化工进展 ›› 2023, Vol. 42 ›› Issue (10): 5147-5161.DOI: 10.16085/j.issn.1000-6613.2022-2033
催化脱除钢铁副产煤气中COS和H2S的研究进展
袁礼( ), 王学谦(
), 王学谦( ), 李翔, 王郎郎, 马懿星, 宁平(
), 李翔, 王郎郎, 马懿星, 宁平( ), 熊亦然
), 熊亦然
- 昆明理工大学环境科学与工程学院,云南 昆明 650500
-
收稿日期:2022-11-01修回日期:2023-01-31出版日期:2023-10-15发布日期:2023-11-11 -
通讯作者:王学谦,宁平 -
作者简介:袁礼(1991—),男,博士研究生,研究方向为工业废气净化及资源化。E-mail:634105655@qq.com。 -
基金资助:国?家?重点研发计划(2018YFC0213400);国家自然科学基金(52070090);云南科技计划(202101BC070001-009);云南省应用基础研究项目(2019FD043)
Research advances on catalytic removal COS and H2S from by-product gas in iron and steel industry
YUAN Li( ), WANG Xueqian(
), WANG Xueqian( ), LI Xiang, WANG Langlang, MA Yixing, NING ping(
), LI Xiang, WANG Langlang, MA Yixing, NING ping( ), XIONG Yiran
), XIONG Yiran
- Faculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming 650500, Yunnan, China
-
Received:2022-11-01Revised:2023-01-31Online:2023-10-15Published:2023-11-11 -
Contact:WANG Xueqian, NING ping
摘要:
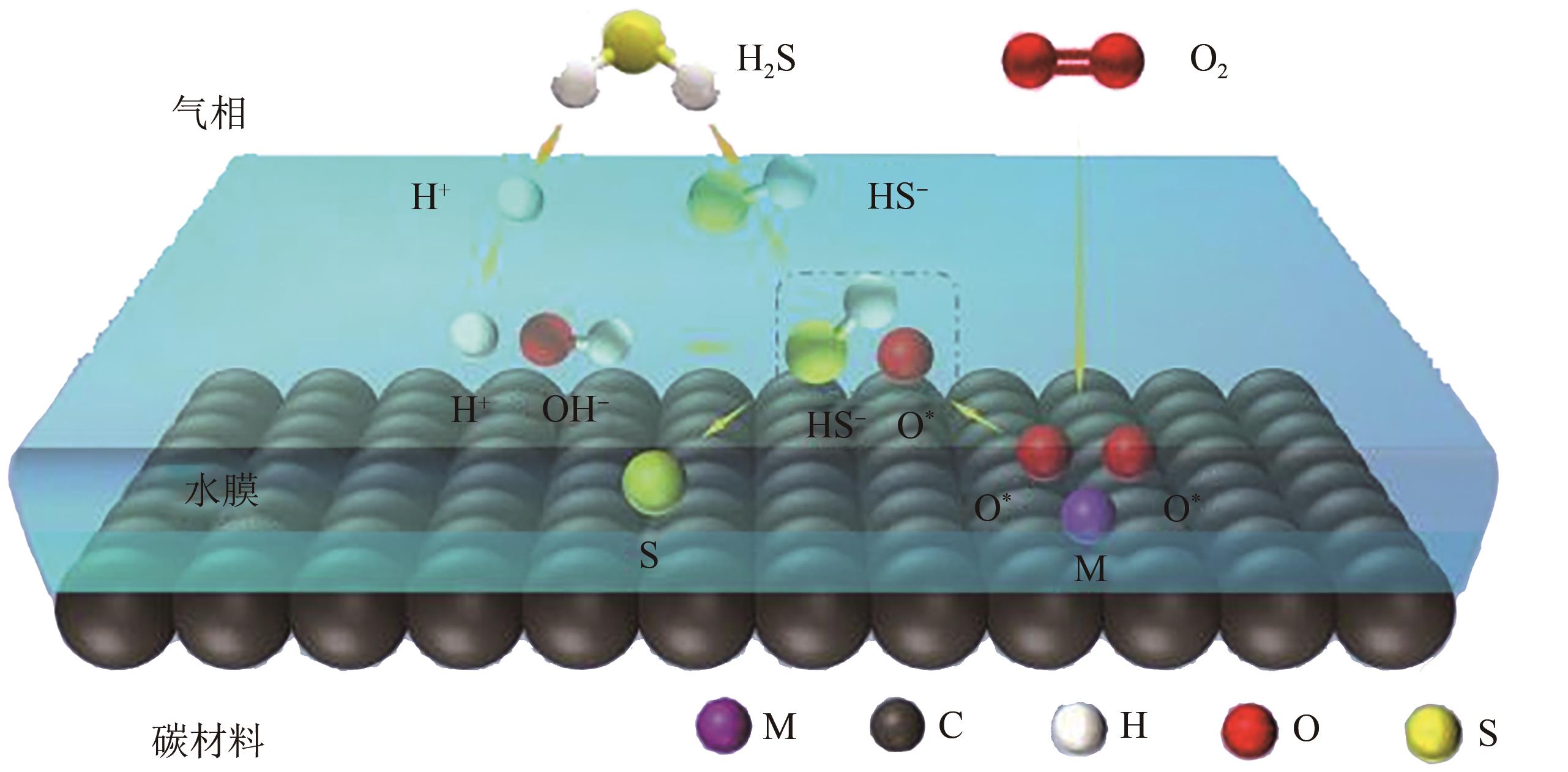
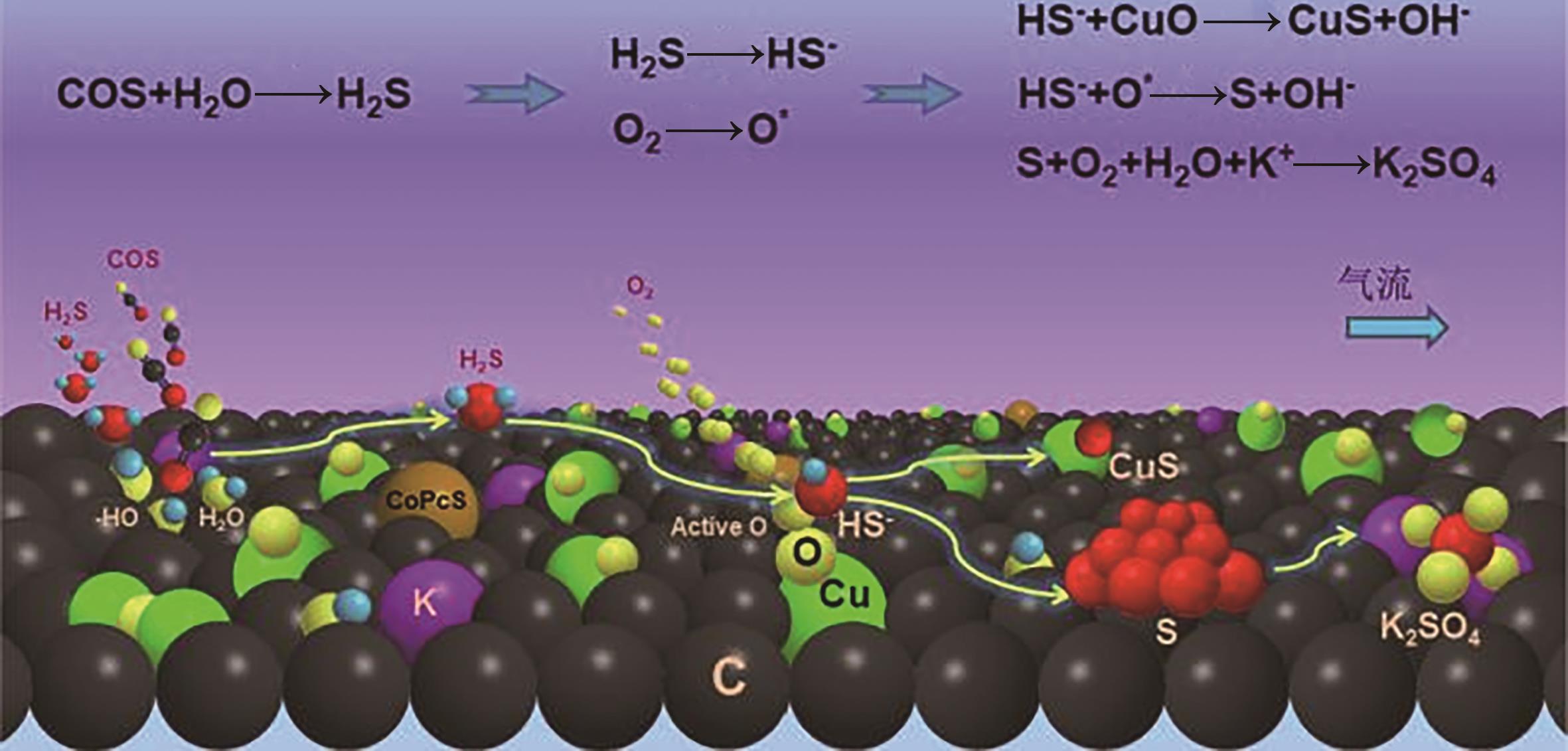
煤气是钢铁生产过程中重要的副产能源,其所含的羰基硫(COS)和硫化氢(H2S)不仅造成环境污染,还在很大程度上限制了煤气的后续利用。因此,COS和H2S的高效脱除是实现煤气清洁利用的必要前提。鉴于目前探究钢铁副产煤气脱硫过程的冗长和复杂性,迫切需开发一种能同步脱除COS和H2S的双功能催化剂。本文首先从单独脱除COS和H2S催化剂入手,分别探讨负载型和非负载型催化剂的研究现状,深入分析了负载型金属氧化物、负载型碳材料、复合金属氧化物和类水滑石在脱硫催化剂中应用情况,并对同步脱除COS和H2S的双功能催化剂的研究现状进行梳理。在此基础上对COS加氢、水解和H2S催化氧化机理进行了归纳,并着重讨论了双功能催化剂上脱除COS和H2S的反应机理,同时对该催化剂的失活和再生研究进行总结。最后,展望了探究其他材料和改性方法在同步催化脱除COS和H2S的可能性,减少副产物对催化剂的负面影响,并从开发高硫容、易再生、提高脱硫精度、真实氛围等关键方面来指导COS和H2S同步脱除技术,为开发同步脱除COS和H2S的双功能催化剂提供参考和借鉴。
中图分类号:
引用本文
袁礼, 王学谦, 李翔, 王郎郎, 马懿星, 宁平, 熊亦然. 催化脱除钢铁副产煤气中COS和H2S的研究进展[J]. 化工进展, 2023, 42(10): 5147-5161.
YUAN Li, WANG Xueqian, LI Xiang, WANG Langlang, MA Yixing, NING ping, XIONG Yiran. Research advances on catalytic removal COS and H2S from by-product gas in iron and steel industry[J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5147-5161.
| 催化剂 | 比表面积/m2·g-1 | 孔体积/cm·g-1 | 平均孔径/nm | 空速/h-1 | 反应温度/℃ | COS/H2S浓度 | 脱除性能 (转化率)/% | 穿透容量 /mg·g-1 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
| Pd/γ-Al2O3 | — | — | — | 3000 | 200 | COS:60mg·kg-1 CS2:55mg·kg-1 | 100 | — | [ |
| K2CO3-TiO2-γ-Al2O3 | — | 0.63 | 6.24 | 9000 | 300 | COS:2000mg·m-3 | 97 | — | [ |
| 3D-A(P14.3)-K | 114.7 | 0.264 | 9.2 | 16000 | 70 | COS:800mg·m-3 | 100 | — | [ |
| 15% Fe2O3 /TiO2 | 61.7 | 0.095 | 6.18 | 6700 | 180 | H2S:2000mg·kg-1 | 96.57 | — | [ |
| Fe/TiO2-x -S | 74.6 | 0.31 | 15.9 | 9000 | 210 | H2S:5000mg·kg-1 | 100 | — | [ |
| CuO/Al2O3 | 215 | 0.45 | 6.6 | 6100 | 50 | H2S:1521±76mg·m-3 | — | 220.92 | [ |
| V2O5/anatase-21 | 63 | 0.24 | 10.4 | 530000 | 200 | H2S:300mg·kg-1 | 100 | — | [ |
表1 金属氧化物基催化剂脱除COS和H2S的总结
| 催化剂 | 比表面积/m2·g-1 | 孔体积/cm·g-1 | 平均孔径/nm | 空速/h-1 | 反应温度/℃ | COS/H2S浓度 | 脱除性能 (转化率)/% | 穿透容量 /mg·g-1 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
| Pd/γ-Al2O3 | — | — | — | 3000 | 200 | COS:60mg·kg-1 CS2:55mg·kg-1 | 100 | — | [ |
| K2CO3-TiO2-γ-Al2O3 | — | 0.63 | 6.24 | 9000 | 300 | COS:2000mg·m-3 | 97 | — | [ |
| 3D-A(P14.3)-K | 114.7 | 0.264 | 9.2 | 16000 | 70 | COS:800mg·m-3 | 100 | — | [ |
| 15% Fe2O3 /TiO2 | 61.7 | 0.095 | 6.18 | 6700 | 180 | H2S:2000mg·kg-1 | 96.57 | — | [ |
| Fe/TiO2-x -S | 74.6 | 0.31 | 15.9 | 9000 | 210 | H2S:5000mg·kg-1 | 100 | — | [ |
| CuO/Al2O3 | 215 | 0.45 | 6.6 | 6100 | 50 | H2S:1521±76mg·m-3 | — | 220.92 | [ |
