化工进展 ›› 2025, Vol. 44 ›› Issue (6): 3592-3617.DOI: 10.16085/j.issn.1000-6613.2024-0671
• 资源与环境化工 • 上一篇
新型多孔材料吸附分离六氟化硫/氮气研究进展
韩沛1( ), 李金键1, 柯天1, 张治国1,2, 鲍宗必1,2, 任其龙1,2, 杨启炜1,2(
), 李金键1, 柯天1, 张治国1,2, 鲍宗必1,2, 任其龙1,2, 杨启炜1,2( )
)
- 1.浙江大学化学工程与生物工程学院,生物质化工教育部重点实验室,浙江 杭州 310029
2.浙江大学衢州研究院,浙江 衢州 324003
-
收稿日期:2024-04-22修回日期:2024-06-06出版日期:2025-06-25发布日期:2025-07-08 -
通讯作者:杨启炜 -
作者简介:韩沛(1999—),男,硕士研究生,研究方向为分子辨识分离工程。E-mail:22128006@zju.edu.cn。 -
基金资助:国家自然科学基金(22288102);国家自然科学基金(22178305);国家自然科学基金(22308312);浙江省自然科学基金(LR21B060002)
Advances in adsorption separation of sulfur hexafluoride/nitrogen by novel porous materials
HAN Pei1( ), LI Jinjian1, KE Tian1, ZHANG Zhiguo1,2, BAO Zongbi1,2, REN Qilong1,2, YANG Qiwei1,2(
), LI Jinjian1, KE Tian1, ZHANG Zhiguo1,2, BAO Zongbi1,2, REN Qilong1,2, YANG Qiwei1,2( )
)
- 1.College of Chemical and Biological Engineering, Key Laboratory of Biomass Chemical Engineering, Zhejiang University, Hangzhou 310029, Zhejiang, China
2.Institute of Zhejiang University-Quzhou, Quzhou 324003, Zhejiang, China
-
Received:2024-04-22Revised:2024-06-06Online:2025-06-25Published:2025-07-08 -
Contact:YANG Qiwei
摘要:
六氟化硫与氮气的混合气体是电力工业中最重要的新兴绝缘介质之一,从废弃设备和废气中分离回收六氟化硫具有重要意义。吸附法避免了高能耗的相变过程,具有绿色节能的特点,然而六氟化硫与氮气均为惰性的非极性分子,因此开发能够精准辨识六氟化硫的吸附剂极具挑战。目前获得研究的吸附分离材料已经从传统的沸石分子筛发展到以金属有机框架材料为主的新型吸附剂,这些材料具有结构的高度可调性,可以有预期地对其进行设计,从而体现出优异的选择性吸附六氟化硫的能力。本文重点总结了这些材料的结构特点、调控策略、分离性能及其机理,同时指出一些尚存的重要问题,如对扩散传质规律与材料稳定性的研究不足、对材料潜力的评价不够全面等,应当在未来的研究中引起重视。
中图分类号:
引用本文
韩沛, 李金键, 柯天, 张治国, 鲍宗必, 任其龙, 杨启炜. 新型多孔材料吸附分离六氟化硫/氮气研究进展[J]. 化工进展, 2025, 44(6): 3592-3617.
HAN Pei, LI Jinjian, KE Tian, ZHANG Zhiguo, BAO Zongbi, REN Qilong, YANG Qiwei. Advances in adsorption separation of sulfur hexafluoride/nitrogen by novel porous materials[J]. Chemical Industry and Engineering Progress, 2025, 44(6): 3592-3617.
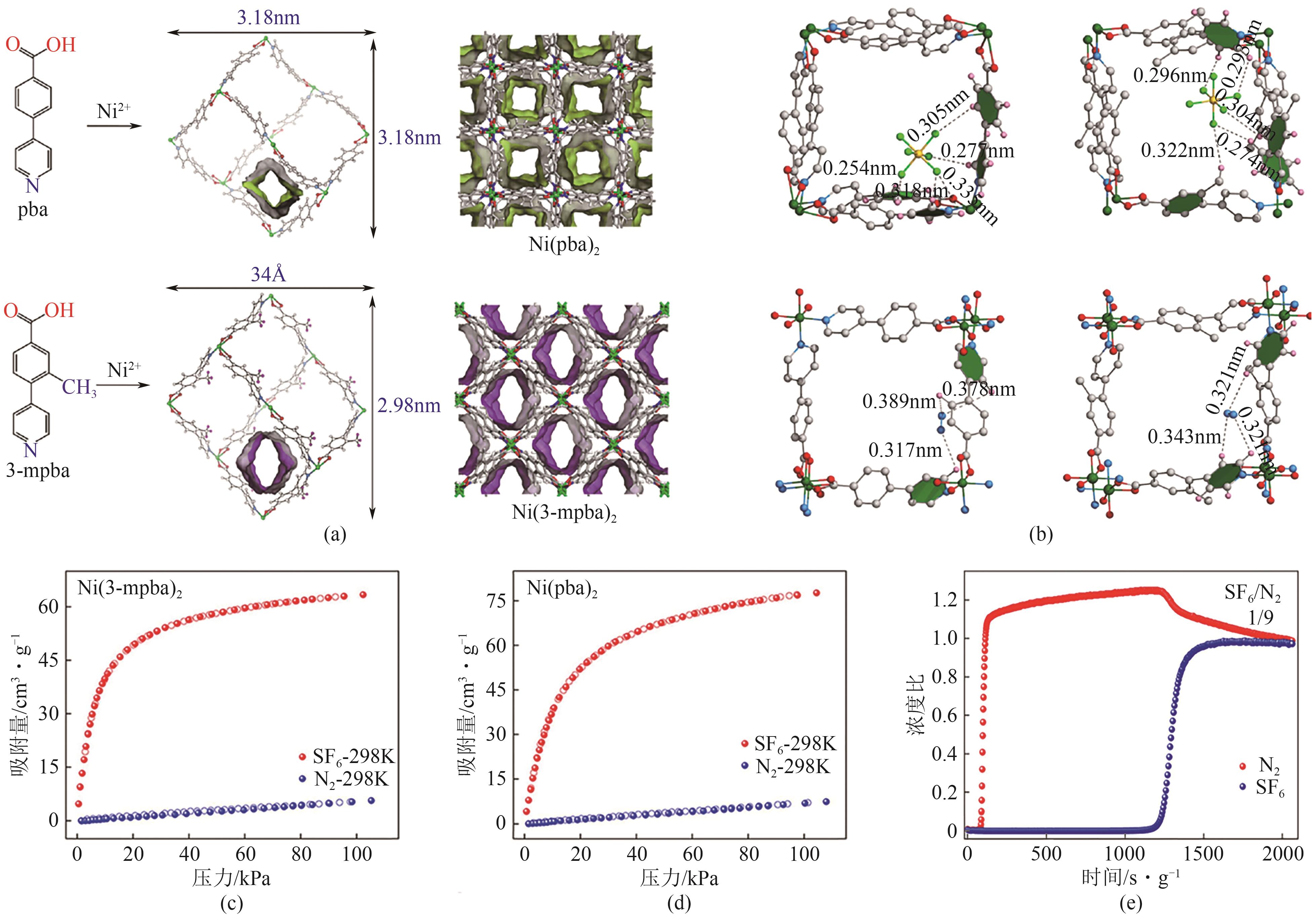
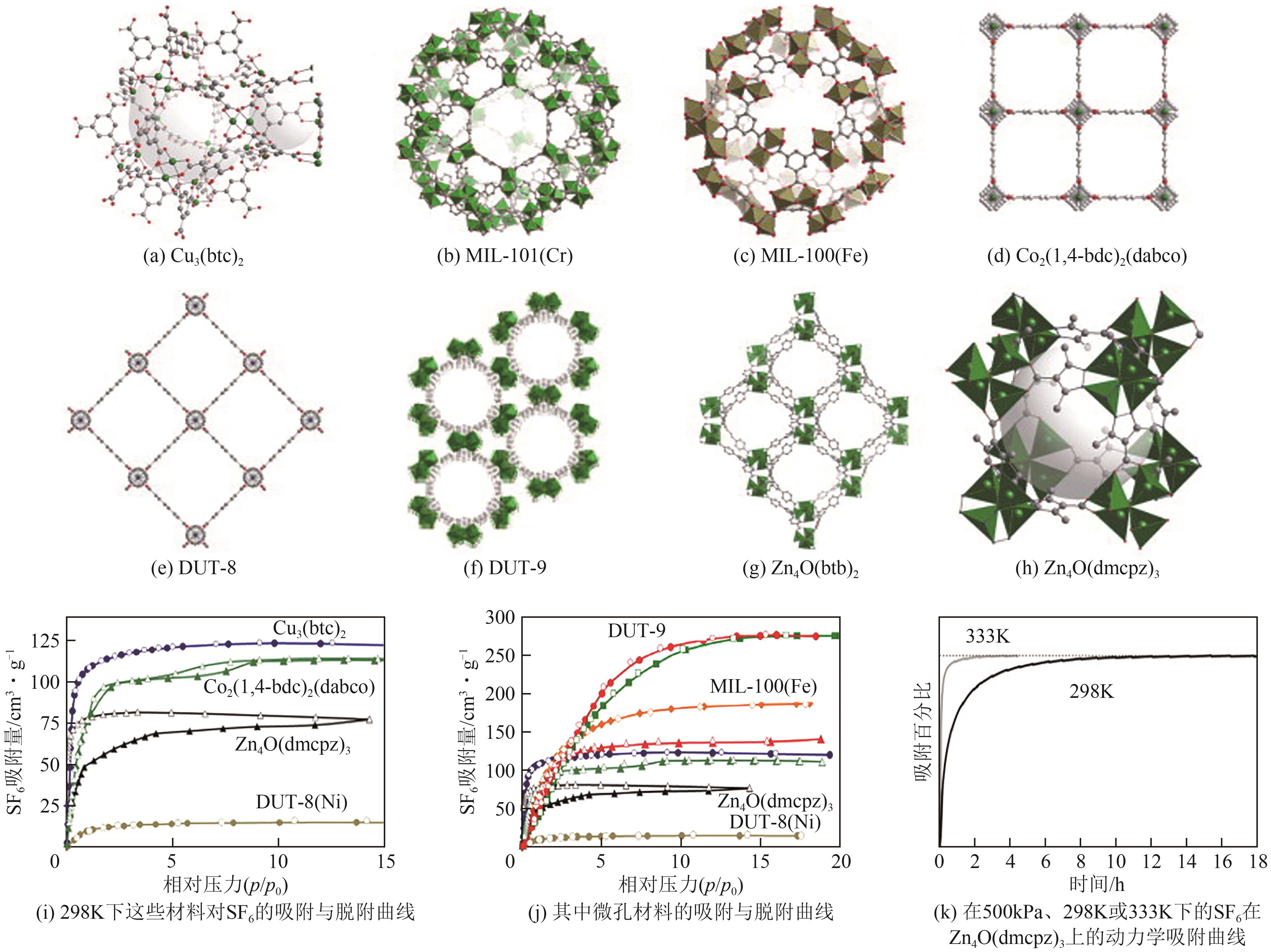
图5 Ni(pba)2和Ni(3-mpba)2的晶体孔结构(a);DFT模拟的Ni(pba)2和Ni(3-mpba)2对SF6和N2作用位点示意图(b);Ni(pba)2(c)和Ni(3-mpba)2(d)在298K下对SF6和N2的吸附等温线;Ni(3-mpba)2在298K下对SF6/N2(10/90)穿透曲线(e)[51]
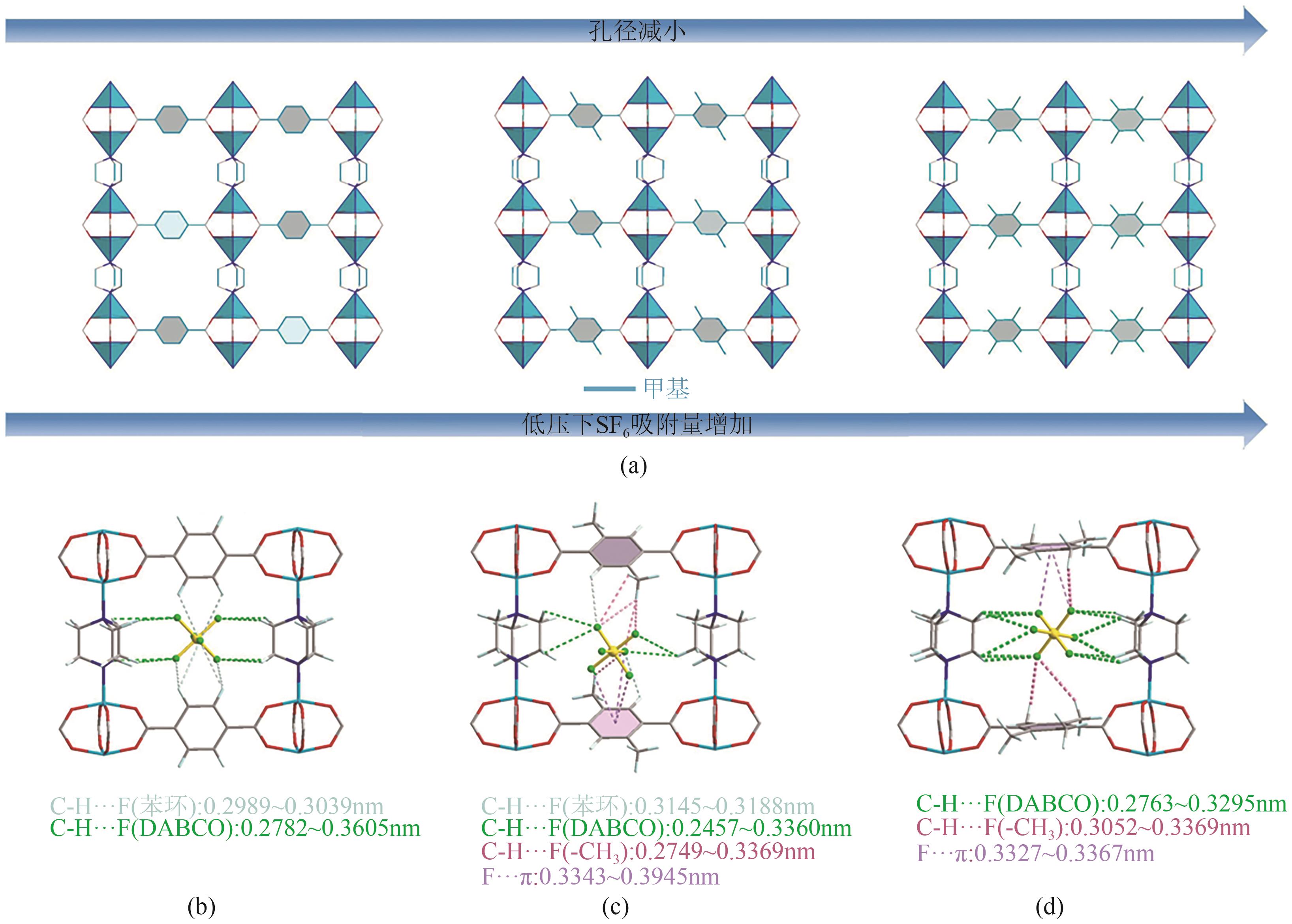
图6 随着甲基官能团的增加,Zn(BDC)(DABCO)0.5、Zn(DMBDC)(DABCO)0.5和Zn(TMBDC)(DABCO)0.5的形貌结构(a);GCMC模拟的SF6与Zn(BDC)(DABCO)0.5(b)、Zn(DMBDC)(DABCO)0.5(c)和Zn(TMBDC)(DABCO)0.5(d)的相互作用[52]
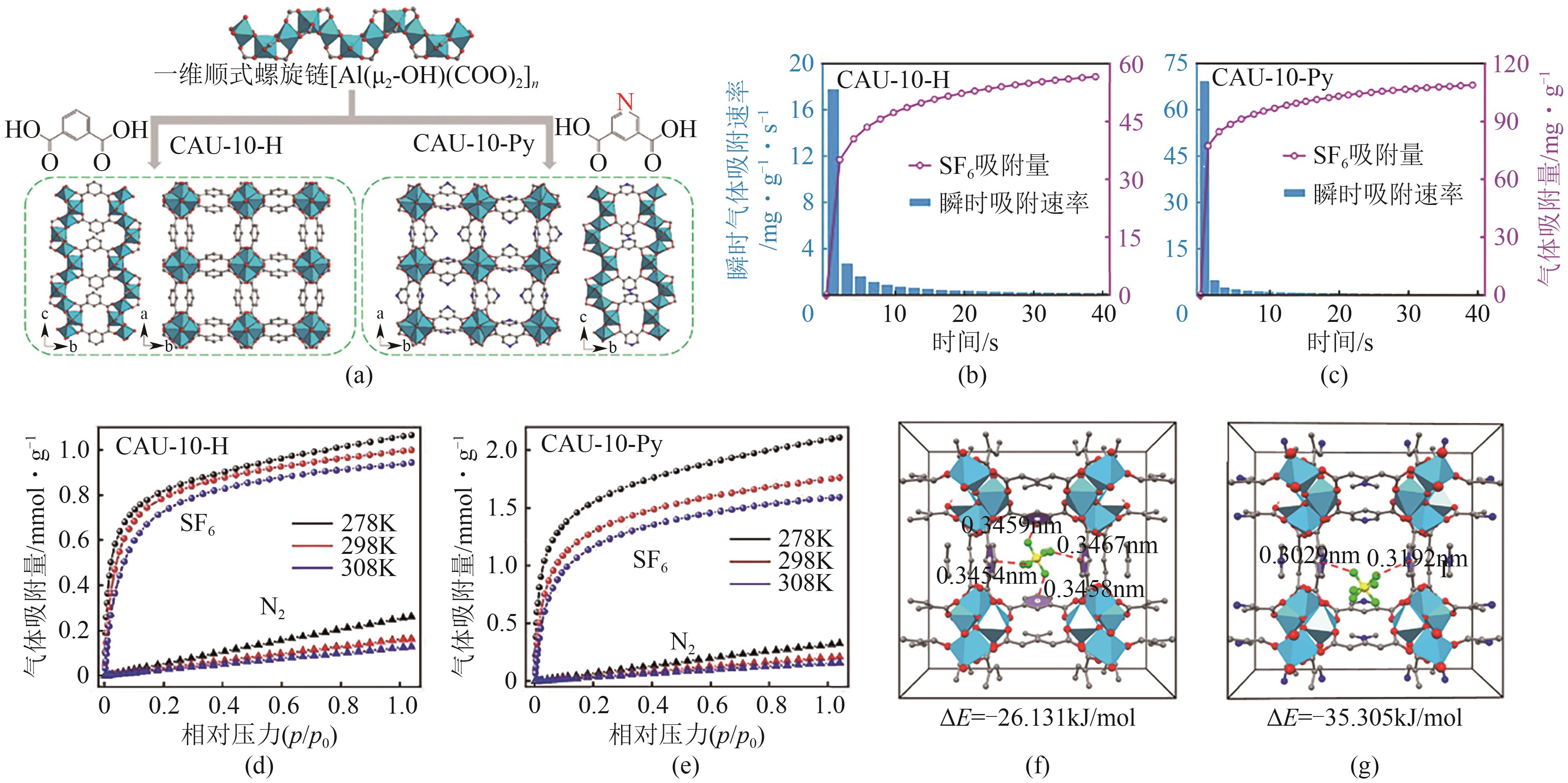
图7 CAU-10-H与CAU-10-Py的晶体结构(a);CAU-10-H(b)与CAU-10-Py(c)在298K、10kPa下对SF6的动力学吸附曲线;CAU-10-H(d)与CAU-10-Py(e)在278K、298K和308K下对SF6和N2的吸附等温线;CAU-10-H(f)与CAU-10-Py(g)与SF6的相互作用[55]
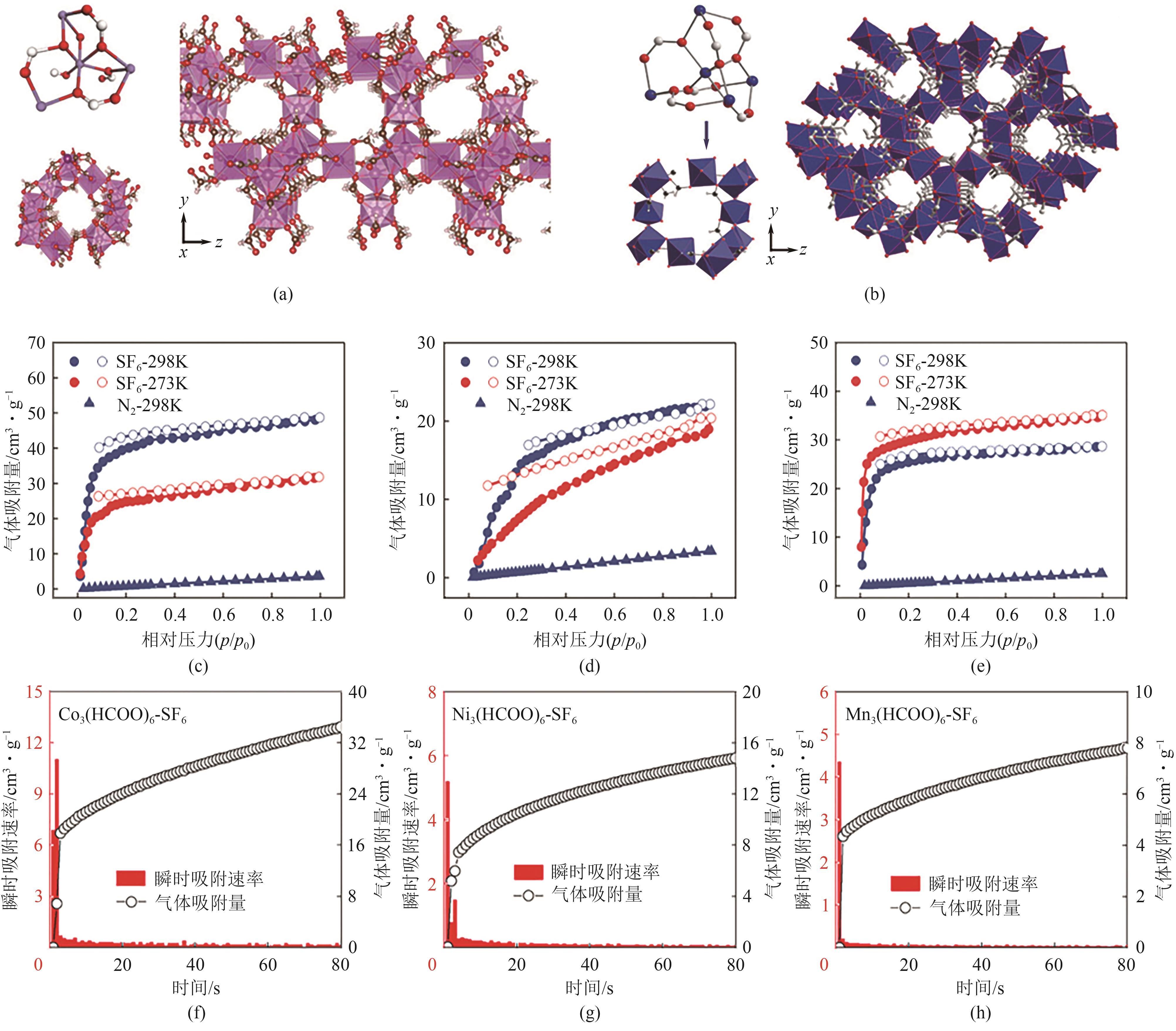
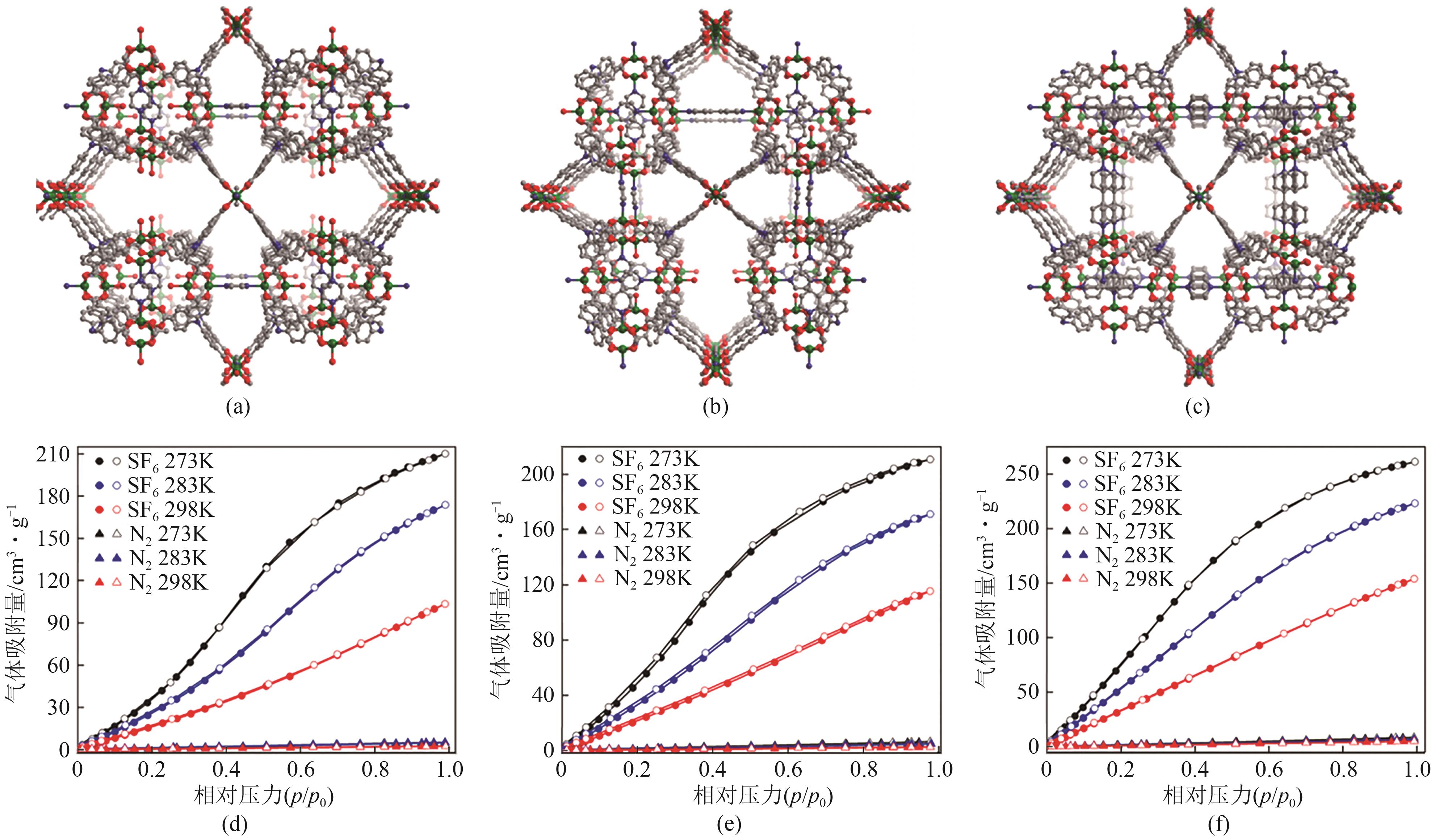
图9 Co3(HCOO)6(a)和Mn3(HCOO)6(b)的金属节点与孔道结构;Co3(HCOO)6(c)、Ni3(HCOO)6(d)和Mn3(HCOO)6(e)在273K和298K下对SF6和N2的吸附等温线;Co3(HCOO)6(f)、Ni3(HCOO)6(g)和Mn3(HCOO)6(h)在298K、10kPa下对SF6的动力学吸附曲线[58]
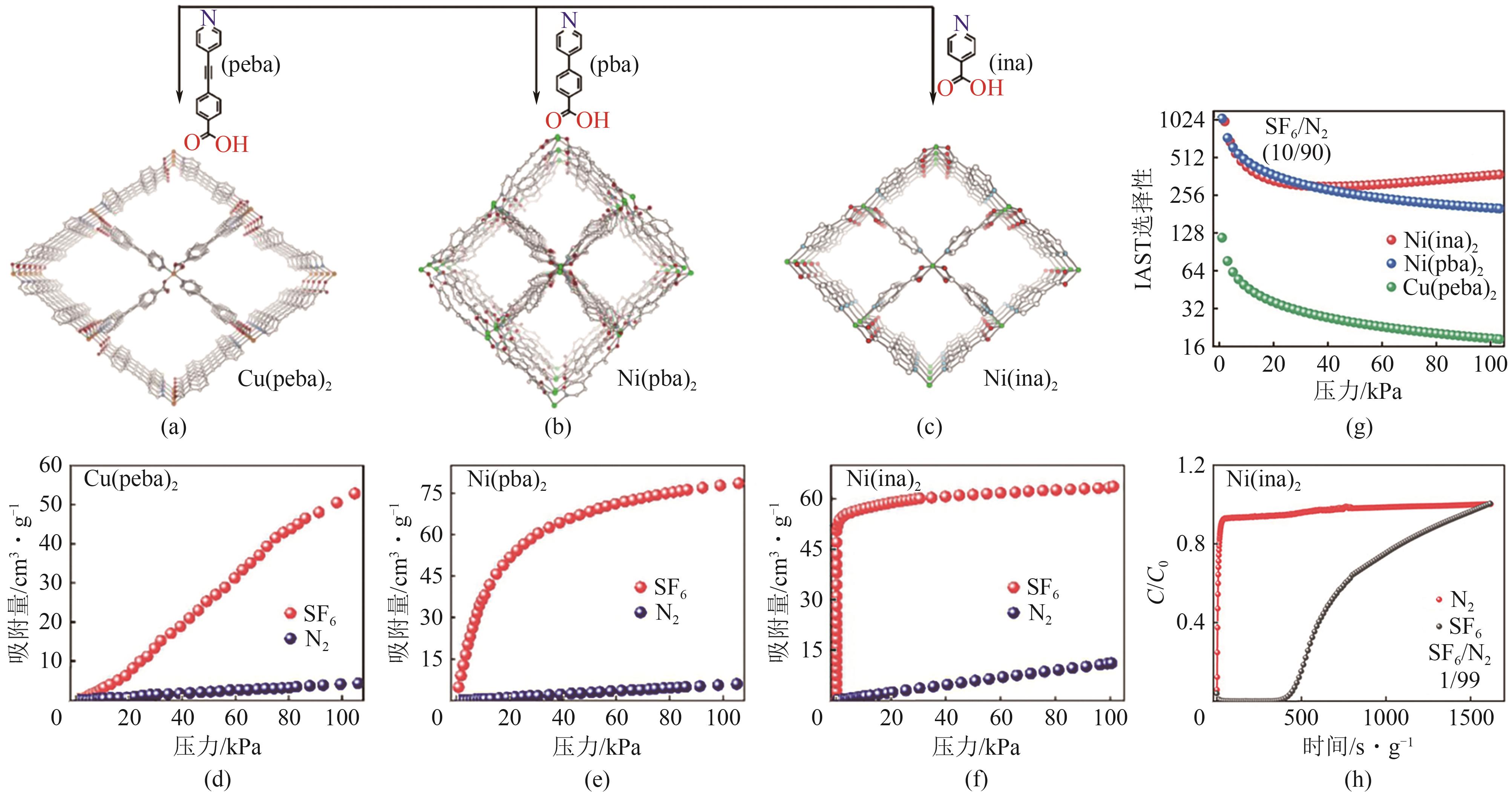
图12 Cu(peba)2(a)、Ni(pba)2(b)与Ni(ina)2(c)的孔结构;Cu(peba)2(d)、Ni(pba)2(e)与Ni(ina)2(f)在298K时的SF6和N2的吸附等温线;Cu(peba)2、Ni(pba)2与Ni(ina)2的IAST选择性(g);Ni(ina)2对SF6/N2(10/90)的穿透曲线(h)[61]
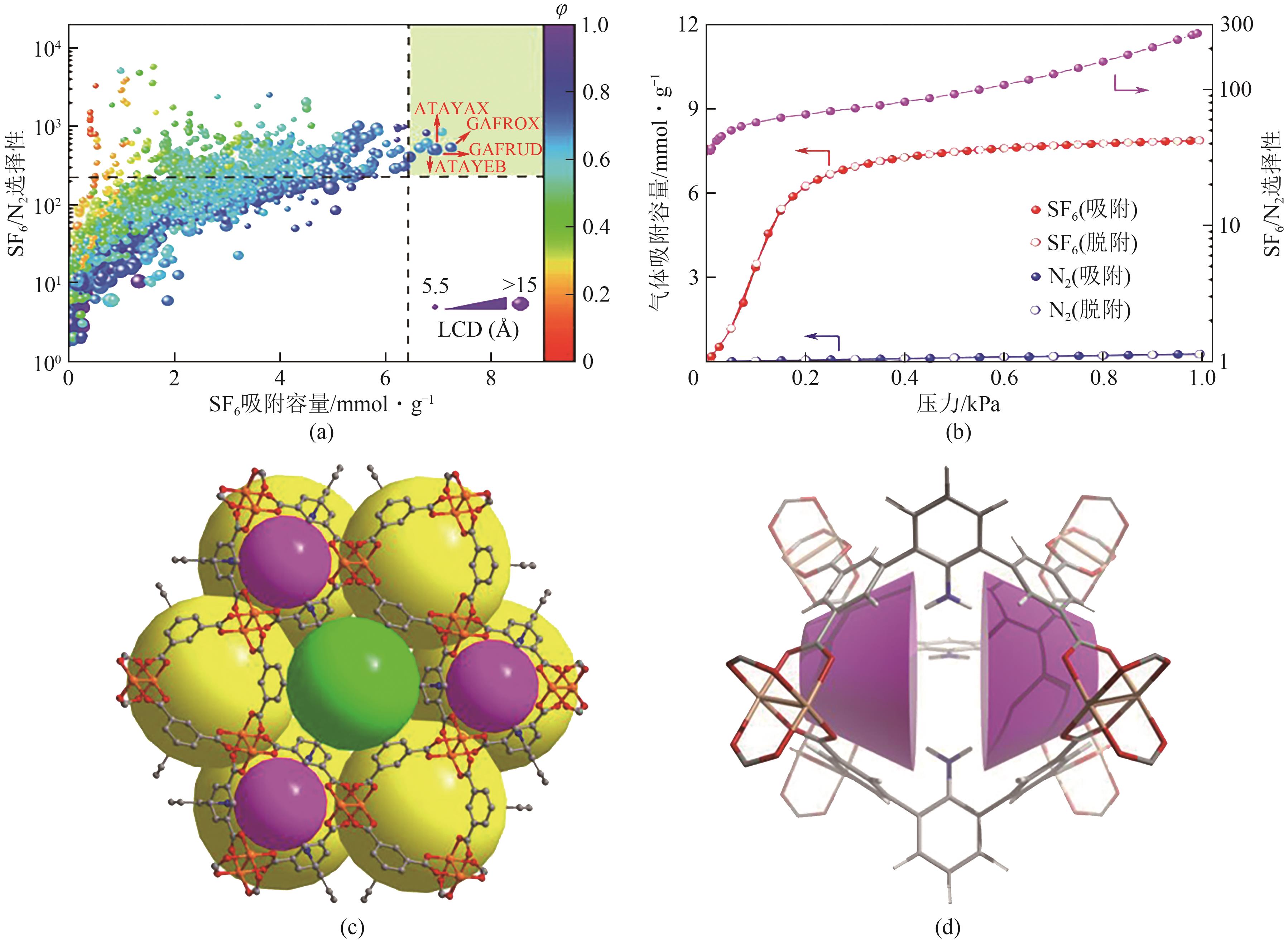
图14 对现有MOF进行的关于吸附容量与IAST选择性的高通量计算筛选(绿色阴影区域为表现最好的MOF)(a);Cu-MOF-NH2在298K下对SF6和N2的吸附等温线实验数据以及计算的IAST选择性(SF6/N2=10/90)(b);Cu-MOF-NH2的晶体结构(紫色、黄色和绿色球体分别表示空腔A、B与C)(c);Cu-MOF-NH2的空腔A中存在的杯芳烃类似微环境(d)[63]
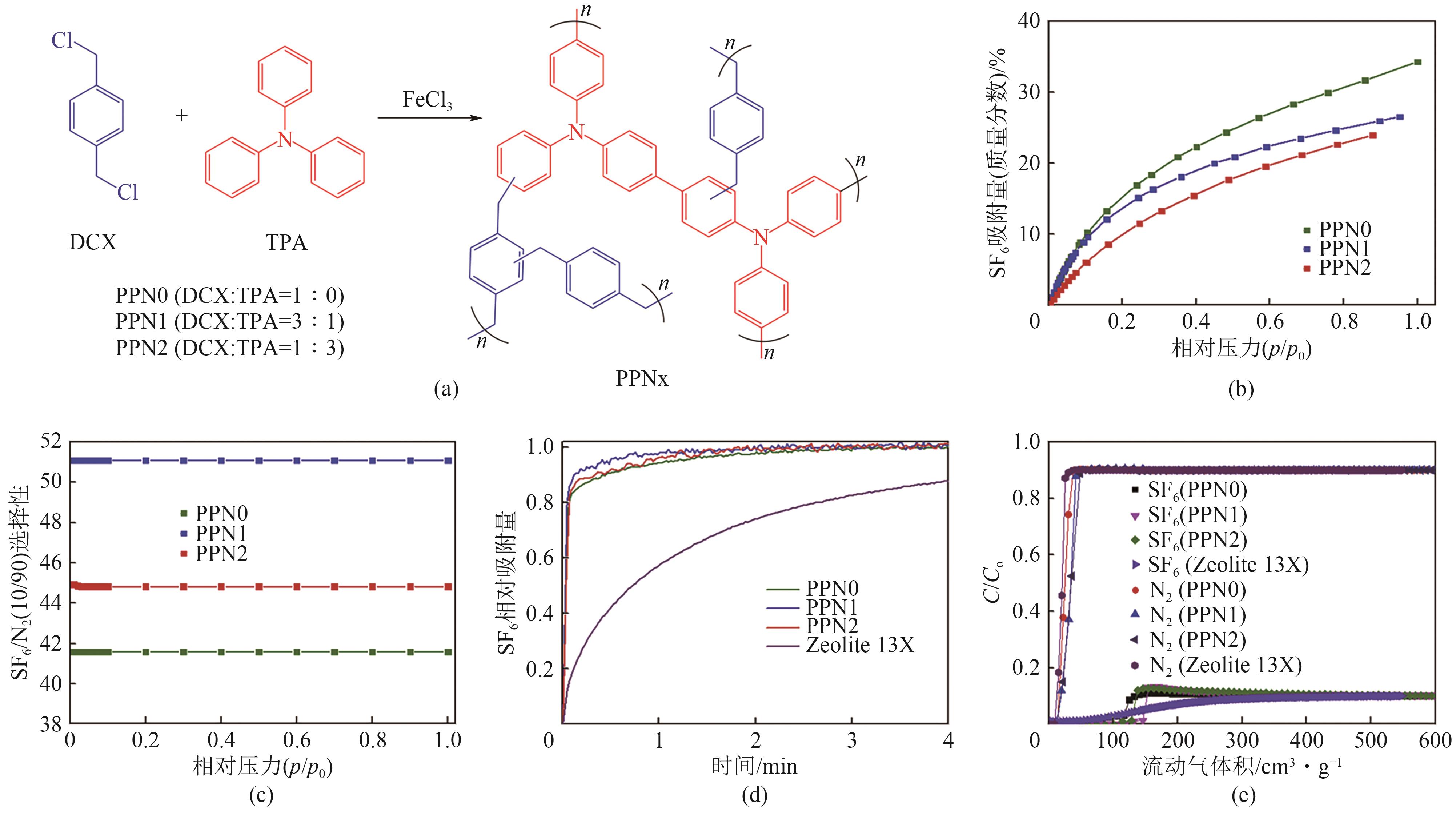
图18 材料PPNx的合成反应过程(a);PPNx在298K下对SF6的吸附等温线(b);PPNx在298K下对SF6/N2(10/90)的IAST选择性(c);13X和PPNx在298K下对SF6的动力学吸附曲线(d);PPNx和13X分子筛在298K下对SF6/N2(10/90)的穿透曲线(e)[71]
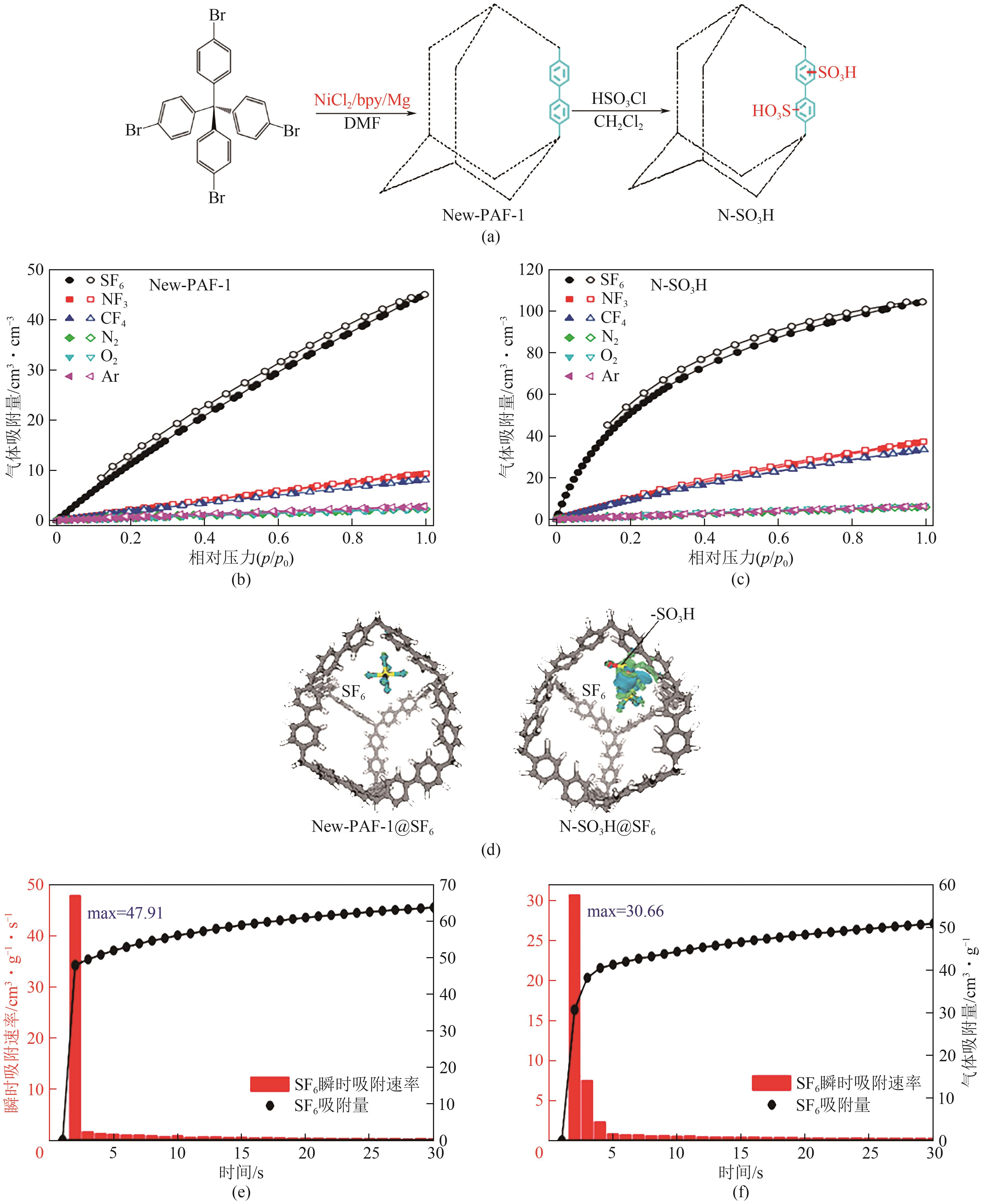
图20 New-PAF-1和N-SO3H合成过程示意图(a);New-PAF-1(b)和N-SO3H(c)对SF6、NF3、CF4、N2、O2和Ar在298K下的吸附等温线;New-PAF-1和N-SO3H与SF6作用的电荷密度差异图(d);New-PAF-1(e)和N-SO3H(f)对SF6的动力学吸附曲线[74]
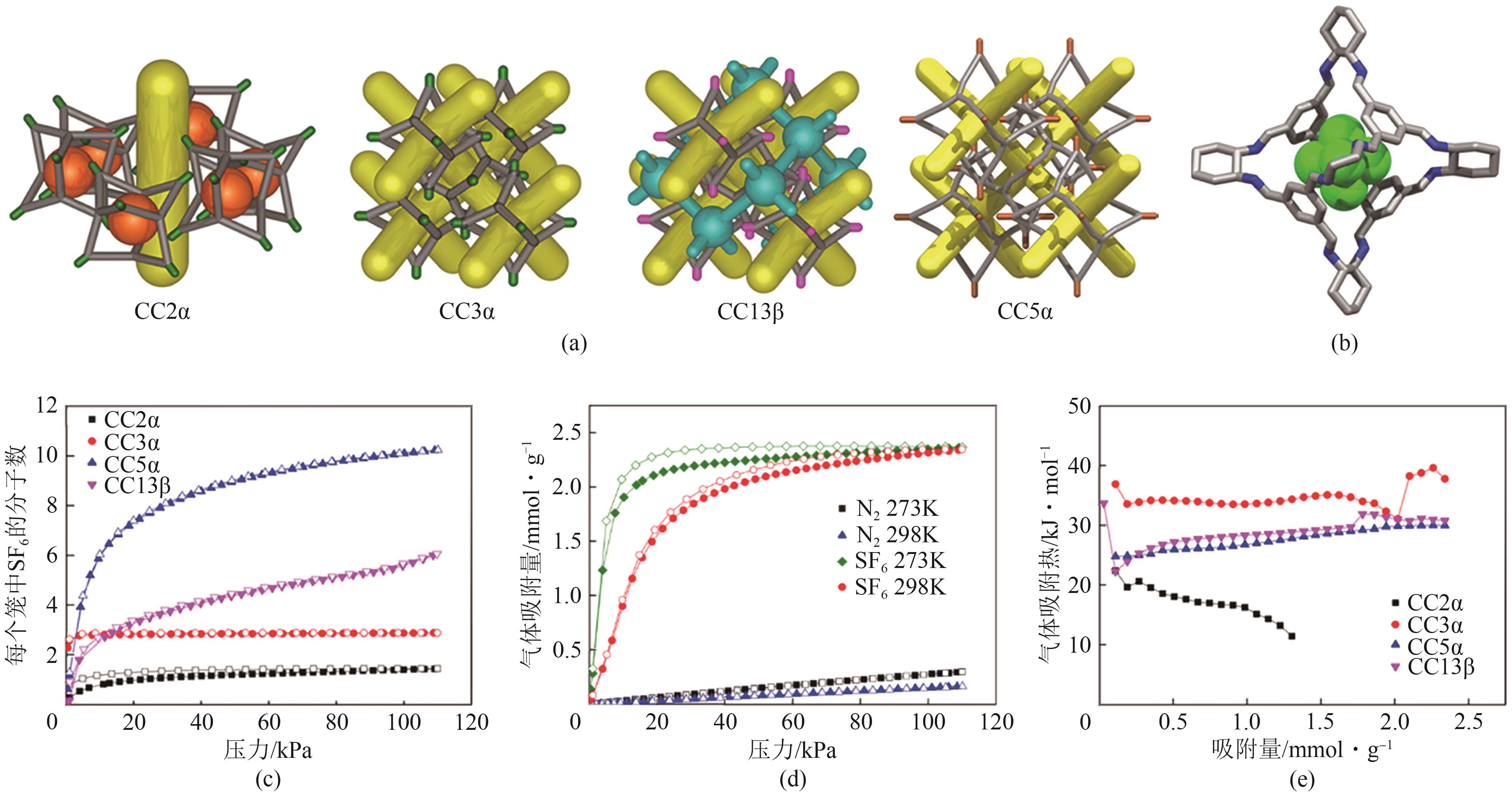
图21 CC2α、CC3α、CC5α和CC13β的单晶结构与孔道表示(a);单晶结构解析的SF6在CC3α腔中的吸附位点(b);CC2α、CC3α、CC5α和CC13β在230K下每个笼内容纳的SF6分子数(c);CC3α在273K和298K下对SF6和N2的吸附等温线(d);CC2α、CC3α、CC5α和CC13β计算得到的SF6吸附热(e)[76]
| 新型多孔材料 | 吸附量/mmol·g-1 | IAST选择性 (SF6/N2=10/90) | 数据来源 | ||
|---|---|---|---|---|---|
| 100kPa,SF6 | 10kPa,SF6 | 100kPa,N2 | |||
| Cu3(btc)2 | 4.77 | — | — | — | [ |
| MIL-100(Fe) | 2.94 | — | — | — | [ |
| MIL-101(Cr) | 2.01 | — | — | — | [ |
| Zn4O(dmcpz)3 | 2.54 | — | — | — | [ |
| Co2(1,4-bdc)2(dabco) | 3.39 | — | — | — | [ |
| DUT-9 | 2.32 | — | — | — | [ |
| Zn4O(btb)2 | 3.12 | — | — | — | [ |
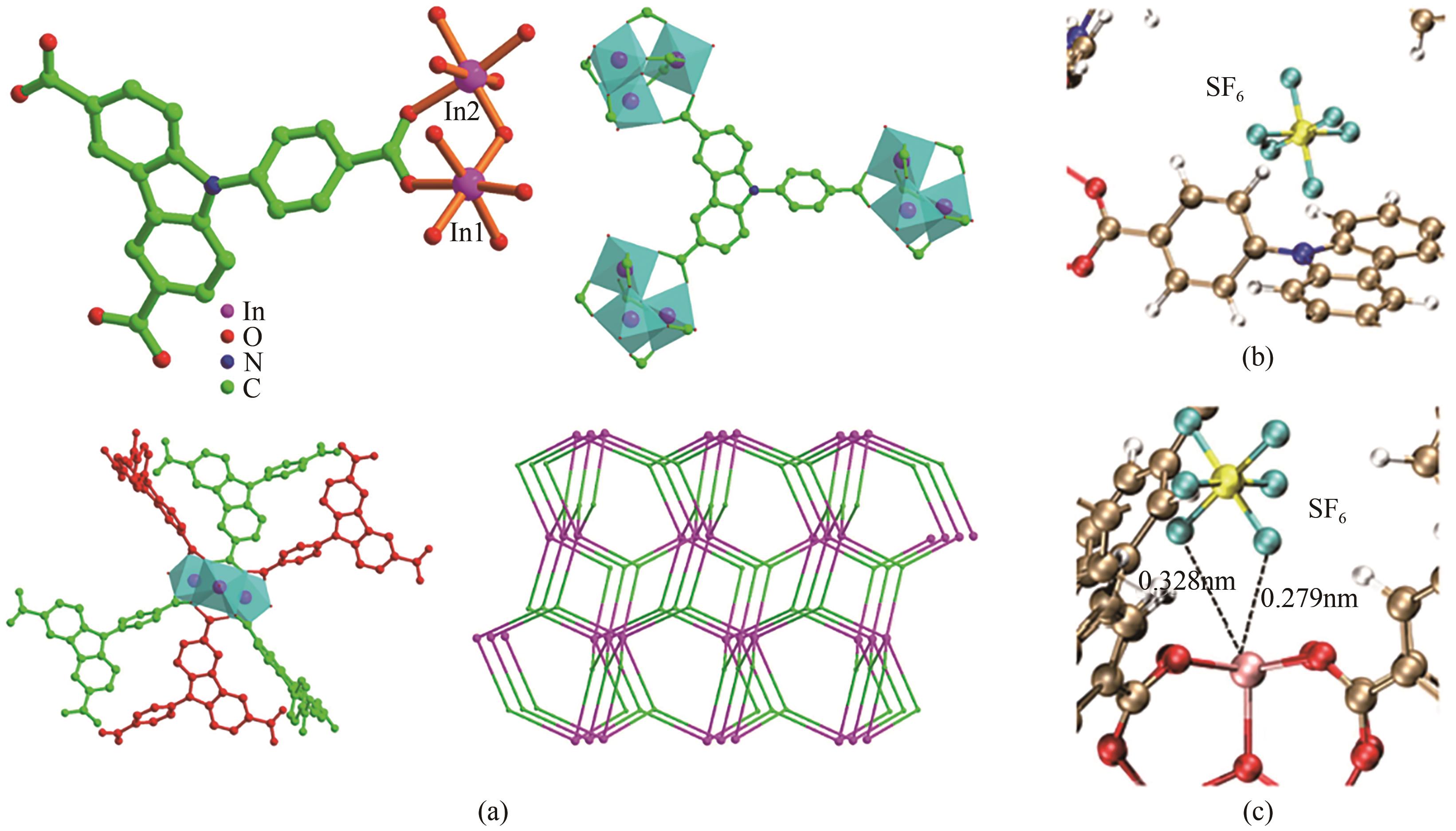
| HKUST-1a | 3.59 | 0.98 | 0.25 | 38 | [ |
| HKUST-1b | 4.02 | 1.19 | 0.25 | 50 | [ |
| HKUST-1c | 4.99 | 1.37 | 0.17 | 70 | [ |
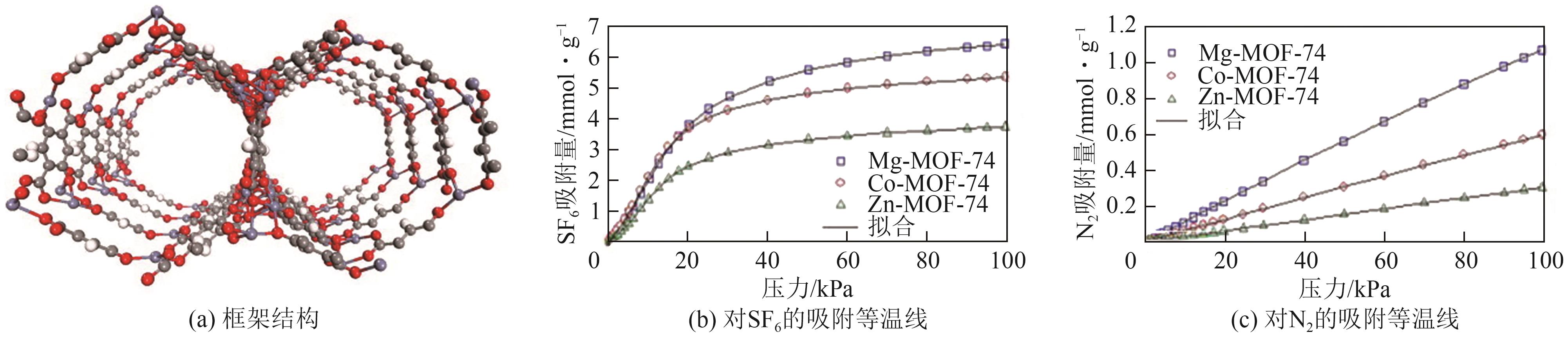
| Zn-MOF-74 | 3.73 | 1.28 | 0.30 | 46 | [ |
| Co-MOF-74 | 5.34 | 1.96 | 0.59 | 38 | [ |
| Mg-MOF-74 | 6.42 | 1.82 | 1.06 | 37 | [ |
| CAU-17 | 1.61① | 1.12① | 0.38① | 31① | [ |
| HBU-21 | 0.95 | 0.35 | 0.04 | 184 | [ |
| UiO-67 | 3.87 | — | 0.14 | 22 | [ |
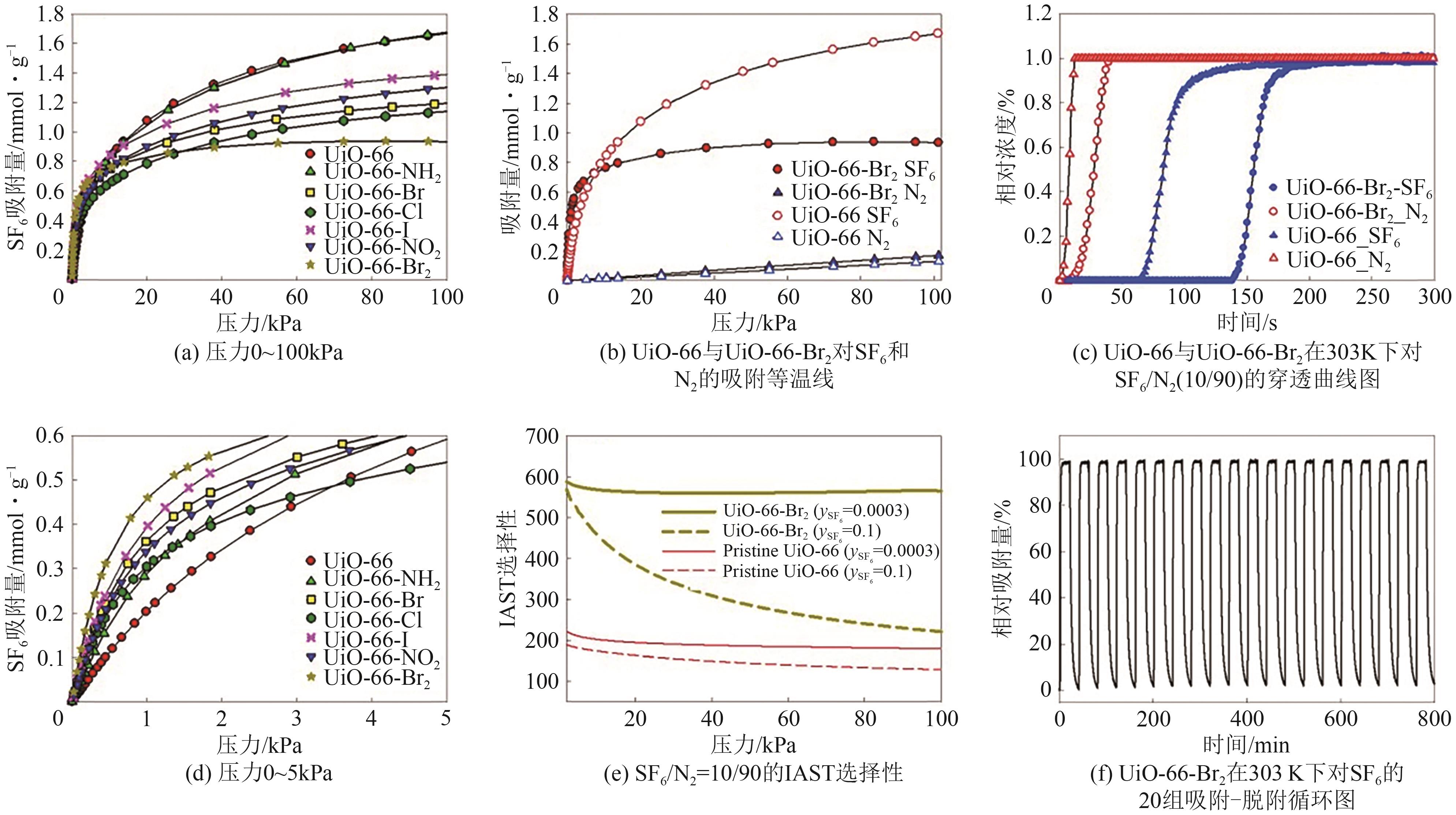
| UiO-66 | 1.67 | 0.80 | 0.12 | 140 | [ |
| UiO-66-NH2 | 1.67 | 0.80 | — | — | [ |
| UiO-66-Br | 1.20 | 0.73 | — | — | [ |
