化工进展 ›› 2025, Vol. 44 ›› Issue (10): 5771-5788.DOI: 10.16085/j.issn.1000-6613.2024-1299
• 材料科学与技术 • 上一篇
金属有机骨架材料吸附SF6的研究进展及展望
- 昆明理工大学化学工程学院,云南 昆明 650000
-
收稿日期:2024-08-07修回日期:2024-10-07出版日期:2025-10-25发布日期:2025-11-10 -
通讯作者:徐志勇 -
作者简介:杨宇(2000—),男,硕士研究生,研究方向为酸性和含氟电子特种气体的捕集。E-mail:919697671@qq.com。 -
基金资助:国家自然科学基金(22278198);国家自然科学基金(21068016)
Research progress and prospect of SF6 adsorption by metal-organic frame materials
YANG Yu( ), ZHAO Wenbo, XU Zhiyong(
), ZHAO Wenbo, XU Zhiyong( )
)
- College of Chemical Engineering, Kunming University of Science and Technology, Kunming 650000, Yunnan, China
-
Received:2024-08-07Revised:2024-10-07Online:2025-10-25Published:2025-11-10 -
Contact:XU Zhiyong
摘要:
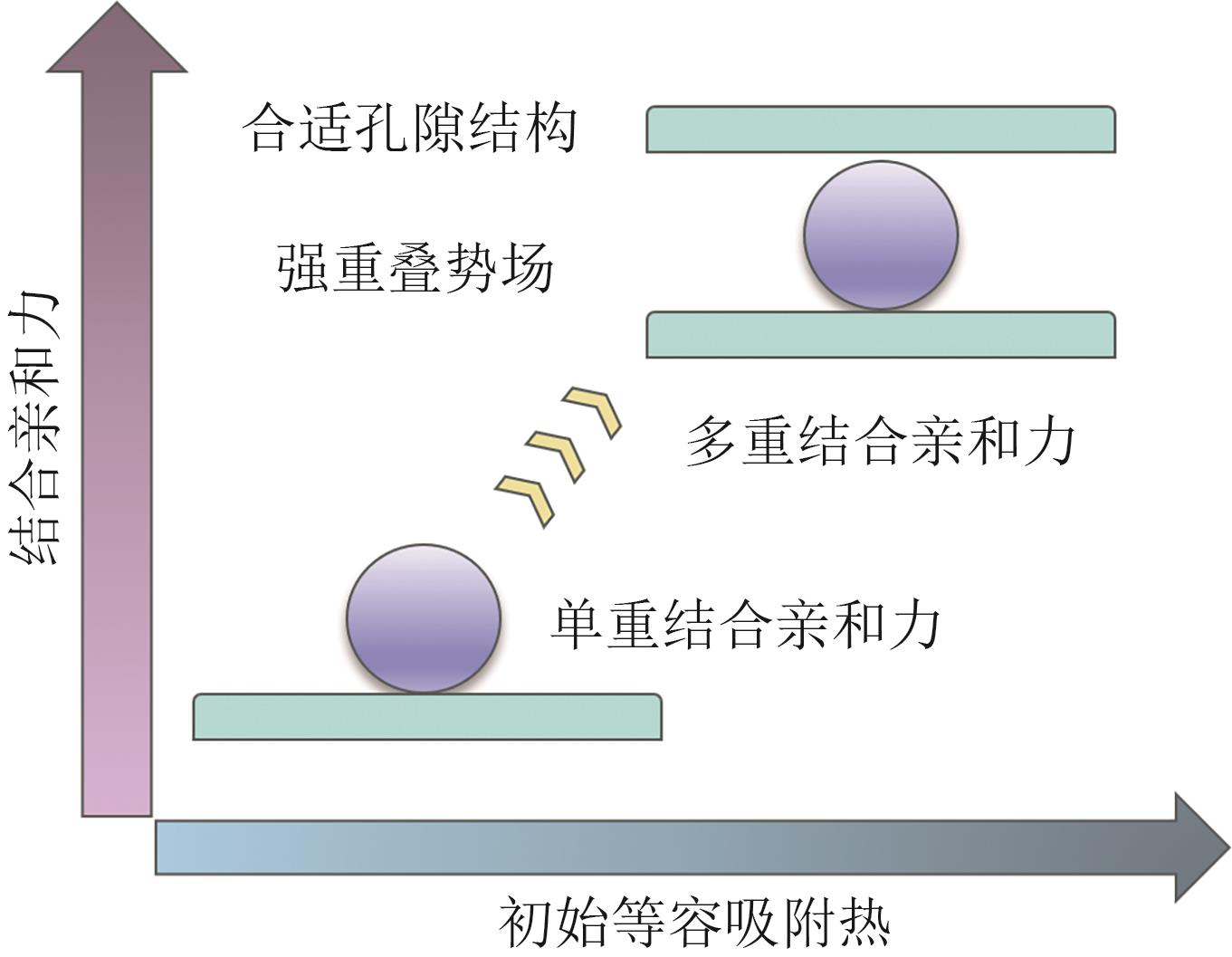
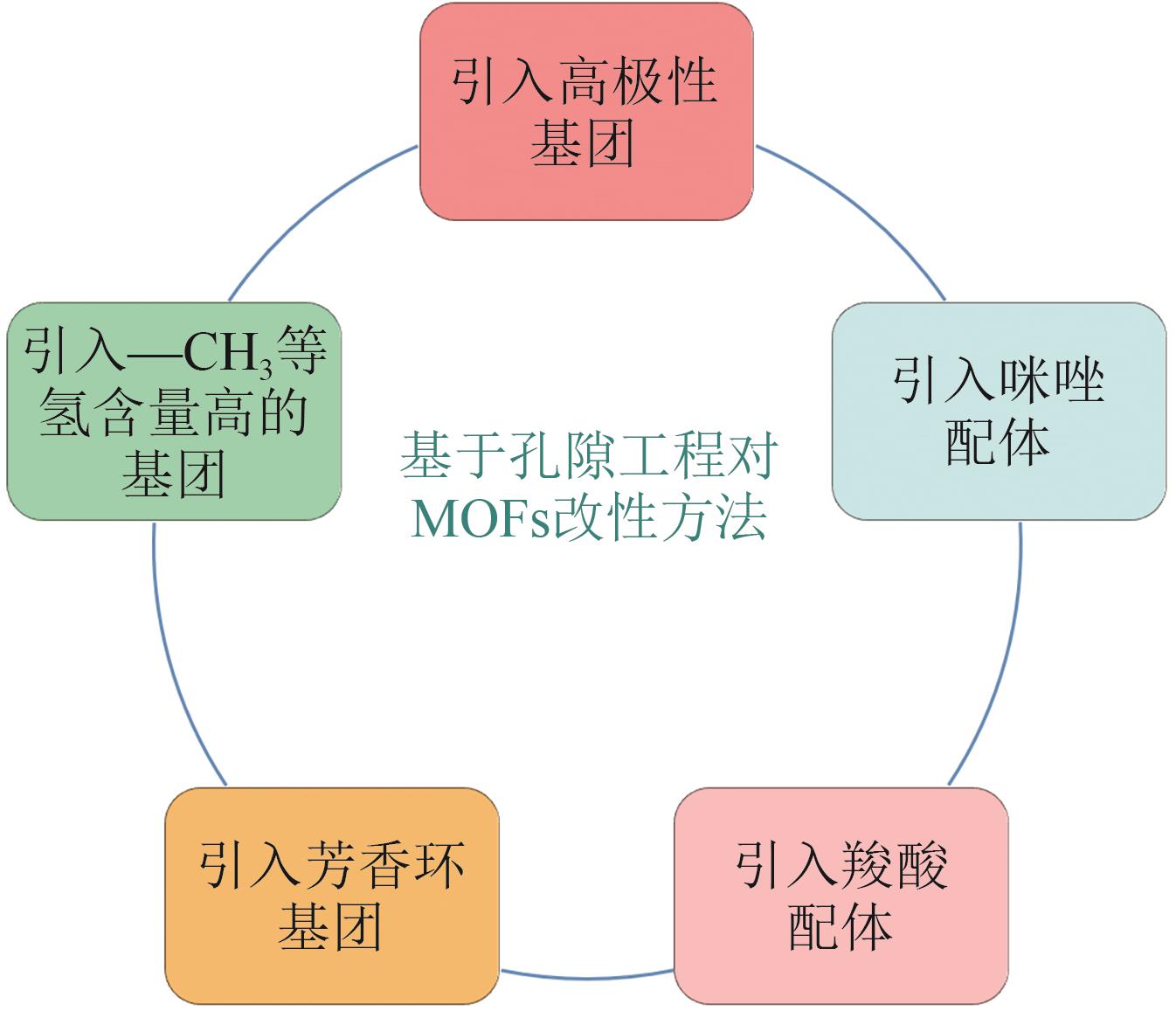
SF6在超大规模集成电路和超高压电力设备等工业技术领域具有重要的应用。但SF6也是最强效的温室气体之一,具有极长的大气寿命和超高的全球变暖潜能值,过度排放对全球变暖和环境恶化存在极高的威胁。因此,在“节能减排”和“双碳”政策的国家战略背景下,从低浓度的SF6/N2混合气体中高效率和低能耗地回收SF6对半导体制造业的发展和环境保护具有重要的意义。在众多的SF6/N2混合气体分离技术中,基于多孔材料的吸附分离是一种节能环保的优选方案。本文介绍了当前对SF6/N2混合气体的分离方法,重点阐述了采用沸石、金属有机骨架(MOFs)和多孔有机聚合物(POCs)以及共价有机框架(COFs)等多孔吸附材料对SF6的吸附分离。在这些吸附材料中,MOFs具有较高的SF6吸附能力、吸附选择性和良好的可再生性能,但由于MOFs对SF6的吸附量和选择性之间权衡性较差,高选择性和高吸附量不能同时存在,所以权衡性差是MOFs吸附SF6气体的一个挑战。因此,本文着重综述了MOFs材料对SF6/N2分离的研究进展及如何解决权衡性问题。最后,对SF6吸附分离领域发展面临的问题进行归纳总结,并对该领域未来的发展方向进行了展望。
中图分类号:
引用本文
杨宇, 赵文波, 徐志勇. 金属有机骨架材料吸附SF6的研究进展及展望[J]. 化工进展, 2025, 44(10): 5771-5788.