| V2O5/anatase-21 | 63 | 0.24 | 10.4 | 530000 | 200 | H2S:300mg·kg-1 | 100 | — | [ |
| 催化剂 | 比表面积/m2·g-1 | 孔体积/cm·g-1 | 平均孔径/nm | 空速/h-1 | 反应温度/℃ | COS/H2S浓度 | 脱除性能 | 穿透容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| NiO-MoS2/AC | — | — | — | 7000 | 280 | COS:500mg/kg-1 | 97.7① | — | [ |
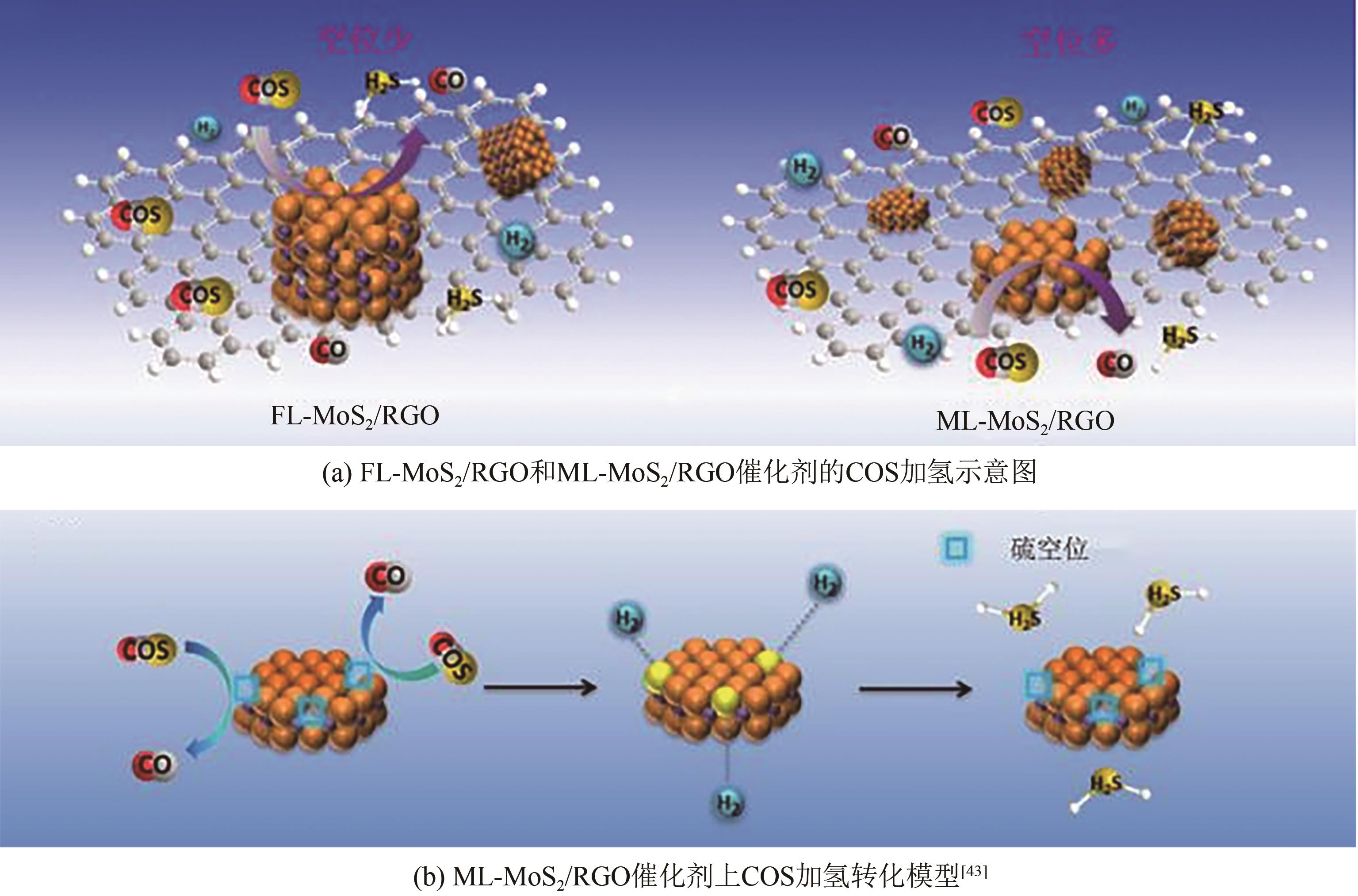
| ML-MoS2/RGO | — | — | — | 32000 | 220 | COS:500mg/kg-1 | 100① | — | [ |
| Cu-Co-K/AC | 540.1 | 0.2969 | 1.099 | 5000 | 60 | COS:2800mg·m-3 | — | 43.34 | [ |
| MnO/AC | — | — | — | 1000 | 70 | COS:900mg·m-3 | 90② | — | [ |
| Fe-Cu-Ce/AC | 323.1 | 0.035 | 1.14 | 1000 | 50 | COS:1582mg·m-3 | 100① | — | [ |
| Mn/AC | 905 | 0.464 | — | 3000 | 180 | H2S:3000mg·kg-1 | — | 142 | [ |
| MCS-MgO-15 | 660 | 1.74 | 12 | 9000 | 30 | H2S:1000mg·kg-1 | — | 2320 | [ |
表2 碳基催化剂脱除COS和H2S的总结
| 催化剂 | 比表面积/m2·g-1 | 孔体积/cm·g-1 | 平均孔径/nm | 空速/h-1 | 反应温度/℃ | COS/H2S浓度 | 脱除性能 | 穿透容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| NiO-MoS2/AC | — | — | — | 7000 | 280 | COS:500mg/kg-1 | 97.7① | — | [ |
| ML-MoS2/RGO | — | — | — | 32000 | 220 | COS:500mg/kg-1 | 100① | — | [ |
| Cu-Co-K/AC | 540.1 | 0.2969 | 1.099 | 5000 | 60 | COS:2800mg·m-3 | — | 43.34 | [ |
| MnO/AC | — | — | — | 1000 | 70 | COS:900mg·m-3 | 90② | — | [ |
| Fe-Cu-Ce/AC | 323.1 | 0.035 | 1.14 | 1000 | 50 | COS:1582mg·m-3 | 100① | — | [ |
| Mn/AC | 905 | 0.464 | — | 3000 | 180 | H2S:3000mg·kg-1 | — | 142 | [ |
| MCS-MgO-15 | 660 | 1.74 | 12 | 9000 | 30 | H2S:1000mg·kg-1 | — | 2320 | [ |
| 催化剂 | 比表面积/m2·g-1 | 孔体积/cm·g-1 | 平均孔径/nm | 空速/h-1 | 反应温度/℃ | COS/H2S浓度 | 脱除性能(转化率)/% | 穿透容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 10Cu-Co3O4 | 37.4 | 0.21 | 17.7 | 6000 | 70 | COS:110mg·m-3 | 100 | — | [ |
| Fe-Cu-Al-O | 96.7 | — | — | 25000 | 40 | H2S:1000mg·kg-1 | — | 113.9 | [ |
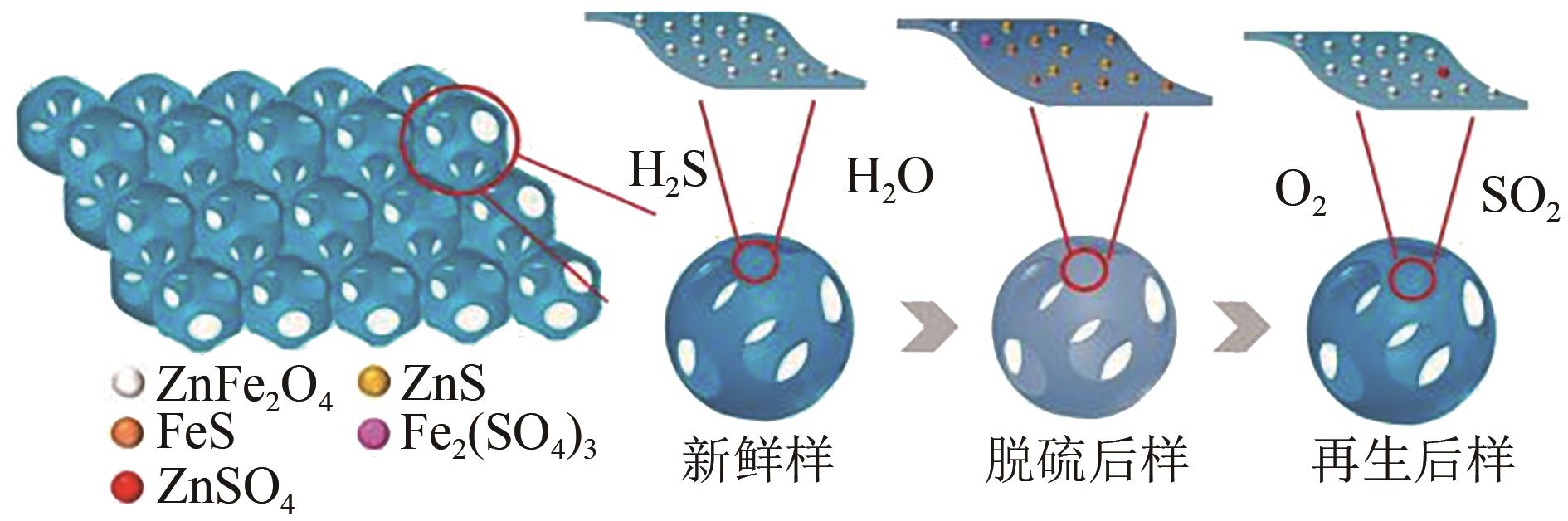
| 3DOM-50ZFS | 229.38 | 0.41 | 7.33 | — | 500 | H2S:1000mg·kg-1 | — | 70 | [ |
| 3DOM-70ZFS | 213.10 | 0.39 | 7.4 | — | 500 | H2S:1000mg·kg-1 | — | 50 | [ |
| ZnAl-3 | — | — | — | 10000 | 500 | H2S:2000mg·kg-1 | — | 268.3 | [ |
| MA-LDH-3∶1 | 34 | 0.04 | 5.26 | 12000 | 130 | COS:110 mg·m-3 | 100 | — | [ |
| MA-LDH-3∶1 | 34 | 0.04 | 5.26 | 12000 | 150 | H2S:5000mg·kg-1 | 100 | — | [ |
| HTLCs-200 | 159.33 | 0.49 | 11.14 | 5000 | 50 | COS:360mg·kg-1 | 95 | — | [ |
表3 非负载型催化剂脱除COS和H2S的总结
| 催化剂 | 比表面积/m2·g-1 | 孔体积/cm·g-1 | 平均孔径/nm | 空速/h-1 | 反应温度/℃ | COS/H2S浓度 | 脱除性能(转化率)/% | 穿透容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 10Cu-Co3O4 | 37.4 | 0.21 | 17.7 | 6000 | 70 | COS:110mg·m-3 | 100 | — | [ |
| Fe-Cu-Al-O | 96.7 | — | — | 25000 | 40 | H2S:1000mg·kg-1 | — | 113.9 | [ |
| 3DOM-50ZFS | 229.38 | 0.41 | 7.33 | — | 500 | H2S:1000mg·kg-1 | — | 70 | [ |
| 3DOM-70ZFS | 213.10 | 0.39 | 7.4 | — | 500 | H2S:1000mg·kg-1 | — | 50 | [ |
| ZnAl-3 | — | — | — | 10000 | 500 | H2S:2000mg·kg-1 | — | 268.3 | [ |
| MA-LDH-3∶1 | 34 | 0.04 | 5.26 | 12000 | 130 | COS:110 mg·m-3 | 100 | — | [ |
| MA-LDH-3∶1 | 34 | 0.04 | 5.26 | 12000 | 150 | H2S:5000mg·kg-1 | 100 | — | [ |
| HTLCs-200 | 159.33 | 0.49 | 11.14 | 5000 | 50 | COS:360mg·kg-1 | 95 | — | [ |
| 催化剂 | 比表面积/m2·g-1 | 孔体积/cm·g-1 | 平均孔径/nm | 空速/h-1 | 反应温度/℃ | COS/H2S浓度 | 脱除性能 | 穿透容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Cu-K-Co/AC | 641.48 | 0.31 | 2.29 | 30000 | 60 | H2S:75mg·m-3 COS:125mg·m-3 | 100② | 218.21 | [ |
