化工进展 ›› 2022, Vol. 41 ›› Issue (8): 4513-4529.DOI: 10.16085/j.issn.1000-6613.2021-2026
零价铁及其耦合技术强化抗生素废水的处理
- 天津大学环境科学与工程学院,天津 300072
-
收稿日期:2021-09-26修回日期:2021-11-29出版日期:2022-08-25发布日期:2022-08-22 -
通讯作者:刘轶文 -
作者简介:朱婷婷(1986—),女,讲师,硕士生导师,研究方向为污水厌氧生物处理与资源化。E-mail:ttzhu@tju.edu.cn 。 -
基金资助:国家自然科学基金(52000135)
Treatment of antibiotic wastewater enhanced by zero-valent iron and its coupling technology
ZHU Tingting( ), SU Zhongxian, ZHAO Tianhang, LIU Yiwen(
), SU Zhongxian, ZHAO Tianhang, LIU Yiwen( )
)
- School of Environmental Science and Engineering, Tianjin University, Tianjin 300072, China
-
Received:2021-09-26Revised:2021-11-29Online:2022-08-25Published:2022-08-22 -
Contact:LIU Yiwen
摘要:
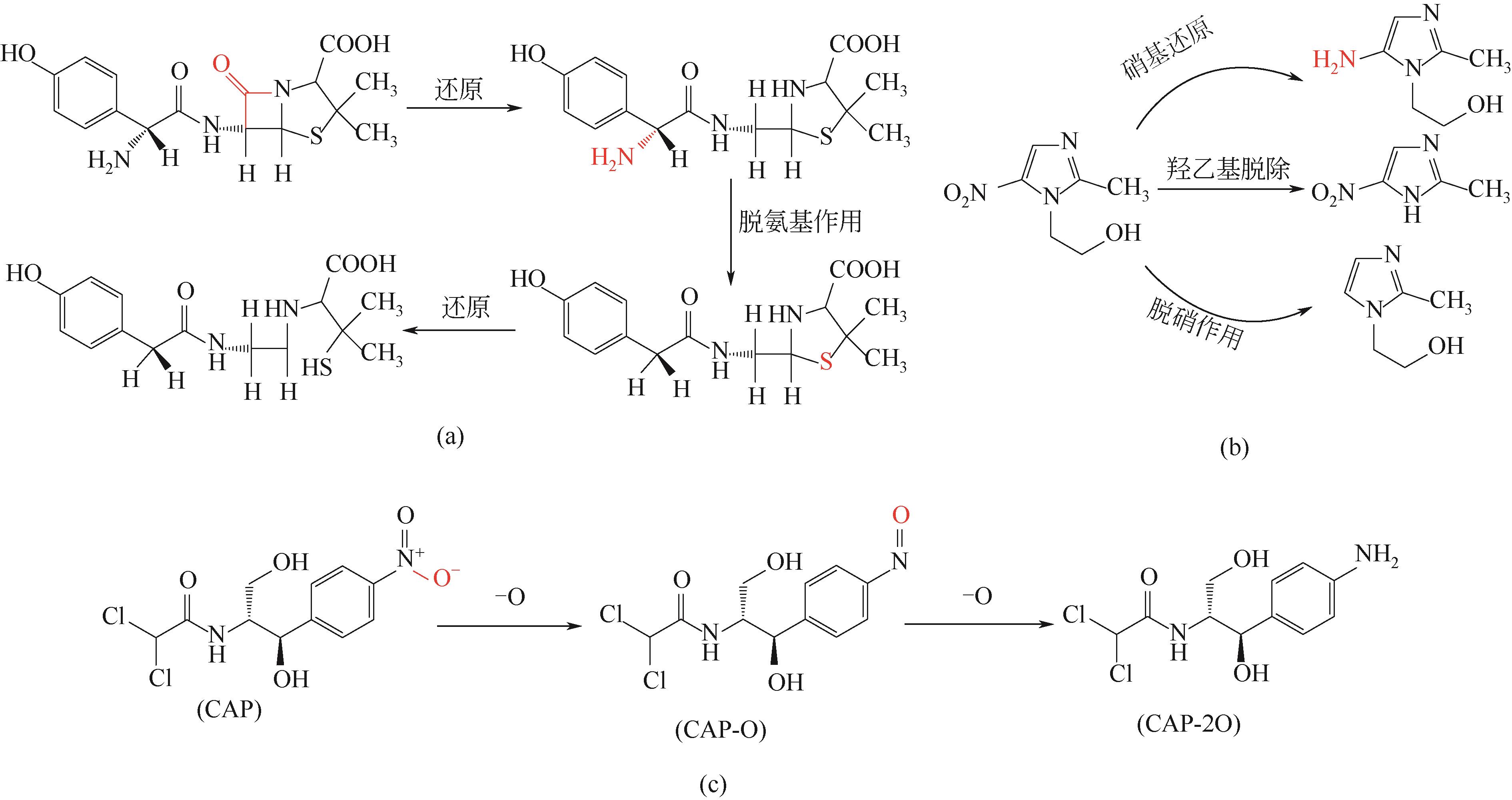
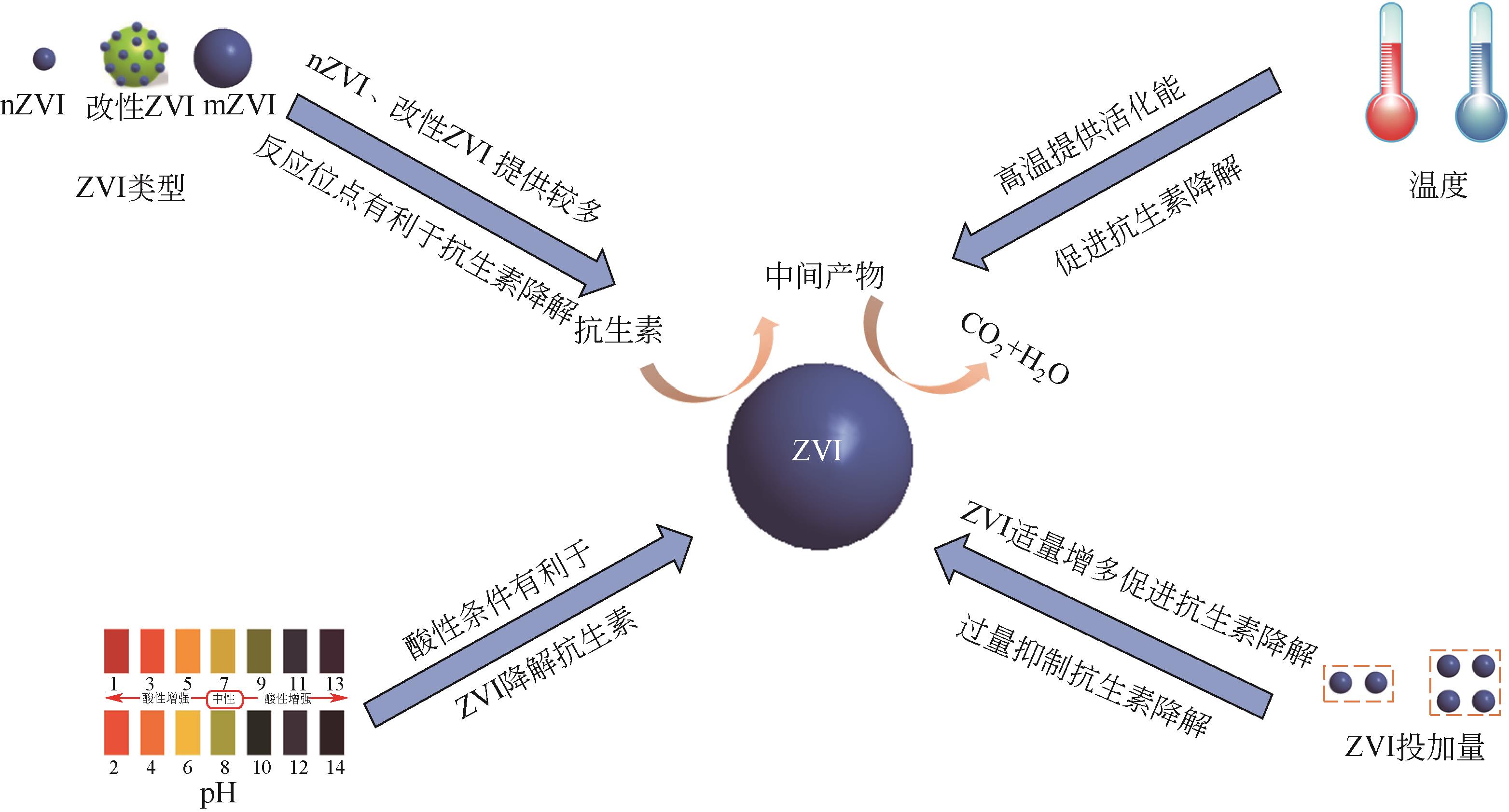
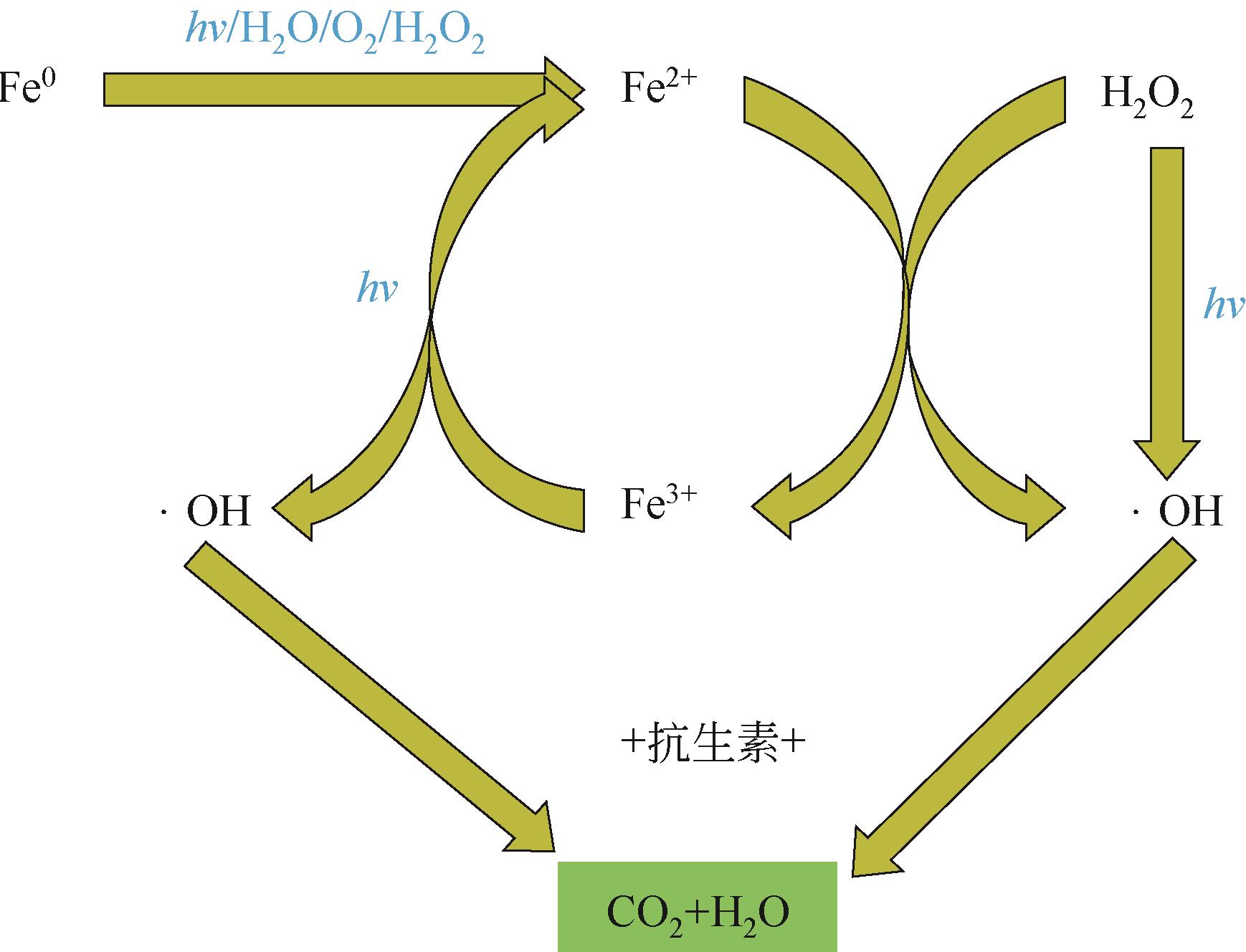
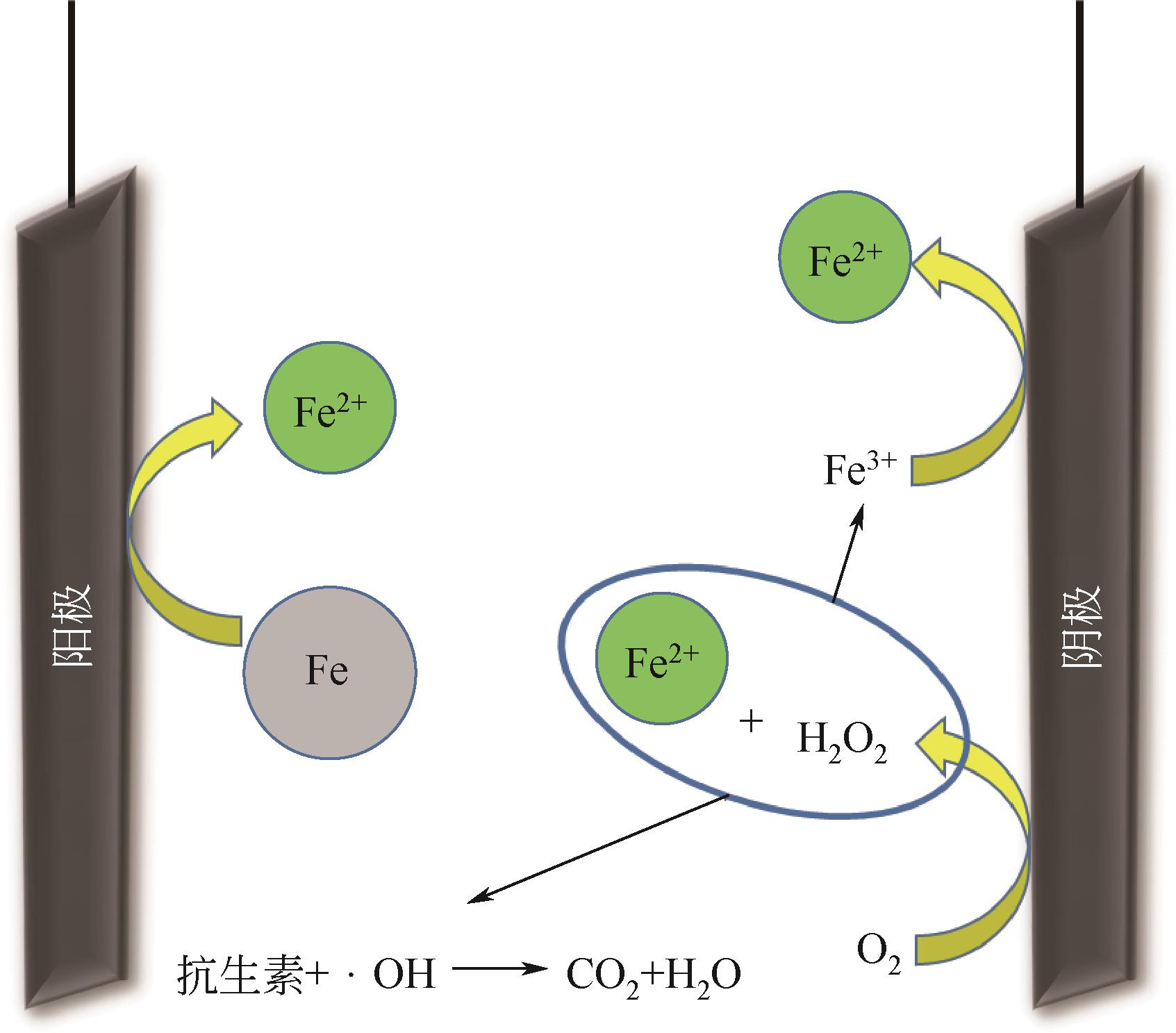
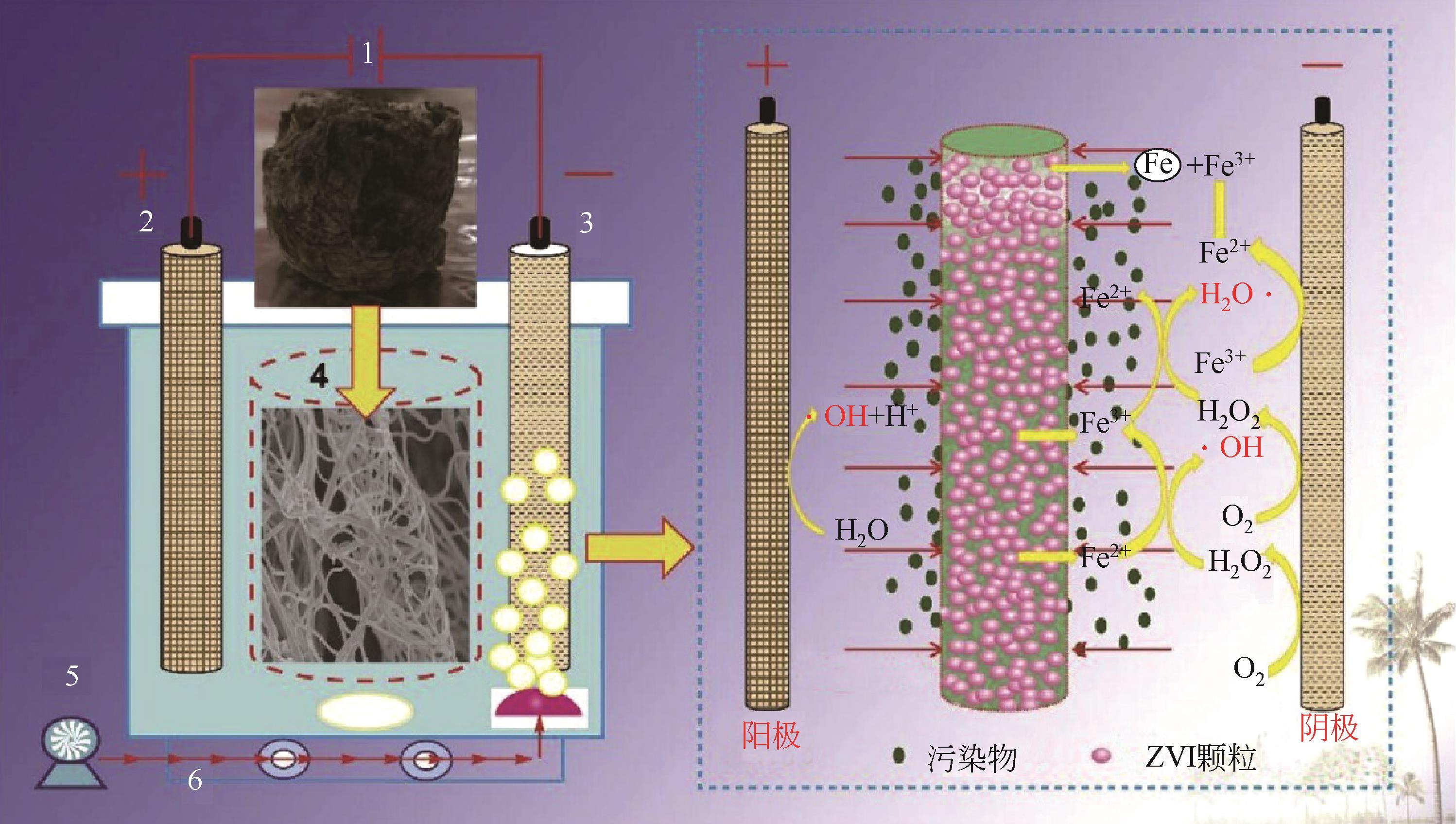
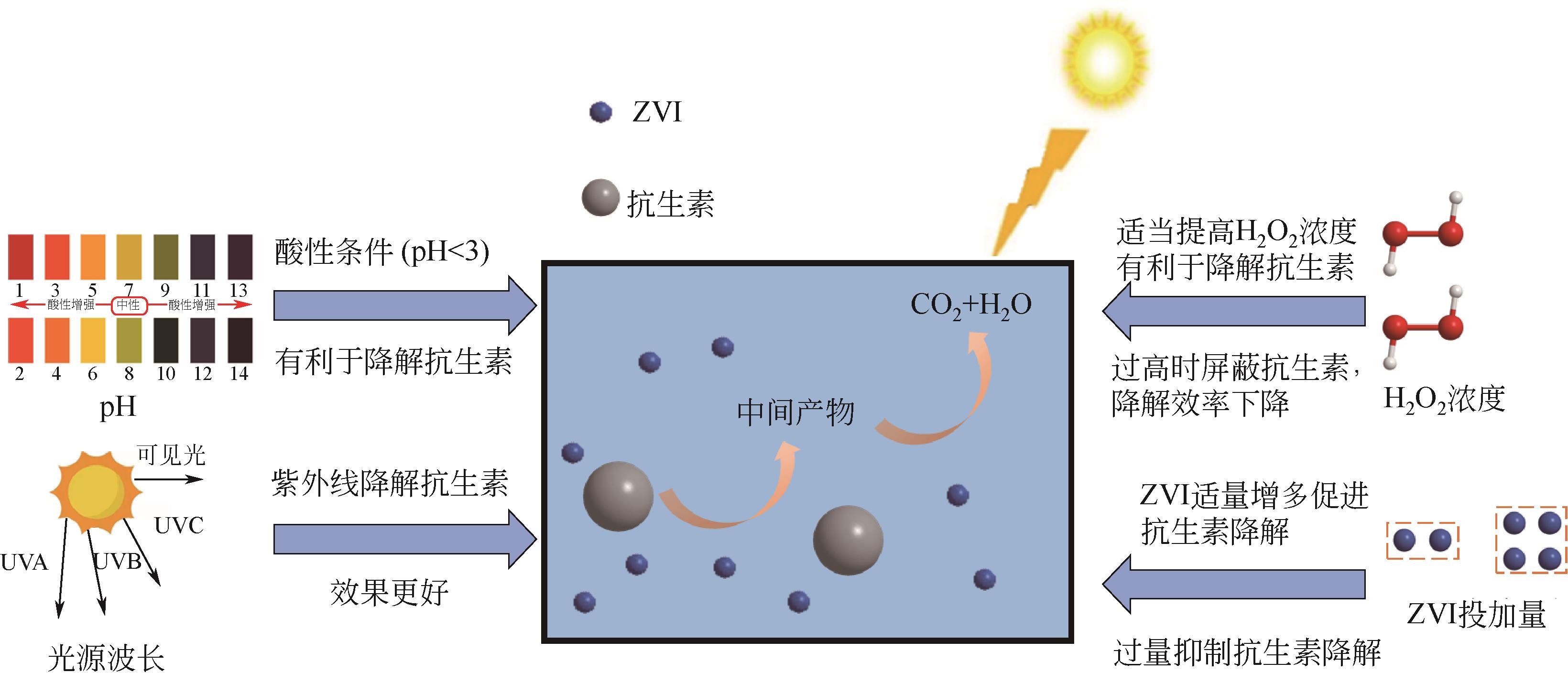
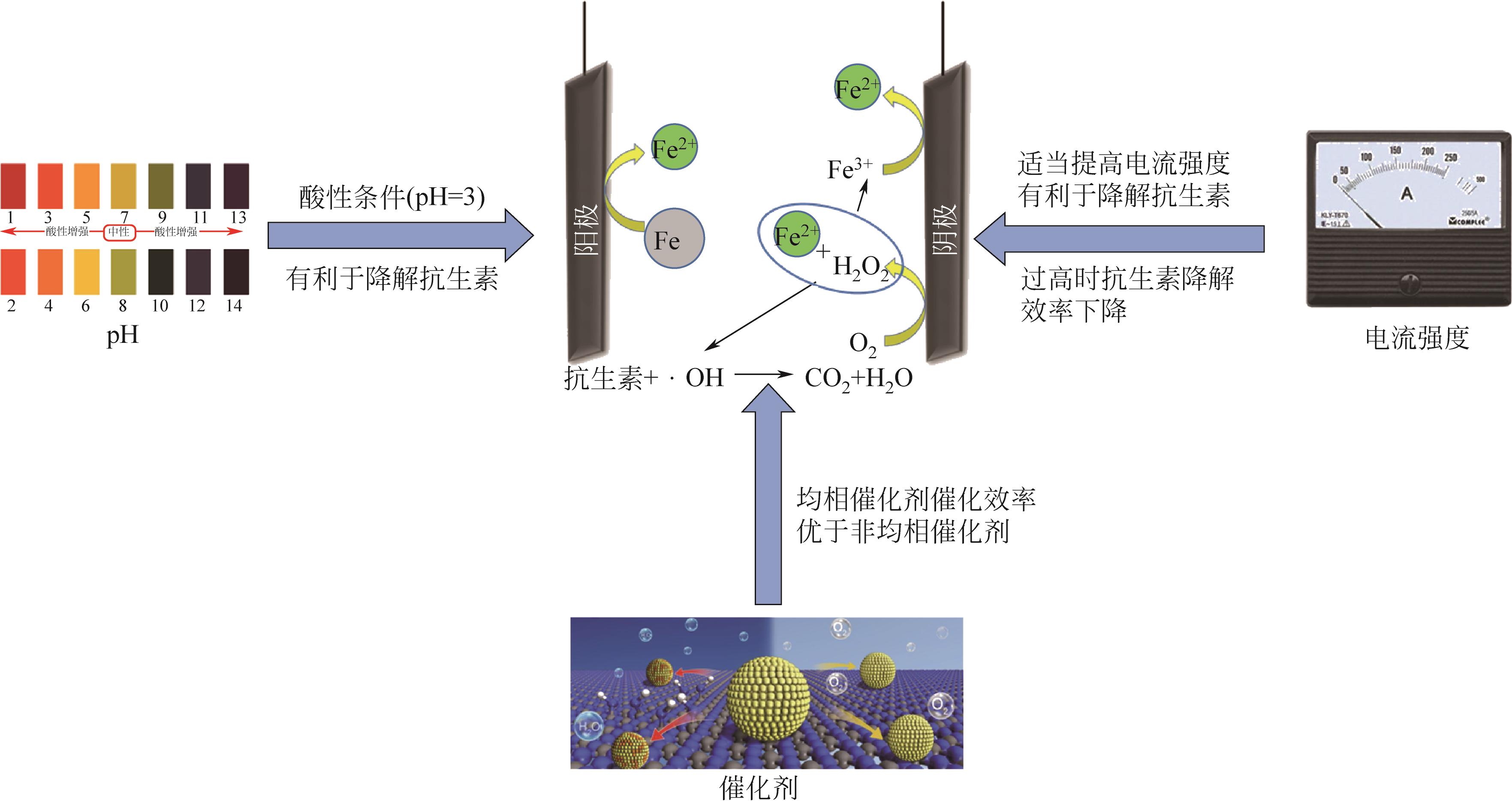
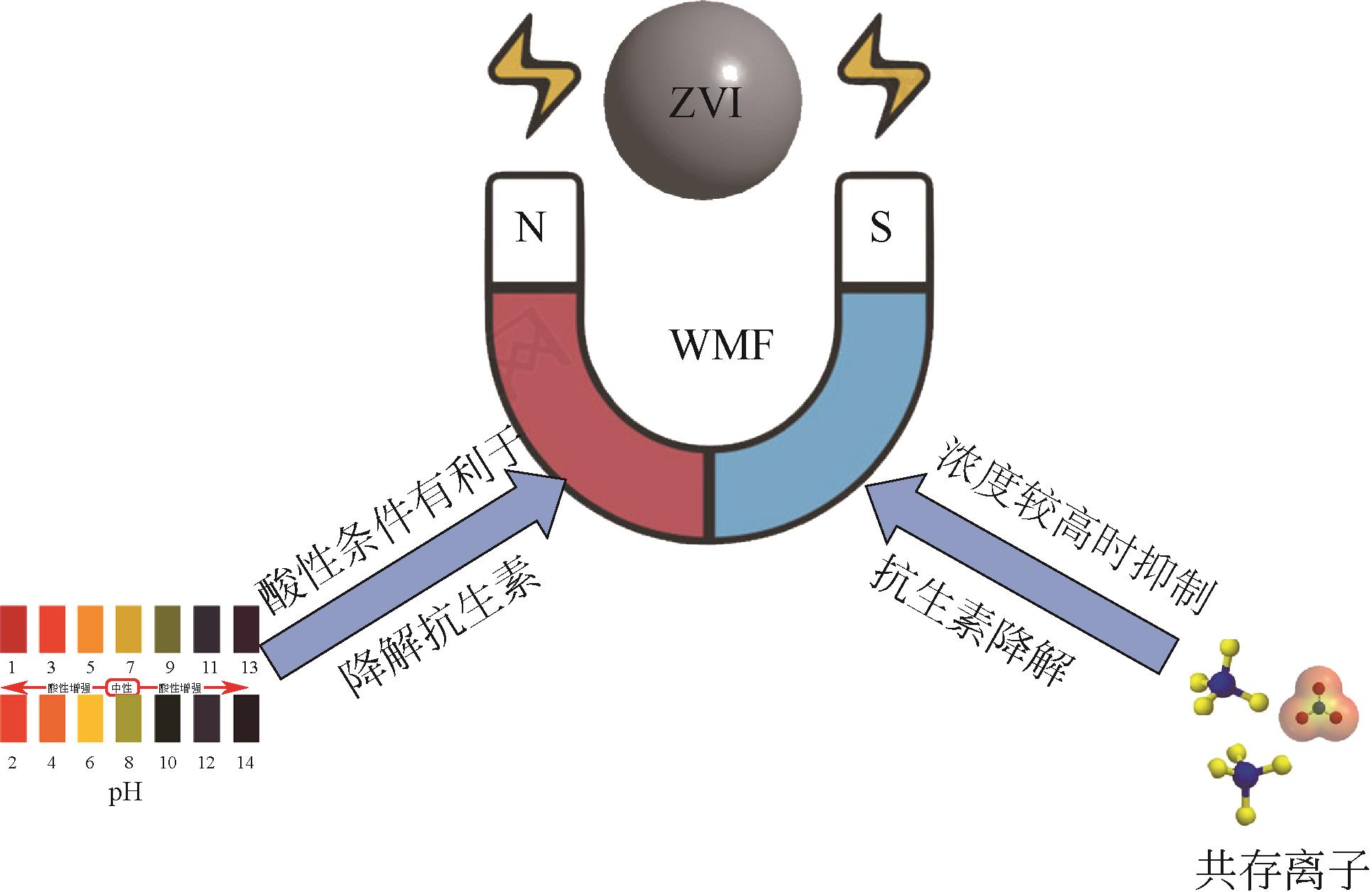
抗生素被广泛应用于治疗疾病、畜牧养殖业及病虫害防治等,然而抗生素大规模的生产及使用,对生态系统造成了持久性破坏。同时,未完全降解的抗生素在环境中逐步积累,导致抗生素抗性基因(ARGs)的富集,对环境造成极大的威胁,因此亟待开发经济、高效且可削减ARGs的抗生素处理方法。零价铁(ZVI)因廉价、易操作、不产生二次污染,被广泛用于含难降解污染物的污水处理过程,并在抗生素废水的处理中进行了广泛研究。本文从ZVI及其耦合技术对抗生素的作用机制与ZVI对厌氧消化的影响等方面,综述ZVI及耦合技术在处理抗生素废水中的应用。文章指出,ZVI主要通过产生羟基自由基(·OH)氧化降解抗生素,此外ZVI被腐蚀后形成的氢氧化物、氧化物也可吸附去除大量抗生素。零价铁-光芬顿与零价铁-电芬顿耦合工艺分别通过光能与电能促进·OH的产生,并实现Fe2+的循环利用。ZVI耦合厌氧生物处理过程中,ZVI可优化微生物群落,提高酶活性,从而促进厌氧消化降解抗生素,并削减部分ARGs。针对以上工艺特点,合成廉价高效的ZVI材料、探索ZVI对厌氧消化过程中ARGs的削减机制将是ZVI及其耦合技术强化抗生素废水处理的研究重点。
中图分类号:
引用本文
朱婷婷, 苏仲弦, 赵天杭, 刘轶文. 零价铁及其耦合技术强化抗生素废水的处理[J]. 化工进展, 2022, 41(8): 4513-4529.
ZHU Tingting, SU Zhongxian, ZHAO Tianhang, LIU Yiwen. Treatment of antibiotic wastewater enhanced by zero-valent iron and its coupling technology[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4513-4529.
| 抗生素 | 结构式 | ZVI材料 | 颗粒大小 | 氧化剂/还原剂 | 操作条件① | 去除率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| 磺胺甲??唑 |  | mZVI | 215μm | H2O2(原位产生) | c0=32,c=3,pH=3,T=25℃ | 100 | [ |
| 环丙沙星 |  | mZVI | 840μm | H2O2(原位产生) | c0=65μmol/L,c=2.6,pH=2.5 | 80.2 | [ |
| 磺胺甲基嘧啶 |  | nZVI | 46nm | Na2S2O8=1mmol/L | c0=10,c=0.028,T=25℃ | 99 | [ |
| 诺氟沙星 |  | nZVI | 50~150nm | H2O2(原位产生) | c0=10,c=3,pH=4.0,T=40℃ | 94 | [ |
| 氯霉素 |  | nZVI | 20~50nm | H2O2(原位产生) | c0=100,c=1,pH=6.8,T=25℃ | 100 | [ |
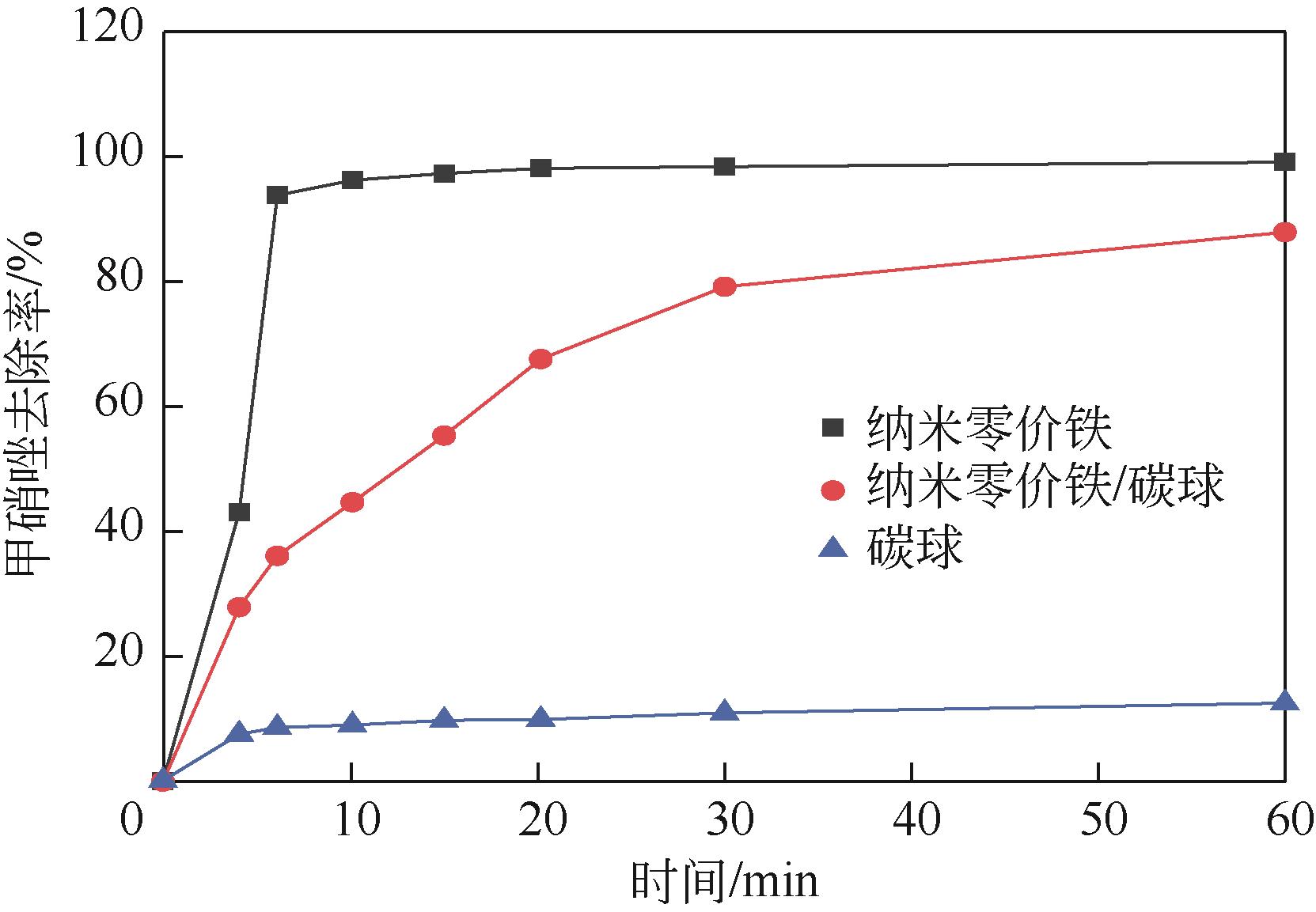
| 甲硝唑 |  | nZVI | 20~40nm | H·(原位产生) | c0=80,c=0.1,pH=5.6,T=25℃ | 100 | [ |
| 氟苯尼考 |  | 硫化物改性的零价铁 | 300nm | H2O2(原位产生) | c0=10,c=10.2,pH=6.5,T=25℃ | 90.2 | [ |
| 四环素 |  | 聚乙烯吡咯烷酮 改性的纳米零价铁 | 10~40nm | nZVI=1g/L | c0=100,c=0.1,pH=6.5,T=25℃ | 98 | [ |
表1 不同ZVI材料对不同抗生素的去除效果
| 抗生素 | 结构式 | ZVI材料 | 颗粒大小 | 氧化剂/还原剂 | 操作条件① | 去除率/% | 参考文献 |
|---|---|---|---|---|---|---|---|
| 磺胺甲??唑 |  | mZVI | 215μm | H2O2(原位产生) | c0=32,c=3,pH=3,T=25℃ | 100 | [ |
| 环丙沙星 |  | mZVI | 840μm | H2O2(原位产生) | c0=65μmol/L,c=2.6,pH=2.5 | 80.2 | [ |
| 磺胺甲基嘧啶 |  | nZVI | 46nm | Na2S2O8=1mmol/L | c0=10,c=0.028,T=25℃ | 99 | [ |
| 诺氟沙星 |  | nZVI | 50~150nm | H2O2(原位产生) | c0=10,c=3,pH=4.0,T=40℃ | 94 | [ |
| 氯霉素 |  | nZVI | 20~50nm | H2O2(原位产生) | c0=100,c=1,pH=6.8,T=25℃ | 100 | [ |
| 甲硝唑 |  | nZVI | 20~40nm | H·(原位产生) | c0=80,c=0.1,pH=5.6,T=25℃ | 100 | [ |
| 氟苯尼考 |  | 硫化物改性的零价铁 | 300nm | H2O2(原位产生) | c0=10,c=10.2,pH=6.5,T=25℃ | 90.2 | [ |
| 四环素 |  | 聚乙烯吡咯烷酮 改性的纳米零价铁 | 10~40nm | nZVI=1g/L | c0=100,c=0.1,pH=6.5,T=25℃ | 98 | [ |
| 抗生素 | 结构式 | 浓度 | 光源 | pH | 氧化剂 | 去除率/% | 文献 |
|---|---|---|---|---|---|---|---|
