化工进展 ›› 2025, Vol. 44 ›› Issue (2): 899-913.DOI: 10.16085/j.issn.1000-6613.2024-0153
新型汞离子吸附材料研究进展
张爱京1( ), 王桢钰1, 肖宁宁2, 宋艳娜1, 李军3, 冯江涛1(
), 王桢钰1, 肖宁宁2, 宋艳娜1, 李军3, 冯江涛1( ), 延卫1
), 延卫1
- 1.西安交通大学能源与动力工程学院,西安市固体废物资源化利用重点实验室,陕西 西安 710049
2.西安交通大学人居环境与建筑工程学院,陕西 西安 710049
3.渭南市环境科学研究中心,陕西 渭南 714000
-
收稿日期:2024-01-19修回日期:2024-03-12出版日期:2025-02-25发布日期:2025-03-10 -
通讯作者:冯江涛 -
作者简介:张爱京(1999—),男,硕士研究生,研究方向为环境吸附材料设计。E-mail:zaj409@stu.xjtu.edu.cn。 -
基金资助:国家自然科学基金(52070155)
Research progress on novel adsorption materials for mercury ion
ZHANG Aijing1( ), WANG Zhenyu1, XIAO Ningning2, SONG Yanna1, LI Jun3, FENG Jiangtao1(
), WANG Zhenyu1, XIAO Ningning2, SONG Yanna1, LI Jun3, FENG Jiangtao1( ), YAN Wei1
), YAN Wei1
- 1.School of Energy and Power Engineering, Xi’an Key Laboratory of Solid Waste Recycling and Resource Recovery, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
2.School of Human Settlements and Civil Engineering, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
3.Weinan Environmental Science Research Center Weinan 714000, Shaanxi, China
-
Received:2024-01-19Revised:2024-03-12Online:2025-02-25Published:2025-03-10 -
Contact:FENG Jiangtao
摘要:
汞污染来源广泛、排放量大,已成为世界上最严重的十大污染源之一。在众多汞离子去除技术中,吸附法由于具有高效且经济的特点,成为了较为成熟且应用广泛的处理技术。性能优异的吸附剂是吸附法的关键,而传统的吸附材料吸附性能有限。基于此,本文介绍了近年来报道的金属有机骨架材料(MOFs)、共价有机框架材料(COFs)、共轭微孔聚合物(CMPs)、导电聚合物、二维过渡金属碳(氮)化物(MXenes)和复合材料等新型汞离子吸附材料,详细论述了这些吸附材料的结构特征及其与汞离子的吸附特性之间的关系。总结了水中汞离子吸附材料的发展前景,指出调节材料结构以及添加特定官能团是进一步研发新材料的方向,希望对新型汞离子吸附材料的设计和开发提供一定参考和启发。
中图分类号:
引用本文
张爱京, 王桢钰, 肖宁宁, 宋艳娜, 李军, 冯江涛, 延卫. 新型汞离子吸附材料研究进展[J]. 化工进展, 2025, 44(2): 899-913.
ZHANG Aijing, WANG Zhenyu, XIAO Ningning, SONG Yanna, LI Jun, FENG Jiangtao, YAN Wei. Research progress on novel adsorption materials for mercury ion[J]. Chemical Industry and Engineering Progress, 2025, 44(2): 899-913.
| 名称 | 优点 | 缺点 |
|---|---|---|
| 化学沉淀法 | 适合处理汞含量高的废水 | 易产生大量废渣、处理不彻底、导致二次污染 |
| 离子交换法 | 适用于处理低浓度废水、无二次污染 | 受水中杂质,交换剂种类、用量和成本的影响大 |
| 电化学法 | 操作简单、可直接回收金属汞 | 耗能高、成本高、对低浓度废水处理效果不佳 |
| 生物法 | 高效率、高选择性 | 培育研发时间长、处理条件较 苛刻 |
| 吸附法 | 高效、工艺简单、可进行深度处理、二次污染小 | 吸附剂用量较大 |
表1 水体中汞离子处理方法优缺点对比[9]
| 名称 | 优点 | 缺点 |
|---|---|---|
| 化学沉淀法 | 适合处理汞含量高的废水 | 易产生大量废渣、处理不彻底、导致二次污染 |
| 离子交换法 | 适用于处理低浓度废水、无二次污染 | 受水中杂质,交换剂种类、用量和成本的影响大 |
| 电化学法 | 操作简单、可直接回收金属汞 | 耗能高、成本高、对低浓度废水处理效果不佳 |
| 生物法 | 高效率、高选择性 | 培育研发时间长、处理条件较 苛刻 |
| 吸附法 | 高效、工艺简单、可进行深度处理、二次污染小 | 吸附剂用量较大 |
| 分类 | 优点 | 缺点 |
|---|---|---|
| 金属有机骨架 | 高比表面积、高孔隙率、结构可调节 | 稳定性较差、容易造成金属离子二次污染 |
| 共价有机框架 | 高比表面积、孔结构规则、稳定性好 | 合成较难、成本 较高、难以大量制备 |
| 共轭微孔聚合物 | 高比表面积、孔隙结构 可调节、材料结构可调节 | 合成较为复杂、一定条件下不稳定 |
| 导电聚合物 | 易于合成/功能化、良好的掺杂/脱掺杂性能、良好的热/机械稳定性 | 低比表面积、难以控制链长度和取向、掺杂物容易脱落 |
| MXenes | 高比表面积、表面具有大量活性官能团、层状结构 | 表面官能团易氧化、机械强度差、易层状堆叠 |
| 复合材料 | 结合多种材料的优点 | 制备复杂、吸附 容量较低 |
表2 不同类型材料优缺点对比
| 分类 | 优点 | 缺点 |
|---|---|---|
| 金属有机骨架 | 高比表面积、高孔隙率、结构可调节 | 稳定性较差、容易造成金属离子二次污染 |
| 共价有机框架 | 高比表面积、孔结构规则、稳定性好 | 合成较难、成本 较高、难以大量制备 |
| 共轭微孔聚合物 | 高比表面积、孔隙结构 可调节、材料结构可调节 | 合成较为复杂、一定条件下不稳定 |
| 导电聚合物 | 易于合成/功能化、良好的掺杂/脱掺杂性能、良好的热/机械稳定性 | 低比表面积、难以控制链长度和取向、掺杂物容易脱落 |
| MXenes | 高比表面积、表面具有大量活性官能团、层状结构 | 表面官能团易氧化、机械强度差、易层状堆叠 |
| 复合材料 | 结合多种材料的优点 | 制备复杂、吸附 容量较低 |
