化工进展 ›› 2022, Vol. 41 ›› Issue (3): 1453-1469.DOI: 10.16085/j.issn.1000-6613.2021-2227
废弃塑料化学回收及升级再造研究进展
- 华东理工大学化工学院,上海 200237
-
收稿日期:2021-10-30修回日期:2021-12-26出版日期:2022-03-23发布日期:2022-03-28 -
通讯作者:张亚运,龙东辉 -
作者简介:陈欢(1997—),男,硕士研究生,研究方向为固体废弃物的光热转化。E-mail:chenhuan9715@163.com 。 -
基金资助:国家自然科学基金(22008073);上海市青年科技人才扬帆计划(20YF1410600)
Recent progresses in chemical recycling and upcycling of waste plastics
CHEN Huan( ), WAN Kun, NIU Bo, ZHANG Yayun(
), WAN Kun, NIU Bo, ZHANG Yayun( ), LONG Donghui(
), LONG Donghui( )
)
- College of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China
-
Received:2021-10-30Revised:2021-12-26Online:2022-03-23Published:2022-03-28 -
Contact:ZHANG Yayun,LONG Donghui
摘要:
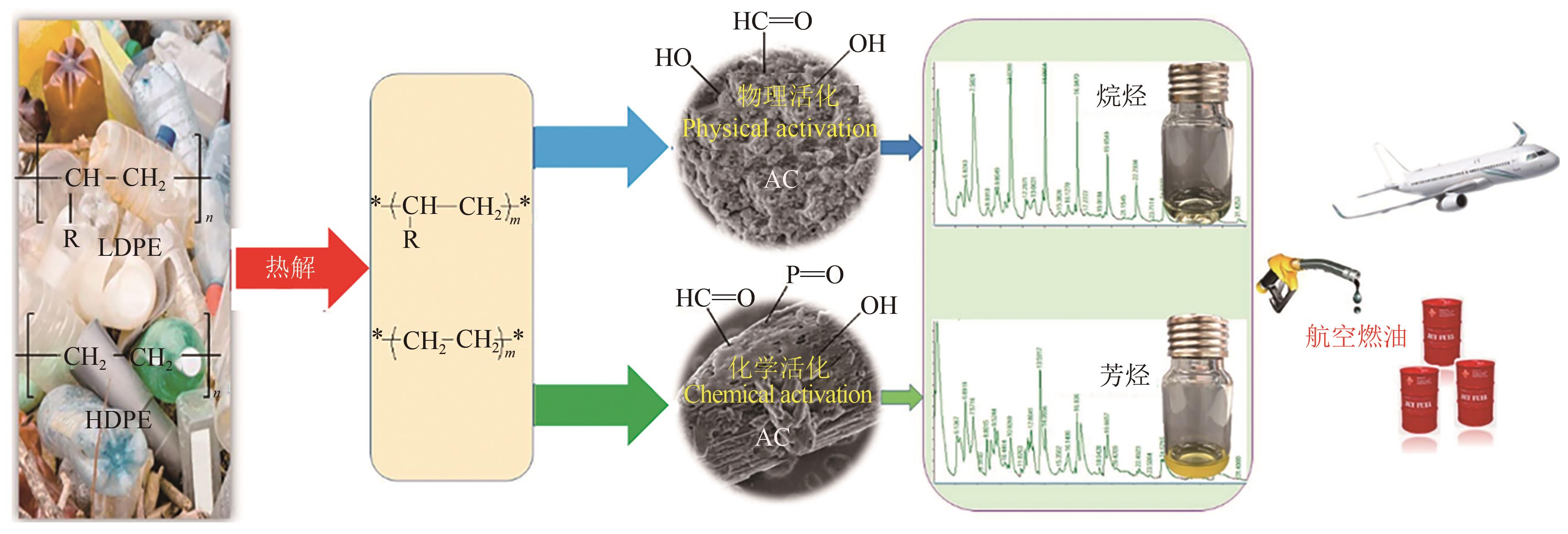
塑料对人类社会进步和经济发展发挥着重要作用,但其大规模生产和不恰当的处置已造成了严重的生态灾难。通过化学回收和化学升级再造的方法将废弃塑料转化为高附加值的产品是实现塑料资源可持续发展的关键技术之一。本综述总结了近年来废弃塑料的回收现状和化学升级再造的途径,包括催化热解、化学解聚、催化氢解、光催化、化学氧化等,着重探讨了反应条件对于产物分布和产率的影响、催化剂的构效关系及反应机理等。针对目前存在的反应条件严苛、催化剂成本高且重复利用性差等问题,提出未来研究方向包括优化工艺条件、弄清催化剂失活机理和开发价格低廉的高效催化剂,有望进一步实现废塑料资源化利用的工业化发展。
中图分类号:
引用本文
陈欢, 万坤, 牛波, 张亚运, 龙东辉. 废弃塑料化学回收及升级再造研究进展[J]. 化工进展, 2022, 41(3): 1453-1469.
CHEN Huan, WAN Kun, NIU Bo, ZHANG Yayun, LONG Donghui. Recent progresses in chemical recycling and upcycling of waste plastics[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1453-1469.