| UiO-66-Cl | 1.15 | 0.62 | — | — | [ |
| UiO-66-I | 1.37 | 0.80 | — | — | [ |
| UiO-66-NO2 | 1.31 | 0.72 | — | — | [ |
| UiO-66-Br2 | 0.92 | 0.73 | 0.14 | 220 | [ |
| Ni(pba)2 | 3.47 | 1.69 | 0.33 | 160 | [ |
| Ni(3-mpba)2 | 2.83 | 1.79 | 0.25 | 221 | [ |
| Co(3-mpba)2 | 3.25 | 1.77 | 0.34 | 161 | [ |
| Zn(BDC)(DABCO)0.5 | 3.48 | 0.57 | 0.15 | 32 | [ |
| Zn(DMBDC)(DABCO)0.5 | 4.77 | 1.40 | 0.15 | 119 | [ |
| Zn(TMBDC)(DABCO)0.5 | 4.61 | 2.48 | 0.33 | 239 | [ |
| Ni(ina)(bdc)0.5 | 2.25 | 0.78 | 0.18 | 53 | [ |
| Ni(3-min)(bdc)0.5 | 1.81 | 1.05 | 0.19 | 91 | [ |
| ZIF-8 | 0.15② | 0.01② | 0.10② | 1.69② | [ |
| ZIF-70.06-80.94 | 0.20② | 0.02② | 0.17② | 1.31② | [ |
| ZIF-70.20-80.80 | 1.40② | 0.29② | 0.17② | 21② | [ |
| ZIF-70.26-80.74 | 2.08② | 0.57② | 0.15② | 40② | [ |
| ZIF-70.37-80.63 | 1.81② | 0.48② | 0.14② | 34② | [ |
| ZIF-70.47-80.53 | 1.56② | 0.34② | 0.12② | 27② | [ |
| ZIF-70.52-80.48 | 1.19② | 0.27② | 0.15② | 22② | [ |
| ZIF-70.90-80.10 | 0.65② | 0.13② | 0.11② | 13② | [ |
| ZIF-70.98-80.02 | 0.51② | 0.13② | 0.09② | 15② | [ |
| ZIF-7 | 0.46② | 0.13② | 0.05② | 22② | [ |
| CAU-10 | 1.00 | 0.68 | 0.16 | 123 | [ |
| CAU-10-Py | 1.76 | 1.13 | 0.20 | 204 | [ |
| Sc-cage-MOF | 1.59 | 0.92 | 0.30 | 8.1 | [ |
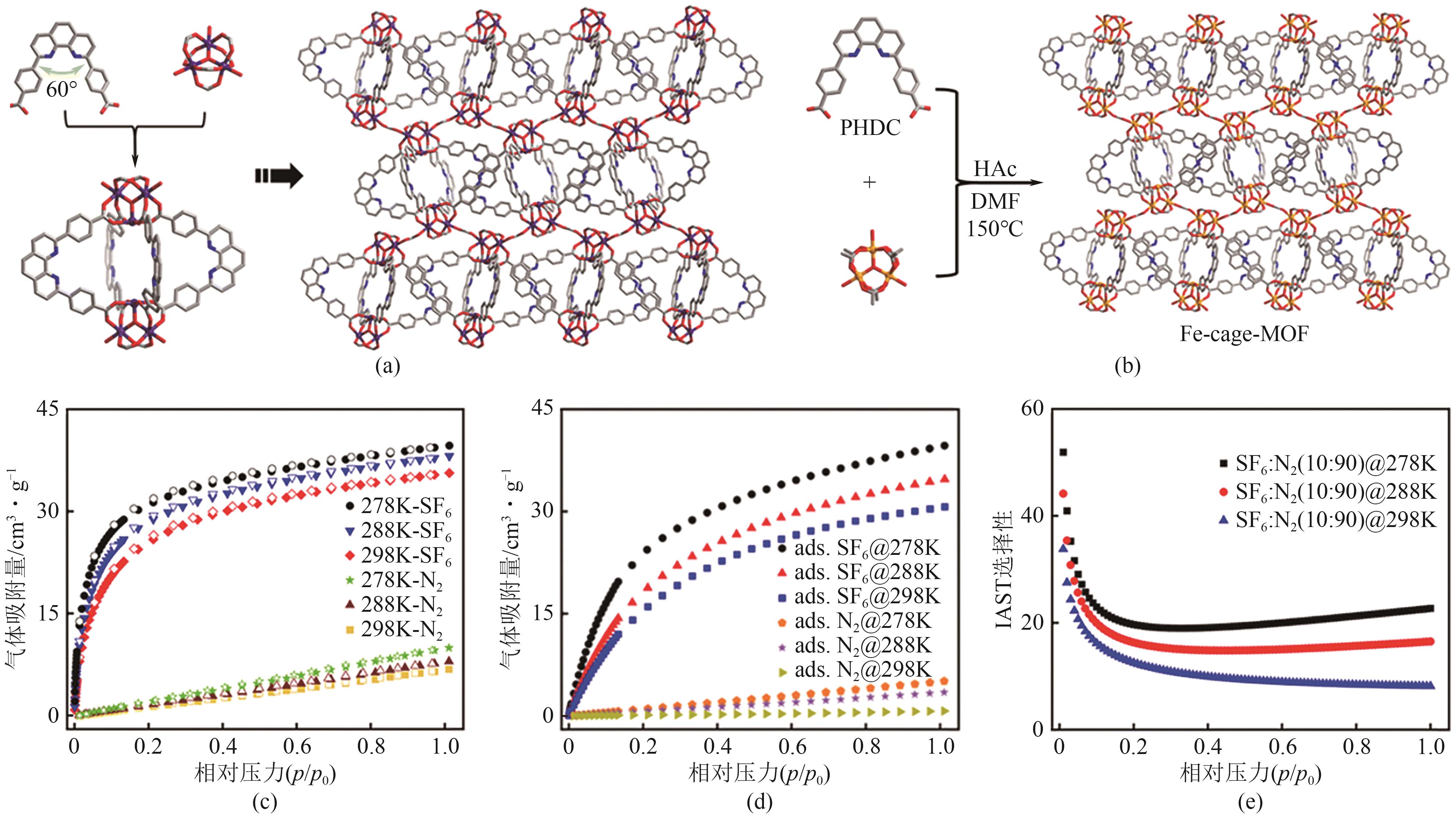
| Fe-cage-MOF | 1.36 | 0.43 | 0.03 | 21 | [ |
| Co3(HCOO)6 | 2.18 | 1.63 | 0.18 | 125 | [ |
| Ni3(HCOO)6 | 1.00 | 0.37 | 0.17 | 19.2 | [ |
| Mn3(HCOO)6 | 1.28 | 1.06 | 0.11 | 263 | [ |
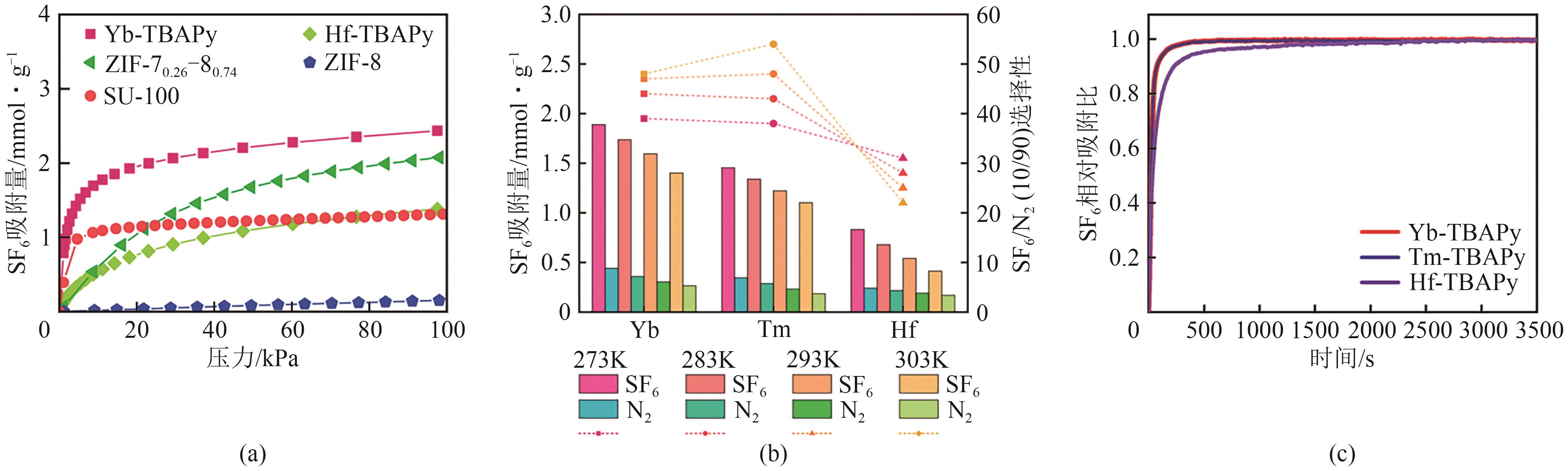
| Hf-TBAPy | 1.38② | 0.54② | 0.21② | 25② | [ |
| Tm-TBAPy | 1.83② | 1.22② | 0.25② | 48② | [ |
| Yb-TBAPy | 2.33② | 1.60② | 0.33② | 47② | [ |
| Ce-TBAPy | 2.16② | 1.47② | 0.31② | 47② | [ |
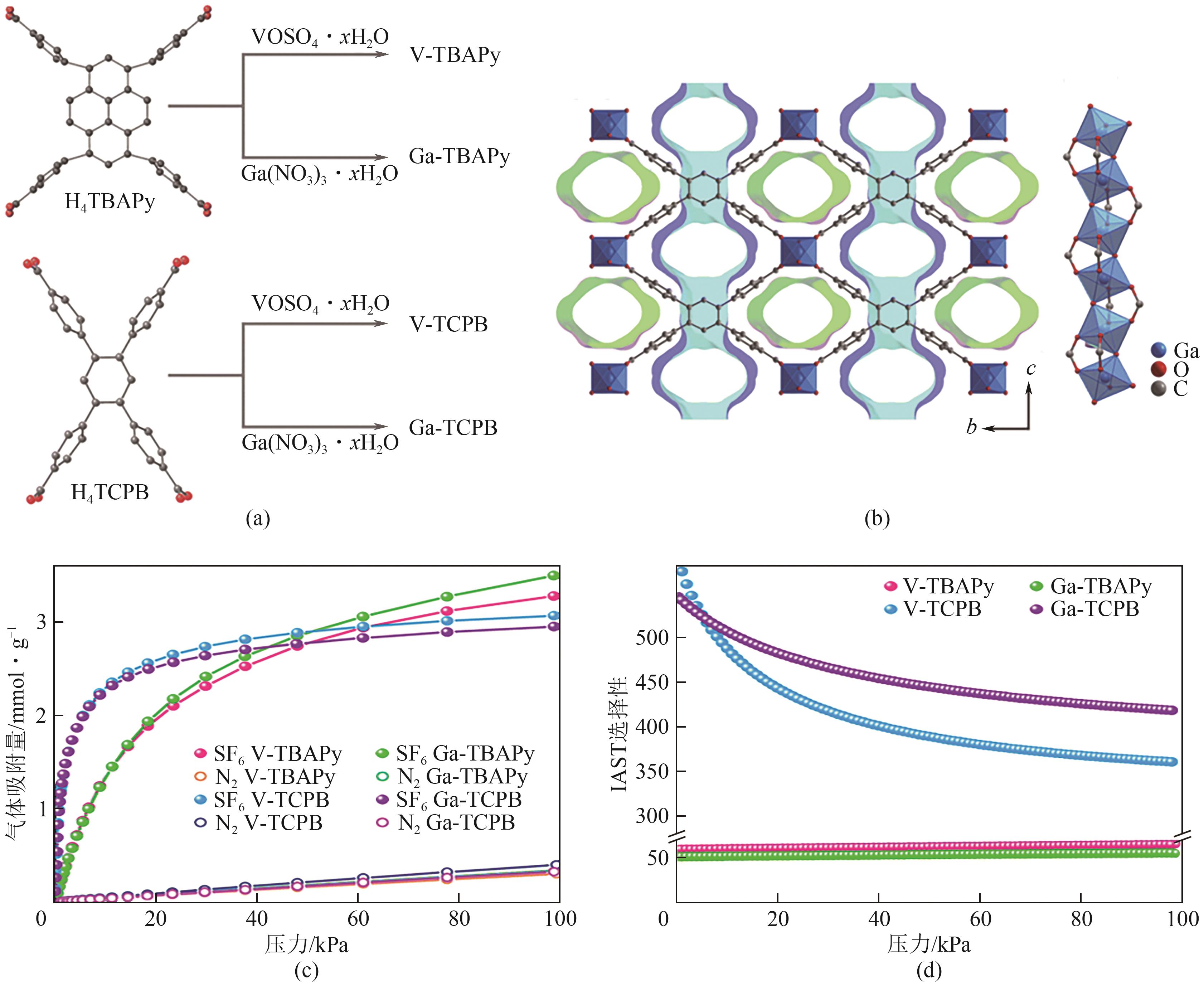
| Ga-TBAPy | 3.50② | 1.33② | 0.36② | 55② | [ |
| V-TBAPy | 3.28② | 1.33② | 0.33② | 65② | [ |
| Ga-TCPB | 3.07② | 2.26② | 0.33② | 418② | [ |
| V-TCPB | 2.95② | 2.29② | 0.36② | 361② | [ |
| Cu(peba)2 | 2.36 | 0.14 | 0.19 | 18 | [ |
| Ni(pba)2 | 3.50 | 1.67 | 0.26 | 201 | [ |
| Ni(ina)2 | 2.84 | 2.39 | 0.49 | 375 | [ |
| SNNU-202 | 4.55 | 0.33 | 0.12 | 9 | [ |
| SNNU-203 | 5.09 | 0.45 | 0.14 | 27 | [ |
| SNNU-204 | 6.92 | 0.72 | 0.13 | 49 | [ |
| Cu-MOF-NH2 | 7.88 | 3.39 | 0.27 | 266 | [ |
| TKL-107 | 3.97 | 1.26 | 0.19 | 180 | [ |
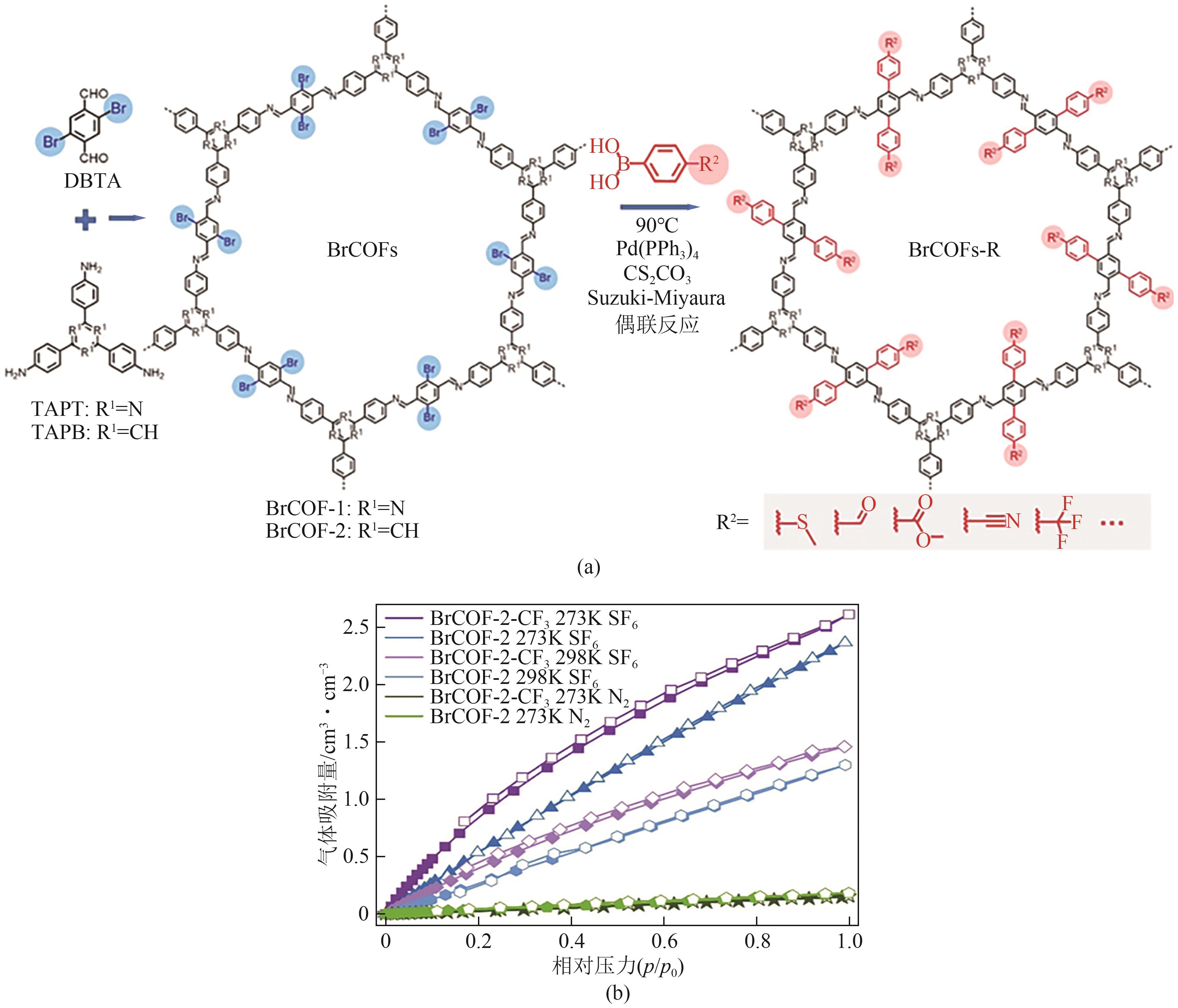
| BrCOF-2 | 1.25 | 0.13 | 0.17① | 17① | [ |
| BrCOF-2-CF3 | 1.45 | 0.24 | 0.16① | 39① | [ |
| 3D-TMTAPB-COF | 2.72 | 1.19 | 0.17 | 335 | [ |
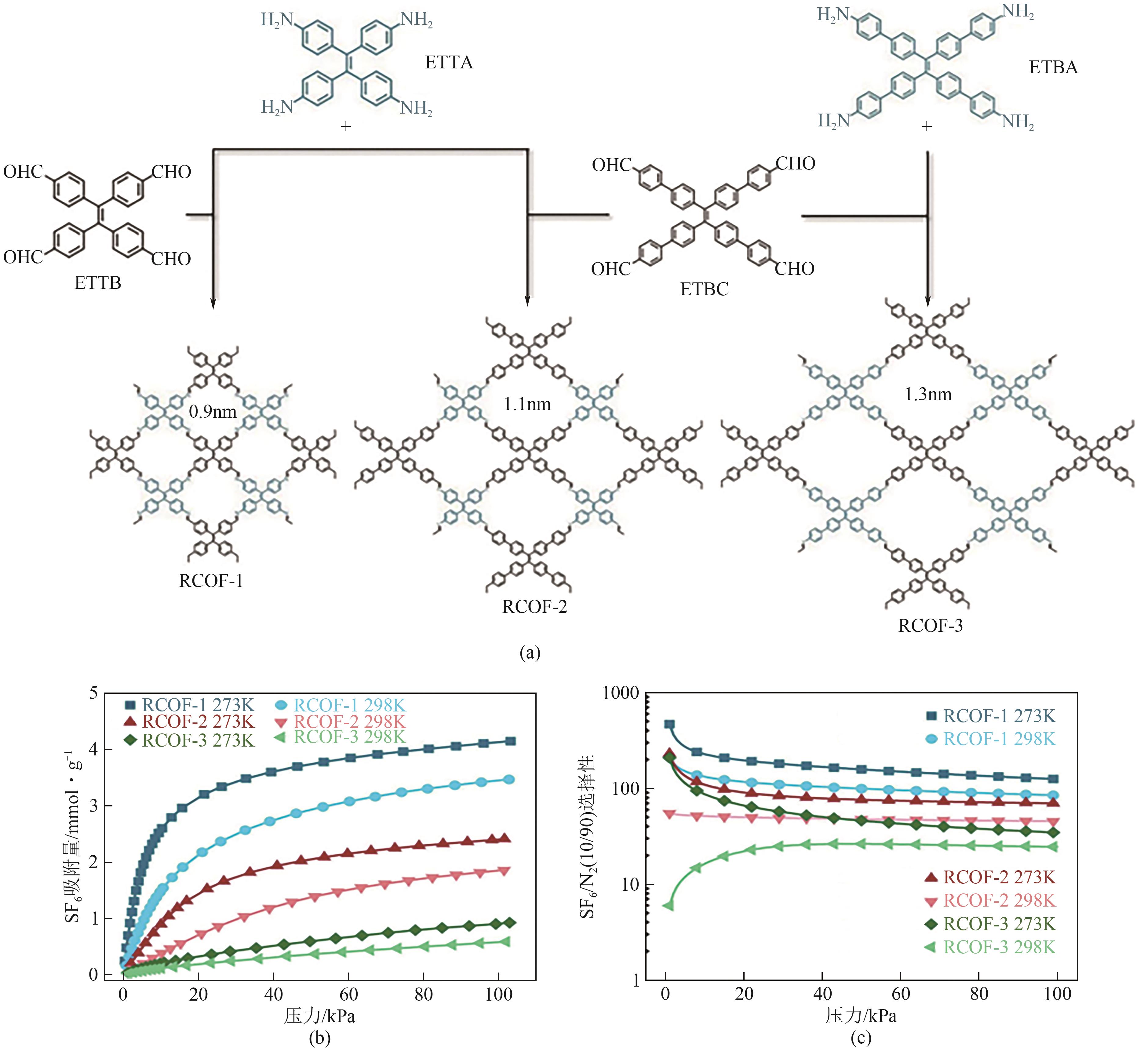
| RCOF-1 | 3.46 | 1.50 | — | 83 | [ |
| RCOF-1-2 | 2.97 | 1.29 | — | 78 | [ |
| RCOF-1-3 | 2.49 | 1.08 | — | 88 | [ |
| RCOF-1-4 | 2.42 | 1.13 | — | 88 | [ |
| RCOF-1-5 | 1.11 | 0.50 | — | 52 | [ |
| RCOF-2 | 1.86 | 0.38 | — | 45 | [ |
| RCOF-3 | 0.58 | 0.12 | — | 25 | [ |
| COF-300 | 3.09 | 2.63 | — | 51 | [ |
| ACOF-1 | 2.15 | 0.65 | — | 54 | [ |
| FCOF-1 | 1.08 | 0.25 | — | 14 | [ |
| KFCOF-1 | 0.99 | 0.31 | — | 32 | [ |
| PPN0 | 1.52 | 0.62 | 0.17 | 42 | [ |
| PPN1 | 1.03 | 0.62 | 0.16 | 51 | [ |
| PPN2 | 0.94 | 0.39 | 0.10 | 45 | [ |
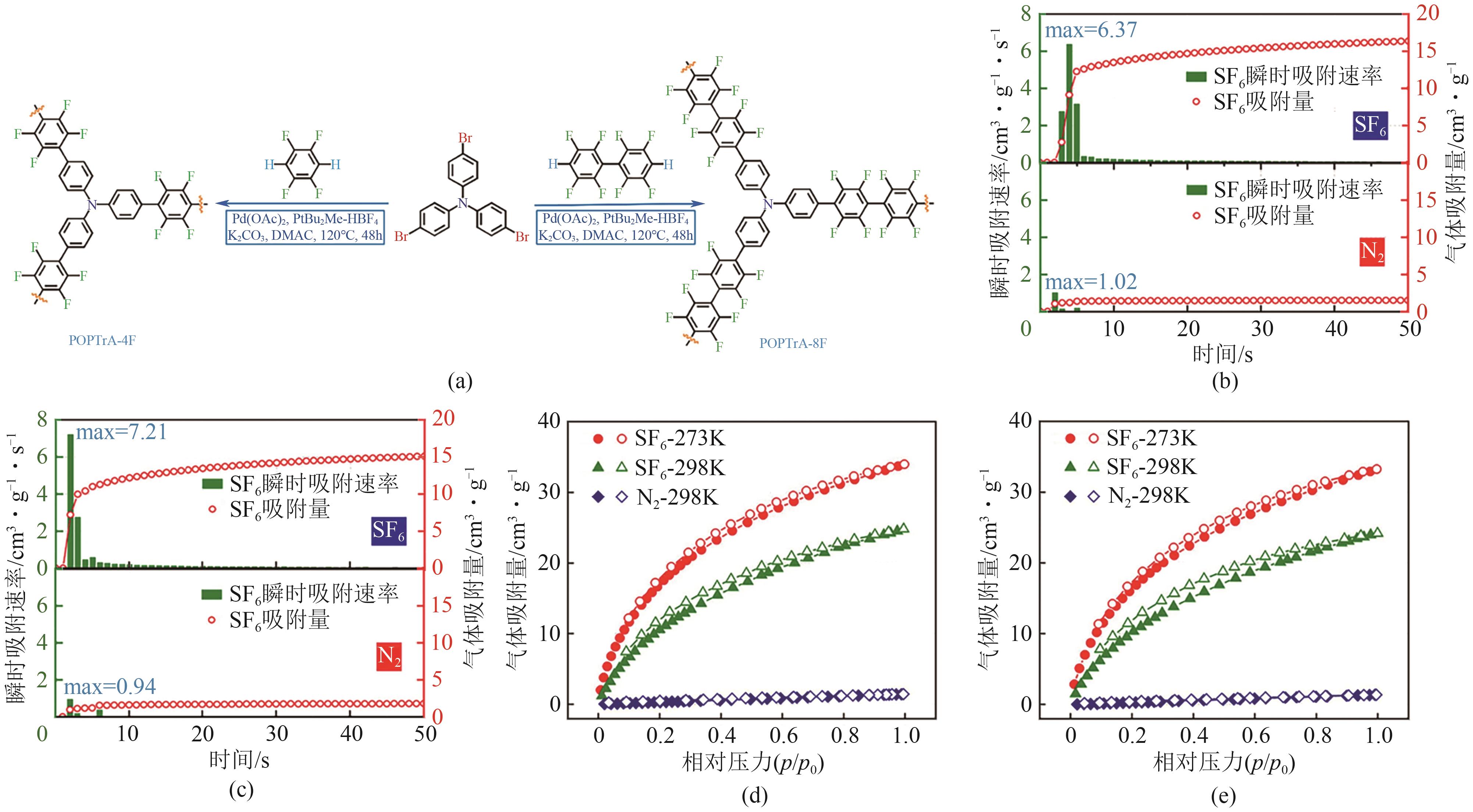
| POPTrA-4F | 1.12 | 0.51 | 0.04 | 62⑤ | [ |
| POPTrA-4F | 1.07 | 0.49 | 0.05 | 27⑤ | [ |
| POPTrB-4F | 1.78 | 0.80 | 0.04 | 63⑤ | [ |
| POPTrB-8F | 1.70 | 0.62 | 0.05 | 52⑤ | [ |
| New-PAF-1 | 45.07④ | 5.6④ | 2.27④ | 35 | [ |
| N-SO3H | 104.5④ | 34.5④ | 5.86④ | 40 | [ |
| PAF-XJTU-1 | 2.10 | — | 0.15 | 27⑤ | [ |
| PAF-XJTU-2 | 2.68 | — | 0.18 | 38⑤ | [ |
| PAF-XJTU-3 | 2.46 | — | 0.18 | 37⑤ | [ |
| PAF-XJTU-4 | 1.61 | — | 0.13 | 24⑤ | [ |
| CC2α | 1.03 | 0.27 | — | — | [ |
| CC3α | 2.29 | 0.88 | 0.14 | 73 | [ |
| CC5α | 1.90 | 0.38 | — | — | [ |
| CC13β | 1.64 | 0.05 | — | — | [ |
| [4[2+3]+6]笼 | 2.46 | 0.79 | — | — | [ |
| SBMOF-1 | 1.02 | 0.92 | 0.17 | 325 | [ |
| SU-100 | 2.07① | 1.86① | 0.51① | 36① | [ |
| CAU-33 | 1.04② | 0.38② | 0.22② | — | [ |
| SU-101 | 1.44② | 0.51② | 0.11② | 40② | [ |
| Ni(ndc)(ted)0.5 | 100④ | 61.9④ | 6.05④ | 750 | [ |
| UU-200 | 1.19② | 0.73② | 0.16② | 45② | [ |
| CTH-18 | 1.92② | 1.53② | 0.50② | 29② | [ |
| Ni(adc)(dabco)0.5 | 2.38 | 2.23 | 0.30 | 919 | [ |
| SIFSIX-2-Cu-i | 7.0③ | — | 0.2③ | 25③ | [ |
| YTU-30 | 68.6④ | 20.8 | 3.3④ | 68 | [ |
表1 目前已报道的吸附SF6及吸附分离SF6/N2的新型多孔材料的主要性能参数
| 新型多孔材料 | 吸附量/mmol·g-1 | IAST选择性 (SF6/N2=10/90) | 数据来源 | ||
|---|---|---|---|---|---|
| 100kPa,SF6 | 10kPa,SF6 | 100kPa,N2 | |||
| Cu3(btc)2 | 4.77 | — | — | — | [ |
| MIL-100(Fe) | 2.94 | — | — | — | [ |
| MIL-101(Cr) | 2.01 | — | — | — | [ |
| Zn4O(dmcpz)3 | 2.54 | — | — | — | [ |
| Co2(1,4-bdc)2(dabco) | 3.39 | — | — | — | [ |
| DUT-9 | 2.32 | — | — | — | [ |
| Zn4O(btb)2 | 3.12 | — | — | — | [ |
| HKUST-1a | 3.59 | 0.98 | 0.25 | 38 | [ |
| HKUST-1b | 4.02 | 1.19 | 0.25 | 50 | [ |
| HKUST-1c | 4.99 | 1.37 | 0.17 | 70 | [ |
| Zn-MOF-74 | 3.73 | 1.28 | 0.30 | 46 | [ |
| Co-MOF-74 | 5.34 | 1.96 | 0.59 | 38 | [ |
