化工进展 ›› 2022, Vol. 41 ›› Issue (9): 4884-4894.DOI: 10.16085/j.issn.1000-6613.2021-2399
制备聚酰胺复合膜中界面聚合反应添加剂研究进展
张赛晖1,2( ), 李校阳1,2, 高慧2,3, 王丽丽1,2
), 李校阳1,2, 高慧2,3, 王丽丽1,2
- 1.天津工业大学化学学院,天津 300387
2.省部共建分离膜与膜过程国家重点实验室,天津 300387
3.天津工业大学环境科学与工程学院,天津 300387
-
收稿日期:2021-11-23修回日期:2022-01-23出版日期:2022-09-25发布日期:2022-09-27 -
通讯作者:张赛晖 -
作者简介:张赛晖(1981—),男,博士,讲师,硕士生导师,研究方向为分离膜材料及膜过程。E-mail:zhangsh_tjpu@126.com。 -
基金资助:天津市教委科研计划(2017KJ078)
Recent progress in additives in interfacial polymerization for the preparation of polyamide composite membrane
ZHANG Saihui1,2( ), LI Xiaoyang1,2, GAO Hui2,3, WANG Lili1,2
), LI Xiaoyang1,2, GAO Hui2,3, WANG Lili1,2
- 1.School of Chemistry, Tiangong University, Tianjin 300387, China
2.School of Environmental Science and Engineering, Tiangong University, Tianjin 300387, China
3.State Key Laboratory of Separation Membranes and Membrane Processes, Tiangong University, Tianjin 300387, China
-
Received:2021-11-23Revised:2022-01-23Online:2022-09-25Published:2022-09-27 -
Contact:ZHANG Saihui
摘要:
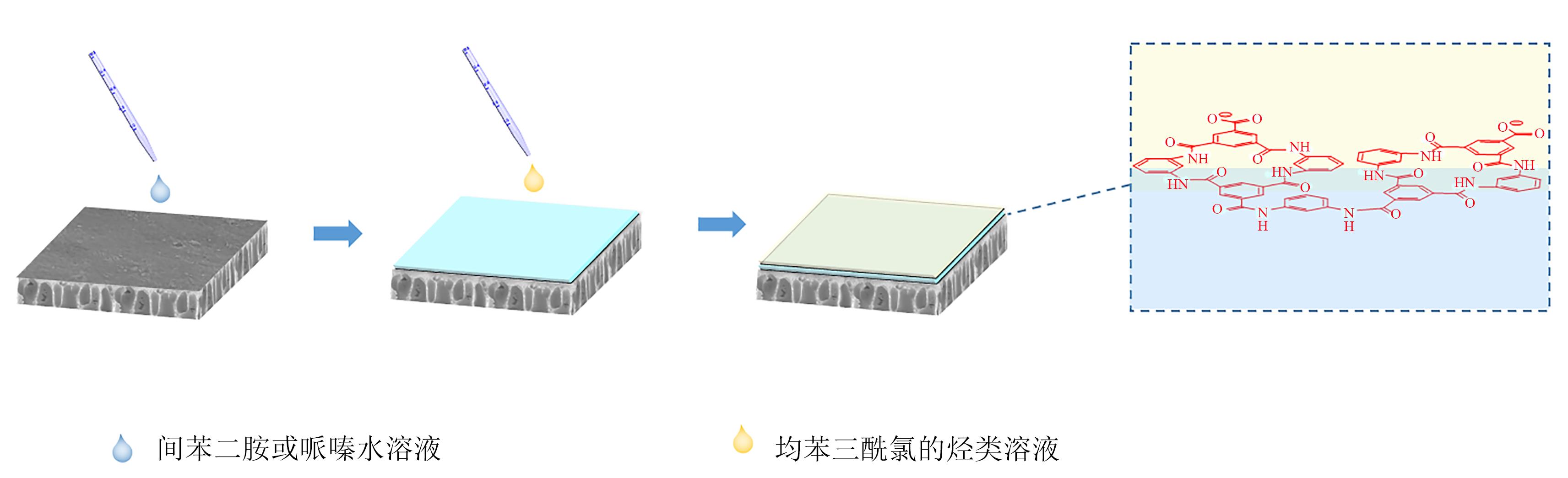
聚酰胺复合膜以其优良的稳定性及良好的分离选择性成为水处理和化工分离领域应用范围最广的分离材料之一。聚酰胺复合膜一般采用界面聚合法制备,由于界面聚合反应活性高、反应参数多,致使界面层结构难以控制,膜的渗透性或选择性不理想。因此,如何有效调控膜结构,实现膜的高渗透性或选择性是目前面临的重要挑战。近期诸多研究表明,在水相或有机相中引入添加剂可以改变油水界面张力进而调控单体界面扩散速率及界面分布,或通过改变反应机理影响聚合反应速率,最终实现对界面层结构和膜性能的调控。本文从添加剂种类、性质和调控作用等角度总结了近年来添加剂对复合膜结构及性能调控的研究进展,分析了现有研究存在的问题,并建议从微观层面探究界面过程的物理化学性质以及开发高时间分辨率原位表征方法等。
中图分类号:
引用本文
张赛晖, 李校阳, 高慧, 王丽丽. 制备聚酰胺复合膜中界面聚合反应添加剂研究进展[J]. 化工进展, 2022, 41(9): 4884-4894.
ZHANG Saihui, LI Xiaoyang, GAO Hui, WANG Lili. Recent progress in additives in interfacial polymerization for the preparation of polyamide composite membrane[J]. Chemical Industry and Engineering Progress, 2022, 41(9): 4884-4894.