| 氟苯尼考 |  | 0.2mmol | 高压汞灯(253.7nm) | 3.0 | 5mmol H2O2 | 99 | [ |
| 甲砜霉素 |  | 98 | |||||
| 恩诺沙星 |  | 1g/L | 高压汞灯(253.7nm) | 3.0 | 20mmol H2O2 | 100 | [ |
| 培氟沙星 |  | 1g/L | 20mmol H2O2 | 79.1 | |||
| 磺胺喹??啉 |  | 4mg/L | 5mmol H2O2 | 100 | |||
| 磺胺甲基嘧啶 |  | 20mg/L | 60Co γ射线源 | 4.1 | H2O2(原位产生) | 99 | [ |
| 甲基嘧啶 |  | 2~20mg/L | 长波紫外灯 | 4.5 | H2O2(原位产生) | 100 | [ |
| 四环素 |  | 40mg/L | 短波紫外灯 | 3±0.2 | 5mmol H2O2 | 99.45 | [ |
| 阿莫西林 |  | 104mg/L | 紫外灯(365nm) | 3.0 | 15~40mmol H2O2 | 100 | [ |
| 双氯西林 |  | 100mg/L | 365nm | 3.0 | 10mmol H2O2 | 100 | [ |
表2 ZVI-PF对不同抗生素的去除效果
| 抗生素 | 结构式 | 浓度 | 光源 | pH | 氧化剂 | 去除率/% | 文献 |
|---|---|---|---|---|---|---|---|
| 氟苯尼考 |  | 0.2mmol | 高压汞灯(253.7nm) | 3.0 | 5mmol H2O2 | 99 | [ |
| 甲砜霉素 |  | 98 | |||||
| 恩诺沙星 |  | 1g/L | 高压汞灯(253.7nm) | 3.0 | 20mmol H2O2 | 100 | [ |
| 培氟沙星 |  | 1g/L | 20mmol H2O2 | 79.1 | |||
| 磺胺喹??啉 |  | 4mg/L | 5mmol H2O2 | 100 | |||
| 磺胺甲基嘧啶 |  | 20mg/L | 60Co γ射线源 | 4.1 | H2O2(原位产生) | 99 | [ |
| 甲基嘧啶 |  | 2~20mg/L | 长波紫外灯 | 4.5 | H2O2(原位产生) | 100 | [ |
| 四环素 |  | 40mg/L | 短波紫外灯 | 3±0.2 | 5mmol H2O2 | 99.45 | [ |
| 阿莫西林 |  | 104mg/L | 紫外灯(365nm) | 3.0 | 15~40mmol H2O2 | 100 | [ |
| 双氯西林 |  | 100mg/L | 365nm | 3.0 | 10mmol H2O2 | 100 | [ |
| 抗生素 | 结构式 | 浓度 | 阳极 | 阴极 | pH | 电流 | 去除率/% | 文献 |
|---|---|---|---|---|---|---|---|---|
| 左氧氟沙星 |  | 50mg/L | Ti/RuO2-IrO2 | 氮掺杂的石墨毡 | 3.0 | 100~400mA | 90 | [ |
| 阿莫西林 |  | 36.5mg/L | Ti4O7 | 碳毡 | 3.0 | 10~120mA | 100 | [ |
| 磺胺二甲嘧啶 |  | 28.8mg/L | 硼掺杂金刚石 | 石墨毡 | 3.0 | 2.08~4.16mA/cm2 | 98.5 | [ |
| 依诺沙星 |  | 50mg/L | 铂 | 碳毡 | 3.0 | 300mA | 97 | [ |
| 诺氟沙星 |  | 0.25mmol | 硼掺杂金刚石 | 碳毡 | 3.0 | 60mA | 97.7 | [ |
| 左氧氟沙星 |  | 200mg/L | RuO2/Ti | 活性炭纤维 | 3.0 | 6.67mA/cm2 | 61 | [ |
表3 ZVI-EF对不同抗生素的去除效果
| 抗生素 | 结构式 | 浓度 | 阳极 | 阴极 | pH | 电流 | 去除率/% | 文献 |
|---|---|---|---|---|---|---|---|---|
| 左氧氟沙星 |  | 50mg/L | Ti/RuO2-IrO2 | 氮掺杂的石墨毡 | 3.0 | 100~400mA | 90 | [ |
| 阿莫西林 |  | 36.5mg/L | Ti4O7 | 碳毡 | 3.0 | 10~120mA | 100 | [ |
| 磺胺二甲嘧啶 |  | 28.8mg/L | 硼掺杂金刚石 | 石墨毡 | 3.0 | 2.08~4.16mA/cm2 | 98.5 | [ |
| 依诺沙星 |  | 50mg/L | 铂 | 碳毡 | 3.0 | 300mA | 97 | [ |
| 诺氟沙星 |  | 0.25mmol | 硼掺杂金刚石 | 碳毡 | 3.0 | 60mA | 97.7 | [ |
| 左氧氟沙星 |  | 200mg/L | RuO2/Ti | 活性炭纤维 | 3.0 | 6.67mA/cm2 | 61 | [ |
| 1 | ZHU Tingting, SU Zhongxian, LAI Wenxia, et al. Insights into the fate and removal of antibiotics and antibiotic resistance genes using biological wastewater treatment technology[J]. Science of the Total Environment, 2021, 776: 145906. |
| 2 | YANG Lihua, YING Guangguo, SU Haochang, et al. Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata[J]. Environmental Toxicology and Chemistry, 2008, 27(5): 1201-1208. |
| 3 | 段晓丹. 滥用抗生素的危害及科学使用抗生素[J]. 当代医学, 2012, 18(24): 19-20. |
| DUAN Xiaodan. The harm of abusing antibiotics and use antibiotics scientifically[J]. Contemporary Medicine, 2012, 18(24): 19-20. | |
| 4 | 孙敏. 零价铁对污水好氧生物处理及污泥水热处理过程中四环素抗性基因的影响研究[D]. 上海: 东华大学, 2019. |
| SUN Min. Effect of zero-valent iron on tetracycline resistance gene in aerobic biological treatment of sewage and hydrothermal treatment of sludge[D]. Shanghai: Donghua University, 2019. | |
| 5 | 王倩芝. 纳米零价铁对养殖场废弃物堆肥过程中抗生素抗性基因的影响[D]. 杨凌: 西北农林科技大学, 2020. |
| WANG Qianzhi. Effects of nano-zerovalent iron on antibiotic resistance genes during livestock waste composting[D]. Yangling: Northwest A & F University, 2020. | |
| 6 | MA Liping, LI Bing, ZHANG Tong. New insights into antibiotic resistome in drinking water and management perspectives: a metagenomic based study of small-sized microbes[J]. Water Research, 2019, 152: 191-201. |
