化工进展 ›› 2021, Vol. 40 ›› Issue (9): 4998-5011.DOI: 10.16085/j.issn.1000-6613.2021-0534
锂离子电池正极材料合成及改性
王策1,2( ), 王国庆1,2, 王二锐1,2, 吴天昊1,2, 尉海军1,2(
), 王国庆1,2, 王二锐1,2, 吴天昊1,2, 尉海军1,2( )
)
- 1.北京工业大学材料与制造学部先进电池材料与器件研究所,北京 100124
2.新型功能材料教育部重点实验室,北京 100124
-
收稿日期:2021-03-17修回日期:2021-04-03出版日期:2021-09-05发布日期:2021-09-13 -
通讯作者:尉海军 -
作者简介:王策(1994—),男,博士研究生,主要从事锂离子电池正极材料方面的研究。E-mail:ce-wang@emails.bjut.edu.cn 。
Synthesis and modification of lithium-ion battery cathode materials
WANG Ce1,2( ), WANG Guoqing1,2, WANG Errui1,2, WU Tianhao1,2, YU Haijun1,2(
), WANG Guoqing1,2, WANG Errui1,2, WU Tianhao1,2, YU Haijun1,2( )
)
- 1.Institute of Advanced Battery Materials and Devices,Faculty of Materials and Manufacturing,Beijing University of Technology, Beijing 100124, China
2.Key Laboratory of Advanced Functional Materials,Ministry of Education, Beijing 100124, China
-
Received:2021-03-17Revised:2021-04-03Online:2021-09-05Published:2021-09-13 -
Contact:YU Haijun
摘要:
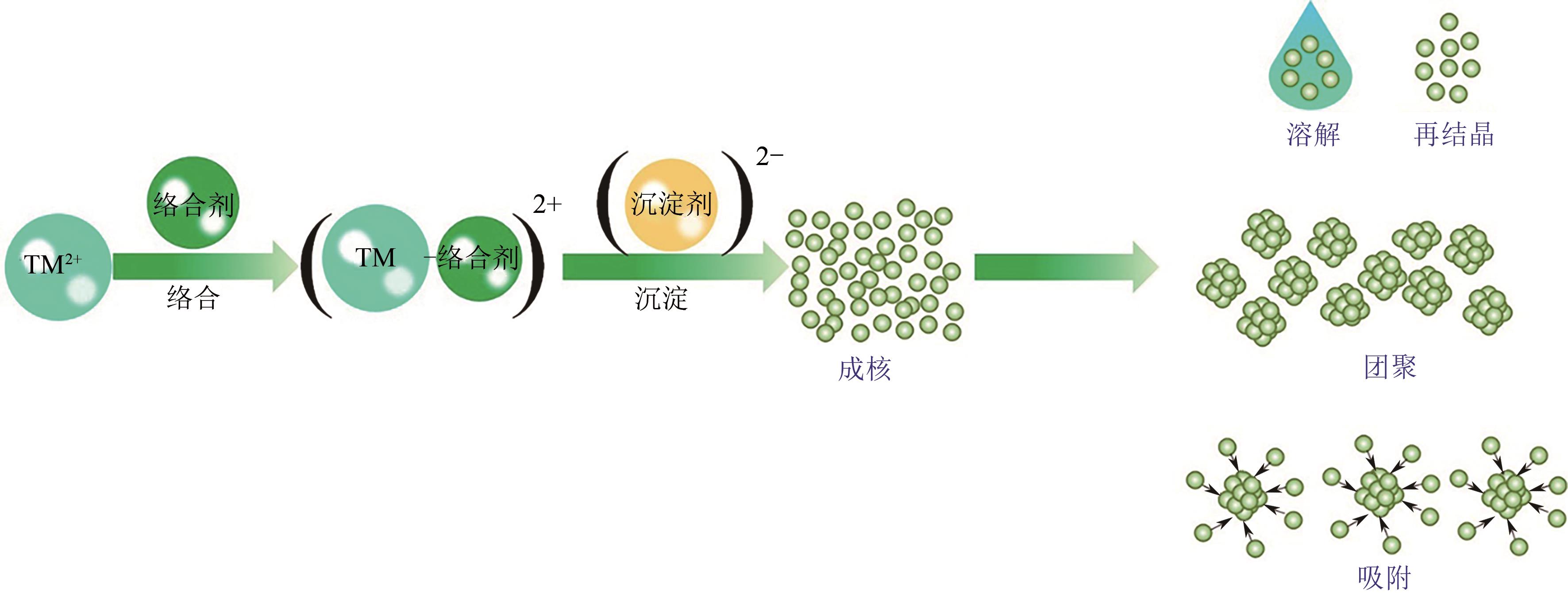
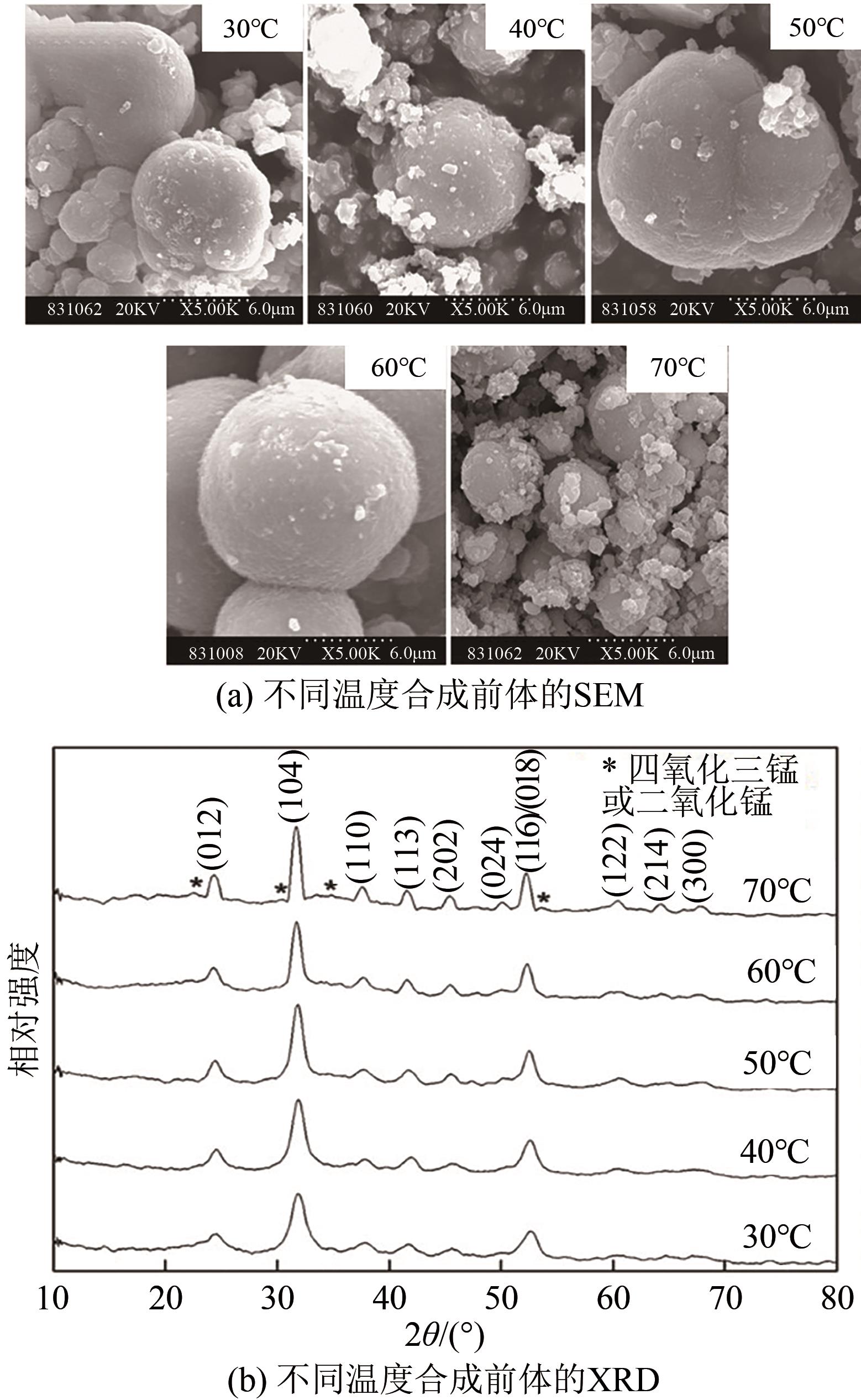
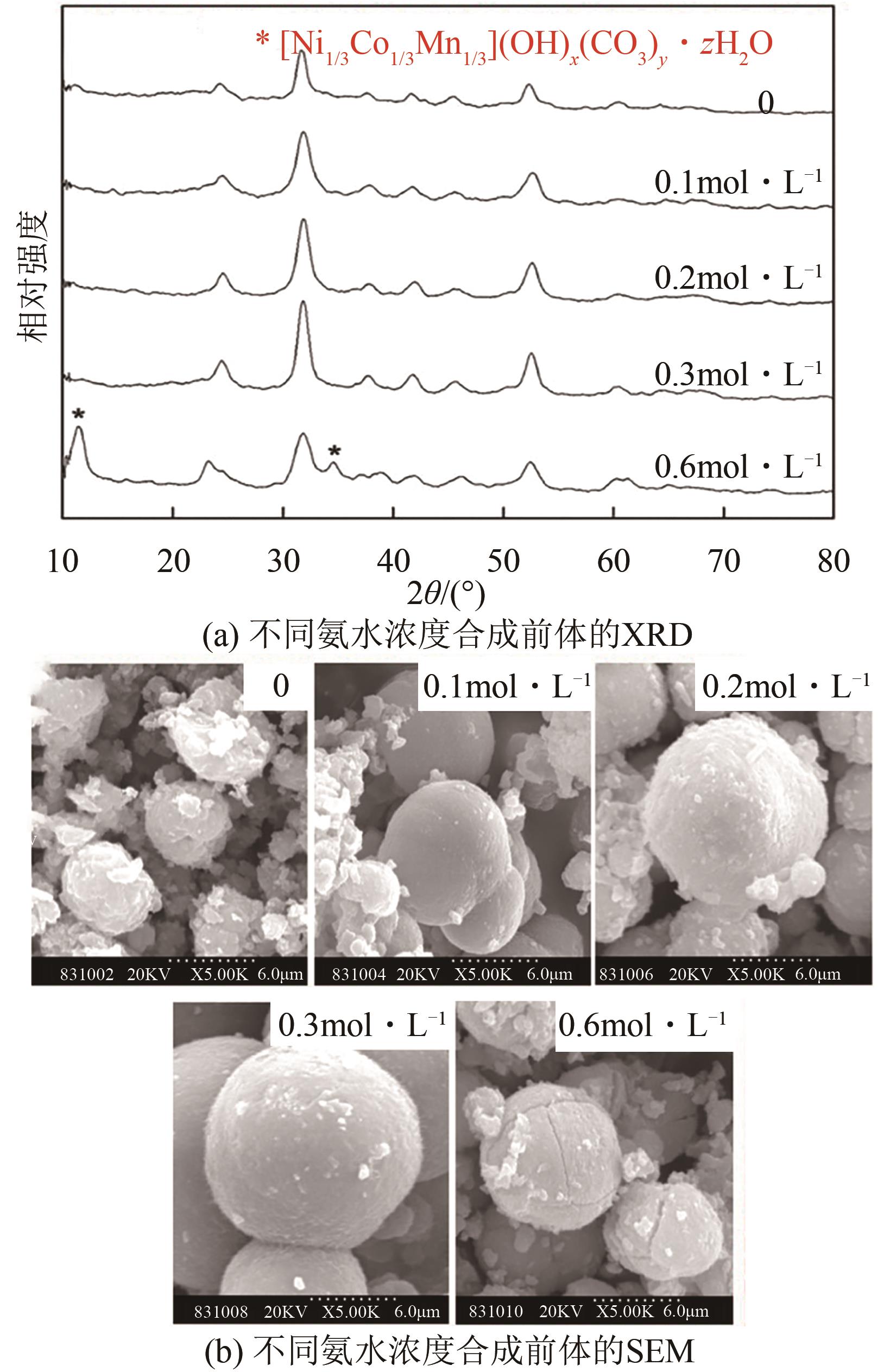
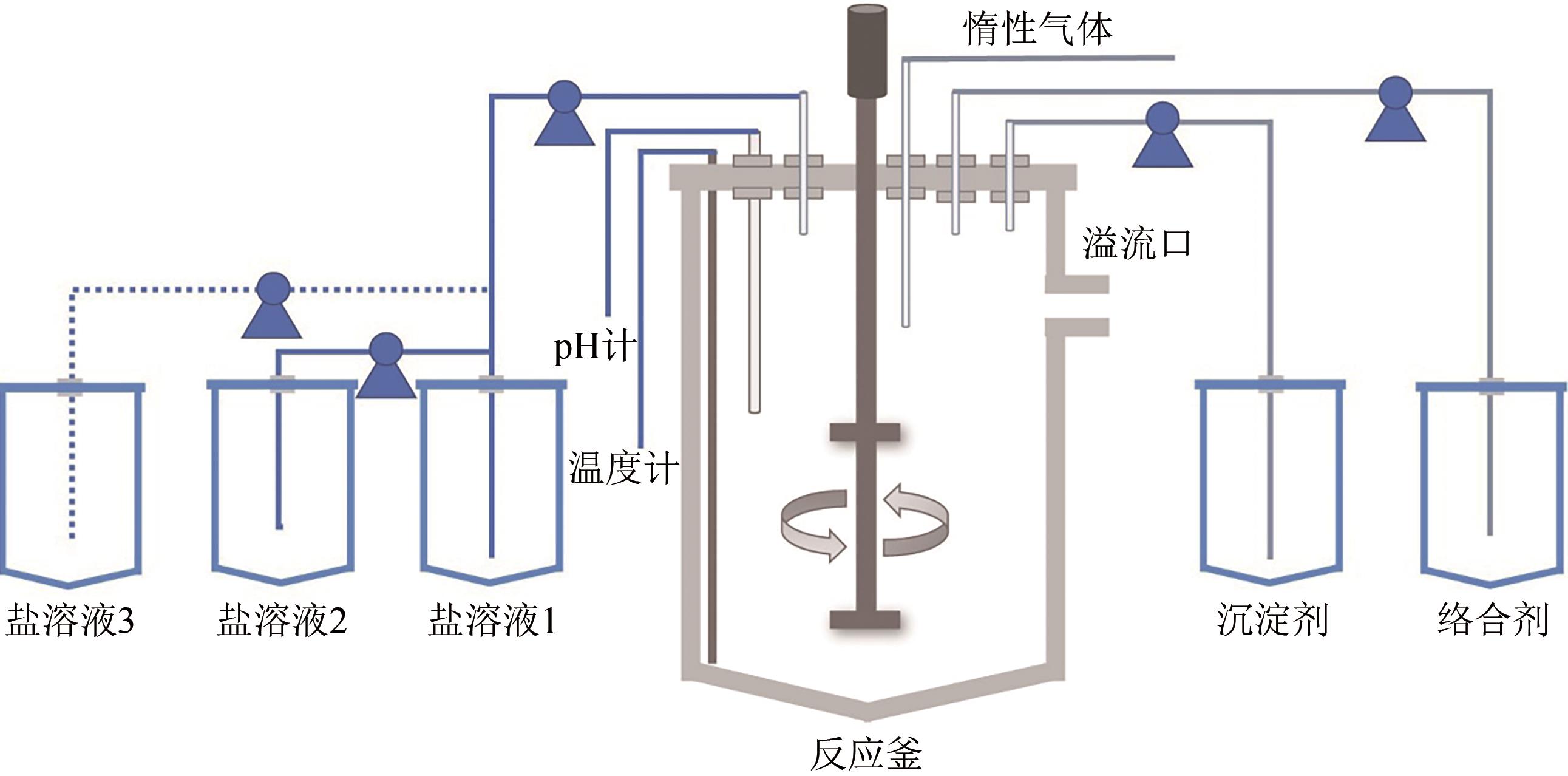
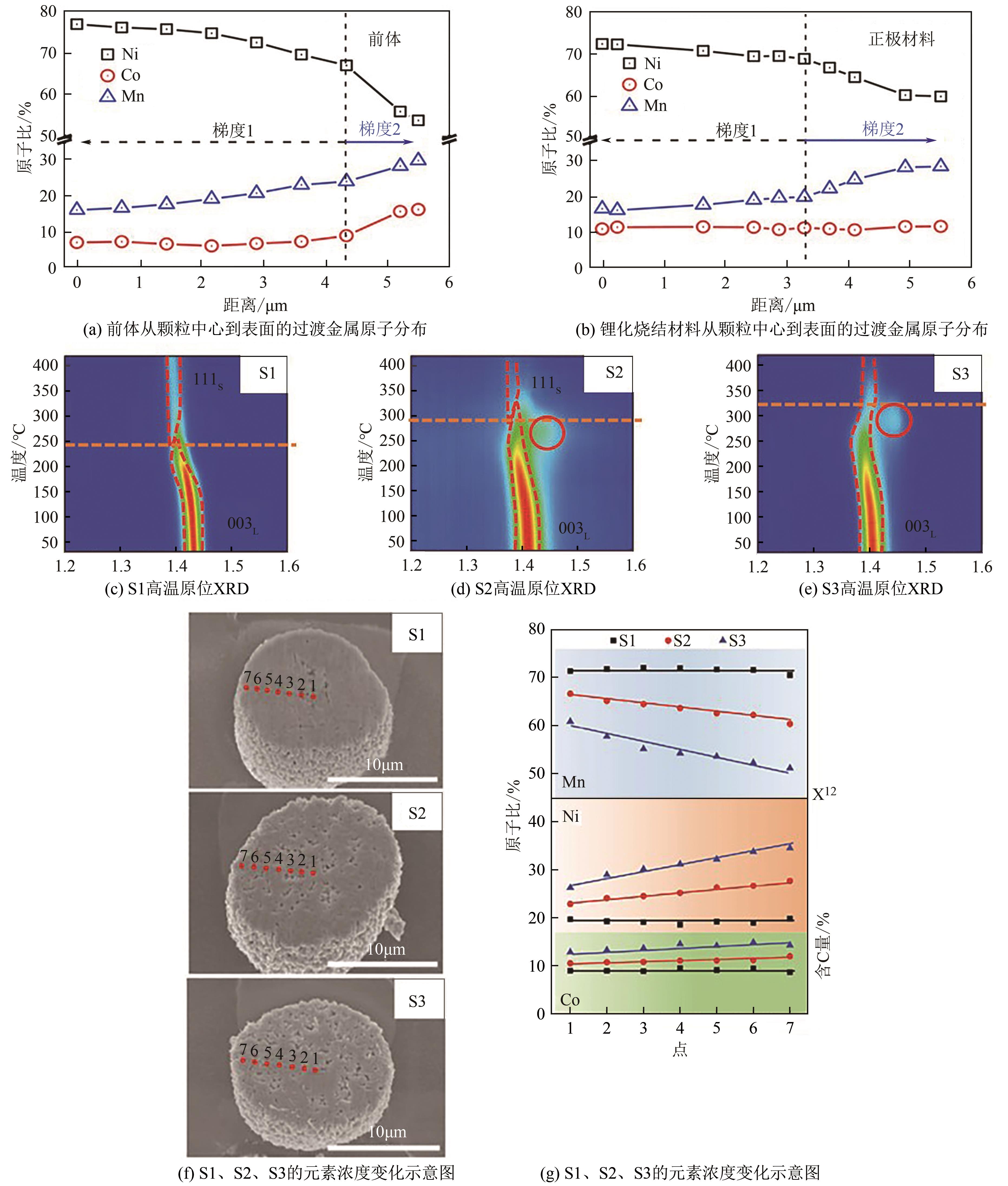
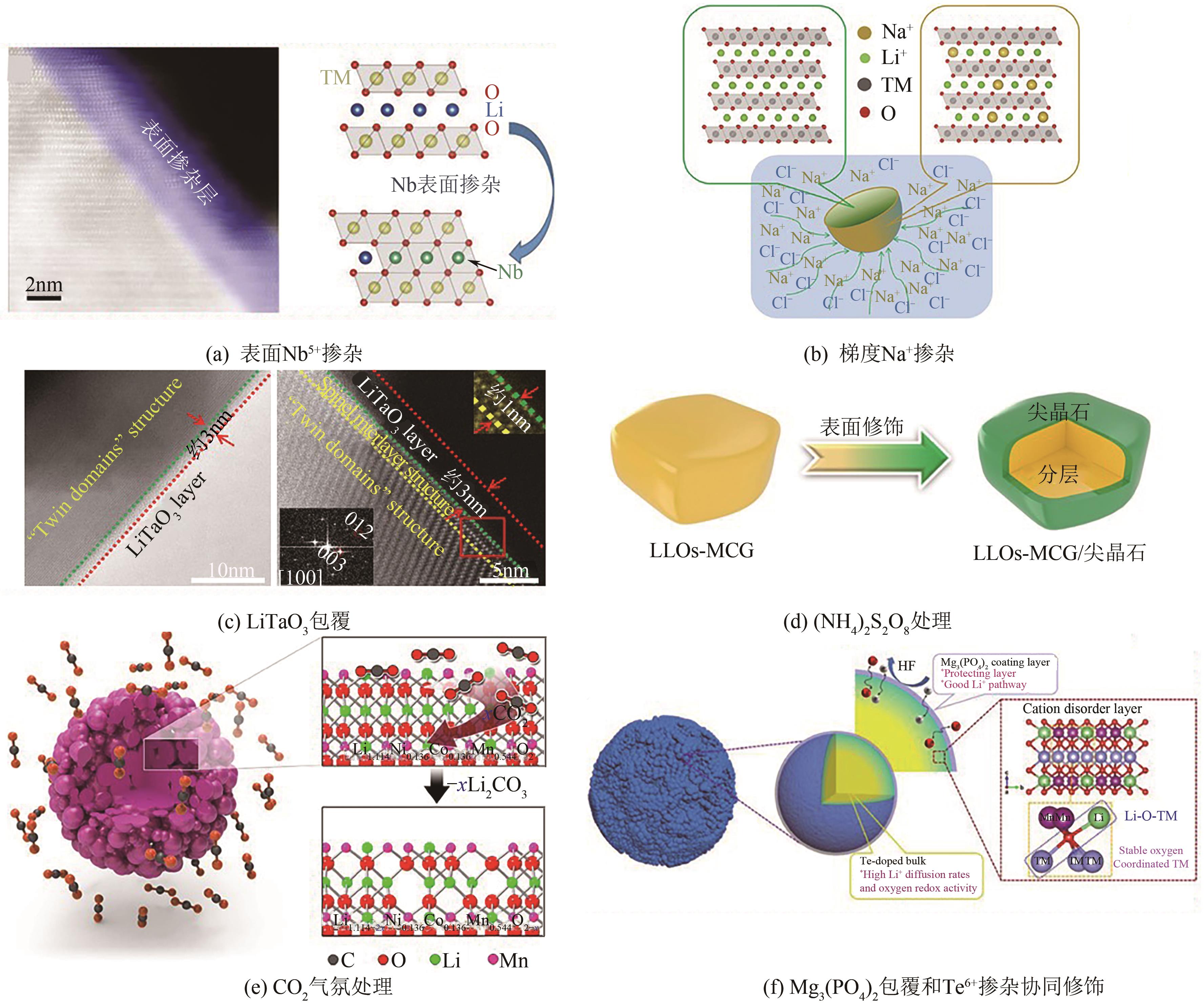
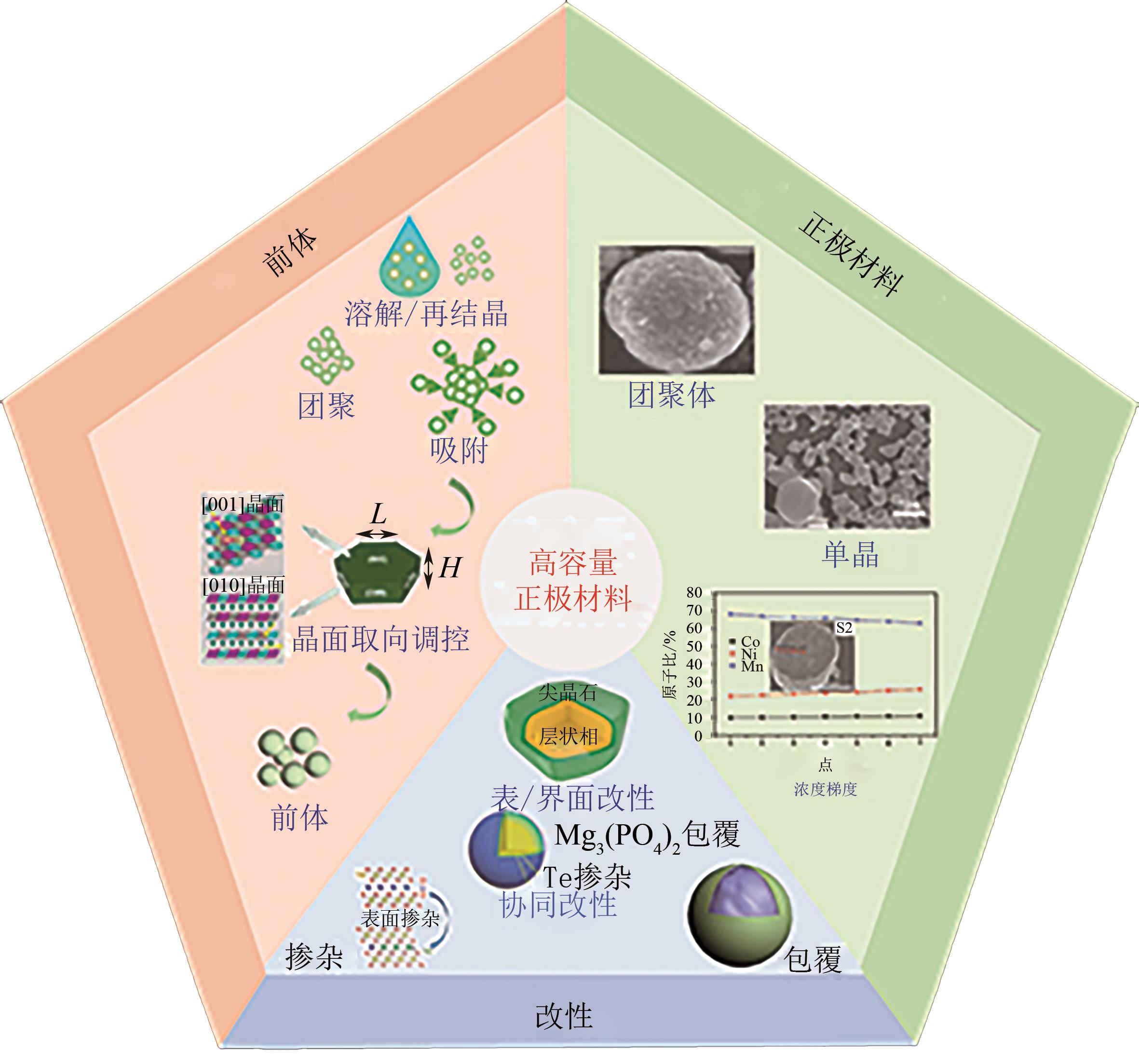
电动汽车续航里程的提升主要依赖于锂离子电池的能量密度,其中发展高容量的正极材料成为关键。富锂锰基层状氧化物(LLOs)和高镍三元层状氧化物(NCM,Ni≥80%)等高容量正极材料成为了研究热点,其前体的开发对正极材料电化学性能的发挥有重要的影响。本文从工业化的角度对共沉淀法制备LLOs和NCM正极材料前体的反应过程和影响因素进行了介绍,分析了球形团聚体、单晶和浓度梯度等正极材料的结构和性能,并详细阐述了正极材料中晶面取向调控、掺杂及表界面处理等改性策略的原理及优缺点。文章指出,综合来看单晶材料表现出较好的循环稳定性和热稳定性,但倍率性能有待进一步提升。浓度梯度正极材料不仅保持了高容量特性,还兼顾良好的结构稳定性和热稳定性,有望突破高容量正极材料进一步发展的技术瓶颈。最后,基于本文作者课题组在高容量正极材料方面的研究,对正极材料的未来发展趋势给出了一些建议。
中图分类号:
引用本文
王策, 王国庆, 王二锐, 吴天昊, 尉海军. 锂离子电池正极材料合成及改性[J]. 化工进展, 2021, 40(9): 4998-5011.
WANG Ce, WANG Guoqing, WANG Errui, WU Tianhao, YU Haijun. Synthesis and modification of lithium-ion battery cathode materials[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4998-5011.
| 1 | XU K. Electrolytes and interphases in Li-ion batteries and beyond[J]. Chem. Rev., 2014, 114(23): 11503-11618. |
| 2 | CROY J R, BALASUBRAMANIAN M, GALLAGHER K G, et al. Review of the U.S. Department of Energy’s “deep dive” effort to understand voltage fade in Li- and Mn-rich cathodes[J]. Accounts Chem. Res., 2015, 48(11): 2813-2821. |
