NGUYEN Van-Truc, Thi-Dieu-Hien VO, NGUYEN Thanh-Binh, et al. Adsorption of norfloxacin from aqueous solution on biochar derived from spent coffee ground: Master variables and response surface method optimized adsorption process[J]. Chemosphere, 2022, 288: 132577. |
| 83 | LUO Xuewen, SHEN Minxian, LIU Junhong, et al. Resource utilization of piggery sludge to prepare recyclable magnetic biochar for highly efficient degradation of tetracycline through peroxymonosulfate activation[J]. Journal of Cleaner Production, 2021, 294: 126372. |
| 84 | ZHANG Jishi, ZHAO Wenqian, ZHANG Huiwen, et al. Recent achievements in enhancing anaerobic digestion with carbon- based functional materials[J]. Bioresource Technology, 2018, 266: 555-567. |
| 85 | LOGAN Bruce E, ROSSI Ruggero, RAGAB Ala’a, et al. Electroactive microorganisms in bioelectrochemical systems[J]. Nature Reviews Microbiology, 2019, 17(5): 307-319. |
| 86 | ZHAO Jing, LI Yu, EUVERINK Gert Jan Willem. Effect of bioaugmentation combined with activated charcoal on the mitigation of volatile fatty acids inhibition during anaerobic digestion[J]. Chemical Engineering Journal, 2022, 428: 131015. |
| 87 | HUANG Jinrong, CHEN Xiong, HU Binbin, et al. Bioaugmentation combined with biochar to enhance thermophilic hydrogen production from sugarcane bagasse[J]. Bioresource Technology, 2022, 348: 126790. |
| 88 | ZHANG Zhaohan, GAO Peng, CHENG Jiaqi, et al. Enhancing anaerobic digestion and methane production of tetracycline wastewater in EGSB reactor with GAC/NZVI mediator[J]. Water Research, 2018, 136: 54-63. |
| 89 | YANG Yu, GUO Jialiang, HU Zhiqiang. Impact of nano zero valent iron (NZVI) on methanogenic activity and population dynamics in anaerobic digestion[J]. Water Research, 2013, 47(17): 6790-6800. |
| 90 | Chengxin LYU, SHEN Yanwen, LI Chao, et al. Redox-active biochar and conductive graphite stimulate methanogenic metabolism in anaerobic digestion of waste-activated sludge: Beyond direct interspecies electron transfer[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(33): 12626-12636. |
| 91 | WANG Gaojun, GAO Xin, LI Qian, et al. Redox-based electron exchange capacity of biowaste-derived biochar accelerates syntrophic phenol oxidation for methanogenesis via direct interspecies electron transfer[J]. Journal of Hazardous Materials, 2020, 390: 121726. |
| 92 | SHEN Yanwen, YU Yamei, ZHANG Yue, et al. Role of redox-active biochar with distinctive electrochemical properties to promote methane production in anaerobic digestion of waste activated sludge[J]. Journal of Cleaner Production, 2021, 278: 123212. |
| 93 | WANG Gaojun, ZHU Jinglin, XING Yao, et al. When dewatered swine manure-derived biochar meets swine wastewater in anaerobic digestion: A win-win scenario towards highly efficient energy recovery and antibiotic resistance genes attenuation for swine manure management[J]. Science of the Total Environment, 2022, 803: 150126. |
| 94 | WANG Gaojun, CHU Yuxi, ZHU Jinglin, et al. Multi-faceted influences of biochar addition on swine manure digestion under tetracycline antibiotic pressure[J]. Bioresource Technology, 2022, 346: 126352. |