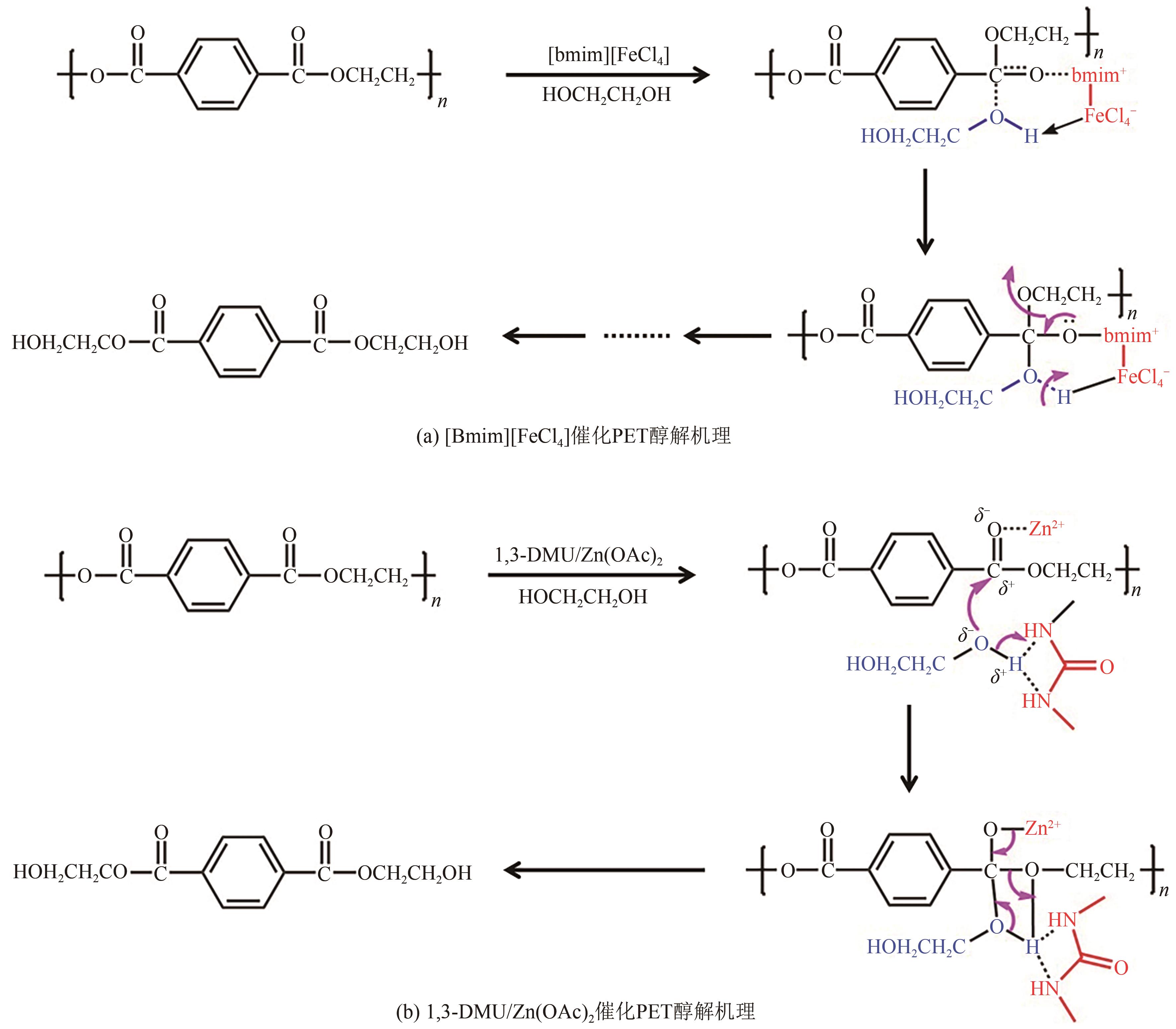
| EG/PET | 催化剂 | 催化剂/PET | 温度/℃ | 时间/min | PET 转化率/% | BHET 收率/% | 文献 |
|---|---|---|---|---|---|---|---|
| 4/1(mL/g) | [Bmim]Cl | 0.5(质量比) | 175 | 180 | 100 | 64 | [ |
| 4/1(质量比) | [Bmim][FeCl4] | 0.2(质量比) | 140 | 240 | 100 | 59.2 | [ |
| 11.7/1(质量比) | [Bmim]2[CoCl4] | 0.17(质量比) | 170 | 90 | 100 | 81.1 | [ |
| 4/1(质量比) | [Ch]3[PO]4 | 0.2(质量比) | 180 | 180 | 100 | 60.6 | [ |
| 4/1(质量比) | [Ch][OAc] | 0.05(质量比) | 180 | 480 | 94.3 | 85.2 | [ |
| 4/1(质量比) | 尿素/ZnCl2 | 0.05(质量比) | 170 | 30 | 100 | 82.8 | [ |
| 4/1(质量比) | 1,3-二甲基脲/ZnCl2 | 0.05(质量比) | 190 | 20 | 100 | 82 | [ |
| 6.7/1(质量比) | [Bmim-Fe][(OAc)3]/膨润土 | 0.33(质量比) | 190 | 180 | 100 | 44 | [ |
| 10/1(mL/g) | Fe3O4@SiO2@(mim)[FeCl4] | 0.15(质量比) | 180 | 1440 | N/A | 100 | [ |
| 5/1(质量比) | 乙酰胺/ZnCl2@ZIF-8 | 0.004(质量比) | 195 | 25 | 100 | 83.2 | [ |
表1 不同催化剂催化醇解PET的性能对比
| EG/PET | 催化剂 | 催化剂/PET | 温度/℃ | 时间/min | PET 转化率/% | BHET 收率/% | 文献 |
|---|---|---|---|---|---|---|---|
| 4/1(mL/g) | [Bmim]Cl | 0.5(质量比) | 175 | 180 | 100 | 64 | [ |
| 4/1(质量比) | [Bmim][FeCl4] | 0.2(质量比) | 140 | 240 | 100 | 59.2 | [ |
| 11.7/1(质量比) | [Bmim]2[CoCl4] | 0.17(质量比) | 170 | 90 | 100 | 81.1 | [ |
| 4/1(质量比) | [Ch]3[PO]4 | 0.2(质量比) | 180 | 180 | 100 | 60.6 | [ |
| 4/1(质量比) | [Ch][OAc] | 0.05(质量比) | 180 | 480 | 94.3 | 85.2 | [ |
| 4/1(质量比) | 尿素/ZnCl2 | 0.05(质量比) | 170 | 30 | 100 | 82.8 | [ |
| 4/1(质量比) | 1,3-二甲基脲/ZnCl2 | 0.05(质量比) | 190 | 20 | 100 | 82 | [ |
| 6.7/1(质量比) | [Bmim-Fe][(OAc)3]/膨润土 | 0.33(质量比) | 190 | 180 | 100 | 44 | [ |
| 10/1(mL/g) | Fe3O4@SiO2@(mim)[FeCl4] | 0.15(质量比) | 180 | 1440 | N/A | 100 | [ |
| 5/1(质量比) | 乙酰胺/ZnCl2@ZIF-8 | 0.004(质量比) | 195 | 25 | 100 | 83.2 | [ |
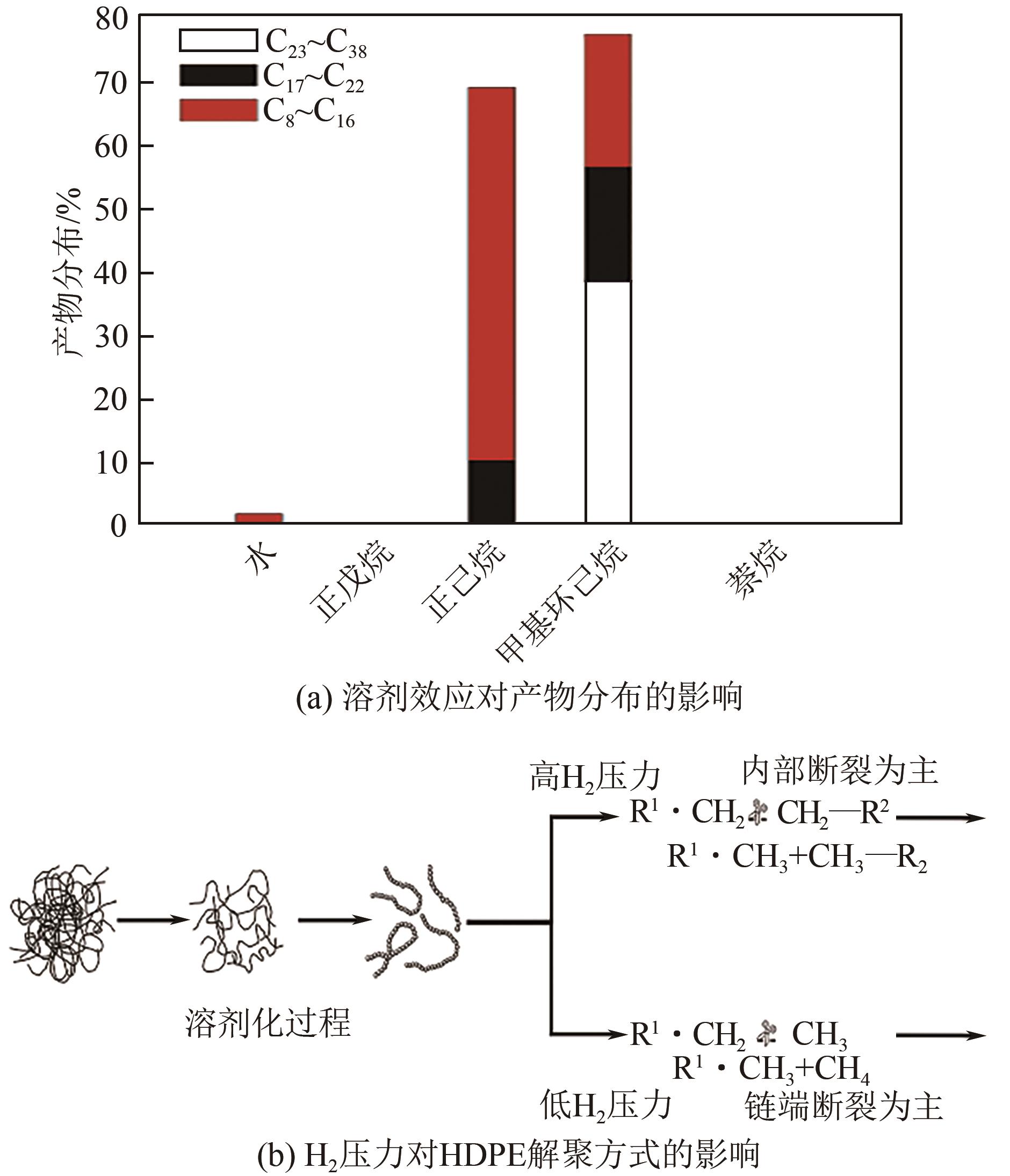
| 塑料 | 催化剂 | 反应条件 | 产物 | 收率/% |
|---|---|---|---|---|
| LDPE[ | Ru/C | 200~250℃,20bar H2,16h | C7~C45液体烷烃 | >90 |
| HDPE[ | Ru/C | 220℃,60bar H2,1h,正己烷 | C8~C16液体烷烃 | 60.8 |
| PE[ | Pt/SrTiO3 | 300℃,12bar H2,96h | 润滑油和蜡 | 42~97 |
| PE[ | Ru/CeO2 | 200~240℃,20~35bar H2,8144h | C5~C21液体烷烃,C22~C45蜡 | 83~92 |
| PP[ | Ru/TiO2 | 250℃,30bar H2,16h | 润滑油 | >80 |
| LDPE[ | Pt/WO3/ZrO2-HY | 225℃,30bar H2,2 h | 柴油和汽油 | 85 |
| PE[ | mSiO2/Pt/SiO2 | 250℃,13.8 bar H2,24h | 柴油和润滑油 | 38 |
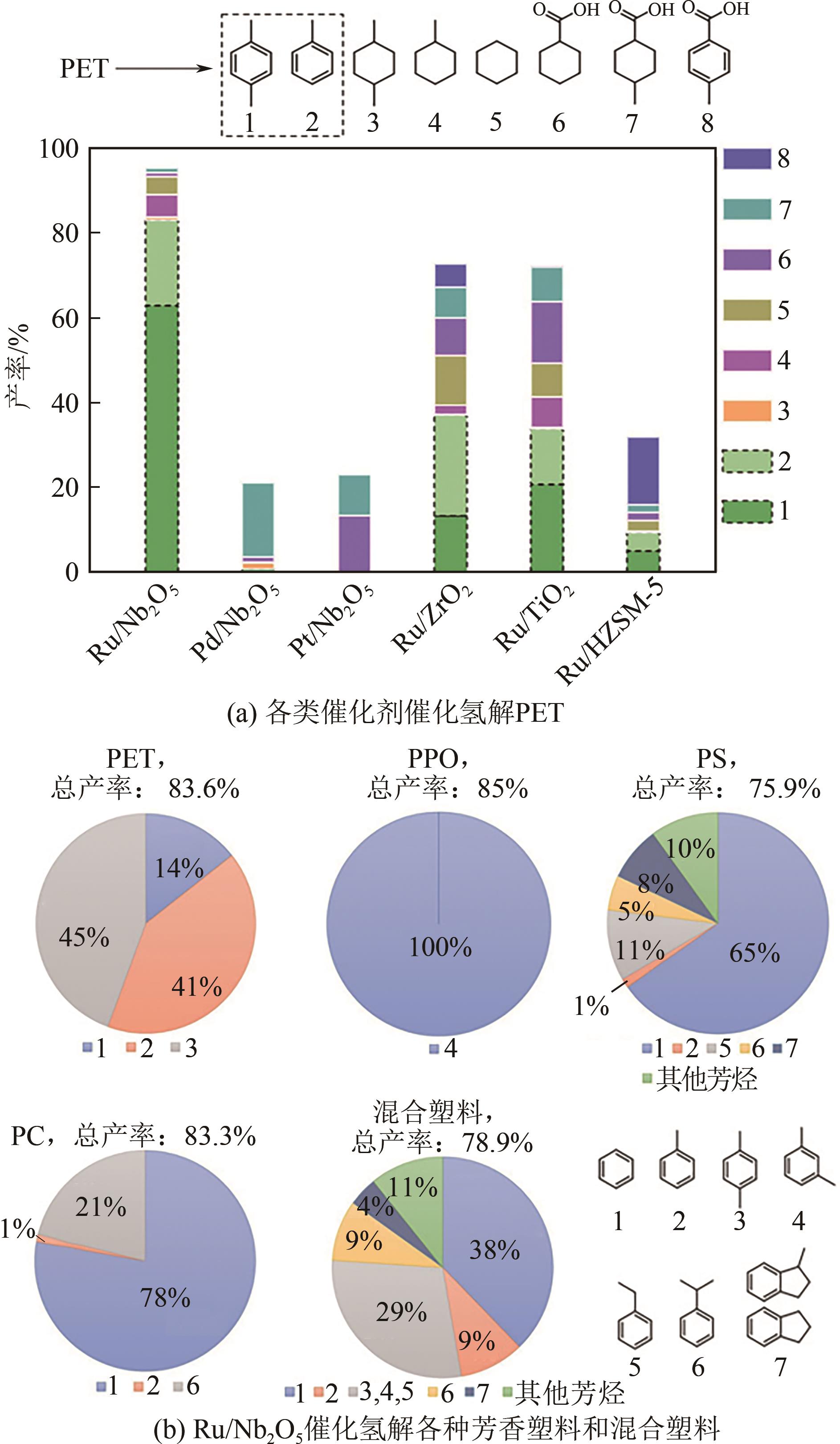
| PET[ | Ru/Nb2O5 | 280~320℃,3bar H2,8~16h | 芳烃 | 75~85 |
| PET64] | Ru/Nb2O5 | 220℃,20bar N2,12h,乙二醇 | 苯、甲苯、二甲苯 | 91 |
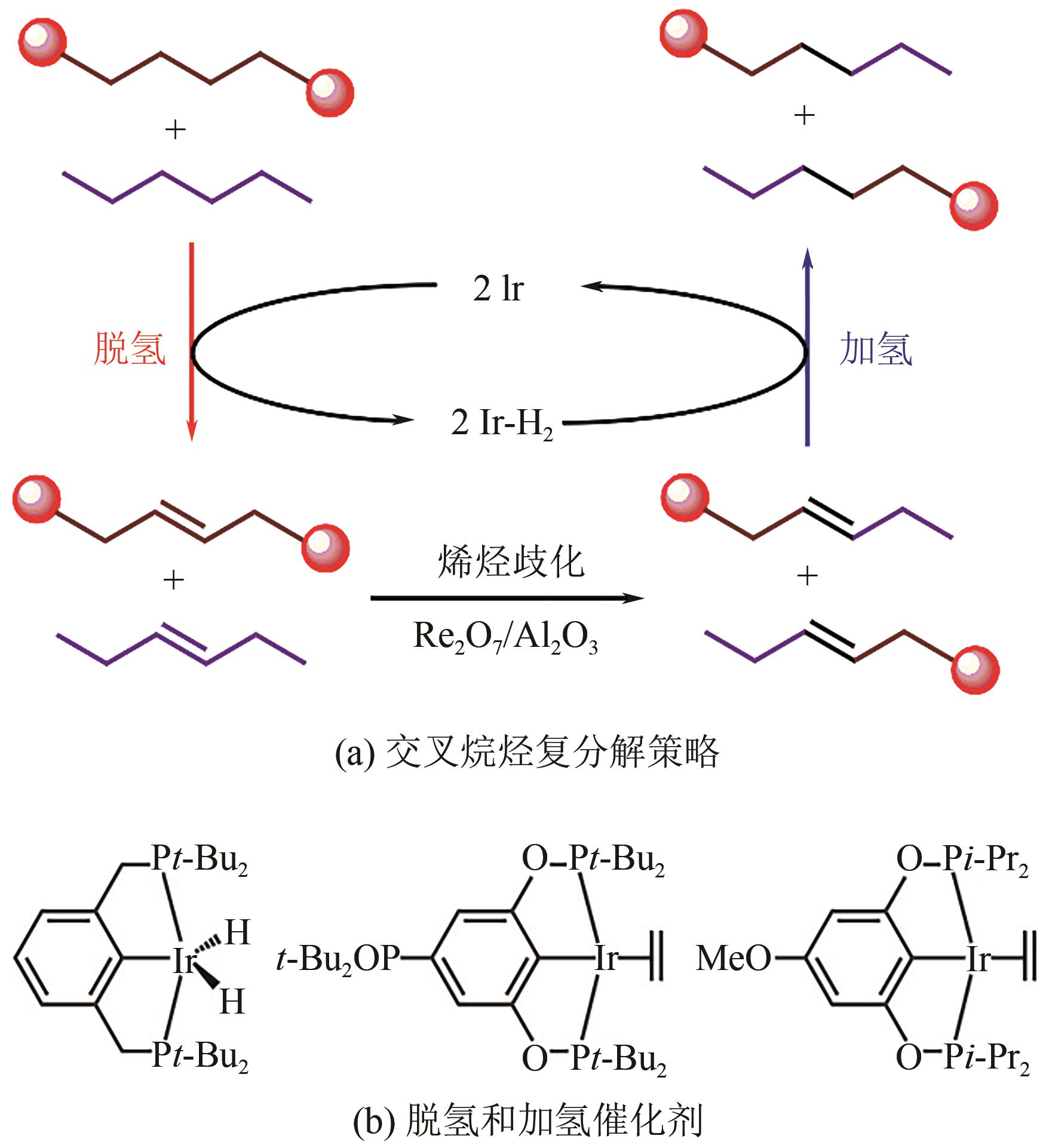
| PE[ | ( t-BuPCP)Ir,Re2O7/γ-Al2O3 | 150~175℃,72~96h,正己烷 | 液体油和蜡 | 98 |
| PE[ | SnPt/γ-Al2O3,Re2O7/γ-Al2O3 | 200℃,20~40bar He,15h | 低分子量齐聚物 | N/A |
| PE[ | Pt/γ-Al2O3 | 280℃,24h | 烷基芳烃和蜡 | 80 |
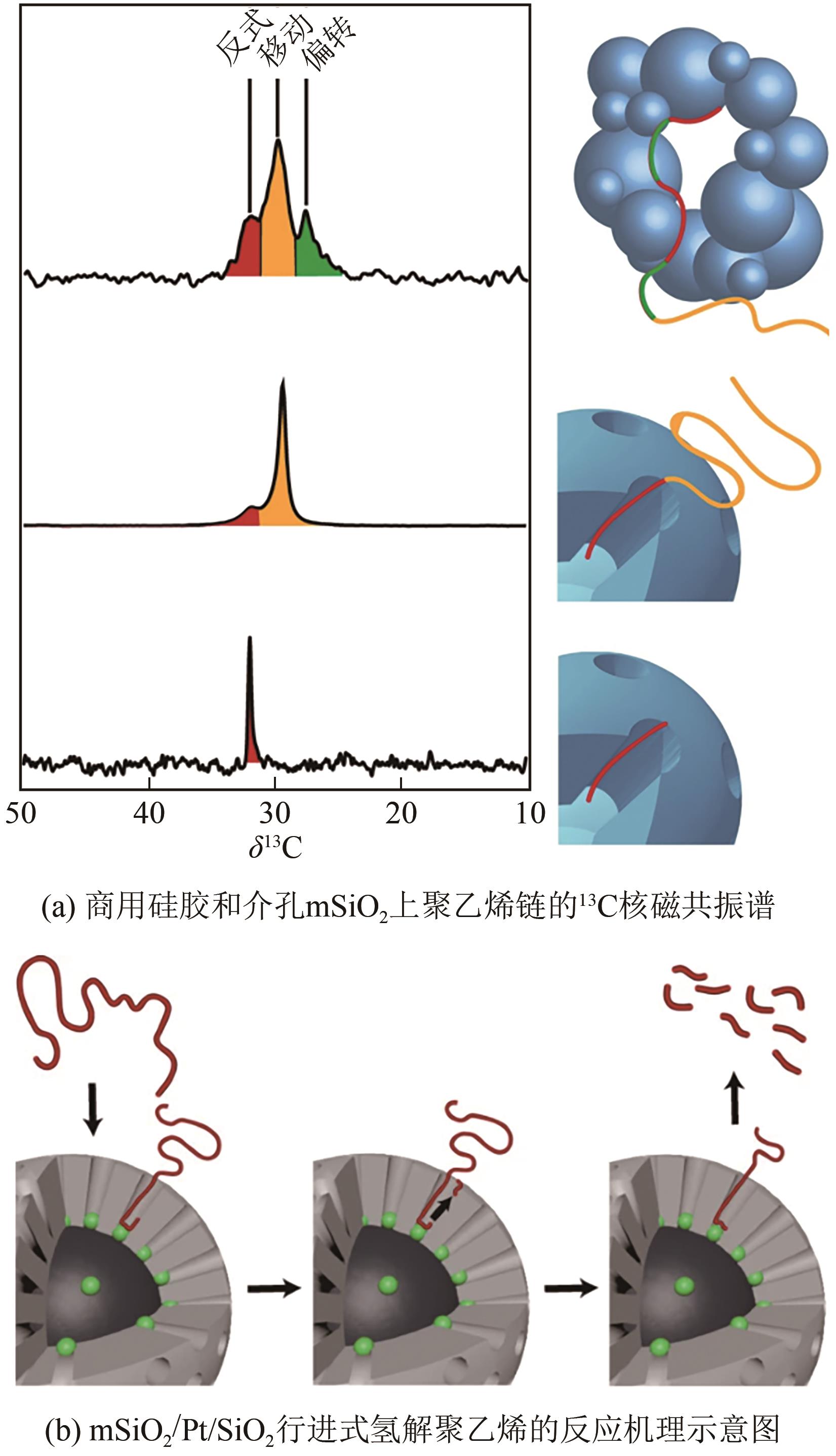
表2 不同催化剂催化氢解废塑料的最佳操作条件及产物对比
