化工进展 ›› 2023, Vol. 42 ›› Issue (1): 247-254.DOI: 10.16085/j.issn.1000-6613.2022-0513
核壳结构在甲烷干重整中的应用
- 东南大学能源与环境学院,江苏 南京 210096
-
收稿日期:2022-03-29修回日期:2022-06-21出版日期:2023-01-25发布日期:2023-02-20 -
通讯作者:边洲峰 -
作者简介:邓少碧(1999—),女,硕士研究生,研究方向为催化重整制氢。E-mail:1984599571@qq.com。 -
基金资助:国家自然科学基金青年科学基金(5200060197);江苏省基础研究计划青年项目(BK20200370)
Application of core-shell structure catalyst in dry reforming of methane
DENG Shaobi( ), BIAN Zhoufeng(
), BIAN Zhoufeng( )
)
- School of Energy and Environment, Southeast University, Nanjing 210096, Jiangsu, China
-
Received:2022-03-29Revised:2022-06-21Online:2023-01-25Published:2023-02-20 -
Contact:BIAN Zhoufeng
摘要:
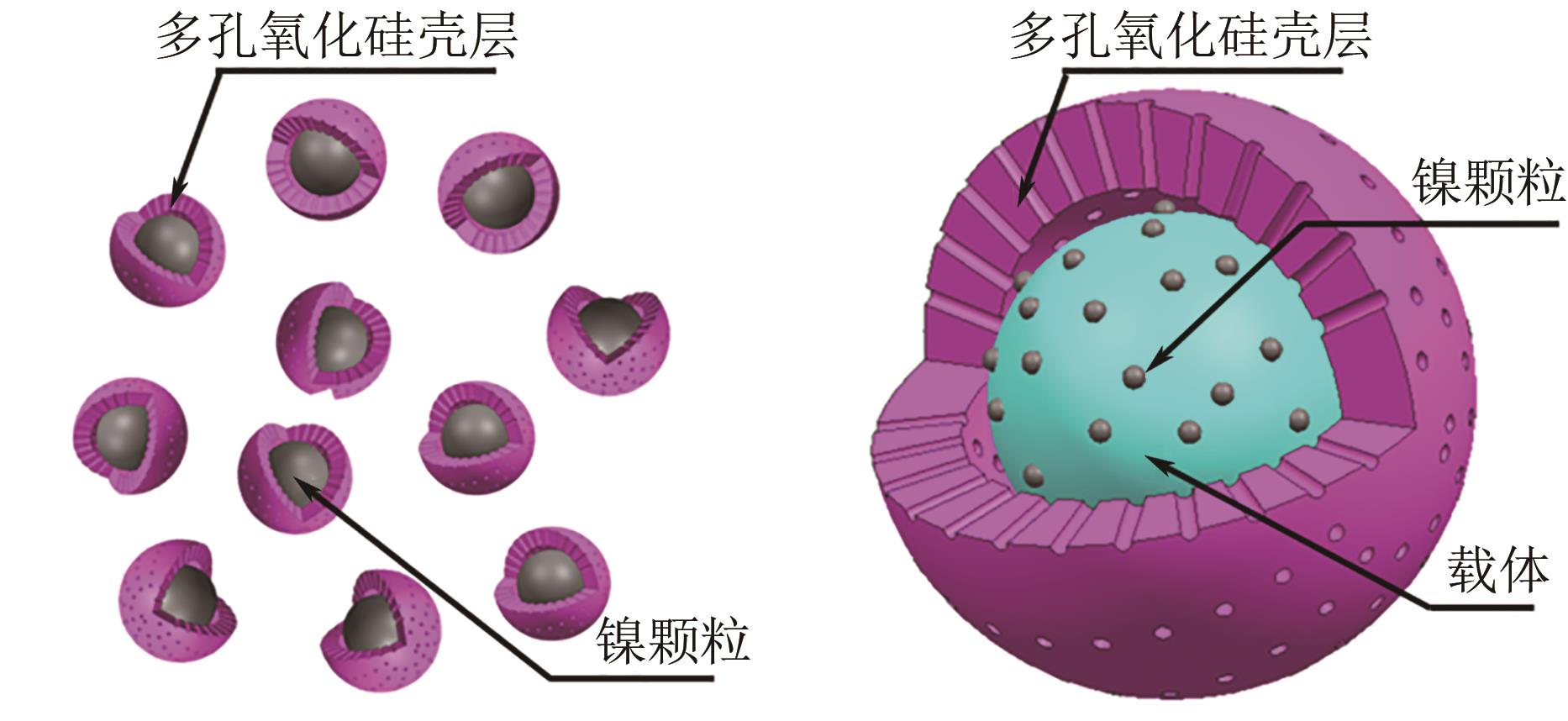
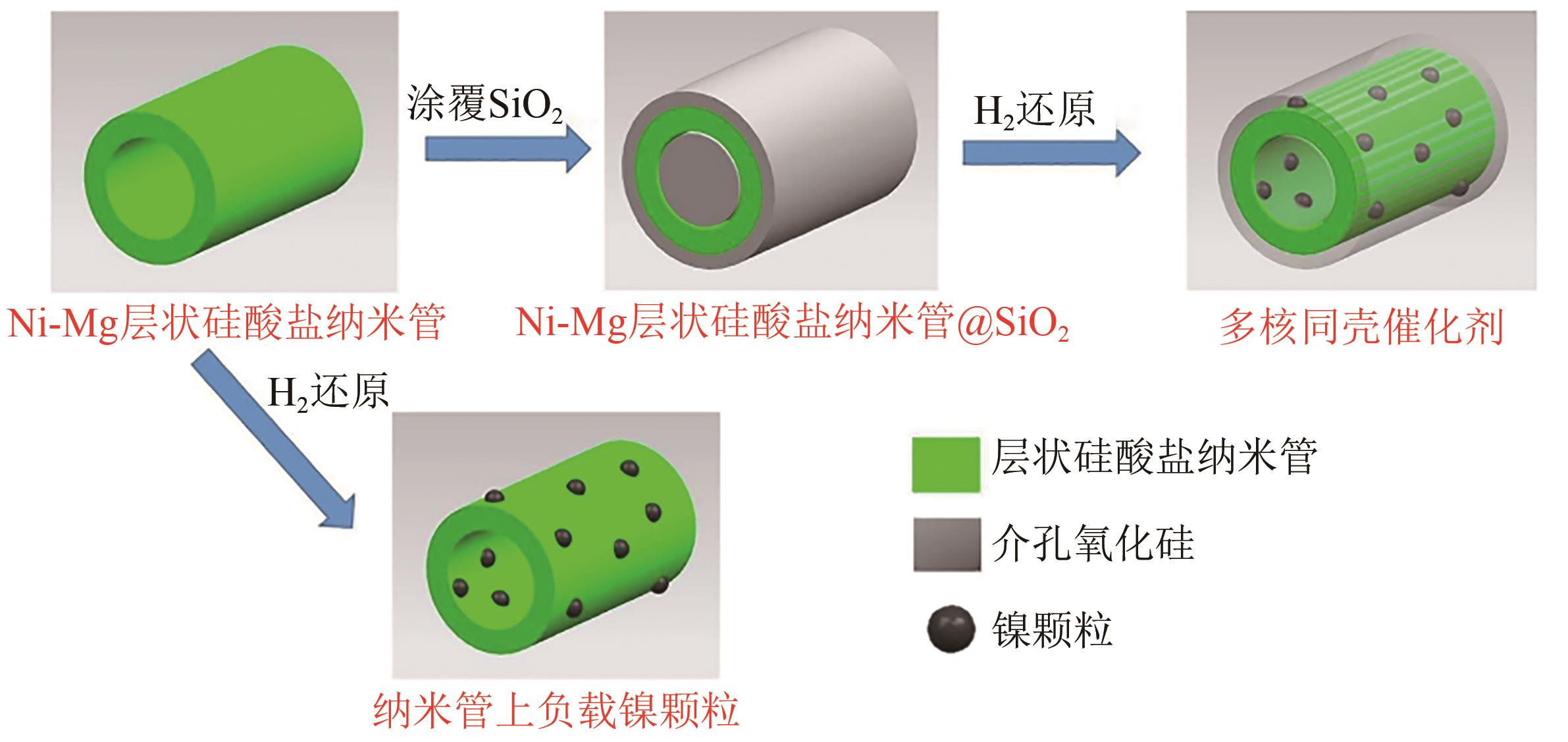
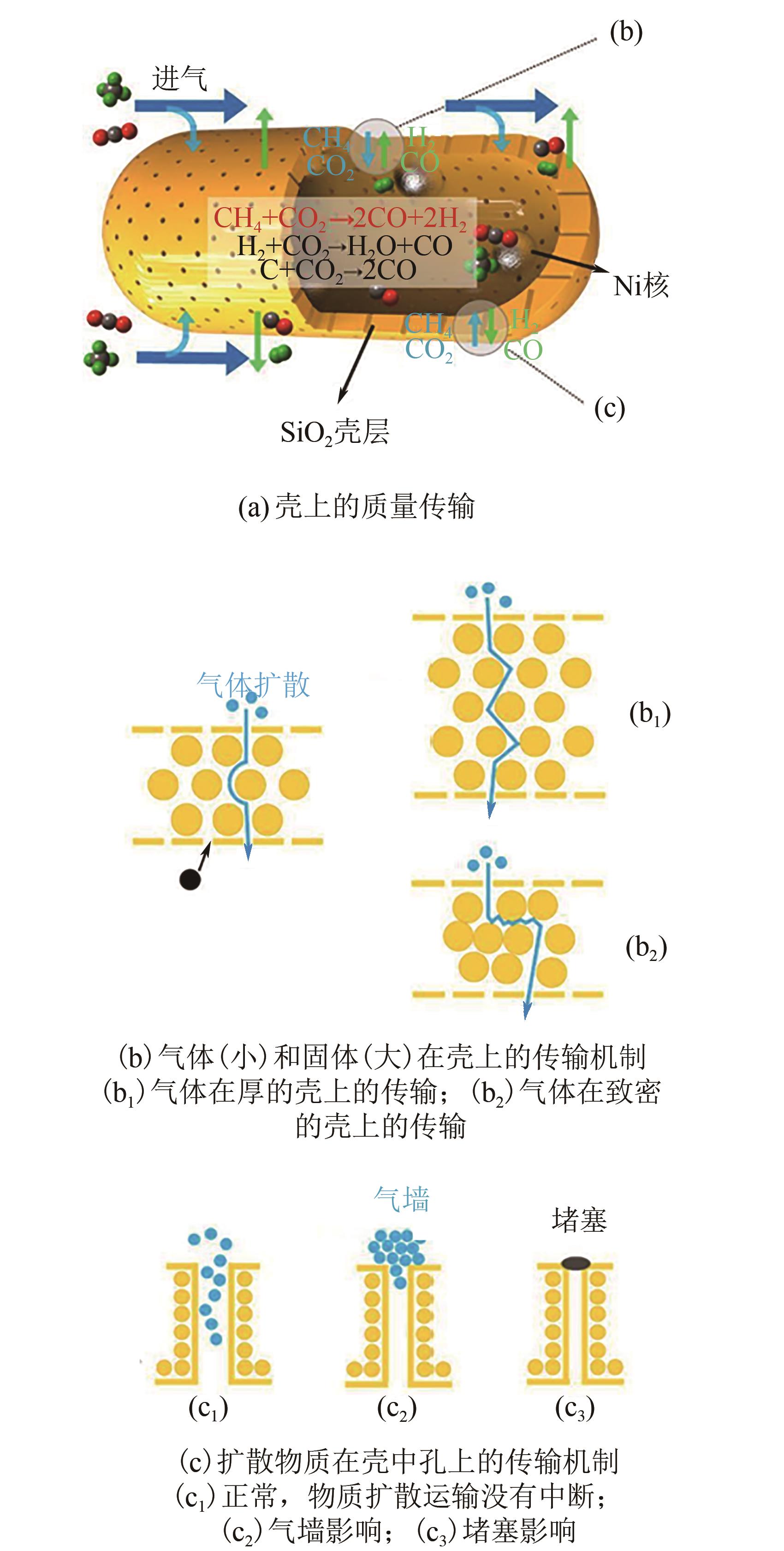
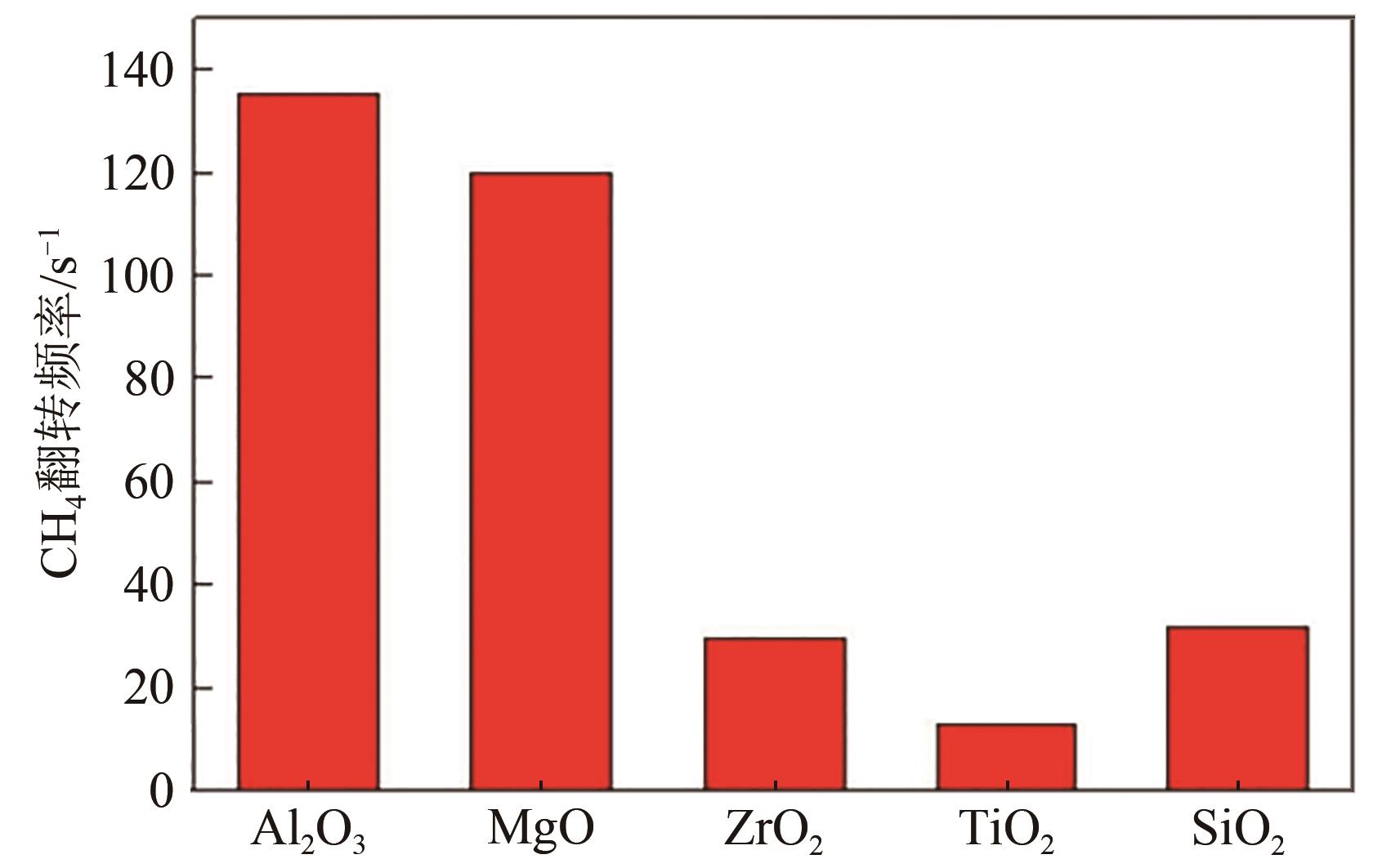
甲烷干重整可以将两种温室气体(CO2和CH4)转化为合成气,传统负载型催化剂存在金属烧结、碳沉积的问题,导致失活。核壳结构催化剂具有空间限域效应,能有效解决以上问题。本文根据壳的种类,将核壳结构分为SiO2壳层、Al2O3壳层和其他壳层三类,并分别从制备方法、形貌结构、催化特性的角度介绍了研究现状。文中指出:氧化硅壳层的优势是制备简单,壳层易于调控,热稳定性高;Al2O3壳层能够提供碱性位点,增强CO2吸附与反应;CeO2壳层则可以提供氧空位,促进CO2活化和积炭的气化。据此,本文展望了核壳结构在未来的几个研究方向:对壳层材料的拓展与研究;对蛋黄壳、三明治等新型核壳结构的研究;精准调节核壳结构的形态并研究构效关系;大规模制备和工业应用等。
中图分类号:
引用本文
邓少碧, 边洲峰. 核壳结构在甲烷干重整中的应用[J]. 化工进展, 2023, 42(1): 247-254.