| 95 | AHMED Mohammad Boshir, ZHOU John L, Huu Hao NGO, et al. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges[J]. Science of the Total Environment, 2015, 532: 112-126. |
| 1 | WANG Chengxian, LIU Jianfeng, LI Qiumin, et al. A review of the effects of antibiotics on the anaerobic digestion of swine waste[J]. Current Opinion in Environmental Science & Health, 2022, 25: 100312. |
| 2 | WANG Xiaotong, LIN Yufei, ZHENG Yang, et al. Antibiotics in mariculture systems: A review of occurrence, environmental behavior, and ecological effects[J]. Environmental Pollution, 2022, 293: 118541. |
| 3 | D G Joakim LARSSON, DE PEDRO Cecilia, PAXEUS Nicklas. Effluent from drug manufactures contains extremely high levels of pharmaceuticals[J]. Journal of Hazardous Materials, 2007, 148(3): 751-755. |
| 4 | ANDREA Visca, ANNA Barra Caracciolo, PAOLA Grenni, et al. Anaerobic digestion and removal of sulfamethoxazole, enrofloxacin, ciprofloxacin and their antibiotic resistance genes in a full-scale biogas plant[J]. Antibiotics, 2021, 10(5): 502. |
| 5 | GUO Xinyan, YAN Zheng, ZHANG Yi, et al. Behavior of antibiotic resistance genes under extremely high-level antibiotic selection pressures in pharmaceutical wastewater treatment plants[J]. Science of the Total Environment, 2018, 612: 119-128. |
| 6 | VALENTINA Mazzurco Miritana, GIULIA Massini, ANDREA Visca, et al. Effects of sulfamethoxazole on the microbial community dynamics during the anaerobic digestion process[J]. Frontiers in Microbiology, 2020, 11: 537783. |
| 7 | SYAFIUDDIN Achmad, BOOPATHY Raj. Role of anaerobic sludge digestion in handling antibiotic resistant bacteria and antibiotic resistance genes—A review[J]. Bioresource Technology, 2021, 330: 124970. |
| 8 | 谢丽, 张艺蝶, 朱雯喆, 等. 林可霉素和3种大环内酯类抗生素对厌氧消化的影响[J]. 同济大学学报(自然科学版), 2021, 49(2):254-263. |
| XIE Li, ZHANG Yidie, ZHU Wenzhe, et al. Effect of lincomycin and three macrolide antibiotics on anaerobic digestion[J]. Journal of Tongji University (Natural Science), 2021, 49(2): 254-263. | |
| 9 | 吴爽爽, 解诗雨, 李佳佳, 等. 畜禽粪便中常见抗生素去除的研究进展[J]. 天津农学院学报, 2019, 26(2): 89-92. |
| WU Shuangshuang, XIE Shiyu, LI Jiajia, et al. Research progress on composting removal of antibiotics in livestock manure[J]. Journal of Tianjin Agricultural University, 2019, 26(2): 89-92. | |
| 10 | 聂宇, 陈娅婷, 孙照勇, 等. 污水/城市污泥中抗生素对厌氧消化的影响研究进展[J]. 应用与环境生物学报, 2020, 26(2): 479-488. |
| NIE Yu, CHEN Yating, SUN Zhaoyong, et al. Research progress on the effects of antibiotics on anaerobic digestion in sewage and municipal sludge[J]. Chinese Journal of Applied and Environmental Biology, 2020, 26(2): 479-488. | |
| 96 | ZHANG Jingxin, CHUA Qing Wei, MAO Feijian, et al. Effects of activated carbon on anaerobic digestion methanogenic metabolism, mechanisms of antibiotics and antibiotic resistance genes removal[J]. Bioresource Technology Reports, 2019, 5: 113-120. |