| Na(0.45)-SAC | 455.05 | 0.22 | 1.89 | 7200 | 60 | H2S:200mg·kg-1 COS:300 mg·kg-1 | 100② | 116.60 | [ |
| Cu-Fe/TSAC | 554 | 0.29 | 0.3~1.6 | 10000 | 60 | H2S:500mg·kg-1 COS:400mg·kg-1 CS2:60mg·kg-1 | 100② | 231.28 | [ |
| 2DNHPC-10 | 567 | 0.50 | 12.1 | 12000 | 180 | H2S:5000mg·kg-1 | 100① | — | [ |
| 2DNHPC-10 | 567 | 0.50 | 12.1 | 12000 | 150 | COS:110mg·m-3 | 100① | — | [ |
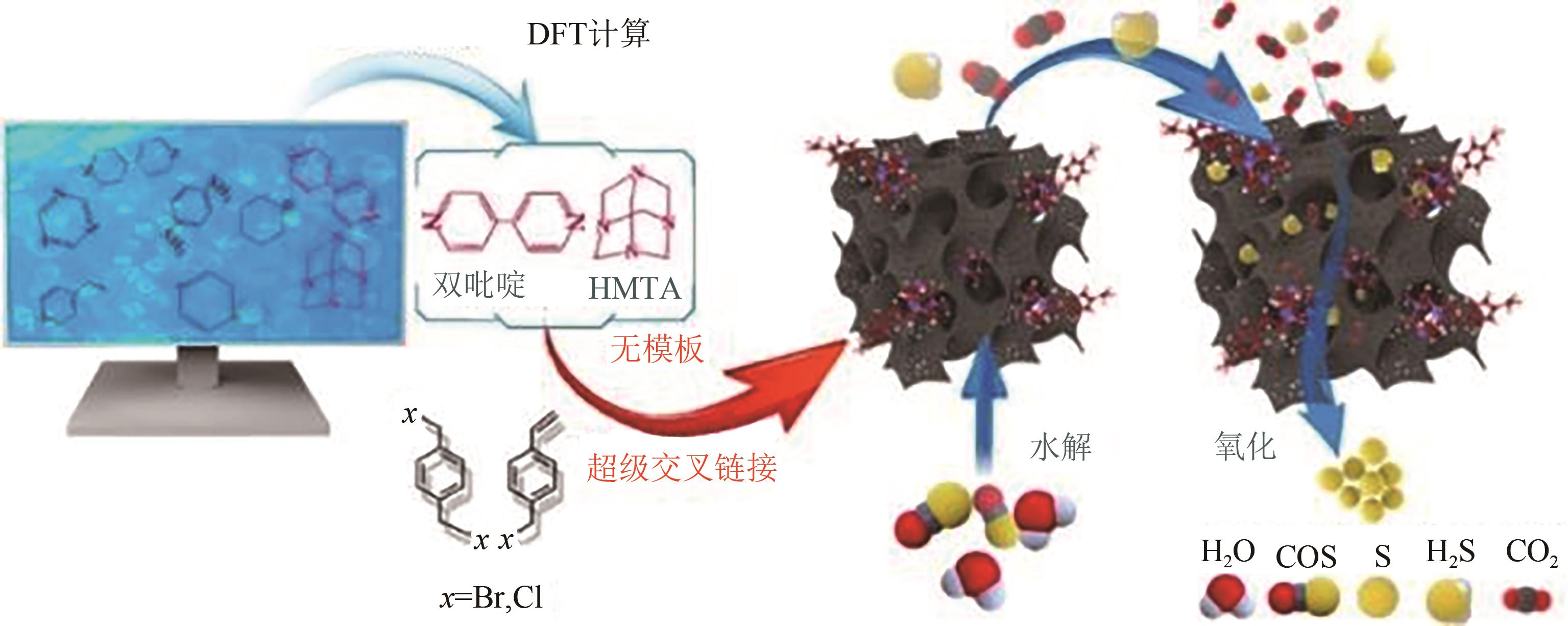
| N-HPP-HMTA | 1397 | 0.83 | 0~140 | 6000 | 50 | H2S:5000mg·kg-1 | 100① | — | [ |
| N-HPP-HMTA | 1397 | 0.83 | 0~140 | 6000 | 180 | COS:100mg·m-3 | 100① | — | [ |
表4 碳基催化剂同步脱除COS和H2S的总结
| 催化剂 | 比表面积/m2·g-1 | 孔体积/cm·g-1 | 平均孔径/nm | 空速/h-1 | 反应温度/℃ | COS/H2S浓度 | 脱除性能 | 穿透容量/mg·g-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Cu-K-Co/AC | 641.48 | 0.31 | 2.29 | 30000 | 60 | H2S:75mg·m-3 COS:125mg·m-3 | 100② | 218.21 | [ |
| Na(0.45)-SAC | 455.05 | 0.22 | 1.89 | 7200 | 60 | H2S:200mg·kg-1 COS:300 mg·kg-1 | 100② | 116.60 | [ |
| Cu-Fe/TSAC | 554 | 0.29 | 0.3~1.6 | 10000 | 60 | H2S:500mg·kg-1 COS:400mg·kg-1 CS2:60mg·kg-1 | 100② | 231.28 | [ |
| 2DNHPC-10 | 567 | 0.50 | 12.1 | 12000 | 180 | H2S:5000mg·kg-1 | 100① | — | [ |
| 2DNHPC-10 | 567 | 0.50 | 12.1 | 12000 | 150 | COS:110mg·m-3 | 100① | — | [ |
| N-HPP-HMTA | 1397 | 0.83 | 0~140 | 6000 | 50 | H2S:5000mg·kg-1 | 100① | — | [ |
| N-HPP-HMTA | 1397 | 0.83 | 0~140 | 6000 | 180 | COS:100mg·m-3 | 100① | — | [ |
| 1 | ZHANG Yongliang, TIAN Zexing, CHEN Xinnan, et al. Technology-environment-economy assessment of high-quality utilization routes for coke oven gas[J]. International Journal of Hydrogen Energy, 2022, 47(1): 666-685. |
| 2 | 上官方钦, 干磊, 周继程, 等. 钢铁工业副产煤气资源化利用分析及案例[J]. 钢铁, 2019, 54(7): 114-120. |
| SHANGGUAN Fangqin, GAN Lei, ZHOU Jicheng, et al. Analysis and case on material conversion utilization of by-product gases in steel industry[J]. Iron & Steel, 2019, 54 (7): 114-120. | |
| 3 | 周守毅. 钢铁企业副产煤气中硫化物的测定[J]. 环境科学与技术, 2017, 40(S1): 252-254. |
| ZHOU Shouyi. Determiningsulfur compound in by-product gas of iron and steel enterprises[J]. Environmental Science & Technology, 2017, 40 (S1): 252-254. | |
| 4 | CAO Rui, NING Ping, WANG Xueqian, et al. Low-temperature hydrolysis of carbonyl sulfide in blast furnace gas using Al2O3-based catalysts with high oxidation resistance[J]. Fuel, 2022, 310: 122295. |
| 5 | YANG Dongdong, CHEN Guoming, FU Jianmin, et al. The mitigation performance of ventilation on the accident consequences of H2S-containing natural gas release[J]. Process Safety and Environmental Protection, 2021, 148: 1327-1336. |
| 6 | 生态环境部. 关于推进实施钢铁行业超低排放的意见[J]. 中国钢铁业, 2019, (6): 5-8. |
| Ministry of Ecological Environment. Opinions on promoting the implementation of ultra low emission in iron and steel industry[J]. China Steel, 2019, (6): 5-8. | |
| 7 | 李翔, 王学谦, 李鹏飞, 等. 高炉煤气特征组分分析及其对脱硫过程的影响研究进展[J]. 化工进展, 2021, 40(12): 6629-6639. |
| LI Xiang, WANG Xueqian, LI Pengfei, et al. Progress on characteristic components analysis of blast furnace gas and its influence on desulfurization process[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6629-6639. | |
| 8 | 王明飞, 陈鹏, 陶雷, 等. 有机硫水解催化剂研究进展[J]. 材料导报, 2022, 36(17): 40-48. |
| WANG Mingfei, CHEN Peng, TAO Lei, et al. Research progress of organic sulfur hydrolysis catalyst[J]. Materials Reports, 2022, 36(17): 40-48. | |