| 3 | WANG J, HE X, PAILLARD E, et al. Lithium- and manganese-rich oxide cathode materials for high-energy lithium ion batteries[J]. Adv. Energy Mater., 2016, 6(21): 1600906. |
| 4 | LIU W, OH P, LIU X, et al. Nickel-reiche lithium-übergangsmetall-schichtverbindungen für hochenergie-lithium ion enakkumulatoren[J]. Angewandte Chemie, 2015, 127(15): 4518-4536. |
| 5 | GALLAGHER K G, CROY J R, BALASUBRAMANIAN M, et al. Correlating hysteresis and voltage fade in lithium-and manganese-rich layered transition-metal oxide electrodes[J]. Electrochemistry Communications, 2013, 33: 96-98. |
| 6 | SHARPE R, HOUSE R A, CLARKE M J, et al. Redox chemistry and the role of trapped molecular O2 in Li-rich disordered rocksalt oxyfluoride cathodes[J]. J. Am. Chem. Soc., 2020, 142(52): 21799-21809. |
| 7 | SINGER A, ZHANG M, HY S, et al. Nucleation of dislocations and their dynamics in layered oxide cathode materials during battery charging[J]. Nat. Energy, 2018, 3(8): 641-647. |
| 8 | HOUSE R A, REES G J, PÉREZ-OSORIO M A, et al. First-cycle voltage hysteresis in Li-rich 3d cathodes associated with molecular O2 trapped in the bulk[J]. Nat. Energy, 2020, 5(10): 777-785. |
| 9 | SATHIYA M, ABAKUMOV A M, FOIX D, et al. Origin of voltage decay in high-capacity layered oxide electrodes[J]. Nat. Mater., 2015, 14(2): 230-238. |
| 10 | BOIVIN E, GUERRINI N, HOUSE R A, et al. The role of Ni and Co in suppressing O-loss in Li-rich layered cathodes[J]. Adv. Funct. Mater., 2021, 31(2): 2003660. |
| 11 | XU M, FEI L F, LU W, et al. Engineering hetero-epitaxial nanostructures with aligned Li-ion channels in Li-rich layered oxides for high-performance cathode application[J]. Nano Energy, 2017, 35: 271-280. |
| 12 | FENG X, GAO Y R, BEN L B, et al. Enhanced electrochemical performance of Ti-doped Li1.2Mn0.54Co0.13Ni0.13O2 for lithium-ion batteries[J]. J. Power Sources, 2016, 317: 74-80. |
| 13 | ZHU Y, CHOI S H, FAN X, et al. Recent progress on spray pyrolysis for high performance electrode materials in lithium and sodium rechargeable batteries[J]. Adv. Energy Mater., 2017, 7(7): 1601578. |
| 14 | LEE M H, KANG Y J, MYUNG S T, et al. Synthetic optimization of Li[Ni1/3Co1/3Mn1/3]O2viaco-precipitation[J]. Electrochim. Acta, 2004, 50(4): 939-948. |