| Mg-MOF-74 | 6.42 | 1.82 | 1.06 | 37 | [ |
| CAU-17 | 1.61① | 1.12① | 0.38① | 31① | [ |
| HBU-21 | 0.95 | 0.35 | 0.04 | 184 | [ |
| UiO-67 | 3.87 | — | 0.14 | 22 | [ |
| UiO-66 | 1.67 | 0.80 | 0.12 | 140 | [ |
| UiO-66-NH2 | 1.67 | 0.80 | — | — | [ |
| UiO-66-Br | 1.20 | 0.73 | — | — | [ |
| UiO-66-Cl | 1.15 | 0.62 | — | — | [ |
| UiO-66-I | 1.37 | 0.80 | — | — | [ |
| UiO-66-NO2 | 1.31 | 0.72 | — | — | [ |
| UiO-66-Br2 | 0.92 | 0.73 | 0.14 | 220 | [ |
| Ni(pba)2 | 3.47 | 1.69 | 0.33 | 160 | [ |
| Ni(3-mpba)2 | 2.83 | 1.79 | 0.25 | 221 | [ |
| Co(3-mpba)2 | 3.25 | 1.77 | 0.34 | 161 | [ |
| Zn(BDC)(DABCO)0.5 | 3.48 | 0.57 | 0.15 | 32 | [ |
| Zn(DMBDC)(DABCO)0.5 | 4.77 | 1.40 | 0.15 | 119 | [ |
| Zn(TMBDC)(DABCO)0.5 | 4.61 | 2.48 | 0.33 | 239 | [ |
| Ni(ina)(bdc)0.5 | 2.25 | 0.78 | 0.18 | 53 | [ |
| Ni(3-min)(bdc)0.5 | 1.81 | 1.05 | 0.19 | 91 | [ |
| ZIF-8 | 0.15② | 0.01② | 0.10② | 1.69② | [ |
| ZIF-70.06-80.94 | 0.20② | 0.02② | 0.17② | 1.31② | [ |
| ZIF-70.20-80.80 | 1.40② | 0.29② | 0.17② | 21② | [ |
| ZIF-70.26-80.74 | 2.08② | 0.57② | 0.15② | 40② | [ |
| ZIF-70.37-80.63 | 1.81② | 0.48② | 0.14② | 34② | [ |
| ZIF-70.47-80.53 | 1.56② | 0.34② | 0.12② | 27② | [ |
| ZIF-70.52-80.48 | 1.19② | 0.27② | 0.15② | 22② | [ |
| ZIF-70.90-80.10 | 0.65② | 0.13② | 0.11② | 13② | [ |
| ZIF-70.98-80.02 | 0.51② | 0.13② | 0.09② | 15② | [ |
| ZIF-7 | 0.46② | 0.13② | 0.05② | 22② | [ |
| CAU-10 | 1.00 | 0.68 | 0.16 | 123 | [ |
| CAU-10-Py | 1.76 | 1.13 | 0.20 | 204 | [ |
| Sc-cage-MOF | 1.59 | 0.92 | 0.30 | 8.1 | [ |
| Fe-cage-MOF | 1.36 | 0.43 | 0.03 | 21 | [ |
| Co3(HCOO)6 | 2.18 | 1.63 | 0.18 | 125 | [ |
| Ni3(HCOO)6 | 1.00 | 0.37 | 0.17 | 19.2 | [ |
| Mn3(HCOO)6 | 1.28 | 1.06 | 0.11 | 263 | [ |
| Hf-TBAPy | 1.38② | 0.54② | 0.21② | 25② | [ |
| Tm-TBAPy | 1.83② | 1.22② | 0.25② | 48② | [ |
| Yb-TBAPy | 2.33② | 1.60② | 0.33② | 47② | [ |
| Ce-TBAPy | 2.16② | 1.47② | 0.31② | 47② | [ |
| Ga-TBAPy | 3.50② | 1.33② | 0.36② | 55② | [ |
| V-TBAPy | 3.28② | 1.33② | 0.33② | 65② | [ |
| Ga-TCPB | 3.07② | 2.26② | 0.33② | 418② | [ |
| V-TCPB | 2.95② | 2.29② | 0.36② | 361② | [ |
| Cu(peba)2 | 2.36 | 0.14 | 0.19 | 18 | [ |
| Ni(pba)2 | 3.50 | 1.67 | 0.26 | 201 | [ |
| Ni(ina)2 | 2.84 | 2.39 | 0.49 | 375 | [ |
| SNNU-202 | 4.55 | 0.33 | 0.12 | 9 | [ |
| SNNU-203 | 5.09 | 0.45 | 0.14 | 27 | [ |
| SNNU-204 | 6.92 | 0.72 | 0.13 | 49 | [ |
| Cu-MOF-NH2 | 7.88 | 3.39 | 0.27 | 266 | [ |
| TKL-107 | 3.97 | 1.26 | 0.19 | 180 | [ |
| BrCOF-2 | 1.25 | 0.13 | 0.17① | 17① | [ |
| BrCOF-2-CF3 | 1.45 | 0.24 | 0.16① | 39① | [ |
| 3D-TMTAPB-COF | 2.72 | 1.19 | 0.17 | 335 | [ |
| RCOF-1 | 3.46 | 1.50 | — | 83 | [ |
| RCOF-1-2 | 2.97 | 1.29 | — | 78 | [ |
| RCOF-1-3 | 2.49 | 1.08 | — | 88 | [ |
| RCOF-1-4 | 2.42 | 1.13 | — | 88 | [ |
| RCOF-1-5 | 1.11 | 0.50 | — | 52 | [ |
| RCOF-2 | 1.86 | 0.38 | — | 45 | [ |
| RCOF-3 | 0.58 | 0.12 | — | 25 | [ |
| COF-300 | 3.09 | 2.63 | — | 51 | [ |
| ACOF-1 | 2.15 | 0.65 | — | 54 | [ |
| FCOF-1 | 1.08 | 0.25 | — | 14 | [ |
| KFCOF-1 | 0.99 | 0.31 | — | 32 | [ |
| PPN0 | 1.52 | 0.62 | 0.17 | 42 | [ |
| PPN1 | 1.03 | 0.62 | 0.16 | 51 | [ |
| PPN2 | 0.94 | 0.39 | 0.10 | 45 | [ |
| POPTrA-4F | 1.12 | 0.51 | 0.04 | 62⑤ | [ |
| POPTrA-4F | 1.07 | 0.49 | 0.05 | 27⑤ | [ |
| POPTrB-4F | 1.78 | 0.80 | 0.04 | 63⑤ | [ |
| POPTrB-8F | 1.70 | 0.62 | 0.05 | 52⑤ | [ |
| New-PAF-1 | 45.07④ | 5.6④ | 2.27④ | 35 | [ |
| N-SO3H | 104.5④ | 34.5④ | 5.86④ | 40 | [ |
| PAF-XJTU-1 | 2.10 | — | 0.15 | 27⑤ | [ |
| PAF-XJTU-2 | 2.68 | — | 0.18 | 38⑤ | [ |
| PAF-XJTU-3 | 2.46 | — | 0.18 | 37⑤ | [ |
| PAF-XJTU-4 | 1.61 | — | 0.13 | 24⑤ | [ |
| CC2α | 1.03 | 0.27 | — | — | [ |
| CC3α | 2.29 | 0.88 | 0.14 | 73 | [ |
| CC5α | 1.90 | 0.38 | — | — | [ |
| CC13β | 1.64 | 0.05 | — | — | [ |
| [4[2+3]+6]笼 | 2.46 | 0.79 | — | — | [ |
| SBMOF-1 | 1.02 | 0.92 | 0.17 | 325 | [ |
| SU-100 | 2.07① | 1.86① | 0.51① | 36① | [ |
| CAU-33 | 1.04② | 0.38② | 0.22② | — | [ |
| SU-101 | 1.44② | 0.51② | 0.11② | 40② | [ |
| Ni(ndc)(ted)0.5 | 100④ | 61.9④ | 6.05④ | 750 | [ |
| UU-200 | 1.19② | 0.73② | 0.16② | 45② | [ |
| CTH-18 | 1.92② | 1.53② | 0.50② | 29② | [ |
| Ni(adc)(dabco)0.5 | 2.38 | 2.23 | 0.30 | 919 | [ |
| SIFSIX-2-Cu-i | 7.0③ | — | 0.2③ | 25③ | [ |
| YTU-30 | 68.6④ | 20.8 | 3.3④ | 68 | [ |
| 活性炭与分子筛 | 吸附量/mmol·g-1 | IAST选择性 (SF6/N2=10/90) | 数据来源 | ||
|---|---|---|---|---|---|
| 100kPa,SF6 | 10kPa,SF6 | 100kPa,N2 | |||
| CNHs | 3.56⑥ | 1.30⑥ | 0.36⑥ | 44⑥ | [ |
| 13X | 1.96 | 0.99 | 0.33 | 56.5 | [ |
| 商用AC | 2.50 | 1.00 | — | 45.0 | [ |
| PC-CaCit | 3.61 | 1.05 | 0.33 | 30 | [ |
| PC-MgCit | 3.34 | 0.92 | 0.31 | 30 | [ |
| 高硅沸石 | 1.99① | 1.73① | — | — | [ |
| ACK0 | 2.71 | 0.99 | 0.23 | 481 | [ |
| ACK1 | 3.10 | 1.96 | 0.23 | 684 | [ |
| ACK2 | 3.03 | 1.96 | 0.40 | 591 | [ |
| ACK3 | 3.26 | 1.98 | 0.42 | 473 | [ |
| ACK4 | 2.68 | 1.52 | 0.40 | 260 | [ |
| PC-700 | 2.67 | 1.74 | 0.40 | 150 | [ |