| 塑料 | 催化剂 | 反应条件 | 产物 | 收率/% |
|---|---|---|---|---|
| LDPE[ | Ru/C | 200~250℃,20bar H2,16h | C7~C45液体烷烃 | >90 |
| HDPE[ | Ru/C | 220℃,60bar H2,1h,正己烷 | C8~C16液体烷烃 | 60.8 |
| PE[ | Pt/SrTiO3 | 300℃,12bar H2,96h | 润滑油和蜡 | 42~97 |
| PE[ | Ru/CeO2 | 200~240℃,20~35bar H2,8144h | C5~C21液体烷烃,C22~C45蜡 | 83~92 |
| PP[ | Ru/TiO2 | 250℃,30bar H2,16h | 润滑油 | >80 |
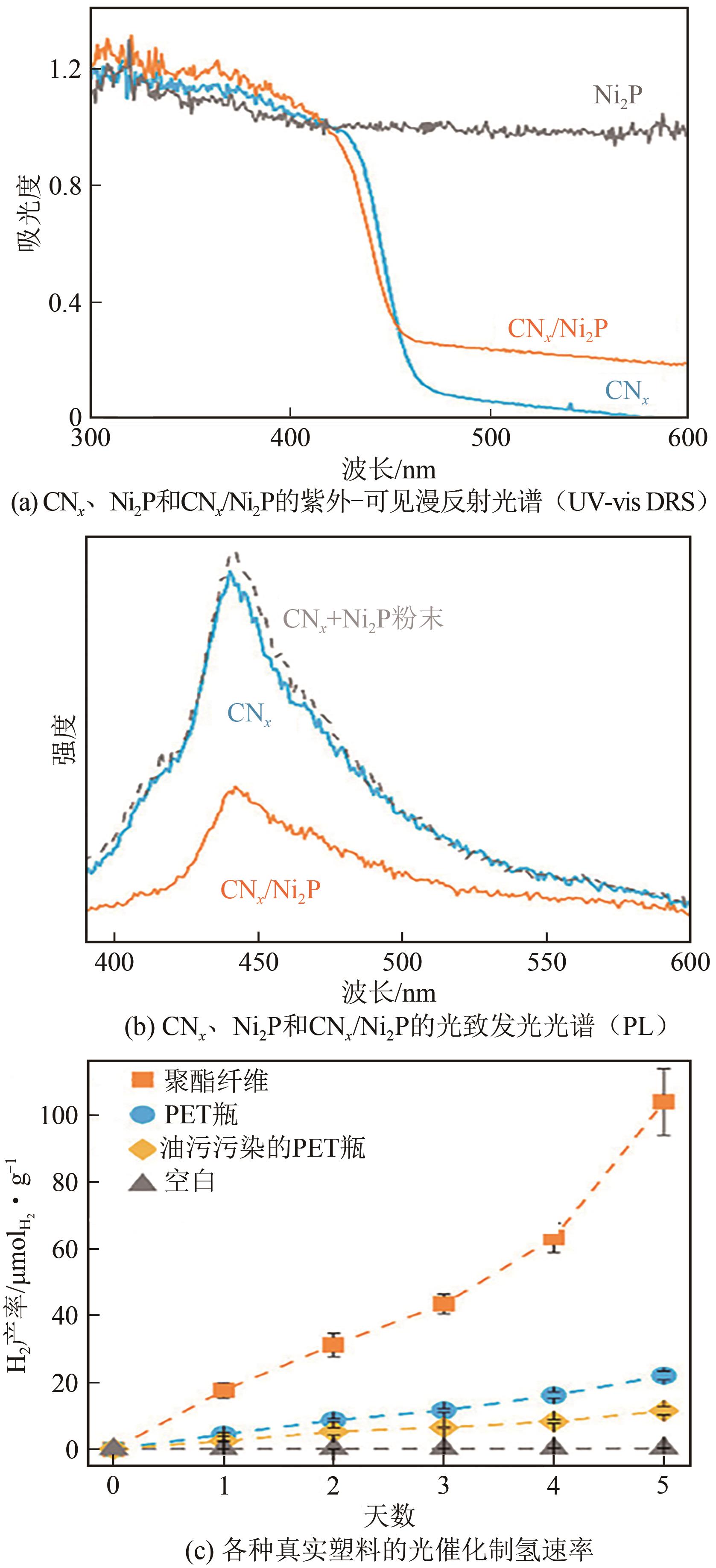
| LDPE[ | Pt/WO3/ZrO2-HY | 225℃,30bar H2,2 h | 柴油和汽油 | 85 |
| PE[ | mSiO2/Pt/SiO2 | 250℃,13.8 bar H2,24h | 柴油和润滑油 | 38 |
| PET[ | Ru/Nb2O5 | 280~320℃,3bar H2,8~16h | 芳烃 | 75~85 |
| PET64] | Ru/Nb2O5 | 220℃,20bar N2,12h,乙二醇 | 苯、甲苯、二甲苯 | 91 |
| PE[ | ( t-BuPCP)Ir,Re2O7/γ-Al2O3 | 150~175℃,72~96h,正己烷 | 液体油和蜡 | 98 |
| PE[ | SnPt/γ-Al2O3,Re2O7/γ-Al2O3 | 200℃,20~40bar He,15h | 低分子量齐聚物 | N/A |
| PE[ | Pt/γ-Al2O3 | 280℃,24h | 烷基芳烃和蜡 | 80 |
| 1 | CHEN X, WANG Y D, ZHANG L. Recent progress in the chemical upcycling of plastic wastes[J]. ChemSusChem, 2021, 14(19): 4137-4151. |
| 2 | NARAYAN R. Carbon footprint of bioplastics using biocarbon content analysis and life-cycle assessment[J]. MRS Bulletin, 2011, 36(9): 716-721. |
| 3 | GEYER R, JAMBECK J R, LAW K L. Production, use, and fate of all plastics ever made[J]. Science Advances, 2017, 3(7): e1700782. |
| 4 | VOLLMER I, JENKS M J F, ROELANDS M C P, et al. Beyond mechanical recycling: giving new life to plastic waste[J]. Angewandte Chemie International Edition, 2020, 59(36): 15402-15423. |
| 5 | HE P J, CHEN L Y, SHAO L M, et al. Municipal solid waste (MSW) landfill: a source of microplastics? -Evidence of microplastics in landfill leachate[J]. Water Research, 2019, 159: 38-45. |
| 6 | SERRANO D P, AGUADO J, ESCOLA J M. Developing advanced catalysts for the conversion of polyolefinic waste plastics into fuels and chemicals[J]. ACS Catalysis, 2012, 2(9): 1924-1941. |
| 7 | RAHIMI A, GARCÍA J M. Chemical recycling of waste plastics for new materials production[J]. Nature Reviews Chemistry, 2017, 1: 46. |
| 8 | CHEN H, WAN K, ZHANG Y Y, et al. Waste to wealth: chemical recycling and chemical upcycling of waste plastics for a great future[J]. ChemSusChem, 2021, 14(19): 4123-4136. |
| 9 | FAGNANI D E, TAMI J L, COPLEY G, et al. 100th Anniversary of macromolecular science viewpoint: redefining sustainable polymers[J]. ACS Macro Letters, 2021, 10(1): 41-53. |
| 10 | ZHANG F, ZHAO Y T, WANG D D, et al. Current technologies for plastic waste treatment: a review[J]. Journal of Cleaner Production, 2021, 282: 124523. |
| 11 | LI Q Y, FARAMARZI A, ZHANG S, et al. Progress in catalytic pyrolysis of municipal solid waste[J]. Energy Conversion and Management, 2020, 226: 113525. |