| 97 | GAO Pin, GU Chaochao, WEI Xin, et al. The role of zero valent iron on the fate of tetracycline resistance genes and class 1 integrons during thermophilic anaerobic co-digestion of waste sludge and kitchen waste[J]. Water Research, 2017, 111: 92-99. |
| 98 | SUN Wei, GU Jie, WANG Xiaojuan, et al. Impacts of biochar on the environmental risk of antibiotic resistance genes and mobile genetic elements during anaerobic digestion of cattle farm wastewater[J]. Bioresource Technology, 2018, 256: 342-349. |
| 99 | XIANG Yinping, YANG Zhaohui, ZHANG Yanru, et al. Influence of nanoscale zero-valent iron and magnetite nanoparticles on anaerobic digestion performance and macrolide, aminoglycoside, β-lactam resistance genes reduction[J]. Bioresource Technology, 2019, 294: 122139. |
| 11 | ZHANG Junya, SUI Qianwen, ZHONG Hui, et al. Impacts of zero valent iron, natural zeolite and Dnase on the fate of antibiotic resistance genes during thermophilic and mesophilic anaerobic digestion of swine manure[J]. Bioresource Technology, 2018, 258: 135-141. |
| 12 | CHENG Dongle, Huu Hao NGO, GUO Wenshan, et al. Improving sulfonamide antibiotics removal from swine wastewater by supplying a new pomelo peel derived biochar in an anaerobic membrane bioreactor[J]. Bioresource Technology, 2021, 319: 124160. |
| 13 | AYDIN Sevcan, INCE Bahar, CETECIOGLU Zeynep, et al. Combined effect of erythromycin, tetracycline and sulfamethoxazole on performance of anaerobic sequencing batch reactors[J]. Bioresource Technology, 2015, 186: 207-214. |
| 14 | YAO Linlin, WANG Yanxin, TONG Lei, et al. Occurrence and risk assessment of antibiotics in surface water and groundwater from different depths of aquifers: A case study at Jianghan Plain, central China[J]. Ecotoxicology and Environmental Safety, 2017, 135: 236-242. |
| 15 | ZHANG Min, LIU Yousheng, ZHAO Jianliang, et al. Occurrence, fate and mass loadings of antibiotics in two swine wastewater treatment systems[J]. Science of the Total Environment, 2018, 639: 1421-1431. |
| 16 | Klaus KÜMMERER. Antibiotics in the aquatic environment—A review—Part I[J]. Chemosphere, 2009, 75(4): 417-434. |
| 17 | YANG Yuan, HUANG Wenli, HUANG Weiwei. Antibiotic inhibition on anaerobic digestion of animal manure and controlling strategies: A short review[J]. Clean - Soil, Air, Water, 2019, 47(1): 1700653. |
| 18 | World Health Organization Geneva. Critically important antimicrobials for human medicine, 6th revision[EB/OL]. [2022-03-25]. . |
| 19 | LEKAGUL Angkana, TANGCHAROENSATHIEN Viroj, YEUNG Shunmay. Patterns of antibiotic use in global pig production: A systematic review[J]. Veterinary and Animal Science, 2019, 7: 100058. |
| 20 | SINGH Rachna, SINGH Akhand Pratap, KUMAR Sunil, et al. Antibiotic resistance in major rivers in the world: A systematic review on occurrence, emergence, and management strategies[J]. Journal of Cleaner Production, 2019, 234: 1484-1505. |
| 21 | ROBLES-JIMENEZ Lizbeth E, Aranda-Aguirre Edgar, CASTELAN-ORTEGA Octavio A, et al. Worldwide traceability of antibiotic residues from livestock in wastewater and soil: A systematic review[J]. Animals, 2021, 12(1): 60. |