DENG Shaobi, BIAN Zhoufeng. Application of core-shell structure catalyst in dry reforming of methane[J]. Chemical Industry and Engineering Progress, 2023, 42(1): 247-254.
| 1 | GAO Wanlin, LIANG Shuyu, WANG Rujie, et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| 2 | 邵斌, 孙哲毅, 章云, 等. 二氧化碳转化为合成气及高附加值产品的研究进展[J]. 化工进展, 2022, 41(3): 1136-1151. |
| SHAO Bin, SUN Zheyi, ZHANG Yun, et al. Recent progresses in CO2 to syngas and high value-added products[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1136-1151. | |
| 3 | 周伟, 成康, 张庆红, 等. 合成气转化中的接力催化[J]. 科学通报, 2021, 66(10): 1157-1169. |
| ZHOU Wei, CHENG Kang, ZHANG Qinghong, et al. Relay catalysis in the conversion of syngas[J]. Chinese Science Bulletin, 2021, 66(10): 1157-1169. | |
| 4 | ZAIN M M, MOHAMED A R. An overview on conversion technologies to produce value added products from CH4 and CO2 as major biogas constituents[J]. Renewable and Sustainable Energy Reviews, 2018, 98: 56-63. |
| 5 | 阮勇哲, 卢遥, 王胜平. 甲烷干重整Ni基催化剂失活及抑制失活研究进展[J]. 化工进展, 2018, 37(10): 3850-3857. |
| RUAN Yongzhe, LU Yao, WANG Shengping. Progress in deactivation and anti-deactivation of nickel-based catalysts for methane dry reforming[J]. Chemical Industry and Engineering Progress, 2018, 37(10): 3850-3857. | |
| 6 | 林俊明, 岑洁, 李正甲, 等. Ni基重整催化剂失活机理研究进展[J]. 化工进展, 2022, 41(1): 201-209. |
| LIN Junming, CEN Jie, LI Zhengjia, et al. Development on deactivation mechanism of Ni-based reforming catalysts[J]. Chemical Industry and Engineering Progress, 2022, 41(1): 201-209. | |
| 7 | 史健, 祝星, 李孔斋, 等. 甲烷干重整及金属-载体相互作用[J]. 石油学报(石油加工), 2020, 36(6): 1407-1418. |
| SHI Jian, ZHU Xing, LI Kongzhai, et al. Dry reforming of methane and metal-support interactions[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2020, 36(6): 1407-1418. | |
| 8 | 郑幼松, 邹宗鹏, 吕莉, 等. 甲烷干重整抗失活镍基催化剂研究进展[J]. 天然气化工(C1化学与化工), 2021, 46(6): 1-8, 16. |
| ZHENG Yousong, ZOU Zongpeng, Li LYU, et al. Research progress of anti-deactivation nickel based catalysts for dry reforming of methane[J]. Natural Gas Chemical Industry, 2021, 46(6): 1-8, 16. | |
| 9 | LI Xinyu, LI Di, TIAN Hao, et al. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles[J]. Applied Catalysis B: Environmental, 2017, 202: 683-694. |
| 10 | SORCAR S, DAS J, KOMARALA E P, et al. Design of coke-free methane dry reforming catalysts by molecular tuning of nitrogen-rich combustion precursors[J]. Materials Today Chemistry, 2022, 24: 100765. |
| 11 | SASSON B J, HE T, NESTLER E, et al. Utilizing bimetallic catalysts to mitigate coke formation in dry reforming of methane[J]. Journal of Energy Chemistry, 2022, 68: 124-142. |