| PC-750 | 4.09 | 2.17 | 0.45 | 436 | [ |
| PC-800 | 4.82 | 1.64 | 0.30 | 331 | [ |
| MFI-1 | 1.45 | 0.86 | 0.96 | 106 | [ |
| MFI-2 | 1.28 | 0.71 | 0.89 | 78 | [ |
| Carbosieve G | 3.30② | 1.76② | — | — | [ |
| Westvaco | 1.96③ | 0.61② | — | — | [ |
| Maxsorb | 5.40④ | 1.87④ | — | — | [ |
| 模板衍生碳 | 0.75 | 0.27 | — | — | [ |
| AC-1 | 2.86⑤ | — | 0.29⑤ | — | [ |
| ZX | 1.54⑤ | — | 0.25⑤ | — | [ |
表2 目前已报道的吸附SF6及吸附分离SF6/N2的活性炭与分子筛的主要性能参数
| 活性炭与分子筛 | 吸附量/mmol·g-1 | IAST选择性 (SF6/N2=10/90) | 数据来源 | ||
|---|---|---|---|---|---|
| 100kPa,SF6 | 10kPa,SF6 | 100kPa,N2 | |||
| CNHs | 3.56⑥ | 1.30⑥ | 0.36⑥ | 44⑥ | [ |
| 13X | 1.96 | 0.99 | 0.33 | 56.5 | [ |
| 商用AC | 2.50 | 1.00 | — | 45.0 | [ |
| PC-CaCit | 3.61 | 1.05 | 0.33 | 30 | [ |
| PC-MgCit | 3.34 | 0.92 | 0.31 | 30 | [ |
| 高硅沸石 | 1.99① | 1.73① | — | — | [ |
| ACK0 | 2.71 | 0.99 | 0.23 | 481 | [ |
| ACK1 | 3.10 | 1.96 | 0.23 | 684 | [ |
| ACK2 | 3.03 | 1.96 | 0.40 | 591 | [ |
| ACK3 | 3.26 | 1.98 | 0.42 | 473 | [ |
| ACK4 | 2.68 | 1.52 | 0.40 | 260 | [ |
| PC-700 | 2.67 | 1.74 | 0.40 | 150 | [ |
| PC-750 | 4.09 | 2.17 | 0.45 | 436 | [ |
| PC-800 | 4.82 | 1.64 | 0.30 | 331 | [ |
| MFI-1 | 1.45 | 0.86 | 0.96 | 106 | [ |
| MFI-2 | 1.28 | 0.71 | 0.89 | 78 | [ |
| Carbosieve G | 3.30② | 1.76② | — | — | [ |
| Westvaco | 1.96③ | 0.61② | — | — | [ |
| Maxsorb | 5.40④ | 1.87④ | — | — | [ |
| 模板衍生碳 | 0.75 | 0.27 | — | — | [ |
| AC-1 | 2.86⑤ | — | 0.29⑤ | — | [ |
| ZX | 1.54⑤ | — | 0.25⑤ | — | [ |
| [1] | RAVISHANKARA A R, SOLOMON S, TURNIPSEED A A, et al. Atmospheric lifetimes of long-lived halogenated species[J]. Science, 1993, 259(5092): 194-199. |
| [2] | CHRISTOPHOROU L G, VANBRUNT R J. SF6/N2 mixtures: basic and HV insulation properties[J]. IEEE Transactions on Dielectrics and Electrical Insulation, 1995, 2(5): 952-1003. |
| [3] | MONTZKA S A, DLUGOKENCKY E J, BUTLER J H. Non-CO2 greenhouse gases and climate change[J]. Nature, 2011, 476(7358): 43-50. |
| [4] | STENNING A H, MARTIN C B. An analytical and experimental study of air lift pump performance[J]. Journal of Engineering for Power, 1968, 90: 106-110. |
| [5] | XU Gang, LIANG Fei Fei, YANG Yong Ping, et al. An improved CO2 separation and purification system based on cryogenic separation and distillation theory[J]. Energies, 2014, 7(5): 3484-3502. |
| [6] | MALEK A H, FAROOQ S. Hydrogen purification from refinery fuel gas by pressure swing adsorption[J]. AIChE Journal, 1998, 44(9): 1985-1992. |
| [7] | TAKASE Atsushi, KANOH Hirofumi, OHBA Tomonori. Wide carbon nanopores as efficient sites for the separation of SF6 from N2 [J]. Scientific Reports, 2015, 5: 11994. |
| [8] | CHIANG Yu Chun, WU Po Yun. Adsorption equilibrium of sulfur hexafluoride on multi-walled carbon nanotubes[J]. Journal of Hazardous Materials, 2010, 178(/2/3): 729-738. |
| [9] | CAO D V, SIRCAR S. Heat of adsorption of pure sulfur hexafluoride on micro-mesoporous adsorbents[J]. Adsorption-journal of the International Adsorption Society, 2001, 7(1): 73-80. |
| [10] | DUNNE J A, MARIWALS R, RAO M, et al. Calorimetric heats of adsorption and adsorption isotherms. 1. O2, N2, Ar, CO2, CH4, C2H6 and SF6 on silicalite[J]. Langmuir, 1996, 12(24): 5888-5895. |
| [11] | YAGHI O M, LI G M, LI H L. Selective binding and removal of guests in a microporous metal-organic framework[J]. Nature, 1995, 378(6558): 703-706. |
| [12] | FAN Weidong, ZHANG Xiurong, KANG Zixi, et al. Isoreticular chemistry within metal-organic frameworks for gas storage and separation[J]. Coordination Chemistry Reviews, 2021, 443: 213968. |
| [13] | FURUKAWA Hiroyasu, YAGHI Omar M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications[J]. Journal of the American Chemical Society, 2009, 131(25): 8875-8883. |
| [14] | DING Sanyuan, WANG Wei. Covalent organic frameworks (COFs): From design to applications[J]. Chemical Society Reviews, 2013, 42(2): 548-568. |
| [15] | LIU Ying, DAI Juanjuan, ZHANG Zhiguo, et al. Crystal structure transformation in hydrogen-bonded organic frameworks via ion exchange[J]. Chemistry: An Asian Journal, 2021, 16(23): 3978-3984. |
| [16] | LIN Ruibiao, HE Yabing, LI Peng, et al. Multifunctional porous hydrogen-bonded organic framework materials[J]. Chemical Society Reviews, 2019, 48(5): 1362-1389. |
| [17] | HASELL Tom, COOPER Andrew I. Porous organic cages: soluble, modular and molecular pores[J]. Nature Reviews Materials, 2016, 1(9): 16053. |
| [18] | LIU Ying, WU Hui, GUO Lidong, et al. Hydrogen-bonded metal-nucleobase frameworks for efficient separation of xenon and krypton[J]. Angewandte Chemie International Edition, 2022, 61(11): 202117609. |
| [19] | LI Li Bo, LIN Rui Biao, KRISHNA Rajamani, et al. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites[J]. Science, 2018, 362(6413): 443-446. |