| 9 | 刘雪珂, 张丽, 刘芬, 等. NHD/MDEA/H2O复合脱硫液催化水解羰基硫[J]. 化工学报, 2020, 71(11): 5286-5293. |
| LIU Xueke, ZHANG Li, LIU Fen, et al. Catalytic hydrolysis of carbonyl sulfide with application of NHD/MDEA/H2O[J]. CIESC Journal, 2020, 71(11): 5286-5293. | |
| 10 | KHABAZIPOUR Maryam, ANBIA Mansoor. Removal of hydrogen sulfide from gas streams using porous materials: A review[J]. Industrial & Engineering Chemistry Research, 2019, 58(49): 22133-22164. |
| 11 | 梁键星, 李咸伟, 刘道清, 等. 协同催化水解羰基硫和二硫化碳的低温催化剂的研究进展[J]. 材料导报, 2021, 35(21): 21028-21036. |
| LIANG Jianxing, LI Xianwei, LIU Daoqing, et al. A review of catalysts with activities for simultaneous hydrolyses of carbonyl sulfide and carbon disulfide at low temperatures[J]. Materials Reports, 2021, 35(21): 21028-21036. | |
| 12 | ZHANG Xiaoyang, CUI Lin, AN Donghai, et al. H2S-selective catalytic oxidation to sulfur over iron oxide sorbent supported on semi-coke[J]. Energy & Fuels, 2020, 34(2): 2315-2322. |
| 13 | CUPTA Nishesh Kumar, Jiyeol BAE, KIM Suho, et al. Fabrication of Zn-MOF/ZnO nanocomposites for room temperature H2S removal: Adsorption, regeneration, and mechanism[J]. Chemosphere, 2021, 274: 129789. |
| 14 | YANG Jae Hwan. Hydrogen sulfide removal technology: A focused review on adsorption and catalytic oxidation[J]. Korean Journal of Chemical Engineering, 2021, 38(4): 674-691. |
| 15 | WANG Yifan, DING Long, LONG Hongming, et al. Carbonyl sulfur removal from blast furnace gas: Recent progress, application status and future development[J]. Chemosphere, 2022, 307: 136090. |
| 16 | 于勇, 朱廷钰, 刘霄龙. 中国钢铁行业重点工序烟气超低排放技术进展[J]. 钢铁, 2019, 54(9): 1-11. |
| YU Yong, ZHU Tingyu, LIU Xiaolong. Progress of ultra-low emission technology for key processes of iron and steel industry in China[J]. Iron & Steel, 2019, 54(9): 1-11. | |
| 17 | REZVANI Mohammad Ali, SHATERIAN Maryam, AGHMASHEH Masomeh. Catalytic oxidative desulphurization of gasoline using amphiphilic polyoxometalate@polymer nanocomposite as an efficient, reusable, and green organic-inorganic hybrid catalyst[J]. Environmental Technology, 2020, 41(10): 1219-1231. |
| 18 | LI Haifeng, SU Sheng, HU Song, et al. Effect of preparation conditions on Mn x O y /Al2O3 sorbent for H2S removal from high-temperature synthesis gas[J]. Fuel, 2018, 223: 115-124. |
| 19 | 刘娜, 宁平, 李凯, 等. HCN、COS和CS2催化水解及其水解产物协同净化的研究进展[J]. 化工进展, 2018, 37(1): 301-310. |
| LIU Na, NING Ping, LI Kai, et al. Research progress in catalytic hydrolysis of HCN,COS and CS2 and synergetic purification of hydrolysates[J]. Chemical Industry and Engineering Progress, 2018, 37(1): 301-310. | |
| 20 | ZHANG Ge, YANG Fan, LIU Xiaodong, et al. Tuning surface chemical property in hierarchical porous carbon via nitrogen and phosphorus doping for deep desulfurization[J]. Separation and Purification Technology, 2022, 280: 119923. |
| 21 | 孙秋霞, 卫藩婧, 李宇杰, 等. Mo/MgO-Al2O3催化剂的载体结构对COS加氢性能的影响[J]. 太原理工大学学报, 2020, 51(6): 794-800. |
| SUN Qiuxia, WEI Fanjing, LI Yujie, et al. Effect of support structure on COS hydrogenation property on Mo/MgO-Al2O3 catalyst[J]. Journal of Taiyuan University of Technology, 2020, 51(6): 794-800. | |
| 22 | 杜彩霞. 有机硫加氢转化催化剂的使用[J]. 工业催化, 2003, 11(9): 13-17. |
| DU Caixia. Application techniques of organic sulfur hydroconversion catalysts[J]. Industrial Catalysis, 2003, 11(9): 13-17. | |