| 7 | 杨莲. 抗生素抗性基因在城镇污水处理系统的分布与去除机制研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. |
| YANG Lian. Distribution and removal mechanism of antibiotic resistance genes in municipal wastewater treatment system[D]. Harbin: Harbin Institute of Technology, 2019. | |
| 8 | JARA C C, FINO D, SPECCHIA V, et al. Electrochemical removal of antibiotics from wastewaters[J]. Applied Catalysis B: Environmental, 2007, 70(1/2/3/4): 479-487. |
| 9 | MCKINNEY C W, PRUDEN A. Ultraviolet disinfection of antibiotic resistant bacteria and their antibiotic resistance genes in water and wastewater[J]. Environmental Science & Technology, 2012, 46(24): 13393-13400. |
| 10 | FANG Zhanqiang, QIU Xiuqi, CHEN Jinhong, et al. Degradation of metronidazole by nanoscale zero-valent metal prepared from steel pickling waste liquor[J]. Applied Catalysis B: Environmental, 2010, 100(1/2): 221-228. |
| 11 | WANG Xiangyu, LIU Peng, MA Jun, et al. Preparation of novel composites based on hydrophilized and functionalized polyacrylonitrile membrane-immobilized nZVI for reductive transformation of metronidazole[J]. Applied Surface Science, 2017, 396: 841-850. |
| 12 | ZHANG Junya, SUI Qianwen, ZHONG Hui, et al. Impacts of zero valent iron, natural zeolite and Dnase on the fate of antibiotic resistance genes during thermophilic and mesophilic anaerobic digestion of swine manure[J]. Bioresource Technology, 2018, 258: 135-141. |
| 13 | 陆贤, 郭美婷, 张伟贤. 纳米零价铁对耐四环素菌耐药特性的影响[J]. 中国环境科学, 2017, 37(1): 381-385. |
| LU Xian, GUO Meiting, ZHANG Weixian. Influence of nanoscale zero-valent iron (nZVI) on resistance character of tetracycline resistant bacteria[J]. China Environmental Science, 2017, 37(1): 381-385. | |
| 14 | KOBAYASHI M, KUROSU S, YAMAGUCHI R, et al. Removal of antibiotic sulfamethoxazole by zero-valent iron under oxic and anoxic conditions: removal mechanisms in acidic, neutral and alkaline solutions[J]. Journal of Environmental Management, 2017, 200: 88-96. |
| 15 | PERINI J A D L, SILVA B F, NOGUEIRA R F P. Zero-valent iron mediated degradation of ciprofloxacin—Assessment of adsorption, operational parameters and degradation products[J]. Chemosphere, 2014, 117: 345-352. |
| 16 | LIN C C, CHEN Y H. Feasibility of using nanoscale zero-valent iron and persulfate to degrade sulfamethazine in aqueous solutions[J]. Separation and Purification Technology, 2018, 194: 388-395. |
| 17 | DIAO Zenghui, QIAN Wei, LEI Zexiang, et al. Insights on the nitrate reduction and norfloxacin oxidation over a novel nanoscale zero valent iron particle: reactivity, products, and mechanism[J]. Science of the Total Environment, 2019, 660: 541-549. |
| 18 | XIA Siqing, GU Zaoli, ZHANG Zhiqiang, et al. Removal of chloramphenicol from aqueous solution by nanoscale zero-valent iron particles[J]. Chemical Engineering Journal, 2014, 257: 98-104. |
| 19 | CAO Zhen, LIU Xue, XU Jiang, et al. Removal of antibiotic florfenicol by sulfide-modified nanoscale zero-valent iron[J]. Environmental Science & Technology, 2017, 51(19): 11269-11277. |
| 20 | 杨晓丹, 王玉如, 李敏睿. 纳米零价铁的制备、改性及对废水中重金属和有机污染物的去除[J]. 化工进展, 2019, 38(7): 3412-3424. |
| YANG Xiaodan, WANG Yuru, LI Minrui. Preparation, modification of nanoscale zero valent iron and its application for the removal of heavy metals and organic pollutants from wastewater[J]. Chemical Industry and Engineering Progress, 2019, 38(7): 3412-3424. | |