| [20] | ZHOU Jingyi, KE Tian, STEINKE Felix, et al. Tunable confined aliphatic pore environment in robust metal-organic frameworks for efficient separation of gases with a similar structure[J]. Journal of the American Chemical Society, 2022, 144(31): 14322-14329. |
| [21] | ZENG Heng, XIE Mo, WANG Ting, et al. Orthogonal-array dynamic molecular sieving of propylene/propane mixtures[J]. Nature, 2021, 595(7868): 542-548. |
| [22] | ZHU Xiaoqian, KE Tian, ZHOU Jingyi, et al. Vertex strategy in layered 2D MOFs: Simultaneous improvement of thermodynamics and kinetics for record C2H2/CO2 separation performance[J]. Journal of the American Chemical Society, 2023, 145(16): 9254-9263. |
| [23] | ZHENG Fang, CHEN Rundao, DING Zexiang, et al. Interlayer symmetry control in flexible-robust layered metal-organic frameworks for highly efficient C2H2/CO2 Separation[J]. Journal of the American Chemical Society, 2023, 145(36): 19903-19911. |
| [24] | WANG Pengfei, TENG Ying, ZHU Jinlong, et al. Review on the synergistic effect between metal-organic frameworks and gas hydrates for CH4 storage and CO2 separation applications[J]. Renewable & Sustainable Energy Reviews, 2022, 167: 112807. |
| [25] | Felix SAHAYARAJ A, Joy PRABU H, MANIRAJ J, et al. Metal-organic frameworks (MOFs): The next generation of materials for catalysis, gas storage, and separation[J]. Journal of Inorganic and Organometallic Polymers and Materials, 2023, 33(7): 1757-1781. |
| [26] | WU Dan, ZHANG Pengfeng, YANG Guoping, et al. Supramolecular control of MOF pore properties for the tailored guest adsorption/separation applications[J]. Coordination Chemistry Reviews, 2021, 434: 213709. |
| [27] | NANDASIRI Manjula I, JAMBOVANE Sachin R, Peter MCGRAIL B, et al. Adsorption, separation, and catalytic properties of densified metal-organic frameworks[J]. Coordination Chemistry Reviews, 2016, 311: 38-52. |
| [28] | LEE Gyudong, YOO Dong Kyu, AHMED Imteaz, et al. Metal-organic frameworks composed of nitro groups: Preparation and applications in adsorption and catalysis[J]. Chemical Engineering Journal, 2023, 451: 138538. |
| [29] | LEE Jeong Yong, FARHA Omar K, ROBERTS John, et al. Metal-organic framework materials as catalysts[J]. Chemical Society Reviews, 2009, 38(5): 1450-1459. |
| [30] | SHEN Yu, PAN Ting, WANG Liu, et al. Programmable logic in metal-organic frameworks for catalysis[J]. Advanced Materials, 2021, 33(46): 2007442. |
| [31] | LIU Chunsen, LI Jingjing, PANG Huan. Metal-organic framework-based materials as an emerging platform for advanced electrochemical sensing[J]. Coordination Chemistry Reviews, 2020, 410: 213222. |
| [32] | RICE Allison M, MARTIN Corey R, GALITSKIY Vladimir A, et al. Photophysics modulation in photoswitchable metal-organic frameworks[J]. Chemical Reviews, 2020, 120(16): 8790-8813. |
| [33] | ALLENDORF Mark D, DONG Renhao, FENG Xinliang, et al. Electronic devices using open framework materials[J]. Chemical Reviews, 2020, 120(16): 8581-8640. |
| [34] | LIANG Weibin, WIED Peter, CARRARO Francesco, et al. Metal-organic framework-based enzyme biocomposites[J]. Chemical Reviews, 2021, 121(3): 1077-1129. |
| [35] | NATARAJAN Srinivasan, MAHATA Partha. Metal-organic framework structures—How closely are they related to classical inorganic structures?[J]. Chemical Society Reviews, 2009, 38(8): 2304-2318. |
| [36] | THALLAPALLY Praveen K, GRATE Jay W, MOTKURI Radha Kishan. Facile xenon capture and release at room temperature using a metal-organic framework: A comparison with activated charcoal[J]. Chemical Communications, 2012, 48(3): 347-349. |
| [37] | WANG Lisa J, DENG Hexiang, FURUKAWA Hiroyasu, et al. Synthesis and characterization of metal-organic framework-74 containing 2, 4, 6, 8, and 10 different metals[J]. Inorganic Chemistry, 2014, 53(12): 5881-5883. |
| [38] | EDDAOUDI M, KIM J, ROSI N, et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage[J]. Science, 2002, 295(5554): 469-472. |
| [39] | YANG Huajun, PENG Fang, DANG Candy, et al. Ligand charge separation to build highly stable quasi-isomer of MOF-74-Zn[J]. Journal of the American Chemical Society, 2019, 141(25): 9808-9812. |
| [40] | PEI Jiyan, WANG Jiaxin, SHAO Kai, et al. Engineering microporous ethane-trapping metal-organic frameworks for boosting ethane/ethylene separation[J]. Journal of Materials Chemistry A, 2020, 8(7): 3613-3620. |
| [41] | COLOMBO Valentina, MONTORO Carmen, MASPERO Angelo, et al. Tuning the adsorption properties of isoreticular pyrazolate-based metal-organic frameworks through ligand modification[J]. Journal of the American Chemical Society, 2012, 134(30): 12830-12843. |
| [42] | LIU Jian, THALLAPALLY Praveen K, STRACHAN Denis. Metal-organic frameworks for removal of Xe and Kr from nuclear fuel reprocessing plants[J]. Langmuir, 2012, 28(31): 11584-11589. |