YANG Yu, ZHAO Wenbo, XU Zhiyong. Research progress and prospect of SF6 adsorption by metal-organic frame materials[J]. Chemical Industry and Engineering Progress, 2025, 44(10): 5771-5788.
| 吸附气体 | 全球变暖潜能值 | 动力学直径/Å | 标准熔点/℃ | 标准沸点/℃ | 极化率/cm3 | 四极矩/esu·cm2 |
|---|---|---|---|---|---|---|
| SF6 | 22800 | 5.13 | -64 | -50 | 6.54×10-24 | 0 |
| N2 | 0 | 3.64 | -198 | -210 | 1.74×10-24 | 1.52×10-26 |
| CO2 | 1 | 3.30 | -78 | — | 2.91×10-24 | 4.30×10-26 |
表1 SF6和N2、CO2的气体特性[17-19]
| 吸附气体 | 全球变暖潜能值 | 动力学直径/Å | 标准熔点/℃ | 标准沸点/℃ | 极化率/cm3 | 四极矩/esu·cm2 |
|---|---|---|---|---|---|---|
| SF6 | 22800 | 5.13 | -64 | -50 | 6.54×10-24 | 0 |
| N2 | 0 | 3.64 | -198 | -210 | 1.74×10-24 | 1.52×10-26 |
| CO2 | 1 | 3.30 | -78 | — | 2.91×10-24 | 4.30×10-26 |
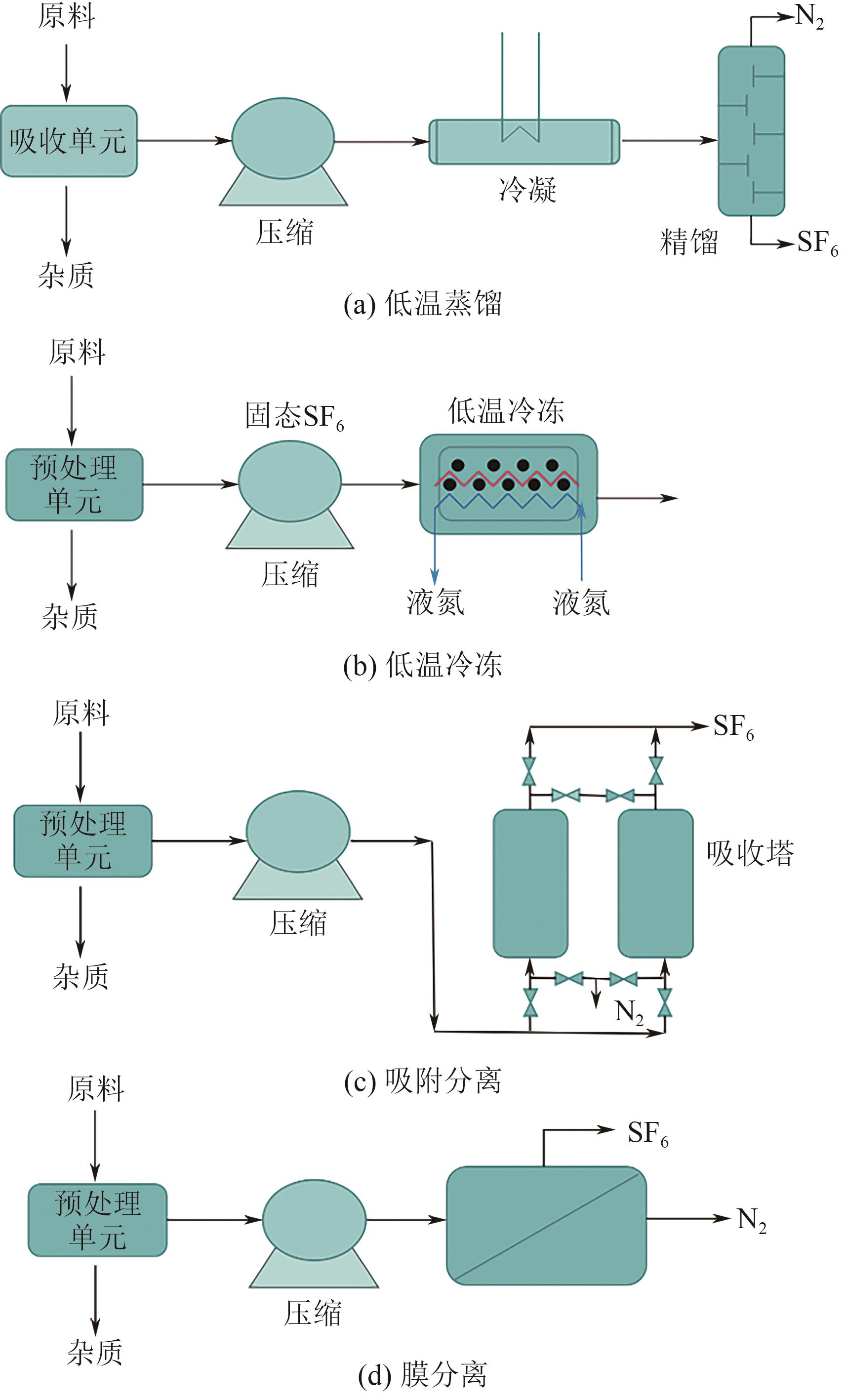
| 分离方法 | 工作原理 | 典型工况 | SF6回收率/% | 所用材料/溶剂 | 优点 | 缺点 | 已工业化的技术 | 文献 |
|---|---|---|---|---|---|---|---|---|
| 低温蒸馏技术 | SF6和N2之间的沸点差异 | 0.4MPa,-30℃ | 99.0 | 冷媒 | 可以达到较高的SF6纯度(99.999%,体积分数);通过热集成可以提高能源效率 | 投资和运营成本高,设备庞大,能耗高 | 由KEPCO开发的模型 | [ |
| 低温冷冻技术 | SF6和N2之间的熔点差异 | -196℃ | 99.0 | 液氮 | SF6冻结后融化成液相,其他杂质可以通过过滤器去除 | 要将SF6从气体转变为固体,需要采用非常特殊的低温 | 由ABB开发的模型 由KEPCO开发的模型 | [ |
| 膜分离技术 | 利用混合气体在通过膜时传递速率的不同 | 101325Pa或更高 | 90.0 | 聚合物膜、无机膜、混合基质膜 | 由于不涉及相变,具有较高的膜比表面积 | 进料中的杂质会降低分离效率 | 膜分离装置(Solvay Fluor and Derivate GmbH) | [ |
| 离子液体吸收技术 | 独特的阴阳离子与气体分子之间的相互作用 | — | 90.0 | 离子溶剂 | 高稳定性、溶解性能可调节、不易挥发、可再生 | 成本高、黏度高、制备工艺复杂 | 离子液体烟气脱硫 | [ |
| 吸附分离技术 | 通过吸附剂与气体混合物各组分之间的相互作用力 | 101325Pa或更高 | 80.0~90.0 | 沸石分子筛、金属有机骨架、活性炭 | 使用高比表面积的吸附剂可以吸附大量的SF6 | SF6吸附-解吸循环可能需要较高的吸附压力 | 沸石13X(Solvay Fluor and Derivate GmbH) | [ |
表2 SF6分离和回收的现有技术的比较
| 分离方法 | 工作原理 | 典型工况 | SF6回收率/% | 所用材料/溶剂 | 优点 | 缺点 | 已工业化的技术 | 文献 |
|---|---|---|---|---|---|---|---|---|
| 低温蒸馏技术 | SF6和N2之间的沸点差异 | 0.4MPa,-30℃ | 99.0 | 冷媒 | 可以达到较高的SF6纯度(99.999%,体积分数);通过热集成可以提高能源效率 | 投资和运营成本高,设备庞大,能耗高 | 由KEPCO开发的模型 | [ |
| 低温冷冻技术 | SF6和N2之间的熔点差异 | -196℃ | 99.0 | 液氮 | SF6冻结后融化成液相,其他杂质可以通过过滤器去除 | 要将SF6从气体转变为固体,需要采用非常特殊的低温 | 由ABB开发的模型 由KEPCO开发的模型 | [ |
| 膜分离技术 | 利用混合气体在通过膜时传递速率的不同 | 101325Pa或更高 | 90.0 | 聚合物膜、无机膜、混合基质膜 | 由于不涉及相变,具有较高的膜比表面积 | 进料中的杂质会降低分离效率 | 膜分离装置(Solvay Fluor and Derivate GmbH) | [ |
| 离子液体吸收技术 | 独特的阴阳离子与气体分子之间的相互作用 | — | 90.0 | 离子溶剂 | 高稳定性、溶解性能可调节、不易挥发、可再生 | 成本高、黏度高、制备工艺复杂 | 离子液体烟气脱硫 | [ |
| 吸附分离技术 | 通过吸附剂与气体混合物各组分之间的相互作用力 | 101325Pa或更高 | 80.0~90.0 | 沸石分子筛、金属有机骨架、活性炭 | 使用高比表面积的吸附剂可以吸附大量的SF6 | SF6吸附-解吸循环可能需要较高的吸附压力 | 沸石13X(Solvay Fluor and Derivate GmbH) | [ |
| 材料 | BET比表面积/m2·g-1 | IAST选择性SF6/N2(10∶90) | SF6吸附量(10kPa)/mmol·g-1 | SF6吸附量(100kPa)/mmol·g-1 | Qst/kJ·mol-1 | 参考文献 |
|---|---|---|---|---|---|---|
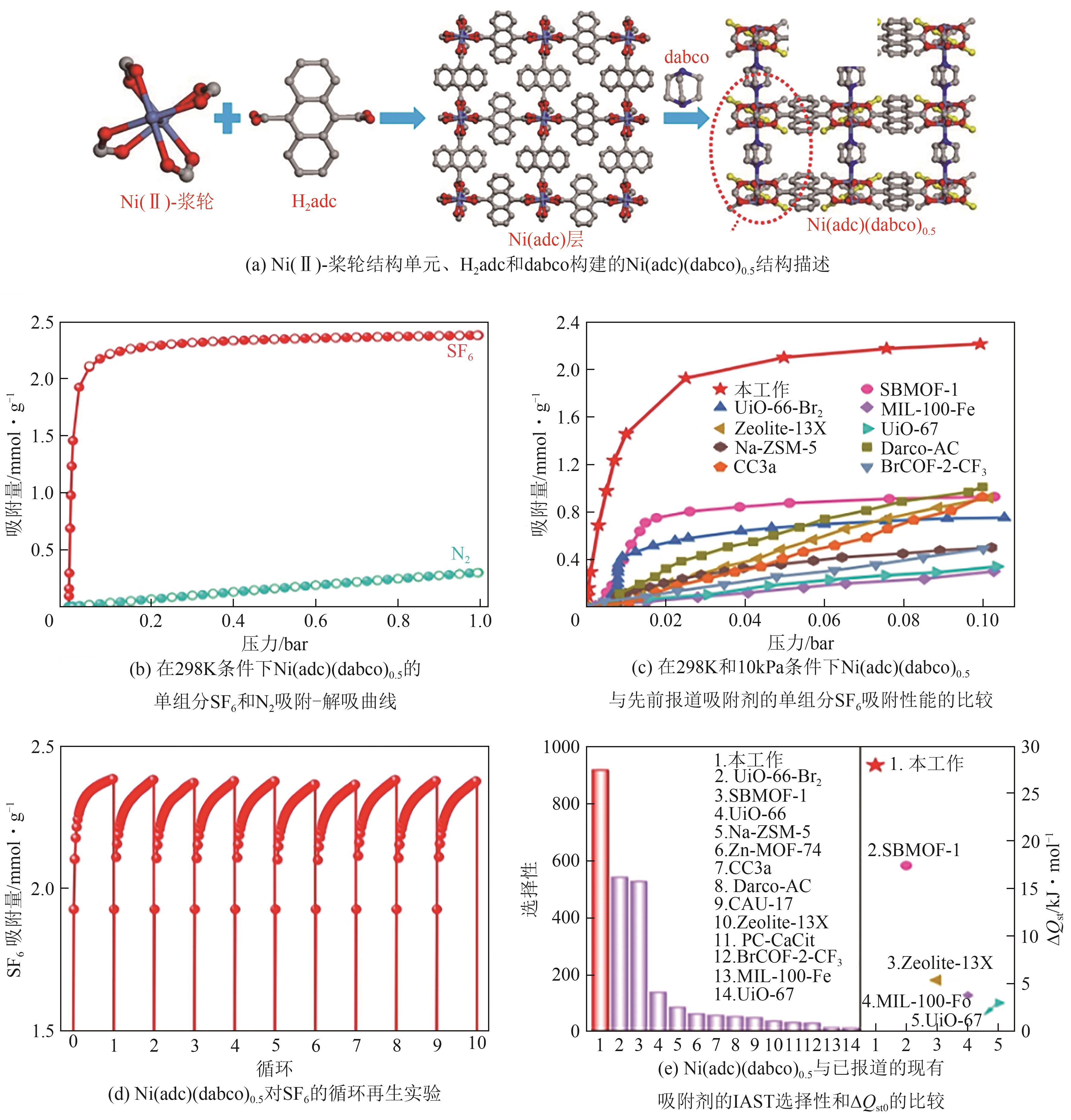
| Ni(adc)(dabco)0.5 | 743.9 | 948.2 | 2.23 | 2.38 | 47.6 | [ |
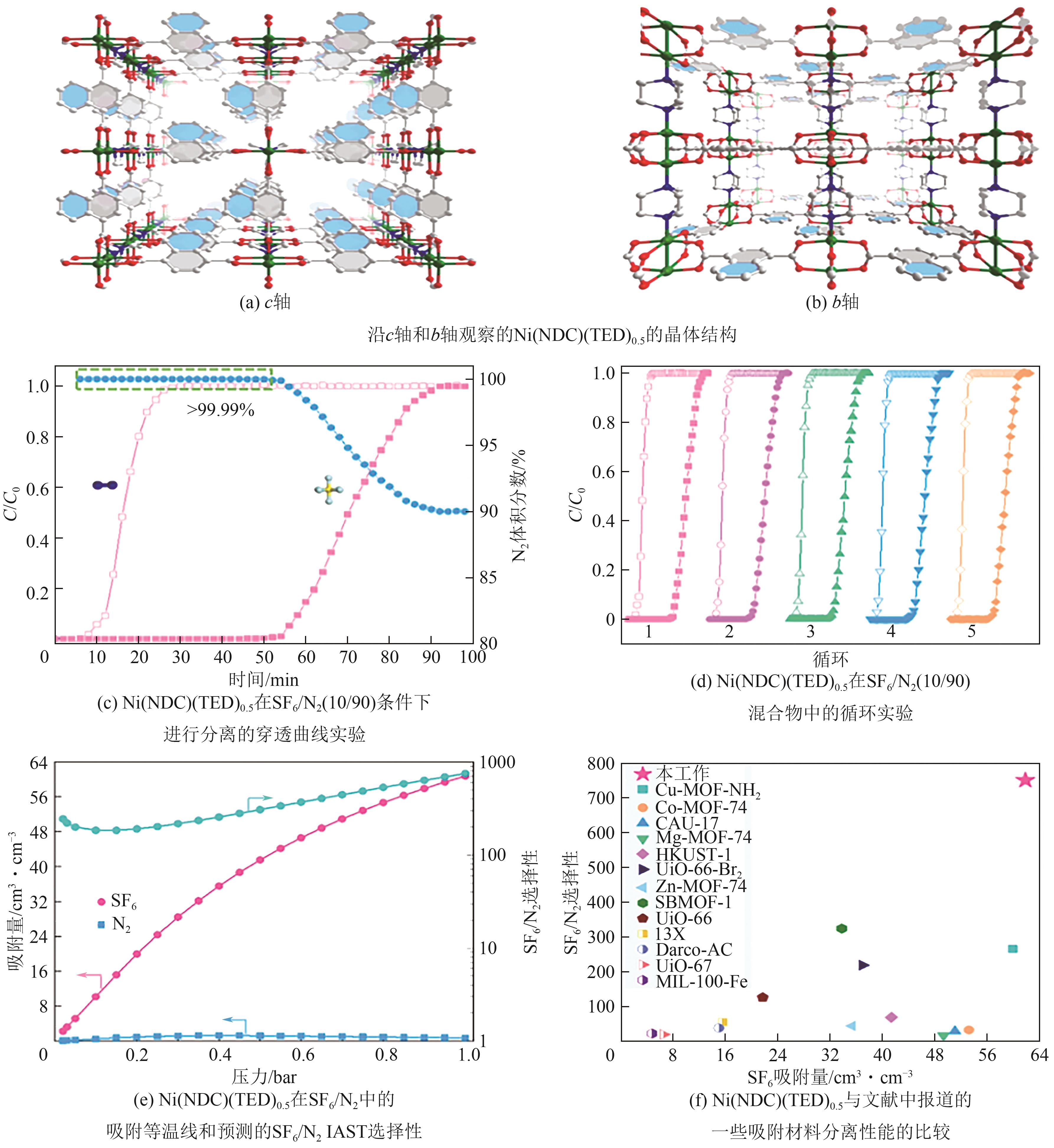
| Ni(NDC)(TED)0.5 | 1306.6 | 748 | 2.76 | 4.37 | 32.15 | [ |
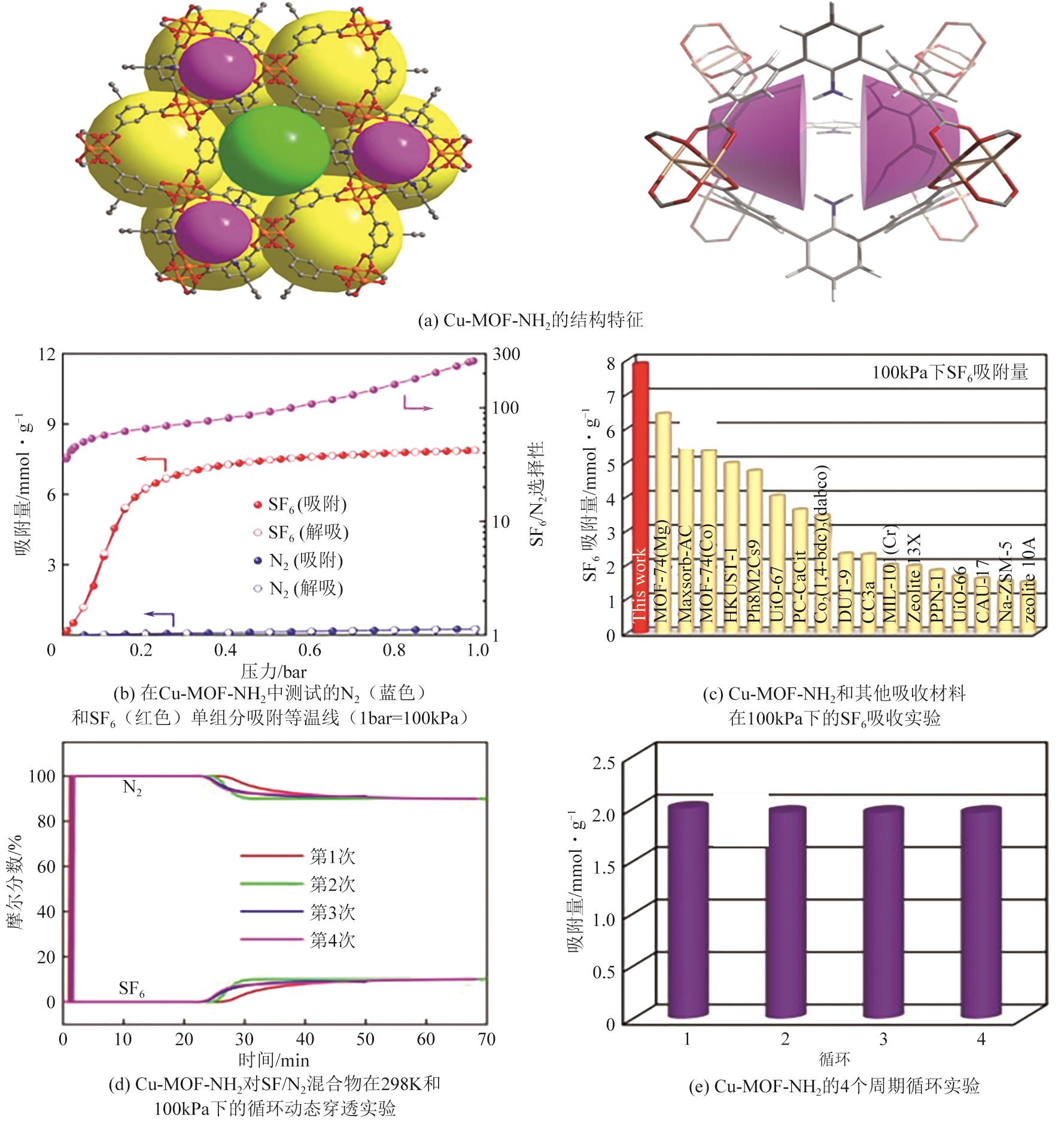
| Cu-MOF-NH2 | 2145 | 266 | 3.39 | 7.88 | 55.2 | [ |
| V-TCPB | 1107 | 360.7 | 2.29 | 3.07 | 30.08 | [ |
| GA-TCPB | 1144 | 418.5 | 2.26 | 2.95 | 30.44 | [ |
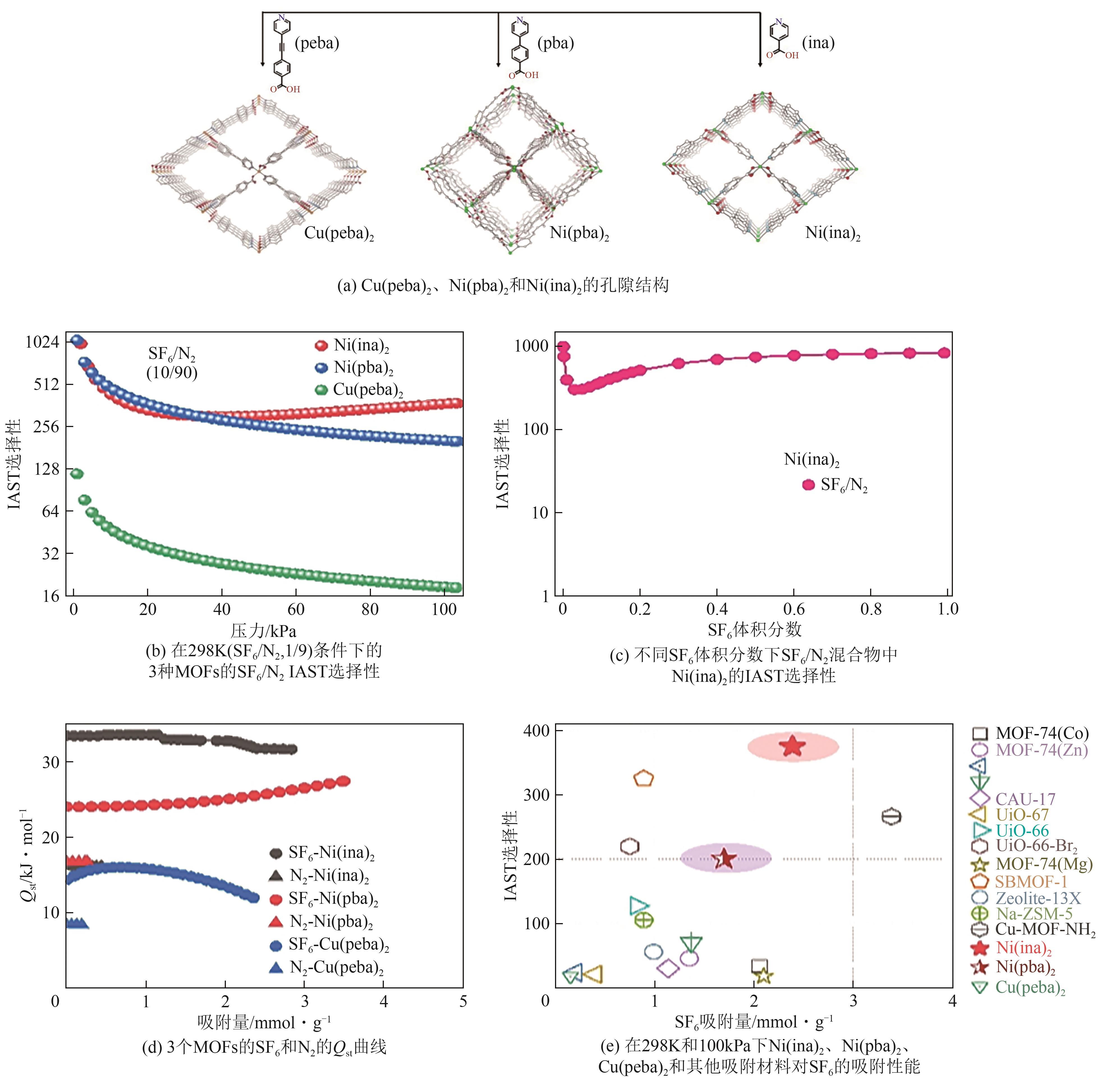
| Ni(ina)2 | 470 | 375.1 | 2.39 | 2.84 | 33.4 | [ |
| SBMOF-1 | 150 | 325.0 | 0.93 | 1.02 | 32.5 | [ |
| Ni(3-mpba)2 | 835.1 | 221 | — | 2.83 | 38.4 | [ |
| Ni(pba)2 | 807.2 | 156.5 | 1.69 | 3.47 | 38.2 | [ |
| CAU-10-H | 684.4 | 122.6 | 0.68 | 1.00 | 24.9 | [ |
| CAU-10-Py | 935.9 | 203.6 | 1.13 | 1.76 | 32.6 | [ |
| HBU-21 | 381.44 | 184.05 | 1.34 | — | 24.52 | [ |
| MIL-101 | — | 70 | — | 2.23 | — | [ |
| DUT-9 | — | 70 | — | 2.32 | — | [ |
| Mg-MOF-74 | 1631 | 19 | 1.34 | 6.42 | — | [ |
| Co-MOF-74 | 1219 | 37 | 1.32 | 5.34 | — | [ |
| Zn-MOF-74 | 992 | 45 | 1.02 | 3.76 | 42.3 | [ |
| HKUST-1a | 1090 | 37 | 0.86 | 3.46 | 57.4 | [ |
| HKUST-1b | 1135 | 49 | 1.32 | 4.12 | 34.5 | [ |
| HKUST-1c | 1328 | 70 | 1.50 | 4.98 | 30.2 | [ |
| CAU-17 | 530 | 31 | — | 1.61 | 27.6 | [ |
| SU-100 | 385 | 36 | — | 2.07 | 20.4 | [ |
| ZIF-7-8s | 1624 | 40 | — | 2.08 | — | [ |
| UiO-66-Br2 | 616 | 220 | 0.8 | 1.63 | 45 | [ |
| Zn(TMBDC)(DABCO)0.5 | 975.9 | 239 | 2.48 | 4.61 | 46 | [ |
| UiO-66-Br2@PS/DVB | 59 | — | — | 0.18 | — | [ |
| YTU-29-NH2 | 1269.5 | 36.7 | — | 4.26 | 28.9 | [ |
| Ni(3-min)(bdc)0.5 | 628 | 91 | — | 2.25 | 31.3 | [ |
| GNU-3a | 930.08 | 317.6 | — | 2.63 | 22.9 | [ |
| SNNU-204 | 2170 | 49 | — | 6 | 21.0 | [ |
| YTU-30 | 714.0 | 68 | — | 1.65 | 27 | [ |
| Sc-cage-MOF | 580 | 22.7 | — | 1.59 | 30.7 | [ |