| 22 | 2020年中国兽用抗菌药使用情况报告[N]. 中国畜牧兽医报, 2021-11-14(3). |
| Report on the use of veterinary antibiotics in China in 2020[N]. Chinese Animal Husbandry and Veterinary News, 2021-11-14(3). | |
| 23 | SARMAH Ajit K, MEYER Michael T, BOXALL Alistair B A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment[J]. Chemosphere, 2006, 65(5): 725-759. |
| 24 | Jia LYU, YANG Linsheng, ZHANG Lan, et al. Antibiotics in soil and water in China—A systematic review and source analysis[J]. Environmental Pollution, 2020, 266: 115147. |
| 25 | CETECIOGLU Zeynep, INCE Bahar, GROS Meritxell, et al. Biodegradation and reversible inhibitory impact of sulfamethoxazole on the utilization of volatile fatty acids during anaerobic treatment of pharmaceutical industry wastewater[J]. Science of the Total Environment, 2015, 536: 667-674. |
| 26 | XIONG Yanghui, Harb Moustapha, HONG Peiying. Performance and microbial community variations of anaerobic digesters under increasing tetracycline concentrations[J]. Applied Microbiology and Biotechnology, 2017, 101(13): 5505-5517. |
| 27 | ZHAO Lin, JI Yi, SUN Peizhe, et al. Effects of individual and combined zinc oxide nanoparticle, norfloxacin, and sulfamethazine contamination on sludge anaerobic digestion[J]. Bioresource Technology, 2019, 273: 454-461. |
| 28 | 马清佳, 田哲, 员建, 等. 9种抗生素对污泥高温厌氧消化的急性抑制[J]. 环境工程学报, 2018, 12(7): 2084-2093. |
| MA Qingjia, TIAN Zhe, YUAN Jian, et al. Acute inhibition of nine antibiotics on sludge thermophilic anaerobic digestion[J]. Chinese Journal of Environmental Engineering, 2018, 12(7): 2084-2093. | |
| 29 | ZHU Wenzhe, BU Fan, XU Jun, et al. Influence of lincomycin on anaerobic digestion: Sludge type, biogas generation, methanogenic pathway and resistance mechanism[J]. Bioresource Technology, 2021, 329: 124913. |
| 30 | WANG Mengmeng, REN Peng, WANG Yafei, et al. Erythromycin stimulates rather than inhibits methane production in anaerobic digestion of antibiotic fermentation dregs[J]. Science of the Total Environment, 2022, 807: 151007. |
| 31 | WEI Min, WANG Changmei, ZHAO Xingling, et al. Effective difference of oxytetracycline concentrations on anaerobic batch digestion of pig manure[J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2020, 42(17): 2082-2089. |
| 32 | ZHI Suli, LI Qian, YANG Fengxia, et al. How methane yield, crucial parameters and microbial communities respond to the stimulating effect of antibiotics during high solid anaerobic digestion[J]. Bioresource Technology, 2019, 283: 286-296. |
| 33 | YUDY Andrea Londoño, DIANA Catalina Rodríguez, GUSTAVO Peñuela. The operation of two EGSB reactors under the application of different loads of oxytetracycline and florfenicol[J]. Water Science and Technology, 2012, 66(12): 2578-2585. |
| 34 | CETECIOGLU Z, ORHON D. How do sulfamethoxazole and tetracycline affect the utilization of short chain fatty acids under anaerobic conditions?[J]. Journal of Environmental Chemical Engineering, 2018, 6(1): 1305-1313. |