| 添加剂种类 | 添加剂名称 | 作用机理以及对分离层的影响 | 油相/水相 | 膜性能 | 参考文献 |
|---|---|---|---|---|---|
| 共溶剂 | 甲醇、乙醇、乙二醇、 木糖醇等 | 提高间苯二胺(MPD)单体的扩散速率 | 水相 | 水通量提高5~10倍 | [ |
| 二甲基亚砜(DMSO) | 改变界面张力,提高反应速率 | 水相 | 水通量大幅度提高,脱盐率略有下降 | [ | |
| 六亚甲基磷酰胺(HMPA) | 提高MPD向有机相的扩散速率 | 水相 | 水通量为3.33L/(m2·h·bar),NaCl截留率为98.27% | [ | |
| 丙酮、乙酸乙酯、N,N二甲基 甲酰胺(DMF)等 | 减小MPD和均苯三甲酰氯(TMC)之间的溶解度差异和界面张力 | 油相 | 水通量显著提高 | [ | |
| 甲酸乙酯 | 使两相之间形成一层混溶区,影响MPD到有机相的扩散 | 油相 | 水通量增加2.6倍 | [ | |
| 极性有机 化合物 | 磷酸三苯酯(TPP) | 与TMC形成配合物,增加TMC的亲水性,提高MPD在有机相中的溶解度 | 油相 | 水通量增加,没有明显的脱盐率损失 | [ |
| 1,3-丙烷磺内酯(PS) | 通过氢键作用阻碍MPD的扩散 | 油相 | 水通量为3.13L/(m2·h·bar),NaCl截留率为99.39% | [ | |
| 冠醚 | 促进MPD单体向有机相的扩散 | 水相 | 水通量提高了164% | [ | |
| 葡萄糖、蔗糖、棉子糖 | 通过氢键进入聚酰胺(PA)层 | 水相 | 水通量提高86% | [ | |
| 单宁酸(TA) | 通过质子化反应和氢键阻止哌嗪(PIP)向有机界面扩散 | 水相 | 水通量增加了13.4%,Na2SO4和MgSO4的截留率分别为97.0%和94.4% | [ | |
| 甘油 | 增加黏度,抑制PIP向反应界面的扩散 | 水相 | 水通量提高了51%,Na2SO4的截留率保持在99.4%以上 | [ | |
| 多巴胺 | 氨基和邻苯二酚基团与TMC的酰氯基团发生反应 | 水相 | 水通量为3.4L/(m2·h·bar),NaCl截留率为99.4% | [ | |
| 氨基酸 | 氨基酸中氨基及羟基基团与TMC发生反应,降低PA层的致密性 | 水相 | 水通量增大,脱盐率降低 | [ | |
| 精氨酸 | 与MPD分子结成亲水性离子对,降低其扩散速率 | 水相 | 水通量从2.90L/(m2·h·bar)增至3.38 L/(m2·h·bar),NaCl截留率从96.34%提高到98.36% | [ | |
| 表面活性剂 | 十二烷基硫酸钠(SDS) | 降低界面张力,提高MPD的扩散速率 | 水相 | 水通量为4.76L/(m2·h·bar),NaCl截留率为93.6% | [ |
| 十六烷基三甲基氯化铵 (HTAC) | 增加IP反应速率 | 水相 | 水通量为0.93L/(m2·h·bar),NaCl截留率为95.1% | [ | |
| Tritonx-100 | 吸收PIP,提高其扩散速率 | 油相 | 水通量为5.3L/(m2·h·bar) | [ | |
| 十六烷基三甲基溴化铵 (CTAB) | 促进 PIP 扩散到有机相中,形成更厚、更致密的薄膜 | 水相 | 水通量从0.83L/(m2·h·bar)增至2.09 L/(m2·h·bar),脱盐率不变 | [ | |
| 无机盐 | NaCl | 改变界面张力,促进TMC水解 | 水相 | 水通量达21.5L/(m2·h·bar),Na2SO4截留率为99.10% | [ |
| LiCl | 与TMC的羰基络合,促进TMC的水解 | 水相 | 水通量显著提高 | [ | |
| CaCl2 | 水相 | 水通量为(2.44±0.05)L/(m2·h·bar),NaCl 截留率为97.9%±0.3% | [ | ||
| CuSO4、NiSO4、MgSO4、 Al2(SO4)3 | 金属离子与聚乙烯亚胺(PEI)发生络合,影响IP反应 | 水相 | NaCl截留率在94%以上,MgSO4截留率达到97%以上 | [ | |
| NaHCO₃、NH4HCO3 | 消耗聚合反应产生的HCl并产生CO2,加快反应速率,形成较疏松的PA表面 | 水相 | NaHCO3质量分数为1.5%,NH4HCO3质量分数为0.5%时,具有较好的水通量和脱盐率 | [ | |
| Na3PO4 | 消耗聚合反应产生的HCl,加快反应速率 | 水相 | 水通量约为4.83 L/(m2·h·bar),MgSO4截留率约为93.5% | [ | |
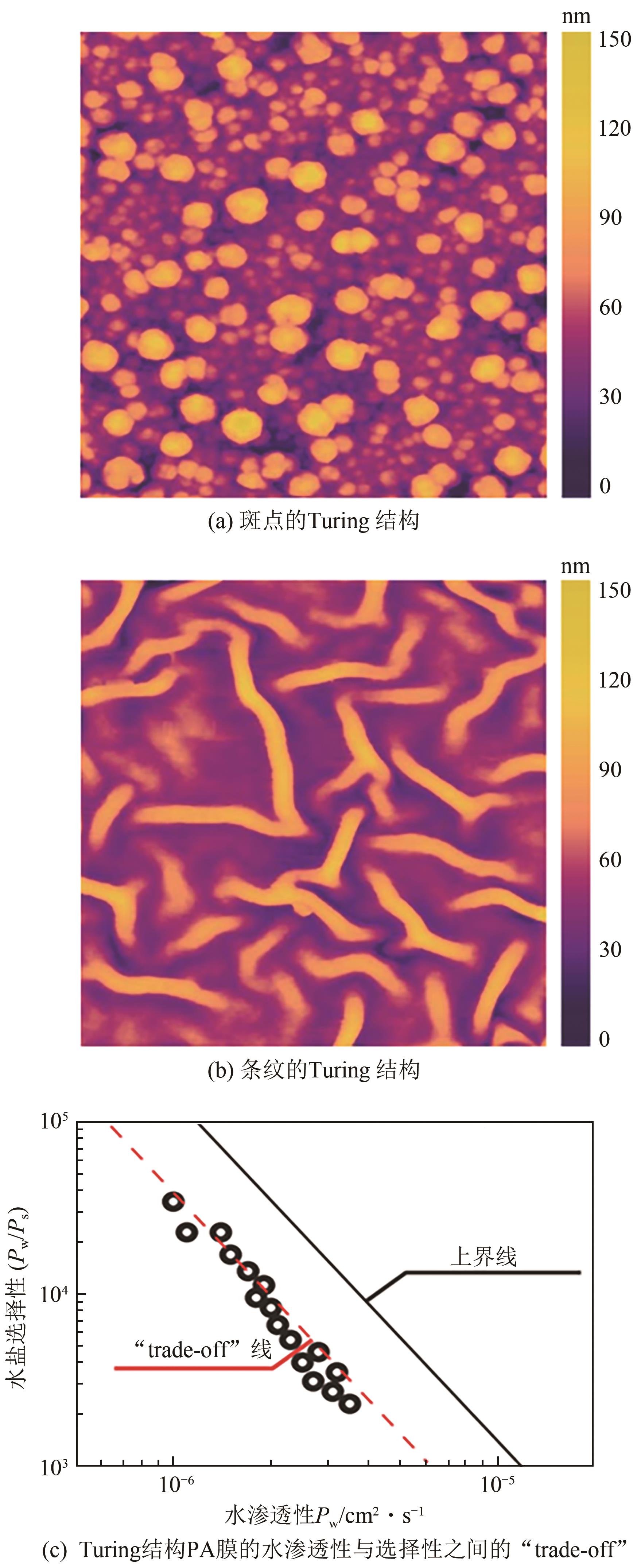
| 亲水性 大分子 | PVA | 降低水相单体的扩散速率 | 水相 | 形成Turing结构,水通量提高,脱盐率稳定 | [ |
| PEG | 提高PIP的扩散速率 | 水相 | PEG分子量为200Da所制备的膜,水通量提高了41% | [ | |
| PVP | 降低PIP扩散速率,降低IP反应速率 | 水相 | 水通量约为14.1~23.8L/(m2·h·bar),Na2SO4截留率大于98.4% | [ | |
| 纳米材料 | SiO2 | 干扰IP聚合过程,降低PIP与TMC反应速率,导致交联度低及PA层缺陷 | 油相 | 水通量为(9.72±0.78)L/(m2·h·bar),不同盐截留率分别为Na2SO4 99.0%±0.6%、MgCl2 67.3%±1.3%、MgSO4 96.5%±2.6%、NaCl 42.6%±2.3% | [ |
| TiO2 | 限制MPD的扩散 | 水相 | 水通量为2.94L/(m2·h·bar),NaCl截留率为94.2% | [ | |
| Cu-Al层状双氢氧化物 | 降低PIP的扩散速率 | 油相 | 水通量为7.01L/(m2·h·bar),不同盐的截留率分别为Na2SO4 96.8%、MgCl2 95.6%、MgSO4 95.4%、NaCl 60.8% | [ | |
| 纳米Ag | 吸附MPD,导致交联度增加 | 水相 | 水通量增加了两倍 | [ | |
| 沸石 | 促进MPD向界面的扩散 | 油相 | 水通量增加了一倍,NO3-截留率从63%提高到85% | [ | |
| 氧化石墨烯(GO) | 降低PIP的扩散速率 | 水相 | 水通量达到8L/(m2·h·bar),Na2SO4截留率为96.1% | [ | |
| 氮掺杂GO量子点 (N-GOQD) | 削弱MPD和TMC单体之间的反应,降低PA的交联度 | 水相 | 水通量提高了近3倍,脱盐率保持不变 | [ | |
| GO-羧酸型超支化聚合物 (HBE-COOH) | 降低PIP的扩散速率 | 水相 | 水通量为8L/(m2·h·bar),脱盐率维持不变 | [ | |
| 羧基化GO(CFGO) | 阻碍PIP向TMC的扩散,影响聚合反应速率 | 水相 | 水通量为11.04L/(m2·h·bar),NaCl截留率为25.0% | [ | |
| 多壁碳纳米管(MWNT) | 吸附MPD,稳定聚合物网络,降低溶胀性 | 水相 | 脱盐率保持不变,提高膜的耐氯性 | [ | |
| UiO-66 | 增加TMC-正己烷溶液的黏度,阻碍了PIP扩散到有机相中,减慢IP速率,形成低交联度的PA层 | 油相 | 水通量达14.64L/(m2·h·bar) | [ | |
| MIL-101(Cr)@GO | 影响MPD与TMC反应,使IP反应不完全,降低PA的交联度 | 水相 | 水通量从1.02L/(m2·h·bar)增加到1.90L/(m2·h·bar) | [ | |
| 磺酸化氧化石墨烯(SGO)@ UiO-66 | 通过化学反应和氢键吸附MPD,减弱其与TMC聚合反应 | 油相 | 水通量提高了41% | [ | |
| 季铵化SiO2@多金属氧酸盐(QNP@WPOM) | 限制MPD的扩散 | 水相 | 水通量从2.19L/(m2·h·bar)增至5.07 L/(m2·h·bar) | [ |
表1 添加剂对IP反应的调控机理及对膜性能的影响
| 添加剂种类 | 添加剂名称 | 作用机理以及对分离层的影响 | 油相/水相 | 膜性能 | 参考文献 |
|---|---|---|---|---|---|
| 共溶剂 | 甲醇、乙醇、乙二醇、 木糖醇等 | 提高间苯二胺(MPD)单体的扩散速率 | 水相 | 水通量提高5~10倍 | [ |
| 二甲基亚砜(DMSO) | 改变界面张力,提高反应速率 | 水相 | 水通量大幅度提高,脱盐率略有下降 | [ | |
| 六亚甲基磷酰胺(HMPA) | 提高MPD向有机相的扩散速率 | 水相 | 水通量为3.33L/(m2·h·bar),NaCl截留率为98.27% | [ | |