| UU-200 | 115 | 44.81 | — | 1.19 | — | [ |
| BUT-53 | 866 | 2485 | — | 3.62 | 23.8 | [ |
表3 MOFs的吸附性能参数
| 材料 | BET比表面积/m2·g-1 | IAST选择性SF6/N2(10∶90) | SF6吸附量(10kPa)/mmol·g-1 | SF6吸附量(100kPa)/mmol·g-1 | Qst/kJ·mol-1 | 参考文献 |
|---|---|---|---|---|---|---|
| Ni(adc)(dabco)0.5 | 743.9 | 948.2 | 2.23 | 2.38 | 47.6 | [ |
| Ni(NDC)(TED)0.5 | 1306.6 | 748 | 2.76 | 4.37 | 32.15 | [ |
| Cu-MOF-NH2 | 2145 | 266 | 3.39 | 7.88 | 55.2 | [ |
| V-TCPB | 1107 | 360.7 | 2.29 | 3.07 | 30.08 | [ |
| GA-TCPB | 1144 | 418.5 | 2.26 | 2.95 | 30.44 | [ |
| Ni(ina)2 | 470 | 375.1 | 2.39 | 2.84 | 33.4 | [ |
| SBMOF-1 | 150 | 325.0 | 0.93 | 1.02 | 32.5 | [ |
| Ni(3-mpba)2 | 835.1 | 221 | — | 2.83 | 38.4 | [ |
| Ni(pba)2 | 807.2 | 156.5 | 1.69 | 3.47 | 38.2 | [ |
| CAU-10-H | 684.4 | 122.6 | 0.68 | 1.00 | 24.9 | [ |
| CAU-10-Py | 935.9 | 203.6 | 1.13 | 1.76 | 32.6 | [ |
| HBU-21 | 381.44 | 184.05 | 1.34 | — | 24.52 | [ |
| MIL-101 | — | 70 | — | 2.23 | — | [ |
| DUT-9 | — | 70 | — | 2.32 | — | [ |
| Mg-MOF-74 | 1631 | 19 | 1.34 | 6.42 | — | [ |
| Co-MOF-74 | 1219 | 37 | 1.32 | 5.34 | — | [ |
| Zn-MOF-74 | 992 | 45 | 1.02 | 3.76 | 42.3 | [ |
| HKUST-1a | 1090 | 37 | 0.86 | 3.46 | 57.4 | [ |
| HKUST-1b | 1135 | 49 | 1.32 | 4.12 | 34.5 | [ |
| HKUST-1c | 1328 | 70 | 1.50 | 4.98 | 30.2 | [ |
| CAU-17 | 530 | 31 | — | 1.61 | 27.6 | [ |
| SU-100 | 385 | 36 | — | 2.07 | 20.4 | [ |
| ZIF-7-8s | 1624 | 40 | — | 2.08 | — | [ |
| UiO-66-Br2 | 616 | 220 | 0.8 | 1.63 | 45 | [ |
| Zn(TMBDC)(DABCO)0.5 | 975.9 | 239 | 2.48 | 4.61 | 46 | [ |
| UiO-66-Br2@PS/DVB | 59 | — | — | 0.18 | — | [ |
| YTU-29-NH2 | 1269.5 | 36.7 | — | 4.26 | 28.9 | [ |
| Ni(3-min)(bdc)0.5 | 628 | 91 | — | 2.25 | 31.3 | [ |
| GNU-3a | 930.08 | 317.6 | — | 2.63 | 22.9 | [ |
| SNNU-204 | 2170 | 49 | — | 6 | 21.0 | [ |
| YTU-30 | 714.0 | 68 | — | 1.65 | 27 | [ |
| Sc-cage-MOF | 580 | 22.7 | — | 1.59 | 30.7 | [ |
| UU-200 | 115 | 44.81 | — | 1.19 | — | [ |
| BUT-53 | 866 | 2485 | — | 3.62 | 23.8 | [ |
| [1] | LEE How Ming, CHANG Moo Been, WU KUAN Yu. Abatement of sulfur hexafluoride emissions from the semiconductor manufacturing process by atmospheric-pressure plasmas[J]. Journal of the Air & Waste Management Association, 2004, 54(8): 960-970. |
| [2] | GUPTA Nishesh Kumar, VIKRANT Kumar, KIM Kwang Soo, et al. Regeneration strategies for metal-organic frameworks post acidic gas capture[J]. Coordination Chemistry Reviews, 2022, 467: 214629. |
| [3] | SEEGER Martin. Perspectives on research on high voltage gas circuit breakers[J]. Plasma Chemistry and Plasma Processing, 2015, 35(3): 527-541. |
| [4] | MAISS Manfred, BRENNINKMEIJER Carl A M. Atmospheric SF6: Trends, sources, and prospects[J]. Environmental Science and Technology, 1998, 32(20): 3077-3086. |
| [5] | FANG Xuekun, HU Xia, Greet JANSSENS-MAENHOUT, et al. Sulfur hexafluoride (SF6) emission estimates for China: An inventory for 1990—2010 and a projection to 2020[J]. Environmental Science & Technology, 2013, 47(8): 3848-3855. |
| [6] | HASELL Tom, MIKLITZ Marcin, STEPHENSON Andrew, et al. Porous organic cages for sulfur hexafluoride separation[J]. Journal of the American Chemical Society, 2016, 138(5): 1653-1659. |
| [7] | SKARMOUTSOS Ioannis, KOUKARAS Emmanuel N, GALIOTIS Costas, et al. Porous carbon nanotube networks and pillared graphene materials exhibiting high SF6 adsorption uptake and separation selectivity of SF6/N2 fluid mixtures: A comparative molecular simulation study[J]. Microporous and Mesoporous Materials, 2020, 307: 110464. |
| [8] | WANG Shaomin, MU Xuantong, LIU Haoran, et al. Pore-structure control in metal-organic frameworks (MOFs) for capture of the greenhouse gas SF6 with record separation[J]. Angewandte Chemie International Edition, 2022, 61(33): e202207066. |
| [9] | BEROUAL Abderrahmane, HADDAD Abderrahmane Manu. Recent advances in the quest for a new insulation gas with a low impact on the environment to replace sulfur hexafluoride (SF6) gas in high-voltage power network applications[J]. Energies, 2017, 10(8): 1216. |
| [10] | INAMI Kiyoshi, MAEDA Yasuhiro, HABUCHI Yoshitaka, et al. Problems of the application of N2/SF6 mixtures to gas-insulated bus[J]. Electrical Engineering in Japan, 2001, 137(4): 25-31. |
| [11] | FU Hongru, JIANG Yuying, LUO Jiahua, et al. A robust heterometallic Cd(Ⅱ)/Ba(Ⅱ)-organic framework with exposed amino group and active sites exhibiting excellent CO2/CH4 and C2H2/CH4 separation[J]. Chinese Journal of Structural Chemistry, 2022, 41(3): 2203287-2203292. |