| 15 | BARAI P, FENG Z G, KONDO H, et al. Multiscale computational model for particle size evolution during coprecipitation of Li-ion battery cathode precursors[J]. J. Phys. Chem. B, 2019, 123(15): 3291-3303. |
| 16 | BOMMEL A VAN, DAHN J R. Analysis of the growth mechanism of coprecipitated spherical and dense nickel, manganese, and cobalt-containing hydroxides in the presence of aqueous ammonia[J]. Chem. Mater., 2009, 21(8): 1500-1503. |
| 17 | PARK S H, SHIN H S, MYUNG S T, et al. Synthesis of nanostructured Li[Ni1/3Co1/3Mn1/3]O2via a modified carbonate process[J]. Chem. Mater., 2005, 17(1): 6-8. |
| 18 | SHEN Y, WU Y, XUE H, et al. Insight into the coprecipitation-controlled crystallization reaction for preparing lithium-layered oxide cathodes[J]. ACS Appl. Mater. Inter., 2021, 13(1): 717-726. |
| 19 | DONG H, KOENIG G M. A review on synthesis and engineering of crystal precursors produced via coprecipitation for multicomponent lithium-ion battery cathode materials[J]. CrystEngComm, 2020, 22(9): 1514-1530. |
| 20 | WANG D P, BELHAROUAK I, KOENIG G M, et al. Growth mechanism of Ni0.3Mn0.7CO3 precursor for high capacity Li-ion battery cathodes[J]. J. Mater. Chem., 2011, 21(25): 9290. |
| 21 | 张翔,王春雷,孔继周,等. 浅析共沉淀法合成锂电池层状Li-Ni-Co-Mn-O正极材料[J]. 化工进展, 2014, 33(11): 2991-2999. |
| ZHANG Xiang, WANG Chunlei, KONG Jizhou, et al. A preliminary analysis on the synthesis of layered Li-Ni-Co-Mn-O cathode material by co-precipitation method[J]. Chemical Industry and Engineering Progress, 2014, 33(11): 2991-2999. | |
| 22 | QIN X G, LIU G Q. Grain growth simulation based on potts model with different parameters[J]. Materials Science Forum, 2005, 475/476/477/478/479: 3173-3176. |
| 23 | 苏继桃,苏玉长,赖智广,等. 共沉淀法制备镍、钴、锰复合碳酸盐的热力学分析[J]. 硅酸盐学报, 2006, 34(6): 695-698. |
| SU Jitao, SU Yuchang, LAI Zhiguang, et al. Thermodynamic analysis of preparation of multiple carbonate of Ni, Co and Mn by coprecipitation method[J]. Journal of the Chinese Ceramic Society, 2006, 34(6): 695-698. | |
| 24 | 肖新颜,叶永清. 共沉淀法合成Ni1/3Co1/3Mn1/3(OH)2的热力学分析[J]. 华南理工大学学报(自然科学版), 2010(4): 30-34, 44. |