| 丙酮、乙酸乙酯、N,N二甲基 甲酰胺(DMF)等 | 减小MPD和均苯三甲酰氯(TMC)之间的溶解度差异和界面张力 | 油相 | 水通量显著提高 | [ | |
| 甲酸乙酯 | 使两相之间形成一层混溶区,影响MPD到有机相的扩散 | 油相 | 水通量增加2.6倍 | [ | |
| 极性有机 化合物 | 磷酸三苯酯(TPP) | 与TMC形成配合物,增加TMC的亲水性,提高MPD在有机相中的溶解度 | 油相 | 水通量增加,没有明显的脱盐率损失 | [ |
| 1,3-丙烷磺内酯(PS) | 通过氢键作用阻碍MPD的扩散 | 油相 | 水通量为3.13L/(m2·h·bar),NaCl截留率为99.39% | [ | |
| 冠醚 | 促进MPD单体向有机相的扩散 | 水相 | 水通量提高了164% | [ | |
| 葡萄糖、蔗糖、棉子糖 | 通过氢键进入聚酰胺(PA)层 | 水相 | 水通量提高86% | [ | |
| 单宁酸(TA) | 通过质子化反应和氢键阻止哌嗪(PIP)向有机界面扩散 | 水相 | 水通量增加了13.4%,Na2SO4和MgSO4的截留率分别为97.0%和94.4% | [ | |
| 甘油 | 增加黏度,抑制PIP向反应界面的扩散 | 水相 | 水通量提高了51%,Na2SO4的截留率保持在99.4%以上 | [ | |
| 多巴胺 | 氨基和邻苯二酚基团与TMC的酰氯基团发生反应 | 水相 | 水通量为3.4L/(m2·h·bar),NaCl截留率为99.4% | [ | |
| 氨基酸 | 氨基酸中氨基及羟基基团与TMC发生反应,降低PA层的致密性 | 水相 | 水通量增大,脱盐率降低 | [ | |
| 精氨酸 | 与MPD分子结成亲水性离子对,降低其扩散速率 | 水相 | 水通量从2.90L/(m2·h·bar)增至3.38 L/(m2·h·bar),NaCl截留率从96.34%提高到98.36% | [ | |
| 表面活性剂 | 十二烷基硫酸钠(SDS) | 降低界面张力,提高MPD的扩散速率 | 水相 | 水通量为4.76L/(m2·h·bar),NaCl截留率为93.6% | [ |
| 十六烷基三甲基氯化铵 (HTAC) | 增加IP反应速率 | 水相 | 水通量为0.93L/(m2·h·bar),NaCl截留率为95.1% | [ | |
| Tritonx-100 | 吸收PIP,提高其扩散速率 | 油相 | 水通量为5.3L/(m2·h·bar) | [ | |
| 十六烷基三甲基溴化铵 (CTAB) | 促进 PIP 扩散到有机相中,形成更厚、更致密的薄膜 | 水相 | 水通量从0.83L/(m2·h·bar)增至2.09 L/(m2·h·bar),脱盐率不变 | [ | |
| 无机盐 | NaCl | 改变界面张力,促进TMC水解 | 水相 | 水通量达21.5L/(m2·h·bar),Na2SO4截留率为99.10% | [ |
| LiCl | 与TMC的羰基络合,促进TMC的水解 | 水相 | 水通量显著提高 | [ | |
| CaCl2 | 水相 | 水通量为(2.44±0.05)L/(m2·h·bar),NaCl 截留率为97.9%±0.3% | [ | ||
| CuSO4、NiSO4、MgSO4、 Al2(SO4)3 | 金属离子与聚乙烯亚胺(PEI)发生络合,影响IP反应 | 水相 | NaCl截留率在94%以上,MgSO4截留率达到97%以上 | [ | |
| NaHCO₃、NH4HCO3 | 消耗聚合反应产生的HCl并产生CO2,加快反应速率,形成较疏松的PA表面 | 水相 | NaHCO3质量分数为1.5%,NH4HCO3质量分数为0.5%时,具有较好的水通量和脱盐率 | [ | |
| Na3PO4 | 消耗聚合反应产生的HCl,加快反应速率 | 水相 | 水通量约为4.83 L/(m2·h·bar),MgSO4截留率约为93.5% | [ | |
| 亲水性 大分子 | PVA | 降低水相单体的扩散速率 | 水相 | 形成Turing结构,水通量提高,脱盐率稳定 | [ |
| PEG | 提高PIP的扩散速率 | 水相 | PEG分子量为200Da所制备的膜,水通量提高了41% | [ | |
| PVP | 降低PIP扩散速率,降低IP反应速率 | 水相 | 水通量约为14.1~23.8L/(m2·h·bar),Na2SO4截留率大于98.4% | [ | |
| 纳米材料 | SiO2 | 干扰IP聚合过程,降低PIP与TMC反应速率,导致交联度低及PA层缺陷 | 油相 | 水通量为(9.72±0.78)L/(m2·h·bar),不同盐截留率分别为Na2SO4 99.0%±0.6%、MgCl2 67.3%±1.3%、MgSO4 96.5%±2.6%、NaCl 42.6%±2.3% | [ |
| TiO2 | 限制MPD的扩散 | 水相 | 水通量为2.94L/(m2·h·bar),NaCl截留率为94.2% | [ | |
| Cu-Al层状双氢氧化物 | 降低PIP的扩散速率 | 油相 | 水通量为7.01L/(m2·h·bar),不同盐的截留率分别为Na2SO4 96.8%、MgCl2 95.6%、MgSO4 95.4%、NaCl 60.8% | [ | |
| 纳米Ag | 吸附MPD,导致交联度增加 | 水相 | 水通量增加了两倍 | [ | |
| 沸石 | 促进MPD向界面的扩散 | 油相 | 水通量增加了一倍,NO3-截留率从63%提高到85% | [ | |
| 氧化石墨烯(GO) | 降低PIP的扩散速率 | 水相 | 水通量达到8L/(m2·h·bar),Na2SO4截留率为96.1% | [ | |
| 氮掺杂GO量子点 (N-GOQD) | 削弱MPD和TMC单体之间的反应,降低PA的交联度 | 水相 | 水通量提高了近3倍,脱盐率保持不变 | [ | |
| GO-羧酸型超支化聚合物 (HBE-COOH) | 降低PIP的扩散速率 | 水相 | 水通量为8L/(m2·h·bar),脱盐率维持不变 | [ | |
| 羧基化GO(CFGO) | 阻碍PIP向TMC的扩散,影响聚合反应速率 | 水相 | 水通量为11.04L/(m2·h·bar),NaCl截留率为25.0% | [ | |
| 多壁碳纳米管(MWNT) | 吸附MPD,稳定聚合物网络,降低溶胀性 | 水相 | 脱盐率保持不变,提高膜的耐氯性 | [ | |
| UiO-66 | 增加TMC-正己烷溶液的黏度,阻碍了PIP扩散到有机相中,减慢IP速率,形成低交联度的PA层 | 油相 | 水通量达14.64L/(m2·h·bar) | [ | |
| MIL-101(Cr)@GO | 影响MPD与TMC反应,使IP反应不完全,降低PA的交联度 | 水相 | 水通量从1.02L/(m2·h·bar)增加到1.90L/(m2·h·bar) | [ | |
| 磺酸化氧化石墨烯(SGO)@ UiO-66 | 通过化学反应和氢键吸附MPD,减弱其与TMC聚合反应 | 油相 | 水通量提高了41% | [ | |
| 季铵化SiO2@多金属氧酸盐(QNP@WPOM) | 限制MPD的扩散 | 水相 | 水通量从2.19L/(m2·h·bar)增至5.07 L/(m2·h·bar) | [ |
| 1 | FREITAS T V, SOUSA E A, FUZARI JR G C, et al. Different morphologies of polyaniline nanostructures synthesized by interfacial polymerization[J]. Materials Letters, 2018, 224: 42-45. |
| 2 | 王磊, 呼佳瑞, 苗瑞, 等. 聚酰胺复合纳滤膜的制备与表征[J]. 膜科学与技术, 2014, 34(5): 16-21. |
| WANG Lei, HU Jiarui, MIAO Rui, et al. Preparation and characterization of thin-film composite polyamide nanofiltration membranes[J]. Membrane Science and Technology, 2014, 34(5): 16-21, 26. | |
| 3 | 顾红霞. 界面聚合制备聚酰胺复合纳滤膜[D]. 北京: 北京化工大学, 2012. |
| GU Hongxia. Polyamide composite nanofiltration membranes prepared by interfacial polymerization[D]. Beijing: Beijing University of Chemical Technology, 2012. | |