| 12 | KOSLOSKI-OH S C, WOOD Z A, MANJARREZ Yet al. Catalytic methods for chemical recycling or upcycling of commercial polymers[J]. Materials Horizons, 2021, 8(4): 1084-1129. |
| 13 | BAGRI R, WILLIAMS P T. Catalytic pyrolysis of polyethylene[J]. Journal of Analytical and Applied Pyrolysis, 2002, 63(1): 29-41. |
| 14 | CALDEIRA V P S, PERAL A, LINARES M, et al. Properties of hierarchical Beta zeolites prepared from protozeolitic nanounits for the catalytic cracking of high-density polyethylene[J]. Applied Catalysis A: General, 2017, 531: 187-196. |
| 15 | AGUADO J, SERRANO D P, MIGUEL G SAN, et al. Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system[J]. Journal of Analytical and Applied Pyrolysis, 2007, 79(1/2): 415-423. |
| 16 | RATNASARI D K, NAHIL M A, WILLIAMS P T. Catalytic pyrolysis of waste plastics using staged catalysis for production of gasoline range hydrocarbon oils[J]. Journal of Analytical and Applied Pyrolysis, 2017, 124: 631-637. |
| 17 | ELORDI G, OLAZAR M, LOPEZ G, et al. Catalytic pyrolysis of HDPE in continuous mode over zeolite catalysts in a conical spouted bed reactor[J]. Journal of Analytical and Applied Pyrolysis, 2009, 85(1/2): 345-351. |
| 18 | XUE Y, JOHNSTON P, BAI X L. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics[J]. Energy Conversion and Management, 2017, 142: 441-451. |
| 19 | MARK L O, CENDEJAS M C, HERMANS I. The use of heterogeneous catalysis in the chemical valorization of plastic waste[J]. ChemSusChem, 2020, 13(22): 5808-5836. |
| 20 | RENZINI M S, SEDRAN U, PIERELLA L B. H-ZSM-11 and Zn-ZSM-11 zeolites and their applications in the catalytic transformation of LDPE[J]. Journal of Analytical and Applied Pyrolysis, 2009, 86(1): 215-220. |
| 21 | ZHANG X S, LEI H W, YADAVALLI G, et al. Gasoline-range hydrocarbons produced from microwave-induced pyrolysis of low-density polyethylene over ZSM-5[J]. Fuel, 2015, 144: 33-42. |
| 22 | DING K, LIU S S, HUANG Y, et al. Catalytic microwave-assisted pyrolysis of plastic waste over NiO and HY for gasoline-range hydrocarbons production[J]. Energy Conversion and Management, 2019, 196: 1316-1325. |
| 23 | FAN L L, SU Z Y, WU J B, et al. Integrating continuous-stirred microwave pyrolysis with ex-situ catalytic upgrading for linear low-density polyethylene conversion: effects of parameter conditions[J]. Journal of Analytical and Applied Pyrolysis, 2021, 157: 105213. |
| 24 | DANISH M, AHMAD T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application[J]. Renewable and Sustainable Energy Reviews, 2018, 87: 1-21. |
| 25 | UTETIWABO W, YANG L, TUFAIL M K, et al. Electrode materials derived from plastic wastes and other industrial wastes for supercapacitors[J]. Chinese Chemical Letters, 2020, 31(6): 1474-1489. |