| [12] | Michelle ÅHLÉN, AMOMBO NOA Francoise M, Lars ÖHRSTRÖM, et al. Pore size effect of 1,3,6,8-tetrakis(4-carboxyphenyl)pyrene-based metal-organic frameworks for enhanced SF6 adsorption with high selectivity[J]. Microporous and Mesoporous Materials, 2022, 343: 112161. |
| [13] | FURMANIAK Sylwester, TERZYK Artur P, GAUDEN Piotr A, et al. Simulation of SF6 adsorption on the bundles of single walled carbon nanotubes[J]. Microporous and Mesoporous Materials, 2012, 154: 51-55. |
| [14] | YAN Le, ZHENG Hui-Ting, SONG Liang, et al. Methyl-functionalized flexible ultra-microporous MOF for efficient SF6/N2 mixture separation[J]. Chemical Engineering Journal, 2023, 472: 145145. |
| [15] | YANG Shanqing, HU Tongliang, CHEN Banglin. Microporous metal-organic framework materials for efficient capture and separation of greenhouse gases[J]. Science China Chemistry, 2023, 66(8): 2181-2203. |
| [16] | ZHAO Yanlong, ZHANG Xin, LI Muzi, et al. Non-CO2 greenhouse gas separation using advanced porous materials[J]. Chemical Society Reviews, 2024, 53(4): 2056-2098. |
| [17] | LI Jianrong, KUPPLER Ryan J, ZHOU Hongcai. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. |
| [18] | SIRCAR Shivaji. Basic research needs for design of adsorptive gas separation processes[J]. Industrial & Engineering Chemistry Research, 2006, 45(16): 5435-5448. |
| [19] | BAKER Richard W. Membrane technology and applications[M]. Membrane Technology & Applications, 2004, 39(7): 85. |
| [20] | SHI Qi, WANG Jing, SHANG Hua, et al. Effective CH4 enrichment from N2 by SIM-1 via a strong adsorption potential SOD cage[J]. Separation and Purification Technology, 2020, 230: 115850. |
| [21] | MA Shengqian, SUN Daofeng, WANG Xisen, et al. A mesh-adjustable molecular sieve for general use in gas separation[J]. Angewandte Chemie International Edition, 2007, 46(14): 2458-2462. |
| [22] | LI Tong, JIA Xiaoxia, CHEN Hui, et al. Tuning the pore environment of MOFs toward efficient CH4/N2 separation under humid conditions[J]. ACS Applied Materials & Interfaces, 2022, 14(13): 15830-15839. |
| [23] | ISMAIL N H, SALLEH W N W, ISMAIL A F, et al. Hydrophilic polymer-based membrane for oily wastewater treatment: A review[J]. Separation and Purification Technology, 2020, 233: 116007. |
| [24] | BAKER Richard W, LOKHANDWALA Kaaeid. Natural gas processing with membranes: An overview[J]. Industrial & Engineering Chemistry Research, 2008, 47(7): 2109-2121. |
| [25] | REINE Travis A, Bruce ELDRIDGE R. Absorption equilibrium and kinetics for ethylene-ethane separation with a novel solvent[J]. Industrial & Engineering Chemistry Research, 2005, 44(19): 7505-7510. |
| [26] | MA Chen, WANG Xingjie, WANG Xun, et al. Novel glucose-based adsorbents (Glc-As) with preferential adsorption of ethane over ethylene and high capacity[J]. Chemical Engineering Science, 2017, 172: 612-621. |
| [27] | HAMEDI Homa, KARIMI Iftekhar A, GUNDERSEN Truls. Optimal cryogenic processes for nitrogen rejection from natural gas[J]. Computers & Chemical Engineering, 2018, 112: 101-111. |
| [28] | 李晓波, 胡保安, 顾平. 动态膜分离技术研究进展[J]. 膜科学与技术, 2007, 27(4): 91-95. |
| LI Xiaobo, HU Baoan, GU Ping. Research progress of dynamic membrane separation technology[J]. Science and technology of Membrane, 2007, 27(4): 91-95. | |
| [29] | LI Yuxiao, WANG Ting, LIU Dahuanet al. Fabrication of ultrathin membranes using 2D-MOF nanosheets for tunable gas separation[J]. Chemistry: An Asian Journal, 2021, 16(21): 3413-3418. |
| [30] | 林刚, 陈晓惠, 金石, 等. 气体膜分离原理、动态与展望[J]. 低温与特气, 2003, 21(2): 13-18. |
| LIN Gang, CHEN Xiaohui, JIN Shi, et al. Principle, dynamics and prospect of gas membrane separation[J]. Low Temperature and Specialty Gases, 2003, 21(2): 13-18. | |
| [31] | HAO Shuang, JIA Zhiqian, WEN Jianping, et al. Progress in adsorptive membranes for separation—A review[J]. Separation and Purification Technology, 2021, 255: 117772. |
| [32] | KIM Kyeongsook, KIM Kwang Sin, LEE Jeong Eun, et al. Status of SF6 separation/refining technology development for electric industry in Korea[J]. Separation and Purification Technology, 2018, 200: 29-35. |
| [33] | Sung-Hwan BAE, KIM Ja-Hee, Han-Seung LIM. A study on new power business model using power information technology[J]. Journal of Electrical Engineering & Technology, 2010, 5(3): 379-388. |