| 4 | YU Chuang, LI Haiyan, ZHANG Xiru, et al. Polyamide thin-film composite membrane fabricated through interfacial polymerization coupled with surface amidation for improved reverse osmosis performance[J]. Journal of Membrane Science, 2018, 566: 87-95. |
| 5 | 高慧. 大分子水相添加剂对聚酰胺复合膜结构调控机理研究[D]. 天津: 天津工业大学, 2021. |
| GAO Hui. Research on the mechanism of macromolecular aqueous phase additives on the structure regulation of polyamide composite membrane[D]. Tianjin: Tiangong University, 2021. | |
| 6 | LIU Yanling, ZHAO Yangying, WANG Xiaomao, et al. Effect of varying piperazine concentration and post-modification on prepared nanofiltration membranes in selectively rejecting organic micropollutants and salts[J]. Journal of Membrane Science, 2019, 582: 274-283. |
| 7 | WEI Jing, LIU Xin, QIU Changquan, et al. Influence of monomer concentrations on the performance of polyamide-based thin film composite forward osmosis membranes[J]. Journal of Membrane Science, 2011, 381(1/2): 110-117. |
| 8 | KHORSHIDI B, THUNDAT T, FLECK B A, et al. A novel approach toward fabrication of high performance thin film composite polyamide membranes[J]. Scientific Reports, 2016, 6: 22069. |
| 9 | LIU Meihong, YU Sanchuan, TAO Jie, et al. Preparation, structure characteristics and separation properties of thin-film composite polyamide-urethane seawater reverse osmosis membrane[J]. Journal of Membrane Science, 2008, 325(2): 947-956. |
| 10 | PARK S J, KWON S J, KWON H E, et al. Aromatic solvent-assisted interfacial polymerization to prepare high performance thin film composite reverse osmosis membranes based on hydrophilic supports[J]. Polymer, 2018, 144: 159-167. |
| 11 | 曹阳, 任玉灵, 郭世伟, 等. 聚酰胺薄层复合膜的界面聚合制备过程调控研究进展[J]. 化工进展, 2020, 39(6): 2125-2134. |
| CAO Yang, REN Yuling, GUO Shiwei, et al. Research progress on process optimization for preparation of polyamide thin-film composite membrane by interfacial polymerization[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2125-2134. | |
| 12 | 秘一芳, 安全福. 界面聚合聚酰胺纳滤膜渗透选择性能优化的研究进展[J]. 化工进展, 2020, 39(6): 2093-2104. |
| MI Yifang, AN Quanfu. Progress in optimization of polyamide nanofiltration membranes prepared by interfacial polymerization for perm-selectivity[J]. Chemical Industry and Engineering Progress, 2020, 39(6): 2093-2104. | |
| 13 | SHI Mengqi, WANG Zhi, ZHAO Song, et al. A support surface pore structure re-construction method to enhance the flux of TFC RO membrane[J]. Journal of Membrane Science, 2017, 541: 39-52. |
| 14 | GOHIL J M, RAY P. A review on semi-aromatic polyamide TFC membranes prepared by interfacial polymerization: potential for water treatment and desalination[J]. Separation and Purification Technology, 2017, 181: 159-182. |
| 15 | CHENG Jun, ZHANG Zhiqiang, SHI Wenxin, et al. A novel polyester composite nanofiltration membrane prepared by interfacial polymerization catalysed by 4-dimethylaminopyridine: enhanced the water permeability and anti-fouling ability[J]. Polymer, 2018, 153: 24-32. |
| 16 | TAN Zhe, CHEN Shengfu, PENG Xinsheng, et al. Polyamide membranes with nanoscale Turing structures for water purification[J]. Science, 2018, 360(6388): 518-521. |
| 17 | KARAN S, JIANG Z W, LIVINGSTON A G. Sub–10nm polyamide nanofilms with ultrafast solvent transport for molecular separation[J]. Science, 2015, 348(6241): 1347-1351. |
| 18 | YANG Zhe, ZHOU Zhiwen, GUO Hao, et al. Tannic acid/Fe3+ nanoscaffold for interfacial polymerization: toward enhanced nanofiltration performance[J]. Environmental Science & Technology, 2018, 52(16): 9341-9349. |
| 19 | WANG Xinghua, WANG Wei. Effect of rejection performance on hollow fiber composite reverse osmosis membrane by alcohols additives[J]. Modern Applied Science, 2010, 4(11): 147. |