| 23 | OKAMOTO Yasuaki, OCHIAI Keiji, KAWANO Masatoshi, et al. Evaluation of the maximum potential activity of Co-Mo/Al2O3 catalysts for hydrodesulfurization[J]. Journal of Catalysis, 2004, 222(1): 143-151. |
| 24 | KAMP Eva, THIELERT Holger, VON MORSTEIN Olaf, et al. Investigation on the simultaneous removal of COS, CS2 and O2 from coke oven gas by hydrogenation on a Pd/Al2O3 catalyst[J]. Catalysis Science & Technology, 2020, 10(9): 2961-2969. |
| 25 | 刘艳霞, 上官炬, 王泽鑫, 等. TiO2改性γ-Al2O3基催化剂的中温水解羰基硫活性[J]. 化工进展, 2018, 37(10): 3885-3894. |
| LIU Yanxia, SHANGGUAN Ju, WANG Zexin, et al. Moderate temperature COS hydrolysis activity of γ-Al2O3 based catalyst modified by TiO2 [J]. Chemical Industry and Engineering Progress, 2018, 37(10): 3885-3894. | |
| 26 | RENDA Simona, BARBA Daniela, PALMA Vincenzo. Recent solutions for efficient carbonyl sulfide hydrolysis: A review[J]. Industrial & Engineering Chemistry Research, 2022, 61(17): 5685-5697. |
| 27 | GEORGE Z M. Kinetics of cobalt-molybdate-catalyzed reactions of SO2 with H2S and COS and the hydrolysis of COS[J]. Journal of Catalysis, 1974, 32(2): 261-271. |
| 28 | TAN Shishao, LI Chunhu, LIANG Shengzhao, et al. Compensation effect in catalytic hydrolysis of carbonyl sulfide at lower temperature compensation effect in COS hydrolysis[J]. Catalysis Letters, 1991, 8(2/3/4): 155-167. |
| 29 | SAUR O, BENSITEL M, SAAD A, et al. The structure and stability of sulfated alumina and titania[J]. Journal of Catalysis, 1986, 99(1): 104-110. |
| 30 | JIN Hongkun, AN Zhongyi, LI Qichao, et al. Catalysts of ordered mesoporous alumina with a large pore size for low-temperature hydrolysis of carbonyl sulfide[J]. Energy & Fuels, 2021, 35(10): 8895-8908. |
| 31 | HE Enyun, HUANG Guan, FAN Huiling, et al. Macroporous alumina- and titania-based catalyst for carbonyl sulfide hydrolysis at ambient temperature[J]. Fuel, 2019, 246: 277-284. |
| 32 | ZHANG Xin, TANG Yuyin, QU Siqiu, et al. H2S-selective catalytic oxidation: Catalysts and processes[J]. ACS Catalysis, 2015, 5(2): 1053-1067. |
| 33 | 邢向文, 张肖阳, 汤吉昀, 等. 基于Fe2O3/TiO2催化剂的H2S选择性催化氧化反应研究[J]. 煤炭学报, 2021, 46(S2): 1088-1095. |
| XING Xiangwen, ZHANG Xiaoyang, TANG Jiyun, et al. Selective catalytic oxidation of H2S over Fe2O3/TiO2 catalysts[J]. Journal of China Coal Society, 2021, 46 (S2): 1088-1095. | |
| 34 | LIU Xinmei, MENG Xin, ZHAO Jiangtao. Synthesis of nanocrystalline iron oxides with mesostructure as desulfurizer[J]. Materials Letters, 2013, 92(2): 255-258. |
| 35 | TERÖRDE R J A M, VAN DEN BRINK P J, VISSER L M, et al. Selective oxidation of hydrogen sulfide to elemental sulfur using iron oxide catalysts on various supports[J]. Catalysis Today, 1993, 17(1/2): 217-224. |
| 36 | ZHENG Xiaohai, LI Yanli, YOU Weilong, et al. Construction of Fe-doped TiO2- x ultrathin nanosheets with rich oxygen vacancies for highly efficient oxidation of H2S[J]. Chemical Engineering Journal, 2022, 430: 132917. |
| 37 | AYESH Ahmad I, ABU-HANI Ayah F S, MAHMOUD Saleh T, et al. Selective H2S sensor based on CuO nanoparticles embedded in organic membranes[J]. Sensors and Actuators B: Chemical, 2016, 231(3): 593-600. |
| 38 | 尹梦雪, 樊飞跃, 赵龙, 等. Cu/Al2O3低温催化氧化H2S研究[J]. 环境科学研究, 2021, 34(5): 1071-1078. |
| YIN Mengxue, FAN Feiyue, ZHAO Long, et al. Catalytic oxidation of H2S by Cu/Al2O3 at low temperature[J]. Research of Environmental Sciences, 2021, 34 (5): 1071-1078. | |