| MOFs分类 | 优点 | 缺点 |
|---|---|---|
| 拉瓦锡骨架材料 | 结晶度好、结构可调节、发达的孔隙结构、高比表面积 | 生产成本高、脆性、难以加工 |
| 类沸石咪唑骨架材料 | 高孔隙率、高比表面积、结构可调节、稳定性较好 | 吸附汞离子后容易形成二次金属离子污染、孔径大小不易调控 |
| UiO系列MOFs | 高比表面积、结构可调节、高度稳定性、环境适应性好 | 需要制造表面缺陷 结构来提高吸附容量 |
表3 不同MOFs材料优缺点对比
| MOFs分类 | 优点 | 缺点 |
|---|---|---|
| 拉瓦锡骨架材料 | 结晶度好、结构可调节、发达的孔隙结构、高比表面积 | 生产成本高、脆性、难以加工 |
| 类沸石咪唑骨架材料 | 高孔隙率、高比表面积、结构可调节、稳定性较好 | 吸附汞离子后容易形成二次金属离子污染、孔径大小不易调控 |
| UiO系列MOFs | 高比表面积、结构可调节、高度稳定性、环境适应性好 | 需要制造表面缺陷 结构来提高吸附容量 |
| 分类 | 材料名称 | 吸附容量 /mg·g-1 | Kd/mL·g-1 | 参考 文献 |
|---|---|---|---|---|
| MOFs | ||||
| MILs | SH-MIL-101 | 250.0 | [ | |
| MIL88A-SH | 1111.1 | 1.0×105 | [ | |
| SH-MIL-68(In) | 450.0 | [ | ||
| ZIFs | ZnS-ZIF-8 | 925.9 | [ | |
| ZIF-67 | 1740 | 2.3×104 | [ | |
| ZIF-8 | 2195.1 | 1.8×104 | [ | |
| UiO | UiO-66-SH | 785.0 | [ | |
| UiO-66-DMTD | 670.5 | [ | ||
| COFs | COF-S-SH | 1350 | 2.3×109 | [ |
| COF-SH | 1283 | [ | ||
| JNU-3 | 960 | 1.42×106 | [ | |
| JNU-4 | 561 | [ | ||
| TpTch-90 | 4270 | [ | ||
| TpTch-120 | 4277 | 4.6×106 | [ | |
| CMPs | HCMP-1 | 604 | [ | |
| CMPA-M | 975 | [ | ||
| CMPPDA | 1582.6 | 1.4×105 | [ | |
| 导电聚合物 | PPy/MAA | 1736.8 | [ | |
| PPy@L-Cyst | 2042.7 | [ | ||
| PPyCE | 684.59 | 1.79×105 | [ | |
| PPD4CBA | 1400 | 1.27×105 | [ | |
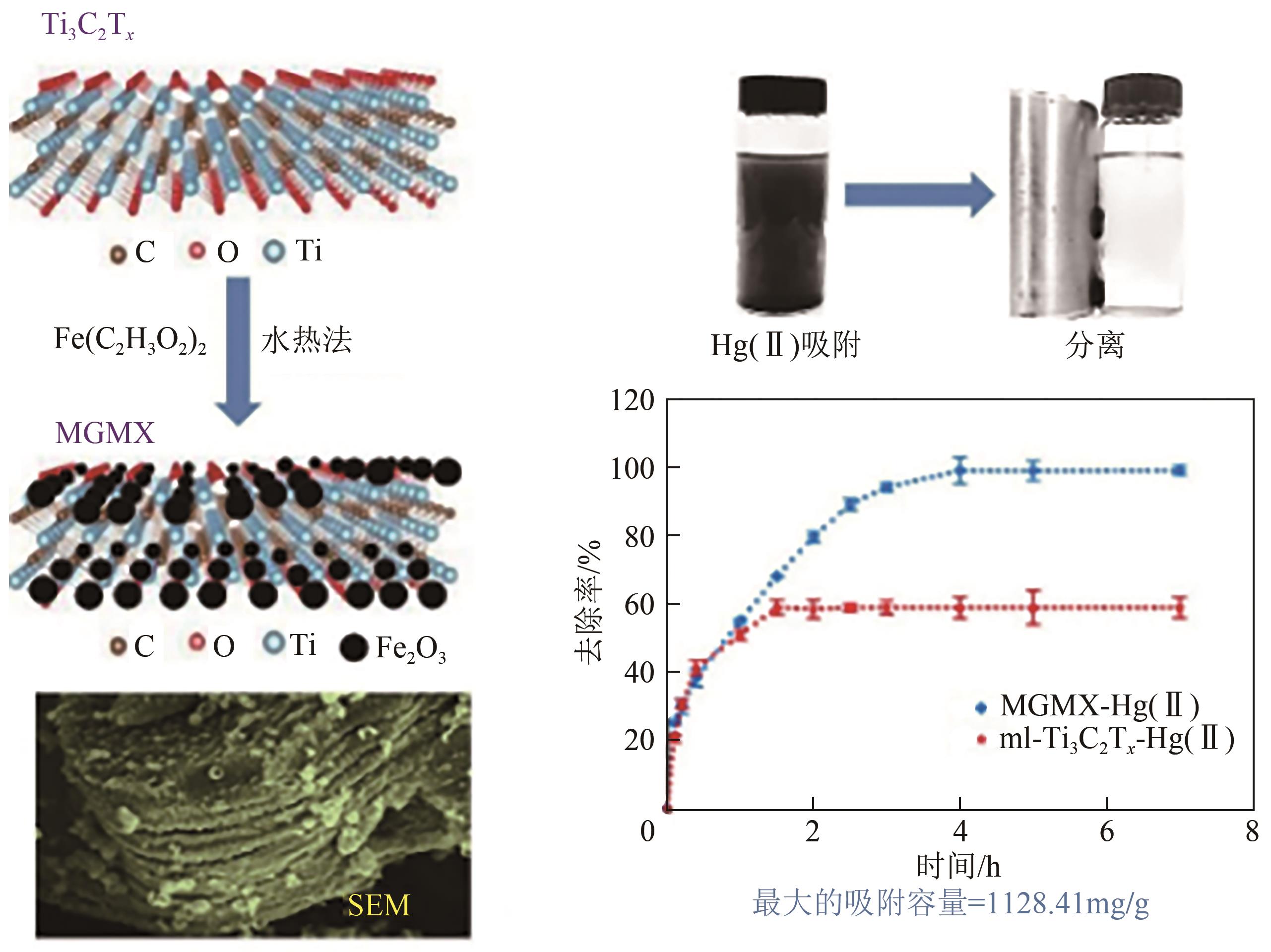
| MXenes | Ti3C2T x -MXenes/Fe3O4 | 1128.41 | [ | |
| 二维MXenes | 1057.33 | [ | ||
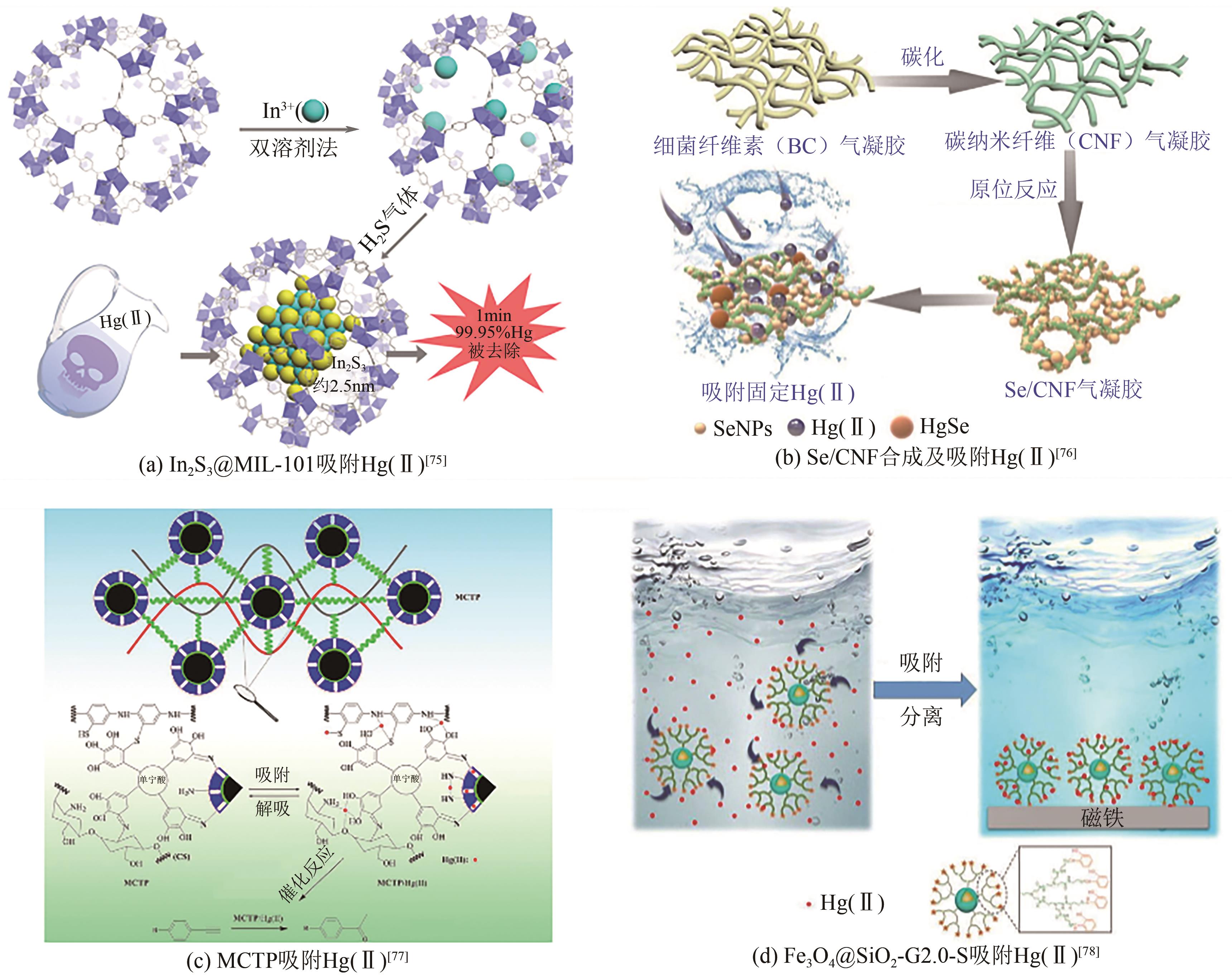
| 复合材料 | In2S3@MIL-101 | 518 | 2.2×107 | [ |
| Se/CNF | 943.4 | [ | ||
| MCTP | 515.46 | [ | ||
| Fe3O4@SiO2-G2.0-S | 605.8 | [ | ||
表4 不同材料的吸附性能
| 分类 | 材料名称 | 吸附容量 /mg·g-1 | Kd/mL·g-1 | 参考 文献 |
|---|---|---|---|---|
| MOFs | ||||
| MILs | SH-MIL-101 | 250.0 | [ | |
| MIL88A-SH | 1111.1 | 1.0×105 | [ | |
| SH-MIL-68(In) | 450.0 | [ | ||
| ZIFs | ZnS-ZIF-8 | 925.9 | [ | |
| ZIF-67 | 1740 | 2.3×104 | [ | |
| ZIF-8 | 2195.1 | 1.8×104 | [ | |
| UiO | UiO-66-SH | 785.0 | [ | |
| UiO-66-DMTD | 670.5 | [ | ||
| COFs | COF-S-SH | 1350 | 2.3×109 | [ |
| COF-SH | 1283 | [ | ||
| JNU-3 | 960 | 1.42×106 | [ | |
| JNU-4 | 561 | [ | ||
| TpTch-90 | 4270 | [ | ||
| TpTch-120 | 4277 | 4.6×106 | [ | |
| CMPs | HCMP-1 | 604 | [ | |
| CMPA-M | 975 | [ | ||
| CMPPDA | 1582.6 | 1.4×105 | [ | |
| 导电聚合物 | PPy/MAA | 1736.8 | [ | |
| PPy@L-Cyst | 2042.7 | [ | ||
| PPyCE | 684.59 | 1.79×105 | [ | |
| PPD4CBA | 1400 | 1.27×105 | [ | |
| MXenes | Ti3C2T x -MXenes/Fe3O4 | 1128.41 | [ | |
| 二维MXenes | 1057.33 | [ | ||
| 复合材料 | In2S3@MIL-101 | 518 | 2.2×107 | [ |
| Se/CNF | 943.4 | [ | ||
| MCTP | 515.46 | [ | ||
| Fe3O4@SiO2-G2.0-S | 605.8 | [ | ||
| 1 | ZHENG Keyang, ZENG Zhijun, TIAN Qianwen, et al. Epidemiological evidence for the effect of environmental heavy metal exposure on the immune system in children[J]. Science of the Total Environment, 2023, 868: 161691. |
| 2 | MAO Lulu, REN Wenbo, LIU Xitao, et al. Tracking the multiple Hg sources in sediments in a typical river-lake basin by isotope compositions and mixing models[J]. Journal of Hazardous Materials, 2023, 459: 132166. |