| 26 | ZHANG Y Y, DUAN D L, LEI H W, et al. Jet fuel production from waste plastics via catalytic pyrolysis with activated carbons[J]. Applied Energy, 2019, 251:113337. |
| 27 | WAN K, CHEN H, ZHENG F J, et al. Tunable production of jet-fuel range alkanes and aromatics by catalytic pyrolysis of LDPE over biomass-derived activated carbons[J]. Industrial & Engineering Chemistry Research, 2020, 59(39): 17451-17461. |
| 28 | SUN K, HUANG Q X, MENG X D, et al. Catalytic pyrolysis of waste polyethylene into aromatics by H3PO4-activated carbon[J]. Energy & Fuels, 2018, 32(9): 9772-9781. |
| 29 | DUAN D L, FENG Z Q, DONG X Y, et al. Improving bio-oil quality from low-density polyethylene pyrolysis: effects of varying activation and pyrolysis parameters[J]. Energy, 2021, 232: 121090. |
| 30 | SUN K, HUANG Q X, CHI Y, et al. Effect of ZnCl2-activated biochar on catalytic pyrolysis of mixed waste plastics for producing aromatic-enriched oil[J]. Waste Management, 2018, 81: 128-137. |
| 31 | LEE K H. Composition of aromatic products in the catalytic degradation of the mixture of waste polystyrene and high-density polyethylene using spent FCC catalyst[J]. Polymer Degradation and Stability, 2008, 93(7): 1284-1289. |
| 32 | VOLLMER I, JENKS M J F, MAYORGA GONZÁLEZ R, et al. Plastic waste conversion over a refinery waste catalyst[J]. Angewandte Chemie International Edition, 2021, 60(29): 16101-16108. |
| 33 | MANOS G, YUSOF I Y, PAPAYANNAKOS N, et al. Catalytic cracking of polyethylene over clay catalysts. Comparison with an ultrastable Y zeolite[J]. Industrial & Engineering Chemistry Research, 2001, 40(10): 2220-2225. |
| 34 | LI K X, LEI J X, YUAN G A, et al. Fe-, Ti-, Zr- and Al-pillared clays for efficient catalytic pyrolysis of mixed plastics[J]. Chemical Engineering Journal, 2017, 317: 800-809. |
| 35 | ZHANG Z B, HIROSE T, NISHIO S, et al. Chemical recycling of waste polystyrene into styrene over solid acids and bases[J]. Industrial & Engineering Chemistry Research, 1995, 34(12): 4514-4519. |
| 36 | 冯时宇, 李洋, 李凯, 等. 塑料废弃物热催化制备碳纳米管的研究进展[J]. 环境工程, 2021, 39(4): 107-114. |
| FENG Shiyu, LI Yang, LI Kai, et al. Progress in preparation of carbon nanotubes by thermal catalysis of waste plastics[J]. Environmental Engineering, 2021, 39(4): 107-114. | |
| 37 | WU C F, NAHIL M A, MISKOLCZI N, et al. Production and application of carbon nanotubes, as a co-product of hydrogen from the pyrolysis-catalytic reforming of waste plastic[J]. Process Safety and Environmental Protection, 2016, 103: 107-114. |