| [43] | SENKOVSKA Irena, BAREA Elisa, NAVARRO Navarro Jorge Andres, et al. Adsorptive capturing and storing greenhouse gases such as sulfur hexafluoride and carbon tetrafluoride using metal-organic frameworks[J]. Microporous and Mesoporous Materials, 2012, 156: 115-120. |
| [44] | CHUAH Chong Yang, Kun Li GOH, Tae Hyun BAE. Hierarchically structured HKUST-1 nanocrystals for enhanced SF6 capture and recovery[J]. Journal of Physical Chemistry C, 2017, 121(12): 6748-6755. |
| [45] | KIM Min Bum, LEE Seung Joon, LEE Chang Yeon, et al. High SF6 selectivities and capacities in isostructural metal-organic frameworks with proper pore sizes and highly dense unsaturated metal sites[J]. Microporous and Mesoporous Materials, 2014, 190: 356-361. |
| [46] | Milan KÖPPEN, DHAKSHINAMOORTHY Amarajothi, Ken INGE A, et al. Synthesis, transformation, catalysis, and gas sorption investigations on the bismuth metal-organic framework CAU-17[J]. European Journal of Inorganic Chemistry, 2018, (30): 3496-3503. |
| [47] | YAN Jiangwen, GANG Shuqi, LIU Ziyue, et al. An In(Ⅲ)-MOF based on pore engineering for efficient capture SF6 from SF6/N2 mixture[J]. Separation and Purification Technology, 2023, 327: 124929. |
| [48] | KIM Min Bum, YOON Tae Ung, HONG Do Young, et al. High SF6/N2 selectivity in a hydrothermally stable zirconium-based metal-organic framework[J]. Chemical Engineering Journal, 2015, 276: 315-321. |
| [49] | KIM Min Bum, KIM Tea Hoon, YOON Tae Ung, et al. Efficient SF6/N2 separation at high pressures using a zirconium-based mesoporous metal-organic framework[J]. Journal of Industrial and Engineering Chemistry, 2020, 84: 179-184. |
| [50] | KIM Min Bum, KIM Kyung Min, KIM Tea Hoon, et al. Highly selective adsorption of SF6 over N2 in a bromine-functionalized zirconium-based metal-organic framework[J]. Chemical Engineering Journal, 2018, 339: 223-229. |
| [51] | ZHENG Sutao, JIANG Runyuan, JIANG Yu, et al. Methyl-functionalized microporous metal-organic framework for efficient SF6/N2 separation[J]. Separation and Purification Technology, 2023, 318: 123957. |
| [52] | YAN Le, ZHENG Huiting, SONG Liang, et al. Methyl-functionalized flexible ultra-microporous MOF for efficient SF6/N2 mixture separation[J]. Chemical Engineering Journal, 2023, 472: 145145. |
| [53] | LIU Haoran, WANG Shaomin, DONG Yongli, et al. Control of pore environment in nickel-based metal-organic frameworks for SF6/N2 separation[J]. Chinese Journal of Structural Chemistry, 2023, 42(2): 100022. |
| [54] | Michelle ÅHLÉN, JAWORSKI Aleksander, STROMME Maria, et al. Selective adsorption of CO2 and SF6 on mixed-linker ZIF-7-8s: The effect of linker substitution on uptake capacity and kinetics[J]. Chemical Engineering Journal, 2021, 422: 130117. |
| [55] | HU Yongqi, WANG Lingyao, Ruihan NAN, et al. Pore engineering in cost-effective and stable Al-MOFs for efficient capture of the greenhouse gas SF6 [J]. Chemical Engineering Journal, 2023, 471: 144851. |
| [56] | WANG Hao, SHI Le, XIONG Zhangyi, et al. A two-dimensional metal-organic framework assembled from scandium-based cages for the selective capture of sulfur hexafluoride[J]. Chemical Communications, 2024, 60(17): 2397-2400. |
| [57] | WANG Hao, SHI Le, CAO Honghao, et al. Synthesis of an iron-based metal-organic framework with octahedral cages for the selective capture of sulfur hexafluoride[J]. CrystEngComm, 2024, 26(13): 1912-1916. |
| [58] | WU Yue, YAN Tong, ZHANG Wenxiang, et al. Adsorption interface-induced H…F charge transfer in ultramicroporous metal-organic frameworks for perfluorinated gas separation[J]. Industrial & Engineering Chemistry Research, 2022, 61(36): 13603-13611. |
| [59] | Michelle ÅHLÉN, Francoise M Amombo NOA, Lars ÖHRSTRÖM, et al. Pore size effect of 1,3,6,8-tetrakis(4-carboxyphenyl)pyrene-based metal-organic frameworks for enhanced SF6 adsorption with high selectivity[J]. Microporous and Mesoporous Materials, 2022, 343: 112161. |
| [60] | Michelle AHLÉN, ZHOU Yi, HEDBOM Daniel, et al. Efficient SF6 capture and separation in robust gallium- and vanadium-based metal-organic frameworks[J]. Journal of Materials Chemistry A, 2023, 11(48): 26435-26441. |
| [61] | WANG Shaomin, MU Xuantong, LIU Haoran, et al. Pore-structure control in metal-organic frameworks (MOFs) for capture of the greenhouse gas SF6 with record separation[J]. Angewandte Chemie-International Edition, 2022, 61(33): 202207066. |
| [62] | LI Yongpeng, NI Jingjing, LI Shuo, et al. Rational pore-window size control in three Cu-MOFs with different pore environments for efficient capture of the greenhouse gas SF6 [J]. Journal of Solid State Chemistry, 2024, 329: 124443. |
| [63] | REN Jiahao, CHANG Miao, ZENG Wenjiang, et al. Computer-aided discovery of MOFs with calixarene-analogous microenvironment for exceptional SF6 capture[J]. Chemistry of Materials, 2021, 33(13): 5108-5114. |