| 39 | PONGTHAWORNSAKUN Boontida, PHATYENCHUEN Suvijak, PANPRANOT Joongjai, et al. The low temperature selective oxidation of H2S to elemental sulfur on TiO2 supported V2O5 catalysts[J]. Journal of Environmental Chemical Engineering, 2018, 6(1): 1414-1423. |
| 40 | QIN F, BROSSEAU C. A review and analysis of microwave absorption in polymer composites filled with carbonaceous particles[J]. Journal of Applied Physics, 2012, 111(6): 061301. |
| 41 | GEORGIADIS Amvrosios, CHARISIOU Nikolaos, GOULA Maria. Removal of hydrogen sulfide from various industrial gases: A review of the most promising adsorbing materials[J]. Catalysts, 2020, 10(5): 521. |
| 42 | 张海鹰, 王旭珍, 赵宗彬, 等. 载Mo和Ni-Mo酚醛树脂基活性炭加氢催化转化羰基硫的研究[J]. 燃料化学学报, 2009, 37(5): 618-623. |
| ZHANG Haiying, WANG Xuzhen, ZHAO Zongbin, et al. Hydrodesulfurization of carbonyl sulfide over the catalysts Mo or Ni-Mo supported on phenolic resin-based activated carbon[J]. Journal of Fuel Chemistry and Technology, 2009, 37(5): 618-623. | |
| 43 | YANG Lan, WANG Xuzhen, LIU Yang, et al. Monolayer MoS2 anchored on reduced graphene oxide nanosheets for efficient hydrodesulfurization[J]. Applied Catalysis B: Environmental, 2017, 200: 211-221. |
| 44 | 张晓东, 易红宏, 唐晓龙, 等. 羰基硫、CS2水解催化剂及脱硫机理研究现状与进展[J]. 化工新型材料, 2020, 48(5): 266-270. |
| ZHANG Xiaodong, YI Honghong, TANG Xiaolong, et al. Research progress of COS, CS2 catalytic hydrolysis and desulfurization mechanism[J]. New Chemical Materials, 2020, 48(5): 266-270. | |
| 45 | WANG Xueqian, QIU Juan, NING Ping, et al. Adsorption/desorption of low concentration of carbonyl sulfide by impregnated activated carbon under micro-oxygen conditions[J]. Journal of Hazardous Materials, 2012, 229/230: 128-136. |
| 46 | 王红妍, 易红宏, 唐晓龙, 等. 改性活性炭催化水解羰基硫[J]. 中南大学学报(自然科学版), 2011, 42(3): 848-852. |
| WANG Hongyan, YI Honghong, TANG Xiaolong, et al. Catalytic hydrolysis of carbonyl sulfide over modified activated carbon[J]. Journal of Central South University (Science and Technology), 2011, 42(3): 848-852. | |
| 47 | NING Ping, YU Lili, YI Honghong, et al. Effect of Fe/Cu/Ce loading on the coal-based activated carbons for hydrolysis of carbonyl sulfide[J]. Journal of Rare Earths, 2010, 28(2): 205-210. |
| 48 | BANDOSZ Teresa J. Effect of pore structure and surface chemistry of virgin activated carbons on removal of hydrogen sulfide[J]. Carbon, 1999, 37(3): 483-491. |
| 49 | SUN Minghui, WANG Xuzhen, ZHAO Zongbin, et al. Review of H2S selective oxidation over carbon-based materials at low temperature: from pollutant to energy storage materials[J]. New Carbon Materials, 2022, 37(4): 675-694. |
| 50 | 方惠斌, 赵建涛, 黄戒介, 等. 活性炭担载金属氧化物用于热煤气脱硫[J]. 化学工程, 2010, 38(10): 56-59. |
| FANG Huibin, ZHAO Jiantao, HUANG Jiejie, et al. Activated carbon-supported metal oxides for hot gas desulfurization[J]. Chemical Engineering(China), 2010, 38(10): 56-59. | |
| 51 | ZHANG Zixiao, WANG Jitong, LI Wencheng, et al. Millimeter-sized mesoporous carbon spheres for highly efficient catalytic oxidation of hydrogen sulfide at room temperature[J]. Carbon, 2016, 96: 608-615. |
| 52 | YANG Chao, YANG Song, FAN Huilin, et al. A sustainable design of ZnO-based adsorbent for robust H2S uptake and secondary utilization as hydrogenation catalyst[J]. Chemical Engineering Journal, 2020, 382: 122892. |