| 38 | YAO D D, WU C F, YANG H P, et al. Co-production of hydrogen and carbon nanotubes from catalytic pyrolysis of waste plastics on Ni-Fe bimetallic catalyst[J]. Energy Conversion and Management, 2017, 148: 692-700. |
| 39 | YAO D D, ZHANG Y S, WILLIAMS P T, et al. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: influence of catalyst composition and operational parameters[J]. Applied Catalysis B: Environmental, 2018, 221: 584-597. |
| 40 | JIA J B, VEKSHA A, LIM T T, et al. In situ grown metallic nickel from X-Ni (X=La, Mg, Sr) oxides for converting plastics into carbon nanotubes: influence of metal-support interaction[J]. Journal of Cleaner Production, 2020, 258: 120633. |
| 41 | ABOUL-ENEIN A A, AWADALLAH A E. Production of nanostructure carbon materials via non-oxidative thermal degradation of real polypropylene waste plastic using La2O3 supported Ni and Ni-Cu catalysts[J]. Polymer Degradation and Stability, 2019, 167: 157-169. |
| 42 | JIE X Y, LI W S, SLOCOMBE D, et al. Microwave-initiated catalytic deconstruction of plastic waste into hydrogen and high-value carbons[J]. Nature Catalysis, 2020, 3(11): 902-912. |
| 43 | ZHENG X, ZHANG R Q, FANG P T, et al. Advances in ionic liquid-catalyzed poly(ethylene terephthalate) degradation[J]. Scientia Sinica Chimica, 2021, 51(10): 1330-1342. |
| 44 | WANG H, LI Z X, LIU Y Q, et al. Degradation of poly(ethylene terephthalate) using ionic liquids[J]. Green Chemistry, 2009, 11(10): 1568-1575. |
| 45 | WANG H, LIU Y Q, LI Z X, et al. Glycolysis of poly(ethylene terephthalate) catalyzed by ionic liquids[J]. European Polymer Journal, 2009, 45(5): 1535-1544. |
| 46 | ALNAQBI M A, MOHSIN M A, BUSHEER R M, et al. Microwave assisted glycolysis of poly(ethylene terephthalate) catalyzed by 1-butyl-3-methylimidazolium bromide ionic liquid[J]. Journal of Applied Polymer Science, 2015, 132(12): 41666. |
| 47 | WANG H, YAN R Y, LI Z X, et al. Fe-containing magnetic ionic liquid as an effective catalyst for the glycolysis of poly(ethylene terephthalate)[J]. Catalysis Communications, 2010, 11(8): 763-767. |
| 48 | WANG Q, GENG Y R, LU X M, et al. First-row transition metal-containing ionic liquids as highly active catalysts for the glycolysis of poly(ethylene terephthalate) (PET)[J]. ACS Sustainable Chemistry & Engineering, 2015, 3(2): 340-348. |
| 49 | SUN J, LIU D J, YOUNG R P, et al. Solubilization and upgrading of high polyethylene terephthalate loadings in a low-costing bifunctional ionic liquid[J]. ChemSusChem, 2018, 11(4): 781-792. |
| 50 | LIU Y C, YAO X Q, YAO H Y, et al. Degradation of poly(ethylene terephthalate) catalyzed by metal-free choline-based ionic liquids[J]. Green Chemistry, 2020, 22(10): 3122-3131. |