| 35 | AZIZ Asad, SENGAR Ashish, BASHEER Farrukh, et al. Anaerobic digestion in the elimination of antibiotics and antibiotic-resistant genes from the environment—A comprehensive review[J]. Journal of Environmental Chemical Engineering, 2022, 10(1): 106423. |
| 36 | STONE James J, CLAY Sharon A, ZHU Zhenwei, et al. Effect of antimicrobial compounds tylosin and chlortetracycline during batch anaerobic swine manure digestion[J]. Water Research, 2009, 43(18): 4740-4750. |
| 37 | 冯高, 张昱晨, 苟敏, 等. 丁酸氧化菌群对抗生素及活性炭协同作用响应[J]. 生物技术通报, 2019, 35(8): 64-76. |
| FENG Gao, ZHANG Yuchen, GOU Min, et al. Response of butyrate-oxidizing microbial community to the co-effects of antibiotics and activated carbon[J]. Biotechnology Bulletin, 2019, 35(8): 64-76. | |
| 38 | HUANG Xiaoding, XU Qiuxiang, WU Yanxin, et al. Effect of clarithromycin on the production of volatile fatty acids from waste activated sludge anaerobic fermentation[J]. Bioresource Technology, 2019, 288: 121598. |
| 39 | Do Thi MAI, STUCKEY David C, Seungdae OH. Effect of ciprofloxacin on methane production and anaerobic microbial community[J]. Bioresource Technology, 2018, 261: 240-248. |
| 40 | ZHU Kongyun, ZHANG Lei, WANG Xuexue, et al. Inhibition of norfloxacin on anaerobic digestion: Focusing on the recoverability and shifted microbial communities[J]. Science of the Total Environment, 2021, 752: 141733. |
| 41 | DU Tie, FENG Lei, ZHEN Xiaofei. Microbial community structures and antibiotic biodegradation characteristics during anaerobic digestion of chicken manure containing residual enrofloxacin[J]. Journal of Environmental Science and Health, 2022, 57(2): 102-113. |
| 42 | BAUER A, LIZASOAIN J, NETTMANN E, et al. Effects of the antibiotics chlortetracycline and enrofloxacin on the anaerobic digestion in continuous experiments[J]. BioEnergy Research, 2014, 7(4): 1244-1252. |
| 43 | CETECIOGLU Z, INCE B, ORHON D, et al. Anaerobic sulfamethoxazole degradation is driven by homoacetogenesis coupled with hydrogenotrophic methanogenesis[J]. Water Research, 2016, 90: 79-89. |
| 44 | ZENG Shuting, SUN Jing, CHEN Ziwei, et al. The impact and fate of clarithromycin in anaerobic digestion of waste activated sludge for biogas production[J]. Environmental Research, 2021, 195: 110792. |
| 45 | LUO Yunlong, GUO Wenshan, Huu Hao NGO, et al. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment[J]. Science of the Total Environment, 2014, 473/474: 619-641. |
| 46 | AKASHDEEP Singh Oberoi, JIA Yanyan, ZHANG Huiqun, et al. Insights into the fate and removal of antibiotics in engineered biological treatment systems: A critical review[J]. Environmental Science & Technology, 2019, 53(13): 7234-7264. |
| 47 | ZHANG Huiqun, KHANAL Samir Kumar, JIA Yanyan, et al. Fundamental insights into ciprofloxacin adsorption by sulfate-reducing bacteria sludge: Mechanisms and thermodynamics[J]. Chemical Engineering Journal, 2019, 378: 122103. |
| 48 | SILVA Ana R, CAVALEIRO Ana J, et al. Detoxification of ciprofloxacin in an anaerobic bioprocess supplemented with magnetic carbon nanotubes: Contribution of adsorption and biodegradation mechanisms[J]. International Journal of Molecular Sciences, 2021, 22(6): 2932. |