| XIAO Xinyan, YE Yongqing. Thermodynamic analysis of synthesis of Ni1/3Co1/3Mn1/3(OH)2via coprecipitation[J]. Journal of South China University of Technology(Natural Science Edition), 2010, 38(4): 30-34, 44. | |
| 25 | ZHANG S, DENG C, FU B L, et al. Synthetic optimization of spherical Li[Ni1/3Mn1/3Co1/3]O2 prepared by a carbonate co-precipitation method[J]. Powder Technol., 2010, 198(3): 373-380. |
| 26 | ZUBAIR M, WANG E R, WANG Y Z, et al. Suppression of voltage decay through adjusting tap density of lithium-rich layered oxides for Lithium ion battery[J]. J. Mater. Sci. Technol., 2020, 58: 107-113. |
| 27 | FU F, YAO Y Z, WANG H Y, et al. Structure dependent electrochemical performance of Li-rich layered oxides in lithium-ion batteries[J]. Nano Energy, 2017, 35: 370-378. |
| 28 | WANG Y Z, WANG E R, ZHANG X, et al. High-voltage “single-crystal” cathode materials for lithium-ion batteries[J]. Energ. Fuel., 2021, 35(3): 1918-1932. |
| 29 | LIM B B, YOON S J, PARK K J, et al. Advanced concentration gradient cathode material with two-slope for high-energy and safe lithium batteries[J]. Adv. Funct. Mater., 2015, 25(29): 4673-4680. |
| 30 | YOON C S, PARK K J, KIM U H, et al. High-energy Ni-rich Li[NixCoyMn1-x-y]O2 cathodes via compositional partitioning for next-generation electric vehicles[J]. Chem. Mater., 2017, 29(24): 10436-10445. |
| 31 | SUN Y K, KIM D H, YOON C S, et al. A novel cathode material with a concentration-gradient for high-energy and safe lithium-ion batteries[J]. Adv. Funct. Mater., 2010, 20(3): 485-491. |
| 32 | WEI G Z, LU X, KE F S, et al. Crystal habit-tuned nanoplate material of Li[Li1/3-2x/3NixMn2/3-x/3]O2 for high-rate performance lithium-ion batteries[J]. Adv. Mater., 2010, 22(39): 4364-4367. |
| 33 | SU Y F, CHEN G, CHEN L, et al. Exposing the {010} planes by oriented self-assembly with nanosheets to improve the electrochemical performances of Ni-Rich Li[Ni0.8Co0.1Mn0.1]O2 microspheres[J]. ACS Appl. Mater. Inter., 2018, 10(7): 6407-6414. |
| 34 | CHEN L, SU Y F, CHEN S, et al. Hierarchical Li1.2NiMn0.6O2 nanoplates with exposed {010} planes as high-performance cathode material for lithium-ion batteries[J]. Adv. Mater., 2014, 26(39): 6756-6760. |
| 35 | 杨宗璐,鲍慈光,阎智英. 表面活性剂对结晶过程影响的研究进展[J]. 云南化工, 1994, 3: 25-29. |