| 53 | MU Guanyu, ZENG Yan, ZHENG Yong, et al. Oxygen vacancy defects engineering on Cu-doped Co3O4 for promoting effective COS hydrolysis[J]. Green Energy & Environment, 2021. |
| 54 | LIU Dai, CHEN Shaoyun, FEI Xiaoyao, et al. Regenerable CuO-based adsorbents for low temperature desulfurization application[J]. Industrial & Engineering Chemistry Research, 2015, 54(14): 3556-3562. |
| 55 | LI Lu, ZHANG Hongbo, ZHOU Pin, et al. Three dimensional ordered macroporous zinc ferrite composited silica sorbents with promotional desulfurization and regeneration activity at mid-high temperature[J]. Applied Surface Science, 2019, 470: 177-186. |
| 56 | KARIM Ansaf V, HASSANI Aydin, EGHBALI Paria, et al. Nanostructured modified layered double hydroxides (LDHs)-based catalysts: A review on synthesis, characterization, and applications in water remediation by advanced oxidation processes[J]. Current Opinion in Solid State and Materials Science, 2022, 26(1): 100965. |
| 57 | CHANG Bingwei, WU Mengmeng, MI Jie. Pyrolysis kinetics of ZnAl LDHs and its calcined products for H2S removal[J]. Journal of Thermal Analysis and Calorimetry, 2018, 132(1): 581-589. |
| 58 | ZHANG Guanqing, KAN Xun, ZHENG Yong, et al. A solid thermal and fast synthesis of MgAl-hydrotalcite nanosheets and their applications in the catalytic elimination of carbonyl sulfide and hydrogen sulfide[J]. New Journal of Chemistry, 2021, 45(7): 3535-3545. |
| 59 | YI Honghong, ZHAO Shunzheng, TANG Xiaolong, et al. Influence of calcination temperature on the hydrolysis of carbonyl sulfide over hydrotalcite-derived Zn-Ni-Al catalyst[J]. Catalysis Communications, 2011, 12(15): 1492-1495. |
| 60 | LI Xiang, WANG Xueqian, WANG Langlang, et al. Efficient removal of carbonyl sulfur and hydrogen sulfide from blast furnace gas by one-step catalytic process with modified activated carbon[J]. Applied Surface Science, 2022, 579: 152189. |
| 61 | MA Mingyu, LI Changming, LI Yunjia, et al. Resource utilization of spent activated coke as an efficient material for simultaneous removal of COS and H2S[J]. Energy & Fuels, 2022, 36(9): 4837-4846. |
| 62 | SUN Xin, RUAN Haotian, SONG Xin, et al. Research into the reaction process and the effect of reaction conditions on the simultaneous removal of H2S, COS and CS2 at low temperature[J]. RSC Advances, 2018, 8(13): 6996-7004. |
| 63 | LIANG Shijing, LIU Fujian, JIANG Lilong. Recent advances on nitrogen-doped metal-free materials for the selective catalytic oxidation of hydrogen sulfide[J]. Current Opinion in Green and Sustainable Chemistry, 2020, 25: 100361. |
| 64 | LIANG Shijing, MI Jinxing, LIU Fujian, et al. Efficient catalytic elimination of COS and H2S by developing ordered mesoporous carbons with versatile base N sites via a calcination induced self-assembly route[J]. Chemical Engineering Science, 2020, 221: 115714. |
| 65 | KAN Xun, ZHANG Guanqing, LUO Yingying, et al. Efficient catalytic removal of COS and H2S over graphitized 2D micro-meso-macroporous carbons endowed with ample nitrogen sites synthesized via mechanochemical carbonization[J]. Green Energy & Environment, 2022, 7(5): 983-995. |