| 3 | DIAS Daniel, BESSA José, Susana GUIMARÃES, et al. Inorganic mercury intoxication: A case report[J]. Forensic Science International, 2016, 259: e20-e24. |
| 4 | VENKATESWARLU Sada, YOON Minyoung. Surfactant-free green synthesis of Fe3O4 nanoparticles capped with 3,4-dihydroxyphenethylcarbamodithioate: Stable recyclable magnetic nanoparticles for the rapid and efficient removal of Hg(Ⅱ) ions from water[J]. Dalton Transactions, 2015, 44(42): 18427-18437. |
| 5 | POUIL Simon, STEVENSON Louise M, Leroy GOÑEZ-RODRÍGUEZ, et al. Stannous chloride as a tool for mercury stripping in contaminated streams: Experimental assessment of toxicity in an invertebrate model species[J]. Chemosphere, 2022, 296: 133762. |
| 6 | TUNSU Cristian, WICKMAN Björn. Effective removal of mercury from aqueous streams via electrochemical alloy formation on platinum[J]. Nature Communications, 2018, 9: 4876. |
| 7 | SINGH Shalini, KUMAR Vipin, GUPTA Pratishtha, et al. The trafficking of HgⅡ by alleviating its toxicity via Citrobacter sp. IITISM25 in batch and pilot-scale investigation[J]. Journal of Hazardous Materials, 2022, 433: 128711. |
| 8 | XIAO Mingling, LAI Xiaofang, HE Jun, et al. Highly efficient removal of aqueous Hg(Ⅱ) by FeS micro-flakes[J]. Science of the Total Environment, 2023, 870: 162013. |
| 9 | FU Fenglian, WANG Qi. Removal of heavy metal ions from wastewaters: A review[J]. Journal of Environmental Management, 2011, 92(3): 407-418. |
| 10 | Ji-Hyun LIM, KANG Hee-Man, KIM Lee-Hyung, et al. Removal of heavy metals by sawdust adsorption: Equilibrium and kinetic studies[J]. Environmental Engineering Research, 2008, 13(2): 79-84. |
| 11 | FARHA Omar K, ERYAZICI Ibrahim, JEONG Nak Cheon, et al. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit?[J]. Journal of the American Chemical Society, 2012, 134(36): 15016-15021. |
| 12 | FÉREY G, MELLOT-DRAZNIEKS C, SERRE C, et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area[J]. Science, 2005, 309(5743): 2040-2042. |
| 13 | CHEN Junying, QIN Chencheng, MOU Yi, et al. Linker regulation of iron-based MOFs for highly effective Fenton-like degradation of refractory organic contaminants[J]. Chemical Engineering Journal, 2023, 459: 141588. |
| 14 | RIOU D, ROUBEAU O, FÉREY G. Composite microporous compounds. Part Ⅰ: Synthesis and structure determination of two new vanadium alkyldiphosphonates (MIL-2 and MIL-3) with three-dimensional open frameworks[J]. Microporous and Mesoporous Materials, 1998, 23(1/2): 23-31. |
| 15 | LIVAGE Carine, EGGER Chrystelle, NOGUES Marc, et al. Hybrid open frameworks (MIL-n). Part 5 Synthesis and crystal structure of MIL-9: A new three-dimensional ferrimagnetic cobalt(Ⅱ) carboxylate with a two-dimensional array of edge-sharing Co octahedra with 12-membered rings[J]. Journal of Materials Chemistry, 1998, 8(12): 2743-2747. |
| 16 | SAKAI Motomu, HORI Hayata, MATSUMOTO Takaya, et al. One-pot synthesis method of MIL-96 monolith and its CO2 adsorption performance[J]. ACS Applied Materials & Interfaces, 2023, 15(18): 22395-22402. |