| 51 | WANG Q, YAO X Q, GENG Y R, et al. Deep eutectic solvents as highly active catalysts for the fast and mild glycolysis of poly(ethylene terephthalate)(PET)[J]. Green Chemistry, 2015, 17(4): 2473-2479. |
| 52 | LIU B, FU W Z, LU X M, et al. Lewis acid-base synergistic catalysis for polyethylene terephthalate degradation by 1, 3-dimethylurea/Zn(OAc)2 deep eutectic solvent[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(3): 3292-3300. |
| 53 | AL-SABAGH A M, YEHIA F Z, ESHAQ G, et al. Ionic liquid-coordinated ferrous acetate complex immobilized on bentonite as a novel separable catalyst for PET glycolysis[J]. Industrial & Engineering Chemistry Research, 2015, 54(50): 12474-12481. |
| 54 | CANO I, MARTIN C, FERNANDES J A, et al. Paramagnetic ionic liquid-coated SiO2@Fe3O4 nanoparticles—The next generation of magnetically recoverable nanocatalysts applied in the glycolysis of PET[J]. Applied Catalysis B: Environmental, 2020, 260: 118110. |
| 55 | WANG R, WANG T L, YU G R, et al. A new class of catalysts for the glycolysis of PET: deep eutectic solvent@ZIF-8 composite[J]. Polymer Degradation and Stability, 2021, 183: 109463. |
| 56 | RORRER J E, BECKHAM G T, LESHKOV Y R. Conversion of polyolefin waste to liquid alkanes with Ru-based catalysts under mild conditions[J]. JACS Au, 2021, 1(1): 8-12. |
| 57 | JIA C H, XIE S Q, ZHANG W L, et al. Deconstruction of high-density polyethylene into liquid hydrocarbon fuels and lubricants by hydrogenolysis over Ru catalyst[J]. Chem Catalysis, 2021, 1(2): 437-455. |
| 58 | CELIK G, KENNEDY R M, HACKLER R A, et al. Upcycling single-use polyethylene into high-quality liquid products[J]. ACS Central Science, 2019, 5(11): 1795-1803. |
| 59 | NAKAJI Y, TAMURA M, MIYAOKA S, et al. Low-temperature catalytic upgrading of waste polyolefinic plastics into liquid fuels and waxes[J]. Applied Catalysis B: Environmental, 2021, 285: 119805. |
| 60 | KOTS P A, LIU S B, VANCE B C, et al. Polypropylene plastic waste conversion to lubricants over Ru/TiO2 catalysts[J]. ACS Catalysis, 2021, 11(13): 8104-8115. |
| 61 | LIU S B, KOTS P A, VANCE B C, et al. Plastic waste to fuels by hydrocracking at mild conditions[J]. Science Advances, 2021, 7(17), No. eabf8283. DOI:10.1126/sciadv.abf8283 |
| 62 | TENNAKOON A, WU X, PATERSON A L, et al. Catalytic upcycling of high-density polyethylene via a processive mechanism[J]. Nature Catalysis, 2020, 3(11): 893-901. |
| 63 | JING Y X, WANG Y Q, FURUKAWA S, et al. Towards the circular economy: converting aromatic plastic waste back to arenes over a Ru/Nb2O5 catalyst[J]. Angewandte Chemie International Edition, 2021, 60(10): 5527-5535. |
| 64 | LU S L, JING Y X, FENG B, et al. H2-free plastic conversion: converting PET back to BTX by unlocking hidden hydrogen[J]. ChemSusChem, 2021, 14(19): 4242-4250. |