| 12 | TORREZ-HERRERA J J, KORILI S A, GIL A. Bimetallic (Pt-Ni) La-hexaaluminate catalysts obtained from aluminum saline slags for the dry reforming of methane[J]. Chemical Engineering Journal, 2022, 433: 133191. |
| 13 | AHMAD Y H, MOHAMED A T, KUMAR A, et al. Solution combustion synthesis of Ni/La2O3 for dry reforming of methane: Tuning the basicity via alkali and alkaline earth metal oxide promoters[J]. RSC Advances, 2021, 11(53): 33734-33743. |
| 14 | DELIR K N P, BEKHEET M F, BONMASSAR N, et al. Elucidating the role of earth alkaline doping in perovskite-based methane dry reforming catalysts[J]. Catalysis Science & Technology, 2022, 12(4): 1229-1244. |
| 15 | DAS S, PÉREZ-RAMÍREZ J, GONG J L, et al. Core-shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2 [J]. Chemical Society Reviews, 2020, 49(10): 2937-3004. |
| 16 | 蔡雨露, 田静卓, 张晓雪, 等. 镍基核壳结构催化剂的制备及其在甲烷二氧化碳催化重整中的应用[J]. 天然气化工(C1化学与化工), 2020, 45(1): 103-107. |
| CAI Yulu, TIAN Jingzhuo, ZHANG Xiaoxue, et al. Preparation of nickel-based core-shell catalysts and their application in carbon dioxide reforming of methane[J]. Natural Gas Chemical Industry, 2020, 45(1): 103-107. | |
| 17 | ZHANG Junshe, LI Fanxing. Coke-resistant Ni@SiO2 catalyst for dry reforming of methane[J]. Applied Catalysis B: Environmental, 2015, 176/177: 513-521. |
| 18 | PENG Honggen, ZHANG Xianhua, ZHANG Li, et al. One-pot facile fabrication of multiple nickel nanoparticles confined in microporous silica giving a multiple-cores@shell structure as a highly efficient catalyst for methane dry reforming[J]. ChemCatChem, 2017, 9(1): 127-136. |
| 19 | PANG Yijun, ZHONG Aihua, XU Zhijia, et al. How do core-shell structure features impact on the activity/stability of the Co-based catalyst in dry reforming of methane?[J]. ChemCatChem, 2018, 10(13): 2845-2857. |
| 20 | YANG Juanjuan, WANG Jiaqi, ZHAO Jingjing, et al. CO2 conversion via dry reforming of methane on a core-shell Ru@SiO2 catalyst[J]. Journal of CO2 Utilization, 2022, 57: 101893. |
| 21 | ZHAO Yu, LI Hui, LI Hexing. NiCo@SiO2 core-shell catalyst with high activity and long lifetime for CO2 conversion through DRM reaction[J]. Nano Energy, 2018, 45: 101-108. |
| 22 | LIU Wenming, LI Le, LIN Sixue, et al. Confined Ni-In intermetallic alloy nanocatalyst with excellent coking resistance for methane dry reforming[J]. Journal of Energy Chemistry, 2022, 65: 34-47. |
| 23 | BIAN Zhoufeng, KAWI Sibudjing. Sandwich-likesilica@Ni@silica multicore-shell catalyst for the low-temperature dry reforming of methane: Confinement effect against carbon formation[J]. ChemCatChem, 2018, 10(1): 320-328. |