| [34] | XIONG Wenjie, SHI Mingzhen, ZHANG Xiaomin, et al. The efficient conversion of H2S into mercaptan alcohols mediated in protic ionic liquids under mild conditions[J]. Green Chemistry, 2021, 23(20): 7969-7975. |
| [35] | ZHANG Xiaomin, XIONG Wenjie, SHI Mingzhen, et al. Task-specific ionic liquids as absorbents and catalysts for efficient capture and conversion of H2S into value-added mercaptan acids[J]. Chemical Engineering Journal, 2021, 408: 127866. |
| [36] | SOSA Julio E, SANTIAGO Rubén, Daniel HOSPITAL-BENITO, et al. Process evaluation of fluorinated ionic liquids as F-gas absorbents[J]. Environmental Science & Technology, 2020, 54(19): 12784-12794. |
| [37] | JI Jialan, XIONG Wenjie, ZHANG Xiaomin, et al. Reversible absorption of NF3 with high solubility in Lewis acidic ionic liquids[J]. Chemical Engineering Journal, 2022, 440: 135902. |
| [38] | LIU Ping, CAI Kaixing, LIU Mao, et al. Deep eutectic solvents with multiple hydroxyl sites for efficient and reversible absorption of SF6 [J]. Journal of Molecular Liquids, 2022, 356: 119052. |
| [39] | CHIN Cynthia, KAMIN Zykamilia, BAHRUN Mohd Hardyianto Vai, et al. The production of industrial-grade oxygen from air by pressure swing adsorption[J]. International Journal of Chemical Engineering, 2023, 2023: 2308227. |
| [40] | CHUAH Chong Yang, LEE Yechan, Tae-Hyun BAE. Potential of adsorbents and membranes for SF6 capture and recovery: A review[J]. Chemical Engineering Journal, 2021, 404: 126577. |
| [41] | ROMERO M D, OVEJERO G, RODRÍGUEZ A, et al. Enhancement of the basic properties in FAU zeolites by impregnation with cesium hydroxide[J]. Microporous and Mesoporous Materials, 2005, 81(1/2/3): 313-320. |
| [42] | SUN Xin, KING Jonathan, ANTHONY Jennifer L. Molecular sieve synthesis in the presence of tetraalkylammonium and dialkylimidazolium molten salts[J]. Chemical Engineering Journal, 2009, 147(1): 2-5. |
| [43] | MATITO-MARTOS I, MARTIN-CALVO A, GUTIÉRREZ-SEVILLANO J J, et al. Zeolite screening for the separation of gas mixtures containing SO2, CO2 and CO[J]. Physical Chemistry Chemical Physics, 2014, 16(37): 19884-19893. |
| [44] | BERNAL M P, CORONAS J, MENÉNDEZ M, et al. Separation of CO2/N2 mixtures using MFI-type zeolite membranes[J]. AIChE Journal, 2004, 50(1): 127-135. |
| [45] | HIMENO Shuji, TOMITA Toshihiro, SUZUKI Kenji, et al. Synthesis and permeation properties of a DDR-type zeolite membrane for separation of CO2/CH4 gaseous mixtures[J]. Industrial & Engineering Chemistry Research, 2007, 46(21): 6989-6997. |
| [46] | GARCÍA-PÉREZ E, PARRA J B, ANIA C O, et al. A computational study of CO2, N2, and CH4 adsorption in zeolites[J]. Adsorption, 2007, 13(5): 469-476. |
| [47] | CHEN Sirui, SHEN Yuanhui, GUAN Zhongbo, et al. Adsorption properties of SF6 on zeolite NaY, 13X, activated carbon, and silica gel[J]. Journal of Chemical & Engineering Data, 2020, 65(8): 4044-4051. |
| [48] | MATITO-MARTOS I, ÁLVAREZ-OSSORIO J, GUTIÉRREZ-SEVILLANO J J, et al. Zeolites for the selective adsorption of sulfur hexafluoride[J]. Physical Chemistry Chemical Physics, 2015, 17(27): 18121-18130. |
| [49] | JAGIELLO Jacek, BANDOSZ Teresa J, PUTYERA Karol, et al. Adsorption near ambient temperatures of methane, carbon tetrafluoride, and sulfur hexafluoride on commercial activated carbons[J]. Journal of Chemical & Engineering Data, 1995, 40(6): 1288-1292. |
| [50] | SUN Rui, TAI Cheuk-Wai, Maria STRØMME, et al. Hierarchical porous carbon synthesized from novel porous amorphous calcium or magnesium citrate with enhanced SF6 uptake and SF6/N2 selectivity[J]. ACS Applied Nano Materials, 2019, 2(2): 778-789. |
| [51] | CHIANG Yuchun, WU Poyun. Adsorption equilibrium of sulfur hexafluoride on multi-walled carbon nanotubes[J]. Journal of Hazardous Materials, 2010, 178(1/2/3): 729-738. |
| [52] | KANG Dong Won, KANG Minjung, HONG Chang Seop, et al. Post-synthetic modification of porous materials: Superprotonic conductivities and membrane applications in fuel cells[J]. Journal of Materials Chemistry A, 2020, 8(16): 7474-7494. |
| [53] | LEE Daeyeon, LEE Sangho, Younghu SON, et al. Uncoordinated tetrazole ligands in metal-organic frameworks for proton-conductivity studies[J]. Bulletin of the Korean Chemical Society, 2022, 43(7): 912-917. |