| YANG Zonglu, BAO Ciguang, YAN Zhiying. The development of studies on the influence of surfactants on crystallization[J]. Yunnan Chemical Technology, 1994, 3: 25-29. | |
| 36 | FU F, XU G, WANG Q, et al. Synthesis of single crystalline hexagonal nanobricks of LiNi1/3Co1/3Mn1/3O2 with high percentage of exposed {010} active facets as high rate performance cathode material for lithium-ion battery[J]. J. Mater. Chem. A, 2013, 1(12): 3860. |
| 37 | WANG J, YUAN G X, ZHANG M H, et al. The structure, morphology, and electrochemical properties of Li1+xNi1/6Co1/6Mn4/6O2.25+x/2 (0.1≤x≤0.7) cathode materials[J]. Electrochim. Acta, 2012, 66: 61-66. |
| 38 | LIU P F, ZHANG H, HE W, et al. Lithium deficiencies engineering in Li-rich layered oxide Li1.098Mn0.533Ni0.113Co0.138O2 for high-stability cathode[J]. J. Am. Chem. Soc., 2019, 141(27): 10876-10882. |
| 39 | LEE J, ZHANG Q H, KIM J, et al. Controlled atomic solubility in Mn-rich composite material to achieve superior electrochemical performance for Li-ion batteries[J]. Adv. Energy Mater., 2020, 10(5): 1902231. |
| 40 | WANG R, QIAN G Y, LIU T C, et al. Tuning Li-enrichment in high-Ni layered oxide cathodes to optimize electrochemical performance for Li-ion battery[J]. Nano Energy, 2019, 62: 709-717. |
| 41 | LOGAN E R, HEBECKER H, MA X, et al. A comparison of the performance of different morphologies of LiNi0.8Mn0.1Co0.1O2 using isothermal microcalorimetry, ultra-high precision coulometry, and long-term cycling[J]. J. Electrochem. Soc., 2020, 167(6): 060530. |
| 42 | WANG Y, WANG L, GUO X, et al. Thermal stability enhancement through structure modification on the microsized crystalline grain surface of lithium-rich layered oxides[J]. ACS Appl. Mater. Inter., 2020, 12(7): 8306-8315. |
| 43 | LANGDON J, MANTHIRAM A. A perspective on single-crystal layered oxide cathodes for lithium-ion batteries[J]. Energy Storage Mater., 2021, 37: 143-160. |
| 44 | LI Y, XU R, REN Y, et al. Synthesis of full concentration gradient cathode studied by high energy X-ray diffraction[J]. Nano Energy, 2016, 19: 522-531. |
| 45 | WU T, LIU X, ZHANG X, et al. Full concentration gradient-tailored Li-rich layered oxides for high-energy lithium-ion batteries[J]. Adv. Mater., 2021, 33(2): 2001358. |