| 17 | HUANGFU Chengyu, YU Shuning, TONG Bo, et al. Efficient lithium extraction from aqueous solutions by MIL-100(Fe): A study on adsorption kinetics, thermodynamics and mechanism[J]. Separation and Purification Technology, 2023, 322: 124365. |
| 18 | CHEN Xiaojuan, YAO Liang, HE Juhua, et al. Enhanced degradation of tetracycline under natural sunlight through the synergistic effect of Ag3PO4/MIL-101(Fe) photocatalysis and Fenton catalysis: Mechanism, pathway, and toxicity assessment[J]. Journal of Hazardous Materials, 2023, 449: 131024. |
| 19 | CEVALLOS-MENDOZA Jaime E, CEDEÑO-MUÑOZ Jeffrey Saúl, NAVIA-MENDOZA Jennifer Maria, et al. Development of hybrid MIL-53(Al)@CBS for ternary adsorption of tetracyclines antibiotics in water: Physical interpretation of the adsorption mechanism[J]. Bioresource Technology, 2024, 396: 130453. |
| 20 | ZHAO Shiyong, LI Yanhui, WANG Mingzhen, et al. Efficient adsorption of Congo red by micro/nano MIL-88A (Fe, Al, Fe-Al)/chitosan composite sponge: Preparation, characterization, and adsorption mechanism[J]. International Journal of Biological Macromolecules, 2023, 239: 124157. |
| 21 | YUAN Shuai, FENG Liang, WANG Kecheng, et al. Stable metal-organic frameworks: Design, synthesis, and applications[J]. Advanced Materials, 2018, 30(37): 1704303. |
| 22 | REN Xueying, WANG Chongchen, LI Yang, et al. Ag(Ⅰ) removal and recovery from wastewater adopting NH2-MIL-125 as efficient adsorbent: A 3Rs (reduce, recycle and reuse) approach and practice[J]. Chemical Engineering Journal, 2022, 442: 136306. |
| 23 | PENG Yongwu, ZHAO Meiting, CHEN Bo, et al. Hybridization of MOFs and COFs: A new strategy for construction of MOF@COF core-shell hybrid materials[J]. Advanced Materials, 2018, 30(3):1705454. |
| 24 | LI Zongchen, LIU Xuemin, JIN Wei, et al. Adsorption behavior of arsenicals on MIL-101(Fe): The role of arsenic chemical structures[J]. Journal of Colloid and Interface Science, 2019, 554: 692-704. |
| 25 | GHANBARI Taravat, ABNISA Faisal,WAN DAUD Wan Mohd Ashri. A review on production of metal organic frameworks (MOF) for CO2 adsorption[J]. Science of the Total Environment, 2020, 707: 135090. |
| 26 | FANG Ying, YANG Zhaoguang, LI Haipu, et al. MIL-100(Fe) and its derivatives: From synthesis to application for wastewater decontamination[J]. Environmental Science and Pollution Research, 2020, 27(5): 4703-4724. |
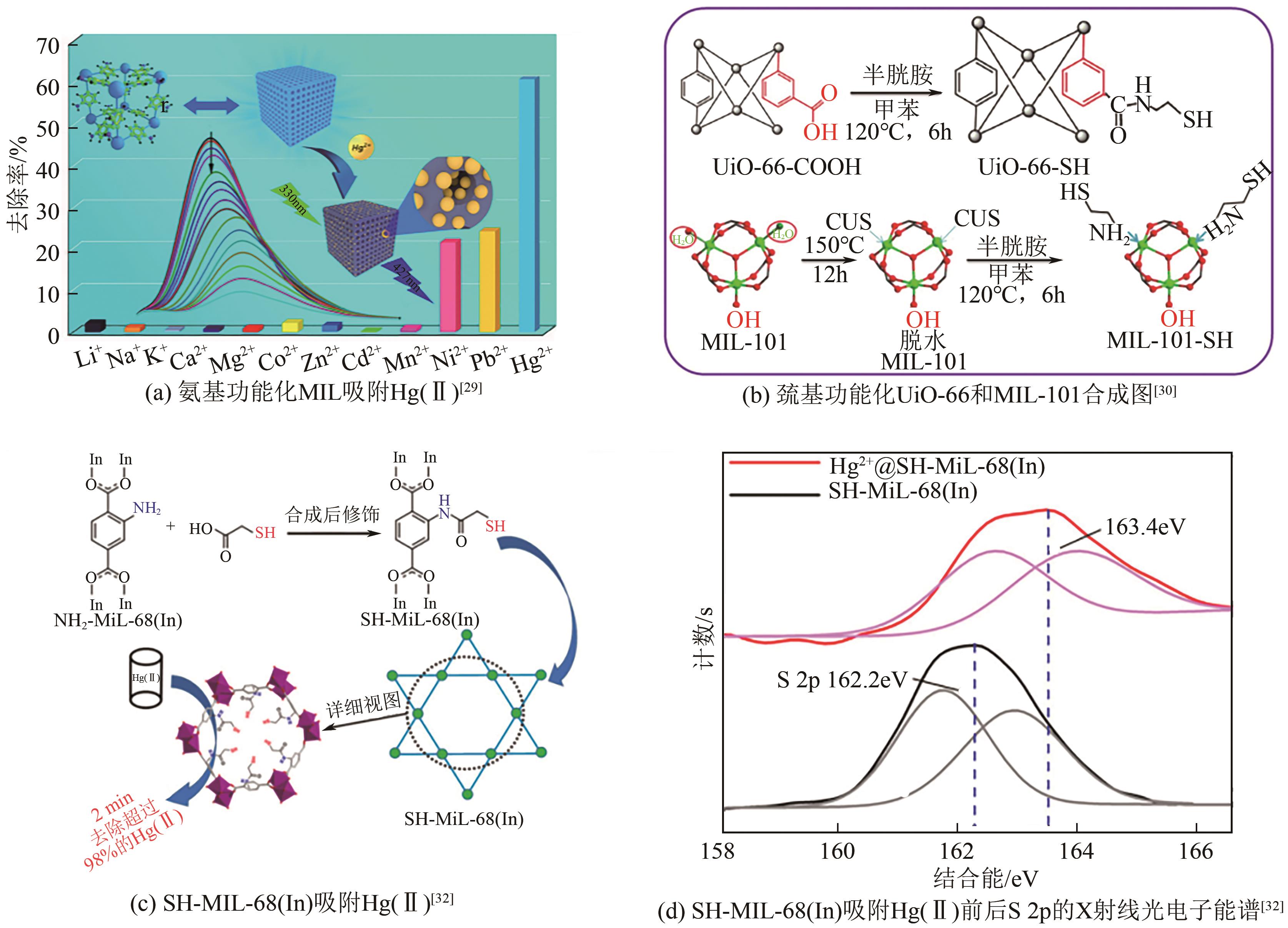
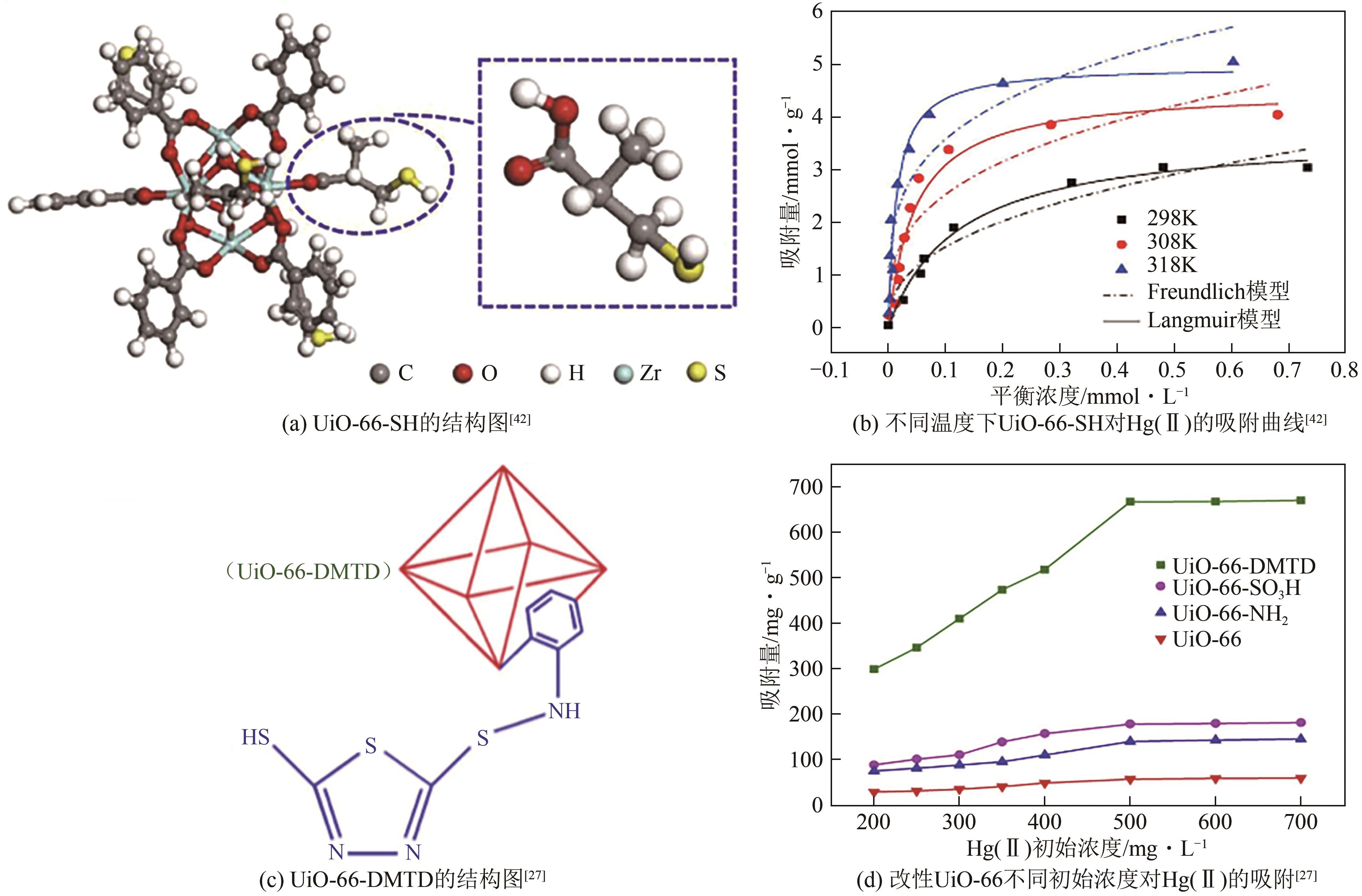
| 27 | FU Likang, WANG Shixing, LIN Guo, et al. Post-functionalization of UiO-66-NH2 by 2,5-dimercapto-1,3,4-thiadiazole for the high efficient removal of Hg(Ⅱ) in water[J]. Journal of Hazardous Materials, 2019, 368: 42-51. |
| 28 | HADAVIFAR Mojtaba, BAHRAMIFAR Nader, YOUNESI Habibollah, et al. Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups[J]. Chemical Engineering Journal, 2014, 237: 217-228. |
| 29 | ZHANG Liang, WANG Jing, DU Ting, et al. NH2-MIL-53(Al) metal-organic framework as the smart platform for simultaneous high-performance detection and removal of Hg2+ [J]. Inorganic Chemistry, 2019, 58(19): 12573-12581. |