| 66 | MI Jinxing, LIU Fujian, CHEN Wei, et al. Design of efficient, hierarchical porous polymers endowed with tunable structural base sites for direct catalytic elimination of COS and H2S[J]. ACS Applied Materials & Interfaces, 2019, 11(33): 29950-29959. |
| 67 | YAO Xiaoqian, LI Yongwang. Density functional theory study on the hydrodesulfurization reactions of COS and CS2 with Mo3S9 model catalyst[J]. Journal of Molecular Structure: Theochem, 2009, 899(1/2/3): 32-41. |
| 68 | 李春虎, 郭汉贤, 谈世韶. 碱改性γ-Al2O3催化剂表面碱强度分布与COS水解活性的研究[J]. 分子催化, 1994, 8(4): 305-314. |
| LI Chunhu, GUO Hanxian, TAN Shishao. Study on the alkalized γ-Al2O3 catalyst for its base strength distribution and catalytic activity[J]. Journal of Molecular Catalysis (China), 1994, 8(4): 305-314. | |
| 69 | GU Jianan, LIANG Jianxing, HU Songjie, et al. Enhanced removal of COS from blast furnace gas via catalytic hydrolysis over Al2O3-based catalysts: Insight into the role of alkali metal hydroxide[J]. Separation and Purification Technology, 2022, 295: 121356. |
| 70 | 尹梦雪, 樊飞跃, 赵龙, 等. 硫化氢催化氧化技术的研究进展[J]. 环境工程技术学报, 2020, 10(3): 475-481. |
| YIN Mengxue, FAN Feiyue, ZHAO Long, et al. Research progress of catalytic oxidation technologies of hydrogen sulfide[J]. Journal of Environmental Engineering Technology, 2020, 10(3): 475-481. | |
| 71 | KANE Tanushree, GUERRERO CABALLERO Jesús, Axel LÖFBERG. Chemical looping selective oxidation of H2S using V2O5 impregnated over different supports as oxygen carriers[J]. ChemCatChem, 2020, 12(9): 2569-2579. |
| 72 | PHATYENCHUEN Suvijak, PONGTHAWORNSAKUN Boontida, PANPRANOT Joongjai, et al. Effect of transition metal dopants (M= Nb, La, Zr, and Y) on the M-TiO2 supported V2O5 catalysts in the selective oxidation of H2S to elemental sulfur[J]. Journal of Environmental Chemical Engineering, 2018, 6(5): 5655-5661. |
| 73 | JANGAM Kalyani, CHEN Yu-Yen, QIN Lang, et al. Perspectives on reactive separation and removal of hydrogen sulfide[J]. Chemical Engineering Science: X, 2021, 11: 100105. |
| 74 | Jürgen KLEIN, HENNING Klaus-Dirk. Catalytic oxidation of hydrogen sulphide on activated carbons[J]. Fuel, 1984, 63(8): 1064-1067. |
| 75 | 梁美生, 李春虎, 郭汉贤, 等. 红外光谱法对COS水解催化剂氧中毒行为的研究[J]. 燃料化学学报, 2002, 30(4): 347-352. |
| LIANG Meisheng, LI Chunhu, GUO Hanxian, et al. Ftir study on oxygen poisoning behavior of COS hydrolysis catalyst[J]. Journal of Fuel Chemistry and Technology, 2002, 30(4): 347-352. | |
| 76 | Hanki EOM, JANG Younghee, CHOI Sung Yeol, et al. Application and regeneration of honeycomb-type catalysts for the selective catalytic oxidation of H2S to sulfur from landfill gas[J]. Applied Catalysis A: General, 2020, 590: 117365. |
| 77 | CHEN Shanshan, GUO Yiyue, ZHANG Jianan, et al. CuFe2O4/activated carbon adsorbents enhance H2S adsorption and catalytic oxidation from humidified air at room temperature[J]. Chemical Engineering Journal, 2022, 431: 134097. |
| 78 | 李勇. 铁盐改性污泥-秸秆基活性炭及其脱除H2S性能研究[D]. 武汉: 华中科技大学, 2017. |
| LI Yong. Ferric salt modified sludge-stalk based activated carbon and removal of H2S[D]. Wuhan: Huazhong University of Science and Technology, 2017. | |
| 79 | 阮昊天. 烟杆基生物炭载体催化剂同时脱除H2S、COS和CS2的研究[D]. 昆明: 昆明理工大学, 2017. |
| RUAN Haotian. Simultaneous removal of H2S, COS and CS2 with tobacco stem biochar carrier catalyst[D]. Kunming: Kunming University of Science and Technology, 2017. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||