| 46 | LIN R, HU E, LIU M, et al. Anomalous metal segregation in lithium-rich material provides design rules for stable cathode in lithium-ion battery[J]. Nat. Commun., 2019, 10(1): 1650. |
| 47 | GAO Y R, WANG X F, MA J, et al. Selecting substituent elements for Li-rich Mn-based cathode materials by density functional theory (DFT) calculations[J]. Chem. Mater., 2015, 27(9): 3456-3461. |
| 48 | ZUBAIR M, LI G Y, WANG B Y, et al. Electrochemical kinetics and cycle stability improvement with Nb doping for lithium-rich layered oxides[J]. ACS Appl. Energy Mater., 2019, 2(1): 503-512. |
| 49 | RYU H H, PARK N Y, YOON D R, et al. New class of Ni-rich cathode materials Li[NixCoyB1-x-y]O2 for next lithium batteries[J]. Adv. Energy Mater., 2020, 10(25): 2000495. |
| 50 | KIM U H, PARK G T, SON B K, et al. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge[J]. Nat. Energy, 2020, 5(11): 860-869. |
| 51 | KIM U H, PARK G T, CONLIN P, et al. Cation ordered Ni-rich layered cathode for ultra-long battery life[J]. Energ. Environ. Sci., 2021, 14(3): 1573-1583. |
| 52 | LIU S, LIU Z P, SHEN X, et al. Surface doping to enhance structural integrity and performance of Li-rich layered oxide[J]. Adv. Energy Mater., 2018, 8(31): 1802105. |
| 53 | QING R, SHI J, XIAO D, et al. Enhancing the kinetics of Li-rich cathode materials through the pinning effects of gradient surface Na+ doping[J]. Adv. Energy Mater., 2016, 6(6): 1501914. |
| 54 | DUAN J G, HU G R, CAO Y B, et al. Enhanced electrochemical performance and storage property of LiNi0.815Co0.15Al0.035O2via Al gradient doping[J]. J. Power Sources, 2016, 326(15):322-330. |
| 55 | KONG D F, HU J T, CHEN Z F, et al. Ti-gradient doping to stabilize layered surface structure for high performance high-Ni oxide cathode of Li-ion battery[J]. Adv. Energy Mater., 2019, 9(41): 1901756. |
| 56 | ZHENG J M, GU M, XIAO J, et al. Corrosion/fragmentation of layered composite cathode and related capacity/voltage fading during cycling process[J]. Nano Lett., 2013, 13(8): 3824-3830. |
| 57 | WANG Z Y, LIU E Z, GUO L C, et al. Cycle performance improvement of Li-rich layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 by ZrO2 coating[J]. Surf. Coat. Tech., 2013, 235: 570-576. |