| 30 | LIU Fengtai, XIONG Wenjing, FENG Xinrui, et al. Highly recyclable cysteamine-modified acid-resistant MOFs for enhancing Hg(Ⅱ) removal from water[J]. Environmental Technology, 2020, 41(23): 3094-3104. |
| 31 | SINGH Neha, SRIVASTAVA Ila, DWIVEDI Jaya, et al. Ultrafast removal of ppb levels of Hg(Ⅱ) and volatile Hg(0) using post modified metal organic framework[J]. Chemosphere, 2021, 270: 129490. |
| 32 | LI Gaopeng, ZHANG Kun, ZHANG Pengfeng, et al. Thiol-functionalized pores via post-synthesis modification in a metal-organic framework with selective removal of Hg(Ⅱ) in water[J]. Inorganic Chemistry, 2019, 58(5): 3409-3415. |
| 33 | FURUKAWA Hiroyasu, CORDOVA Kyle E, Michael O’KEEFFE, et al. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341(6149): 1230444. |
| 34 | BUTT Saeed Fraz, SAFDAR Muddasar, LEWIS Allana, et al. Superhydrophobic ZIF-67 with exceptional hydrostability[J]. Materials Today Advances, 2023, 20: 100448. |
| 35 | HAYASHI Hideki, CÔTÉ Adrien P, FURUKAWA Hiroyasu, et al. Zeolite A imidazolate frameworks[J]. Nature Materials, 2007, 6: 501-506. |
| 36 | PAN Yichang, HERYADI Dodi, ZHOU Feng, et al. Tuning the crystal morphology and size of zeolitic imidazolate framework-8 in aqueous solution by surfactants[J]. CrystEngComm, 2011, 13(23): 6937-6940. |
| 37 | KANETI Yusuf Valentino, DUTTA Saikat, HOSSAIN Md S A, et al. Strategies for improving the functionality of zeolitic imidazolate frameworks: Tailoring nanoarchitectures for functional applications[J]. Advanced Materials, 2017, 29(38): 1700213. |
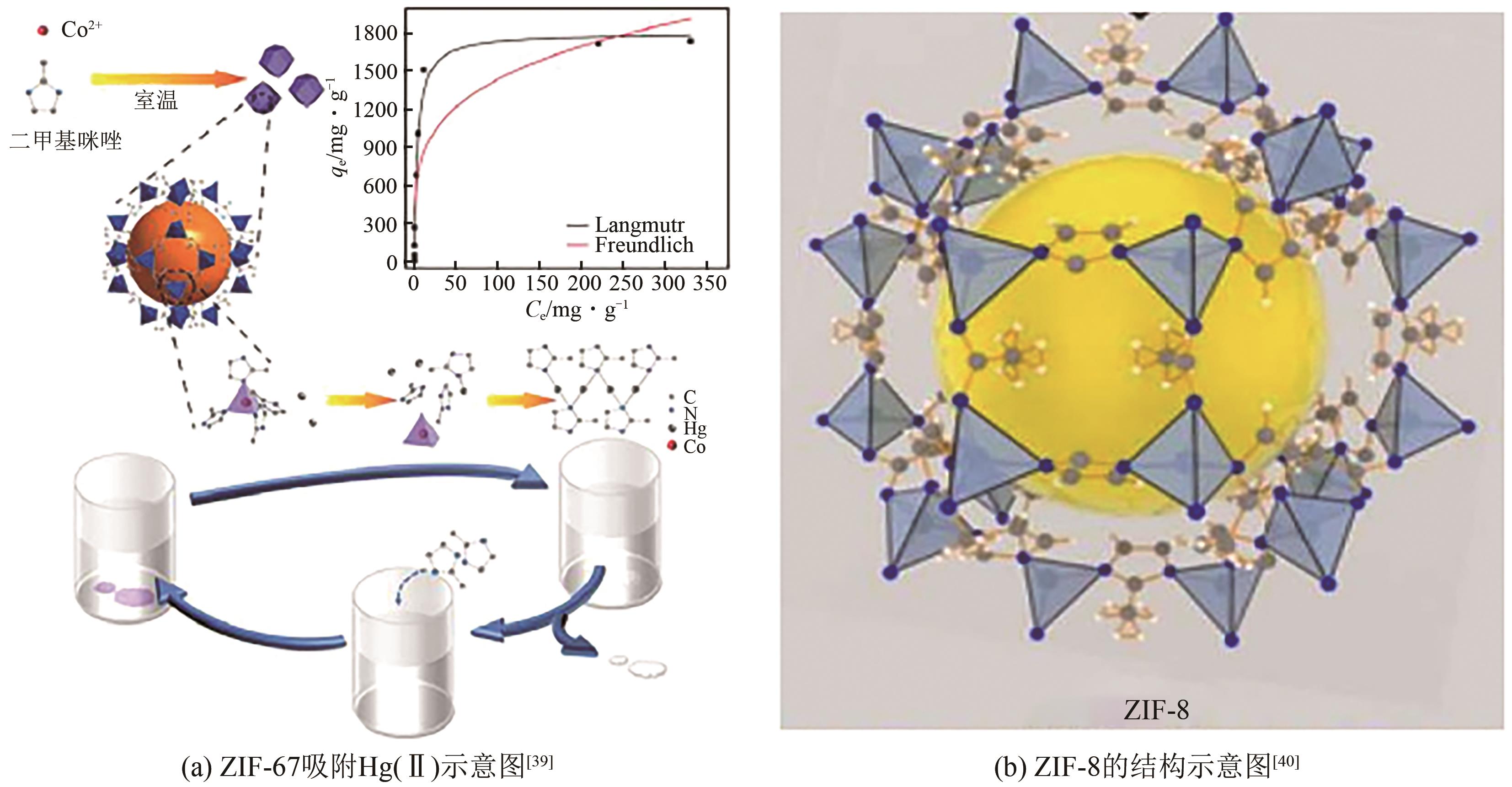
| 38 | LIU Fengtai, XIONG Wenjing, FENG Xinrui, et al. A novel monolith ZnS-ZIF-8 adsorption material for ultraeffective Hg(Ⅱ) capture from wastewater[J]. Journal of Hazardous Materials, 2019, 367: 381-389. |
| 39 | ZHOU Jiacheng, ZHANG Hao, XIE Tianying, et al. Highly efficient Hg2+ removal via a competitive strategy using a co-based metal organic framework ZIF-67[J]. Journal of Environmental Sciences, 2022, 119: 33-43. |
| 40 | LI Mingzhi, DUAN Yu, WEI Junqi, et al. One-step synthesis of ZIF-8 and anchoring it on SCB for rapid and high-capacity capture of mercury from aqueous solution[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108852. |
| 41 | CAVKA Jasmina Hafizovic, Søren JAKOBSEN, OLSBYE Unni, et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability[J]. Journal of the American Chemical Society, 2008, 130(42): 13850-13851. |
| 42 | LI Jie, LIU Yang, AI Yuejie, et al. Combined experimental and theoretical investigation on selective removal of mercury ions by metal organic frameworks modified with thiol groups[J]. Chemical Engineering Journal, 2018, 354: 790-801. |
| 43 | CHAI Xu, DONG Huaqi, ZHANG Zenggao, et al. A novel Zr-MOF modified by 4,6-diamino-2-mercaptopyrimidine for exceptional Hg(Ⅱ) removal[J]. Journal of Water Process Engineering, 2022, 46: 102606. |
| 44 | WANG Zhifang, ZHANG Yushu, WANG Ting, et al. Organic flux synthesis of covalent organic frameworks[J]. Chem, 2023, 9(8): 2178-2193. |
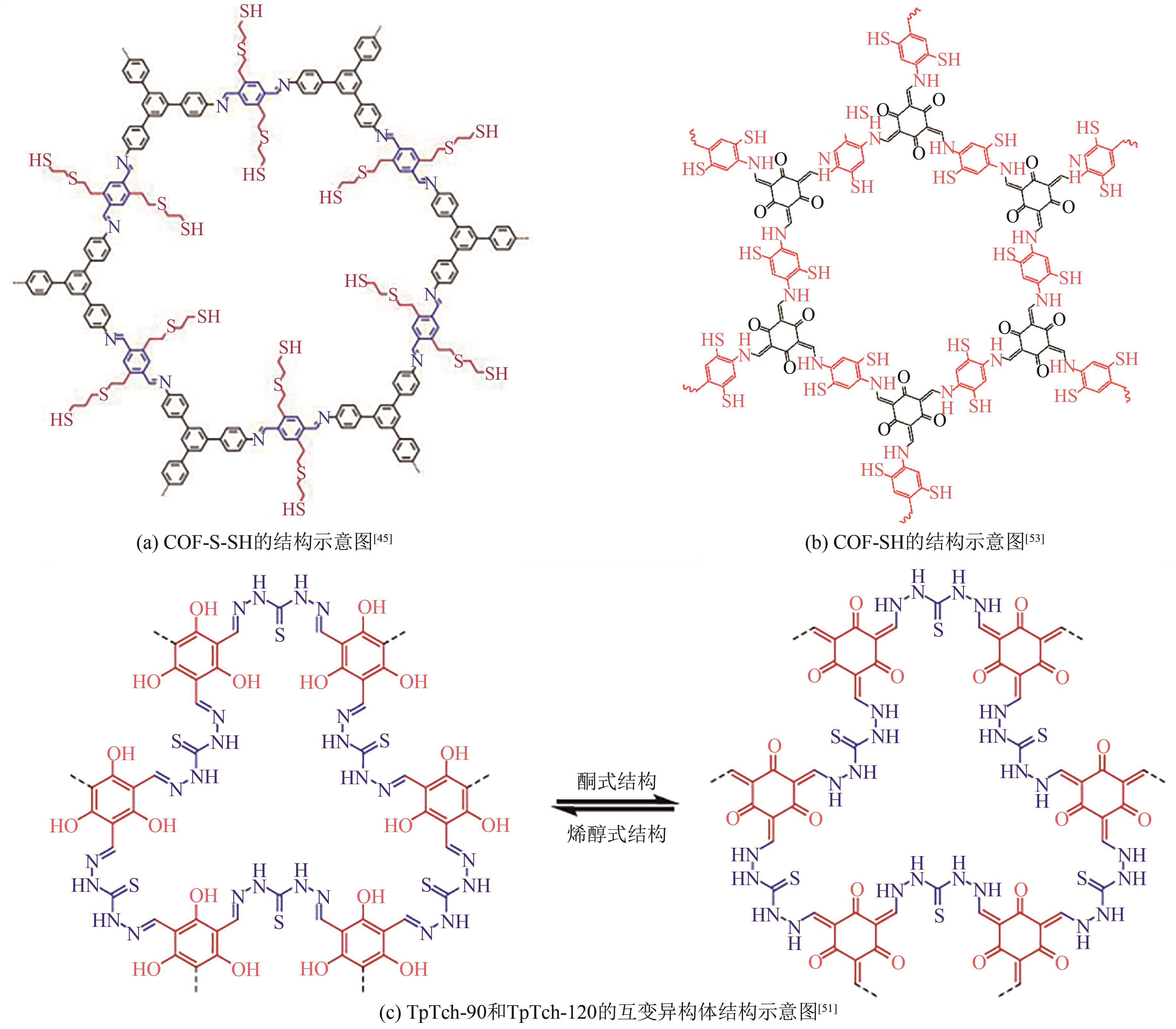