| [54] | KANG Dong Won, Minki JUN, KIM Jun, et al. Double hypercrosslinked porous organic polymer-derived electrocatalysts for a water splitting device[J]. ACS Applied Energy Materials, 2022, 5(3): 3269-3274. |
| [55] | Seyoung KOO, KANG Dong Won. Emerging porous solids and sonochemistry[J]. Crystengcomm, 2023, 25(43): 5994-6005. |
| [56] | WANG Luyao, HUANG Hengcong, ZHANG Xiaoyu, et al. Designed metal-organic frameworks with potential for multi-component hydrocarbon separation[J]. Coordination Chemistry Reviews, 2023, 484: 215111. |
| [57] | SUN Zhiqiang, LIAO Yiren, ZHAO Shilin, et al. Research progress in metal-organic frameworks (MOFs) in CO2 capture from post-combustion coal-fired flue gas: Characteristics, preparation, modification and applications[J]. Journal of Materials Chemistry A, 2022, 10(10): 5174-5211. |
| [58] | YOON Jung Heum, LEE Woo Ram, LEE Jeong Tae, et al. Design and synthesis of novel lanthanide MOFs by unique in situ organic and inorganic reactions[J]. Bulletin of the Korean Chemical Society, 2022, 43(9): 1136-1140. |
| [59] | CHOI In-Hwan, GU Ja-Min, KIM Hyun-Chul, et al. Gas sorption properties of a new Zn-BTB metal-organic framework with permanent porosity[J]. Bulletin of the Korean Chemical Society, 2023, 44(9): 780-787. |
| [60] | SENKOVSKA Irena, BAREA Elisa, NAVARRO Jorge Andrés Rodríguez, et al. Adsorptive capturing and storing greenhouse gases such as sulfur hexafluoride and carbon tetrafluoride using metal-organic frameworks[J]. Microporous & Mesoporous Materials, 2012, 156: 115-120. |
| [61] | HORCAJADA Patricia, Suzy SURBLÉ, SERRE Christian, et al. Synthesis and catalytic properties of MIL-100(Fe), an iron(Ⅲ) carboxylate with large pores[J]. Chemical Communications, 2007(27): 2820-2822. |
| [62] | Jinhee BAE, PARK Sun Ho, MOON Dohyun, et al. Crystalline hydrogen bonding of water molecules confined in a metal-organic framework[J]. Communications Chemistry, 2022, 5: 51. |
| [63] | SONG Dahae, Jinhee BAE, JI Hoon, et al. Coordinative Reduction of metal nodes enhances the hydrolytic stability of a paddlewheel metal-organic framework[J]. Journal of the American Chemical Society, 2019, 141(19): 7853-7864. |
| [64] | CHUI Stephen S Y, Samuel M F LO, CHARMANT Jonathan P H, et al. A chemically functionalizable nanoporous material[Cu3(TMA)2(H2O)3] n [J]. Science, 1999, 283(5405): 1148-1150. |
| [65] | WANG Hepeng, Jürgen GETZSCHMANN, SENKOVSKA Irena, et al. Structural transformation and high pressure methane adsorption of Co2(1,4-bdc)2dabco[J]. Microporous and Mesoporous Materials, 2008, 116(1/2/3): 653-657. |
| [66] | KIM Hyojin, HONG Chang Seop. MOF-74-type frameworks: Tunable pore environment and functionality through metal and ligand modification[J]. CrystEngComm, 2021, 23(6): 1377-1387. |
| [67] | KIM Min-Bum, LEE Seung-Joon, LEE Chang Yeon, et al. High SF6 selectivities and capacities in isostructural metal-organic frameworks with proper pore sizes and highly dense unsaturated metal sites[J]. Microporous & Mesoporous Materials, 2014, 190: 356-361. |
| [68] | CHUAH Chong Yang, Kunli GOH, Tae-Hyun BAE. Hierarchically structured HKUST-1 nanocrystals for enhanced SF6 capture and recovery[J]. The Journal of Physical Chemistry C, 2017, 121(12): 6748-6755. |
| [69] | KIM Min-Bum, KIM Kyung-Min, Hoon KIM-Tea, et al. Highly selective adsorption of SF6 over N2 in a bromine-functionalized zirconium-based metal-organic framework[J]. Chemical Engineering Journal, 2018, 339: 223-229. |
| [70] | MIAO Xiaoyu, SUI Jincheng, WENG Sen, et al. Construction of hierarchical porous UiO-66-Br2@PS/DVB-packed columns by high internal phase emulsion strategy for enhanced separation of CF4/N2 and SF6/N2 [J]. ACS Applied Materials & Interfaces, 2024,16(18): 24083-24093. |
| [71] | REN Jiahao, CHANG Miao, ZENG Wenjiang, et al. Computer-aided discovery of MOFs with calixarene-analogous microenvironment for exceptional SF6 capture[J]. Chemistry of Materials, 2021, 33(13): 5108-5114. |
| [72] | LI Yongpeng, NI Jingjing, ZHANG Xiaojie, et al. Pore environmental modification by amino groups in robust microporous MOFs for SF6 capturing and SF6/N2 separation[J]. Inorganic Chemistry, 2024, 63(29): 13568-13575. |
| [73] | HU Yongqi, WANG Lingyao, Ruihan NAN, et al. Pore engineering in cost-effective and stable Al-MOFs for efficient capture of the greenhouse gas SF6 [J]. Chemical Engineering Journal, 2023, 471: 144851. |