| 58 | SETENI B, RAPULENYANE N, NGILA J C, et al. Coating effect of LiFePO4 and Al2O3 on Li1.2Mn0.54Ni0.13Co0.13O2 cathode surface for lithium ion batteries[J]. J. Power Sources, 2017, 353: 210-220. |
| 59 | ZHANG X F, BELHAROUAK I, LI L, et al. Structural and electrochemical study of Al2O3 and TiO2 coated Li1.2Ni0.13Mn0.54Co0.13O2 cathode material using ALD[J]. Adv. Energy Mater., 2013, 3(10): 1299-1307. |
| 60 | GUO S H, YU H J, LIU P, et al. Surface coating of lithium-manganese-rich layered oxides with delaminated MnO2 nanosheets as cathode materials for Li-ion batteries[J]. J. Mater. Chem. A, 2014, 2(12): 4422. |
| 61 | ZHENG J, GU M, XIAO J, et al. Functioning mechanism of AlF3 coating on the Li- and Mn-rich cathode materials[J]. Chem. Mater., 2014, 26(22): 6320-6327. |
| 62 | NIU B B, LI J L, LIU Y Y, et al. Re-understanding the function mechanism of surface coating: modified Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathodes with YF3 for high performance lithium-ions batteries[J]. Ceram Int., 2019, 45(9): 12484-12494. |
| 63 | GAN Q M, QIN N, WANG Z Y, et al. Revealing mechanism of Li3PO4 coating suppressed surface oxygen release for commercial Ni-rich layered cathodes[J]. ACS Appl. Energy Mater., 2020, 3(8): 7445-7455. |
| 64 | SU Y F, YUAN F Y, CHEN L, et al. Enhanced high-temperature performance of Li-rich layered oxide via surface heterophase coating[J]. J. Energy Chem., 2020, 51: 39-47. |
| 65 | 王兆翔,马君,高玉瑞,等. 稳定富锂层状氧化物正极材料的结构与性能[J]. 化学进展, 2019, 31(11): 1591-1614. |
| WANG Zhaoxiang, MA Jun, GAO Yurui, et al. Stabilizing structure and performances of lithium rich layer-structured oxide cathode materials[J]. Progress in Chemistry, 2019, 31(11): 1591-1614. | |
| 66 | WANG E R, ZHAO Y, XIAO D D, et al. Composite nanostructure construction on the grain surface of Li-rich layered oxides[J]. Adv. Mater., 2020, 32(49): 1906070. |
| 67 | CHEN S Z, XIE Y X, CHEN W, et al. Enhanced electrochemical performance of Li-rich cathode materials by organic fluorine doping and spinel Li1+xNiyMn2-yO4 coating[J]. ACS Sustain. Chem. Eng., 2019, 8(1): 121-128. |
| 68 | LI Q Y, NING D, ZHOU D, et al. The effect of oxygen vacancy and spinel phase integration on both anionic and cationic redox in Li-rich cathode materials[J]. J. Mater. Chem. A, 2020, 8(16): 7733-7745. |