| 45 | SUN Qi, AGUILA Briana, PERMAN Jason, et al. Postsynthetically modified covalent organic frameworks for efficient and effective mercury removal[J]. Journal of the American Chemical Society, 2017, 139(7): 2786-2793. |
| 46 | HUANG Yan, HAO Xiaoqian, MA Shuanglong, et al. Covalent organic framework-based porous materials for harmful gas purification[J]. Chemosphere, 2022, 291(Pt 1): 132795. |
| 47 | GAO Ruiqi, BAI Junxian, SHEN Rongchen, et al. 2D/2D covalent organic framework/CdS Z-scheme heterojunction for enhanced photocatalytic H2 evolution: Insights into interfacial charge transfer mechanism[J]. Journal of Materials Science & Technology, 2023, 137: 223-231. |
| 48 | TRAXLER Michael, GISBERTZ Sebastian, PACHFULE Pradip, et al. Acridine-functionalized covalent organic frameworks (COFs) as photocatalysts for metallaphotocatalytic C—N cross-coupling[J]. Angewandte Chemie International Edition, 2022, 61(21): e202117738. |
| 49 | HAO Liqin, JIA Shuping, QIAO Xueling, et al. Pore geometry and surface engineering of covalent organic frameworks for anhydrous proton conduction[J]. Angewandte Chemie International Edition, 2023, 62(6): e202217240. |
| 50 | Mähringer ANDRE, MEDINA DANA D. Taking stock of stacking[J]. Nature Chemistry, 2020, 12(11): 985-987. |
| 51 | CÔTÉ Adrien P, BENIN Annabelle I, OCKWIG Nathan W, et al. Porous, crystalline, covalent organic frameworks[J]. Science, 2005, 310(5751): 1166-1170. |
| 52 | DING Sanyuan, DONG Ming, WANG Yawen, et al. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(Ⅱ)[J]. Journal of the American Chemical Society, 2016, 138(9): 3031-3037. |
| 53 | MA Zhiyao, LIU Fuyang, LIU Nengsheng, et al. Facile synthesis of sulfhydryl modified covalent organic frameworks for high efficient Hg(Ⅱ) removal from water[J]. Journal of Hazardous Materials, 2021, 405: 124190. |
| 54 | QIAN Hailong, ZHU Mengsi, DU Meilan, et al. Engineering linkage as functional moiety into irreversible thiourea-linked covalent organic framework for ultrafast adsorption of Hg(Ⅱ)[J]. Journal of Hazardous Materials, 2022, 427: 128156. |
| 55 | HUSSAIN Muzammil, MAILE Nagesh, TAHIR Khurram, et al. Flexible thiourea-based covalent organic frameworks for ultrahigh mercury removal from aqueous solutions[J]. Chemical Engineering Journal, 2022, 446: 137410. |
| 56 | 王玉冰, 陈杰, 延卫, 等. 共轭微孔聚合物的制备与应用[J]. 化学进展, 2021, 33(5): 838-854. |
| WANG Yubing, CHEN Jie, YAN Wei, et al. Preparation and application of conjugated microporous polymers[J]. Progress in Chemistry, 2021, 33(5): 838-854. | |
| 57 | JIANG Jiaxing, SU Fabing, TREWIN Abbie, et al. Conjugated microporous poly(aryleneethynylene) networks[J]. Angewandte Chemie International Edition, 2007, 46(45): 8574-8578. |
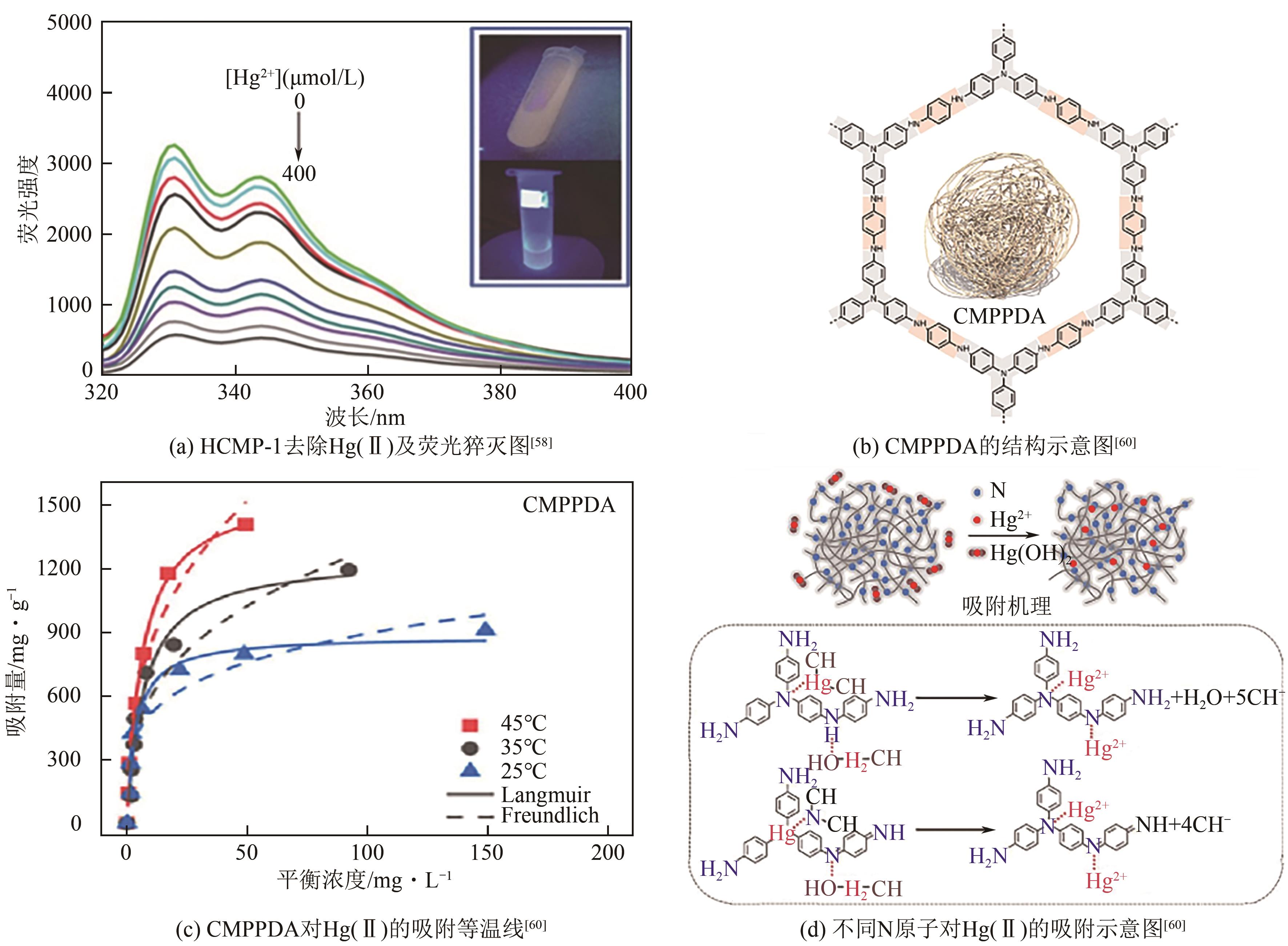
| 58 | XIANG Lu, ZHU Yunlong, GU Shuai, et al. A luminescent hypercrosslinked conjugated microporous polymer for efficient removal and detection of mercury ions[J]. Macromolecular Rapid Communications, 2015, 36(17): 1566-1571. |