| 21 | CHEN Jinhong, QIU Xiuqi, FANG Zhanqiang, et al. Removal mechanism of antibiotic metronidazole from aquatic solutions by using nanoscale zero-valent iron particles[J]. Chemical Engineering Journal, 2012, 181/182: 113-119. |
| 22 | GUO Yige, HUANG Wenli, CHEN Bin, et al. Removal of tetracycline from aqueous solution by MCM-41-zeolite A loaded nano zero valent iron: synthesis, characteristic, adsorption performance and mechanism[J]. Journal of Hazardous Materials, 2017, 339: 22-32. |
| 23 | WANG Xiangyu, DU Yi, MA Jun. Novel synthesis of carbon spheres supported nanoscale zero-valent iron for removal of metronidazole[J]. Applied Surface Science, 2016, 390: 50-59. |
| 24 | YU Jie, HOU Xiaoli, HU Xiantao, et al. Efficient degradation of chloramphenicol by zero-valent iron microspheres and new insights in mechanisms[J]. Applied Catalysis B: Environmental, 2019, 256: 117876. |
| 25 | CAO Jinyan, XIONG Zhaokun, LAI Bo. Effect of initial pH on the tetracycline (TC) removal by zero-valent iron: adsorption, oxidation and reduction[J]. Chemical Engineering Journal, 2018, 343: 492-499. |
| 26 | WENG Xiulan, CAI Wanling, LIN Shen, et al. Degradation mechanism of amoxicillin using clay supported nanoscale zero-valent iron[J]. Applied Clay Science, 2017, 147: 137-142. |
| 27 | LIU Xue, CAO Zhen, YUAN Zilin, et al. Insight into the kinetics and mechanism of removal of aqueous chlorinated nitroaromatic antibiotic chloramphenicol by nanoscale zero-valent iron[J]. Chemical Engineering Journal, 2018, 334: 508-518. |
| 28 | SHAHWAN T, SIRRIAH S A, NAIRAT M, et al. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes[J]. Chemical Engineering Journal, 2011, 172(1): 258-266. |
| 29 | ZHOU Yuzhou, WANG Ting, ZHI Dan, et al. Applications of nanoscale zero-valent iron and its composites to the removal of antibiotics: a review[J]. Journal of Materials Science, 2019, 54(19): 12171-12188. |
| 30 | MOUSSAVI G, REZAEI M, POURAKBAR M. Comparing VUV and VUV/Fe2+ processes for decomposition of cloxacillin antibiotic: degradation rate and pathways, mineralization and by-product analysis[J]. Chemical Engineering Journal, 2018, 332: 140-149. |
| 31 | QIU Wenhui, ZHENG Ming, SUN Jing, et al. Photolysis of enrofloxacin, pefloxacin and sulfaquinoxaline in aqueous solution by UV/H2O2, UV/Fe(Ⅱ), and UV/H2O2/Fe(Ⅱ) and the toxicity of the final reaction solutions on zebrafish embryos[J]. Science of the Total Environment, 2019, 651: 1457-1468. |
| 32 | NOUBACTEP C, SCHÖNER A. Metallic iron for environmental remediation: learning from electrocoagulation[J]. Journal of Hazardous Materials, 2010, 175(1/2/3): 1075-1080. |
| 33 | HANAY Ö, YıLDıZ B, ASLAN S, et al. Removal of tetracycline and oxytetracycline by microscale zerovalent iron and formation of transformation products[J]. Environmental Science and Pollution Research International, 2014, 21(5): 3774-3782. |
| 34 | ASLAN S, YALÇIN K, HANAY Ö, et al. Removal of tetracyclines from aqueous solution by nanoscale Cu/Fe bimetallic particle[J]. Desalination and Water Treatment, 2016, 57(31): 14762-14773. |
| 35 | LIU Ning, SIJAK S, ZHENG Ming, et al. Aquatic photolysis of florfenicol and thiamphenicol under direct UV irradiation, UV/H2O2 and UV/Fe(Ⅱ) processes[J]. Chemical Engineering Journal, 2015, 260: 826-834. |
| 36 | YANG Qi, CHEN Dan, CHU Libing, et al. Enhancement of ionizing radiation-induced catalytic degradation of antibiotics using Fe/C nanomaterials derived from Fe-based MOFs[J]. Journal of Hazardous Materials, 2020, 389: 122148. |