| 65 | JIA X Q, QIN C, FRIEDBERGER T, et al. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions[J]. Science Advances, 2016, 2(6): e1501591. |
| 66 | ELLIS L D, ORSKI S V, KENLAW G A, et al. Tandem heterogeneous catalysis for polyethylene depolymerization via an olefin-intermediate process[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(2): 623-628. |
| 67 | ZHANG F, ZENG M H, YAPPERT R D, et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization[J]. Science, 2020, 370(6515): 437-441. |
| 68 | KAWAI T, SAKATA T. Photocatalytic hydrogen production from water by the decomposition of poly-vinylchloride, protein, algae, dead insects, and excrement[J]. Chemistry Letters, 1981, 10(1): 81-84. |
| 69 | UEKERT T, KUEHNEL M F, WAKERLEY D W, et al. Plastic waste as a feedstock for solar-driven H2 generation[J]. Energy & Environmental Science, 2018, 11(10): 2853-2857. |
| 70 | UEKERT T, KASAP H, REISNER E. Photoreforming of nonrecyclable plastic waste over a carbon nitride/nickel phosphide catalyst[J]. Journal of the American Chemical Society, 2019, 141(38): 15201-15210. |
| 71 | JIAO X C, ZHENG K, CHEN Q X, et al. Photocatalytic conversion of waste plastics into C2 fuels under simulated natural environment conditions[J]. Angewandte Chemie International Edition, 2020, 59(36): 15497-15501. |
| 72 | CHEN L Y, MALOLLARI K G, ULIANA A, et al. Selective, catalytic oxidations of C—H bonds in polyethylenes produce functional materials with enhanced adhesion[J]. Chem, 2021, 7(1): 137-145. |
| 73 | LEWIS S E, WILHELMY B E, LEIBFARTH F A. Upcycling aromatic polymers through C—H fluoroalkylation[J]. Chemical Science, 2019, 10(25): 6270-6277. |
| 74 | LEWIS S E, WILHELMY B E, LEIBFARTH F A. Organocatalytic C—H fluoroalkylation of commodity polymers[J]. Polymer Chemistry, 2020, 11(30): 4914-4919. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [6] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [7] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [8] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [9] | 张启, 赵红, 荣峻峰. 质子交换膜燃料电池中氧还原反应抗毒性电催化剂研究进展[J]. 化工进展, 2023, 42(9): 4677-4691. |
| [10] | 王伟涛, 鲍婷玉, 姜旭禄, 何珍红, 王宽, 杨阳, 刘昭铁. 醛酮树脂基非金属催化剂催化氧气氧化苯制备苯酚[J]. 化工进展, 2023, 42(9): 4706-4715. |
| [11] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [12] | 吴海波, 王希仑, 方岩雄, 纪红兵. 3D打印催化材料开发与应用进展[J]. 化工进展, 2023, 42(8): 3956-3964. |
| [13] | 向阳, 黄寻, 魏子栋. 电催化有机合成反应的活性和选择性调控研究进展[J]. 化工进展, 2023, 42(8): 4005-4014. |
| [14] | 王耀刚, 韩子姗, 高嘉辰, 王新宇, 李思琪, 杨全红, 翁哲. 铜基催化剂电还原二氧化碳选择性的调控策略[J]. 化工进展, 2023, 42(8): 4043-4057. |
| [15] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||