| 20 | KHORSHIDI B, SOLTANNIA B, THUNDAT T, et al. Synthesis of thin film composite polyamide membranes: effect of monohydric and polyhydric alcohol additives in aqueous solution[J]. Journal of Membrane Science, 2017, 523: 336-345. |
| 21 | LEE J, WANG R, BAE T H. A comprehensive understanding of co-solvent effects on interfacial polymerization: interaction with trimesoyl chloride[J]. Journal of Membrane Science, 2019, 583: 70-80. |
| 22 | LI Can, LI Shuxuan, Li LYU, et al. High solvent-resistant and integrally crosslinked polyimide-based composite membranes for organic solvent nanofiltration[J]. Journal of Membrane Science, 2018, 564: 10-21. |
| 23 | LIU Zhengang, LIU Zhenxia, ZHANG Fei, et al. An experimental study of the effects of different transverse trenches on depositing and temperature on a plate with film cooling holes[J]. Aerospace Science and Technology, 2019, 88: 40-50. |
| 24 | LIANG Zhixia, YUN Yanbin, JI Quanju, et al. Effects of organic solvents and different aqueous-phase additives on the polyamide (PA) thin-film composite (TFC) membranes for forward osmosis[J]. Desalination and Water Treatment, 2018, 119: 45-52. |
| 25 | WU F J, LIU X J, AU C. Effects of DMSO and glycerol additives on the property of polyamide reverse osmosis membrane[J]. Water Science and Technology, 2016, 74(7): 1619-1625. |
| 26 | DUAN Meirong, WANG Zhi, XU Jun, et al. Influence of hexamethyl phosphoramide on polyamide composite reverse osmosis membrane performance[J]. Separation and Purification Technology, 2010, 75(2): 145-155. |
| 27 | KAMADA T, OHARA T, SHINTANI T, et al. Controlled surface morphology of polyamide membranes via the addition of co-solvent for improved permeate flux[J]. Journal of Membrane Science, 2014, 467: 303-312. |
| 28 | YANG Shanshan, ZHEN Hongyan, SU Baowei. Polyimide thin film composite (TFC) membranes via interfacial polymerization on hydrolyzed polyacrylonitrile support for solvent resistant nanofiltration[J]. RSC Advances, 2017, 7(68): 42800-42810. |
| 29 | MACHODI M J, DARAMOLA M O. Synthesis and performance evaluation of PES/chitosan membranes coated with polyamide for acid mine drainage treatment[J]. Scientific Reports, 2019, 9: 17657. |
| 30 | YOU Meng, WANG Binfei, AN Liyi, et al. Different roles of aqueous and organic additives in the morphology and performance of polyamide thin-film composite membranes[J]. Chemical Engineering Research and Design, 2021, 165: 1-11. |
| 31 | AL-HOBAIB A S, AL-SUHYBANI M S, AL-SHEETAN K M, et al. New RO TFC membranes by interfacial polymerization in n-dodecane with various co-solvents[J]. Membranes, 2016, 6(2): 24. |
| 32 | 刘兆峰, 魏玉林, 张大鹏, 等. 甲酸乙酯改性聚酰胺正渗透复合膜的制备及表征[J]. 水处理技术, 2017, 43(11): 14-18. |
| LIU Zhaofeng, WEI Yulin, ZHANG Dapeng, et al. Preparation and characterization of ethyl acetate modified polyamide forward osmosis composite membrane[J]. Technology of Water Treatment, 2017, 43(11): 14-18. | |
| 33 | KIM I C, JEONG B R, KIM S J, et al. Preparation of high flux thin film composite polyamide membrane: the effect of alkyl phosphate additives during interfacial polymerization[J]. Desalination, 2013, 308: 111-114. |
| 34 | KIM S M, HONG S, NGUYEN B T DUY, et al. Effect of additives during interfacial polymerization reaction for fabrication of organic solvent nanofiltration (OSN) membranes[J]. Polymers, 2021, 13(11): 1716. |
| 35 | ZHANG Yang, MIAO Xiaopei, PAN Guoyuan, et al. Highly improved permeation property of thin-film-composite polyamide membrane for water desalination[J]. Journal of Polymer Research, 2016, 24(1): 1-12. |
| 36 | AKBARI A, OSTADMORADI N, ROSTAMI S M M, et al. Role of organic acids in flux enhancement of polyamide nanofiltration membranes[J]. Chemical Engineering & Technology, 2017, 40(1): 76-87. |
| 37 | SHEN L, YI M, JAPIP S, et al. Breaking through permeability-selectivity trade-off of thin-film composite membranes assisted with crown ethers[J]. AIChE Journal, 2020, 67: e17173. |