| 37 | MOREIRA F C, BOAVENTURA R A R, BRILLAS E, et al. Degradation of trimethoprim antibiotic by UVA photoelectro-Fenton process mediated by Fe( Ⅲ )-carboxylate complexes[J]. Applied Catalysis B: Environmental, 2015, 162: 34-44. |
| 38 | KAKAVANDI B, TAKDASTAN A, JAAFARZADEH N, et al. Application of Fe3O4@C catalyzing heterogeneous UV-Fenton system for tetracycline removal with a focus on optimization by a response surface method[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2016, 314: 178-188. |
| 39 | ELMOLLA E S, CHAUDHURI M. Degradation of the antibiotics amoxicillin, ampicillin and cloxacillin in aqueous solution by the photo-Fenton process[J]. Journal of Hazardous Materials, 2009, 172(2/3): 1476-1481. |
| 40 | VILLEGAS-GUZMAN P, SILVA-AGREDO J, FLOREZ O, et al. Selecting the best AOP for isoxazolyl penicillins degradation as a function of water characteristics: effects of pH, chemical nature of additives and pollutant concentration[J]. Journal of Environmental Management, 2017, 190: 72-79. |
| 41 | LIU Xiaocheng, YANG Danxing, ZHOU Yaoyu, et al. Electrocatalytic properties of N-doped graphite felt in electro-Fenton process and degradation mechanism of levofloxacin[J]. Chemosphere, 2017, 182: 306-315. |
| 42 | OTURAN N, GANIYU S O, RAFFY S, et al. Sub-stoichiometric titanium oxide as a new anode material for electro-Fenton process: application to electrocatalytic destruction of antibiotic amoxicillin[J]. Applied Catalysis B: Environmental, 2017, 217: 214-223. |
| 43 | SOPAJ F, OTURAN N, PINSON J, et al. Effect of the anode materials on the efficiency of the electro-Fenton process for the mineralization of the antibiotic sulfamethazine[J]. Applied Catalysis B: Environmental, 2016, 199: 331-341. |
| 44 | ANNABI C, FOURCADE F, SOUTREL I, et al. Degradation of enoxacin antibiotic by the electro-Fenton process: optimization, biodegradability improvement and degradation mechanism[J]. Journal of Environmental Management, 2016, 165: 96-105. |
| 45 | ÖZCAN A, ATıLıR ÖZCAN A, DEMIRCI Y. Evaluation of mineralization kinetics and pathway of norfloxacin removal from water by electro-Fenton treatment[J]. Chemical Engineering Journal, 2016, 304: 518-526. |
| 46 | GONG Yuexiang, LI Jiuyi, ZHANG Yanyu, et al. Partial degradation of levofloxacin for biodegradability improvement by electro-Fenton process using an activated carbon fiber felt cathode[J]. Journal of Hazardous Materials, 2016, 304: 320-328. |
| 47 | ZHAO Ting, ZHENG Ming, FU Caixia, et al. Effect of low-level H2O2 and Fe(Ⅱ) on the UV treatment of tetracycline antibiotics and the toxicity of reaction solutions to zebrafish embryos[J]. Chemical Engineering Journal, 2020, 394: 125021. |
| 48 | WANG Xiangyu, WANG Anqi, LU Mengyang, et al. Synthesis of magnetically recoverable Fe0/graphene-TiO2 nanowires composite for both reduction and photocatalytic oxidation of metronidazole[J]. Chemical Engineering Journal, 2018, 337: 372-384. |
| 49 | GRČIĆ I, PAPIĆ S, ŽIŽEK K, et al. Zero-valent iron (ZVI) Fenton oxidation of reactive dye wastewater under UV-C and solar irradiation[J]. Chemical Engineering Journal, 2012, 195/196: 77-90. |
| 50 | ZEPP R G, FAUST B C, HOIGNE J. Hydroxyl radical formation in aqueous reactions (pH 3-8) of iron(Ⅱ) with hydrogen peroxide: the photo-Fenton reaction[J]. Environmental Science & Technology, 1992, 26(2): 313-319. |