| 24 | BIAN Z F, SURYAWINATA I Y, KAWI S. Highly carbon resistant multicore-shell catalyst derived from Ni-Mg phyllosilicate nanotubes@silica for dry reforming of methane[J]. Applied Catalysis B: Environmental, 2016, 195: 1-8. |
| 25 | ZHAO Xiaoyuan, LI Hongrui, ZHANG Jianping, et al. Design and synthesis of NiCe@m-SiO2 yolk-shell framework catalysts with improved coke-and sintering-resistance in dry reforming of methane[J]. International Journal of Hydrogen Energy, 2016, 41(4): 2447-2456. |
| 26 | LI Ziwei, MO Liuye, KATHIRASER Yasotha, et al. Yolk-satellite-shell structured Ni-Yolk@Ni@SiO2 nanocomposite: Superb catalyst toward methane CO2 reforming reaction[J]. ACS Catalysis, 2014, 4(5): 1526-1536. |
| 27 | PANG Yijun, DOU Yixuan, ZHONG Aihua, et al. Nanostructured Ru-Co@SiO2: Highly efficient yet durable for CO2 reforming of methane with a desirable H2/CO ratio[J]. Applied Catalysis A: General, 2018, 555: 27-35. |
| 28 | WANG Changzhen, WU Hao, Xiangyu JIE, et al. Yolk-shell nanocapsule catalysts as nanoreactors with various shell structures and their diffusion effect on the CO2 reforming of methane[J]. ACS Applied Materials & Interfaces, 2021, 13(27): 31699-31709. |
| 29 | LI Yunhua, WANG Yaquan, ZHANG Xiangwen, et al. Thermodynamic analysis of autothermal steam and CO2 reforming of methane[J]. International Journal of Hydrogen Energy, 2008, 33(10): 2507-2514. |
| 30 | CHAI Ruijuan, ZHAO Guofeng, ZHANG Zhiqiang, et al. High sintering-/coke-resistance Ni@SiO2/Al2O3/FeCrAl-fiber catalyst for dry reforming of methane: One-step, macro-to-nano organization via cross-linking molecules[J]. Catalysis Science & Technology, 2017, 7(23): 5500-5504. |
| 31 | NAKAMURA J, AIKAWA K, SATO K, et al. Role of support in reforming of CH4 with CO2 over Rh catalysts[J]. Catalysis Letters, 1994, 25(3/4): 265-270. |
| 32 | HUANG Qiong, FANG Xiuzhong, CHENG Qinzhen, et al. Synthesis of a highly active and stable nickel-embedded alumina catalyst for methane dry reforming: On the confinement effects of alumina shells for nickel nanoparticles[J]. ChemCatChem, 2017, 9(18): 3563-3571. |
| 33 | BAKTASH E, LITTLEWOOD P, SCHOMäCKER R, et al. Alumina coated nickel nanoparticles as a highly active catalyst for dry reforming of methane[J]. Applied Catalysis B: Environmental, 2015, 179: 122-127. |
| 34 | WANG S B, LU G Q, MILLAR G J. Carbon dioxide reforming of methane to produce synthesis gas over metal-supported catalysts: state of the art[J]. Energy & Fuels, 1996, 10(4): 896-904. |