| 38 | 沈倩, 徐孙杰, 许振良. 糖基掺杂聚酰胺纳滤膜制备及其性能研究[J]. 膜科学与技术, 2020, 40(1): 117-122. |
| SHEN Qian, XU Sunjie, XU Zhenliang. Fabrication and properties of carbohydrate doped thin film composite nanofiltration membranes[J]. Membrane Science and Technology, 2020, 40(1): 117-122. | |
| 39 | ZHANG Na, SONG Xiangju, CHEN Yiqiang, et al. A facile and economic route assisted by trace tannic acid to construct a high-performance thin film composite NF membrane for desalination[J]. Environmental Science: Water Research & Technology, 2021, 7(5): 956-968. |
| 40 | ZHU Chengye, LIU Chang, YANG Jing, et al. Polyamide nanofilms with linearly-tunable thickness for high performance nanofiltration[J]. Journal of Membrane Science, 2021, 627: 119142. |
| 41 | 岳鑫业, 李俊俊, 王铭, 等. 亲水性添加剂多巴胺对聚酰胺反渗透膜性能的影响[J]. 水处理技术, 2014, 40(8): 25-28. |
| YUE Xinye, LI Junjun, WANG Ming, et al. Effect of hydrophilic additive dopamine on polyamide reverse osmosis membrane for brackish water desalination[J]. Technology of Water Treatment, 2014, 40(8): 25-28. | |
| 42 | 凡祖伟, 刘让同, 蓝伟光. 聚哌嗪酰胺复合纳滤膜的性能调控[J]. 净水技术, 2020, 39(2): 125-128. |
| FAN Zuwei, LIU Rangtong, LAN Weiguang. Performance control of polypiperazine-amide composite nanofiltration membrane[J]. Water Purification Technology, 2020, 39(2): 125-128. | |
| 43 | CHEN Dandan, CHEN Qiang, LIU Tianyu, et al. Influence of L-arginine on performances of polyamide thin-film composite reverse osmosis membranes[J]. RSC Advances, 2019, 9(35): 20149-20160. |
| 44 | GUAN Jingyuan, FAN Lin, LIU Yanan, et al. Incorporating arginine-FeⅢ complex into polyamide membranes for enhanced water permeance and antifouling performance[J]. Journal of Membrane Science, 2020, 602: 117980. |
| 45 | KHORSHIDI B, THUNDAT T, PERNITSKY D, et al. A parametric study on the synergistic impacts of chemical additives on permeation properties of thin film composite polyamide membrane[J]. Journal of Membrane Science, 2017, 535: 248-257. |
| 46 | 邱实, 吴礼光, 张林, 等. 界面聚合工艺条件对反渗透复合膜性能的影响[J]. 化工学报, 2008, 59(8): 2027-2034. |
| QIU Shi, WU Liguang, ZHANG Lin, et al. Effect of process conditions of interfacial polymerization on performance of reverse osmosis composite membrane[J]. CIESC Journal, 2008, 59(8): 2027-2034. | |
| 47 | MANSOURPANAH Y, MADAENI S S, RAHIMPOUR A. Fabrication and development of interfacial polymerized thin-film composite nanofiltration membrane using different surfactants in organic phase; study of morphology and performance[J]. Journal of Membrane Science, 2009, 343(1/2): 219-228. |
| 48 | QIU Shi, WU Liguang, ZHANG Lin, et al. Preparation of reverse osmosis composite membrane with high flux by interfacial polymerization of MPD and TMC[J]. Journal of Applied Polymer Science, 2009, 112(4): 2066-2072. |
| 49 | LIU Zhixiao, WANG Tao, WANG Daming, et al. Regulating the morphology of nanofiltration membrane by thermally induced inorganic salt crystals for efficient water purification[J]. Journal of Membrane Science, 2021, 617: 118645. |
| 50 | SHEN Ke, LI Peiyun, ZHANG Tonghui, et al. Salt-tuned fabrication of novel polyamide composite nanofiltration membranes with three-dimensional Turing structures for effective desalination[J]. Journal of Membrane Science, 2020, 607: 118153. |
| 51 | LIU Peng, YU Jiaozhu. Influence of modifying interfacial polymerization compositions on the performance of composite forward osmosis hollow fiber membranes[J]. Journal of Polymer Research, 2019, 26(3): 60. |
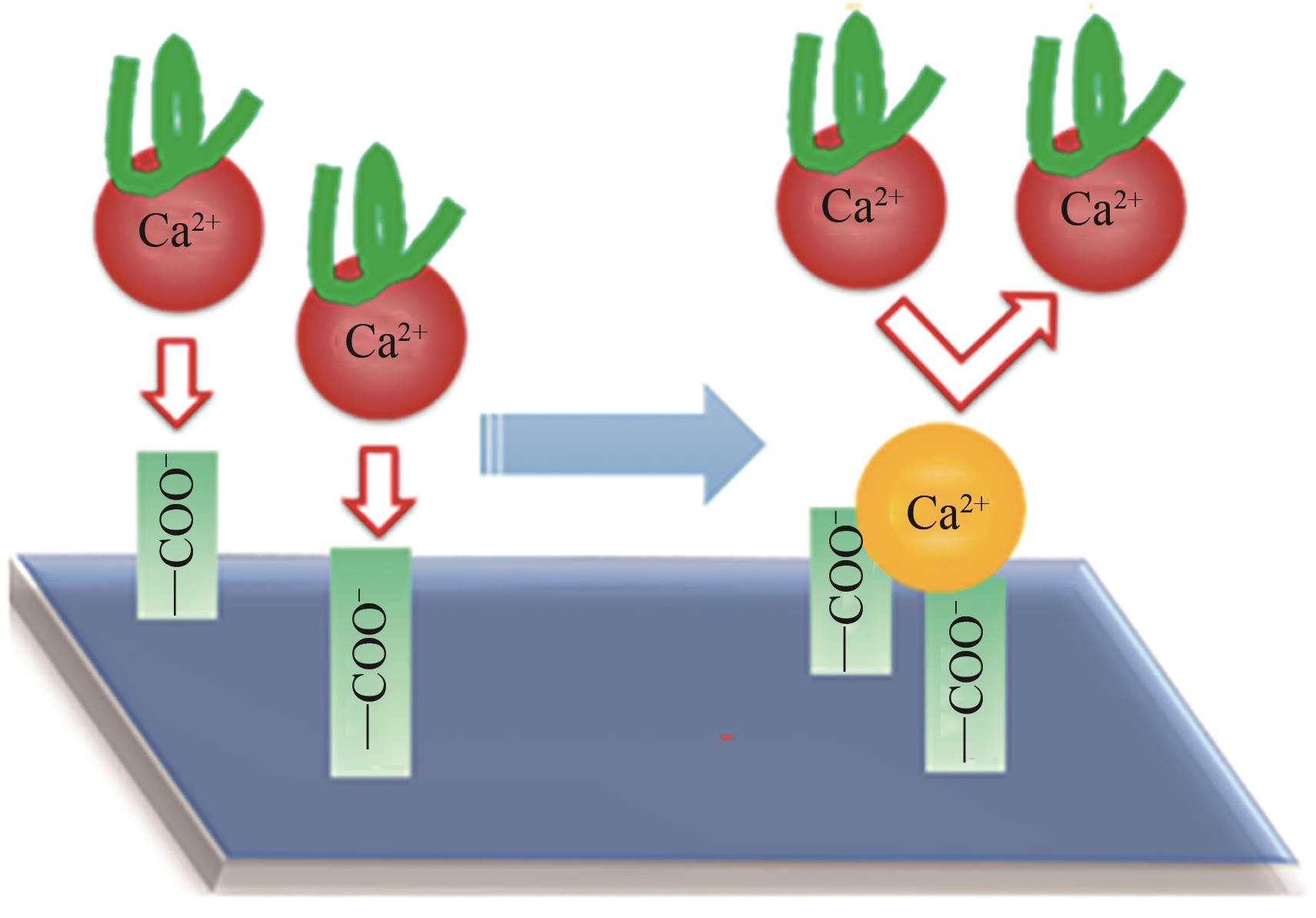
| 52 | HAO Xiujuan, GAO Shanshan, TIAN Jiayu, et al. Calcium-carboxyl intrabridging during interfacial polymerization: a novel strategy to improve antifouling performance of thin film composite membranes[J]. Environmental Science & Technology, 2019, 53(8): 4371-4379. |