| 51 | CHEN Yiping, YANG Liming, CHEN J P, et al. Electrospun spongy zero-valent iron as excellent electro-Fenton catalyst for enhanced sulfathiazole removal by a combination of adsorption and electro-catalytic oxidation[J]. Journal of Hazardous Materials, 2019, 371: 576-585. |
| 52 | LIANG Xiaoyan, CHENG Jianhua, YANG Cao, et al. Factors influencing aqueous perfluorooctanoic acid (PFOA) photodecomposition by VUV irradiation in the presence of ferric ions[J]. Chemical Engineering Journal, 2016, 298: 291-299. |
| 53 | PAN Yuwei, ZHANG Ying, ZHOU Minghua, et al. Enhanced removal of antibiotics from secondary wastewater effluents by novel UV/pre-magnetized Fe0/H2O2 process[J]. Water Research, 2019, 153: 144-159. |
| 54 | GODINI H, SHEIKHMOHAMMADI A, ABBASPOUR L, et al. Energy consumption and photochemical degradation of Imipenem/Cilastatin antibiotic by process of UVC/ Fe2+/ H2O2 through response surface methodology[J]. Optik, 2019, 182: 1194-1203. |
| 55 | MARTÍNEZ-COSTA J I, RIVERA-UTRILLA J, LEYVA-RAMOS R, et al. Individual and simultaneous degradation of the antibiotics sulfamethoxazole and trimethoprim in aqueous solutions by Fenton, Fenton-like and photo-Fenton processes using solar and UV radiations[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2018, 360: 95-108. |
| 56 | NIE Minghua, YAN Caixia, XIONG Xiaoying, et al. Degradation of chloramphenicol using a combination system of simulated solar light, Fe2+ and persulfate[J]. Chemical Engineering Journal, 2018, 348: 455-463. |
| 57 | LIU Xiaocheng, ZHOU Yaoyu, ZHANG Jiachao, et al. Insight into electro-Fenton and photo-Fenton for the degradation of antibiotics: mechanism study and research gaps[J]. Chemical Engineering Journal, 2018, 347: 379-397. |
| 58 | MALAKOOTIAN M, KANNAN K, GHARAGHANI M A, et al. Removal of metronidazole from wastewater by Fe/charcoal micro electrolysis fluidized bed reactor[J]. Journal of Environmental Chemical Engineering, 2019, 7(6): 103457. |
| 59 | ZHAO Hongying, QIAN Lin, GUAN Xiaohong, et al. Continuous bulk FeCuC aerogel with ultradispersed metal nanoparticles: an efficient 3D heterogeneous electro-Fenton cathode over a wide range of pH 3-9[J]. Environmental Science & Technology, 2016, 50(10): 5225-5233. |
| 60 | CAMPOS S, SALAZAR R, ARANCIBIA-MIRANDA N, et al. Nafcillin degradation by heterogeneous electro-Fenton process using Fe, Cu and Fe/Cu nanoparticles[J]. Chemosphere, 2020, 247: 125813. |
| 61 | MALAKOOTIAN M, AHMADIAN M. Ciprofloxacin removal by electro-activated persulfate in aqueous solution using iron electrodes[J]. Applied Water Science, 2019, 9(5): 1-10. |
| 62 | XIONG Xinmei, SUN Bo, ZHANG Jing, et al. Activating persulfate by Fe0 coupling with weak magnetic field: performance and mechanism[J]. Water Research, 2014, 62: 53-62. |
| 63 | FENG Pian, GUAN Xiaohong, SUN Yuankui, et al. Weak magnetic field accelerates chromate removal by zero-valent iron[J]. Journal of Environmental Sciences, 2015, 31: 175-183. |
| 64 | JIANG Xiao, QIAO Junlian, LO I M C, et al. Enhanced paramagnetic Cu2+ ions removal by coupling a weak magnetic field with zero valent iron[J]. Journal of Hazardous Materials, 2015, 283: 880-887. |
| 65 | WANG Wenbing, ZHAO Pingping, HU Yifan, et al. Application of weak magnetic field coupling with zero-valent iron for remediation of groundwater and wastewater: a review[J]. Journal of Cleaner Production, 2020, 262: 121341. |
| 66 | DU Juanshan, GUO Wanqian, CHE Di, et al. Weak magnetic field for enhanced oxidation of sulfamethoxazole by Fe0/H2O2 and Fe0/persulfate: performance, mechanisms, and degradation pathways[J]. Chemical Engineering Journal, 2018, 351: 532-539. |