| [64] | HE Yanjing, CAO Xiaohao, ZHANG Zhengqing, et al. Discovery of high-performing metal-organic frameworks for efficient SF6/N2 separation: a combined computational screening, machine learning, and experimental study[J]. Industrial & Engineering Chemistry Research, 2023, 62(19): 7642-7649. |
| [65] | LIAO Qiaobo, KE Can, HUANG Xin, et al. A versatile method for functionalization of covalent organic frameworks via Suzuki-Miyaura cross-coupling[J]. Angewandte Chemie International Edition, 2021, 60(3): 1411-1416. |
| [66] | YIN Ying, ZHANG Ya, ZHOU Xu, et al. Single-crystal three-dimensional covalent organic framework constructed from 6-connected triangular prism node[J]. Journal of the American Chemical Society, 2023, 145(41): 22329-22334. |
| [67] | LIAO Qiaobo, XU Haocheng, KE Can, et al. Rational regulating pore structures of covalent organic frameworks for sulfur hexafluoride capture and separation[J]. Separation and Purification Technology, 2023, 306: 122595. |
| [68] | ZHENG Xianqiang, SHEN Yanlong, WANG Shitao, et al. Selective adsorption of SF6 in covalent- and metal-organic frameworks[J]. Chinese Journal of Chemical Engineering, 2021, 39: 88-95. |
| [69] | SEKIZKARDES Ali K, WANG Ping, HOFFMAN James, et al. Amine-functionalized porous organic polymers for carbon dioxide capture[J]. Materials Advances, 2022, 3(17): 6668-6686. |
| [70] | TAN Liangxiao, TAN Bi’en. Hypercrosslinked porous polymer materials: design, synthesis, and applications[J]. Chemical Society Reviews, 2017, 46(11): 3322-3356. |
| [71] | CHUAH Chong Yang, YANG Yan Qin, Tae Hyun BAE. Hierarchically porous polymers containing triphenylamine for enhanced SF6 separation[J]. Microporous and Mesoporous Materials, 2018, 272: 232-240. |
| [72] | ZHANG Wenxiang, LI Yinhui, WU Yue, et al. Fluorinated porous organic polymers for efficient recovery perfluorinated electronic specialty gas from exhaust gas of plasma etching[J]. Separation and Purification Technology, 2022, 287: 120561. |
| [73] | ZHANG Wenxiang, WU Yue, LI Yinhui, et al. Fluorine-functionalized porous organic polymers for durable F-gas capture from semiconductor etching exhaust[J]. Macromolecules, 2022, 55(4): 1435-1444. |
| [74] | WANG Shanshan, WU Yue, ZHANG Ying, et al. HF resistant porous aromatic frameworks for electronic special gases separation[J]. Langmuir, 2022, 38(28): 8667-8676. |
| [75] | WU Yue, LI Xiaoyu, LI Yinhui, et al. Porous aromatic frameworks as HF resistant adsorbents for SF6 separation at elevated pressure[J]. Separation and Purification Technology, 2023, 315: 123657. |
| [76] | HASELL Tom, MIKLITZ Marcin, STEPHENSON Andrew, et al. Porous organic cages for sulfur hexafluoride separation[J]. Journal of the American Chemical Society, 2016, 138(5): 1653-1659. |
| [77] | ZHU Qiang, QU Hang, AVCI Gokay, et al. Computationally guided synthesis of a hierarchical [4[2+3]+6] porous organic ‘cage of cages’[J]. Nature Synthesis, 2024, 3: 825-834. |
| [78] | WANG Tongge, CHANG Miao, YAN Tong’an, et al. Calcium-based metal-organic framework for efficient capture of sulfur hexafluoride at low concentrations[J]. Industrial & Engineering Chemistry Research, 2021, 60(16): 5976-5983. |
| [79] | GRAPE Erik Svensson, XU Hongyi, CHEUNG Ocean, et al. Breathing metal-organic framework based on flexible inorganic building units[J]. Crystal Growth & Design, 2020, 20(1): 320-329. |
| [80] | Michelle ÅHLÉN, KAPACA Elina, HEDBOM Daniel, et al. Gas sorption properties and kinetics of porous bismuth-based metal-organic frameworks and the selective CO2 and SF6 sorption on a new bismuth trimesate-based structure UU-200[J]. Microporous and Mesoporous Materials, 2022, 329: 111548. |
| [81] | YANG Mingshan, CHANG Miao, YAN Tong’an, et al. A nickel-based metal-organic framework for efficient SF6/N2 separation with record SF6 uptake and SF6/N2 selectivity[J]. Separation and Purification Technology, 2022, 295: 121340. |
| [82] | Francoise Amombo M NOA, CHEUNG Ocean, Michelle AHLÉN, et al. A hexagon based Mn(Ⅱ) rod metal-organic framework-structure, SF6 gas sorption, magnetism and electrochemistry[J]. Chemical Communications, 2023, 59(15): 2106-2109. |
| [83] | CHANG Miao, Yan Tong’an, WEI Yan, et al. Metal-organic framework-based single-molecule SF6 trap for record SF6 capture[J]. Chemistry of Materials, 2022, 34(20): 9134-9143. |
| [84] | SKARMOUTSOS Ioannis, EDDAOUDI Mohamed, MAURIN Guillaume. Highly tunable sulfur hexafluoride separation by interpenetration control in metal organic frameworks[J]. Microporous and Mesoporous Materials, 2019, 281: 44-49. |
| [85] | LI Yongpeng, ZHANG Xiaojie, NI Jingjing, et al. Design of a highly-stable cobalt (Ⅱ) porous framework based on aromatic stacking strategy for efficient SF6 capture and SF6/N2 mixture separation[J]. Separation and Purification Technology, 2024, 343: 126995. |