| 53 | GHOSH B, GHOSH A K, BINDAL R C. Influence of metal salts in reaction medium on performance enhancement of novel aliphatic-aromatic-based polyamide thin-film composite osmosis membranes[J]. Separation Science and Technology, 2019, 54(8): 1363-1375. |
| 54 | 靳伟光. 界面聚合法制备高通量TFC纳滤膜及性能研究[D]. 天津: 天津大学, 2018. |
| JIN Weiguang. Fabrication and properties of TFC nanofiltration membrane by interfacial polymerization for high water flux[D]. Tianjin: Tianjin University, 2018. | |
| 55 | SUN Yongli, JIN Weiguang, ZHANG Luhong, et al. Sodium bicarbonate as novel additive for fabrication of composite nanofiltration membranes with enhanced permeability[J]. Journal of Applied Polymer Science, 2018, 135(23): 46363. |
| 56 | LI Xindong, WANG Lei, HUANG Wanfu, et al. Preparation and characterization of hydrophilic composite nanofiltration membrane by interfacial polymerization[J]. Journal of Engineering Science and Technology Review, 2016, 9(3): 74-79. |
| 57 | HIROSE M, ITO H, KAMIYAMA Y. Effect of skin layer surface structures on the flux behaviour of RO membranes[J]. Journal of Membrane Science, 1996, 121(2): 209-215. |
| 58 | LIU Meihong, ZHOU Choumou, DONG Bingyan, et al. Enhancing the permselectivity of thin-film composite poly(vinyl alcohol) (PVA) nanofiltration membrane by incorporating poly(sodium-p-styrene-sulfonate) (PSSNa)[J]. Journal of Membrane Science, 2014, 463: 173-182. |
| 59 | CHAE H R, KIM I C. Enhancement in permeability of piperazine-based thin-film composite membrane via surface roughening using a highly organic-soluble additive[J]. Journal of Applied Polymer Science, 2019, 136(36): 47913. |
| 60 | YANG Xi. Monitoring the interfacial polymerization of piperazine and trimesoyl chloride with hydrophilic interlayer or macromolecular additive by in situ FT-IR spectroscopy[J]. Membranes, 2020, 10(1): 12. |
| 61 | AN Quanfu, LI Feng, JI Yanli, et al. Influence of polyvinyl alcohol on the surface morphology, separation and anti-fouling performance of the composite polyamide nanofiltration membranes[J]. Journal of Membrane Science, 2011, 367(1/2): 158-165. |
| 62 | ANG M B M Y, PEREIRA J M, TRILLES C A, et al. Performance and antifouling behavior of thin-film nanocomposite nanofiltration membranes with embedded silica spheres[J]. Separation and Purification Technology, 2019, 210: 521-529. |
| 63 | KEDCHAIKULRAT P, VANKELECOM I F J, FAUNGNAWAKIJ K, et al. Effects of colloidal TiO2 and additives on the interfacial polymerization of thin film nanocomposite membranes[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 601: 125046. |
| 64 | TAJUDDIN M H, YUSOF N, WAN AZELEE I, et al. Development of copper-aluminum layered double hydroxide in thin film nanocomposite nanofiltration membrane for water purification process[J]. Frontiers in Chemistry, 2019, 7: 3. |
| 65 | YANG Zhe, GUO Hao, YAO Zhikan, et al. Hydrophilic silver nanoparticles induce selective nanochannels in thin film nanocomposite polyamide membranes[J]. Environmental Science & Technology, 2019, 53(9): 5301-5308. |
| 66 | GHAEE A, ZERAFAT M M, ASKARI P, et al. Fabrication of polyamide thin-film nanocomposite membranes with enhanced surface charge for nitrate ion removal from water resources[J]. Environmental Technology, 2017, 38(6): 772-781. |
| 67 | KARAMI P, KHORSHIDI B, SHAMAEI L, et al. Nanodiamond-enabled thin-film nanocomposite polyamide membranes for high-temperature water treatment[J]. ACS Applied Materials & Interfaces, 2020, 12(47): 53274-53285. |
| 68 | TIAN Long, JIANG Yongxiang, LI Shuxuan, et al. Graphene oxide interlayered thin-film nanocomposite hollow fiber nanofiltration membranes with enhanced aqueous electrolyte separation performance[J]. Separation and Purification Technology, 2020, 248: 117153. |
| 69 | 张彦君, 张少峰, 赵长伟, 等. SGO改性复合纳滤膜的制备及分盐性能[J]. 环境科学, 2019, 40(2): 724-729. |