| 67 | LI Jinxiang, SHI Zhong, MA Bin, et al. Improving the reactivity of zerovalent iron by taking advantage of its magnetic memory: implications for arsenite removal[J]. Environmental Science & Technology, 2015, 49(17): 10581-10588. |
| 68 | XU Bingqian, JIANG Wan, WANG Lianjun, et al. Yolk-shell structured Fe@void@mesoporous silica with high magnetization for activating peroxymonosulfate[J]. Chinese Chemical Letters, 2020, 31(7): 2003-2006. |
| 69 | SUI Qianwen, ZHANG Junya, CHEN Meixue, et al. Distribution of antibiotic resistance genes (ARGs) in anaerobic digestion and land application of swine wastewater[J]. Environmental Pollution, 2016, 213: 751-759. |
| 70 | INCE B, COBAN H, TURKER G, et al. Effect of oxytetracycline on biogas production and active microbial populations during batch anaerobic digestion of cow manure[J]. Bioprocess and Biosystems Engineering, 2013, 36(5): 541-546. |
| 71 | ROMERO-GÜIZA M S, VILA J, MATA-ALVAREZ J, et al. The role of additives on anaerobic digestion: a review[J]. Renewable and Sustainable Energy Reviews, 2016, 58: 1486-1499. |
| 72 | 张景新. 铁强化微生物——电催化厌氧污水处理技术的研究[D]. 大连: 大连理工大学, 2013. |
| ZHANG Jingxin. Iron enhanced microbial——Electrocatalysis of anaerobic wastewater treatment[D]. Dalian: Dalian University of Technology, 2013. | |
| 73 | 由晓刚. 纳米零价铁(NZVI)对污泥厌氧消化过程的影响研究[D]. 青岛: 青岛科技大学, 2020. |
| YOU Xiaogang. Effect of nano-zerovalent iron (nZVI) on anaerobic digestion of sludge[D]. Qingdao: Qingdao University of Science & Technology, 2020. | |
| 74 | MENG Xusheng, ZHANG Yaobin, LI Qi, et al. Adding Fe0 powder to enhance the anaerobic conversion of propionate to acetate[J]. Biochemical Engineering Journal, 2013, 73: 80-85. |
| 75 | ZHANG Jingxin, ZHANG Yaobin, QUAN Xie, et al. Bioaugmentation and functional partitioning in a zero valent iron-anaerobic reactor for sulfate-containing wastewater treatment[J]. Chemical Engineering Journal, 2011, 174(1): 159-165. |
| 76 | ZHOU Haidong, CAO Zhengcao, ZHANG Minquan, et al. Zero-valent iron enhanced in-situ advanced anaerobic digestion for the removal of antibiotics and antibiotic resistance genes in sewage sludge[J]. Science of the Total Environment, 2021, 754: 142077. |
| 77 | LUO Yunlong, GUO Wenshan, NGO H H, et al. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment[J]. Science of the Total Environment, 2014, 473/474: 619-641. |
| 78 | CARBALLA M, FINK G, OMIL F, et al. Determination of the solid-water distribution coefficient (Kd) for pharmaceuticals, estrogens and musk fragrances in digested sludge[J]. Water Research, 2008, 42(1/2): 287-295. |
| 79 | YAN Qing, GAO Xu, CHEN Youpeng, et al. Occurrence, fate and ecotoxicological assessment of pharmaceutically active compounds in wastewater and sludge from wastewater treatment plants in Chongqing, the Three Gorges Reservoir Area[J]. Science of the Total Environment, 2014, 470/471: 618-630. |
| 80 | OBEROI A S, JIA Yanyan, ZHANG Huiqun, et al. Insights into the fate and removal of antibiotics in engineered biological treatment systems: a critical review[J]. Environmental Science & Technology, 2019, 53(13): 7234-7264. |
| 81 | CLARA M, STRENN B, GANS O, et al. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants[J]. Water Research, 2005, 39(19): 4797-4807. |