| 59 | LOU Xiaoyu, CHEN Jie, XIONG Zhuo, et al. Porosity design on conjugated microporous poly(aniline)-S for exceptional mercury(Ⅱ) removal[J]. ACS Applied Materials & Interfaces, 2021, 13(51): 61653-61660. |
| 60 | WANG Yubing, LI Shanshan, WU Xiaoxi, et al. Nitrogen-based conjugated microporous polymers for efficient Hg(Ⅱ) removal from water: Performance and mechanism[J]. Chemical Engineering Journal, 2023, 471: 144659. |
| 61 | ZHANG Yongzheng, CAO Zhenjiang, LIU Sijin, et al. Charge-enriched strategy based on MXene-based polypyrrole layers toward dendrite-free zinc metal anodes[J]. Advanced Energy Materials, 2022, 12(13): 2103979. |
| 62 | WEI Jiang, JIA Jia, LIAO Tong. Highly selective adsorption of dyes by functional hypercrosslinked-polymers prepared in a facile and chemically stable manner[J]. Journal of Environmental Chemical Engineering, 2023, 11(5): 110555. |
| 63 | LI Bin, LIU Shude, GENG Yifei, et al. Achieving stable zinc metal anode via polyaniline interface regulation of Zn ion flux and desolvation[J]. Advanced Functional Materials, 2024, 34(5): 2214033. |
| 64 | KHOKHAR Deepali, JADOUN Sapana, ARIF Rizwan, et al. Facile synthesis of the chemically oxidative grafted copolymer of 2,6-diaminopyridine (DAP) and thiophene (Th) for optoelectronic and antioxidant studies[J]. Journal of Molecular Structure, 2022, 1248: 131453. |
| 65 | AHMAD Nafees, ANAE Jerry, KHAN Mohammad Zain, et al. Visible light-conducting polymer nanocomposites as efficient photocatalysts for the treatment of organic pollutants in wastewater[J]. Journal of Environmental Management, 2021, 295: 113362. |
| 66 | ZHOU Tingting, LIANG Qianwei, ZHOU Xin, et al. Enhanced removal of toxic hexavalent chromium from aqueous solution by magnetic Zr-MOF@polypyrrole: Performance and mechanism[J]. Environmental Science and Pollution Research, 2021, 28(11): 13084-13096. |
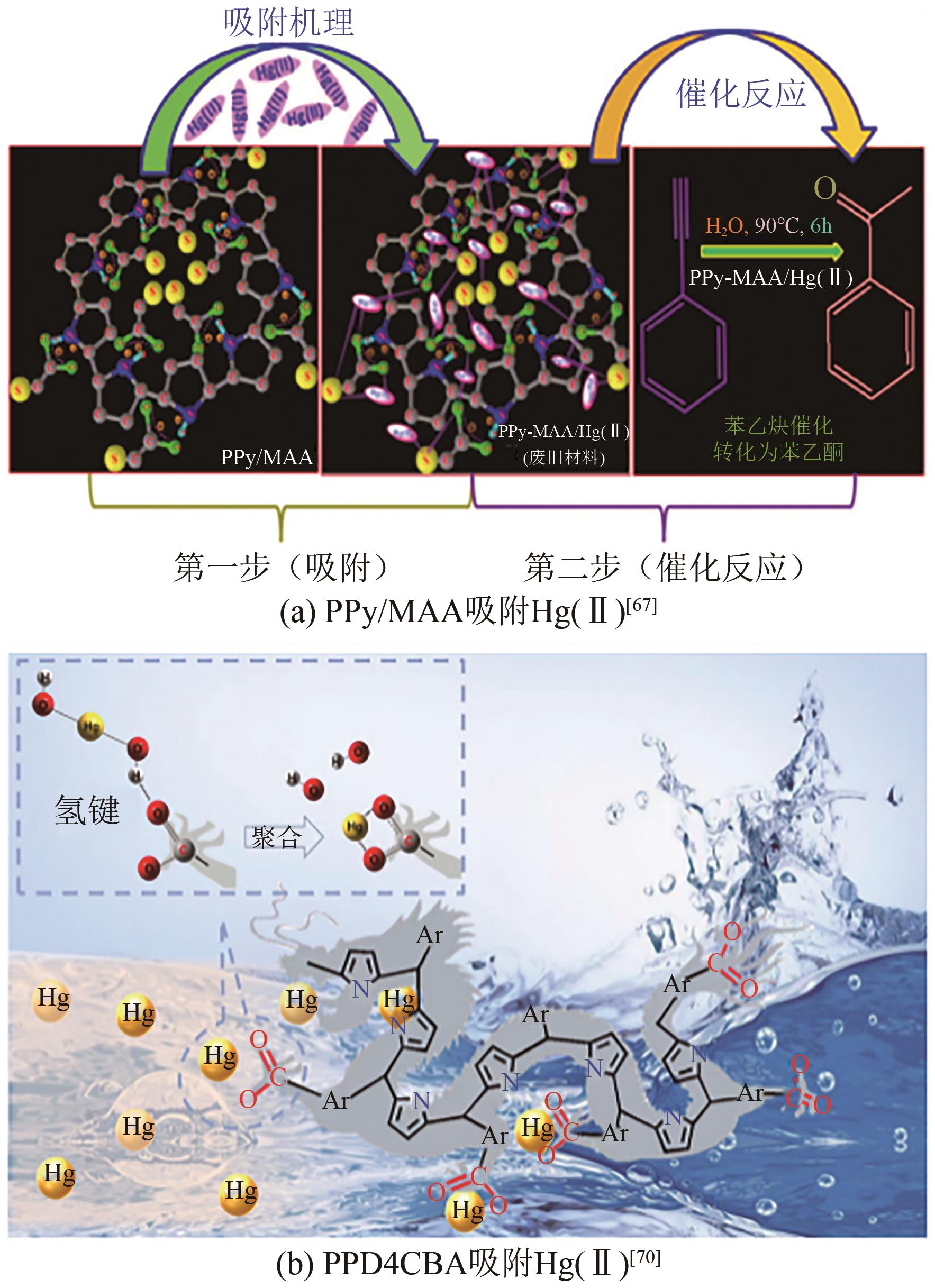
| 67 | Raghunath DAS, GIRI Somnath, MULIWA Anthony M, et al. High-performance Hg(Ⅱ) removal using thiol-functionalized polypyrrole (PPy/MAA) composite and effective catalytic activity of Hg(Ⅱ)-adsorbed waste material[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 7524-7536. |
| 68 | BALLAV Niladri, Raghunath DAS, GIRI Somnath, et al. L-cysteine doped polypyrrole (PPy@L-Cyst): A super adsorbent for the rapid removal of Hg2+ and efficient catalytic activity of the spent adsorbent for reuse[J]. Chemical Engineering Journal, 2018, 345: 621-630. |
| 69 | GUO Ziyu, WANG Zhenyu, LIU Jinbo, et al. Efficient mercury(Ⅱ) capture by functionalized poly(pyrrole methane)s: The role of chloro and imino groups[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 658: 130694. |
| 70 | WANG Zhenyu, LIU Yunpeng, ZHANG Wenlong, et al. Selective mercury adsorption and enrichment enabled by phenylic carboxyl functionalized poly(pyrrole methane)s chelating polymers[J]. Science of the Total Environment, 2023, 858: 159870. |
| 71 | WANG Lin, SONG Huan, YUAN Liyong, et al. Efficient U(Ⅵ) reduction and sequestration by Ti2CT x MXene[J]. Environmental Science & Technology, 2018, 52(18): 10748-10756. |