| [74] | ZHENG Sutao, JIANG Runyuan, JIANG Yu, et al. Methyl-functionalized microporous metal-organic framework for efficient SF6/N2 separation[J]. Separation and Purification Technology, 2023, 318: 123957. |
| [75] | LIU Hao ran, WANG Shao min, DONG Yong li, et al. Control of pore environment in nickel-based metal-organic frameworks for SF6/N2 separation[J]. Chinese Journal of Structural Chemistry, 2023, 42(2): 100022. |
| [76] | CHANG Miao, WEI Yan, LIU Dahuan, et al. a general strategy for instantaneous and continuous synthesis of ultrasmall metal-organic framework nanoparticles[J]. Angewandte Chemie International Edition, 2021, 60(50): 26390-26396. |
| [77] | TOYODA M, MURASE H, IMAI T, et al. SF6 Reclaimer from SF6/N2 mixtures by gas separation with molecular sieving effect[J]. IEEE Power Engineering Review, 2002, 22(6): 61. |
| [78] | WANG Tongge, CHANG Miao, YAN Tongan, et al. Calcium-based metal-organic framework for efficient capture of sulfur hexafluoride at low concentrations[J]. Industrial & Engineering Chemistry Research, 2021, 60(16): 5976-5983. |
| [79] | CHANG Miao, YAN Tongan, WEI Yan, et al. Metal-organic framework-based single-molecule SF6 trap for record SF6 capture[J]. Chemistry of Materials, 2022, 34(20): 9134-9143. |
| [80] | YANG Mingshan, CHANG Miao, YAN Tongan, et al. A nickel-based metal-organic framework for efficient SF6/N2 separation with record SF6 uptake and SF6/N2 selectivity[J]. Separation and Purification Technology, 2022, 295: 121340. |
| [81] | LI Shi ming, ZHANG Qiang, JIANG Hong chan, et al. Constructing local nanomolecular trap in a scalable, low-cost, and ultramicroporous metal-organic framework for efficient capture of greenhouse gases SF6 and CO2 [J]. Chemical Engineering Journal, 2024, 496: 154026. |
| [82] | LI Yong peng, NI Jing jing, LI Shuo, et al. Rational pore-window size control in three Cu-MOFs with different pore environments for efficient capture of the greenhouse gas SF6 [J]. Journal of Solid State Chemistry, 2024, 329: 124443. |
| [83] | LI Yong peng, ZHANG Xiaojie, NI Jing jing, et al. Design of a highly-stable cobalt (Ⅱ) porous framework based on aromatic stacking strategy for efficient SF6 capture and SF6/N2 mixture separation[J]. Separation and Purification Technology, 2024, 343: 126995. |
| [84] | Milan KÖPPEN, DHAKSHINAMOORTHY Amarajothi, Ken INGE A, et al. Synthesis, transformation, catalysis, and gas sorption investigations on the bismuth metal-organic framework CAU-17[J]. European Journal of Inorganic Chemistry, 2018, 2018(30): 3496-3503. |
| [85] | GRAPE Erik, ROOTH Victoria, XU Hongyi, et al. Breathing metal-organic frameworks based on flexible inorganic building units[J]. Acta Crystallographica Section A: Foundations and Advances, 2019, 75(a2): e478. |
| [86] | WANG Hao, SHI Le, XIONG Zhangyi, et al. A two-dimensional metal-organic framework assembled from scandium-based cages for the selective capture of sulfur hexafluoride[J]. Chemical Communications, 2024, 60(17): 2397-2400. |
| [87] | Michelle ÅHLÉN, JAWORSKI Aleksander, Maria STRØMME, et al. Selective adsorption of CO2 and SF6 on mixed-linker ZIF-7-8s: The effect of linker substitution on uptake capacity and kinetics[J]. Chemical Engineering Journal, 2021, 422(21): 130117. |
| [88] | LIU Ping, ZHAO Tianxiang, CAI Kaixing, et al. Rapid mechanochemical construction of HKUST-1 with enhancing water stability by hybrid ligands assembly strategy for efficient adsorption of SF6 [J]. Chemical Engineering Journal, 2022, 437: 135364. |
| [89] | Jae-Hoon CHA, Seong-Bin GA, LEE Seung-Jun, et al. Integrated material and process evaluation of metal-organic frameworks database for energy-efficient SF6/N2 separation[J]. Chemical Engineering Journal, 2021, 426: 131787. |
| [90] | AMOMBO NOA Francoise M, CHEUNG Ocean, Michelle ÅHLÉN, et al. A hexagon based Mn(Ⅱ) rod metal-organic framework-structure, SF6 gas sorption, magnetism and electrochemistry[J]. Chemical Communications, 2023, 59(15): 2106-2109. |
| [91] | YAN Jiangwen, GANG Shuqi, LIU Ziyue,et al. An In(Ⅲ)-MOF based on pore engineering for efficient capture SF6 from SF6/N2 mixture[J]. Separation and Purification Technology, 2023, 327: 124929. |
| [92] | Michelle ÅHLÉN, ZHOU Yi, HEDBOM Daniel, et al. Efficient SF6 capture and separation in robust gallium-and vanadium-based metal-organic frameworks[J]. Journal of Materials Chemistry A, 2023, 11(48): 26435-26441. |