| 35 | GOULD T, IZAR A, WEIMER A, et al. Stabilizing Ni catalysts by molecular layer deposition for harsh, dry reforming conditions[J]. ACS Catalysis, 2014, 4: 2714-2717. |
| 36 | ZHAO Yu, KANG Yunqing, LI Hui, et al. CO2 conversion to synthesis gas via DRM on the durable Al2O3/Ni/Al2O3 sandwich catalyst with high activity and stability[J]. Green Chemistry, 2018, 20(12): 2781-2787. |
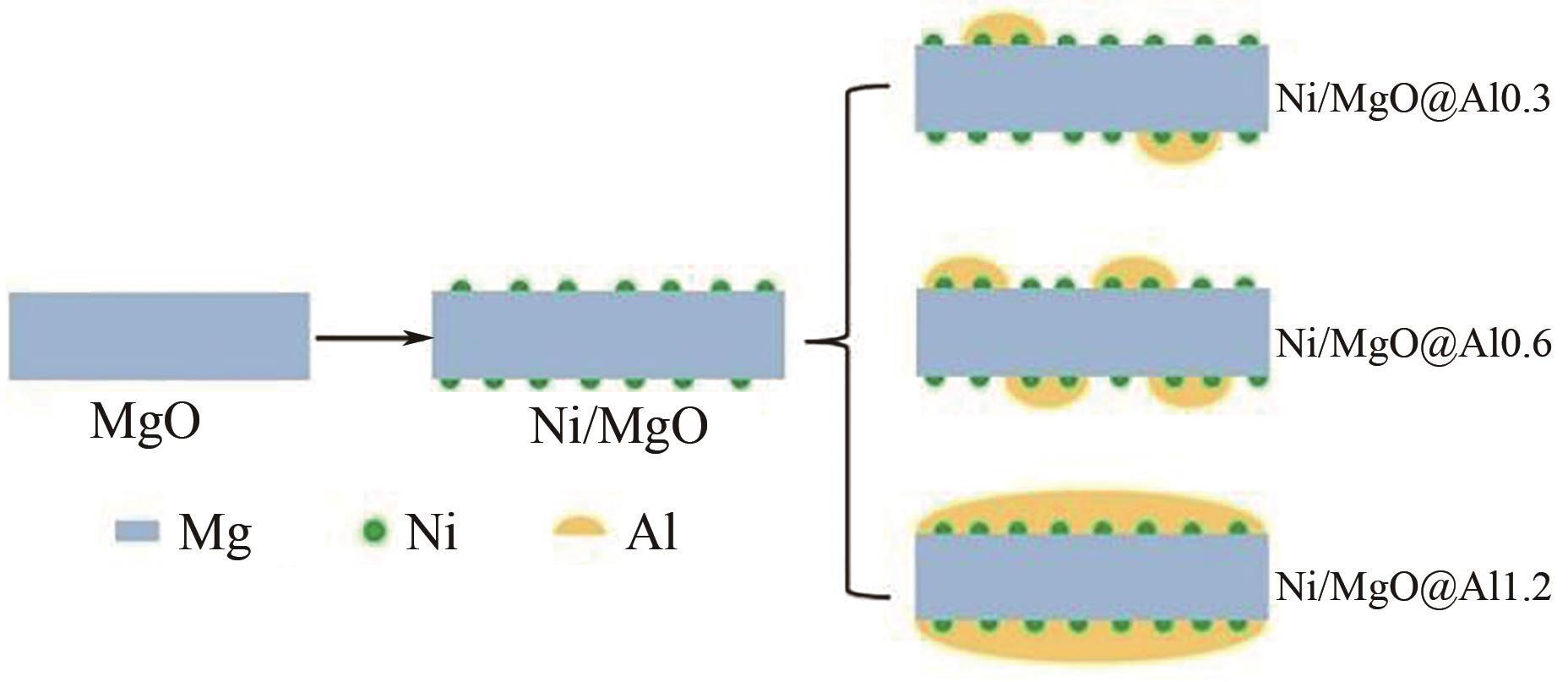
| 37 | DAI Hui, ZHU Yongqing, XIONG Siqi, et al. Dry reforming of methane over Ni/MgO@Al catalysts with unique features of sandwich structure[J]. Chemistryselect, 2021, 6(48): 13862-13872. |
| 38 | HAN J W, PARK J S, CHOI M S, et al. Uncoupling the size and support effects of Ni catalysts for dry reforming of methane[J]. Applied Catalysis B: Environmental, 2017, 203: 625-632. |
| 39 | TANG Chengli, LV Liping, ZHANG Limei, et al. High carbon-resistance Ni@CeO2 core-shell catalysts for dry reforming of methane[J]. Kinetics and Catalysis, 2017, 58(6): 800-808. |
| 40 | HAN Kaihang, YU Weishu, XU Leilei, et al. Reducing carbon deposition and enhancing reaction stability by ceria for methane dry reforming over Ni@SiO2@CeO2 catalyst[J]. Fuel, 2021, 291: 120182. |
| 41 | LI Ziwei, SIBUDJING Kawi. Facile synthesis of multi-Ni-core@Ni phyllosilicate@CeO2 shell hollow spheres with high oxygen vacancy concentration for dry reforming of CH4 [J]. ChemCatChem, 2018, 10(14): 2994-3001. |
| 42 | 任枭雄, 邱泽刚, 李志勤. ZrO2制备方法研究进展[J]. 煤化工, 2021, 49(1): 18-22. |
| REN Xiaoxiong, QIU Zegang, LI Zhiqin. Research progress on preparation method of ZrO2 [J]. Coal Chemical Industry, 2021, 49(1): 18-22. | |
| 43 | DOU Jian, ZHANG Riguang, HAO Xiaobin, et al. Sandwiched SiO2@Ni@ZrO2 as a coke resistant nanocatalyst for dry reforming of methane[J]. Applied Catalysis B: Environmental, 2019, 254: 612-623. |
| 44 | DAI Chengyi, ZHANG Shaohua, ZHANG Anfeng, et al. Hollow zeolite encapsulated Ni-Pt bimetals for sintering and coking resistant dry reforming of methane[J]. Journal of Materials Chemistry A: Materials for Energy and Sustainability, 2015, 3(32): 16461-16468. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [7] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [8] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [9] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [10] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [11] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [12] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [13] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [14] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [15] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||