| [86] | YANG Yanqin, Kun Li GOH, CHUAH Chong Yang, et al. Sub-Angstrom-level engineering of ultramicroporous carbons for enhanced sulfur hexafluoride capture[J]. Carbon, 2019, 155: 56-64. |
| [87] | SUN Rui, TAI Cheuk Wai, STROMME Maria, et al. Hierarchical porous carbon synthesized from novel porous amorphous calcium or magnesium citrate with enhanced SF6 uptake and SF6/N2 selectivity[J]. ACS Applied Nano Materials, 2019, 2(2): 778-89. |
| [88] | S Sun MATTHEW, SHAH D B., H Xu HEATHER, et al. Adsorption equilibria of C1 to C4 alkanes, CO2, and SF6 on silicalite [J]. Journal of Physical Chemistry B, 1998, 102(8): 1466-1473. |
| [89] | WANG Jian, FU Wenxu, WANG Limei, et al. Modulation of pore structure in a microporous carbon for enhanced adsorption of perfluorinated electron specialty gases with efficient separation[J]. Chemical Engineering Journal, 2023, 477: 147128. |
| [90] | FU Wenxu, WANG Jian, LI Yulin, et al. Highly-efficient separation of SF6/N2 and NF3/N2 with record selectivity on one-step synthesized carbon nanosheet[J]. Separation and Purification Technology, 2024, 330: 125496. |
| [91] | CHUAH Chong Yang, YU Suyeon, NA Kyungsu, et al. Enhanced SF6 recovery by hierarchically structured MFI zeolite[J]. Journal of Industrial and Engineering Chemistry, 2018, 62: 64-71. |
| [92] | JAGIELLO Jacek, BANDOSZ Teresa J, PUTYERA Karol, et al. Adsorption near ambient temperatures of methane, carbon tetrafluoride, and sulfur hexafluoride on commercial activated carbons [J]. Journal of Chemical and Engineering Data, 1995, 40(6): 1288-1292. |
| [93] | JAGIELLO Jacek, BANDOSZ Teresa J, PUTYERA Karol, et al. Micropore structure of template-derived carbons studied using adsorption of gases with different molecular diameters[J]. Journal of the Chemical Society, Faraday Transactions, 1995, 91(17): 2929-2933. |
| [94] | CHO Wan Seon, LEE Kwang Hoon, CHANG Hyang Ja, et al. Evaluation of pressure-temperature swing adsorption for sulfur hexafluoride (SF6) recovery from SF6 and N2 gas mixture[J]. Korean Journal of Chemical Engineering, 2011, 28: 2196-2201. |
| [95] | 朱峰, 宋玉梅, 许一力, 等. 应用金属-有机框架材料吸附分离六氟化硫中八氟丙烷的方法: CN114471468A[P]. 2022-05-13. |
| ZHU Feng, SONG Yu Mei, XU Yi Li, et al. Method for adsorption and separation of octafluoropropane from sulfur hexafluoride using metal-organic framework materials: CN114471468A[P]. 2022-05-13. | |
| [96] | 唐炬, 曾福平, 梁鑫, 等. 两种吸附剂对SF6分解特征组分吸附的实验与分析[J]. 中国电机工程学报, 2013(31): 211-219. |
| Tang Ju, ZENG Fu Ping, LIANG Xin, et al. Experimental and analytical study on the adsorption of characteristic components of SF6 decomposition by two adsorbents[J]. Proceedings of the CSEE, 2013(31): 211-219. | |
| [97] | 刘耀. 沸石多孔材料对SF6分解气体的气敏性和分离性能仿真研究[D]. 哈尔滨: 哈尔滨理工大学, 2022. |
| LIU Yao. Simulation reaserch on insulating gas adsorption and catalytic performance of porous materials with zeolite structure[D]. Harbin University of Science and Technology, 2022. | |
| [98] | 钟理鹏, 汲胜昌, 李金宇, 等. 吸附剂对SF6典型分解产物含量及变化规律的影响[J]. 西安交通大学学报, 2015, 49(2): 86-92. |
| ZHONG Lipeng, JI Shengchang, LI Jinyu, et al. The influence of adsorbents on the content and variation pattern of typical decomposition products of SF6 [J]. Journal of Xi’an Jiaotong University, 2015, 49(2): 86-92. | |
| [99] | 朱登军, 杨镇宁, 杨勇, 等. SF6开关设备微水含量优化处理[J]. 电气开关, 2018, 56(5): 11-13. |
| ZHU Dengjun, YANG Zhenning, YANG Yong, et al. Optimization treatment of micro water content in SF6 switchgear[J]. Electric Switchgear, 2018, 56(5): 11-13. |
| [1] | 罗伊雯, 赵亮, 张宇豪, 刘东阳, 高金森, 徐春明. 轻烃分离材料和机理的研究进展[J]. 化工进展, 2025, 44(5): 2938-2954. |
| [2] | 王雪莉, 杨卫亚, 张会成, 王少军, 凌凤香. 金属有机框架(MOF)基混合基质膜界面改性方法及其气体分离性能[J]. 化工进展, 2025, 44(2): 928-940. |
| [3] | 李斯文, 雷敏, 刘玉霜, 董兆琪, 薛丽丽, 赵建社. 离子液体多酸在燃油氧化脱硫中的研究进展[J]. 化工进展, 2024, 43(6): 3322-3335. |
| [4] | 金彬浩, 朱小倩, 柯天, 张治国, 鲍宗必, 任其龙, 苏宝根, 杨启炜. 芳香烃/环烷烃吸附分离材料研究进展[J]. 化工进展, 2024, 43(4): 1863-1881. |
| [5] | 王璧琮, 潘大伟, 谢锐, 巨晓洁, 刘壮, 汪伟, 褚良银. 复合酶@ZIF-8的制备及其黑米花青素提取性能[J]. 化工进展, 2024, 43(3): 1403-1411. |
| [6] | 薛丽丽, 吴嘉琪, 李壮壮, 李斯文, 王伟, 赵建社. MOFs限域多酸团簇的合成及其成型化研究进展[J]. 化工进展, 2024, 43(12): 6968-6982. |
| [7] | 肖翩翩, 卓超越, 钟瑾荣, 张跃飞. 用于CO2捕获的金属有机框架材料改性研究进展[J]. 化工进展, 2024, 43(12): 6944-6956. |
| [8] | 陈乐, 种海玲, 张致慧, 何明阳, 陈群. CTAB改性Cu-BTC材料的合成及其吸附分离二甲苯异构体的性能[J]. 化工进展, 2024, 43(1): 455-464. |
| [9] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [10] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [11] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [12] | 杨志强, 曾纪珺, 马义丁, 尉涛, 赵波, 刘英哲, 张伟, 吕剑, 李兴文, 张博雅, 唐念, 李丽, 孙东伟. 六氟化硫替代气体的研究现状及未来发展趋势[J]. 化工进展, 2023, 42(8): 4093-4107. |
| [13] | 蒋博龙, 崔艳艳, 史顺杰, 常嘉城, 姜楠, 谭伟强. 过渡金属Co3O4/ZnO-ZIF氧还原催化剂Co/Zn-ZIF模板法制备及其产电性能[J]. 化工进展, 2023, 42(6): 3066-3076. |
| [14] | 朱雅静, 徐岩, 简美鹏, 李海燕, 王崇臣. 金属有机框架材料用于海水提铀的研究进展[J]. 化工进展, 2023, 42(6): 3029-3048. |
| [15] | 毛梦雷, 孟令玎, 高蕊, 孟子晖, 刘文芳. 多孔框架材料固定化酶研究进展[J]. 化工进展, 2023, 42(5): 2516-2535. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||