| 82 | PAN Xiaofang, LYU N, LI Chunxing, et al. Impact of nano zero valent iron on tetracycline degradation and microbial community succession during anaerobic digestion[J]. Chemical Engineering Journal, 2019, 359: 662-671. |
| 83 | LI Bing, ZHANG Tong. Biodegradation and adsorption of antibiotics in the activated sludge process[J]. Environmental Science & Technology, 2010, 44(9): 3468-3473. |
| 84 | YU Bao, HUANG Xiaoting, ZHANG Dongling, et al. Response of sludge fermentation liquid and microbial community to nano zero-valent iron exposure in a mesophilic anaerobic digestion system[J]. RSC Advances, 2016, 6(29): 24236-24244. |
| 85 | ZHANG Yanru, YANG Zhaohui, XIANG Yinping, et al. Evolutions of antibiotic resistance genes (ARGs), class 1 integron-integrase (intI1) and potential hosts of ARGs during sludge anaerobic digestion with the iron nanoparticles addition[J]. Science of the Total Environment, 2020, 724: 138248. |
| 86 | GAO Pin, GU Chaochao, WEI Xin, et al. The role of zero valent iron on the fate of tetracycline resistance genes and class 1 integrons during thermophilic anaerobic co-digestion of waste sludge and kitchen waste[J]. Water Research, 2017, 111: 92-99. |
| 87 | 杨帆, 徐雯丽, 钱雅洁, 等. 零价铁对污泥厌氧消化过程中四环素抗性基因水平转移的作用影响[J]. 环境科学, 2018, 39(4): 1748-1755. |
| YANG Fan, XU Wenli, QIAN Yajie, et al. Effect of zero valent iron on the horizontal gene transfer of tetracycline resistance genes during anaerobic sludge digestion process[J]. Environmental Science, 2018, 39(4): 1748-1755. | |
| 88 | GUI Mengyao, CHEN Qian, NI Jinren. Effect of sulfamethoxazole on aerobic denitrification by strain Pseudomonas stutzeri PCN-1[J]. Bioresource Technology, 2017, 235: 325-331. |
| 89 | 杨娇雪. 水体中吡咯杀菌剂及药物的降解机理及生态毒性评估[D]. 济南: 山东大学, 2020. |
| YANG Jiaoxue. Degradation mechanism and eco-toxicity assessment of pyrrole fungicides and pharmaceuticals in the aquatic environment[D]. Jinan: Shandong University, 2020. | |
| 90 | SUN Liang, SONG Haiou, LI Qiang, et al. Fe/Cu bimetallic catalysis for reductive degradation of nitrobenzene under oxic conditions[J]. Chemical Engineering Journal, 2016, 283: 366-374. |
| [1] | 陈翔宇, 卞春林, 肖本益. 温度分级厌氧消化工艺的研究进展[J]. 化工进展, 2023, 42(9): 4872-4881. |
| [2] | 王雪婷, 顾霞, 徐先宝, 赵磊, 薛罡, 李响. 水热预处理对餐厨垃圾厌氧发酵产戊酸的影响[J]. 化工进展, 2023, 42(9): 4994-5002. |
| [3] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| [4] | 奚永兰, 王成成, 叶小梅, 刘洋, 贾昭炎, 曹春晖, 韩挺, 张应鹏, 田雨. 微纳米气泡在厌氧消化中的应用研究进展[J]. 化工进展, 2023, 42(8): 4414-4423. |
| [5] | 刘洋, 叶小梅, 苗晓, 王成成, 贾昭炎, 曹春晖, 奚永兰. 农村有机生活垃圾干发酵氨胁迫下中试工艺[J]. 化工进展, 2023, 42(7): 3847-3854. |
| [6] | 庄捷, 薛锦辉, 赵斌成, 张文艺. 猪粪厌氧消化进程中重金属与腐殖质的有机结合机制[J]. 化工进展, 2023, 42(6): 3281-3291. |
| [7] | 李白雪, 信欣, 朱羽蒙, 刘琴, 刘鑫. SASD-A体系构建及进水不同S/N对脱氮工艺的影响机制[J]. 化工进展, 2023, 42(6): 3261-3271. |
| [8] | 杨竞莹, 施万胜, 黄振兴, 谢利娟, 赵明星, 阮文权. 改性纳米零价铁材料制备的研究进展[J]. 化工进展, 2023, 42(6): 2975-2986. |
| [9] | 李华华, 李逸航, 金北辰, 李隆昕, 成少安. 厌氧氨氧化-生物电化学耦合废水处理系统的研究进展[J]. 化工进展, 2023, 42(5): 2678-2690. |
| [10] | 黄越, 赵立欣, 姚宗路, 于佳动, 李再兴, 申瑞霞, 安柯萌, 黄亚丽. 木质纤维类废弃物定向生物转化乳酸、乙酸研究进展[J]. 化工进展, 2023, 42(5): 2691-2701. |
| [11] | 范思涵, 于国熙, 来超超, 何欢, 黄斌, 潘学军. 非生物改性对厌氧微生物产物光化学活性影响[J]. 化工进展, 2023, 42(4): 2180-2189. |
| [12] | 王玉, 余广炜, 江汝清, 黎长江, 林佳佳, 邢贞娇. 餐厨厌氧沼渣生物炭吸附盐酸环丙沙星[J]. 化工进展, 2023, 42(4): 2160-2170. |
| [13] | 朱紫旋, 陈俊江, 张星星, 李祥, 刘文如, 吴鹏. 基于短程反硝化厌氧氨氧化新型污水生物脱氮工艺的研究进展[J]. 化工进展, 2023, 42(4): 2091-2100. |
| [14] | 赵星程, 贾方旭, 蒋伟彧, 陈佳熠, 刘晨雨, 姚宏. 氧化还原介体介导厌氧氨氧化生物脱氮的研究进展[J]. 化工进展, 2023, 42(3): 1606-1617. |
| [15] | 孟晓山, 汤子健, 陈琳, 呼和涛力, 周政忠. 厌氧消化系统酸化预警及调控技术研究进展[J]. 化工进展, 2023, 42(3): 1595-1605. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||