| [93] | Michelle ÅHLÉN, KAPACA Elina, HEDBOM Daniel, et al. Gas sorption properties and kinetics of porous bismuth-based metal-organic frameworks and the selective CO2 and SF6 sorption on a new bismuth trimesate-based structure UU-200[J]. Microporous and Mesoporous Materials, 2022, 329: 111548. |
| [94] | ZHANG Xin, ZHAO Yanlong, LI Xiangyu, et al. Recovery of high-purity SF6 from humid SF6/N2 mixture within a Co(Ⅱ)-Pyrazolate framework[J]. Journal of the American Chemical Society, 2024, 146(28): 19303-19309. |
| [95] | RIDDELL Imogen A, SMULDERS Maarten M J, CLEGG Jack K, et al. Encapsulation, storage and controlled release of sulfur hexafluoride from a metal-organic capsule[J]. Chemical Communications, 2011, 47(1): 457-459. |
| [96] | KITAO Takashi, ZHANG Yuanyuan, KITAGAWA Susumu, et al. Hybridization of MOFs and polymers[J]. Chemical Society Reviews, 2017, 46(11): 3108-3133. |
| [97] | TIAN Jian, THALLAPALLY Praveen K, Peter MCGRAIL B. Porous organic molecular materials[J]. CrystEngComm, 2012, 14(6): 1909-1919. |
| [98] | ZHANG Gang, MASTALERZ Michael. Organic cage compounds-from shape-persistency to function[J]. Chemical Society Reviews, 2014, 43(6): 1934-1947. |
| [99] | MASTALERZ Michael. Shape-persistent organic cage compounds by dynamic covalent bond formation[J]. Angewandte Chemie International Edition, 2010, 49(30): 5042-5053. |
| [100] | AVELLANEDA Antonio, VALENTE Peter, BURGUN Alexandre, et al. Kinetically controlled porosity in a robust organic cage material[J]. Angewandte Chemie International Edition, 2013, 52(13): 3746-3749. |
| [101] | HASELL Tom, MIKLITZ Marcin, STEPHENSON Andrew, et al. Porous organic cages for sulfur hexafluoride separation[J]. Journal of the American Chemical Society, 2016, 138(5): 1653-1659. |
| [102] | LI Xinle, YANG Chongqing, SUN Bing, et al. Expeditious synthesis of covalent organic frameworks: A review[J]. Journal of Materials Chemistry A, 2020, 8(32): 16045-16060. |
| [103] | LIANG Rongran, JIANG Shuyan, Ru-Han A, et al. Two-dimensional covalent organic frameworks with hierarchical porosity[J]. Chemical Society Reviews, 2020, 49(12): 3920-3951. |
| [104] | SUN Tao, XIE Jian, GUO Wei, et al. Covalent-organic frameworks: Advanced organic electrode materials for rechargeable batteries[J]. Advanced Energy Materials, 2020, 10(19): 1904199. |
| [105] | FURUKAWA Hiroyasu, YAGHI Omar M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications[J]. Journal of the American Chemical Society, 2009, 131(25): 8875-8883. |
| [106] | ZHENG Xianqiang, SHEN Yanlong, WANG Shitao, et al. Selective adsorption of SF6 in covalent- and metal-organic frameworks[J]. Chinese Journal of Chemical Engineering, 2021, 39: 88-95. |
| [107] | WANG Shanshan, WU Yue, ZHANG Ying, et al. HF resistant porous aromatic frameworks for electronic special gases separation[J]. Langmuir, 2022, 38(28): 8667-8676. |
| [1] | 杨证禄, 杨立峰, 路晓飞, 锁显, 张安运, 崔希利, 邢华斌. 机器学习加速多孔吸附剂筛选发现的研究进展[J]. 化工进展, 2025, 44(8): 4288-4301. |
| [2] | 梁书玮, 俞杰, 谢钟音, 裴鉴禄, 林中鑫, 陈泽翔. 共价有机框架吸附放射性气态碘的研究进展[J]. 化工进展, 2025, 44(7): 3965-3975. |
| [3] | 李佩燚, 孙波龙, 刘瑞岩, 周歆尧, 刘瑞林, 胡园园, 徐功涛, 李新平. 海藻酸钠/二氧化钛复合多孔材料的制备及油水分离应用[J]. 化工进展, 2025, 44(6): 3053-3061. |
| [4] | 傅钰, 李晓宇, 伍岳, 陶春珲, 段然, 张文祥, 马和平. 电子级CF4中痕量NF3杂质的吸附脱除[J]. 化工进展, 2025, 44(6): 3570-3578. |
| [5] | 韩沛, 李金键, 柯天, 张治国, 鲍宗必, 任其龙, 杨启炜. 新型多孔材料吸附分离六氟化硫/氮气研究进展[J]. 化工进展, 2025, 44(6): 3592-3617. |
| [6] | 张新宇, 陶梦滢, 于小婷, 赵钟兴, 赵祯霞. 介孔金属有机骨架固定化漆酶及其活性艳蓝KN-R降解性能[J]. 化工进展, 2025, 44(3): 1758-1767. |
| [7] | 张爱京, 王桢钰, 肖宁宁, 宋艳娜, 李军, 冯江涛, 延卫. 新型汞离子吸附材料研究进展[J]. 化工进展, 2025, 44(2): 899-913. |
| [8] | 焦芮, 周涛, 孙寒雪, 李吉焱, 朱照琪, 李安. 多孔材料用于废水中放射性核素吸附的研究进展[J]. 化工进展, 2025, 44(1): 354-366. |
| [9] | 耿秀梅, 张逢, 张翔, 单美霞, 张亚涛. 用于CO2分离的Pebax基混合基质膜稳定性研究进展[J]. 化工进展, 2024, 43(9): 4996-5012. |
| [10] | 石佳博, 张宇轩, 陈雪峰, 谭蕉君. 单宁酸-纳米协同改性胶原纤维多孔材料的制备及其油水分离性能[J]. 化工进展, 2024, 43(8): 4624-4629. |
| [11] | 王丽娜, 武金升. 共价有机框架材料的合成与应用研究进展[J]. 化工进展, 2024, 43(7): 3834-3856. |
| [12] | 王涛, 高翔, 高继峰, 邓海全, 余显涌, 周振华, 唐玲, 吕航. 改性Cu-BTC基混合基质膜在CO2分离中的应用[J]. 化工进展, 2024, 43(6): 3240-3246. |
| [13] | 金彬浩, 朱小倩, 柯天, 张治国, 鲍宗必, 任其龙, 苏宝根, 杨启炜. 芳香烃/环烷烃吸附分离材料研究进展[J]. 化工进展, 2024, 43(4): 1863-1881. |
| [14] | 路广军, 韩晋钢, 陈英, 马志斌. 镁渣基多孔材料的制备及其对废水中Pb2+的吸附性能[J]. 化工进展, 2024, 43(4): 2126-2134. |
| [15] | 何兰, 高助威, 亓欣雨, 李成欣, 王世豪, 刘钟馨. 三聚氰胺海绵疏水改性及在油水分离领域的研究进展[J]. 化工进展, 2024, 43(2): 984-1000. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||