| ZHANG Yanjun, ZHANG Shaofeng, ZHAO Changwei, et al. Preparation of sulfonated graphene oxide modified composite nanofiltration membrane and application in salts separation[J]. Environmental Science, 2019, 40(2): 724-729. | |
| 70 | REZAEI M T, VALIZADEH S, NAJI L. Influences of sulfonation level on the nanofiltration performance of sulfonated graphene oxide polyamide nanocomposite membranes[J]. Thin Solid Films, 2021, 728: 138688. |
| 71 | FATHIZADEH M, TIEN H N, KHIVANTSEV K, et al. Polyamide/nitrogen-doped graphene oxide quantum dots (N-GOQD) thin film nanocomposite reverse osmosis membranes for high flux desalination[J]. Desalination, 2019, 451: 125-132. |
| 72 | XIE Quanling, ZHANG Shishen, MA Hanjun, et al. A novel thin-film nanocomposite nanofiltration membrane by incorporating 3D hyperbranched polymer functionalized 2D graphene oxide[J]. Polymers, 2018, 10(11): 1253. |
| 73 | ZHANG Huijuan, LI Bin, PAN Jiefeng, et al. Carboxyl-functionalized graphene oxide polyamide nanofiltration membrane for desalination of dye solutions containing monovalent salt[J]. Journal of Membrane Science, 2017, 539: 128-137. |
| 74 | CRUZ-SILVA R, INUKAI S, ARAKI T, et al. High performance and chlorine resistant carbon nanotube/aromatic polyamide reverse osmosis nanocomposite membrane[J]. MRS Advances, 2016, 1(20): 1469-1476. |
| 75 | XU Ping, HONG Jun, XU Zhenzhen, et al. MWCNTs-COOK-assisted high positively charged composite membrane: accelerating Li+ enrichment and Mg2+ removal[J]. Composites Part B: Engineering, 2021, 212: 108686. |
| 76 | XIAO Fan, HU Xiaoyu, CHEN Yingbo, et al. Porous Zr-based metal-organic frameworks (Zr-MOFs)-incorporated thin-film nanocomposite membrane toward enhanced desalination performance[J]. ACS Applied Materials & Interfaces, 2019, 11(50): 47390-47403. |
| 77 | SONG Na, SUN Yongchao, XIE Xiao, et al. Doping MIL-101(Cr)@GO in polyamide nanocomposite membranes with improved water flux[J]. Desalination, 2020, 492: 114601. |
| 78 | HE Miaolu, WANG Lei, ZHANG Zhe, et al. Stable forward osmosis nanocomposite membrane doped with sulfonated graphene oxide@metal-organic frameworks for heavy metal removal[J]. ACS Applied Materials & Interfaces, 2020, 12(51): 57102-57116. |
| 79 | SHAKERI A, SALEHI H, GHORBANI F, et al. Polyoxometalate based thin film nanocomposite forward osmosis membrane: superhydrophilic, anti-fouling, and high water permeable[J]. Journal of Colloid and Interface Science, 2019, 536: 328-338. |
| [1] | 张祚群, 高扬, 白超杰, 薛立新. 二次界面聚合同步反扩散原位生长ZIF-8纳米粒子制备聚酰胺混合基质反渗透(RO)膜[J]. 化工进展, 2023, 42(S1): 364-373. |
| [2] | 向硕, 卢鹏, 石伟年, 杨鑫, 何燕, 朱立业, 孔祥微. 二维WS2纳米片的规模化可控制备及其摩擦学性能[J]. 化工进展, 2023, 42(9): 4783-4790. |
| [3] | 李雪佳, 李鹏, 李志霞, 晋墩尚, 郭强, 宋旭锋, 宋芃, 彭跃莲. 亲水和疏水改性膜的抗结垢和润湿能力的对比[J]. 化工进展, 2023, 42(8): 4458-4464. |
| [4] | 徐杰, 夏隆博, 罗平, 邹栋, 仲兆祥. 面向膜蒸馏过程的全疏膜制备及其应用进展[J]. 化工进展, 2023, 42(8): 3943-3955. |
| [5] | 潘宜昌, 周荣飞, 邢卫红. 高效分离同碳数烃的先进微孔膜:现状与挑战[J]. 化工进展, 2023, 42(8): 3926-3942. |
| [6] | 王报英, 王皝莹, 闫军营, 汪耀明, 徐铜文. 聚合物包覆膜在金属分离回收中的研究进展[J]. 化工进展, 2023, 42(8): 3990-4004. |
| [7] | 陆诗建, 刘苗苗, 杨菲, 张俊杰, 陈思铭, 刘玲, 康国俊, 李清方. 改良型CO2湿壁塔内气液两相流动规律及传质特性[J]. 化工进展, 2023, 42(7): 3457-3467. |
| [8] | 冯江涵, 宋钫. 阴离子交换膜电解池的研究进展[J]. 化工进展, 2023, 42(7): 3501-3509. |
| [9] | 陈香李, 李倩倩, 张甜, 李彪, 李康康. 自愈合油水分离膜的研究进展[J]. 化工进展, 2023, 42(7): 3600-3610. |
| [10] | 任重远, 何金龙, 袁清. 分子筛膜晶间缺陷控制与修复技术研究进展[J]. 化工进展, 2023, 42(5): 2454-2463. |
| [11] | 王林, 辛梅华, 李明春, 陈琦, 毛扬帆. 季铵化/磺化壳聚糖的制备及其抗生物被膜活性[J]. 化工进展, 2023, 42(5): 2577-2585. |
| [12] | 于捷, 张文龙. 锂离子电池隔膜的发展现状与进展[J]. 化工进展, 2023, 42(4): 1760-1768. |
| [13] | 赵珍珍, 郑喜, 王雪琪, 王涛, 冯英楠, 任永胜, 赵之平. 聚酰胺复合膜微孔支撑基底的研究进展[J]. 化工进展, 2023, 42(4): 1917-1933. |
| [14] | 叶海星, 陈宇昊, 陈仪, 孙海翔, 牛青山. 镁锂分离复合纳滤膜研究进展[J]. 化工进展, 2023, 42(4): 1934-1943. |
| [15] | 常晓青, 彭东来, 李东洋, 张延武, 王景, 张亚涛. MOFs基丙烯/丙烷高效分离混合基质膜研究进展[J]. 化工进展, 2023, 42(4): 1961-1973. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||