| 72 | LIAO Mingjia, ZHENG Zhili, JIANG Haiyang, et al. MXenes as emerging adsorbents for removal of environmental pollutants[J]. Science of the Total Environment, 2024, 912: 169014. |
| 73 | SHAHZAD Asif, RASOOL Kashif, MIRAN Waheed, et al. Mercuric ion capturing by recoverable titanium carbide magnetic nanocomposite[J]. Journal of Hazardous Materials, 2018, 344: 811-818. |
| 74 | HU Xiaolan, CHEN Changhong, ZHANG Dawei, et al. Kinetics, isotherm and chemical speciation analysis of Hg(Ⅱ) adsorption over oxygen-containing MXene adsorbent[J]. Chemosphere, 2021, 278: 130206. |
| 75 | LIANG Linfeng, LIU Luyao, JIANG Feilong, et al. Incorporation of In2S3 nanoparticles into a metal-organic framework for ultrafast removal of Hg from water[J]. Inorganic Chemistry, 2018, 57(9): 4891-4897. |
| 76 | ZHAO Dongmin, LI Zhuoyan, ZHU Kaini, et al. Highly dispersed amorphous nano-selenium functionalized carbon nanofiber aerogels for high-efficient uptake and immobilization of Hg(Ⅱ) ions[J]. Journal of Hazardous Materials, 2024, 465: 133162. |
| 77 | FU Yong, SUN Yu, ZHENG Yutong, et al. New network polymer functionalized magnetic-mesoporous nanoparticle for rapid adsorption of Hg(Ⅱ) and sequential efficient reutilization as a catalyst[J]. Separation and Purification Technology, 2021, 259: 118112. |
| 78 | ZHOU Yunzhe, LUAN Liping, TANG Bentian, et al. Fabrication of Schiff base decorated PAMAM dendrimer/magnetic Fe3O4 for selective removal of aqueous Hg(Ⅱ)[J]. Chemical Engineering Journal, 2020, 398: 125651. |
| 79 | HUANG Lijin, HE Man, CHEN Beibei, et al. A mercapto functionalized magnetic Zr-MOF by solvent-assisted ligand exchange for Hg2+ removal from water[J]. Journal of Materials Chemistry A, 2016, 4(14): 5159-5166. |
| [1] | 王丽娜, 武金升. 共价有机框架材料的合成与应用研究进展[J]. 化工进展, 2024, 43(7): 3834-3856. |
| [2] | 王涛, 高翔, 高继峰, 邓海全, 余显涌, 周振华, 唐玲, 吕航. 改性Cu-BTC基混合基质膜在CO2分离中的应用[J]. 化工进展, 2024, 43(6): 3240-3246. |
| [3] | 苏士焜, 刘唐, 金也, 郑金玉. 氢气纯化吸附材料研究进展[J]. 化工进展, 2024, 43(10): 5612-5632. |
| [4] | 候林丽, 张梦玲, 郎锋祥, 郑希怡, 刘利民. 共价有机框架材料在环境修复领域中吸附有机污染物的研究进展[J]. 化工进展, 2024, 43(10): 5837-5856. |
| [5] | 汪尚, 姚瑶, 王佳, 董迪迪, 常刚刚. CoZn-MOF衍生多级孔取向碳载CoP及其析氢性能[J]. 化工进展, 2024, 43(1): 447-454. |
| [6] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [7] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [8] | 潘宜昌, 周荣飞, 邢卫红. 高效分离同碳数烃的先进微孔膜:现状与挑战[J]. 化工进展, 2023, 42(8): 3926-3942. |
| [9] | 毛梦雷, 孟令玎, 高蕊, 孟子晖, 刘文芳. 多孔框架材料固定化酶研究进展[J]. 化工进展, 2023, 42(5): 2516-2535. |
| [10] | 常晓青, 彭东来, 李东洋, 张延武, 王景, 张亚涛. MOFs基丙烯/丙烷高效分离混合基质膜研究进展[J]. 化工进展, 2023, 42(4): 1961-1973. |
| [11] | 陈飞, 丁玉栋, 马丽娇, 朱恂, 程旻, 廖强. 微波合成MOF-808及其水蒸气捕集特性[J]. 化工进展, 2023, 42(12): 6461-6468. |
| [12] | 曹明敏, 韩铖乐, 杨芳, 陈玉焕. 离子液体/金属有机框架复合材料捕集分离CO2[J]. 化工进展, 2023, 42(11): 5831-5841. |
| [13] | 马文杰, 姚卫棠. 共价有机框架(COFs)在锂离子电池中的应用[J]. 化工进展, 2023, 42(10): 5339-5352. |
| [14] | 张金辉, 张焕, 朱新锋, 宋忠贤, 康海彦, 刘红盼, 邓炜, 侯广超, 李桂亭, 黄真真. UiO-66复合材料用于典型有机污染物吸附和光催化氧化的研究进展[J]. 化工进展, 2023, 42(1): 445-456. |
| [15] | 边宇, 张百超, 郑红. 多级孔COFs材料的设计、合成及应用[J]. 化工进展, 2022, 41(9): 4866-4883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||