| 49 | CHEE Xiang Chen, AZMI Aris, Ling Yong EE, et al. A review of antibiotic removal from domestic wastewater using the activated sludge process: Removal routes, kinetics and operational parameters[J]. Environmental Science and Pollution Research, 2022, 29(4): 4787-4802. |
| 50 | ZHU Tingting, SU Zhongxian, LAI Wenxia, et al. Insights into the fate and removal of antibiotics and antibiotic resistance genes using biological wastewater treatment technology[J]. Science of the Total Environment, 2021, 776: 145906. |
| 51 | 靳红梅, 黄红英, 管永祥, 等. 规模化猪场废水处理过程中四环素类和磺胺类抗生素的降解特征[J]. 生态与农村环境学报, 2016, 32(6): 978-985. |
| JIN Hongmei, HUANG Hongying, GUAN Yongxiang, et al. Characteristics of degradation tetracyclines and sulfonamides during wastewater treating processes in an intensive swine farm[J]. Journal of Ecology and Rural Environment, 2016, 32(6): 978-985. | |
| 52 | 杨侠, 李茹莹. pH对污泥厌氧消化过程中抗生素降解迁移的影响[J]. 环境科学学报, 2018, 38(4): 1446-1452. |
| YANG Xia, LI Ruying. Effects of pH on antibiotics degradation and migration during anaerobic sludge digestion[J]. Acta Scientiae Circumstantiae, 2018, 38(4): 1446-1452. | |
| 53 | YANG Shufan, Faisal I HAI, PRICE William E, et al. Occurrence of trace organic contaminants in wastewater sludge and their removals by anaerobic digestion[J]. Bioresource Technology, 2016, 210: 153-159. |
| 54 | 吴秀茹. 大环内酯类抗生素的厌氧降解及其生态毒性研究[D]. 开封: 河南大学, 2018. |
| WU Xiuru. Study on anaerobic degradation and ecotoxicity of macrolide antibiotics[D]. Kaifeng: Henan University, 2018. |
| [1] | 奚永兰, 王成成, 叶小梅, 刘洋, 贾昭炎, 曹春晖, 韩挺, 张应鹏, 田雨. 微纳米气泡在厌氧消化中的应用研究进展[J]. 化工进展, 2023, 42(8): 4414-4423. |
| [2] | 庄捷, 薛锦辉, 赵斌成, 张文艺. 猪粪厌氧消化进程中重金属与腐殖质的有机结合机制[J]. 化工进展, 2023, 42(6): 3281-3291. |
| [3] | 孟晓山, 汤子健, 陈琳, 呼和涛力, 周政忠. 厌氧消化系统酸化预警及调控技术研究进展[J]. 化工进展, 2023, 42(3): 1595-1605. |
| [4] | 应璐瑶, 王荣昌. 菌藻共生系统削减抗生素类污染物的去除途径及胁迫响应[J]. 化工进展, 2023, 42(1): 469-479. |
| [5] | 刘亚利, 张宏伟, 康晓荣. 微塑料对污泥厌氧消化的影响和机理[J]. 化工进展, 2022, 41(9): 5037-5046. |
| [6] | 朱婷婷, 苏仲弦, 赵天杭, 刘轶文. 零价铁及其耦合技术强化抗生素废水的处理[J]. 化工进展, 2022, 41(8): 4513-4529. |
| [7] | 鲍艳, 郑茜, 郭茹月. 柔性可降解压力传感器关键制备材料的研究进展[J]. 化工进展, 2022, 41(7): 3624-3635. |
| [8] | 邵明帅, 张超, 吴华南, 王宁, 陈钦冬, 徐期勇. 水热耦合厌氧消化技术处理餐厨垃圾沼渣沼液及工艺能耗分析[J]. 化工进展, 2022, 41(5): 2733-2742. |
| [9] | 郑小梅, 林茹晶, 周文静, 徐泠, 张洪宁, 张昕颖, 谢丽. 微生物电解池辅助CO2甲烷化阴极材料的研究进展[J]. 化工进展, 2022, 41(5): 2476-2486. |
| [10] | 阮敏, 孙宇桐, 黄忠良, 李辉, 张轩, 吴希锴, 赵成, 姚世蓉, 张拴保, 张巍, 黄兢. 污泥预处理-厌氧消化体系的能源经济性评价[J]. 化工进展, 2022, 41(3): 1503-1516. |
| [11] | 王勇, 姜明昊, 王怡霖, 徐婧婷, 支硕. 层状双金属氢氧化物的构筑及其处理水体中抗生素的研究进展[J]. 化工进展, 2022, 41(2): 803-815. |
| [12] | 齐亚兵. 活化过硫酸盐高级氧化法降解抗生素的研究进展[J]. 化工进展, 2022, 41(12): 6627-6643. |
| [13] | 翟重渊, 赵丹荻, 何亚鹏, 黄惠, 陈步明, 郭忠诚. 掺硼金刚石阳极电催化降解新兴抗生素类污染物研究进展[J]. 化工进展, 2022, 41(12): 6615-6626. |
| [14] | 姜记威, 张诗轩, 曾文炉, 李凤祥. 生物炭基材料在抗生素废水处理中的研究进展[J]. 化工进展, 2021, 40(S2): 389-401. |
| [15] | 吴文瞳, 张玲玲, 李子富, 王晨希, 余春松, 王庆国. 高级氧化技术降解抗生素及去除耐药性的研究进展[J]. 化工进展, 2021, 40(8): 4551-4561. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||