| 69 | JOHNSON C S, KIM J, LEFIEF C, et al. The significance of the Li2MnO3 component in ‘composite’ xLi2MnO3·(1-x)LiMn0.5Ni0.5O2 electrodes[J]. Electrochem. Commun., 2004, 6 (10): 1085-1091. |
| 70 | QIU B, ZHANG M, WU L, et al. Gas-solid interfacial modification of oxygen activity in layered oxide cathodes for lithium-ion batteries[J]. Nat. Commun., 2016, 7: 12108. |
| 71 | YANG H P, WU H H, GE M Y, et al. Simultaneously dual modification of Ni-rich layered oxide cathode for high-energy lithium-ion batteries[J]. Adv. Funct. Mater., 2019, 29 (13): 1808825. |
| 72 | YU R Z, BANIS M N, WANG C H, et al. Tailoring bulk Li+ ion diffusion kinetics and surface lattice oxygen activity for high-performance lithium-rich manganese-based layered oxides[J]. Energy Storage Mater., 2021, 37: 509-520. |
| [1] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [2] | 王帅旗, 王从新, 王学林, 田志坚. 无溶剂快速合成ZSM-12分子筛[J]. 化工进展, 2023, 42(7): 3561-3571. |
| [3] | 王昊, 霍进达, 曲国瑞, 杨家琪, 周世伟, 李博, 魏永刚. 退役锂电池正极材料资源化回收技术研究进展[J]. 化工进展, 2023, 42(5): 2702-2716. |
| [4] | 于捷, 张文龙. 锂离子电池隔膜的发展现状与进展[J]. 化工进展, 2023, 42(4): 1760-1768. |
| [5] | 马文杰, 姚卫棠. 共价有机框架(COFs)在锂离子电池中的应用[J]. 化工进展, 2023, 42(10): 5339-5352. |
| [6] | 王玥, 郑晓洪, 陶天一, 刘秀庆, 李丽, 孙峙. 废锂离子电池正极材料中锂元素选择性回收的研究进展[J]. 化工进展, 2022, 41(8): 4530-4543. |
| [7] | 程明强, 汝娟坚, 华一新, 王丁, 耿笑, 张文文, 黄皓铭, 王道祥. 低共熔溶剂在废旧锂离子电池正极材料回收中的研究进展[J]. 化工进展, 2022, 41(6): 3293-3305. |
| [8] | 马续, 邹明贵, 崔巍巍, 付安然, 廖小龙, 巩桂芬. 一种水性负极黏结剂的合成及性能[J]. 化工进展, 2022, 41(6): 3138-3145. |
| [9] | 龚鑫, 刘小冬, 温福山, 师楠, 刘东. 中间相炭微球乳化-聚合法制备及电化学性能[J]. 化工进展, 2022, 41(5): 2379-2388. |
| [10] | 段曼华, 程丹, 肖伟, 杨占旭. 聚丙烯腈/聚酯无纺布微孔复合锂电隔膜的制备及性能[J]. 化工进展, 2022, 41(5): 2615-2622. |
| [11] | 温嘉玮, 杨晨林, 成建凤, 黄国勇, 郭学益. 尖晶石型三元高电压正极材料的合成及其性能[J]. 化工进展, 2022, 41(11): 5968-5976. |
| [12] | 胡华坤, 薛文东, 蒋朋, 李勇. 锂离子电池安全添加剂的研究进展[J]. 化工进展, 2022, 41(10): 5441-5455. |
| [13] | 邱治文, 吴爱民, 王杰, 黄昊. Si基锂离子电池负极材料研究进展[J]. 化工进展, 2021, 40(S1): 253-269. |
| [14] | 王志鸿, 朱华威, 余海峰, 江浩, 李春忠. 共沉淀法制备高镍氧化物正极材料前体研究进展[J]. 化工进展, 2021, 40(9): 5097-5106. |
| [15] | 宋洁尘, 夏青, 徐宇兴, 谭强强. 全固态锂离子电池的研究进展与挑战[J]. 化工进展, 2021, 40(9): 5045-5060. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||